readdata
书法比赛奖状readdata

马静雯同学:在2012年春季学校书法比赛中,以端正的字体,特被评为一等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日朱晓燕同学:在2012年春季学校书法比赛中,以端正的字体,特被评为一等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日刘菲同学:在2012年春季学校书法比赛中,以端正的字体,特被评为一等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日黄金草同学:在2012年春季学校书法比赛中,以端正的字体,特被评为一等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日张梦丽同学:在2012年春季学校书法比赛中,以端正的字体,特被评为一等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日周家铭同学:在2012年春季学校书法比赛中,以端正的字体,特被评为二等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日武钰菡同学:在2012年春季学校书法比赛中,以端正的字体,特被评为二等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日王萌萌同学:在2012年春季学校书法比赛中,以端正的字体,特被评为二等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日武庭玉同学:在2012年春季学校书法比赛中,以端正的字体,特被评为二等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日刘佳欣同学:在2012年春季学校书法比赛中,以端正的字体,特被评为二等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日郭敬凯同学:在2012年春季学校书法比赛中,以端正的字体,特被评为三等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日王之文同学:在2012年春季学校书法比赛中,以端正的字体,特被评为三等奖,特发此状,以资鼓励。
曲梁镇实验小学2012年3月23日李梦阳同学:在2012年春季学校书法比赛中,以端正的字体,特被评为三等奖,特发此状,以资鼓励。
副本readdata
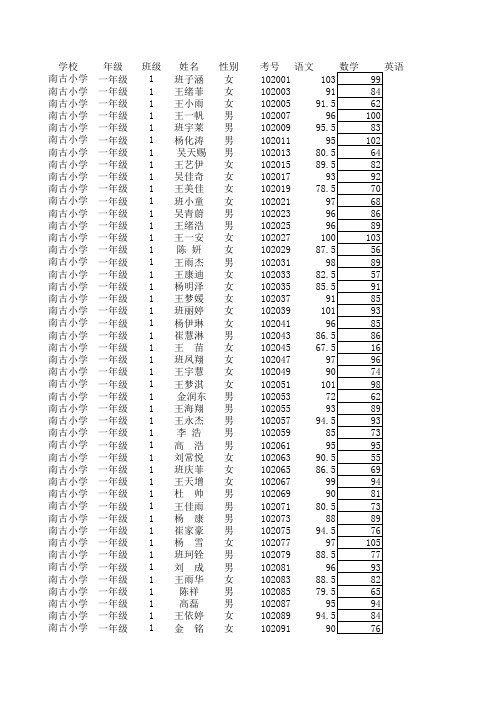
学校年级班级姓名性别考号语文南古小学一年级1班子涵女102001南古小学一年级1王绪菲女102003南古小学一年级1王小雨女102005南古小学一年级1王一帆男102007南古小学一年级1班宇莱男102009南古小学一年级1杨化涛男102011南古小学一年级1 吴天赐男102013南古小学一年级1王艺伊女102015南古小学一年级1吴佳奇女102017南古小学一年级1王美佳女102019南古小学一年级1班小童女102021南古小学一年级1吴青蔚男102023南古小学一年级1王绪浩男102025南古小学一年级1王一安女102027南古小学一年级1陈 妍女102029南古小学一年级1王雨杰男102031南古小学一年级1王康迪女102033南古小学一年级1杨明泽女102035南古小学一年级1王梦媛女102037南古小学一年级1班丽婷女102039南古小学一年级1杨伊琳女102041南古小学一年级1崔慧淋男102043南古小学一年级1王 苗女102045南古小学一年级1班凤翔女102047南古小学一年级1王宇慧女102049南古小学一年级1王梦淇女102051南古小学一年级1 金润东男102053南古小学一年级1王海翔男102055南古小学一年级1王永杰男102057南古小学一年级1李 浩男102059南古小学一年级1高 浩男102061南古小学一年级1刘常悦女102063南古小学一年级1班庆菲女102065南古小学一年级1王天增女102067南古小学一年级1杜 帅男102069南古小学一年级1王佳雨男102071南古小学一年级1杨 康男102073南古小学一年级1崔家豪男102075南古小学一年级1杨 雪女102077南古小学一年级1班珂铨男102079南古小学一年级1刘 成男102081南古小学一年级1王雨华女102083南古小学一年级1陈祥男102085南古小学一年级1高磊男102087南古小学一年级1王依婷女102089南古小学一年级1金 铭女102091南古小学一年级2高凡女102084南古小学二年级1王俪蒙女南古小学二年级1陈浩洋男南古小学二年级1王美晴女南古小学二年级1王可可男南古小学二年级1王统泽男南古小学二年级1赵迪女南古小学二年级1王文博男南古小学二年级1王帅迪女南古小学二年级1班晓欣女南古小学二年级1贾文鑫女南古小学二年级1禚城源男南古小学二年级1王国豪男南古小学二年级1王馨女南古小学二年级1班硕男南古小学二年级1刘星雨女南古小学二年级1高源女南古小学二年级1杨静女南古小学二年级1李宝旺男南古小学二年级1王艺凯男南古小学二年级1李冉女南古小学二年级1王超男南古小学二年级1班红伟男南古小学二年级1王新雨女南古小学二年级1王文旭男南古小学二年级1王佳乐男南古小学二年级1李贞强男南古小学二年级1班红齐男南古小学二年级1王春升男南古小学二年级1李笑女南古小学二年级1班彦凯男南古小学二年级1王凯旋男南古小学二年级1王婧霏女南古小学二年级1王凯男南古小学二年级1王思尧男南古小学二年级1李康男南古小学二年级1门雯雯女南古小学二年级2李欢 女南古小学二年级3王安庆男南古小学二年级3李爽女南古小学二年级3李辉男南古小学二年级3王润男南古小学二年级3陈佳慧女南古小学二年级3李守庆男南古小学二年级3王悦男南古小学二年级3王轩女南古小学二年级3刘文轩男南古小学二年级3张馨玲女南古小学二年级3班文静女南古小学二年级3吴金航男南古小学二年级3王志男南古小学二年级3王旭男南古小学二年级3陈婷女南古小学二年级3曹硕男南古小学二年级3王玉凤女南古小学二年级3邸翔男南古小学二年级3王冰雨女南古小学二年级3王红女南古小学二年级3王统玉男南古小学二年级3李昊霖男南古小学二年级3王佳慧女南古小学二年级3班俊杰男南古小学二年级3陈璇女南古小学二年级3王玥涵女南古小学二年级3崔豪杰男南古小学二年级3班瑞阳男南古小学三年级1吴一帆男3020018510188南古小学三年级1缪志翔男30200382.54467南古小学三年级1王童童女30200587.59987南古小学三年级1禚洪甲男302007688785南古小学三年级1王文琪女302009889795南古小学三年级1杨瑞男302011899798南古小学三年级1卓宝娟女30201380.59798南古小学三年级1班牛牛女30201582.577.591南古小学三年级1程天浩男3020178499.591南古小学三年级1陈星男3020197783.585南古小学三年级1王丽娟女30202186.510196南古小学三年级1李林倩女30202391.58991南古小学三年级1杨兰兰女30202586.59893南古小学三年级1王睿智男30202765.59364南古小学三年级1王听雨女302029839493南古小学三年级1杨大圆男30203180.58970南古小学三年级1王梦琪女30203388.510283南古小学三年级1李钲豪男302035719185南古小学三年级1陈庆豪男3020379210290南古小学三年级1王玥女302039879581南古小学三年级1王清雯女3020418910395南古小学三年级1王淑婷女30204379.58883南古小学三年级1王浩然男30204590.59798南古小学三年级1陈俊女30204786.89685南古小学三年级1王瑞斌男30204986.59596南古小学三年级1班硕男302051839996南古小学三年级1王姝雯女302053828385南古小学三年级1班慧女302055869891南古小学三年级1武岳男302057819387南古小学三年级1王田雨女30205987.5104103南古小学三年级1班彦琪男302061638469南古小学三年级1晁旭男302063769695南古小学三年级1王润涛男302065779097南古小学三年级1赵雪如女30206780.59486南古小学三年级1吴文熙男302069878379南古小学三年级1王统潇男30207186.590.590南古小学三年级1李悦女30207386.510082南古小学三年级1陈泰宇男30207587.510293南古小学三年级1班淑倩女302077829687南古小学三年级1王凯男3020798910478南古小学三年级1吴超男30208182.597.571南古小学三年级1卓旭男30208381.59397南古小学三年级1崔丽媛女30208576.59285南古小学三年级1班红源女30208787.5101103南古小学三年级1班鸣泽男3020898910195南古小学三年级1班凯杰男3020918810195南古小学三年级1王琪女3020939197100南古小学三年级1王晓凤女30209591.59994南古小学三年级1王艺蔓女302097899581南古小学三年级1杨宇春男30209984.59280南古小学三年级2徐启迪男302002909098南古小学三年级2班凡婷女30200494.59686南古小学三年级2王皓磊男302006949674南古小学三年级2李二征男3020085675.569南古小学三年级2王倩女30201087.59994南古小学三年级2王文强男30201271.578.573南古小学三年级2王皓月女3020148610298南古小学三年级2王宁男302016829786南古小学三年级2王熒女30201891.59697南古小学三年级2刘琦女30202066.570.564南古小学三年级2班优优女30202290.510092南古小学三年级2李康佳男302024552963南古小学三年级2王璐琦男30202683.58085南古小学三年级2王统正男3020287575.572南古小学三年级2王俪颖女30203087.59886南古小学三年级2王煜男302032819966南古小学三年级2王爱雯女3020349690101南古小学三年级2王宸男30203688.58677南古小学三年级2陈祥凤女30203893.59393南古小学三年级2班伟强男302040817759南古小学三年级2高瑜女30204294.59983南古小学三年级2李贞豪男302044879491南古小学三年级2武天祥男302046546159南古小学三年级2王浩源男30204888.590101南古小学三年级2王统旺男302050796871南古小学三年级2李沅澄男30205285.59877南古小学三年级2王一龙男302054727671南古小学三年级2王晓慧女30205694.59481南古小学三年级2李一飞男30205861.56454南古小学三年级2禚曦然女30206092.57899南古小学三年级2李大征男302062667260南古小学三年级2王家豪男302064808761南古小学三年级2陆红女30206676.58074南古小学三年级2李才女30206880.581.582南古小学三年级2班原宁男30207085.58783南古小学三年级2赵秋雨女30207285.58766南古小学三年级2班雨辰女30207484.59594南古小学三年级2王江雨男30207670.561.546南古小学三年级2李婷女30207893.510099南古小学三年级2杨羽女302080799688南古小学三年级2崔力文女302082939993南古小学三年级2怡霏女30208480.57392南古小学三年级2班晴女302086859295南古小学三年级2李芮女3020888810399南古小学三年级2李宝源男30209072.59187南古小学三年级2吴奕鑫男30209287.58393南古小学三年级2高旭辉男30209460.573.574南古小学三年级2于浩男302096788982南古小学三年级2班梦晓女30209843.54447南古小学四年级1王柯茗男402001939590南古小学四年级1王恒云女402003869777南古小学四年级1刘敏女40200587.510187南古小学四年级1王雨捷男40200774.54643南古小学四年级1陈柏文男4020099310086南古小学四年级1武硕男402011828881