2015 (Purinergic Signal) P2X7 and Fyn mediate ATP-induced oligodendrocyte progenitor cell migration
细胞生物学C名词解释

细胞生物学C名词解释【1】细胞膜与物质的转运一、名词解释1. cell membrane(细胞膜)也称质膜,是指包围在细胞质外周的一层由蛋白质、脂类和糖类等物质组成的生物膜。
2. biological membrane/biomembrane(生物膜)细胞膜和细胞内各种膜性结构的统称。
3. unit membrane(单位膜)不同的生物膜在电镜下呈现一种较为一致的“两暗夹一明”的3层结构,即电子密度较高的内外两层(2nm×2)夹着电子密度较低的中间层(3.5nm)。
4. amphipathic molecule(两亲性分子/双亲媒性分子)像磷脂分子那样的,既具有亲水的极性头部,又具有疏水的非极性尾部的分子。
5. head group(头部基团)磷脂分子中亲水的小基团位于分子的末端与带负电的磷酸基团一起形成的高度水溶性的结构域,极性很强。
6. integral/intrinsic/transmenbraneprotein(整合/内在/穿膜蛋白)有的膜蛋白通过α-螺旋(也有β-片层)一次或多次穿膜而镶嵌在脂双层中。
7. extrinsic/peripheral protein(外在/周边蛋白)是一类与细胞膜结合疏松(非共价键)、不插入脂双层的蛋白质,分布于质膜的胞内侧或胞外侧。
8. lipid anchored/linked protein(脂锚定/连接蛋白)与外在蛋白类似位于膜的两侧、不穿膜,但以共价键和脂双层中的脂质分子结合。
9. the fluidity of cell membrane(细胞膜的流动性)是指构成细胞膜的膜脂和膜蛋白处于不断的运动状态,是保证细胞正常功能的重要条件。
10. liposome(脂质体)脂质分子在水相中会自发形成脂质双分子层。
为了避免其两端疏水尾部和水的接触,游离端往往可以闭合形成一种自我封闭的稳定的中空结构,称为脂质体。
11. phase transition(相变)由于温度的改变导致膜状态的改变。
神经递质.ppt

Outline
Neurotransmitter categories Neurotransmitter chemistry Some important neurotransmitters
Basic Concepts of NT
Neurotransmitter
Endogenous signaling molecules that alter the behaviour of neurons or effector cells.
A diversity of subunits come together to form functional ionotropic receptors
Two Families of Postsynaptic Receptors
Transmitter-gated ion channels
Agent of transmission Synaptic delay
Ion current Virtually absent
Direction of transmission
Usually bidirectional
unidirectional
Signal Transmission at Chemical Synapses
Neuromodulator
Endogenous signaling molecules that regulate the behaviour of neurons or effector cells.
Criteria for neurotransmitter
The molecule must be synthesized and stored in the presynaptic neuron.
Hippo-YAP信号通路及其在脑胶质瘤中的研究 20151125

Hippo-YAP信号通路及其在脑胶质瘤中的研究谢鹏1, 耿德诚1, 姜洋1综述周秀萍2审校1.徐州医学院研究生学院神经外科在读研究生,地址:徐州医学院淮西校区神经系统疾病研究所江苏徐州2.周秀萍,博士,院聘教授,硕导,徐州医学院神经系统疾病研究所副所长,教育部“新世纪优秀人才支持计划”培养对象,省“333”中青年科学技术带头人,省“六大人才高峰”培养对象江苏省徐州市泉山区淮海西路84号徐州医学院西校区生化楼3楼神经外科实验室邮箱:***************.cn摘要:恶性脑胶质瘤是最常见的原发性脑肿瘤,即使采用手术联合放、化疗等综合治疗措施,效果也不理想,发病率和致死率极高,因此,积极探寻脑胶质瘤发生发展的分子机制,发现潜在的分子治疗靶点有着重要意义。
Hippo-YAP信号通路是通过调控细胞增殖、凋亡和分化来调控器官大小的一个新兴信号通路,YAP是其核心效应分子,Hippo通路通过调控YAP的磷酸化和核定位来发挥作用。
生理状态下,Hippo-YAP信号通路的调节处于动态平衡中,保证细胞增殖和凋亡的平衡及内环境的稳态。
Hippo-YAP通路失调,尤其是YAP水平升高在人类多种肿瘤中均有发现,下调YAP抑制肿瘤细胞的增殖,提示YAP可能是恶性肿瘤潜在的治疗靶点。
本文综述了Hippo-YAP信号通路组成和作用及其在脑胶质瘤中的研究,为寻找脑胶质瘤治疗新靶点提供新思路。
关键词:Hippo-YAP信号通路;YAP;脑胶质瘤脑胶质瘤是最常见的原发性颅脑肿瘤,由大脑和脊髓的胶质细胞异常恶性增生所致。
目前,世界卫生组织(WHO)根据脑胶质瘤的组织病理分化程度及细胞异型性特点,将胶质瘤按照恶性程度由低到高分为4个级别,1级恶性程度最低、预后最好,4级恶性程度最高、预后最差。
由于恶性脑胶质瘤细胞易浸润到正常脑组织,通过手术不能完全切除,故复发率较高[1]。
尽管对恶性胶质瘤采用了手术、替莫唑胺化疗和放疗的综合治疗,效果依然不理想,联合放化疗后一年生存率也仅为57%[2],因此,积极探寻脑胶质瘤发生发展的分子机制有着重要的理论与临床意义。
藁本内酯抑制氧糖剥夺再灌注损伤神经元凋亡的机制

藁本内酯抑制氧糖剥夺再灌注损伤神经元凋亡的机制来自中国医科大学附属盛京医院的毕国荣团队最新研究发现,在体外培养PC12细胞模拟神经细胞氧糖剥夺再灌注损伤的模型中,自噬激活可以抑制氧糖剥夺再灌注诱导的细胞凋亡。
藁本内酯是当归、川芎等传统中药的主要有效成分之一。
在氧糖剥夺再灌注诱导的PC12细胞凋亡中,藁本内酯可通过激活自噬抑制凋亡起到细胞保护作用。
缺血性脑血管疾病具有高致残率和高致死率的特点。
目前针对缺血的标准治疗是快速实现再灌注,但是,缺血再灌注损伤会加重组织损伤。
因此,需要针对缺血再灌注损伤探索新的靶分子进而开展新的治疗策略。
自噬是细胞在应激情况下所做出的一种非损伤性应答反应。
当受到各种生理或病理刺激因子的作用时,自噬介导的降解反应对稳定细胞形态和结构,对维持细胞正常功能具有重要意义。
此外,自噬缺陷可导致细胞碎片不能被及时清除,继而诱导细胞凋亡。
既往研究证实,可通过阻止线粒体自噬发挥拮抗细胞凋亡的作用。
因此,以自噬调控作为潜在靶点对缺血脑血管疾病治疗具有重要意义。
此次,毕国荣等的实验发现,藁本内酯能够抑制氧糖剥夺再灌注诱导的PC12细胞凋亡。
首先发现,藁本内酯可以通过增加Bcl-2,抑制Bax表达抑制氧糖剥夺再灌注诱导的PC12细胞凋亡。
此外,藁本内酯的这种抗细胞凋亡保护作用,可被自噬抑制剂3-甲基腺嘌呤抑制,说明藁本内酯可能通过调节自噬阴性细胞凋亡。
同时还发现藁本内酯可以上调自噬相关蛋白LC3-II以及p-LKB1和p-AMPK 的表达水平,并通过激活LKB1-AMPK-mTOR通路调节自噬,抑制凋亡发挥对氧糖剥夺再灌注诱导的细胞损伤保护作用,说明藁本内酯是治疗神经系统缺血再灌注损伤的潜在药物。
文章发表在《中国神经再生研究(英文版)》杂志2020年3期。
文章摘要:最近的研究证实自噬对脑损伤具有一定的保护作用,藁本内酯是一种从传统中药川芎中分离出来的生物活性物质,具有保护神经细胞的药理作用,但是藁本内酯的神经保护作用是否通过影响细胞自噬的机制而发挥,目前尚不清楚。
华中科技大学学术期刊分类目录(T-D)_最新权威版
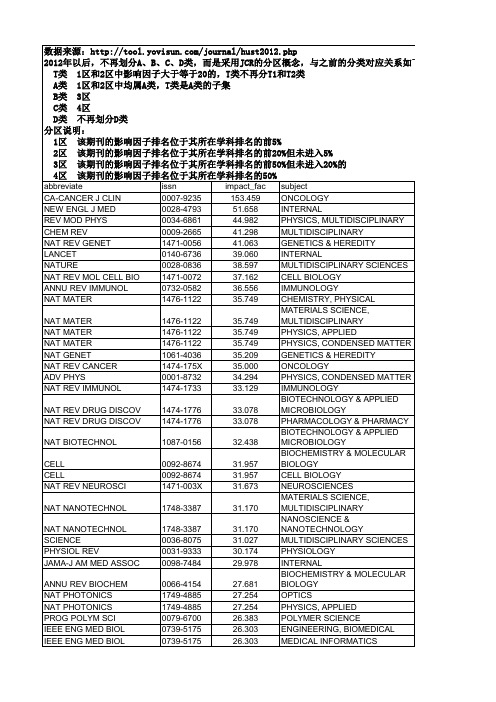
24.755 23.917 23.654 23.565 23.333 23.194 22.929 22.864 22.864 22.864 22.490 22.345 22.333 21.757 21.543 21.543 20.833 20.761 20.614 19.966 19.795 19.547 19.352 18.571 18.571 18.038 17.983 17.983 17.949 17.689 17.689 17.689 17.436 17.313 17.215 16.417 16.238 16.179 16.008 16.008 15.766 15.575 15.518 15.389 15.389 15.389 15.333 15.333 15.280 15.280 15.265 15.253 15.251 15.202
ONCOLOGY CLINICAL NEUROLOGY PLANT SCIENCES BIOCHEMICAL RESEARCH METHODS ASTRONOMY & ASTROPHYSICS MATERIALS SCIENCE, MULTIDISCIPLINARY PHYSICS, MULTIDISCIPLINARY BIOCHEMISTRY & MOLECULAR BIOLOGY CELL BIOLOGY MEDICINE, RESEARCH & EXPERIMENTAL MICROBIOLOGY PHARMACOLOGY & PHARMACY PHYSICS, PARTICLES & FIELDS CHEMISTRY, MULTIDISCIPLINARY PHARMACOLOGY & PHARMACY TOXICOLOGY CHEMISTRY, MULTIDISCIPLINARY CELL BIOLOGY NEUROSCIENCES INFECTIOUS DISEASES IMMUNOLOGY PHYSIOLOGY PHYSICS, MULTIDISCIPLINARY BEHAVIORAL SCIENCES NEUROSCIENCES ONCOLOGY CELL BIOLOGY DEVELOPMENTAL BIOLOGY ECOLOGY CHEMISTRY, MULTIDISCIPLINARY MAபைடு நூலகம்ERIALS SCIENCE, MULTIDISCIPLINARY NANOSCIENCE & NANOTECHNOLOGY GENETICS & HEREDITY MICROBIOLOGY MEDICINE, GENERAL & INTERNAL MICROBIOLOGY ASTRONOMY & ASTROPHYSICS MATERIALS SCIENCE, MULTIDISCIPLINARY BEHAVIORAL SCIENCES NEUROSCIENCES NEUROSCIENCES PSYCHOLOGY CLINICAL NEUROLOGY ECOLOGY EVOLUTIONARY BIOLOGY GENETICS & HEREDITY CHEMISTRY, PHYSICAL PHYSICS, CONDENSED MATTER BIOCHEMISTRY & MOLECULAR BIOLOGY CELL BIOLOGY PSYCHOLOGY MEDICINE, GENERAL & INTERNAL NEUROSCIENCES CARDIAC & CARDIOVASCULAR SYSTEMS
J. Exp. Bot.-2013-Banasiak-1005-15

Journal of Experimental Botany , Vol. 64, No. 4, pp. 1005–1015, 2013doi:10.1093/jxb/ers380 Advance Access publication 10 January, 2013© The Author [2013]. Published by Oxford University Press [on behalf of the Society for Experimental Biology]. All rights reserved. For permissions, please email: journals.permissions@Abbreviations: ABA, abscisic acid; ABC, ATP-binding cassette; β-AS, β-amyrin synthase; EV , empty vector; GUS, β-glucuronidase; IFS, isoflavone synthase; LC/ESI/MS, liquid chromatography/electrospray ionization/mass spectrometry analysis; MAPK, mitogen-activated protein kinase; MeJA, methyl jasmonate; PAL, phenylalanine ammonia-lyase; PAMP , pathogen-associated molecular pattern; PDR, pleiotropic drug resistance; RNAi, RNA interference; SA, salicylic acid; SD, standard deviation.ReseaRch papeRA Medicago truncatula ABC transporter belonging to subfamily G modulates the level of isoflavonoidsJoanna Banasiak 1, Wanda Biała 2, Anna Staszków 1, Barbara Swarcewicz 1, Ewa K ępczy ńska 3, Marek Figlerowicz 1 and Michał Jasi ński 1,2*1Institute of Bioorganic Chemistry PAS, Noskowskiego 12/14, 61–704 Pozna ń, Poland 2Department of Biochemistry and Biotechnology, Pozna ń University of Life Sciences, Woły ńska 35, 60–637 Pozna ń, Poland 3Department of Plant Biotechnology, Faculty of Natural Science, University of Szczecin, Waska 13, 71–415 Szczecin, Poland * To whom correspondence should be addressed. E-mail: jasinski@ibch.poznan.plReceived 8 October 2012; Revised 22 November 2012; Accepted 12 December 2012AbstractFull-sized ATP-binding cassette (ABC) transporters of the G subfamily (ABCG) are considered to be essential compo-nents of the plant immune system. These proteins have been proposed to be implicated in the active transmembrane transport of various secondary metabolites. Despite the importance of ABCG-based transport for plant–microbe interactions, these proteins are still poorly recognized in legumes. The experiments described here demonstrated that the level of Medicago truncatula ABCG10 (MtABCG10) mRNA was elevated following application of fungal oli-gosaccharides to plant roots. Spatial expression pattern analysis with a reporter gene revealed that the MtABCG10 promoter was active in various organs, mostly within their vascular tissues. The corresponding protein was located in the plasma membrane. Silencing of MtABCG10 in hairy roots resulted in lower accumulation of the phenylpropa-noid pathway-derived medicarpin and its precursors. PCR-based experiments indicated that infection with Fusarium oxysporum , a root-infecting pathogen, progressed faster in MtABCG10-silenced composite plants (consisting of wild-type shoots on transgenic roots) than in the corresponding controls. Based on the presented data, it is proposed that in Medicago , full-sized ABCG transporters might modulate isoflavonoid levels during the defence response associ-ated with de novo synthesis of phytoalexins.Key words: ABCG transporters, immune system, isoflavonoids, Medicago truncatula , PDR transporters, phytoalexin.IntroductionATP-binding cassette (ABC) transporters form one of the largest and most evolutionarily conserved families of proteins in all kingdoms. They possess a conserved domain-based structure and are classified into eight subfamilies (ABCA–H) (Verrier et al ., 2008). Full-sized ABC transporters of the ABCG subfamily [formerly called pleiotropic drug resist-ance (PDR)] have been identified in plants, fungi, oomycetes, brown algae, and slime molds (Kang et al ., 2011).Several full-sized ABCG proteins play a role in the response to biotic stress, especially in a non-specific manner, which con-fers protection against a wide group of pathogens. For example, RNA interference (RNAi)-mediated silencing of NpPDR1 causes Nicotiana plumbaginifolia to be more sensitive to infec-tion with fungal (Botrytis cinerea and Fusarium oxysporum ) and oomycete pathogens (Phytophthora nicotianae ) (Bultreys et al ., 2009). Expression of Nicotiana tabacum NtPDR1 was shownat Huazhong Agricultural University on May 13, 2013/Downloaded from1006|Banasiak et al.to be induced by treatment with various general elicitors such as flagellin Psto, yeast extract, and INF1 elicitin (Sasabe et al., 2002). I n Arabidopsis, the mRNA level of AtPDR8/PEN3/ AtABCG36 is elevated during plant infection with virulent and avirulent strains of the bacterial pathogen Pseudomonas syrin-gae. Knockout of AtPDR8 decreases Arabidopsis resistance to inappropriate pathogenic fungi (Kobae et al., 2006; Stein et al., 2006). Expression of Arabidopsis PDR12/AtABCG40, signifi-cantly increases after infection with compatible (Sclerotina sclerotiorum) and incompatible (Alternaria brassicicola) fungal pathogens, as well as after treatment with salicylic acid (SA), ethylene, or methyl jasmonate (MeJA) (Campbell et al., 2003). In wheat (Triticum