复旦大学物理化学AII 15-1 The phase rule and its deduction 2015
复旦大学物理化学AII 12-9 Chemical potential(2)

图解法准确,但费时费力,其他方法? 方法2:已知实际气体状态方程
例:pVm= RT +Bp
Vm/RT-1/p = B/RT
p B f Bp ln dp 0 RT p RT
f pe
2015/4/13
Bp RT
物理化学II
12
The second law and the third law
须选取参考态。逸度绝对值参考态的选取
选取 p 0时, f /p 1的状态为参考态,表述为:
limp0( f/p) = p/p =1
物理化学II
8
Hale Waihona Puke The second law and the third law
Chemical potential (2)
单组分实际气体逸度的计算
逸度主要适用于实际气体的混合物,它对于研究实
Chemical potential (2)
如何从 Z 计算 f ?
p V f 1 m,实 ln ( )dp 0 p RT p
pVm,实 ZRT
代人可得
p Z 1 f ln ( )dp 0 p p
把压力换成对比压力
Z 1 f ln ( )d 0 p
根据查得Z,做 (Z-1) / 对 图,积分可求得 。
物理化学II
3
The second law and the third law
Chemical potential (2)
实际纯气体化学势表达式: p
(T, p) = (T) +RTln(f/p)
= (T) + RTln(p/p) + RTln (f = p) =理想气体+RTln
复旦大学物理化学AII 11-3 Enthalpy and thermal capacity

System lose= PindV Surroundings gain= PexdV Surroundings lose= PexdV
Compression System gain= PindV
Wsys
V2
V1
P in dV
V2
V1
nRT dV V
Expansion work(PexdV)is the work (or the capability of doing work ) gained or lost by the surroundings.
U = QP + WP = QP – V1 PexdV
V2
= QP – Pex(V2 – V1)
So, QP = U + Pex(V2-V1) = U2-U1 + PexV2 - PexV1 = (U2 + PexV2) – (U1 + PexV1) = (U2 + P2V2) – (U1 + P1V1 )
Physical Chemistry II
7
The first law of thermodynamics and thermochemistry
Enthalpy and heat capacity
Heat effect at constant pressure
Pin = Pex = C,dP = 0
2015/3/20
Physical Chemistry II
12
The first law of thermodynamics and thermochemistry
Enthalpy and heat capacity
复旦大学物理化学AII 12-6 Relation among thermodynamic functions and their applications (1)

S p ( )T ( )V V T
( S V )T ( ) p p T
M and N are the functions of x and y.
M 2 z N 2 z ( )x , ( )y y yx x xy M N ( )x ( ) y y x
Physical Chemistry II
14
The second law and the third law
(2)
A
A U TS
A Wmax (dT 0, Reversible)
(dA)T ,R Wmax
The change in Helmholtz energy is equal to the maximum work accompanying a process at constant temperature.
H U pV
H Q p
(dp 0,Wadd 0)
Physical Chemistry II
The second law and the third law
Relations among thermodynamics functions and their applications (1)
(2)
Because
dH TdS Vdp
H U pV
dH dU pdV Vdp
dU TdS pdV
Thus
dH TdS Vdp
Physical Chemistry II
7
The second law and the third law
Relations among thermodynamics functions and their applications (1)
复旦大学物理化学AII 09-3 理想气体的热力学过程

焓 和 热 容
等压热容 与等容热 容的关系
2015/3/9
U V Cp CV p dT V T T p
物理化学II
2
Q p
热力学第一定律和热化学
理想气体的热力学过程
§3 理想气体的热力学过程 焦耳实验(1843) ----- 理想气体内能与何有关?