南古小学四年级1王璇女4020139410098南古小学四年级1李宝宝男402015768259南古小学四年级1班雨晴女402017949792南古小学四年级1王凯女40201982.59058南古小学四年级1杨蒙女40202184.510381南古小学四年级1班子翔男40202367.55231南古小学四年级1王文慧女40202594.510387南古小学四年级1朱珠男40202775.59265南古小学四年级1古岳男40202987.510390南古小学四年级1王勇翔男40203175.59049南古小学四年级1班红洋女40203387.59983南古小学四年级1王绪强男402035698546南古小学四年级1王谦男4020379310493南古小学四年级1陈瑞雪女402039779471南古小学四年级1吴淑锟男4020419310478南古小学四年级1王统阳男402043768063南古小学四年级1王瑶建男402045809788南古小学四年级1王孜女40204789.59272南古小学四年级1王晴涵女402049929994南古小学四年级1赵亿媛女40205187.59582南古小学四年级1班红菊女40205387.59873南古小学四年级1王枫男402055789865南古小学四年级1王竞笛女402057779574南古小学四年级1颜菲女40205994.510287南古小学四年级1王皓冬男402061849959南古小学四年级1王玉芝女402063969992南古小学四年级1高慧女40206590.510176南古小学四年级1班贺凯男40206790.510277南古小学四年级1王绪莲女402069879749南古小学四年级1王鹏飞男40207184.59961南古小学四年级1李云燕女40207392.59688南古小学四年级1陈雨亭女402075899977南古小学四年级1王依凡男40207781.58746南古小学四年级2班士超男402002827644南古小学四年级2王迪男40200490.510272南古小学四年级2王明月女40200681.59562南古小学四年级2王静女40200888.5102100南古小学四年级2杨竣茹女402010817865南古小学四年级2王艳梅女402012889575南古小学四年级2班璐阳女402014929067南古小学四年级2崔文杰男4020169410285南古小学四年级2王绪炜男4020189210285南古小学四年级2王启笛男40202082.59649南古小学四年级2王鑫男40202285.59552南古小学四年级2陆超男40202477.55447南古小学四年级2卢韵萱女40202610110599南古小学四年级2王瑞通男402028728645南古小学四年级2班艳女402030889384南古小学四年级2王绪芹女4020328610173南古小学四年级2吴爽女402034879993南古小学四年级2杨梦瑶女402036919676南古小学四年级2班凯男402038827745南古小学四年级2陈悦女4020409310090南古小学四年级2王意今男402042798674南古小学四年级2王正阳男4020448910071南古小学四年级2王景灏男40204690.59452南古小学四年级2王晓迪女402048949877南古小学四年级2班亚斌男40205079.510061南古小学四年级2高菡女402052869889南古小学四年级2王薪羽男40205422.51031南古小学四年级2陆宁女4020569610386南古小学四年级2班妞妞女402058739448南古小学四年级2郑湘祺女402060909377南古小学四年级2班庆伟男402062644626南古小学四年级2班鲁山男402064899770南古小学四年级2缪春艳女40206686.510276南古小学四年级2班圣男402068789643南古小学四年级2吴杰女40207088.510069南古小学四年级2王雪女402072878764南古小学四年级2班洪新男40207470.59755南古小学四年级2金锴男402076909483南古小学四年级2班永琪男402078829166南古小学四年级2班杰克男402079879152南古小学四年级2崔小凡男402080757544南古小学四年级2李京达男40208185.584.562南古小学四年级2杨世川男402082859289南古小学五年级1杨同兴男50200174.599.559南古小学五年级1李晨男5020038210075南古小学五年级1曹一凡女50200582.5101.5103南古小学五年级1班仕涛男5020078495.581南古小学五年级1王玉豪男5020098167.576南古小学五年级1陈梦菲男50201168.54041南古小学五年级1陈雨婷女5020137567.546南古小学五年级1李阿丹女50201588.5102.5102南古小学五年级1王振东男50201781.59686南古小学五年级1班振强男5020197982.569南古小学五年级1王钰婷女502021959695南古小学五年级1王统捷男5020232328.570南古小学五年级1李根男502025859786南古小学五年级1王静女502027809378南古小学五年级1陆薇女50202987101.591南古小学五年级1王永豪男50203179.589.571南古小学五年级1王绪征男502033663957南古小学五年级1王莹女502035827376南古小学五年级1李晴女5020377496103南古小学五年级1王凯男50203988.5103100南古小学五年级1赵婧女50204189.510386南古小学五年级1杨峻豪男50204387.59978南古小学五年级1王恒雨女5020457995105南古小学五年级1陈梓鑫男50204789.59891南古小学五年级1王方舟男5020493436.546南古小学五年级1王扬男50205190105103南古小学五年级1王文雅女502053698777南古小学五年级1赵婧妤女50205579.56388南古小学五年级1王佳莹女50205788102102南古小学五年级1王芝霖女50205977.52673南古小学五年级1班翔男502061788057南古小学五年级1王昆宏男502063654374南古小学五年级1相文博男502065818886南古小学五年级1王晴女50206786.587.566南古小学五年级1陆鑫男502069729969南古小学五年级1王渊之男50207187.597.589南古小学五年级1吴棒男50207381223南古小学五年级1钟子豪男50207579.59880南古小学五年级1徐勇杰男50207792.594.587南古小学五年级1金志远男502079949082南古小学五年级1王梦龙男502081849768南古小学五年级1王永男5020837485.565南古小学五年级1李蕊女502085908791南古小学五年级2班如月女50200293.5102101南古小学五年级2刘常乐男50200493.510491南古小学五年级2吴心雨女50200692104.597南古小学五年级2王婧女50200889.510198南古小学五年级2王慧鑫女5020109010394南古小学五年级2刘可心女50201287.59994南古小学五年级2吴美颖女502014829987南古小学五年级2高敏女50201691102.596南古小学五年级2禚越女5020189591.582南古小学五年级2于圣统男5020208610079南古小学五年级2禚宝轩男50202295102.594南古小学五年级2王佳浩男50202491.5102.5100南古小学五年级2杨潇男5020269610484南古小学五年级2王赵丽女5020289091.583南古小学五年级2王倩女5020308293.575南古小学五年级2黄金龙男50203288.59572南古小学五年级2赵鹏程男502034588348南古小学五年级2王粤明男50203687101.589南古小学五年级2王恒宇男502038859989南古小学五年级2王程翔男50204091101.571南古小学五年级2王俪颖女50204287100.595南古小学五年级2王林男5020448388.586南古小学五年级2朱超男5020468810493南古小学五年级2李志楠男5020487810182南古小学五年级2王绪超男5020509096101南古小学五年级2杜树飞男5020527897.574南古小学五年级2王统富男50205474.595.591南古小学五年级2葛勇男5020568090.592南古小学五年级2王蕾女502058979884南古小学五年级2朱佳佳男5020606679.550南古小学五年级2吴琦男502062819966南古小学五年级2武小暄女5020648295.593南古小学五年级2刘晴女5020667322.537南古小学五年级2马建通男50206882.598.550南古小学五年级2王凯男502070789046南古小学五年级2王友涵男50207260.575.550南古小学五年级2王宇女50207485.59549南古小学五年级2刘诗雨女5020769097.598南古小学五年级2于娜女50207876.572.571南古小学五年级2王文康男50208084.5100.581南古小学五年级2马建康男50208280.594.558南古小学五年级2李鑫男50208489.589.571南古小学五年级2王兴行男5020861250.564南古小学五年级2班彦可男50208775.59871南古小学五年级2王依凡女502088839889。
readdata浪子的故事

读《浪子的故事》有感-----焦庄小学石蕊近日,我读到《浪子的故事》,这个离家、落魄到归家的故事使我受到深深的触动。
故事的情节大致为:一个人有两个儿子,小儿子问父亲要家产,然后带着父亲给予的所有财产往远方去了。
他在那里任意放荡,浪费资财,花尽了所有钱财,就穷苦起来。
无奈去投靠别人,他被打发去喂猪,恨不得拿喂猪的食物物来充饥。
此时,他突然想起他父亲家中口粮有余,便踏上了回归之旅。
父亲老远看见他,就跑去抱住他,亲吻他,激动地老泪纵横。
给他上好的衣服、戒指,并宰了肥牛犊,全家来庆祝儿子的喜从天降!浪子的经历是许多年轻人的写照,许多年轻人在家这个“温柔乡”呆久了,便不顾一切地想冲出束缚他的这个牢笼。
他们总以为远方有别致的风景,迷人的诱惑。
而到远方才发现,他们要经历流离和迷失,孤单和困惑。
等他们享尽一切欢乐才发现,唯有家才是那个拥有一切的丰富之地。
此时,想到《论语·里仁》中讲到:“父母在,不远游,游必有方。
”。
孔子的教导是子女要对健在的父母尽孝心,外出游学追求自己的目标要有方向,不令父母牵挂。
浪子故事的转折点在于---当他沦落到要拿喂猪的东西来充饥时的幡然醒悟:“我父亲家中有多少的雇工,口粮有余,我倒要在这里饿死吗?”。
联想到当今快速的经济发展热潮,许多人走在背井离乡,到外地去谋发展的路上。
在我看来,我不反对年轻人的奋斗。
年轻理当有抱负,有理想,有追求,不免有时要经受挫折、痛苦甚至冷眼、厌弃。
紧要的是能够回转,返回那可以避风的家,那个心灵的自由之乡,重镇旗鼓。
父亲在故事中描述较少,在儿子要家业的时候,他没说什么;当儿子回来时,相离还很远,他就跑去迎接。
他对这失而复得的儿子没有丝毫的责备,反而是更加地疼爱。
他把上好的袍子给他穿上,把戒指给他戴上,把鞋子给他穿上;他还要宰了肥牛犊来共同庆祝,吃喝欢乐。
心理意想对话解释说:袍子代表保护,戒指象征身份,鞋子象征地位。
这说明父亲对儿子的爱在乎全然的饶恕。
他没有责怪儿子不懂事向他要家产,没批评儿子不争气,花光了所有才回来。
ABB电机说明书readdata.jsp

三相异步电动机使用维护说明书上海ABB电机有限公司2007年6月ABB电机使用维护说明书一、产品介绍1、适用范围本说明书适用于ABB各标准系列及其所派生的各种系列电机(防爆系列电机除外)。
机座中心高:56-355。
(对一些特殊应用场合或有特殊设计考虑的型号电机还需参阅其它特别的指导说明)。
二、一般要求1、起动1.1 收货检验∙收货后,立即检验电机有无外部损伤,检验所有的铭牌数据,尤其是电压和绕组的连接方式(Y 或△)。
∙用手旋转转轴,检测电机空转情况,如果电机装有锁定装置,注意将其打开。
1.2 绝缘性能检测∙电机初次使用之前,绕组有可能受潮,都要测量其绝缘阻值。
∙25℃时测量的绝缘电阻值应超过参考值,20×URi ≥ MΩ1000+2PU=电压 V, P=输出功率 kW[注意]测量后绕组要立即放电,避免电击。
∙周围环境温度每升高20℃,电阻的参考值减少一半。
