静电纺丝_最终版
静电纺丝

静电纺丝技术制备聚合物超细纤维1.电纺过程处在导电体尖端的聚合物熔体或溶液液滴,在高压电场的作用下,流体克服表面张力的作用形成直径微小的射流,快速地射向电场的另一极。
射流在从电场的一极向另一极的高速运动过程中,迅速地冷却凝固,或者溶剂迅速挥发而固化,最终以直径超细的纤维形式沉积在电场的另一极。
图1. (左)静电纺丝示意图和(右)电纺纤维的扫描电镜图2.静电纺丝技术的起源“静电纺丝”一词来源于“electrospinning”或更早一些的“electrostatic spinning”,国内一般简称为“静电纺”、“电纺”等。
1934年,Formalas发明了用静电力制备聚合物纤维的实验装置并申请了专利,其专利公布了聚合物溶液如何在电极间形成射流,这是首次详细描述利用高压静电来制备纤维装置的专利,被公认为是静电纺丝技术制备纤维的开端。
但是,从科学基础来看,这一发明可视为静电雾化或电喷的一种特例,其概念可以追溯到1745年。
静电雾化与静电纺丝的最大区别在于二者采用的工作介质不同,静电雾化采用的是低粘度的牛顿流体,而静电纺丝采用的是较高粘度的非牛顿流体。
这样,静电雾化技术的研究也为静电纺丝体系提供了一定的理论依据和基础。
对静电纺丝过程的深入研究涉及到静电学、电流体力学、流变学、空气动力学等领域。
20世纪30年代到80年代期间,静电纺丝技术发展较为缓慢,科研人员大多集中在静电纺丝装置的研究上,发布了一系列的专利,但是尚未引起广泛的关注。
进入90年代,美国阿克隆大学Reneker研究小组对静电纺丝工艺和应用展开了深入和广泛的研究。
特别是近年来,随着纳米技术的发展,静电纺丝技术获得了快速发展,世界各国的科研界和工业界都对此技术表现出了极大的兴趣。
此段时期,静电纺丝技术的发展大致经历了四个阶段:1)第一阶段主要研究不同聚合物的可纺性和纺丝过程中工艺参数对纤维直径及性能的影响以及工艺参数的优化等;2)第二阶段主要研究静电纺纳米纤维成分的多样化及结构的精细调控;3)第三个阶段主要研究静电纺纤维在能源、环境、生物医学、光电等领域的应用;4)第四阶段主要研究静电纺纤维的批量化制造问题。
静电纺丝技术

静电纺丝技术静电纺丝技术是利用高压静电作用使聚合物溶液或熔体带电并发生形变,在喷头末端处形成悬垂的锥状液滴,当液滴表面静电斥力大于其表面张力时,液滴表面就会喷射出高速飞行的射流,并在较短的时间内经电场力拉伸、溶剂挥发、聚合物固化形成纤维。
所获得的静电纺纤维直径小、比表面积大,同时纤维膜还具有孔径小、孔隙率高、孔道连通性好等优势,在过滤、传感、医疗卫生以及自清洁等领域具有广泛的应用。
1静电纺丝的起源与发展静电纺丝起源于200多年前人们对静电雾化过程的研究。
1745年,Bose通过对毛细管末端的水表面施加高电势,发现其表面将会有微细射流喷出,从而形成高度分散的气溶胶,并得出该现象是由液体表面的机械压力与电场力失衡所引起的。
1882年,Rayleigh指出当带电液滴表面的电荷斥力超过其表面张力时,就会在其表面形成微小的射流,并对该现象进行理论分析总结,得到射流形成的临界条件。
1902年,Cooley与Morton申请了第一个利用电荷对不同挥发性液体进行分散的专利。
随后Zeleny研究了毛细管端口处液体在高压静电作用下的分裂现象,通过观察总结出几种不同的射流形成模型,认为当液滴内压力与外界施加压力相等时,液滴将处于不稳定状态。
基于上述的基础研究,1929年,Hagiwara公开了一种以人造蚕丝胶体溶液为原料,通过高压静电制备人造蚕丝的专利。
1934年,Formhals设计了一种利用静电斥力来生产聚合物纤维的装置并申请了专利,该专利首次详细介绍了聚合物在高压电场作用下形成射流的原因,这被认为是静电纺丝技术制备纤维的开端。
从此,静电纺丝技术成为了一种制备超细纤维的有效可行方法。
1966年,Simons发明了一种生产静电纺纤维的装置,获得了具有不同堆积形态的纤维膜。
20世纪60年代,Taylor在研究电场力诱导液滴分裂的过程中发现,随着电压升高,带电液体会在毛细管末端逐渐形成一个半球形状的悬垂液滴,当液滴表面电荷斥力与聚合物溶液表面张力达到平衡时,带电液滴会变成圆锥形;当电荷斥力超过表面张力时,就会从圆锥形聚合物液滴表面喷射出液体射流。
静电纺丝

Carbohydrate Polymers 92 (2013) 1012–1017Contents lists available at SciVerse ScienceDirectCarbohydratePolymersj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /c a r b p olAntimicrobial activity of carboxymethyl chitosan/polyethylene oxide nanofibers embedded silver nanoparticlesMoustafa M.G.Fouda a ,b ,∗,M.R.El-Aassar c ,Salem S.Al-Deyab aaPetrochemical Research Chair,Chemistry Department,College of Science,King Saud University,P.O.Box 2455,Riyadh 11451,Saudi Arabia bTextile Research Division,National Research Center,Dokki,Cairo,P.O.Box 12622,Giza 12522,Egypt cPolymer Materials Research Department,Institute of Advanced Technology and New Material,City of Scientific Research and Technology Applications,New Borg El-Arab City 21934,Alexandria,Egypta r t i c l ei n f oArticle history:Received 22September 2012Received in revised form 10October 2012Accepted 19October 2012Available online 26 October 2012Keywords:ChitosanPolyethylene oxide Silver nanoparticles Electrospinning Antimicrobiala b s t r a c tA facile method to synthesize silver nanoparticles (AgNPs)using carboxymethyl chitosan (CMCTS),which act as reducing agent for silver ions as well as protecting agent for the formed AgNPs,is reported.CMCTS embedded AgNPs are mixed with polyethylene oxide (PEO).The blend polymers containing AgNPs are electrospun resulting in blend nano-fiber mats.The formation of AgNPs has been confirmed using UV–vis and TEM.The diameter range of 12–18nm of well-dispersed AgNPs with a concentration of 100ppm was obtained.The electrospun mats are characterized using SEM,EDX as well as TGA.Antimicrobial activity against different species of pathogenic/nonpathogenic;Staphylococcus aureus ATCC 25923,Pseudomonas aeruginosa ATCC 27853and Escherichia coli ATCC 25922in addition to the fungus Candida albicans ATCC 10231was studied.The results show excellent antimicrobial activity compared with nanofibers without AgNPs and AgNPs alone.© 2012 Elsevier Ltd. All rights reserved.1.IntroductionIn electrospinning,the simplest and most cost-effective method of fabricating polymer nanofibres,various polymers have been electrospun into ultrafine fibers with diameters range of 20–400nm (Huang,Zhang,&Kotaki,2003).In electrospinning,the polymer solution is placed into syringe with millimeter size nozzle.Strong electric field is applied on a droplet of polymer solution held by its surface tension at the tip of a syringe’s needle.As a result,the pendent drop becomes highly electrified and the induced charges are distributed over its surface.Increasing the intensity of electric field,the surface of the liquid drop will be distorted to a conical shape known as the Taylor cone (Taylor,1969).Once the electric field strength exceeds a threshold value,the repulsive electric