APIC 原料药亚硝胺风险评估报告模板-2020年2月(中英文对照版)
供应商风险评估报告文书
供应商风险评估报告文书目的确认供应商审计的范围及程度,识别供应商及物料采购选择质量风险,对风险进行分级,根据等级大小,进行分析、评估,确保关键风险要素能够得到有效控制,以降低供应商带来的质量风险,并为及时更换供应商提供依据。
范围公司生产品种所涉及的原辅料、包材的供应商均在此风险评估的范围内,重点是评估供应商的质量管理体系和所采购物料的风险等级。
责任质量管理部、供销部内容1供应商风险评估:包括质量保证能力评估、供货历史评估、维护性评估。
2采购物料风险评估:分为关键性物料、影响产品质量物料、不影响产品质量物料等三级质量风险评估。
3风险评估小组组员及职责4风险评估程序启动质量风险管理风险管理工具5 供应商质量风险评估项目、风险分析原则及标准:一、项目确定原则:1.供应商系统设计性能检测项目2.生产工艺设计储存条件对系统的要求3.《洁净厂房设计规范》GB50073-20014. 《药品生产质量管理规范》2010版二、评估标准:根据我公司生产所用的供应商,对供应商相关资质、机构与人员、厂房和设施设备、物料管理、生产管理、质量管理、运输与交货七个重点项目用帕累托图分析法进行分析。
分析供应商所存在的问题,分为3类,A类属于关键问题,累计分数在0~80%;B类问题属于次要问题,累计分数在80%~90%;C类问题属于一般问题,累计分数在90%~100%。
年月日至年月日,风险评估小组人员对供应商系统按照重点项目进行风险评估,各关键要素的风险分析,评估及结果见下表:评分标准0分-------- 未有文件;1分-------- 手写的程序或文件(未受控) ;2 分-------- 不足夠,需要改善;3 分-------- 备注,需要关注;4分-------------- 满意;N/A -------------不适用. 风险评估表表2 供应商存在问题排列表按以上风险评估得出的审计范围和程度,在验证过程中,应对经评估确定的关键点,进行供应商审计,以证明供应商系统各关键要素能够得到有效控制,能够保证从供应商购买合格的原辅料。
APIC原料药厂清洁验证指南(201405-中英文).docx

APIC原料药厂清洁验证指南(201405 中英文)ACTIVE PHARMACEUTICAL INGREDIENTS COMMITTEE (APIC)GUIDANCE ON ASPECTS OF CLEANING VALIDATIONIN ACTIVE PHARMACEUTICAL INGREDIENT PLANTSAPIC 原料药工厂中清洁验证指南May 2014Table of Contents1.0 FOREWORD 前言The original version of this guidance document has now been updated by the APIC Cleaning Validation Task Force on behalf of the Active Pharmaceutical Ingredient Committee (APIC) of CEFIC.本指南文件的原版本现已由APIC清洁验证工作组代表CEFIC的APIC委员会进行了更新。
The Task Force members are:- 以下是工作组的成员Annick Bonneure, APIC, BelgiumTom Buggy, DSM Sinochem Pharmaceuticals, The NetherlandsPaul Clingan, MacFarlan Smith, UKAnke Grootaert, Janssen Pharmaceutica, BelgiumPeter Mungenast, Merck KGaA, Germany.Luisa Paulo, Hovione FarmaCiencia SA, PortugalFilip Quintiens, Genzyme, BelgiumClaude Vandenbossche, Ajinomoto Omnichem, BelgiumJos van der Ven, Aspen Oss B.V., The NetherlandsStefan Wienken, BASF, Germany.With support and review from:- 以下为提供支持和进行审核的人员Pieter van der Hoeven, APIC, BelgiumAnthony Storey, Pfizer, U.K.Rainer Fendt, BASF, Germany.The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of attention from regulators, companies and customers alike.原料药生产工厂的清洁验证一直是法规人员、公司和客户等关注的问题。
中英对照-APIC原料药厂清洁验证指南:7.0分组法(括号法)

APIC 201405原料药厂清洁验证指南:7.0 分组法(括号法)和最差情况分级(中英文)2014-07-15julia翻译蒲公英7.0 Bracketing and Worst Case Rating 分组法(括号法)和最差情况分级7.1 Introduction 介绍The cleaning processes of multiple product use equipment in API facilities are subject to requirements for cleaning validation. The validation effort could be huge. In order to minimize the amount of validation required, a worst case approach for the validation can be used.原料药工厂中的多产品设备清洁要求进行清洁验证。