∙如果没有达到绝缘电阻的参考值,绕组就必须烘干。
∙烘炉的温度为90℃,时间12-16小时。
∙如果安装了排水塞,烘干时必须将其打开。
∙绕组被海水浸泡后一般要重绕。
1.3 直接起动或 Y/△起动∙标准单速电机的接线盒一般有6个接线螺栓和至少1个接地螺栓。
∙电机通电之前,必须按规定要求可靠接地,不能接零代替接地。
∙电压和绕组连接方式在铭牌上有标注。
1.3.1 直接起动绕组可以采用 Y或△接法,例如660VY,380V△分别表示660V,Y接法和380V,△接法。
1.3.2 Y/△起动∙电源电压必须等于△接法电机的额定电压。
∙拆下接线板上所有的接线片,按Y/△起动装置接线,妥善连接到电机六个接线柱上,并能从起动初期的Y连接跳到启动完成的△接。
∙双速电机和其他特种电机的电源接法必须依照接线盒内的接线图说明。
1.4 接线柱和旋转方向∙如果电源相序U1,V1,W1依次与接线柱U1,V1,W1连接,从电机的驱动端观察转轴,其旋转方向为顺时针。
∙换接电源线中的任意两相就可以改变电机的旋转方向。
readdata.jsp

供应链管理的出现促进了企业资源计划(ERP)的发展。ERP强调对供应链的整体管理,将供应商、制造商、协作商、客户甚至是竞争对手都纳入管理资源的整体系统之中,使业务流程更加紧密集成,对市场反应更加灵敏、快捷,柔性化程度大为提高,风险与成本大大降低。ERP的出现不只是一种管理模式和概念,而已成为与供应链管理体制相匹配的现代化管理软件系统,因此ERP本身就是企业信息化和网络化的重要组成部分。
对于信息管理系统在企业中的作用,我个人将其分为四点:
1 信息管理系统可以调整产品的生产周期,提高产品的分销速度,改善产品服务。这样产品就能更快捷地进入市场成为商品供消费者选择购买。2 信息管理系统可使生产经营状况得以改善。其可以突破区域性市场的限制,销售商可通过各分销中心连接形成销售网,使产品可在各地销售,扩大了消费群。3 信息管理系统降低了产业利润的竞争压力。公司的信息系统工程可以降低产品和服务的成本,而使企业利润相对提高。4 信息管理系统改善了用户服务。该系统可以为企业收集更多的客户信息,使企业可根据已有的信息对客户提供个性化服务从而提高企业的战略优势。总之,信息管理系统为企业的发展提供了竞争优势,同时也为消费者带来了一定的好处,是消费者有了更多的商品选择和快捷优质的商品售前和售后服务。
工化中的特殊作用
企业信息化,是指企业应用现代工业工程的理论、技术与方法,在对业务流程进行改造、重组、优化或再设计的基础上,利用计算机技术、通信技术、网络技术与数据库技术,控制和集成化管理企业的所有资源和生产经营活动中的所有信息,实现企业内外部信息共享和有效利用,提高企业的管理水平、市场应变能力和整体竞争能力的工程化过程。
readdata施乐2255定影不牢

DPC2255打印间歇性有重影
日期:2010.1.6
机型:DPC2255
故障现象:DPC2255打印出现间歇性重影(如下图),出现间隔不定,纸张为普通A4纸。
故障原因:机器在环境和纸张两个因素影响下定影温度不够,造成打印某些纸张出现重影。
解决方法:
1.检查纸张类型。
2.更改纸张设置:管理员菜单——打印设定——纸张的画质处理——普通纸——从
“B”改为“S”。
3.在诊断模式下,在DC131中更改如下NVM:
744-401 从0改为1.(普通纸定影不良个别对应模式开关)
744-402 从2改为10.(定影不良个别对应模式转换温度,相对于Run温度的转换温度)
P.S.以上设置改动,经测试不影响出纸速度。
readdata

DOI: 10.1126/science.1204394, 71 (2011);333 Science , et al.Sung Wng Kim −4e ⋅4+]64O 28Al 24Room-Temperature Stable Electride [Ca Solvated Electrons in High-Temperature Melts and Glasses of theThis copy is for your personal, non-commercial use only.clicking here.colleagues, clients, or customers by , you can order high-quality copies for your If you wish to distribute this article to othershere.following the guidelines can be obtained by Permission to republish or repurpose articles or portions of articles): August 23, 2011 (this infomation is current as of The following resources related to this article are available online at/content/333/6038/71.full.html version of this article at:including high-resolution figures, can be found in the online Updated information and services, /content/suppl/2011/06/29/333.6038.71.DC1.htmlcan be found at:Supporting Online Material /content/333/6038/71.full.html#related found at:can be related to this article A list of selected additional articles on the Science Web sites /content/333/6038/71.full.html#ref-list-1, 1 of which can be accessed free:cites 16 articles This article /content/333/6038/71.full.html#related-urls 1 articles hosted by HighWire Press; see:cited by This article has been/cgi/collection/chemistry Chemistrysubject collections:This article appears in the following registered trademark of AAAS.is a Science 2011 by the American Association for the Advancement of Science; all rights reserved. The title Copyright American Association for the Advancement of Science, 1200 New York Avenue NW, Washington, DC 20005. (print ISSN 0036-8075; online ISSN 1095-9203) is published weekly, except the last week in December, by the Science o n A u g u s t 23, 2011w w w .s c i e n c e m a g .o r g D o w n l o a d e d f r o m6.P.Wollants,M.De Bonte,J.R.Roos,Z.Metallk.70,113(1979).7.K.Ando,T.Omori,I.Ohnuma,R.Kainuma,K.Ishida,Appl.Phys.Lett.95,212504(2009).8.T.Maki,K.Kobayashi,M.Minato,I.Tamura,Scr.Metall.18,1105(1984).9.Y.Tanaka et al .,Science 327,1488(2010).10.R.Kainuma,M.Ise,K.Ishikawa,I.Ohnuma,K.Ishida,p.269,173(1998).11.S.M.Hao,T.Takayama,K.Ishida,T.Nishizawa,Metall.Trans.15,1819(1984).12.Materials and methods are available as supportingmaterial on Science Online.13.T.Maki,in Shape Memory Materials ,K.Otsuka,C.M.Wayman,Eds.(Cambridge Univ.Press,Cambridge,1998),pp.117–132.14.L.Muldawer,F.de Bergevin,J.Chem.Phys.35,1904(1961).15.Y.Sutou,N.Koeda,T.Omori,R.Kainuma,K.Ishida,Acta Mater.57,5759(2009).16.H.Y.Kim,S.Hashimoto,J.I.Kim,H.Hosoda,S.Miyazaki,Mater.Trans.45,2443(2004).17.J.Ma,I.Karaman,R.D.Noebe,Int.Mater.Rev.55,257(2010).18.R.Umino et al .,J.Phase Equilibria Diffus.27,54(2006).19.H.C.Tong,C.M.Wayman,Acta Metall.22,887(1974).20.C.Zener,J.Met.7,619(1955).21.S.S.Yan et al .,Solid State Commun.54,831(1985).22.G.A.Pérez Alcazar,E.Galvão da Silva,C.Paduani,Hyperfine Interact.66,221(1991).23.W.Tang,R.Sandström,Z.G.Wei,S.Miyazaki,Metall.Mater.Trans.A Phys.Metall.Mater.Sci.31,2423(2000).24.C.L.Magee,R.G.Davies,Acta Metall.20,1031(1972).25.E.J.Graesser,F.A.Cozzarelli,J.Eng.Mech.117,2590(1991).26.M.Dolce,D.Cardone,R.Marnetto,Earthquake Eng.Struct.Dynam.29,945(2000).27.A.Abbott,Nature 414,572(2001).Acknowledgments:The authors are grateful to K.R.A Ziebeck,Cavendish Laboratory,University of Cambridge,for helpin critical reading.This work was supported by the Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science and by the Global Center of Excellence Program “Materials Integration (International Center of Education and Research),Tohoku University,”Ministry of Education,Culture,Sports,Science,and Technology.A patent application has been filed on the materials,including the alloy presented herein.Supporting Online Material/cgi/content/full/333/6038/68/DC1Materials and Methods SOM Text Figs.S1to S6Table S1References (28–41)27December 2010;accepted 13May 201110.1126/science.1202232Solvated Electrons in