卡诺循环(Carnot cycle)
1mol 理想气体的卡诺循环在pV图上可以分为四步:
过程1:等温(Th ) 可逆膨胀由 p1V1 到 p2V2 (A B)
V2 W1 nRTh ln V1
U1 0
Qh W1
所作功如AB曲线 下面积的负值:
物理化学II
25
热力学第一定律和热化学
W Qh Qc Qh Qh
(Qc 0)
高温热源T1
卡诺 热机
或
V2 nR(Th Tc ) ln( ) Th Tc Tc V1 1 V2 Th Th nRTh ln( ) V1
W U C dT
T1 V T2
W U CV (T2 T1 )
物理化学II
10
热力学第一定律和热化学
理想气体的热力学过程
理想气体绝热过程焓变
H H dH dp H f (T , p) dT T p p T H dH dT C p dT T p
4
热力学第一定律和热化学
理想气体的热力学过程
焦耳实验推论一:
H U pV
对理想气体,
H U nRT
H H f (T ) p
H 0, V 0 T T
复旦物理化学复习大纲

复旦物理化学复习大纲(内部官方版本)Physical Chemistry基本内容:物理化学是研究物质的结构、性质及其变化的普遍规律的一门学科。
内容的第一部分(物理化学AI)讨论微观结构,主要包括量子力学基本原理、原子、分子和晶体结构、对称性和分子间相互作用以及微观结构的测定原理;将微观原理放在前面讲授,有利于引导学生以原子分子的观点深入领悟物理化学的原理。
第二部分(物理化学AII)讨论平衡体系的性质,从统计热力学入手,建立微观到宏观的桥梁,进一步过渡到热力学,包括热力学三大定律、溶液、化学平衡、相平衡;第三部分(物理化学AIII)讨论变化体系的性质,主要是动力学和电化学,还包括非平衡体系热力学的简单介绍以及界面现象和表面化学。
第一章量子化学基础内容提要:现代化学从分子和原子水平上认识物质本质和化学反应规律的基本理论基础是量子力学。
因此本章中将介绍物理化学中涉及到的量子力学基本原理和基础知识,例如微观粒子的波粒二象性、测不准关系、量子力学基本假定和薛定谔(Schrödinger)方程, 以及用它们来处理微观物体运动的基本方法,并运用这些原理和方法讨论一些典型的简单体系。
学习要求:由于微观物体运动遵循量子力学,所以掌握量子力学基础对学习有关微观结构和运动的章节非常重要。
通过本章学习要求(1) 弄清微观粒子运动的基本特征以及与宏观物体运动规律的区别;(2) 了解量子力学的基本假定以及与此相关的波函数,力学量算符,本征值和平均值等概念;(3) 初步学会用量子力学研究微观物体(如电子、原子和分子等) 运动的方法(即根据研究对象及边界条件建立相应的量子力学运动方程薛定谔方程,及对简单方程求解)。
讲课要点:1−1 量子论的诞生1−2 实物粒子运动状态−波函数1−3 波函数的求解1−4 简单体系第二章原子结构和原子光谱内容提要: 本章将应用量子力学基本原理和定态薛定谔方程讨论原子结构及有关的各种性质和原子光谱。
复旦大学分析化学AII 第八章色谱导论..

tR 2 tR 2 n理 5.54( ) 16( ) Y1 / 2 Wb
t 2 t 2 n有效 5.54( ) 16( ) Y1 / 2 Wb H 有效
2018/10/10
' R
' R
L n有效
2 nm n VR -V 色谱流出曲线方程为:c= exp - (1); 2 VR 2 VR m为组分的进样量;VR为组分的保留体积;n为塔板数;
第十九章 色谱分析法
第一节 色谱法概述
信 号
时间 t
2018/10/10
一.色谱分析法的特点、分类和作用
1.概述
俄国植物学家茨维特在1906年使用的装置: 色谱原型装置,如图。
其中的一相固定不动,称为固定相;
另一相是携带试样混合物流过此固定相的流 体(气体或液体),称为流动相。
2018/10/10
分配系数是色谱分离的依据。
2018/10/10
分配系数 K 的讨论
组分在固定相中的浓度 Cs kd = = 组分在流动相中的浓度 Cm
一定温度下,组分的分配系数K越大,出峰越慢;
试样一定时,K主要取决于固定相性质;
每个组份在各种固定相上的分配系数K不同; 选择适宜的固定相可改善分离效果; 试样中的各组分具有不同的K值是分离的基础; 某组分的K = 0时,即不被固定相保留,最先流出。
液体固定相: 由担体和固定液所组成。 固定液对试样中各组分的溶解和解析能力的不同。
分离机理:气液(液液)两相间的反复多次分配过程。
2018/10/10
1. 气相色谱分离过程
当试样由载气携带进入色谱柱 与固定相接触时,被固定相溶解 或吸附; 随着载气的不断通入,被溶解 或吸附的组分又从固定相中挥发 或脱附; 挥发或脱附下的组分随着载气 向前移动时又再次被固定相溶解 或吸附; 随着载气的流动,溶解、挥发 ,或吸附、脱附的过程反复地进 行。 返回 2018/10/10
复旦大学大学物理A电磁学期末试卷及答案
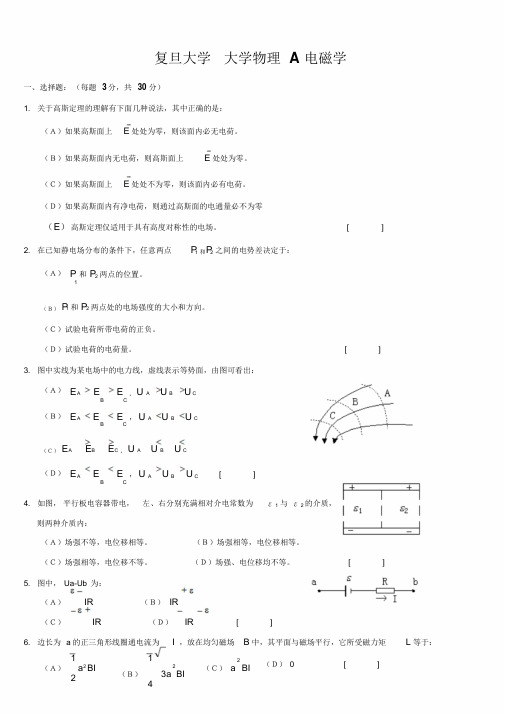
A,B板电势UB0。
现将一带电量为q,面积也是S而厚度可忽略不计的导体片C平行地插
在两极板中间位置(如图所示),则导体片C的电势
U=。
C
27.(3分)如图所示的电路的回路电压方程为。
28.(5分)在安培环路定理i
Bdl0I中,Ii是
L
指;B是指,它是由
决定的。
3.
求通过电源3的电流和R2消耗的功率。
4.一半径为R的塑料圆盘,电荷q均匀分布于表面,圆盘绕通过圆心垂直于盘面的轴转动,角速度为。
求圆盘中心的磁感应强度B。
5.在一半径为R的均匀圆柱体内充满磁感应强度为B的均匀磁场,这磁场以速率
dB
dt
在减小,求如图放置的金属棒ab(abl2R)两端的感生电动势
ab,又问:哪端电位
5.图中,Ua-Ub为:
(A)IR(B)IR
(C)IR(D)IR[]
6.边长为a的正三角形线圈通电流为I,放在均匀磁场B中,其平面与磁场平行,它所受磁力矩L等于:
1
(A)a2BI
2
1
2
(B)3aBI
4
2
(C)aBI
(D)0[]
7. 如图,两个线圈P和Q并联地接到一电动势恒定的电源上,线圈P的自感和电阻
(A)4A。(B)0.44A。
(C)0.33A。(D)0[]
9.在感应电场中电磁感应定律可写成
E
lK
dl
d
dt
,式中
E为感应电场的电场强度。此式表明:
K