清洁工作量会比较大。
为了减少验证的工作量,可以采用最差情形方法进行验证。
By means of a bracketing procedure the substances are grouped.采用分组法时,物质按类进行分组。
A worst case rating procedure is used to select the worst case in each group.然后在每组中采用最差情形分级法选择各组中最差的情况。
Validation of the worst case situation takes place. However, it is of utmost importance that a documented scientific rational for the chosen worst cases exists.对最差情形进行验证。
化妆品原料的、产品安全评估报告、安全评估报告示例(完整版)、(简化版)(范本2021)

附录1化妆品原料的安全评估报告题目:(原料名称)安全评估报告注册人/备案人名称:注册人/备案人地址:评估单位:评估人:评估日期:年月日目录一、摘要 (35)二、原料理化性质 (35)三、评估过程 (35)四、评估结果分析 (36)五、风险控制措施或建议 (37)六、安全评估结论 (37)七、安全评估人员签名 (37)八、安全评估人员简历 (37)九、参考文献 (37)十、附录 (37)一、摘要xx原料(CAS号:xxx),应用于xxx产品中,使用目的xxx,相关毒理学终点有xxx,暴露量为xxx,计算得出MoS值为xxx,可能产生的风险物质为xxx,在xxx的使用情况下不会对人体健康造成危害。
二、原料理化性质1、名称(包括标准中文名称、通用名、商品名、化学名、INCI名、CAS号、EINCES号等):2、物理状态:3、分子结构式和相对分子量:4、化学特性和纯度:5、杂质/残留物:6、溶解度:7、分配系数:8、均质性、稳定性:9、异构体组成:10、其他相关理化指标:11、功能和用途:12、其他(如为矿物、动物、植物来源的原料或香精香料,按照本导则中的要求进行原料特性描述)。
三、评估过程1. 危害识别:1.1 健康危害效应,一般包括:(1)急性毒性(2)刺激性/腐蚀性(3)致敏性(4)光毒性(5)光变态反应(6)遗传毒性(7)重复剂量毒性(8)生殖发育毒性(9)慢性毒性/致癌性(10)毒代动力学(11)人群安全资料(12)其他1.2 危害识别:……2. 剂量反应关系评估:……3. 暴露评估:……4. 风险特征描述:……四、评估结果分析包括对评估过程中资料的完整性、可靠性、科学性的分析,数据不确定性的分析等。
五、风险控制措施或建议……六、安全评估结论……七、安全评估人员签名……八、安全评估人员简历……九、参考文献……十、附录包括检测报告、涉及的原料规格证明等。
若存在风险物质,应提供风险物质评估结论和资料,或风险物质检验报告。
供应商风险评估表范例
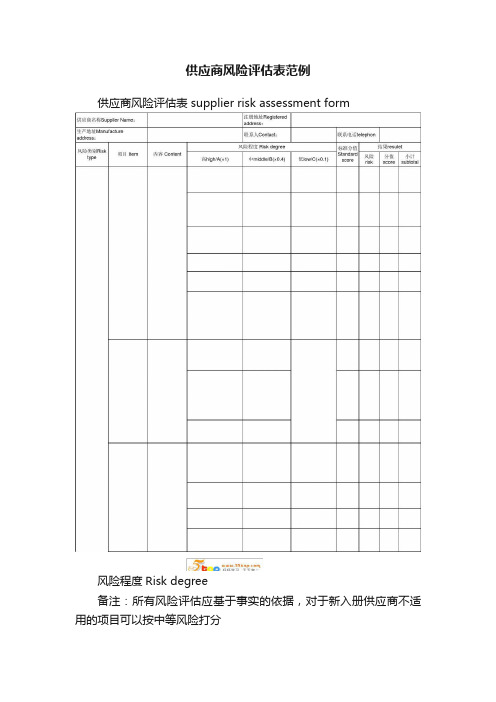
供应商风险评估表范例供应商风险评估表 supplier risk assessment form风险程度Risk degree备注:所有风险评估应基于事实的依据,对于新入册供应商不适用的项目可以按中等风险打分note: All risk evaluation is based on the actual , for new supplier, some item which could not be evalued shall evaluate as middle risk.◇必要时,可对供应商每两年执行1次现场考察或书面审查if necessary, should do on-site visit or writted review every two years.中风险middle risk低风险low risk ◇每批次物料需严格进行检验Each batch of material shall be inspected strictly.◇所有采购材料实时跟踪状态Real-time tracking of all purchasing materials ◇每半年年至少对供应商进行1次实地考察或书面调查one time on-site visit or written review every half a year at least.◇对每批次物料进行检验Each batch of material shall be inspected.◇每年跟踪一至二批材料的供料状态Track of one or two batch of m aterial yearly ◇每年对供应商进行1次实地考察或书面审查one time on-site visit or written review every year ◇对每批次物料进行检验,若供应商的历史交货质量状况良好,可降低抽样检验频率Each batch of material should be inspected, if the supplier's historical delivery quality is in good condition, could reduce the s ampling frequency ◇不跟踪材料的供应状态the supply status of the material is not tracked 评估结论:评估分值在15分以下为低风险,15-30分范围内为中等风险,30分以上为高风险(代理商得分5分以下为低风险,5-8分为中风险,8分以上为高风险)evaluation result: low risk when the evaluated score not more than 15, middle risk when the evaluated score between 15-30, high risk when the evaluated score more than 30(agents scoring 5 point or less is low risk, 5-8 is middle risk, 8 or more is high risk)高风险High risk管理措施Management method 备注:代理商仅考虑商务风险、物流风险和交货风险,不考虑其质量风险。