High-Temperature Melts and Glasses of the Room-TemperatureStable Electride [Ca 24Al 28O 64]4+4e−Sung Wng Kim,1Terumasa Shimoyama,2Hideo Hosono 1,2Solvated electrons in alkali metal-ammonia solutions have attracted attention as a prototype electronic conductor and chemical reducing agent for over a century.However,solvated electrons have not been realized in a high-temperature melt or glass of an oxide system to date.We demonstrated the formation of persistent solvated electrons in both a high-temperature melt and its glass by using the thermally stable electride [Ca 24Al 28O 64]4+⋅4e −(C12A7:e −)and controlling the partial pressure of oxygen.The electrical and structural properties of the resulting melt and glass differ from those of the conventional C12A7:O 2−oxide,exhibiting metallic and hopping conduction,respectively,and a glass transition temperature that is ~160kelvin lower than that of C12A7:O 2−glass.Solvated electrons reside in cage structures in C12A7:e −and form a diamagnetic paired state.Solvated electrons in alkali metal-ammonia solution have been a diverse stem for sci-entific and applied researches (1).Further-more,alkali metal-amine solutions containing solvated electrons can be condensed into ionic solids,known as electrides,in which electrons aretrapped in well-defined structural cavities and/or channels in matrices (2).Organic electrides can be created in which organic complexants,such as crown ethers,bind electrons,but these com-pounds are thermally unstable (2).This draw-back provoked the development of thermallystable electrides based on inorganic compounds,such as calcium aluminum oxide (12CaO ⋅7Al 2O 3,abbreviated as C12A7:O 2−).In this compound,O 2−is a caged species that compensates for the positive charge of the ~0.4-nm cage,and an electride can be formed by displacing this anion (3,4).The caged electrons are protected from air and moisture even near room temperature (RT)because of the small size of the windows (diam-eters of ~0.1nm)connecting the cages.C12A7:e −can be synthesized by means of various chemical and physical processes.We reported that strongly reduced C12A7:O 2−melt crystallizes to form C12A7:e −and that single crys-tals of C12A7:e −can be grown from polycrystal-line C12A7:e −by the floating zone (FZ)melting method under a strongly reducing atmosphere (5,6).These findings imply that the electrons persist in the cages just below melting point (T m )under a strongly reducing atmosphere.Because1Frontier Research Center,Tokyo Institute of Technology,Post Office Box S2-13,4259Nagatsuta,Midori-ku,Yokohama 226-8503,Japan.2Materials and Structures Laboratory,Tokyo In-stitute of Technology,Post Office Box R3-1,4259Nagatsuta,Midori-ku,Yokohama 226-8503,Japan.Fig. 1.(A )The temperature dependence of s of C12A7:e −and C12A7:O 2−melts.The s of C12A7:e −melt decreases as the temperature increases,showing metallic conduction.In contrast,a melt of the mother compound,C12A7:O 2−oxide,shows ionic conduction.Upon melting,the s of C12A7:e −electride decreases to several siemens,which is an order of magnitude lower than that of the crystalline electride just below T m .The sudden de-crease in the s of C12A7:O 2−on melting is ascribed to the disap-pearance of free O 2−ions accom-modated in the cages because of the collapse of the three-dimensional network of sub-nanometer-sized cages that serve as the conduction pathway for oxide ions.The activation of ionic conduction in the C12A7:O 2−melt (6.3eV)is related to viscosity (19),implying that dissociation of O 2−ions from the polymerized network structures determines both the rate of ionic conductionand viscous flow.(B )Density of C12A7:e −and C12A7:O 2−melts as a function of temperature.All data were acquired during heating.The red and pink circles represent the densities of C12A7:e −melts measured under P O 2~10−24and ~10−16atm,respectively.The error range was T 3%for the red and T 2%for the pink and blue datapoints.⋅SCIENCE VOL 3331JULY 201171REPORTSo n A u g u s t 23, 2011w w w .s c i e n c e m a g .o r g D o w n l o a d e d f r o mthe excess electrons in alkali metal-molten salt solutions at high temperature have been studied extensively (7),it is of both fundamental and ap-plied interest whether a high concentration of solv-ated electrons persist in high-temperature melts of typical refractory oxides because the precur-sor C12A7:O 2−,which is a by-product from iron smelting processes,exhibits T m ~1688K (8).Because CaO and Al 2O 3are both stable oxides and typical electrical insulators,the conventional melt of C12A7:O 2−does not contain carrier elec-trons in the molten state.Thus,the question arises:Do solvated electrons exist in the melt of refractory oxide-based C12A7:e −—that is,like the solvated electrons in alkali metal-ammonia so-lutions,but at much higher temperatures?To an-swer this,we studied the physical properties of the melt of C12A7:e −electride under a low-oxygen partial pressure (P O 2).In addition,we examined structural and electrical properties of glasses pre-pared by rapidly quenching the C12A7:e −melt so as to obtain structural information about the melt and realize a new type of amorphous semi-conducting oxide.The electrical conductivity (s )of C12A7:e −at elevated temperature decreases monotonically as the temperature increases up to 1750K (Fig.1A).Although a small decrease in s is observed around the melting point (T m ~1500K)(fig.S3),the