(A)在感应电场中不能像对静电场那样引入电势的概念。
(B)闭合曲线l上
E处处相等。
K
(C)感应电场是保守力场。
50个大学校训翻译

50 个大学校训翻译1、北京大学( 创建于1898年) :爱国进步民主科学Peking University (founded in 1898): Patriotism, Advancement, Democracy and Science2、清华大学( 始建于1911年)自强不息德载物:Tsinghua University (founded as early as 1911): Self-discipline and Social Com mitment3、中国人民大学( Renmin University of China):实事求是Seek Truth From Facts.4、复旦大学( Fudan University ):博学而笃志,切问而近思Rich in Know-ledge and Tenacious of Purpose;Inquiring with Earnestness and Reflecting with Self-practice.5、浙江大学( Zhejiang University ):求实创新:Seek Truth and Be Creative.6、同济大学( Tongji University ):严谨求实,团结创新:Discipline 、Practicality 、Unity and Creativity.7、重庆大学( Chongqing University ):耐劳苦尚俭朴勤学业爱国家:Endurance 、Thrifty 、Diligence 、Patriotism.8、南京大学( Nanjing University ):诚朴雄伟,励学敦行:Be Honest and Intelligent 、Study Hard and Act Sincerely.9、武汉大学(Wuhan University) :自强弘毅,求是拓新:Improve Yoursel 、Carry forward Stamina, Seek Truth and Develop Innovations. 另一译:Get Bestirred 、Develop Perseverance 、Aspire after Truth and Blaze New Trails. 10、山东大学( Shandong University ):气有浩然,学无止境:Noble in Spirit; Boundless in Knowledge. 11、四川大学( Sichuan University ):海纳百川,有容乃大:The Sea Encompasses Hundreds of River; Willingness to Accept All Is Virtuous.12、中山大学( Sun Yat-sen University ):博Study Extensively Enquire学审问慎思明辨笃行:Accurately 、Reflect Carefully, Discriminate Clearly 、and Practise Earnestly.13 、上海外国语大学 ( Shanghai International Studies University :格高志远学贯中外:Integrity 、Vision and Academic Excellence.14 、北京师范大学 ( Beijing Normal University ):学为人师,行为世范Learn to Be an Excellent Teacher; Act as an Exemplary Person.15、中央民族大学( Minzu University of China ):团结求实,文明创新:Seek Truth through Unity and Innovate for Our Civilization.16 、中国传媒大学 ( CommunicationUniversity of China ):立德敬业,博学竞先:Build up the Character for the Career; Compete in Learning.17、北京外国语大学 ( Beijing Foreign Studies University ):兼容并蓄,博学笃行:Learn with an Open Mind to Serve a Great Cause.18、中国科技大学( University of Science and Technology of China ):红专并进,理实交融:Socialist-minded and Professionally Proficient: ;Associating Truth with Fact.19、南开大学( Nankai University ):允公允能,日新月异:Dedication to Public Interests ;Acquisition of AII-round Capability ;and Aspiration for Progress with Each Day.20、中南财经政法大学:博文明理厚德济世Zhongnan University of Economics and Law :Learned, Rational, Virtuous and Devoted. 21、中国海洋大学:海纳百川至人至德1)、Vastocean embraces streams to its tide; Norms received promise one far and wide.