Supplier_Assessment_Report供应商评估报告书(英文)

Potential Supplier Assessment潜在供方评审报告SUPPLIER DATA 供应商资料ASSESSMENT DATA 评审资料Name名称: Date日期:Address地址: Assessed by评审人:Commodity零件类别: Chem化工类 Elec 电器类 Met 金属类Product 产品:Assessment Criteria 评审标准Phone No.: Fax No.传真: Overall Rating Score 总分Supplier Contacts :联系人Position :职位: Overall Rating Result 总结果Comments:评语:Team Leaders’signature’s评审小组组长签字:Assessor Phone No.评审员:RESULTS评审结果** Questions问题Conforms符合Nonconformities不符合PointScoreCorrective Action O/L-DateNo. Element要素Minor轻微Major主要分数纠正措施O/L日期Documentation Review 文件审核All 4.1 Management Responsibility 管理职责 4 4.2 Quality System 质量体系8 4.3 Contract Review 合同评审 1 4.4 Design Control 设计控制 4 4.5 Document and Data Control 文件和资料控制2 4.6 Purchasing 采购 4 4.8 Product Identification and Traceability产品标识及可追溯性1 4.9 Process Control 过程控制 6 4.1Inspection and Testing 检验和试验 64.1 1 Inspection, Measuring and Test Equipment检验,测量和试验设备44.1 3 Control of Nonconforming Product不合格品的控制24.1 4 Corrective and Preventive Action预防和纠正措施54.1 5 Handling, Storage, Packaging and Delivery搬运,储存,包装及交付64.1 6 Control of Quality Records 质量记录的控制14.17Internal Quality Audits 部质量审核 54.18Training 培训 1General Motors Specific 通用汽车具体要求1Total score总分TARGET DATE FOR CORRECTION OF ALL NONCONFORMITIES纠正所有不符合项的目标日期:: ....../....../......Question Scoring问题评分标准Element Scoring要素评分标准分数计算> Supplier not familiar withrequirements of element and has no relevant documentation.>供应商不了解要素的要求并且没有相关文件0> Implementation 80 – 95% complete anddocumented evidence is available.已实施80-95%并且有记录在案的证据可查.6Total Points总分:➢Supplier is familiar withrequirements of element but thereis noevidence/documentation/implement. ➢供应商了解要素要求但没有证据/记录/实施. 1➢Full implementation and confirmedevidence of effectiveness-Supplier metminimum req.➢完全实施并且具有确认实施有效的证据-供应商满足最低要求.7No. ofapplicableelements适用要素数目:➢Supplier is familiarwith requirements ofelement and haspreliminary/draftdocumentation.➢供应商了解要素要求并具有初步/草案性的文件. 2> Analysis of results &continuous improvement can bedemonstrated.有证据表明对实施效果的分析和持续改进8 PointScore分数= Total PointsNo. of applicableelements= Overall Rating:> Documentation is available but implementation is only 0 – 30%complete.➢文件已建立但仅实施了0-30% 3 > Supplier has reached world classperformance and continuous improvement inall areas.