dependence does not change below and above T m ,so the C12A7:e −melt exhibits metallic conduction.In contrast,s increases with tempera-ture in a C12A7:O 2−melt,like that of a conven-tional oxide.The C12A7:e −electride melt exhibits a higher density (r )than that of a C12A7:O 2−melt,by ~10%at 1773K (Fig.1B).This difference suggests that there are marked structural differences between the melts of C12A7:e −and C12A7:O 2−.To clarify this pos-sibility,we rapidly quenched the C12A7:e −melt from ~1873K using a twin-roller at RT and ex-amined the resulting glasses.When the C12A7:e −melt is quenched,it is imperative to maintain a low P O 2in order to prevent oxidation of the melt (figs.S1,S4,and S5).Thus,we attached a homemade chamber containing a twin-roller to the melting system.The quenched black sample (C12A7:e −electride glass)had a thickness of ~50m m (Fig.2A,inset).The x-ray diffraction (XRD)pattern of the glass shows a broad halo,and we only observed a ring pattern of trans-mision electron microscopy (TEM)electron dif-fraction (fig.S2),indicating that the glass was fully amorphous.Figure 2A shows the differen-tial thermal analysis (DTA)of the C12A7:e −glass,revealing a distinct shift in the base line at ~973K that corresponds to the glass transition temperature (T g ).T g is ~160K lower than that of the conventional C12A7:O 2−glass.This differ-ence strongly suggests that the network structure of C12A7:e −glass differs substantially from that of C12A7:O 2−glass.Figure 2B shows the temperature depen-dence of s of C12A7:e −glass.The conductivity near RT is ~10−7S cm −1and increases within-Fig.2.(A )DTA comparison of the samples obtained by quenching C12A7:e −(red line)and C12A7:O 2−melts (black line).The left inset shows a photograph of the C12A7:e −glass,which was sealed in a silica capsule (right inset)under a vacuum to avoid oxidation during heating.The combined XRD,TEM (fig.S2),and DTA results reveal that a glass with a T g of 973K was obtained.(B )The s of C12A7:e −glass.The s increases as the temperature increases,showing semiconducting behavior following log s ºT −1/4.When the glass is crystallized under an atmosphere of P O 2~10−24atm,s shows the same temperature dependence as that of the parent electride at high temperature.The s of C12A7:O 2−glass is lower than the detection limit (10−10S cm −1)at RT.(C )Correlation of the N e in C12A7:e −glasses and that in the starting C12A7:e −electride used for melting.The dashed line shows the value of N e in the starting C12A7:e −electrides.(D )Optical absorption spectra of C12A7:e −glass (red line)and C12A7:O 2−glass (black line).The band at 3.3eV is assigned to F +-like centers containing trapped electrons;their con-centration was determined to be ~5×1018cm −3from an ESR spectrum (inset).Fig.3.Raman spectra of a C12A7:O 2−crystal (c -C12A7:O 2−),C12A7:O 2−glasses (g -C12A7:O 2−),and C12A7:e −glasses (g -C12A7:e −)with different N e .The C12A7:O 2−glasses (B)and (C)were obtained by quenching a melt of C12A7:O 2−oxide under an oxidizing atmosphere of P O 2~1atm by using an alumina crucible and a reducing atmosphere of P O 2~10−16atm by using a carbon crucible (11),respec-tively.The wavelength of the excita-tion laser was 457nm.(Inset)The relationship of the Raman intensity ratio (I 186/I 780)of the 186cm −1band to the 780cm −1band measured with excitation lasers of various wavelengths.The optical absorption spectrum of C12A7:e −glass [sample (G)]is shown to clarify the resonanceeffect.1JULY 2011VOL 333SCIENCE 72REPORTSo n A u g u s t 23, 2011w w w .s c i e n c e m a g .o r g D o w n l o a d e d f r o mcreasing temperature.The temperature dependence of log s is proportional to T −1/4rather than T −1,indicating that electronic conduction is controlled by variable-range hopping (VRH).This tempera-ture dependence implies that the C12A7:e −glass contains a high concentration of localized electrons.Moreover,this change of conduction mechanism —from metallic conduction in the melt to VRH con-duction in the glass —is similar to that reported in an amorphous thin film of metallic sodium-ammonia solution containing solvated electrons (9).Next,we used iodometry (10)to confirm the presence of electrons and quantify the electron concentration (N e )in different C12A7:e −glasses.We prepared glasses by quenching melts of C12A7:e −containing different N e under an atmo-sphere of P O 2~10−24atm.The relation between N e in the polycrystalline C12A7:e −used to form the melts and N e in the resulting C12A7:e −glasses is shown in Fig.2C.The observed linear relation shows that N e is retained in C12A7:e −glasses.That is,C12A7:e −glasses exhibit a maximum N e of 1.1×1021cm −3,which is almost the same as that of recrystallized C12A7:e −from a melt of C12A7:e −with N e of 1.7×1021cm −3.We verified N e values by measuring the weight gain caused by oxidation using thermogravimetry (TG).We checked whether solvated electrons were gen-erated in the preparation of glasses by comparing N e of a glass obtained from a C12A7:O 2−oxide melt under the present P O 2atmosphere and a recrystallized sample.However,N e was negligi-ble in both the glass and crystal of C12A7:O 2−.In other words,the electrons present in the electride glasses and recrystallized electrides are not newly generated but come from the electride melt.This result suggests that electrons caged in C12A7:e −crystals persist in the melt without a severe