(2) 、Vast ocean embraces streams to its tide; Norms received promise well and wide.22、中国政法大学:厚德明法,格物致公China University of Political Science and Law :keeping integrity and law in mind and studying for the people developing moral education, mastering the law, looking for truth and serving the public23、中国地质大学:艰苦朴素,求真务实China University of Geosciences :Work Hard, ;Keep Modest ;Flexibly Unit and pursue progress.24、华中科技大学:团结、求实、创新、进取Huazhong University of Science and Technology: Unity ;Reality ;Strictness and Initiation.25、华中师大:求实创新立德树人huazhong normal university :Seek truth ;make innovations ;enhancemorality ;foster talents.26、西南交大: 勤奋自信遵纪文明Cultivate Talents to Rejuvenate the Chinese Nation and Strive Unceasingly.27、东南大学:止于至善Southeast University: Strive for Perfection.28、上海大学:自强不息Strengthen PersistentlyShanghai University: to make unremitting efforts, and to pursue innovation and the truth.29、南航大学:智周万物,道济天下Nanjing university of aeronautics and astronautics :Acquire knowledge ;serve the people (吴鼎民)团结、俭朴、唯实、创新(老校训)cooperation ;economical ;practical and creative.30、国防科技大学:奉献National University Technology: Dedication求实of Defense andPracticality 31、哈尔滨工业大学:规格严格,功夫到家Harbin Institute of Technology: Strict Standard and Sufficient Effort.32、天津大学:实事求是Tianjin University: Seek Truth from Facts 33、暨南大学:忠信笃敬Jinan University: Loyalty, Credibility, Sincerity, and Piety34、厦门大学:自强不息止于至善XiamenUniversity: Pursue Excellence, Strive for Perfection.35。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Physical Chemistry II
4
Thermodynamics of phase equilibria
The phase rule and its deduction
Phase, number of components, degrees is a form of matter that is homogeneous in
2015/6/15
Physical Chemistry II
5
Thermodynamics of phase equilibria
The phase rule and its deduction
Some basic conceptual issues need to take care when discussing ―phase‖ :
Gas, no matter how many kinds of them are mixed together, there
exists only one gas phase Liquid, depending on its miscibility, one and coexistence of two or three phases are possible Solid, one solid generally constitutes one phase by itself. No matter how well-mixed are two different solid powders, there still exist two phases (Except for solid solution, which is a single phase)
T = T = … =T
(2) Conditions for baric equilibrium: when reaching equilibrium, each phase is at the same pressure
p = p = … =p
2015/6/15
Physical Chemistry II
9
Thermodynamics of phase equilibria
The phase rule and its deduction
Phase, number of components, degrees of freedom
degree of freedom(自由度)
2015/6/15
Physical Chemistry II
6
Thermodynamics of phase equilibria
The phase rule and its deduction
III) In general, mixtures of gases only form a single phase. Since
8
Thermodynamics of phase equilibria
The phase rule and its deduction
(3) Conditions for phase equilibrium: Each substance B in all phases has the same chemical potential, equilibrium phase transition is reached
The phase rule and its deduction
Phase equilibrium is one of the most important parts of