force dominates the surface tension of the liquid and a stable jet emerges from the cone tip.The charged jet is then accelerated toward the target and rapidly thins and dries as a result of elongation and sol-vent evaporation.As the jet diameter decreases,the surface charge density increases and the resulting high repulsive forces split the jet to smaller jets.Ultimately,solidification occurs and fibers are deposited on the surface of the collector as randomly oriented∗Corresponding author at:Petrochemical Research Chair,Chemistry Department,College of Science,King Saud University,P.O.Box 2455,Riyadh 11451,Saudi Arabia.E-mail addresses:m gaballa@ ,mmfoudah@.sa (M.M.G.Fouda).nonwoven mats (Derch,Greiner,&Wendorff,2004).Besides charge density and applied voltage other parameters also influence the final nanofibrous structure and its properties,for example:polymer types and concentration,type of solvent,presence of electrolyte,type and concentration of electrolyte,viscosity,surface tension,tip-to-collector distance,flow rate of the polymer solution,inner diameter of the tip,material of the tip,etc.The field of nanoparticle research has witnessed tremendous growth due to the unique chemical and physical properties from the bulk.Silver nanoparticles have gained considerable attention today due to their potential applications in medical field,since it has been widely used in the production of biodegradable surgical sutures.Recently,electrospun nanofibers embedded AgNPs have a great antimicrobial potential.Different methods have been used to prepare AgNPs,where one of these is chemical reduction method in which the polymer can be used as both reducing and stabilizing agents for the formed AgNPs (Abou-Okeil,2012;Abou-Okeil,Amr,&Abdel-Mohdy,2012;El-Rafie et al.,2011;Hebeish,El-Naggar,et al.,2011;Textor,Fouda,&Mahltig,2010).Chitosan is one of the most important biopolymers,obtained from chitin,the second most abundant natural polysaccharide present on the earth next to cellulose (El-Shafei,Fouda,Knittel,&Schollmeyer,2008;Hebeish,Abdel-Mohdy,et al.,2011).The poor solubility of chitosan in water,due to its rigid crystalline structure,limits its effective utilization in electrospinning process.To overcome this drawback,it is necessary to convert chitosan to water-soluble derivative (El-Shafei et al.,2008).Chemical0144-8617/$–see front matter © 2012 Elsevier Ltd. All rights reserved./10.1016/j.carbpol.2012.10.047M.M.G.Fouda et al./Carbohydrate Polymers92 (2013) 1012–10171013Fig.1.Schematic diagram of the typical electrospinning setup.modification is anticipated to be quite promising.Carboxymethy-lation is one of the chemical methods to prepare water-soluble chi-tosan.This reaction takes place preferentially either at C-6hydroxyl groups or at the NH2-group resulting in N/O–carboxymethyl chi-tosan(CMCTS).Both products are water-soluble and contain an amino group either as the primary(NH2)or as secondary amine (NH CH2COOH).Polyethylene oxide(PEO)is one of the few biodegradable syn-thetic polymers approved for internal use in food,cosmetics, personal care products,and pharmaceutical.PEO is an effective ion conductive polymer(Morgado et al.,1999).Therefore,it is added in order to enhance the spinnability of the modified natural polymer.The objective of this research work is to synthesize well-stabilized AgNPs using CMCTS followed by electrospinning of CMCTS–AgNPs/PEO solution.The structure,morphology and the antimicrobial activity of the resulted nanofiber mats are character-ized.2.Experimental2.1.MaterialsPolyethylene oxide(PEO)(≥95%,average Mw124–186kg/mol) was obtained from Scientific Polymer Products,Inc.Silver nitrate (AgNO3)(99.998%)was purchased from Aldrich,Germany.Chi-tosan,DDA95%was obtained from Aldrich Chemical Co.,Germany. All other solvents and reagents were used as received without any further purification.2.2.Synthesis of carboxymethyl chitosan(CMCTS)The experimental technique adopted for carboxymethylation of chitosan was as follows:certain volume of sodium hydroxide solution(30%,w/v)was added to16g chitosan suspended in iso-propyl alcohol.The mixture was left under stirring for30min at room temperature.To this mixture34g of monochloroacetic acid was added and the content of theflask was subjected to continuous stirring for3h.At the end,the excess alkali was neutralized using glacial acetic acid and chitosan was precipitated by adding acetone. Finally,modified chitosan wasfiltered and washed with isopropyl alcohol/water(70:30)several times and dried at60◦C(El-Shafei et al.,2008).Thefinal product was soluble in water.2.3.Synthesis of silver nanoparticles(AgNPs)Silver nanoparticles(AgNPs)were prepared according to the procedure described by El-Rafie et al.