供应商达到世界级的表现并在各个方面取得持进.9 分数=总分/合适的要素> Documentation is available andimplementation is 30 – 60% complete. ➢文件已建立并实施了解情况50-60% 4 > Supplier is best-in-class, demonstratesignif. Innovation beyond customerrequirements and sets the industrybenchmark.供应商是同类别中的最佳.证据表明供应商有超出客户要求的显著革新并确立该行业的基准.10➢Implementation 60 – 80%complete and there ispreliminary evidence of relevantresults.➢已实施了60-80%并且已有了相应实施效果的初步证据.5Note:注:(1) To Pass or be Recommended the score must >=7.通过评审或被推荐的要求为至少(7)分.(2)To conditional Pass or be Recommended the score must >=6. All nonconformances must becorrectedbefore PPAP and SQE will verify.有条件通过评审或被推荐的要求为至少(6)分.所有的不符合应在产品PPAP 前得到纠正并为SQE验证。
产品中亚硝胺残留风险评价2019

产品亚硝胺杂质残风险分析报告评估报告审批表1评估依据:•按照《质量风险管理标准管理程序》的要求对产品中亚硝胺杂质残留进行风险评估,确保产品中亚硝胺杂质残留符合EMA的要求。
•ICH Q9a•ICH M72评估目的根据EMA发布的《Information on nitrosamines for marketing authorisation holders》以及EDQM 发布的《Announcement to all CEP holders for synthesised APIs regarding presence of nitrosamines》的要求,对产品生产过程进行风险评估,确定产品中是否有亚硝胺杂质残留。
3风险评估3.1根据《Information on nitrosamines for marketing authorisation holders》中描述的亚硝胺的来源:①Nitrosamine impurities can form during API processing under certain processing conditionsand in the presence of some types of raw materials, starting materials, and intermediates.They may not be fully purged in subsequent steps of the API manufacturing process.在某些加工条件下、在某些类型的原料、起始原料和中间体的存在下,API生产过程中可能形成亚硝胺杂质。
在API生产过程的后续步骤中可能无法完全清除它们。
②The use of sodium nitrite (NaNO2), or other nitrites, in the presence of secondary ortertiary amines is a potential cause of nitrosamine formation.在仲胺或叔胺的存在下使用亚硝酸钠(NaNO2)或其他亚硝酸盐是亚硝胺形成的潜在原因。
Q1A(R2)新原料药和制剂的稳定性试验中英文对照版_2020.4.16

Q1A(R2)新原料药和制剂的稳定性试验中英文对照版_2020.4.16STABILITY TESTING OF NEW DRUG SUBSTANCES AND PRODUCTSQ1A(R2)新原料药和制剂的稳定性试验Current Step4version现第四版Dated6February20032003年2月6日制定目录1.INTRODUCTION导言 (3)1.1.Objectives of the Guideline目的 (3)1.2.Scope of the Guideline范围 (3)1.3.General Principles通则 (4)2.GUIDELINES指导 (5)2.1.Drug Substance原料药 (5)2.1.1.General概述 (5)2.1.2.Stress Testing影响因素试验 (5)2.1.3.Selection of Batches批次选择 (6)2.1.4.Container Closure System包装容器系统 (6)2.1.5.Specification质量标准 (7)2.1.6.Testing Frequency检验频次 (7)2.1.7.Storage Conditions贮藏条件 (8)2.1.8.Stability Commitment稳定性承诺 (11)2.1.9.Evaluation评估 (12)2.1.10.Statements/Labeling说明书/标签 (14)2.2.Drug Product制剂 (14)2.2.1.General通则 (15)2.2.2.Photostability Testing光稳定性试验 (15)2.2.3.Selection of Batches批次选择 (15)2.2.4.Container Closure System包装容器系统 (16)2.2.5.Specification质量标准 (16)2.2.6.Testing Frequency检验频次 (17)2.2.7.Storage Conditions贮藏条件 (18)2.2.8.Stability Commitment稳定性承诺 (26)2.2.9.Evaluation评估 (27)2.2.10.Statements/Labeling说明书/标签 (29)3.GLOSSARY术语 (29)4.REFERENCES参考文献 (38)1.INTRODUCTION导言1.1.Objectives of the Guideline目的The following guideline is a revised version of the ICH Q1A guideline and defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the three regions of the EC,Japan,and the United States.It does not seek necessarily to cover the testing for registration in or export to other areas of the world.