aestivum), a putative full-size ABCG trans-porter (LR34) confers a durable resistance to multiple fungal pathogens (Krattinger et al., 2009). Recently, a new opening for the role of ABCG transporters in response to biotic stress has come with the finding of the involvement of the N. tabacum ABCG5 transporter in resistance to Manduca sexta herbivory (Bienert et al., 2012). It was also shown that ABCG32/PEC1 in Arabidopsis and HvABCG31/Eibi1 in barley are required for the formation of a functional cuticle, which can act as the first barrier against abiotic and biotic stresses (Bessire et al., 2011; Chen et al., 2011).Legume ABC transporters additionally attract attention as being possibly implicated in the establishment of symbioses (Sugiyama et al., 2006; Takanashi et al., 2011). Their identi-fication has been accelerated by deciphering of the genome sequences of the model legumes Glycine max and Medicago truncatula (Schmutz et al., 2010; Y oung et al., 2011). Two M. truncatula so-called half-sized ABCG transporters (STR and STR2) that are present in peri-arbuscular membranes were found to be indispensable for arbuscule development in mycorrhizal symbiosis (Zhang et al., 2010). Expression of several legume ABCG genes was shown to be induced upon treatment with molecules such as SA or with pathogenic fungi (Eichhorn et al., 2006; Jasinski et al., 2009).The proposed role of ABCGs in the immune system is modulation of the transmembrane transport of signalling/ defensive compounds. Thus, efforts have been made to iden-tify the phytochemicals transported by the various ABCGs (Badri et al., 2008; Badri et al., 2009). Recently, it was reported that secretion of strigolactones, signalling molecules found in the initiation of arbuscular mycorrhizae, is the contribu-tion of the full-sized ABCG transporter (Kretzschmar et al., 2012). Additionally, Arabidopsis ABCG29 has been found as a p-coumaryl alcohol transporter, and it has been suggested that proper function of this protein has a complex impact on phenolic compounds and glucosinolate levels in this plant (Alejandro et al., 2012). Dysfunction of certain ABCG trans-porters in Arabidopsis results in the accumulation of flavonoid glycosides (kaempeferol and quercetin) in the root tissues (Badri et al., 2012). Flavonoids play a particular role in biotic stress responses. This multifaceted group of plant secondary products can function as antimicrobial agents, UV protect-ants, pollinator attractants, floral pigments, and inducers of the nodulation genes in symbiotic soil bacteria known as rhizobia. A special subclass of flavonoids is composed of iso-flavonoids, which are limited primarily to the Leguminosae. Isoflavonoids are thought to represent the majority of phy-toalexins produced by legume plants (Hassan and Mathesius, 2012). For instance, soybean partial resistance to Fusarium solani seems to be associated with the ability of soybean rootsto produce the phytoalexin glyceollin in response to fungal infection (Lozovaya et al., 2004). It has been proposed thatABC-type transporters can be involved in the secretion of (iso)flavonoids from soybean roots (Sugiyama et al., 2007).In this study, a full-sized ABCG plasma membrane trans-porter from M. truncatula was characterized. Spatial expres-sion pattern analysis with the β-glucuronidase (GUS) reportergene revealed the activity of the MtABCG10 promoter in vari-ous organs including, roots, leaves, flowers, and fruits. Silencingof MtABCG10 in hairy roots resulted in a lower accumulationof isoflavone precursors of the phytoalexin medicarpin. I n addition, faster spreading of F. oxysporum in MtABCG10-silenced Medicago was observed compared with control plants. Materials and methodsPlant materialM. truncatula (Jemalong J5) seedlings were germinated on water-saturated Whatman discs in Petri plates and grown under controlled greenhouse conditions with a mean temperature of 22°C, 50% humidity, and a 16 h photoperiod.Leaf-originated Medicago suspension cell cultures were main-tained in a 16 h photoperiod at 22 °C on an orbital shaker (150 rpm).The cultures were grown in medium (Murashige and Skoog mediumplus Gamborg’s vitamins supplemented with 30 g l–1 of saccharose,2 mg l–1 of 2,4-dichlorophenoxyacetic acid, and 0.25 mg l–1 of kine-tin), and were diluted 1:2 every 2 weeks.Plants with silenced MtABCG10 expression were obtained fromM. truncatula after infection of a radicle with Agrobacterium rhizo-genes Arqua1 (/medicagohandbook).Hairy-root cultures were initiated by cutting off the roots and growing them in the dark at 22 °C on solid Fahraeus medium, sup-plemented with saccharose (10 g l–1), myoinositol (100 mg l–1), thi-amine (10 mg l–1), pyridoxine (1 mg l–1), biotin (1 mg l–1), nicotinicacid (1 mg l–1), and glycine (2 mg l–1). Fragments of hairy roots were transferred onto fresh medium every 3 weeks.Fungal elicitor and MeJA treatmentThe Phoma medicaginis oligosaccharide elicitor was prepared as described previously (Hahn et al., 1992). The concentration of the elic-itor was determined by the phenol/sulphuric acid method (Fry, 1994).Five-d-old Medicago seedlings were transferred to solid 0.5× Gamborg’s medium supplemented with elicitor (25 µg ml–1) or MeJA (10 µM). Water and DMSO were used as controls, respec-tively. Samples were collected at 1, 2, 3, and 6 h after transfer and immediately frozen.For metabolomic analysis, 3-week-old root cultures (250 ± 50 mg)were transferred into liquid medium (5 ml) and acclimatized for 24 h. Samples (hairy roots and medium) were collected at 6, 24, and 72 hafter treatment with elicitor (25 µg ml–1) or water and immediately frozen.Real-time quantitative RT-PCR (qRT-PCR) analysisRNA was isolated from plant material with an RNeasy Extraction kit (Qiagen). Genomic DNA was removed by on-column DNase treatment. Total RNA (500 ng) was converted to cDNA with Omniscript reverse transcriptase (Qiagen), accord-ing to the manufacturer’s protocol. Real-time PCR analysis wasat Huazhong Agricultural University on May 13, 2013/Downloaded fromMedicago ABCG and (iso)flavonoids | 1007performed in Rotor-Gene Q Real Time PCR machine (Corbett Research), using the MESA Green qPCR MasterMix Plus SYBR (Eurogentec). Primers sequences were as follows: MtABCG10:forward, 5’-AACTACTGTTATGTCGACCG-3’, and reverse5’-CACTATCTTTCATTGATGATC-3’; and actin (GenBank no.JQ028731): forward, 5’-TTCTCTCAGTACTTTCCAGC-3’, andreverse 5’-AAGCATCACAATCACTCC-3’. The threshold cyclemethod was used as described by Ruocco et al . (2011).Quantitative transcript abundance analysisRNA was isolated from Medicago roots and converted to cDNA as described for qRT-PCR analysis. The genomic DNA was extracted with a DNeasy kit (Qiagen). PCRs on DNA and cDNA as templates (30 cycles) were performed in an MJ Mini Personal Thermal Cycler (Bio-Rad). The sequences of the primers used for the amplification were as follows: forward, 5’-CATATTGGTATTGGATAGGCG-3’, and reverse 5’-CACTTACACCCATCAAAGC-3’. PCR/RT-PCR products were cloned into pGEM-T Easy (Promega) and 80 ran-domly selected clones were sequenced with Sp6/T7 primers.Preparation of microsomal and plasma membrane fractionsMicrosomal fractions were isolated from 150 mg of Medicago hairy-root culture or 4 g of suspension cell culture as described previously (Jasinski et al ., 2001). The plasma membranes were purified from microsomal fractions of M. truncatula suspension cell cultures by partitioning in an aqueous two-phase partition system (6 ml of phase mixture), as described by Larsson et al . (1987).Western blot analysisProteins (5 µg) were separated by SDS-PAGE and transferred to aPVDF membrane (Millipore) by electroblotting (semi-dry appara-tus; BioRad). The membrane was incubated either with primary pol-yclonal antibodies against a peptide corresponding to Glu2–Glu27of MtABCG10 (Eurogentec) or with primary antibodies specific forthe H +-ATPase (W1G) (Morsomme et al ., 1998). The secondaryantibodies were alkaline phosphatase-conjugated goat anti-rabbitIgG (Sigma).Genetic constructs and plant transformation The promoter region of MtABCG10 (710 bp) was amplified with the primers 5’-ATGAATTCAAGAAGCTGCCACTAAAGC-3’and 5’-TAGGATCCATTTTTTGTGCTGTTGTG-3’. The PCRproduct was cloned via Eco RI and Bam HI restriction sites into thepPR97 binary vector carrying the uidA reporter gene (Szabadoset al ., 1995).The cDNA fragment (139 bp) used for MtABCG10 RNAisilencing was amplified using the primers 5’-GGGGACAAGTTTG T A C A A A A A A G C A G G C T C T A T A A T T T T T A TGGATGAGCC-3’ and 5’-GGGGACCACTTTGTACAAGAAAGCTGGGTTCAAATATGTCAATGCTAGGC-3’, cloned intopDONR TM /Zeo (nvitrogen), and recombined into a modifiedGateway pK7GWI WG2(I I )-p35S ::DsRed binary vector (Limpenset al ., 2005).The binary vectors pPR97 and pK7GWI WG2(I I )-p35S ::DsRedwere transferred into the Agrobacterium tumefaciens strain EHA105and Agrobacterium rhizogenes strain Arqua1, respectively. Finally,the constructs were used to transform M. truncatula according tothe procedures described in M. truncatula handbook (http://www./medicagohandbook ).Transgenic plants carrying the MtABCG10P::GUS reporter con-struct were stained for GUS using 5-bromo-4-chloro-3-indolyl-β-d -glucuronide, according to the protocol described by Gallagher (1992). To identify transgenic roots carrying the silencing construct,the plant material was scanned with a Fluor I mager Fla-5100 (Fujifilm) using a 532 nm green laser and an LPG filter.In situ immunodetection of protoplasts and cells from M. truncatula suspension cultures Protoplasts from 6-d-old leaf-originated Medicago suspensioncell cultures were isolated as described previously (He et al ., 2007),with the exception that the digestion was carried out for 24 h in anenzyme solution consisting of 1.5% cellulase R10 (Serva), 0.6% mac-erozyme R10 (Serva), and 1% Driselase ® (Sigma-Aldrich). Cells werefixed with 3.7% formaldehyde in MS medium/MTSB buffer (50 mM PIPES, 5 mM MgSO 4.7 H 2O, 5 mM EGTA) (1:1) for 30 min and then