(2011)and can be summa-rized as follows:0.5g of CMCTS is dissolved in100ml of distilled water,the temperature of the reaction is raised to60◦C and the pH is adjusted to11.5by using10M NaOH.1ml of AgNO3(1.7M) is added dropwise to the previous solution under continuous stir-ring for almost1h.The formation of silver nanoparticles solution was observed by monitoring the color change(visually,when the color of the solution started to change from its original color to the different degrees of the yellow color,then the reduction reac-tion started to work and silver nanoparticles started to seed).The AgNPs formed are characterized by(UV–vis),transmission electron microscope(TEM).2.4.Electrospinning of CMCTS–AgNPs/PEO solution2g of CMCTS is added to CMCTS solution containing sil-ver nanoparticles(0.5wt%)while stirring.To this solution,PEO (5wt%)is added slowly under continuous stirring till homogeneity occurs.Electrospinning of the prepared blend polymers solution containing AgNPs was carried out using two different methods; typical electrospinning technique and Nanospider technology as a modified electrospinning technique.A schematic diagram of the complete electrospinning apparatus is shown in Fig.1.It consisted of a syringe and stainless needle,a grounded electrode,an iron plate covered by aluminum foil as a collector,and an adjustable high voltage supply.2.5.Testing and analysisUV–vis spectrum was used to prove the formation of AgNPs (Hebeish,El-Naggar,et al.,2011).Particle shapes and sizes of AgNPs were obtained by transmission electron microscope(TEM); JEOL-JEM-1230.Scanning electron microscope(SEM)(JEOL GSM-6610LV)and(JEOL GSM-7600F)field emission scanning electron microscope were used to study the surface characteristics of elec-trospun nanofibers.Specimen in the form offilms was mounted on the specimen stabs and coated with thinfilm of gold by the sputtering method.The micrograph was taken at magnifica-tion of1000×using(KV)accelerating voltage.FT-IR spectra were obtained using FT-IR spectrometer,Bruker,TENSOR Series FT-IR1014M.M.G.Fouda et al./Carbohydrate Polymers 92 (2013) 1012–1017Fig.2.Solid state 13C NMR spectrum typical for O -carboxymethyl chitosan.Spectrometer,Germany,connected to a PC,and the data were analyzed by IR Solution software,where analytical methods are standard in OPUS TM software.2.6.Antimicrobial evaluation of electrospun nanofibersIn order to evaluate the antimicrobial properties of electrospun nanofiber mats with/without AgNPs against microbial pathogens and to compare this effect with the commonly used antibiotics in addition to AgNPs alone as +ve control,the zone of inhibition test was performed against the gram positive bacterium Staphylococ-cus aureus ATCC 25923,the gram negative bacteria Pseudomonas aeruginosa ATCC 27853and Escherichia coli ATCC 25922in addi-tion to the fungus Candida albicans ATCC 10231.To perform the test,several colonies of each strain,obtained from a fresh cul-ture in blood agar plate,were suspended in 5ml of Mueller-Hinton broth to achieve turbidity equal to the 0.5Mac-Farland standards.The suspensions were inoculated with sterile swabs onto 150mm diameter Mueller-Hinton agar plates and after the agar surfaces were allowed to dry,tested disks were applied on each plate.Plates were incubated at 37◦C for 24h and the zones of inhibition (IZ)were measured.Same was performed for the Candida except that it was inoculated in Sabouraud dextrose agar medium and incu-bated for 2–3days at 37◦C.The antimicrobial agents tested in this study were CMCTS–PEO nano-fiber incorporated with silver nano-particles (CMCTS–AgNPs/PEO),the antibiotic Amikacin (AK),ampicillin/clavulinic acid (AMC),100ppm AgNPs solution (10L per disk)as a positive control,in addition to negative controls as empty disks of CMCTS–PEO nano-fibers.3.Results and discussion3.1.Characterization of (CMCTS)by solid state 13C NMRCarboxymethylation of chitosan (CTS)is achieved with monochloroacetic acid and sodium hydroxide.According to El-Shafei et al.