以下指导原则是ICH Q1A的修订版,定义了新原料药和制剂在欧洲、日本、美国三个地区注册所需要的稳定性资料要求。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
REPORT ON THE RISK OF POTENTIAL PRESENCEOF NITROSAMINE IMPURITIES关于潜在存在亚硝胺杂质的风险报告Document ID:文件ID:MATERIAL物料Name: ………………………………………………………………………………………………………………………………名称Material code(s) :……………………………………………………………………………………………………………………材料代码MANUFACTURER (refers to the manufacturing site)制造商(指生产工厂) Name:…………………………………………………………………………………………………………………………………名称Address:………………………………………………………………………………………………………………………………地址Document ID:文件ID Page 1 of 9Delete this page when issuing the letter.发信时删除此页。
Instructions when using this template.使用此模板时的说明。
1.Replace APIC logo by your company logo.将APIC的logo替换为贵公司的logo。
2.In the footer, replace CEFIC logo by “This document was prepared based onAPIC template”.在页脚,使用“此文件基于APIC模板编写”代替CEFIC logo。
3.In various places in the template, text appears in italics in square brackets, e.g. [Option1,Option 2, Option3].Keep the text that applies and remove the text that don’t apply to the situation which is being described.在模板的不同位置,有文字以斜体字出现在方括号内,例如[选项1,选项2,选项3]。
保留适用的文本,删除不适用于所描述情况的文本。
4.In section 3, the detail would depend on the customer with whom the report will beshared. The process description can be replaced by a workflow with the relevantinformation for the assessment or just to reference the filing applicable section.在第3节中,细节将取决于与之共享报告的客户。
工艺描述可以用包含评估相关信息的工作流程图取代,或者只是参考适用的归档章节。
5.In section 3, a description of the process step where the risk assessment was initiated,e.g. from starting material or from intermediate, should be provided with a rationalefor the selection.在第3节中,应说明启动风险评估的工艺步骤,例如从起始材料或中间材料开始,并说明选择的理由。
6.For table in section 6, fill one line per nitrosamine which has been identified asbeing possibly generated during the API manufacturing process.对于第6节的表格,每一个已被确定为在API生产过程中可能产生的亚硝胺填写一行。
1 INTRODUCTION引言This document reports the outcome of the Risk Assessment on the above mentioned API based on the requirements defined in the EMA notices Information on nitrosamines for marketing authorisation holders EMA/189634/2019 and Questions and answers on ”Information on nitrosamines for marketing authorisation holders” EMA/428592/2019 and following revisions and other similar published by other Health Agencies.本文件报告上述API的风险评估结果,评估依据的是EMA/189634/2019《提供上市许可持有人使用的亚硝胺信息》和EMA/428592/2019《有关“提供上市许可持有人使用的亚硝胺信息”的问答》及其后续版本,以及其他卫生机构公布的类似资料。