washed with MS/MTSB. To reduce autofluorescence, the cells were treated with 0.1% NaBH 4. After washing with 0.5× MTSB, the cellwalls were partially digested (except for protoplast immunodetec-tion) with an enzyme cocktail (1.5% cellulase R10, 0.6% macerozyme, and 1% Driselase ® in 0.2 M mannitol, 20 mM KCl, and 20 mM MES, pH 5.8). The plasma membrane was permeabilized with 0.1% Triton X-100 and 0.05% Tween 20 in 0.5× MTSB. The samples were washed with PBS and saturated for 1 h in PBS containing 0.05% Tween 20 and 2% BSA. Finally, the samples were incubated with the primary antibodies anti-MtABCG10 (diluted 1:50), pre-immune serum (1:50), and anti-H +-ATPase (1:400) overnight at 4 °C. The cells were washed as described above and treated with secondary antibody replace by[Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen), diluted 1:600] for 2 h at room temperature. The cells were observed by laser-scanning confocal microscopy (Nikon A1R-si). Nuclei and vacuoles were stained with 0.1 mM DAPI and 0.1% neutral red, respectively .Extraction of phenolic compounds and liquid chromatography/electrospray ionization/mass spectrometry analysis (LC/ESI/MS)Frozen tissue (250 ± 50 mg fresh weight of roots) was ground at 4 °Cwith mortar and pestle and extracted with 4 ml of 80% methanol. Culture medium containing root exudates was subjected to solidphase extraction as described previously (Staszkow et al ., 2011).Dried extracted samples were dissolved in 80% methanol and sub-jected to LC/ESI/MS analysis. Profiles of phenolic compounds wereacquired using an AgilentRR 1200 liquid chromatograph micrOToF-Q mass spectrometer (Bruker Daltonics). Chromatographic separa-tion was performed using an acetonitrile/water gradient. The spectrawere recorded in the targeted mode within the m /z mass range of50–1000. Metabolite profiles were registered in the positive-ion mode. For details, see Staszkow et al . (2011).F . oxysporum infection of composite M. truncatula plants F. oxysporum f. sp. medicaginis strain 179.29 was purchased from theCentraalbureau voor Schimmelcultures, Utrecht, The Netherlands.F. oxysporum was grown on potato dextrose agar at 24 °C with aphotoperiod of 12 h. Suspensions of microconidia were obtained byflooding the Petri dish with sterile water. The spore concentrationwas determined by counting and was then adjusted to 1.3 × 106 sporesml –1. A 100 µl sample of spore suspension was deposit onto the rootsof 4-week-old composite M. truncatula plants grown on Fahraeusmedium. Eighteen empty vector -transformed control compositeplants and 18 MtABCG10-silenced composite plants in three biologi-cal repetitions were analysed. The infection efficiency was determinedas the level of fungal DNA in various Medicago organs. The sameamount of genomic DNA (40 ng) for each sample was used for qPCR.Reactions were conducted (as for qRT-PCR) using F. Oxysporum -specific primers (forward: 5’-ACCGTTGTAGACACCATTGC-3’;reverse: 5’-AGTGCGTAAGTGCTCATCG-3’) for the β-tubulingene (Genbank accession no. DQ092478.1) and M. truncatula spe-cific primers for actin .Results MtABCG10 (GenBank no. XM_003597771) has been described during studies dedicated to full-sized ABCGat Huazhong Agricultural University on May 13, 2013/Downloaded fromMedicago ABCG and (iso)flavonoids|1009(Fig. 2A–C, H, and I). In the leaves, a staining pattern was observed that was restricted to the junctions of three leaflets of the petiole and, to a lesser extent, the veinlets (Fig. 2D, E). In the flowers, GUS activity was observed in the anthers and in the pollen grains (Fig. 2F). In the fruit, GUS expression was seen in the conductive tissues (Fig. 2G).Subcellular localization of MtABCG10In situ immunolocalization of MtABCG10 in cultured Medicago suspension cells showed a clear signal coming from the plasma membrane (Fig. 3A). Under the same parameters of image acquisition, the control with pre-immune serum did not give any signal (Fig. 3B). These images were analogous to those observed for antibodies against H+-ATPase, a plasma membrane marker (Fig. 3C) and control with secondary antibodies (Fig. 3D), respectively. In situ immunolocaliza-tion with culture cells was performed because these do not require embedding, which was found to destroy antigenic epitopes. When protoplasts were used for MtABCG10 immu-nolocalization again, signals from membranes surrounding the cells were observed (Fig. 3E), compared with the control of pre-immune serum (Fig. 3F). The nucleus was visualized by DAPI staining (Fig. 3G). The absence of MtABCG10 from the vacuolar membrane (tonoplast) might be assumed as the vacuole in suspension culture used for immunolocal-ization can readily be stained by neutral red and appeared fragmented into several large vesicles that could not beFig. 2. Expression of the MtABCG10P::GUS reporter construct in M. truncatula. Whole mounts (A–F) of MtABCG10P::GUS transgenic plants were stained for GUS activity. (A–G) Root stele (A–C), leaf (D), young leaf (7-d-old) (E), flower (F), and fruit (G). (H, I) Longitudinal (H) and transverse (I) sections through a root of an MtABCG10P::GUS transgenic plant. Bars, 1.25 cm (A–C); 2.5 cm (D–G); 100 µm (H, I). at Huazhong Agricultural University on May 13, 2013 / Downloaded from1010 | Banasiak et al.misidentified as the plasma membrane. (Fig. 3H ). Finally, Western blot analyses confirmed that MtABCG10 accu-mulates in phase partition-purified plasma membrane frac-tions similarly to H +-ATPase (Fig. 3I ). The specificity of the MtABCG10 antibodies was assayed by Western blotting with protein extracts from MtABCG10-silenced and non-silenced plant material additionally treated or not with fungal oligo-saccharides (Supplementary Fig. S3 at JXB online).RNAi-mediated knockdown of MtABCG10 in Medicago hairy rootsA. rhizogenes -mediated RNAi is a fast and effective method to study gene function in legumes. Transformed hairy roots are a good alternative to stable transgenic lines and can be propagated clonally (Limpens et al ., 2004). To suppress MtABCG10 expression, a 139 bp fragment from the cod-ing region (nt 3156–3295 of the cDNA) was obtained and introduced into the pK7GWIWG2(II)-p35S ::DsRED binary vector (Limpens et al ., 2005). The ability of the RNAi con-struct (MtABCG10 RNAi) to silence the expression of the MtABCG10 gene in the roots was tested. A significant reduc-tion in the MtABCG10/MtABCG10 level was observed when assayed by real-time PCR and Western blotting (Supplementary Fig. S3).Upon elicitation, in the roots of Medicago , induction of MtABCG10 expression proceeded along with that of PAL and IFS (Supplementary Fig. S1). Because one of the pro-posed functions of the full-sized ABCG transporters in plants is the translocation of secondary metabolites (Jasinski et al ., 2001; Badri et al ., 2008; Sugiyama et al ., 2008), a search for phenotypic differences at the metabolome level was initiated. Hairy roots represent a fully differentiated tissue that tends to produce tissue-specific secondary metabolites and thus are a suitable material for such an approach (Pistelli et al ., 2010). Six independent control lines carrying empty vector (EV) and six MtABCG10-silenced lines of hairy roots were analysed. A strong reduction in MtABCG10 levels was not accompa-nied by any visible morphological changes in the transgenic roots (Supplementary Fig. S3). To mimic fungal infection, the transgenic roots were exposed to an elicitor (oligosaccha-rides isolated from P . medicaginis cell walls) that induces theexpression of MtABCG10 and the phenylpropanoid biosyn-thesis pathway.Both control and MtABCG10-silenced root tissue (treated or not with elicitor), as well as root exudates, were analysedby LC/ESI/MS. The profiling and identification of flavonoids and their glycoconjugates in the samples were based on stand-ards, LC retention times, and high-resolution mass spectra.LC/MS analysis revealed that free aglycones like the chalcone isoliquiritigenin and its derivatives liquiritigenin, 5-deoxyisoflavones (e.g. daidzein, formononetin, 2’-hydroksy-formononetin, and vestitone) and medicarpin differentially accumulated in elicited and non-elicited samples (Fig. 4, and Supplementary Figs. 4 and 5 at JXB online). This was also true for naringenin and 5-hydroxyisoflavone (e.g. biochaninA), although to a lesser extent (data not shown). This effect was visible in control and MtABCG10-silenced root tissueFig. 3. MtABCG10 is localized to the plasma membrane. (A–D) In situ immunodetection carried out on M. truncatula cells from suspension culture with secondary Alexa Fluor 488-conjugated antibodies, using primary antibodies against MtABCG10 (A), pre-immune serum (B), primary antibodies against H +-ATPase (C), and a control with secondary antibodies (D). (E, F) In situ immunodetection with protoplasts and secondary Alexa Fluor 488-conjugated antibodies, using primary antibodies against MtABCG10 (E) and pre-immune serum (F). (G, H) Neutral red staining of vacuoles (H) and DAPI staining of the nucleus (G). (I) Protein gel blot analysis of microsomal (Mi) and plasma membrane (PM) fractions (obtained by the two-phase partition method) prepared from M. truncatula cells grown in liquid medium. Protein samples (2.5 µg) were electrophoresed, blotted, and immunodetected using anti-MtABCG10 or anti-H +-ATPase antibodies. PM/Mi indicates the signal ratio for the two membrane fractions. at Huazhong Agricultural University on May 13, 2013/Downloaded fromMedicago ABCG and (iso)flavonoids|1013Owing to the pleiotropic substrate profiles that are often associated with ABCG proteins (e.g. AtABCG36/AtPDR8; Stein et al., 2006; Kim et al., 2007; Strader and Bartel, 2009), it cannot be excluded that MtABCG10 might transport sev-eral different molecules. The latter might be represented by isoliquiritigenin and/or liquiritigenin. These compounds strongly induced the expression of MtABCG10, and it has been shown that certain substrates for ABCG transporters (e.g. sclareolide) induce