(2008)this reaction takes place preferentially either at C-6hydroxyl groups or at the NH 2-group resulting in N/O–carboxymethyl chitosan (CMCTS).The solid state 13C NMR spectrum for a typical N-carboxymethyl chitosan shows sig-nals attributed to the N-carboxymethyl substituent,at 47.7and 168.7ppm,for N CH 2and COOH,respectively (El-Shafei et al.,2008).But in case of our results,the solid state 13C NMR described in Fig.2shows signals at 73and 175ppm which attributed to O CH 2and COOH respectively.This downfield shift of thecarbon indicates the formation of O -carboxymethyl chitosan.The formation of this product agrees with the higher reactivity of hydroxyl group of C 6in this heterogeneous reaction.The N -carboxymethyl substituent is not present because of the absence of peaks at 47and 168ppm for N CH 2and COOH respectively.3.2.Characterization of the synthesized silver nanoparticles (AgNPs)In this research work,CMCTS was used as reducing and as stabi-lizing agent too.The formation of AgNPs could be visualized from changes in the color of the solutions from colorless to light yellow.The reduction of Ag +could occur via the reduction effect of CMCTS at 60◦C and pH 11.5for 30min.Fig.3shows the UV–visible absorp-tion spectra of AgNPs.The surface plasmon absorption bands are centered around 409nm (El-Rafie et al.,2011).The absorption band at 405nm becomes stronger and narrower which means higher conversion of Ag +to Ag 0with smaller nanoparticles size.Fig.4a and b shows the TEM image and the histogram of the size and size distribution of the AgNPs.Results revealed that the size range of prepared AgNPs was between 12and 18nm.3.3.Morphology of the CMCTS–AgNPs/PEO-electrospun nanofibersThe performance and morphology of the electrospun fiber were affected by the electrospinning process parameters.In the present study,two different electrospinning setups were used.In the first setup,a typical electrospinning setup was used (Fig.1)alongwithFig.3.UV–vis spectra of silver nanoparticles embedded in CMCTS.M.M.G.Fouda et al./Carbohydrate Polymers92 (2013) 1012–10171015Fig.4.(a and b)TEM image and the histogram of the size and size distribution of the AgNPs.Nanospider technology.Both electrospinning setup and Nanospi-der are used in order to prepare nanofiber mats,but,Nanospider is used in large scale/sample production of the selected and best nanofiber mats from the results obtained,in addition,no differ-ence in morphology of the resulted mats for both techniques is observed.Generally,the electrospun mat is opaque due to light scattering from thefibrous structure.The obtainedfibers(Fig.5a and b)had cylindrical morphology and nofiber bundles,indicat-ing that applied parameters were adequate for the formation of fibers and proper evaporation of the solvent.On the other hand, the presence of AgNPs in CMCTS has little effect on the electrospun fiber morphology.Thefiber diameter ranged from50to300nm. In addition,after the encapsulation of AgNPs into CMCTS–PEO nanofiber,thefiber diameter decreases compared tofibers consist-ing of CMCTS–PEO without AgNPs,due to the high conductivity, which plays a key role in decreasing of thefiber diameter during electrospinning(Sheikh et al.,2010).The presence of AgNPs results in high electric charge and subsequently high conductivity of the polymer solution which leads to high charge values during elec-trospinning process and possibly forming thinnerfiber diameter (Nirmala et al.,2010;Nirmala,Navamathavan,Kang,El-Newehy, &Kim,2011;Nirmala,Park,et al.,2011).At the same timethe Fig.5.SEM images of electrospun nanofibers containing AgNPs;(a)CMCTS;(b)CMCTS–Ag,(c)CMCTS/PEO–Ag and(d)CMCTS/PEO–Ag(EDX).1016M.M.G.Fouda et al./Carbohydrate Polymers 92 (2013) 1012–1017Fig.6.FT-IR spectra of electrospun nanofibers;(a)PEO and (b)PEO–AgNPs.fibrous structure assures much more loading of AgNPs into the fibers.EDX was used to analyze the elemental constitution of solid samples.Elementary analysis of CMCTS–PEO/silver nanocompos-ite was carried out by using SEM–EDX.Fig.5c displays a spectrum of CMCTS–PEO/silver nanocomposite obtained by elemental micro-probe analysis of EDX.The results show that carbon,oxygen,and Ag were the principal element of CMCTS–PEO/silver nanocomposite.EDX analysis,as a result,provides direct evidence that AgNPs are embedded in the CMCTS–PEO composite.The quantitative analysis of CMCTS–PEO/silver nanocomposite is presented in Table 1.At the same time,Fig.5a and b shows SEM images of CMCTS–PEO/AgNPs nanofibers,which revealed that the AgNPs were evenly distributed in the CMCTS–PEO ultrafine fibers with an average size less than 12–18nm.This suggested that the AgNPs were well stabilized by CMCTS during the preparation of AgNPs.Table 1Stoichiometric ratio of CMCTS–AgNPs.ElementWeight%Atomic%(PEO)/Ag C K 83.9088.17O K 14.8111.68Ag L1.290.15Total100.003.4.FT-IR spectra of electrospun nanofibersFT-IR spectra of electrospun CMCTS–PEO and CMCTS–AgNPs/PEO are shown in Fig.6.The frequencies and assignments for the pristine