thermodynamics in chemistry and chemical engineering. Many processes, for example, dissolution, distillation, extraction, recrystallization, purification and polymorph analysis, needs the knowledge of phase equilibria in heterogeneous systems.
chemical composition and physical state. Two immiscible liquids (or liquid/solid mixtures with different compositions) separated by a distinct boundary or interfaces. The change of macroscopic properties on the interface is very huge. The number of phases in thermodynamic equilibrium with each other is denoted as
I) Phase is homogeneous, but not necessarily continuous. A single phase could be separated into many particles or liquid drops, or dispersed in other phases. Therefore, a clear interface must exist between two different phases, while the existence of an interface does not necessarily mean that there are different phases. II) Being homogeneous but with different properties means that they are not the same phase. For example: iron and sulfur powers can be grinded into very fine and homogeneous state seen with the naked eye, but microscopic observation still reveal two phases. Since no matter how finely grinded, molecularly mixed state will never be reached, i.e., molecular level dispersion not possible, there still exist two phases.
all different types of gases can be molecularly mixed in any ratio, no interface exists between them.
Liquid, depending on its miscibility, there can be one or two phases. Two totally miscible liquids form a single phase, while partly miscible ones form two phases. For example: the benzene-water system consists of two phases, whereas the ethanol-water system consists of only one phase.
Normally, unless they form solid solution, there exist many distinct solid phases. For example: alloy consists of one phase, as alloy is a solid solution formed by molecularly mixed two types of metals.
2015/6/15
Physical Chemistry II
7
Thermodynamics of phase equilibria
The phase rule and its deduction
General requirements for equilibrium in heterogeneous systems In a closed heterogeneous system, heat/ material exchange and work delivery could occur between different phases. For the thermodynamic equilibrium between multiple phases, it actually contains the following four equilibrium conditions: (1) Conditions for thermal equilibrium: consider the system consisting of ,,, phases, when reaching equilibrium, each phase has the same temperature
Phase rule and its deduction Phase diagrams for one-component systems Second-order phase transitions Two-component phase diagrams Three-component phase diagrams
Extraction
2015/6/15
Recrystallization
Physical Chemistry II
2
Thermodynamics of phase equilibria