the expression of their transporter (e.g. NpABC1) (Jasinski et al., 2001).The relatively widespread expression of MtABCG10 in many organs might support the term ‘pleiotropic’, not only for the substrate profile but also for the function fulfilled by MtABCG10 in a particular organ. Apart from its role in roots and an effect on isoflavonoids as revealed by gene silencing, its precise role in other plant parts (e.g. flowers) remains to be elucidated.The translocation of phenolic compounds, especially fla-vonoids and isoflavonoids, is still a matter of debate. Several mechanisms have been proposed, including vesicle-mediated transport and membrane transporter-mediated transport (Hassan and Mathesius, 2012). To date, members of the ABCC [formerly multidrug resistance-associated protein (MRP)] sub-family of ABC proteins have been implicated in phenolic traffic (Goodman et al., 2004; Zhao and Dixon, 2010). However, data describing the transport of genistein in soybean (Sugiyama et al., 2008) and the fact that silencing of MtABCG10 is asso-ciated with changes in isoflavonoid composition bring new insights into the possible role of ABCG proteins in modulating the amount of phenolic compounds in legumes.It is also worth considering that the MtABCG10 substrate may be an unknown signalling molecule that regulates defence mechanisms that rely on isoflavonoid biosynthesis/transport. This regulatory mechanism probably does not affect, at least at the transcriptional level, key enzymes of isoflavonoid biosynthesis, such as PAL or I FS, because their expression profile does not vary in control and silenced lines follow-ing elicitation. The tissue expression pattern of MtABCG10 visualized with the GUS reporter system and its plasma membrane localization suggested that the MtABCG10 pro-tein might translocate such molecules through the stele. Membrane transporters are important players in the regula-tion of metabolite biosynthesis and fluxes (Zhao and Dixon, 2010). In view of the presented data, a new potential role for plant ABCGs as modulators of isoflavonoid levels in legumes during biotic stress can be postulated. Supplementary dataSupplementary data are available at JXB online.Fig. S1 RT-PCR time-course expression analysis of MtABCG10, PAL, IFS, and β-AS in M. truncatula seed-ling roots treated (+) or not (–) with P. medicaginis cell-wall oligosaccharides.Fig. S2 Phylogenetic tree of the MtABCG10 homologues. Fig. S3MtABCG10 silencing in hairy root cultures and specificity of the anti-MtABCG10 antibodies.Fig. S4 Relative levels of selected (iso)flavonoids and isoflavonoid conjugates in M. truncatula control (EV) and MtABCG10-silenced (RNAi10) hairy root cultures.Fig. S5 Relative levels of selected (iso)flavonoids and isoflavonoid conjugates in M. truncatula control (EV) and MtABCG10-silenced (RNAi10) root exudates.Fig. S6 Time-course expression analysis of MtABCG10 inM. truncatula seedlings roots treated (+) or not (–) with isoli-quiritigenin (100 µM) (A) or liquiritigenin (100 µM) (B).Fig. S7 Pictures of control and MtABCG10-silenced plants infected with F. oxysporum.Fig. S8 Time-course expression analysis of MtABCG10 transcript (A) and protein (B) levels in M. truncatula sus-pension cell cultures treated (+) or not (–) with ABA, SA,or MeJA.Fig. S9 Comparison of MtABCG genes expression in con-trol (EV) and MtABCG10-silenced (RNAi10) hairy roots1 h after treatment (+) or (–) with P. medicaginis cell-wall oligosaccharides.Fig. S10 Outline of the biosynthetic pathways leading tothe major classes of flavonoids in M. truncatula.AcknowledgmentsWe thankI. Femiak for excellent technical assistance,M. Stobiecki and D. Muth for their help with LC/MS work, M. Maruniewicz and I. Ziomkiewicz for their helpwith the confocal microscope, P. Bednarek for critical com-ments, M. Boutry for W1G and GPDR antibodies, andE. Limpens for the pK7GWIWG2(II)-p35S::DsRED binary vector. National Science Centre Grants supported this work:2011/03/B/NZ1/02840 and N301 392139.ReferencesAlejandro S, Lee Y, Tohge T, Sudre D, Osorio S, Park J, Bovet L,Lee Y, Geldner N, Fernie AR, Martinoia E. 2012. AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Current Biology22, 1207–1212.Anderson JP, Lichtenzveig J, Gleason C, Oliver RP, Singh KB.2010. The B-3 ethylene response factor MtERF1-1 mediates resistanceto a subset of root pathogens in Medicago truncatula without adversely affecting symbiosis with rhizobia. Plant Physiology154, 861–873.Badri DV, Chaparro JM, Manter DK, Martinoia E, Vivanco JM.2012. Influence of ATP-binding cassette transporters in root exudationof phytoalexins, signals, and in disease resistance. Frontiers in PlantScience3, 149.Badri DV, Loyola-Vargas VM, Broeckling CD, et al. 2008. Alteredprofile of secondary metabolites in the root exudates of ArabidopsisATP-binding cassette transporter mutants. Plant Physiology146,762–771.Badri DV, Quintana N, El Kassis EG, Kim HK, Choi YH,Sugiyama A, Verpoorte R, Martinoia E, Manter DK, VivancoJM. 2009. An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota.Plant Physiology151, 2006–2017.at Huazhong Agricultural University on May 13, 2013/Downloaded from。
依达拉奉右莰醇通过抑制TLR4NF-κB信号通路减轻实验性自身免疫性脑脊髓炎小鼠炎症反应
实验研究依达拉奉右莰醇通过抑制TLR4/NF-κB信号通路减轻实验性自身免疫性脑脊髓炎小鼠炎症反应晚丽,李作孝△摘要:目的探讨依达拉奉右莰醇对实验性自身免疫性脑脊髓炎(EAE)小鼠炎症反应的影响及其机制。
方法30只雌性C57BL/6小鼠随机分为空白组、模型组、依达拉奉右莰醇干预组各10只。
除空白组外,其余2组小鼠均采用髓鞘少突胶质细胞糖蛋白35-55(MOG35-55)多肽诱导EAE模型。
从造模次日开始,依达拉奉右莰醇干预组腹腔注射依达拉奉右莰醇12.5mg/kg,空白组及模型组腹腔注射等量生理盐水,1次/d,连续14d。
观察小鼠发病情况,并行神经功能障碍评分;HE和LFB染色观察脊髓组织病理改变;实时荧光定量PCR检测脑组织匀浆中白细胞介素(IL)-1β、IL-6及肿瘤坏死因子(TNF)-αmRNA表达水平;蛋白免疫印迹法检测脊髓组织中Toll样受体4(TLR4)、核因子κB p65(NF-κB p65)蛋白表达水平。
结果空白组小鼠均未发病,其余2组小鼠不同程度发病。
与模型组相比,依达拉奉右莰醇干预组小鼠的发病潜伏期、高峰期延迟,高峰期神经功能障碍评分降低(P<0.01)。
空白组小鼠脊髓组织未见异常;模型组脊髓组织大量炎性细胞浸润、髓鞘结构紊乱;依达拉奉右莰醇干预组较模型组的炎性细胞浸润减少、髓鞘结构紊乱情况改善。
与空白组相比,其余2组小鼠脑组织匀浆中IL-1β、IL-6、TNF-αmRNA表达水平以及脊髓组织中TLR4、NF-κB p65蛋白表达水平显著升高,以依达拉奉右莰醇干预可逆转建模引起的上述改变(P<0.05)。
结论依达拉奉右莰醇可减轻EAE小鼠炎症反应,其机制可能与抑制TLR4/NF-κB信号通路活化有关。
关键词:脑脊髓炎,自身免疫性,实验性;Toll样受体4;NF-κB;炎症;白细胞介素类;肿瘤坏死因子α;依达拉奉右莰醇;TLR4/NF-κB信号通路中图分类号:R744.51文献标志码:A DOI:10.11958/20212362Edaravone dexborneol reduces inflammation in mice with experimental autoimmuneencephalomyelitis by inhibiting TLR4/NF-κB signaling pathwayWAN Li,LI Zuoxiao△Department of Neurology,the Affiliated Hospital of Southwest Medical University,Luzhou646000,China△Corresponding Author E-mail:****************Abstract:Objective To investigate the effect and mechanism of edaravone dexborneol on the inflammatory response in mice with experimental autoimmune encephalomyelitis(EAE).Methods Thirty female C57BL/6mice were randomly divided into the blank group,the model group and the edaravone dexborneol intervention group,with10mice in each group. Except for the blank group,EAE model was induced by myelin oligodendrocyte glycoprotein35-55(MOG35-55) polypeptide in the other two groups.From the day after modeling,mice in the edaravone dexborneol intervention group were intraperitoneally injected with edaravone dexborneol12.5mg/kg,while the mice in the blank group and the model group were intraperitoneally injected with the equal amount normal saline,once a day for consecutive14days.The behavioral changes of mice were observed,and neurological dysfunction scores were performed.HE and LFB staining were used to detect spinal cord pathological changes.The mRNA expression levels of interleukin(IL)-1β,IL-6and tumor necrosis factor-α(TNF-α)in brain homogenate were detected by real-time fluorescence quantitative PCR.The protein expression levels of Toll-like receptor4(TLR4)and nuclear factorκB p65(NF-κB p65)in spinal cord tissue were detected by Western blot assay.Results None of the mice in the blank group had the disease,and the other two groups of mice had different degrees of pared with the model group,the incubation period and peak period were delayed in the edaravone dexborneol intervention group,and neurological deficit scores in peak period decreased(P<0.01).No abnormality was found in spinal cord tissue structure in mice of the blank group,and a large number of inflammatory cell infiltration,myelin structure 基金项目:泸州市人民政府-西南医科大学科技战略合作基金项目(2018LZXNYD-ZK17)作者单位:西南医科大学附属医院神经内科(邮编646000)作者简介:晚丽(1994),女,硕士在读,主要从事神经免疫方面研究。
3302222a[1]
RESEARCH ARTICLEDelivery of GDNF by an E1,E3/E4deleted adenoviral vector and driven by a GFAP promoter prevents dopaminergic neuron degeneration in a rat model of Parkinson’s diseaseNA Do Thi 1,P Saillour 1,L Ferrero 2,JF Dedieu 2,J Mallet 1and T Paunio 1,31Laboratoire de Genetique Moleculaire de la Neurotransmission et des Processus Neurodegeneratifs,CNRS,Bat.CERVI,Hopital Pitie-Salpetriere,Paris,France;2Gencell SAS,Vitry sur Seine,France;and 3Department of Molecular Medicine,Biomedicum,Helsinki,FinlandA new adenoviral vector (Ad-GFAP-GDNF)(Ad-¼adenovirus,GFAP ¼glial fibrillary acidic protein,GDNF ¼glial cell line-derived neurotrophic factor)was constructed in which (i)the E1,E3/E4regions of Ad5were deleted and (ii)the GDNF transgene is driven by the GFAP promoter.We verified,in vitro,that the recombinant GDNF was expressed in primary cultures of astrocytes.In vivo,the Ad-GFAP-GDNF was injected into the striatum of rats 1week before provoking striatal 6-OHDA lesion.After 1month,the striatal GDNF levels were 37pg/m g total protein.This quantity was at least 120-fold higher than in nontransduced striatum or after injection of the empty adenoviral vector.At 3months after viral injection,GDNF expression decreased,whereas the viral DNA remained unchanged.Furthermore,around 70%of the dopaminergic (DA)neurons were protected from degeneration up to 3months as compared to about 45%in the control groups.In addition,the ampheta-mine-induced rotational behavior was decreased.The results obtained in this study on DA neuron protection and rotational behavior are similar to those previously reported using vectors with viral promoters.In addition to these results,we established that a high level of GDNF was present in the striatum and that the period of GDNF expression was