PEO are indicated asfollows:Fig.7.Diameter inhibition zone (cm)of electrospun nanofibers against Staphylococcus aureus (Sa),Pseudomonas aeruginosa (ps)and Escherichia coli (Ec).M.M.G.Fouda et al./Carbohydrate Polymers92 (2013) 1012–101710172882cm−1due to the CH2group stretching vibration,1097cm−1 and841cm−1due to the C O C asymmetric stretch and bending vibrations.On the other hand,for the electrospun CMCTS–AgNPs nanofiber shows the same characteristic bands,in which the intensity of the bands at2882cm−1and at841cm−1was increased due to the CH2and C O C stretching vibration upon the presence of AgNPs.3.5.Antimicrobial of electrospun nanofibersFig.7shows chart of inhibition zone of the tested antimicrobial samples and the corresponding plates(a,b,c).Results illustrated that S.aureus was the most sensitive microbe against antimicro-bial disk(AMC),CMCTS–PEO–AgNPs nanofiber,and AgNPs solution with inhibition zone30,22and15millimeters(mm)respectively.C.albicans was the least sensitive against all tested antimicrobial agents with IZ of0mm,except for CMCTS–PEO–AgNPs that showed IZ of12mm.It was found that the CMCTS–PEO–AgNPs nanofibers were the most effective silver containing material with IZs of20, 18,15and12against S.aureus,P.aeruginosa,E.coli and C.albicans, respectively.In contrast,the AgNPs showed the least antimicro-bial activity among silver containing nanofibers with IZ of13,7, 6and0mm against S.aureus,P.aeruginosa,E.coli and C.albicans, respectively.It was observed that CMCTS–PEO–AgNPs nanofibers are the most effective silver containing material against all tested microbes.Also,it was found that CMCTS–PEO–AgNPs nanofiber was more than twofold strength of the positive control(AgNPs).How-ever,its efficacy was less than any of the tested antibiotics,but this can be compensated with the less hazardous effect of antibi-otics and the less chance of resistance development compared with silver nanoparticles.4.ConclusionSilver nanoparticles(AgNPs)have been successfully prepared using carboxymethyl chitosan(CMCTS)which acts as both reduc-ing and stabilizing agent for the formed AgNPs.CMCTS–AgNPs with polyethylene oxide(PEO)are well mixed together and subjected to electrospinning process.The resulted nanofiber mats’embedded AgNPs are characterized using different analytical tools.The pres-ence of silver ions in the polymer structure was found to be strongly affecting the electrospun nanofibers diameters due to enhance-ment of electrical conductivity of the nanofibers.The obtained results indicated that the number of Ag+ions that were converted into Ag0increased with increasing the aging time.Antimicrobial activity of the prepared sample was evaluated against different types of microorganisms.It was observed that CMCTS–PEO–AgNPs nanofibers are the most effective silver containing material against all tested microbes.Also,it was found that CMCTS–PEO–AgNPs nanofiber was more than twofold strength of the positive con-trol(AgNPs).Finally,the prepared CMCTS–AgNPs/PEO nanofibers matrix could be properly employed as recommended candidate for many biological applications such as prolonged antimicrobial wound dressing materials.AcknowledgementThe authors extend their appreciation to the Deanship of Scien-tific Research at King Saud University for funding this work through research group no.RGP-VPP-201.ReferencesAbou-Okeil,A.(2012).Ag nanoparticles growing onto cotton fabric using chitosan as a template.Journal of Natural Fibers,9,61–72.Abou-Okeil,A.,Amr,A.,&Abdel-Mohdy,F.A.(2012).Investigation of silver nanopar-ticles synthesis using aminated-beta-cyclodextrin.Carbohydrate Polymers,89, 1–6.Derch,R.,Greiner,A.,&Wendorff,J.H.(Eds.).(2004).Polymer nanofibers prepared by electrospinning.Dekker encyclopedia of nanoscience and nanotechnology.New York:CRC.El-Rafie,M.H.,El-Naggar,M.E.,Ramadan,M.A.,Fouda,M.M.G.,Al-Deyab,S.S., &Hebeish,A.(2011).Environmental synthesis of silver nanoparticles using hydroxypropyl starch and their characterization.Carbohydrate Polymers,86(2), 630–635.El-Shafei,A.M.,Fouda,M.M.G.,Knittel,D.,&Schollmeyer,E.(2008).Antibacte-rial activity of cationically modified cotton fabric with carboxymethyl chitosan.Journal of Applied Polymer Science,110(3),1289–1296.Hebeish,A.,Abdel-Mohdy,F.A.,Fouda,M.M.G.,Elsaid,Z.,Essam,S.,Tammam,G.H.,et al.(2011).Green synthesis of easy care and antimicrobial cotton fabrics.Carbohydrate Polymers,86(4),1684–1691.Hebeish,A.,El-Naggar,M.E.,Fouda,M.M.G.,Ramadan,M.A.,Al-Deyab,S.S.,& El-Rafie,M.H.(2011).Highly effective antibacterial textiles containing green synthesized silver nanoparticles.Carbohydrate Polymers,86(2),936–940. Huang,Z.M.,Zhang,Y.Z.,&Kotaki,M.