deg-radation of N e if a low P O 2of ~10−24atm is maintained during melting and quenching.Optical absorption spectra of C12A7:e −and C12A7:O 2−glasses are shown in Fig.2D.The C12A7:O 2−glass shows no distinct absorption band,whereas the C12A7:e −glass exhibits a broad absorption band at 4.6eV with a shoulder at 3.3eV .The band at 3.3eVis attributed to electrons forming F +-like centers,similar to the C12A7:O 2−glass obtained by melting C12A7:O 2−under a strongly reducing atmosphere in a carbon crucible (11).Electron spin resonance (ESR)measurement (Fig.2D,inset)of the C12A7:e −glass reveals a weak signal at g =1.998that we assigned to the F +-like centers where an electron is trapped at an oxygen ion vacancy and is coordinated by Ca 2+ions (11).ESR measurements gave an N e of ~5×1018cm −3,which is just 0.5%of the N e (~1.1×1021cm −3)of the C12A7:e −glass.From these results,we assume that most of the electrons in the C12A7:e −glasses are responsible for the optical absorption at 4.6eV ,existing in a spin-paired state.Raman spectra of the C12A7:e −glasses with different N e provide structural information to com-pare with those of C12A7:O 2−oxide glasses (Fig.3).There are three distinct differences:First,a sharp band appears at 186cm −1in the spectra of the C12A7:e −glasses,and its intensity increases as N e increases.Second,the band at 430cm −1,which is weak in C12A7:O 2−glass,becomes dis-tinct,and its intensity relative to the 780cm −1band increases with N e .Third,the intensity of the 780cm −1band,which is assigned to the stretch-ing mode of Al −O of a tetrahedral AlO 4unit (12),is greater relative to the 560cm −1band.The sharp band at 186cm −1in the spectra of the electride glasses should be associated with trapped electrons because this band is observed only for the electride glasses with N e >3×1020cm −3and its intensity is proportional to N e .To confirm the relation between the 186cm −1band and trapped electrons,we measured Raman spectra of the electride glass with a N e of 1.1×1021cm −3using several excitation lasers of different wave-lengths.As shown in the inset of Fig.3,the intensity of the 186cm −1band relative to the 780cm −1band increases as the excitation photon energy increases,agreeing well with the optical absorption spectrum.This observation strongly suggests that the 186cm −1band originates from the resonance Raman scattering associated with the absorption band at 4.6eV .Several bands as-sociated with cage vibration are present at around 200cm −1in Raman spectrum of a C12A7crystal (13).Thus,we attribute the sharp band at 186cm −1Fig.4.Model of the melt and glass of C12A7:e −electride.(A and B )The crystal structures of C12A7:O 2−and C12A7:e −electride.The free oxygen anions [blue sphere in the cage of (A)]of C12A7:O 2−can be selectively ex-tracted by several reducing processes,and electrons [green sphere in the cage of (B)]are trapped to maintain charge neutrality,yielding C12A7:e −electride.(C and D )Feasible structures of the melt and glass of C12A7:e −electride,respectively.Upon melting,empty cages collapse to form a dense network struc-ture,and the cages containing elec-trons remain but decrease in size.The wavefunctions of electrons trapped in the cages of the melt percolate to al-low metallic conduction.In the glassy state,electrons in cages have different trapping potential because of structural randomness.The majority of electrons in the glass are trapped in cages to form a diamagnetic state (bipolaron)with a nearby electron,whereas a mi-nority of electrons (~1018cm −3)are trapped at interstitial sites and coordi-nated with Ca 2+ions to form a para-magnetic state similar to an F +-like center inCaO. SCIENCE VOL 3331JULY 201173REPORTSo n A u g u s t 23, 2011w w w .s c i e n c e m a g .o r g D o w n l o a d e d f r o mto vibration associated with the cages containing trapped electrons that generate the absorption band at 4.6eV .The sharpness of this Raman band may be understood in terms of selective excita-tion of the cages containing trapped electrons at the specific excitation wavelength of the laser.Following a previous Raman study on cal-cium aluminate glasses (12),we assigned the band at 560cm −1to the transverse motion of bridging oxygen within 4Al −O −4Al (superscript denotes oxygen coordination number)linkages.The in-crease in the intensity of the 780cm −1band rela-tive to that of the 560cm −1band is explained by the higher concentration of depolymerized tetra-hedral AlO 4units in C12A7:e −glasses as com-pared with C12A7:O 2−glass.Such an anion unit is the dominant structure in a CaO-rich compo-sition.The band at 430cm −1is attributed to edge-or face-sharing AlO 4units that are present in glasses with Al 2O 3-rich compositions (12).The presence of these structural units is consistent with the higher density of the C12A7:e −glass compared with the C12A7:O 2−glass.That is,C12A7:e −glass contains heterogeneous anion structures corresponding to those in CaO-and Al 2O 3-rich compositions (the fraction of such anion structures is very low in conventional C12A7:O 2−glass)in addition to structures with the nominal composition —highly depolymerized structures and a highly polymerized network struc-ture connected by edge-or face-sharing.On the basis of the above results,we pro-pose a structural model for the melt and glass of C12A7:e −electride,as shown in Fig.4.The densities of both electride and oxide