Such evaluation on the potential risk of presence of nitrosamine impurities in the APIs was performed using quality risk management principles, as per ICH Q9 guideline and ICH M7. Manufacturing processes are being reviewed to identify and, if found, to mitigate risk of presence of N-nitrosamine impurities during manufacture and storage of the drug substance.对API中存在亚硝胺杂质的潜在风险的评估是根据ICH Q9指南和ICH M7中的质量风险管理原则进行的。
通过审查生产工艺,以确定并,如有,降低在API的生产和储存过程中出现N-亚硝胺杂质的风险。
2SCOPE范围The Risk Assessment has evaluated the following items as potential sources of nitrosamines or their precursors in line with root causes described in Questions and answers on Information on nitrosamines for marketing authorisation holders EMA/428592/2019:风险评估根据EMA/428592/2019《有关“提供上市许可持有人使用的亚硝胺信息”的问答》中所述的根本原因,评估了下列物品作为亚硝胺或其前体的潜在来源:e of sodium nitrite or other nitrosating agents in presence of secondary, or tertiaryamines, or quaternary ammonium salts within the API manufacturing process either within the same or at different process steps.在API制造过程中,存在仲胺、叔胺或季铵盐的相同或不同的工序中使用亚硝酸钠或其他硝化剂。
e of sodium nitrite or other nitrosating agents in combination with reagents, solvents,and catalysts, which are susceptible to degradation to secondary or tertiary amines, within the same or different process steps.在相同或不同的加工步骤中,将亚硝酸钠或其他硝化剂与易于降解为仲胺或叔胺的试剂、溶剂和催化剂结合使用。
e of contaminated raw materials (e.g. solvents, reagents, catalysts)使用受污染的原料(例如溶剂、试剂、催化剂)e of recovered materials (e.g. solvents, reagents, catalysts)使用回收物料(例如溶剂、试剂、催化剂)e of contaminated starting materials and intermediates supplied by vendors.使用由供应商提供的受污染起始材料和中间体。
6.Potential contamination due to risk for cross-contamination or carry over inmultipurpose plants由于交叉污染风险或在共线工厂结转而造成的潜在污染7.Degradation processes of starting materials, intermediates, or drug substances.起始物料、中间体或药物成分的降解过程。
8.Primary packaging materials.内包装材料。
3 MANUFACTURING / PROCESS STEPS COVERED BY RISK ASSESSEMENT风险评估涵盖的3个制造/加工步骤The Manufacturing Process described below may not contain some process details due to Intellectual Property Protection as per Company Policy.根据公司政策,由于知识产权保护,下面描述的制造过程可能不包含某些工艺细节。
4 RISK ASSESSMENT METHODOLOGY4风险评估方法The Risk Assessment methodology used has been [FMEA / Questionnaire / other]. The risk assessment for potential presence of nitrosamines has been conducted taking into account the following dimensions [severity, probability, detectability].所采用的风险评估方法是[FMEA/调查问卷/其他]。