(2003).A review on polymer nanofibers by electrospinning and their applications in posites Science and Technology,63(15),2223–2253.Morgado,J.,Friend,R.H.,Cacialli,F.,Chuah,B.S.,Moratti,S.C.,&Holmes,A.B.(1999).Journal of Applied Physics,86,6392.Nirmala,R.,Nam,K.T.,Park,S.J.,Shin,Y.S.,Navamathavan,R.,&Kim,H.Y.(2010).Formation of high aspect ratio polyamide-6nanofibers via electrically induced double layer during electrospinning.Applied Surface Science,256,6318–6323. Nirmala,R.,Navamathavan,R.,Kang,H.-S.,El-Newehy,M.H.,&Kim,H.Y.(2011).Preparation of polyamide-6/chitosan composite nanofibers by a single solvent system via electrospinning for biomedical applications.Colloids and Surfaces B: Biointerfaces,83,173–178.Nirmala,R.,Park,H.-M.,Navamathavan,R.,Kang,H.-S.,El-Newehy,M.H.,&Kim,H.Y.(2011).Lecithin blended polyamide-6high aspect ratio nanofibers scaf-folds via electrospinning for human osteoblast cell culture.Materials Science and Engineering C,31,486–493.Sheikh, F. A.,Barakat,N. A.M.,Kanjwal,M. A.,Jeon,S.H.,Kang,H.S.,& Kim,H.Y.(2010).Self synthesize of silver nanoparticles in/on polyurethane nanofibers:Nano-biotechnological approach.Journal of Applied Polymer Science, 115,3189–3198.Taylor,G.I.(1969).Proceedings of the Royal Society of London,313,453.Textor,T.,Fouda,M.M.G.,&Mahltig,B.(2010).Deposition of durable thin silver layers onto polyamides employing a heterogeneous Tollens’reaction.Applied Surface Science,256(8),2337–2342.。
静电纺丝

设计了一套装 置,可以制备 直径在0.051.1微米的丙烯 酸纤维。考察 了纤维直径与 溶液黏度、射 流长度及环境 气体组分之间 的关系。
将聚乙烯和 聚丙烯熔体 纺成连续的 纤维,研究 发现,直径 取决于电场, 操作温度和 熔融体粘度, 与喷丝嘴直 径无明显关 系。
基本设备
静电纺丝的基本设备包括:高压电源、喷丝头和接收装置。纺丝液通 过注射泵从喷丝头中挤出形成小滴,小滴在高压电作用下变成锥形, 在超过某一临界电压后进一步激发形成射流,射流在空气中急剧震荡 和鞭动,从而拉伸细化,最终沉降在接收装置上。
熔体静电纺丝具有溶液静电纺丝无法比拟的优点: 1、不需要有机溶剂,成本低、生产效率高; 2、适用于一些室温下没有合适溶剂的聚合物,如PP、PE等; 3、对熔体电纺建模,有助于更加深入了解电纺机理; 4、如能与现有的熔喷装置相结合,则有很强的工业化应用前景。 同时也存在一定的问题: 1、聚合物熔体粘度高、导电性差,需要较高的电场强度,易发生电场击穿的危险。 2、制备的纤维多在微米级别; 3、装置复杂,需附加高温加热装置,易和高压装置发生静电干扰。
高压静电纺丝的基本过程
静电纺丝的过程可以简单的描述如下:首先在喷丝口处溶液被拉出表面, 沿着直线运动,当运动到一定位置,进入非稳定阶段,开始成螺旋摆动运动,同 时喷射流被进一步拉伸细化。 1、喷射流初始运动阶段 2、喷射流摆动非稳定阶段 在电场力的作用下,喷丝口处的溶液表面布满阳离子或分子中的缺电子 部分,当外加电压较小时,电场力不足以使溶液喷出,喷丝口处的溶液形成 “泰勒锥”,加大电压,当其超过特定的临界值时,带点锥体形成一股带点的 喷射流,沿电场方向加速运动。经过一段稳定的直线运动后,纤维开始不规则 摆动,在接收装置上的落点随机,这一过程中纤维表现出的状态即为非稳定性。
静电纺丝资料
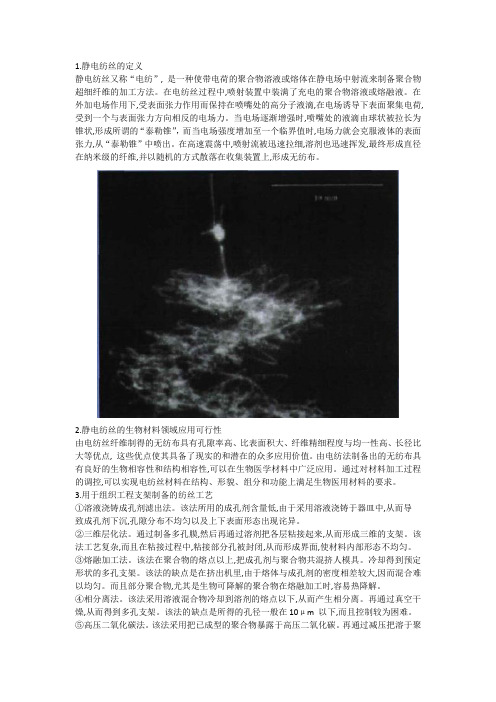
1.静电纺丝的定义静电纺丝又称“电纺”, 是一种使带电荷的聚合物溶液或熔体在静电场中射流来制备聚合物超细纤维的加工方法。
在电纺丝过程中,喷射装置中装满了充电的聚合物溶液或熔融液。
在外加电场作用下,受表面张力作用而保持在喷嘴处的高分子液滴,在电场诱导下表面聚集电荷, 受到一个与表面张力方向相反的电场力。
当电场逐渐增强时,喷嘴处的液滴由球状被拉长为锥状,形成所谓的“泰勒锥”,而当电场强度增加至一个临界值时,电场力就会克服液体的表面张力,从“泰勒锥”中喷出。
在高速震荡中,喷射流被迅速拉细,溶剂也迅速挥发,最终形成直径在纳米级的纤维,并以随机的方式散落在收集装置上,形成无纺布。
2.静电纺丝的生物材料领域应用可行性由电纺丝纤维制得的无纺布具有孔隙率高、比表面积大、纤维精细程度与均一性高、长径比大等优点, 这些优点使其具备了现实的和潜在的众多应用价值。
由电纺法制备出的无纺布具有良好的生物相容性和结构相容性,可以在生物医学材料中广泛应用。
通过对材料加工过程的调控,可以实现电纺丝材料在结构、形貌、组分和功能上满足生物医用材料的要求。
3.用于组织工程支架制备的纺丝工艺①溶液浇铸成孔剂滤出法。
该法所用的成孔剂含量低,由于采用溶液浇铸于器皿中,从而导致成孔剂下沉,孔隙分布不均匀以及上下表面形态出现诧异。
②三维层化法。
通过制备多孔膜,然后再通过溶剂把各层粘接起来,从而形成三维的支架。
该法工艺复杂,而且在粘接过程中,粘接部分孔被封闭,从而形成界面,使材料内部形态不均匀。
③熔融加工法。
该法在聚合物的熔点以上,把成孔剂与聚合物共混挤人模具。
冷却得到预定形状的多孔支架。
该法的缺点是在挤出机里,由于熔体与成孔剂的密度相差较大,因而混合难以均匀。
而且部分聚合物,尤其是生物可降解的聚合物在熔融加工时,容易热降解。
④相分离法。
该法采用溶液混合物冷却到溶剂的熔点以下,从而产生相分离。
再通过真空干燥,从而得到多孔支架。
该法的缺点是所得的孔径一般在10μm 以下,而且控制较为困难。
静电纺丝资料

1.静电纺丝的定义静电纺丝又称“电纺”, 是一种使带电荷的聚合物溶液或熔体在静电场中射流来制备聚合物超细纤维的加工方法。
在电纺丝过程中,喷射装置中装满了充电的聚合物溶液或熔融液。
在外加电场作用下,受表面张力作用而保持在喷嘴处的高分子液滴,在电场诱导下表面聚集电荷, 受到一个与表面张力方向相反的电场力。
当电场逐渐增强时,喷嘴处的液滴由球状被拉长为锥状,形成所谓的“泰勒锥”,而当电场强度增加至一个临界值时,电场力就会克服液体的表面张力,从“泰勒锥”中喷出。
在高速震荡中,喷射流被迅速拉细,溶剂也迅速挥发,最终形成直径在纳米级的纤维,并以随机的方式散落在收集装置上,形成无纺布。
2.静电纺丝的生物材料领域应用可行性由电纺丝纤维制得的无纺布具有孔隙率高、比表面积大、纤维精细程度与均一性高、长径比大等优点, 这些优点使其具备了现实的和潜在的众多应用价值。
由电纺法制备出的无纺布具有良好的生物相容性和结构相容性,可以在生物医学材料中广泛应用。
通过对材料加工过程的调控,可以实现电纺丝材料在结构、形貌、组分和功能上满足生物医用材料的要求。
3.用于组织工程支架制备的纺丝工艺①溶液浇铸成孔剂滤出法。
该法所用的成孔剂含量低,由于采用溶液浇铸于器皿中,从而导致成孔剂下沉,孔隙分布不均匀以及上下表面形态出现诧异。
②三维层化法。
通过制备多孔膜,然后再通过溶剂把各层粘接起来,从而形成三维的支架。
该法工艺复杂,而且在粘接过程中,粘接部分孔被封闭,从而形成界面,使材料内部形态不均匀。
③熔融加工法。
该法在聚合物的熔点以上,把成孔剂与聚合物共混挤人模具。
冷却得到预定形状的多孔支架。
该法的缺点是在挤出机里,由于熔体与成孔剂的密度相差较大,因而混合难以均匀。
而且部分聚合物,尤其是生物可降解的聚合物在熔融加工时,容易热降解。
④相分离法。
该法采用溶液混合物冷却到溶剂的熔点以下,从而产生相分离。
再通过真空干燥,从而得到多孔支架。
该法的缺点是所得的孔径一般在10μm 以下,而且控制较为困难。
静电纺丝——ElectrospinningPPT课件
纺丝工艺参数 施加电压
针头到收集板的距离 纺丝液流量
环境参数 温度 湿度
纺丝室气流速度
材料合成化学
中国科学技术大学
静电纺丝
• 发展历史 • 工作原理 • 装置设备 • 影响因素 • 应用领域
材料合成化学
中国科学技术大学
应用
材料合成化学
中国科学技术大学
制备Li-O2电池正极材料
http://www.aist.go.jp/aist_j/press_release/pr2009/pr20090224 /pr20090224.html
全F面on系g统等地对研静究 静电电纺纺丝丝纳超米细纤 维纤微维观串形珠貌现的影 响象因及素微、观表结征、 过构程作参了数研的究改进
1934
1966
1981
1995
1290909034
Simons申请了 由静电纺丝法 制备超薄、超 细非织造膜的 专利
Reneke多rSp研组iva究分k等聚首合次采物用的流静电 组开始对纺体丝静动过丝电力程。学, 并静描且述电提静纺出电了丝纺静和其 纺丝进行他电研纺方究丝法的。结工艺合参开数发新型 静电纺丝纳迅米速纤维。纳米纤维 发展 纺丝机纳米蜘蛛问世
Electrospinning
静电纺丝
.