melts are greater than the 2.68g cm −3of the crystal com-posed of sub-nanometer-sized cages connected in three dimensions,indicating that the crystal-lographic cages lacking caged species collapse upon melting.Indeed,no cage structure has been observed in many structural analyses of C12A7:O 2−melts and glasses (14,15).However,because the density of the C12A7:e −melt is ~10%greater than that of the C12A7:O 2−melt we need to consider a denser network structure for the electride melt.The appearance of a dis-tinct Raman band from edge-or face-sharing AlO 4groups in the electride glasses is consistent with the observed difference in density.Edge-or face-sharing AlO 4groups form a dense network structure that not only leads to a lower T c of the electride glass than that of C12A7:O 2−glass (Fig.2A)but also facilitates crystallization when the electride melt is quenched.This difference forced us to use a roller-quenching process,even though the viscosity of the C12A7:e −melt is higher than that of the C12A7:O 2−melt (the sta-ble glass formation range for CaO –Al 2O 3sys-tems is restricted to CaO 62to 65%)(16).Next,we considered the structure that traps electrons.The absorption peak from electrons trapped in cages of crystalline C12A7is located at ~2.8eV .Given that a caged electron can be modeled as a “particle in a box ”(17),we con-sidered that the cages containing trapped elec-trons that give rise to the 4.6eV band are smaller than the cages in the crystal.Observations of the spin-paired state and hopping conduction in the glass indicate that two electrons are trapped at adjacent sites.Because of large Coulombic re-pulsion,it is unrealistic that a single shrunken cage accommodates two electrons,so it is more probable that two shrunken cages each contain-ing a trapped electron are connected to each other,forming a peanut-shaped structure:a bipolaron,which is reminiscent of solvated electrons in alkali metal-ammonia solutions (18),although this peanut-shaped bipolaronic structure is still spec-ulative.The metallic conduction of the melt sug-gests that the shrunken cages containing trapped electrons are connected and span the melt,lead-ing to the percolation of the wave functions of the electrons over the melt.Thus,we consider that the melt of C12A7:e −electride at high temper-ature adopts a similar state to an alkali metal-ammonia solution containing solvated electrons in which the electron density is delocalized over the liquid by strongly associated bipolaron struc-tures.That is,solvated electrons persist in the high-temperature melt because they are trapped in sub-nanometer-sized cages like those in a C12A7:e −crystal.The present semiconductive glasses,which were obtained by quenching of C12A7:e −melts,contain a high concentration of interstitial electrons and may be categorized as a previously unidentified class of amorphous elec-tronic materials.C12A7:e −electride is a light metal oxide –based material and is a representative constituent of slag as a vitreous byproduct of the smelting process.The present C12A7:e −melt exhibiting metallic conductivity —metallic slag —and C12A7:e −glass displaying electro-conductivity will provide new applications for oxide melts and glasses.We anticipate that our present work will stimulate further research to exploit similar liquids in otherinorganic-based electrides and elemental electrides under high pressure.References and Notes1.J.C.Thompson,Electrons in Liquid Ammonia (Oxford Univ.Press,Oxford,1976).2.J.L.Dye,Acc.Chem.Res.43,1564(2009).3.S.Matsuishi et al .,Science 301,626(2003).4.S.W.Kim et al .,Nano Lett.7,1138(2007).5.S.W.Kim et al .,J.Am.Chem.Soc.127,1370(2005).6.S.G.Yoon,S.W.Kim,D.H.Yoon,M.Hirano,H.Hosono,J.Nanosci.Nanotechnol.9,7345(2009).7.W.Freyland,in The Metal-Nonmetal Transition Revisited,P.P.Edwards,C.N.Rao,Eds.(Taylor &Francis,London,1995),pp.167−191.8.B.J.Hallstedt,J.Am.Ceram.Soc.73,15(1990).9.N.A.McNeal,A.M.Goldman,Phys.Rev.Lett.38,445(1977).10.T.Yoshizumi,S.Matsuishi,S.-W.Kim,H.Hosono,K.Hayashi,J.Phys.Chem.C 114,15354(2010).11.H.Hosono,N.Asada,Y.Abe,J.Appl.Phys.67,2840(1990).12.P.F.McMillan,B.Piriou,J.Non-Cryst.Solids 55,221(1983).13.K.Kajihara,S.Matsuishi,K.Hayashi,M.Hirano,H.Hosono,J.Phys.Chem.C 111,14855(2007).14.P.F.McMillan et al .,J.Non-Cryst.Solids 195,261(1996).15.Q.Mei et al .,J.Phys.Condens.Matter 20,245107(2008).16.H.Rawson,Inorganic Glass-Forming Systems (AcademicPress,London,1967).17.P.V.Sushko,A.L.Shluger,K.Hayashi,M.Hirano,H.Hosono,Phys.Rev.Lett.91,126401(2003).18.Z.Deng,G.J.Martyna,M.L.Klein,Phys.Rev.Lett.68,2496(1992).19.G.Y.Onoda Jr.,S.D.Brown,J.Am.Ceram.Soc.53,311(1970).Acknowledgments:We thank K.Hayashi and T.Yoshizumiof Tokyo Institute of Technology for the help of iodometric titration.S.Matsuishi and S.Ito of TokyoInstitute of Technology are thanked for useful discussions.Help by T.Tomoda for Raman spectra measurements is acknowledged.This study was supported by the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST),Japan Society for the Promotion of Science,Japan.Supporting Online Material/cgi/content/full/333/6038/71/DC1Materials and Methods Figs.S1to S5Movie S116February 2011;accepted 6May 201110.1126/science.1204394Large Sulfur Isotope Fractionation Does Not Require DisproportionationMin Sub Sim,*Tanja Bosak,Shuhei OnoThe composition of sulfur isotopes in sedimentary sulfides and sulfates traces the sulfur cycle throughout Earth ’s history.In