prolonged after injection of our adenoviral vector.Gene Therapy (2004)11,746–756.doi:10.1038/sj.gt.3302222Published online 15January 2004Keywords:GDNF;Parkinson’s disease;DA neurons;recombinant adenovirusIntroductionParkinson’s disease (PD)is a progressive neurodegen-erative disorder.It is characterized by tremor,bradyki-nesia,rigidity and postural instability that result from a loss of dopaminergic (DA)neurons of the nigrostriatal pathway.The best current therapy of PD is the administration of L -Dopa.However,L -Dopa loses its effectiveness as the disease progresses.Different ther-apeutic approaches are therefore being investigated such as the use of neurotrophic factors,1–4cell/tissue transplantation 5–7and gene transfer of trophic factors using recombinant adenovirus,recombinant adeno-associated virus (AAV)or lentivirus.8,9The ultimate goal of these approaches would be to arrest or to slow down the progressive degenerative process of the disease.Previous research in our laboratory 10,11and in others 12revealed that the progressive degeneration of DA neurons in an adult rat model of PD,13using an E1/E3defective adenovirus (first-generation virus)encoding glial cell line-derived neurotrophic factor (GDNF),under a viral promoter was reduced.The effects obtained in vivo depend on the time and site of administration of recombinant virus.14,15Although these results were encouraging for gene therapy,the first-generation ade-novirus had a toxic effect for transduced cells by inducing the synthesis of viral proteins within these cells.These proteins elicit an immune response in the injected brains,which generates toxicity.It is probable that the toxicity of the E1/E3defective virus is due to the E4region of the type 5adenovirus which is present in the recombinant virus.Yeh et al 16suggested that,in vivo ,a low level of E4expression could be cytotoxic to the recipient cells.In fact,Dedieu et al 17demonstrated that the E1,E3/E4defective adenovirus (third generation)was less toxic for transduced cells than the ‘first-generation’E1/E3defec-tive virus.In addition,the infusion of E1,E3/E4defective virus elicited lower inflammatory responses,improved local tolerance and increased viral DNA persistence in the liver of a lacZ -transgenic mouse.Thus,an E1,E3/E4defective adenovirus represents progression on the path toward preclinical therapy.The aim of our work was to use the E1,E3/E4defective adenovirus to deliver GDNF,a therapeutic gene,into theReceived 17June 2003;accepted 29November 2003;published online 15January 2004Correspondence:J Mallet,Laboratoire de Genetique Moleculaire de la Neurotransmission et des Processus Neurodegeneratifs,Bat.CERVI,Hopital de la Pitie-Salpeˆtriere,83Bd.de l’Hopital,75013Paris,France Gene Therapy (2004)11,746–756&2004Nature Publishing Group All rights reserved 0969-7128/04$/gtbrain of a rat model of PD.The expression of GDNF was targeted to the astrocytes in the lesioned striatum. Astrocytes are the most abundant glial cells in the central nervous system(CNS),and are necessary for the survival of neurons in vivo18and in vitro.19These cells produce and secrete several growth factors,and among them,GDNF and cytokines.20–23In addition,following brain injury,the glialfibrillary acidic protein(GFAP) gene is upregulated in reactive astrocytes.24We therefore used the promoter of the GFAP gene,whose expression in the CNS is restricted to astrocytes,24,25to drive GDNF gene expression.Restriction of GDNF expression to a specific cell type limits the side effects caused by the expression of this gene in surrounding cells,thus facilitating the long-term expression of the transgene. Our results unequivocally showed that the recombinant Ad-GFAP-GDNF,in which the transgene was driven by a glial-specific promoter,prevented DA neurons death after striatal lesions induced by6-OHDA in the rats and improved the drug-induced rotational behavior.As compared to the E1/E3deleted adenovirus used previously in our laboratory,the cytotoxicity in injected animals was much lower.ResultsIn this work we constructed an E1,E3/E4defective recombinant adenovirus encoding rat GDNF under the control of a specific glial promoter,GFAP,by homo-logous recombination in Escherichia coli(see Materials and methods).This defective virus exhibits a large deletion in the E4region,which abrogates the synthesis of all E4-encoded gene products.17The virus was generated in the IGRP2cell line that transcomplements the E4viral function.16In vitro experimentsThe ability of the recombinant Ad-GFAP-GDNF to express GDNF wasfirst tested in primary cultures of astrocytes.The neurotrophic effect of this secreted protein on the survival of DA neurons was performed on mesencephalic cultures.Synthesis of GDNF by various types of cells.To determine whether astrocytes transduced with Ad-GFAP-GDNF express recombinant GDNF,cultivated rat astro-cytes were infected with the recombinant virus as well as an empty control at different doses.Conditioned medium(CM)and cellular pellets were collected at4,6 and8days after infection for analysis by ELISA.The quantity of endogenous GDNF secreted by noninfected astrocytes,or those infected with empty adenovirus was low(120716pg/ml)at all time points tested in CM.The amount of endogenous GDNF was close to the detection limit of the assay(20pg/ml)in the cellular pellet.In astrocytes infected with50viral particles(vp)/cell of Ad-GFAP-GDNF,0.370.05ng/ml of GDNF was secreted in the CM per day.GDNF levels of0.270.04, 0.470.07and0.370.08ng/ml were detected in the cellular pellet at days4,6and8,respectively. However,when astrocytes were infected with Ad-GFAP-GDNF at higher doses(500and103vp/cell), 50–60ng/ml of GDNF was secreted in the CM by105cells per day(Figure1)(P o0.0001for500vp;P¼0.0008for103vp as compared to control).At a dose of5Â103vp/cell,70ng/ml of GDNF was secreted in theCM from day4to day6,and it declined thereafter (Figure1)(P o0.0001as compared to control).In cellular pellets,about4074ng/ml of GDNF was found at thesethree doses at all time points tested.From this result,twodoses of virus,500and5Â103vp/cell,were chosen toinfect the mesencephalic cells.Mesencephalic cells infected with500and5Â103vp/cell of Ad-GFAP-GDNF secreted3079and80720pg/ml of GDNF in the the CM,respectively.In control cultures(cells noninfected by the virus),about35pg/mlof GDNF was found in the CM.In cellular pellets,0.570.1and0.870.12ng/ml ofGDNF were measured in cells infected with500and5Â103vp/cell,respectively,6days after infection.In cellular pellets of control cultures,0.670.09ng/ml ofGDNF was found.These results indicate that recombi-nant GDNF was not effectively synthesized by the mesencephalic cells infected with Ad-GFAP-GDNF.Survival of DA neurons.To test the effect of GDNF onthe survival of neuronal cells,104mesencephalic cellswere plated on a layer of nontransduced astrocytes, astrocytes infected with103vp/cell of Ad-GFAP-GDNFor onto collagen-coated coverslips(control).When plated on transduced astrocytes,457770 tyrosine hydroxylase(TH)-positive neurons were found.This was two-fold lower if plated on noninfected astrocytes(233723).The number of surviving TH-positive neurons was lowest(114718)on collagen-coated coverslips(Figure2).Figure1GDNF levels in cultured astrocytes infected with recombinantvirus.Primary astrocytes were infected with Ad-GFAP-GDNF at differentdoses.d1:500vp/cell;d2:103vp/cell;d3:5Â103vp/cell;C:control, noninfected astrocytes.In all,50–60ng/ml of GDNF was released by105cells per day with both doses d1and d2.At a higher dose(d3),about70ng/ml of GDNF was released by105cells/day until day6after infection.Thenthe quantity of GDNF decreased at day8.*P o0.0001d1versus control atthree times analyzed;**P¼0.008,0.0004,o0.0001d2versus control atthree times analyzed,respectively;***P o0.0001,o0.0001,0.0002d3versus control at three times analyzed,respectively.Degeneration of DA neurons prevented by Ad-GDNFNA Do Thi et al747Gene TherapyIn vivo experimentsThe effect of striatal overexpression of GDNF on the DA neuron survival and motor function in a rat model of PD was investigated by direct in vivo delivery of the transgene,using recombinant Ad-GFAP-GDNF.Body weight.Injection of large doses of recombinant GDNF protein has been found to cause loss of body weight in rats.26No significant differences in weight were observed among the treatment groups over the entire period of experimentation.Thus the quantity of transgenic GDNF detected in the striatum did not affect the body weight of rats.GDNF expression in intact animals.In preliminary experiments ,different doses of virus (107,108,5Â108,109and 3Â109vp/rat)were used to determine an optimal dose both in terms of level of GDNF expression and inflammatory reaction.GDNF expression was assessed by ELISA in striatum obtained from nonlesioned animals that were killed 10days,4,6and 12weeks after vectorinjection.As shown in Table 1,at doses of 108and 5Â108vp/rat,the striatal GDNF content was the highest at all times analyzed as compared to other doses.At a lower dose of the virus (107vp/rat),GDNF levels were 10-fold lower than those measured in rats that had received 108vp of virus,at all time points studied.At doses of 109and 3Â109vp/rat,12–20pg/m g GDNF protein was found (Table 1)in transduced striatum,but a marked,strong inflammatory reaction was observed (data not shown).However,the GDNF protein levels decreased with time at all doses used (Table 1).Analysis with semiquantitative competitive polymer-ase chain reaction (sqc-PCR)showed that the level of viral DNA in injected striatum (108vp/rat)did not change between 10days and 12weeks after viral administration (Figure 3).From these results,we used 108vp/rat in the following experiments.GDNF expression in lesioned rats.The efficacy of adenovirus-mediated GDNF gene transfer was tested on a rat model of PD.13Adult rats were injected stereo-taxically with 108vp of Ad-GFAP-GDNF (G group)into the left striatum as described in Materials