1
中国科学技术大学
静电纺丝
• 发展历史 • 工作原理 • 装置设备 • 影响因素 • 应用领域
材料合成化学
中国科学技术大学
发展历史
A.Formhals在 发明专利中最 早报道静电纺 丝,设计了纺 丝加工的装置
Larrondo等 对聚乙烯和 聚丙烯进行 了熔融静电 纺丝的研究
1三维结构更适合细胞生存 2实现了细胞的储存-释放 3材料可循环利用
静电纺丝法实验报告(3篇)
第1篇一、实验目的1. 熟悉静电纺丝法的原理和操作步骤。
2. 掌握利用静电纺丝法制备纳米纤维的方法。
3. 分析不同参数对纳米纤维形态和性能的影响。
二、实验原理静电纺丝法是一种常用的制备纳米纤维的技术,利用高压电场使高分子溶液或熔体在喷丝头处形成细小的液滴,液滴在电场力、表面张力以及惯性力的共同作用下,拉伸形成纳米纤维。
通过控制实验参数,可以制备出具有不同直径、形态和性能的纳米纤维。
三、实验材料与设备材料:1. 聚乙烯醇(PVA)粉末2. 乙醇3. 纳米氧化锌(ZnO)设备:1. 静电纺丝机2. 电子天平3. 真空干燥箱4. 扫描电子显微镜(SEM)5. 透射电子显微镜(TEM)6. X射线衍射仪(XRD)四、实验步骤1. 配制PVA溶液:称取一定量的PVA粉末,加入适量乙醇溶解,搅拌均匀。
2. 配制纳米氧化锌溶液:称取一定量的纳米氧化锌,加入适量乙醇溶解,搅拌均匀。
3. 混合溶液:将PVA溶液和纳米氧化锌溶液按照一定比例混合均匀。
4. 静电纺丝:将混合溶液注入静电纺丝机,设置合适的电压、喷头与收集器距离等参数,进行静电纺丝。
5. 收集纳米纤维:将静电纺丝制备的纳米纤维收集在铝箔上,干燥。
6. 纳米纤维表征:利用SEM、TEM、XRD等手段对纳米纤维进行表征。
五、实验结果与分析1. SEM分析:从SEM图像可以看出,纳米纤维呈细长条状,直径在100-200nm之间,表面光滑。
2. TEM分析:从TEM图像可以看出,纳米纤维具有明显的纳米级特征,直径在30-50nm之间。
3. XRD分析:从XRD图谱可以看出,纳米纤维具有较好的结晶度,表明纳米氧化锌在纳米纤维中均匀分散。
六、讨论1. 实验结果表明,通过静电纺丝法制备的纳米纤维具有较好的结晶度和均匀的分散性,表明纳米氧化锌在纳米纤维中均匀分散。
2. 实验过程中,电压、喷头与收集器距离等参数对纳米纤维的直径和形态有较大影响。
适当提高电压和缩短喷头与收集器距离,可以制备出更细、更均匀的纳米纤维。
静电纺丝_最终版讲解
1882年 Rayleigh
1915年 Zeleny
1964年 Taylor
研究了到底需 要多少电荷才 能克服液滴的 表面张力使液 滴劈裂的问题, 提出了 “Rayleigh” 极限数值
得出表面张力越 高的液体出现弯 曲不稳定现象时 需要的电压就越 高。
他认为,液体 在电场力的作 用下只受到两 个力,“电场 力和表面张 力”,并提出 了“泰勒锥”。
2)同轴针头 同轴电纺的一个优点在于可以突破单头体系的限制,将一些难以直接电 纺的聚合物通过同轴电纺装置制备纳米纤维。另一个优势是通过将核层选择性 移除,还可以制备中空纳米纤维结构。
3)并列式针头 并列式针头体系是一 种结构简单却易于实现功能 化纳米纤维制备的喷丝头体 系。它将不同的聚合物溶液 通过紧密靠在一起的并列式 针头同时进行射流激发,在 电纺过程中平行射流融合, 得到多根纤维互相连接的束 状单根纤维,因此特别适合 制备双组份聚合物纤维。
喷丝头
喷丝头的作用就是在纺丝过程中产生纺丝小液滴,提供射流激发位点。 一般分为无针头和针头两种不同的喷丝体系,其中针头体系根据针头数量和 形式的不同,还可以进一步分为单头、同轴、并列、多头等不同的形式。
1、无针头体系。核心思想就是在自由聚合物溶液表面形成大量射流激发位点。
2、针头体系。 1)单针头 单针头最常见,根据需要可选择不同型号的针头。
熔体静电纺丝具有溶液静电纺丝无法比拟的优点: 1、不需要有机溶剂,成本低、生产效率高; 2、适用于一些室温下没有合适溶剂的聚合物,如PP、PE等; 3、对熔体电纺建模,有助于更加深入了解电纺机理; 4、如能与现有的熔喷装置相结合,则有很强的工业化应用前景。 同时也存在一定的问题: 1、聚合物熔体粘度高、导电性差,需要较高的电场强度,易发生电场击穿的危险。 2、制备的纤维多在微米级别; 3、装置复杂,需附加高温加热装置,易和高压装置发生静电干扰。
静电纺丝工艺
静电纺丝工艺
一、溶液制备
静电纺丝工艺的第一步是制备纺丝溶液。
这一过程涉及到将聚合物溶解在适当的溶剂中,形成均一的溶液。
为了获得最佳的纺丝效果,需要控制溶液的粘度、浓度以及溶剂的蒸发速率。
通常,聚合物在溶剂中的溶解度越高,溶液的粘度越低,越有利于纺丝。
二、静电纺丝
在制备好纺丝溶液后,通过静电纺丝设备进行纺丝。
静电纺丝过程中,高压电场施加在喷嘴和收集器之间,使得溶液形成细流并射出。
在电场的作用下,细流会逐渐拉伸、细化,最终形成纤维。
这一过程中,可以通过调整电场强度、溶液流量以及喷嘴到收集器的距离等参数来控制纤维的形貌和直径。
三、收集与处理
纺出的纤维会落在收集器上,形成非织造布状的纤维膜。
为了获得实用的纤维材料,通常需要对收集到的纤维进行后处理。
这包括洗涤以去除残留的溶剂和杂质,以及热处理或化学处理以改善纤维的性能。
四、应用与性能测试
静电纺丝工艺制备的纤维材料在许多领域都有广泛的应用,如过滤、增强、生物医学等。
对于不同的应用,纤维材料的性能要求也不同。
因此,需要对制备的纤维材料进行性能测试,以满足实际需求。
常见的性能测试包括纤维直径、形貌、力学性能、热性能、电性能等。
总之,静电纺丝工艺是一种简单、环保的制备纤维材料的方法。
通过合理的工艺参数选择和控制,可以制备出具有优异性能的纤维材料,满足各种应用需求。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