particular,depletions of sulfur-34(34S)in sulfide relative to sulfate exceeding 47per mil (‰)often serve as a proxy for the disproportionation of intermediate sulfur species in addition to sulfate reduction.Here,we demonstrate that a pure,actively growing culture of a marine sulfate-reducing bacterium can deplete 34S by up to 66‰during sulfate reduction alone and in the absence of an extracellular oxidative sulfur cycle.Therefore,similar magnitudes of sulfur isotope fractionation in sedimentary rocks do not unambiguously record the presence of other sulfur-based metabolisms or the stepwise oxygenation of Earth ’s surface environment during the Proterozoic.Dissimilatory microbial sulfate reduction (MSR)uses sulfate (SO 42–)as an elec-tron acceptor and simple organic com-pounds or hydrogen as electron donors,producing sulfide that is depleted in heavy isotopes of sulfur (33S,34S,and 36S)relative to the starting sulfate.For more than 2.5billion years of Earth history,this biological process has controlled the partition-ing of sulfur isotopes between sedimentary sul-fides and sulfates,leaving a sedimentary S isotope record that is commonly used to track the geo-chemical cycling of sulfur,the oceanic budgets of1JULY 2011VOL 333SCIENCE74REPORTSo n A u g u s t 23, 2011w w w .s c i e n c e m a g .o r g D o w n l o a d e d f r o m。
上海牛津版小学英语一年级上册readdata_new.jsp课时练试卷习题

英语课堂的愉快教学头桥小学孙丽萍新课标的逐步实施,英语教师在教学中所充当的角色已经有了新的变化,传统的“传道、授业、解惑”,等教学观念已不再适应现阶段的客观要求了,摆在教师面前的一个严峻话题是:如何引导学生乐学善思,最大限度地发挥其自身主观能动性,变被动接受式学习为积极探索式研究性学习。
在新的教育形式下,只有及时转换角色,不断更新思维方式,以新的教育理念来武装自己,才能适应新阶段的教学,从而达到提高教学质量之目的。
情感教育是一种融知识的传授和情感的激发为一体的教育方式。
它以形象而生动的语言和行为直接作用于学生的内心世界,引起共鸣,使其在一种轻松愉悦的氛围之中,自主地接受教师所传授的知识。
处在生长发育阶段的学生,不仅需要知识的直接性接受,更需要教师无微不至的关怀和呵护。
在实际教学工作中,有些教师对学生的教育方式简单粗暴,在“严爱”的招牌下以罚代教,殊不知这种教育方式会使师生间产生疏远感,甚至造成情绪对立,导致学生放弃所学科目,从而不利于正常有效地开展教学,因为情感是一种很强的内动力,没有情感,就不可能有学习动机。
教师应当利用爱的教育力量,不但激发师生情感共鸣,因为爱是沟通师生心灵的桥梁。
譬如把自己置于学生的位置上去,作换位思考,将心比心,使自己与学生心心相通,学生在爱的力量的感化之下,学习积极性会大大提高;同时应善于发现学生身上的闪光点,在适当的场合给予表扬,可以通过充满情感的眼神、表情、语言,激发学生幸福、快乐,奋发的激情,使学生“亲其师,信其道”,这样你所教的学科他们自然愿意学,尤其对差生他们自卑感较强,信心不足,极少体验表扬滋味,一旦给他们一点温暖、鼓励,效果会更明显。
做好教学情感的最优控制,采取“赏识教育”,形成学生有效学习的策略目标。
依照教材以及学与教要达成的目标,逐步形成了系统的帮助学生有效学习的方法。
如:创设情景与激励情意相结合;理解学生和培养学生相结合;统一要求和个别对待相结合;教法研究与学法指导相结合。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
《记念刘和珍君》(说课稿)
四川省仪陇中学校刘著说教材及本课地位:
《记念刘和珍君》是高中语文必修1第三单元第一课的精读课文。
也是2010级高一师生初次面对新课改所涉及的一篇教学难度较
大的,融写人、叙事、抒情、说理于一体的鲁迅先生的散文力作。
先生以“记念刘和珍君”为切口,实则意在评述“三一八”事变。
事变
始末千头万绪,作家巧妙地化繁为简,举重若轻,以“一斑”而窥“全豹”,高屋建瓴,实属“匠心独运”。
作家创造性地写作,宜引导学
生创造性地学习。
文章线索清晰,可用“悲愤”2字概括。
刘和珍爱读书、爱同胞、
爱学校、爱祖国。
鲁迅的“爱”深沉而博大,具体体现是----关注革
命前途,关爱进步青年,盛赞勇毅精神,痛心群众徒手请愿“府门
喋血”,哀痛青年学子英年早逝,指导革命青年汲取血的教训;怨怒
国人麻木健忘,戳穿“恶意闲人”阴险论调和无耻谎言,展露刽子手
的狰狞面孔和主奴间的反动本性,矛头直刺反动当局;文末号召“真
的猛士”奋然前行,格调高昂地收束全文。
新课标强调了语文课程工具性和人文性,要求学会收集、判断、处
理信息,全面提升高中学生的语文素养;初步形成正确的世界观、
人生观、价值观;具有人文素养、创新精神与实践能力。
鲁迅的《记
念刘和珍君》,感情忧愤,爱憎鲜明,具有经典性人文性的特点。
学会“披文以入情”,也就是把握由“感”入“悟”的审美方。
是一篇指
导学生自主学习、合作学习、探究学习的良好范文。
学情分析:
高一新生对散文欣赏有一定的语文基础,但具体赏析的方法
却很模糊,老师需上本单元(或本课)前切实加以适当的复习指导:
熟读教材明确线索把握内容弄清结构
悟思想情感习方法、技能。
熟读教材易,明线索、理结构难;把握课文内容易,体悟思想、情感,习得方法、技能难。
教学目标的分析及依据:
据此学情,依照新课标“知识和能力、过程和方法、情感态度
和价值观”的三维目标要求,结合本文的特点,确立教学目标如下:
①知识目标:理清作者思想感情发展的脉络,多角度、多层面把握
鲁迅散文《记》的深刻内涵。
②能力目标:培养自主、合作、探究学习的习惯,将习惯慢慢地转
化为能力。
提高语言的感悟力和筛选及概括能力,知识的迁移能力。
③德育目标:理解刘和珍追求真理、不畏强暴、勇敢果决的精神。
④方法目标:动态阅读法;从不同角度和层面引导学生总结个性化
的阅读和创造性的解读方法;引导学生自主、合作、探究学习,初
步学会梳理、积累、运用语文知识的。
教学重点难点:教学过程中学生是主体,教师是主导,考虑到学
生原有的基础,现有的困难以及学习上的心理特征,从而针对性的
确立学习的重难点。
说重点:
理清作者思想感情发展的脉络,领悟文章内容及深刻内涵;
说难点:
夹叙夹议的方法;刘和珍精神的理解。
说教法:
散文教学应重视感悟和熏陶。
在诵读的过程中去感悟,并初步把握文章词句、内容以及情感脉络,为进一步理解课文奠定良好的基础。
据此,本课教学主要采用朗读法、质疑法、讨论法、探究法等。
说学法:
①自主学习法:指导学生朗读、质疑、思考、讨论,理解课文大意。
②探究法:定点探究法、成果汇报法。
③合作学习法(针对教学难点)
说教学手段:
利用多媒体及影像资料。
说教学过程:
一、安排学生课前"自主学习"本课。
(1)用联系的方法,梳理本课中字音、词义易错易混知识点。
譬如:喋血城堞蝴蝶通牒间谍躞蹀长歌当( )哭锐不可
当()螳臂当()车安步当()车
预定(预订)爆发(暴发)噩耗(噩梦)暗淡(黯然)桀
骜不驯(佶屈聱牙)惩创(悲怆)
(2)搜集整合本课的背景资料(可以是简短音像资料),揭露“三
一八”惨案的真相。
二、汇报自学成果(学生可自由交流,然后8分钟举荐学生1人集中交流)。
三、新授导入。
1、“三一八”惨案是在日本帝国主义支持下的段祺瑞政府杀害爱国青年的血的历史,200百多名请愿群众倒在了血泊之中,它又是中
国人残害中国人的一场罪恶,一场耻辱。
刘和珍,这位年仅22岁的大学生,就这样倒在了反动派的枪弹之下!鲁迅先生按捺不住心中的愤怒,毅然写下了这悲愤的文章——《记念刘和珍君》。
2、出示一幅足球场上的一个前仆后继的照片和“三一八”惨案中前仆后继的一个场面。
四、质疑激趣,直奔重点。
目的:通过两份阅读提纲(附表二、四)的拟写,让学生从课文结构、内容、思路、线索整体上把握教材,为后面的局部探索、合作学习、突破教学难点铺垫。
五、指导“研讨与练习”一,强化重点。
作者一方面说“我也早觉得有写一点东西的必要了”,又说“实在无话可说”,文末写到“呜呼,我说不出话”,再找找,结合语境,认真体会作者的复杂情感。
明确:在课文的第一、二、四、五、七部分均有出现。
一部分:写的必要,是因为爱护青年学生----程君劝、爱读书、订《莽原》;无话说,是因为哀痛、愤怒难以言表——虐杀、流言。
二部分:有写的必要(2处),是因为励勇士、醒庸人---忘却的救主降临。
表现痛苦感和责任感。
四部分:有什么可说?是因为万马齐喑的社会现实深感愤懑。
五部分:还有要说的,欲扬先抑,“骨鲠在喉,不吐不快”,剥画皮,露真相,赞英雄,刺魍魉,激愤之
情达到顶峰。
七部分“说不出”哀痛到极点。
作者“不说”是假,欲“说”是真。
哀痛、愤怒、失望、赞赏
之情溢于言表。
小结:全文以“记念”为明线,通篇贯穿“悲愤”之情,夹叙
夹议,形象说理,内涵深刻。
六、微观探幽,合作学习。
1、以课文四、五部分为例分析,文章中,哪些是“叙”,哪些是“议”,如何做到夹叙夹议,叙议结合的?这两段在写法上有何异同?谈谈这样写的艺术效果(分组讨论回答)(明确:听说噩耗和流言及遇难详情,是叙;阐明观点(看法),是议。
广镜头概写和特写细节相结合,谎言不攻自破,正邪对比、反衬,爱憎分明。
)
七、拓展延伸
1、对联:赴国难时代危难淬火—————。
答:悼芳魂勇毅精神闪光。
2、讲讲古今中外,“危难之时显身手”,“不苟且偷生”的人和事。
谭嗣同,中国倘要流血,就从我开始。
/我自横刀向天笑,去留肝胆两昆仑。
夏明翰,《就义诗》—“砍头不要紧,只要主义真。
”
藏羚羊,天敌追赶时飞身做桥让孩子逃命。
唐山地震中的父子,让孩子吮咬破的手指头上的血;
贵州缆车中的父母,双手举起孩子以防孩子被震。
史铁生,双脚残废后终究活下来,而且成为著名作家。
项羽,失败而不偷生,“不肯过江东”。
八、作业
根据教材提供的信息或课外资料,拟写两段(300字)颂扬烈士精神的文字。
教师例文:你是真的猛士,敢于直面惨淡的人生,敢于正视淋漓的鲜血。
你为人民的苦难感到哀痛,你勇于面对黑暗的现实,激发起变革现实的斗志,并以参加这样的斗争作为自己最大的幸福,你永远值得后世的人们赞颂!
你不是一个普通的学生,你是为了中国而死的中国的青年!
虽然你已经踏着淡红的血泊走向了浓黑的悲凉,但你的精神给一代又一代热爱真理的人们一个光明的希望,激励更多的猛士奋然前行!
你让我们看清了一个时代的嘴脸,反动派的凶残,流言家的下劣,还有平庸者的无聊,更让我们看清了自己的苟且偷生!
你让我们陷入深沉的思索,让我们诅咒一个默无声息的民族,不在沉默中爆发,就在沉默中灭亡。
你是一代女性勇毅精神的代表,干练坚决,百折不回,遇事从容,殒身不恤,不畏强暴,追求真理,你是一枝美丽而带刺的蔷薇!
你是一个民族血战前行的历史中普通的一员,你用自己的生命,像木材凝结成煤块一样,留给后人一种精神,让我们坚信:勇毅精神不灭,正义和真理永存!
刘和珍,你虽死犹生!“死去何所道,托体同山阿。
”虽然青山掩埋了你的忠骨,但你的微笑的和蔼的旧影却将一直陪伴我们走向未来!
。