and methods.At 1week after viral injection,rats were anesthetized and received stereotaxic injection of 6-OHDA.Striatum and substantia nigra (SN)were dissected out of animals killed at 4,6and 12weeks after viral injection and the GDNF levels in these tissues were determined by ELISA.As shown in Table 2,the GDNF protein levels were 37–40times higher (37.3and 41pg/m g corresponding to 70and 75ng of GDNF per striatum,respectively)in Ad-GFAP-GDNF-transduced striatum at 4and 6weeks as compared to both control groups (OHDA ¼OH group;empty ¼E group),as well as to the noninjected side.The GDNF quantity was decreased at 12weeks (17.2pg/m g corresponds to 35ng of GDNF per striatum)afterviralFigure 2Survival of mesencephalic cells in culture.The survival TH (þ)neurons was two-fold higher on transduced astrocytes (G)than on noninfected cells (A,P ¼0.002)and four-fold higher as compared to control (C,P ¼0.0003).C:neurons on collagen-coated coverslips used as control;A:neurons on noninfected astrocytes;G:neurons on astrocytes infected with Ad-GFAP-GDNF.Table 1GDNF protein levels measured from transduced striatum of intact animals after Ad-GFAP-GDNF injection Doses 107vp 108vp 5Â108vp 109vp 3Â109vp 10days 3.25723579327814.67215734weeks 1.470.227.87223.47215.37316746weeks 2.570.7277834.77522.27220.147212weeks1.370.1214.67626.37312.5731273GDNF protein levels (pg/m g total protein),measured from injected striatum of intact animals (three animals per point),were high at doses of 108and 5Â108vp/rat as compared to other doses of virus.At 12weeks after viral treatment,the quantity of GDNF decreased with all doses used.Values are means 7s.e.m.Figure 3Viral DNA levels of injected striatum,in nonlesioned rats.The relative viral DNA amount in rats injected with 108vp/rat of Ad-GFAP-GDNF was unchanged from day 10to week 12(three animals per point).P ¼0.5,4weeks versus 10days;P ¼0.9,6weeks versus 10days;P ¼0.9,12weeks versus 10days.Degeneration of DA neurons prevented by Ad-GDNFNA Do Thi et al748Gene Therapyinjection.To explain the decline of GDNF levels in Ad-GFAP-GDNF-injected striatum at a later stage,sqc-PCR was performed to determine the relative quantity of the virus at different times after viral injection.As shown in Figure 4,the viral DNA levels were unchanged during 12weeks of experiment,which suggests a downregulation of GFAP promoter in vivo rather than a loss of injected viral DNA.In the SN,the GDNF protein levels were similar in the injected side as compared to the noninjected side and in all groups of animals (Table 2).The viral DNA levels were not detectable in the SN by sqc-PCR.Amphetamine-induced rotation test.The impact of GDNF overexpression on the behavior of the animals was assessed during 12weeks after viral vector injection.As early as 2weeks after the striatal 6-OHDA lesion (3weeks after viral injection),rats injected with Ad-GFAP-GDNF into the striatum began to display reduced amphetamine-induced rotations.As shown in Figure 5,3,4,6and 12weeks after Ad-GFAP-GDNF treatment,rats exhibited a significant reduction inTable 2GDNF protein levels from striatum and SN of lesioned animalsStriatumSubstantia nigraInjected sideNoninjected sideInjected sideNoninjected sideAd-GFAP-GDNF (G group)4weeks *#137.375.10.2970.070.1570.050.2170.096weeks *#14176.50.3470.130.270.060.2370.0612weeks *#117.274.20.2670.060.2670.040.2270.05Empty virus (E group)4weeks 0.1470.020.0970.010.270.040.1570.036weeks 0.0870.010.0770.0030.1270.020.270.0512weeks0.0970.040.0770.0020.1370.020.1470.056-OHDA alone (OH group)4weeks 0.1270.030.1670.030.1670.020.1470.016weeks 0.1470.020.1570.060.1770.020.1370.0112weeks0.1670.010.1270.020.270.050.270.01GDNF protein levels (pg/m g total protein)from striatum and SN measured by ELISA in lesioned animals treated with Ad-GFAP-GDNF (108vp/rat),with empty adenovirus (108vp/rat)or in naive animals that did not receive treatment before inducing 6-OHDA lesion.Seven animals were used per point.GDNF protein levels decreased with time in transduced striatum (G group)and remained unchanged in the injected striatum of E and OH groups.The GDNF levels were similar in the SN (injected and noninjected side)of all groups.Values are means 7s.e.m.*P o 0.0001different from noninjected side;#P o 0.0001G versus E group;P o 0.0001G versus OHgroup.Figure 4Viral DNA levels of injected striatum in lesioned rats at 4,6and 12weeks after viral treatment.The relative quantity of viral DNA was similar during our experiment from 4to 12weeks (five animals per point).P ¼0.9,6weeks versus 4weeks;P ¼0.6,12weeks versus 4weeks.Figure 5Motor performance of the animals using the drug-induced rotation test.Rats were injected with D -amphetamine (2.5mg/kg,i.p.)and their behavior was recorded for 90min.At 3,4,6and 12weeks after viral injection,rats injected with Ad-GFAP-GDNF (G group)exhibited a significant reduction in ipsilateral rotational behavior compared with control groups (OH and E groups).P ¼0.008,G versus OH group (3weeks);P ¼0.2,G versus E group (3weeks);P o 0.0001,G versus E and OH groups (4weeks);P o 0.0001,G versus E and OH group (6weeks);P ¼0.006,G versus E group (12weeks);P ¼0.0002,G versus OH group (12weeks).Degeneration of DA neurons prevented by Ad-GDNF NA Do Thi et al749Gene Therapyipsilateral rotational behavior compared with control groups(OH and E groups)(P o0.0001).In rats injected with empty virus,the rotation score was not significantly different from that of the animals that received6-OHDA alone at3,4and6weeks after viral injection(P¼0.08,0.4and0.3,respectively).Protection of DA neurons in the SN.Survival of DA neurons was analyzed throughout the SN as described in Materials and parison of the percentage of the TH-positive cells in the SN(average results from the three levels analyzed)revealed that about70%of DA neurons remained in the G group as compared to about 45%in the control groups at all three times examined (P o0.0001,0.01and0.0004,4,6and12weeks, respectively)(Figures6,7a and b).This result suggested that a significant protection of DA neurons was found in animals treated with Ad-GFAP-GDNF.Immunoreactivity in the striatum.Following an injec-tion of6-OHDA into the striatum,there is an immediate toxic damage to the DAfibers and axons followed by a rapid degeneration of their terminals.4,27We used NeuN staining to localize the lesion in the striatum(Figure9d), and immunohistochemistry for the TH to assess the extent of denervation induced by intrastriatal6-OHDA lesions(Figure8a and b).The extent of denervation was prominent in the central and dorsal parts of the injected striatum in all animals analyzed.The intensity of TH-positivefiber staining,measured by optical density,in the injected striatum(average results from the three levels analyzed)was similar in both E and OH groups (Figure8b).It was reduced by about70–75%(P o0.0001) in the injected side versus the noninjected side at4weeks and by about80–85%(P o0.0001)at6and12weeks. The TH intensity was slightly higher in animals of G group(þ7%)at4,6and12weeks as compared to controls,but it did not reach statistical significance (P¼0.2)(Figure8a).Abundant TH immunoreactive profiles(dots)of different sizes(Figure8c and f)were observed in the lesion sites of the striatum.Some of these patterns were scattered throughout the parenchyma in animals of G, OH and E groups.However,in animals treated with Ad-GFAP-GDNF(Figure8c),the number of these TH immunoreactive profiles was increased as compared to control animals at all three times analyzed(Figure8f).At higher magnification,we observed TH-positivefibers with numerous axonal varicosities which displayed different intensity of TH staining(Figure8d,compared with Figure8g(control)).In globus pallidus,we observed the TH immunoreactive area,which appears to correspond to the axonal sprouting of TH-positive fibers in animals treated with Ad-GFAP-GDNF vectors (Figure7c).The TH-positivefibers were also observed in the entopeduncular nucleus of these rats(Figure7d). These patterns were not seen in any of the animals of control groups.The GDNF transgene expression in transduced stria-tum was visualized by anti-GDNF antibody.As shown in Figure9a and b,the striatal astrocytes of animals treated with Ad-GFAP-GDNF were stained with GDNF antibody.We also determined the effect of the viruses on the size of the striatum by analyzing the surface of10sections per brain between the coordinates APþ1.7and APþ0.2. The injected striatal size was not modified in G(P¼0.8), E(P¼0.7)and OH(P¼0.4)groups at4weeks as compared to the noninjected side.However,we observed a nonsignificant atrophy,7%as compared to controlate-ral size,of the injected striatum of G(P¼0.5)andEFigure6Rescue of TH immunoreactive neurons in the SN.Significantincrease in the percentage of TH immunoreactive neurons was observed inlesioned rats treated with Ad-GFAP-GDNF(G group)compared with ratsinjected with empty adenovirus(E group)or with lesioned rats(OHgroup).P o0.0001,G versus E group(4weeks);P¼0.001,G versus OHgroup(4weeks);P¼0.01,G versus E group(6weeks);P¼0.003,Gversus OH group(6weeks);P¼0.0004,G versus E group(12weeks);P o0.0001,G versus OH group(12weeks).Figure7TH staining of injected brain.Many TH-positive neuronsremained in the rostral,middle and caudal parts of the SN in animalstreated with Ad-GFAP-GDNF(a),while fewer cells survived in animalslesioned by6-OHDA(b),12weeks after treatment.The presence of TH-positivefibers(asterisk)was seen in globus pallidus(c)and inentopeduncular nucleus(d),4weeks following Ad-GFAP-GDNF injection(injected side¼left side,arrow;right side¼intact side).Scale barrepresents(a,b)250m m and(c,d)150mm.Degeneration of DA neurons prevented by Ad-GDNFNA Do Thi et al750Gene Therapy(P ¼0.6)groups at 6and 12weeks.In the OH group,very mild atrophy was seen (4%as compared to the controlateral side)at 12weeks,but not significant as assessed by one-way analysis of variance (ANOVA),P ¼0.6.Inflammatory response.Injuries to the brain result in a rapid inflammatory