施加的电场力的数值与液滴的表面张力相等时,液滴就 形成了顶角为49.3°的圆锥,被命名为“泰勒锥”。
电喷技术原理的研究历史
1882年 Rayleigh
1915年 Zeleny
1964年 Taylor
研究了到底需 要多少电荷才 能克服液滴的 表面张力使液 滴劈裂的问题, 提出了 “Rayleigh” 极限数值
环形电极辅助维
静电纺的分类:
根据电纺时纺丝液体系是溶液状态还是熔融状态,可以分为溶液静电纺和熔融 静电纺。
溶液静电纺技术的未来蕴含着无限可能,但是也有一些自身难以克服的缺点: 1、电纺体系中只有10%左右为聚合物,纺丝效率低; 2、某些电纺体系需在强腐蚀性或高剧毒性溶剂中进行; 3、有机溶剂成本高、不易回收,易造成环境污染等。 以上缺点均限制了溶液纺丝的进一步工业化应用,一个可能的解决方法就是熔体 静电纺丝。
3)并列式针头
并列式针头体系是一 种结构简单却易于实现功能 化纳米纤维制备的喷丝头体 系。它将不同的聚合物溶液 通过紧密靠在一起的并列式 针头同时进行射流激发,在 电纺过程中平行射流融合, 得到多根纤维互相连接的束 状单根纤维,因此特别适合 制备双组份聚合物纤维。
并列式针头
4)多针头 在并列式针头装置的基础上,
高压电源
高压电源提供产生纺丝液射流的高压电,电源的两 极分别连接在喷丝头和接收装置。根据电源性质的不同, 可分为直流和交流高压电源两种,都可用于静电纺丝。
直流高压电在电纺过程中通常采用感应充电的方式, 即将直流高压电直接接在喷丝头上,接收装置接地或反之。 电压极性对纺丝过程影响不大,实验室多采用高压正电纺 丝。
熔体静电纺丝具有溶液静电纺丝无法比拟的优点: 1、不需要有机溶剂,成本低、生产效率高; 2、适用于一些室温下没有合适溶剂的聚合物,如PP、PE等; 3、对熔体电纺建模,有助于更加深入了解电纺机理; 4、如能与现有的熔喷装置相结合,则有很强的工业化应用前景。 同时也存在一定的问题: 1、聚合物熔体粘度高、导电性差,需要较高的电场强度,易发生电场击穿的危险。 2、制备的纤维多在微米级别; 3、装置复杂,需附加高温加热装置,易和高压装置发生静电干扰。
首次在专 利中提出 该技术。 他设计了 一套聚合 物溶液在 强电场下 的喷射进 行纺丝的 加工装置。
专利中叙述 了用静电纺 丝技术制备 超细超轻无 纺布的装置, 且发现粘度 高时,纤维 连续,粘度 低时,纤维 短且细。
设计了一套装 置,可以制备 直径在0.051.1微米的丙烯 酸纤维。考察 了纤维直径与 溶液黏度、射 流长度及环境 气体组分之间 的关系。
高压静电纺丝的基本过程
静电纺丝的过程可以简单的描述如下:首先在喷丝口处溶液被拉出表面, 沿着直线运动,当运动到一定位置,进入非稳定阶段,开始成螺旋摆动运动,同 时喷射流被进一步拉伸细化。
1、喷射流初始运动阶段
2、喷射流摆动非稳定阶段
在电场力的作用下,喷丝口处的溶液表面布满阳离子或分子中的缺电子
部分,当外加电压较小时,电场力不足以使溶液喷出,喷丝口处的溶液形成
进一步增大针头间的距离就发展为 多可针头体系,针头数量从2个到 十几个不等,也称为平行电纺。
多针头纺丝体系
接收装置
接收装置用于收集电纺纤维,常规接受装置主要包括平板、滚筒、间隔收 集装置、转盘、金属丝鼓、凝固浴等;根据电纺丝过程中喷丝头及接收装置之间 是否存在相对运动,又可分为静态接收和动态接收两种接收方式。
1、无针头体系。核心思想就是在自由聚合物溶液表面形成大量射流激发位点。
2、针头体系。 1)单针头 单针头最常见,根据需要可选择不同型号的针头。
2)同轴针头 同轴电纺的一个优点在于可以突破单头体系的限制,将一些难以直接电 纺的聚合物通过同轴电纺装置制备纳米纤维。另一个优势是通过将核层选择性 移除,还可以制备中空纳米纤维结构。
他认为,液体
得出表面张力越 在电场力的作
高的液体出现弯 用下只受到两
曲不稳定现象时 个力,“电场
需要的电压就越 力和表面张
高。
力”,并提出
了“泰勒锥”。
静电纺丝技术的发展历史
1934年 Formhals
1966年 Simons
1971年 Baumgarten
1981年
Larrondo 和Manley
静电纺丝
主讲人: 罗磊 小组成员:于磊 逄增媛
contents
1 静电纺丝的基本概念和历史
2 静电纺丝的设备和基本过程
3
静电纺丝的原理
4
静电纺丝的影响因素
5
静电纺丝的应用
相关概念
• 电喷技术:在高压静电场下,导电液滴能够发生高速
喷射的现象。
• 静电纺丝:可以认为是带电射流电雾化的一种变形,
当液体的粘度较小时,射流在受到电场力的作用后破裂 为许多细小的液滴,液滴的直径介于微米和纳米之间, 当液滴的粘度较大时,就会形成纤维。
交流电电纺可显著提高射流鞭动的稳定性,纤维变 粗但有序性增加,同时也可在绝缘的接收装置上有较大的 接收面积。但在纺丝过程中交流电频率不易调整(要考虑 每次的实验条件:温湿度、溶液性质等)。
喷丝头
喷丝头的作用就是在纺丝过程中产生纺丝小液滴,提供射流激发位点。 一般分为无针头和针头两种不同的喷丝体系,其中针头体系根据针头数量和 形式的不同,还可以进一步分为单头、同轴、并列、多头等不同的形式。
将聚乙烯和 聚丙烯熔体 纺成连续的 纤维,研究 发现,直径 取决于电场, 操作温度和 熔融体粘度, 与喷丝嘴直 径无明显关 系。
基本设备
静电纺丝的基本设备包括:高压电源、喷丝头和接收装置。纺丝液通 过注射泵从喷丝头中挤出形成小滴,小滴在高压电作用下变成锥形, 在超过某一临界电压后进一步激发形成射流,射流在空气中急剧震荡 和鞭动,从而拉伸细化,最终沉降在接收装置上。
1、常规接收装置 由于电纺过程中鞭动的不稳定性,收集到的纤维常为无规堆积的无纺布形式。
通过改变接收装置,可以得到其他不同的纤维聚集形式。
平板接收
滚筒接收
2、辅助接收装置 在射流鞭动细化过程中,主要受到电场力的作用,因此通过引入接收装置
改变电场形状或者引入其他场如磁场,就能调控射流运动轨迹,达到可控收集的 目的。