reponse that typically involves recruitment and infiltration of different cell populations.Immunohistochemistry using CD4and CD8antibodies allows one to determine the localization of reactive lymphocytes.CD4immunoreactive cells were most numerous at the injection sites of the adenoviral vector with or without transgene,and at the 6-OHDA lesion in all treatment groups (Figure 9e).They were also scatteredthroughout the parenchyma and close to the blood vessels.A few CD8immunoreactive cells were particu-larly concentrated at the injection sites of the adenoviral vector and around the 6-OHDA lesion (Figure 9f).We did not observe more inflammation in G and E groups as compared to the OH group,with both CD4and CD8antibodies (Table 3).Astrocytic response to injury was assessed by using an antibody against GFAP .Glial fibrillaly acidic protein,an intermediate filament protein,is expressed abundantly in astrocytes during development 28of the CNS and in reactive astrocytes (astrogliosis)following CNS in-jury.24,25Reactive astrocytes,characterized by a signifi-cant increase in the GFAP intensity,cellular hypertrophy and increase in the density of GFAPimmunoreactiveFigure 8TH immunostaining of the striatum,4weeks after viral treatment.On the intact side,the TH staining intensity was high throughout the striatum (a,b,right side).After intrastriatal lesion,the TH staining was almost lost at the site of 6-OHDA injection (b,left side).By contrast,Ad-GFAP-GDNF-treated animals had a more lasting TH intensity on the ipsilateral side (a,left side).High-power magnification of boxed area in (b)showed that the axonal terminals in the striatum were degenerated at the lesion site (e,asterisk),whereas some spared terminals remained (arrow).In these GDNF-treated animals,numerous TH-positive axonal profiles (c,dots)and TH immunoreactive fibers with varicosities and sprouting (d,arrow)were seen in the denervated striatum.In the striatum of 6-OHDA lesioned animals,the TH-positive profiles (f)and TH immunoreactive fibers with varicosities (g)were less numerous.Scale bar represents (a,b)200m m and (c–g)50mm.Figure 9Immunostaining of transduced striatum,4weeks after Ad-GFAP-GDNF treatment.At the injected site,a halo of GDNF was seen with GDNF-positive astrocytes (a);at low magnification astrocytes stained with GDNF antibody (b).Numerous cells stained with CD4(e)and CD8(f)at the injected site.In (d)the site of 6-OHDA lesion was stained by NeuN antibody.Inside the lesion,the neurons were degenerated,while around the lesion the nuclei of neurons were stained by NeuN.Reactive astrocytes were stained by GFAP antibody (c)at the injected site of the striatum.Scale bar represents (a)35m m,(b)200m m,(c)50m m,(d)200m m and (e,f)100mm.Degeneration of DA neurons prevented by Ad-GDNF NA Do Thi et al751Gene Therapyprocesses,were detected throughout the ipsilateral striatum.The GFAP staining was particularly intense at the lesion with a dense network of cell bodies and processes in all study groups (Figure 9c).DiscussionThe aim of the present study was to assess the ability of GDNF,expressed by an improved E1,E3/E4defective recombinant adenovirus in which the GDNF gene is driven by a glial-specific promoter,to preserve the integrity of the nigrostriatal DA system (cell bodies,axonal terminals)and the normal motor function after administration of the virus into the striatum before inducing 6-OHDA lesion.Our interest was also to test the GFAP promoter for PD therapy since this promoter was described to direct specifically transgene expression in astrocytes.24,25In our cell cultures,GDNF protein was not synthe-sized by the mesencephalic cells infected with the recombinant GFAP-GDNF adenovirus,whereas this trophic factor was produced and secreted by the transduced astrocytes (Figure 1).Morelli et al 29observed that only cultured neocortical neurons,infected with a recombinant defective adenovirus vector encoding FasL under the control of the neuronal-specific promoter NSE (RAd-NSE-FasL),released the cytotoxic Fas ligand into the culture supernatant.Neurons transduced with a vector under the control of a glial-specific promoter (RAd-GFAP-FasL)were unable to release the FasL cytotoxic activity.Thus,the expression of the transgene was cell-type restricted when the transcription was directed from a glial-or a neuronal-specific promoter in the adenoviral vector.In vivo ,in a rat model of PD,immunohistochemical experiments,performed in the transduced striatum,demonstrated that the expression of the transgene (GDNF)was confined to astrocytes (Figure 9a and b).This observation was supported by the results obtained from ELISA tests (Table 2).After an intrastriatal injection of Ad-GFAP-GDNF,the GDNF protein levels were high in transduced striatum (37–41pg/m g protein from 4to 6weeks;Table 2).At 12weeks the quantity of GDNF protein decreased,whereas the levels of adenoviral DNAremained unchanged from weeks 4to 12(Figure 4).The decline of the transgene expression could result from the host immune responses to the vector in infected cells 30,31or due to the down regulation of the promoter.12In our study,the decline in the GDNF expression was unequi-vocally the result of a downregulation of the GFAP promoter rather than the loss of adenovirus-infected cells,since the quantity of viral DNA in the transduced striatum did not change during the experiment (Figure 4).Using the RSV promoter to drive the expression of the GDNF,Choi-Lundberg et al 32found that GDNF protein and GDNF DNA levels decreased simultaneously from weeks 1to 7.In our study,although the GDNF protein level decreased,it remained relatively high at 12weeks (17pg/m g protein;Table 2).Armentano et al 33and Dedieu et al 17showed that the deleted E1,E3/E4recombinant adenoviruses were unable to sustain a strong and stable transgene expression when under the control of the CMV and RSV promoters.Thus the long-lasting presence of the recombinant GDNF in our experiment cannot be attributed solely to the E1,E3/E4Ad-GFAP-GDNF backbone.The prolongation of the GDNF expression we obtained was probably the consequence of the activity of the GFAP promoter.Despite a downregulation of the GFAP promoter at 12weeks,its remaining activity would still be sufficient to induce the late and high-level expression of the transgene in the astrocytes.In four animals we even observed GDNF expression in the transduced striatum 5months after Ad-GFAP-GDNF injection (unpublished results).In addition,the recombinant E1,E3/E4defective adenovirus used in the present study appeared to be weakly immunogenic in the brain.We did not observe an increased inflammation in the lesioned brains after GFAP-GDNF or empty virus injection as compared to the OHDA-injected animals (Table 3).Moreover,the transduced striatum sizes were not reduced.These results suggest that this is an improvement of the adenoviral vector compared to the first-generation adenovirus used previously by our group.11Another important result was that approximately 70%(as compared to about 45%in the controls)of the nigral DA neurons were still present at 12weeks when the recombinant Ad-GFAP-GDNF had been injected into the striatum,1week before inducing the intrastriatal 6-OHDA lesion.The ratios of protected neurons did not change with time from week 4to week 12after the viral treatment (Figure 6).As the protection of the DA neurons was not complete,it is possible that the recombinant GDNF,synthesized by striatal transduced astrocytes and transported by retrograde axonal transport,was not sufficient in the SN (Table 2).The results obtained in this work are consistent with previous studies by our group 11and others 9,14using Ad/AAV-GDNF injected in the striatum.In addition,these authors 9,11,14found (1)that the intensity of the TH immunoreactivity was increased in the injected striatum,as compared to control 9,11,14and (2)that the axonal sprouting was present in the striatum and the globus pallidus.9In our rats treated with Ad-GFAP-GDNF,although the sprouting was observed in the striatum and the globus pallidus (Figures 7and 8),the intensity of the striatal TH staining was not modified in the injected side.This may be due to the lowTable 3Semiquantitative estimation of the inflammation in injected animals4weeks 6weeks 12weeks GE OH G E OH G E OH 7404040504242636353+524252424242424342++132414141223030212+++050404041303020203++++000000010000Number of animals from G,E and OH groups where the inflammation was produced in the brains from 4to 12weeks after treatment.For each animal,10CD8and CD4-stained sections were examined and scored as described in Materials and methods.First values in each column (G,E,OH)were estimated on CD8-stained sections,and second values (italic)were estimated on CD4-stainedsections.Degeneration of DA neurons prevented by Ad-GDNFNA Do Thi et al752Gene Therapy。
G蛋白耦联胆汁酸受体激动剂INT777通过激活AMPK信号通路抑制施万细胞成髓鞘过程
南通大学学报(医学版)Journal of Nantong University (Medical Sciences) 2021 : 41 (1)6・D0I:10.16424/32-1807/r.2021.01.002G 蛋白耦联胆汁酸受体激动剂INT777通过激活AMPK 信号通路抑制施万细胞成髓鞘过程*关晋东:丁杰,刘晓宇,孙诚**** [基金项目]国家自然科学基金青年基金资助项目(81701222)** [作者简介]关晋东,男,汉族,生于1995年10月,山西省晋城市人,硕士在读,研究方向:外周神经发育及损伤修复机制的研究。
*** [通信作者] 孙诚,电话**************,E-mail: ********************.cn(南通大学教育部/江苏省神经再生重点实验室/神经再生协同创新中心,南通226001)[摘 要]目的:研究G 蛋白耦联胆汁酸受体(G-protein-coupled bile acid receptor 1, GPBAR1,同时也被称为TGR5) 特异性激动剂6琢-乙基-23(S)-甲基胆酸[6 alpha-ethyl-23(S)-methylcholic acid, INT777[对原代施万细胞髓鞘形成的影响并初步探讨其可能的作用机制遥方法:用5 滋mol/L 的INT777处理二丁酰环腺苷酸(dibutyryl cyclic adenoslne phosphate, dbcAMP )诱导分化原代施万细胞成髓鞘模型,用免疫印迹(Western Blot )方法检测髓磷脂蛋白表达量的变化遥同时,提取 施万细胞总核糖核酸后用定量聚合酶链式反应试验检测INT777对髓鞘形成过程相关分子基因表达的影响遥另外,用Western Blot 方法检测INT777对单磷酸腺苷活化蛋白激酶/核糖体蛋白S6激酶(adenosine 5'-monophosphate-activatedprotein kinase/ribosomal S6 kinase, AMPK/S6K )信号途径的影响遥结果:在dbcAMP 诱导分化原代施万细胞成髓鞘过程中,5 滋mol/L INT777的处理抑制了髓鞘早期生长因子20,八聚体结合转录因子6,髓磷脂蛋白的表达,且5滋mol/L INT777 处理激活了 AMPK 的活性,抑制了雷帕霉素作用靶点信号通路遥结论:INT777抑制dbcAMP 诱导的施万细胞成髓鞘过程,这种抑制作用可能是通过激活施万细胞AMPK 活性、抑制S6K 活性实现的遥[关键词]施万细胞曰髓鞘曰6琢-乙基-23(S)-甲基胆酸曰单磷酸腺苷活化蛋白激酶[中图分类号]R338.1 [文献标志码]A [文章编号]1674-7887(2021)01-0006-05G-protein-coupled bile acid receptor agonists INT777 inhibits myelination of Schwann cells byactivating AMPK signaling pathway*GUAN Jindong **, DING Jie, LIU Xiaoyu, SUN Cheng ***('Key Laboratory of Neuroregeneration of Jiangsu and Ministry ofEducation, Co-innovation Center of Neuroregeneration, Nantong University, Nantong 226001)[Abstract ] Objective: To investigate the effects of G-protein-coupled bile acid receptor 1(GPBAR1, also known as TGR5)specific agonist 6 alpha-ethyl-23(S)-methylcholic acid(INT777) on myelination in primary Schwann cells and the underlying mechanisms. Methods: Primary Schwann cells were treated with 5 滋mol/L INT777 and dibutiryl cyclic adenoslne phosphate (dbcAMP), and the changes of myelin protein zero were detected by Western Blot. Meanwhile, the total RNA was extractedfrom Schwann cells and quantitative real time polymerase chain reaction was employed for detecting myelin gene expression. In addition, the effect of INT777 on the adenosine 5' -monophosphate -activated protein kinase/ribosomal protein S6 kinase (AMPK/S6K) signaling pathway was examined by Western Blot. Results : Treatment with 5 滋mol/L INT777 inhibited theexpression of myelin early growth response -2, octamer -binding transcription factor 6, and myelin protein zero during dbcAMP-induced myelination of differentiated Schwann cells, and treatment with 5 滋mol/L INT777 activated AMPK activity and inhibited mTOR signaling pathway. Conclusion: INT777 attenuates dbcAMP-induced myelination of Schwann cells, whichmay be achieved by activating AMPK and inhibiting S6K activity.[Key words ] Schwann cell; myelination; 6 alpha-ethyl -23(S)-methylcholic acid; adenosine 5'-monophosphate-activated protein kinase外周神经系统(peripheral nervous system, PNS) 在机体内分布广泛且起到介导靶器官与中枢神经系 统信号传递的重要作用。
巨噬细胞极化对动脉粥样硬化发生发展的影响
·综述·巨噬细胞极化对动脉粥样硬化发生发展的影响王立1a,2,陈玉辉1a,2,徐鸿轩1b,2,孟令丙1b,3,刘德平1b,2,3,龚涛1a,2,31.北京医院,a神经内科,b心血管内科国家老年医学中心中国医学科学院老年医学研究院,北京100730;2.国家老年医学中心国家卫生健康委员会北京老年医学研究所;3.中国医学科学院北京协和医学院研究生院[摘要] 动脉粥样硬化的发生和发展主要由局部血管壁炎症和脂质积累引发。
巨噬细胞在病灶演变中发挥着核心作用。
在动脉粥样硬化病变中,巨噬细胞受到各种各样的微环境信号调节,如细胞因子、氧化脂质、亚铁血红素等,从而导致其表型极化。
目前已证实巨噬细胞表型谱主要由T辅助细胞1(Th 1)细胞因子诱导的M1型和由Th 2细胞因子诱导的M2型为主,其中M2型又细分为M2a、M2b、M2c、M2d,此外尚有Mox型、M4型、M(Hb)和Mhem型。
对巨噬细胞表型可塑性机制的研究成为制定抑制或稳定动脉粥样硬化斑块治疗策略的重要内容。
近年来,社会心理因素在动脉粥样硬化发生发展中的作用已成为研究的热点。
慢性心理应激时交感肾上腺髓质(SAM)系统和下丘脑-垂体-肾上腺轴(HPA)的持续激活导致儿茶酚胺类激素和糖皮质激素分泌显著增加,它们对巨噬细胞表型可塑性的调节和功能后果产生重要影响。
该文对动脉粥样硬化斑块中巨噬细胞亚群的特征,及在多种组织中神经内分泌激素如儿茶酚胺类激素和糖皮质激素对巨噬细胞表型可塑性的影响进行综述。
[关键词] 动脉粥样硬化;巨噬细胞;糖皮质激素类;儿茶酚胺类;应激,心理学;综述DOI:10.3969/J.issn.1672 6790.2022.01.032EffectofmacrophagepolarizationontheinitiationandprogressionofatherosclerosisWangLi ,ChenYuhui,XuHongxuan,MengLingbing,LiuDeping,GongTaoDepartmentofNeurology,BeijingHospital,NationalCenterofGerontology;InstituteofGeriatricsMedicine,ChineseAcademyofMedicalSciences,Beijing100730,ChinaCorrespondingauthor:GongTao,Email:gb20598@sina.com[Abstract] Theinitiationandprogressionofatherosclerosisaremainlycausedbyinflammationandlipidaccumulationinthelocalvascular.Macrophagesplayacentralroleinthedevelopmentandprogressionoflesions.Intheatheroscleroticlesions,macrophagesareregulatedbyavarietyofmicroenvironmentalfactors,suchascytokines,oxidizedlipids,andhemeiron,whicheffectthephenotypicpolarization.Nowadays,ithasbeenconfirmedthatthemacrophagephenotypespectrumischaracterizedmainlyclassicalM1macrophagesinducedbyT helper1(Th 1)cytokinesandbythealternativeM2macrophagesinducedbyTh 2cytokines,ofwhichM2macrophagescanbefurtherclassifiedintoM2a,M2b,M2candM2dsubtypes.Andatheroscleroticplaque specificmacrophagephenotypesincludeM4type,M(Hb)orMhemtype,Moxtype.Studyingthemechanismofmacrophagephenotypicplasticityhasbecomeanimportantworktoexploitnewwaytoinhibitandstabilizeatherosclerosis.Inrecentyears,theeffectsofsocialpsychologicalfactorsonthedevelopmentofAShavebecomeahottopic.Thecontinuousactivationofthesympatheticadrenalmedulla(SAM)systemandthehypothalamic pituitary adrenalaxis(HPA)duringpsychosocialstressleadstoasignificantincreaseinthesecretionofcatecholaminesandglucocorticoids,whichplayanimportantroleintheregulationofmacrophagephenotypicplasticityanditsfunctionalconsequences.Thisreviewsummarizestheknowledgeofmacrophagesubsetsinatheroscleroticplaquesandtheinfluenceofneuroendocrinehormonessuchascatecholaminesandglucocorticoidsonthephenotypicplasticityofmacrophagesinvarioustissues.[Keywords] Atherosclerosis;Macrophages;Glucocorticoids;Catecholamines;Stress,psychological;Review基金项目:国家重点研发计划项目(2020YFC2003000);国家自然科学基金项目(51672030);中国医学科学院医学科学创新基金项目(2018 I2M 1 002);中央卫生科研计划项目(W2017BJ11)作者简介:王立,硕士研究生,Email:wangli001@foxmail.com通信作者:龚涛,主任医师,博士研究生导师,Email:gb20598@sina.com 动脉粥样硬化是一种慢性、多因素性疾病,该过程主要由血管壁脂质积聚和随后的动脉壁炎症反应构成,最终导致动脉粥样硬化斑块形成和破裂[1 2]。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ORIGINALARTICLEP2X7receptorsandFynkinasemediateATP-inducedoligodendrocyteprogenitorcellmigration
Ji-FengFeng1&Xiao-FeiGao1&Ying-yanPu1&GeoffreyBurnstock2,3&
ZhenghuaXiang1&ChengHe1
Received:15January2015/Accepted:12June2015#SpringerScience+BusinessMediaDordrecht2015
AbstractRecruitmentofoligodendrocyteprecursorcells(OPCs)tothelesionsisthemostimportanteventforremyelinationaftercentralnervoussystem(CNS)injuryorindemyelinatingdiseases.However,theunderlyingmolecularmechanismisnotfullyunderstood.Inthepresentstudy,wefoundhighconcentrationsofATPcouldincreasethenumberofmigratingOPCsinvitro,whileafterpretreatmentwithox-idizedATP(aP2X7receptorantagonist),thepromotiveeffectwasattenuated.Thepromotiveeffectof2′(3′)-O-(4-benzoylbenzoyl)adenosine5′-triphosphate(BzATP)(aP2X7receptoragonist)wasmorepotentthanATP.Afterin-cubationwithBzATP,theactivityofFyn,onememberoftheSrcfamilyofkinases,wasenhanced.Moreover,theinterac-tionbetweenP2X7andFynwasidentifiedbyco-immunopre-cipitation.AfterblockingtheactivityofFynordown-
regulatingtheexpressionofFyn,themigrationofOPCsin-ducedbyBzATPwasinhibited.ThesedataindicatethatP2X7receptors/FynmaymediateATP-inducedOPCmigrationun-derpathologicalconditions.
KeywordsP2X7receptors.Srcfamilykinase.Fynkinase.Migration.OPCs.ATP
IntroductionIntheadultcentralnervoussystem(CNS),oligodendrocyteprogenitorcells(OPCs),whichareNG2positive,arewidelydistributedinbothwhiteandgraymatter[1,2].AfterCNSinjury,OPCscanquicklymigratetothelesionareas[3,4],differentiateintomatureoligodendrocytes,andformnewmy-elinsheathsaroundtheaxons[5,6]eventuallyimprovingfunctionalrecovery.ThesemigratedNG2positivecellshavealsobeendemonstratedtoplayaroleinsealingthelesionsandinscarformation[7–9].However,themechanismunderlyingOPCmigrationafterinjuryremainslargelyunknown.Adenosine5′-triphosphate(ATP)isnotonlyaneurotrans-mitter[10],butisalsoinvolvedincellproliferation[11],mi-gration[12],survival[13],andapoptosis[14].ExtracellularATPcanactivateP2receptors,whichincludeligand-gatedionchannelreceptors(P2Xreceptors)andmetabotropicreceptors(P2Yreceptors)[15].P2XreceptorsareATP-gatedionchan-nels,mediatingintracellularpotassiumefflux,andextracellu-larsodiumandcalciumionsinflux[16].P2X7receptors,withalongerintracellularC-terminalsequencethanothermembersoftheP2Xreceptorfamily[17],takepartinavarietyofintracellularsignalingtoregulatecellproliferation,migration,differentiation,andapoptosis[18,19].P2X7receptorshavelowaffinityforATP[20],andonlywhenextracellularATPconcentrationsaregreaterthan100μM,P2X7receptorscould
Ji-FengFengandXiao-FeiGaocontributedequallytothiswork.ElectronicsupplementarymaterialTheonlineversionofthisarticle(doi:10.1007/s11302-015-9458-3)containssupplementarymaterial,whichisavailabletoauthorizedusers.
*ZhenghuaXiangxiang-zhenghua@163.com
*ChengHechenghe@smmu.edu.cn
1InstituteofNeuroscienceandKeyLaboratoryofMolecular
NeurobiologyofMinistryofEducation,NeuroscienceResearchCenterofChangzhengHospital,SecondMilitaryMedicalUniversity,Shanghai,China
2AutonomicNeuroscienceCentre,UniversityCollegeMedical
School,RoyalFreeCampus,RowlandHillStreet,LondonNW32PF,UK
3DepartmentofPharmacologyandTherapeutics,TheUniversityof
Melbourne,Melbourne,Australia
PurinergicSignallingDOI10.1007/s11302-015-9458-3beactivated[21–23].FollowingCNSinjury,alargeamountofcelldeathcauseshigherconcentrationsofATPintheex-tracellularenvironmentandtriggertheactivationofP2X7receptorsonneuronsandglialcells[24],therebyplayingim-portantpathophysiologicalroles[25].ManyofstudieshaveindicatedthatactivationofP2X7receptorscanpromotemigrationindifferenttypesofcells,suchasmonocytes[26],tumorcells[27],andepithelialcells[28].P2X7receptorshavealsobeenshowntopromotethemigrationofculturedglialcells[29].Moreover,P2X7recep-toractivationwasdemonstratedtotriggerextracellularcalci-umioninflux,thenactivateintracellularkinases,suchasMAPK[26,28]andAkt[30],orincreasetheexpressionofmatrixmetalloproteinase9[29],therebyfacilitatingcellmi-gration.Moreover,Fynkinase,onememberoftheSrcfamily,isinvolvedinOPCmigration[31,32].Inthepresentstudy,weexaminedtheeffectofahighconcentrationofATPonOPCsinvitro,andshowedthatP2X7receptorscoupletoFynkinasetomediateATP-inducedmigrationofOPCs.
MaterialandmethodsAntibodiesandreagentsCytarabine(Ara-C),adenosine5′-triphosphatedisodiumsalt(ATP),oxidizedATP(oxATP),2′(3′)-O-(4-benzoylbenzoyl)adenosine5′-triphosphatetriethylammoniumsalt(BzATP),andpoly-L-lysine(PLL)werepurchasedfromSigma-
Aldrich(St.Louis,MO,USA).Theantibodiesagainstp-Src(Tyr416)orp-Src(Tyr527)werepurchasedfromCellSignal-ingTechnology(Beverly,MA,USA),anti-NG2fromMillipore(Billerica,MA,USA),anti-FynfromSantaCruz(SantaCruz,TX,USA),anti-P2X7fromAlomone(Jerusa-lem,Israel),anti-MycfromRoche(PaloAlto,CA,USA),andanti-HAandHoechst33258fromSigma-Aldrich.TheHRP-conjugatedsecondaryantibodiesandβ-Actinwerepur-chasedfromKangChen(Shanghai,China),andimmunofluo-rescencesecondaryantibodieswerefromJacksonImmunoResearch(WestGrove,PA,USA).Dulbecco’smod-ifiedeaglemedium(DMEM),fetalbovineserum(FBS),neurobasalmedium,B27,andtrypsinwerepurchasedfromLifeTechnologies(Carlsbad,CA,USA).TheotherreagentsandtheTranswellassaywerepurchasedfromCorning(NY,USA),proteinG-agarosebeadswerefromRoche,andthe0.22-μmmembranefilterswerefromMillipore.
