USP 88 class VI vs ISO 10993
用于WAVE Bioreactor 系统的一次性 Cellbag 生物反应袋

3, 7
P4 P3
4, 8
P1 P2
描述 空气入口滤器 3/16 in × 3/8 in × 2 in硅胶管, 无针取样
1/8 in × 1/4 in × 39 in C-Flex管, female luer 3/16 in × 3/8 in × 2 in硅胶管, 无针取样 空气出口滤器, 止回阀 空气入口滤器
端口
描述
4
表7. Cellbag生物反应袋尺寸和选项 Bioclear 10 膜(续)
Cellbag
Cellbag 2 L, Bioclear 10 film
P7 P9
P6
P5
P8 P1 P2 P3 P4
产品号
28-4122-69 (part number: CB0002L10-01)
端口 描述
1 1/8 in × 1/4 in × 39 in C-Flex管, female Luer 2 N/A 3 3/16 in × 3/8 in × 2 in 硅胶管, 无针取样 4 N/A
表1.Cellbag生物反应袋组件和材料
组件
Bioclear 10 膜
Bioclear 11 膜
倒钩端口 Luer连接 MCP接头 MCX接头 管路适配器 C-Flex™管路 硅胶管 螺帽端口 内部灌注过滤器 y接头 pHOPT传感器 DOOPT II传感器
Tempwell 通气过滤器 CLAVE®接头 ReadyMate™接头
GE医疗中国
数据文件28-9511-36 AG
ReadyToProcessTM
用于WAVE Bioreactor™系统的一次性 Cellbag™生物反应袋
设计与WAVE生物反应器系统一起使用的Cellbag生物反 应袋(图1)为预灭菌的一次性袋子,用于研究、研发 和商业化生产操作中,培养期间的培养基和细胞为非介 入式混合。作为GE 医疗生命科学ReadyToProcess平台 中即用型产品组成部分,Cellbag生物反应袋无需灭菌或 清洗步骤,为细胞生长提供适宜的环境,同时最大限度 减少交叉污染的风险。它是由多层薄的透明USP VI级塑 料制成,且很容易的连接到整套的ReadyToProcess平台 中,来实现即用型的细胞培养、纯化和流体处理。细胞 培养、纯化和流体处理。
医用级abs标准

医用级abs标准
医用级ABS标准是指一种专门用于医疗行业的ABS(丙烯腈
-丁二烯-苯乙烯共聚物)材料标准。
医用级ABS材料需要
符合特定的物理、化学和生物性能要求,以确保其在医疗环境中的安全和可靠性。
下面是一些常见的医用级ABS标准:
1. ISO 10993-1:包括对生物相容性、细胞毒性、致敏性和刺
激性等方面的测试要求。
2. ISO 13485:医疗器械质量管理体系标准,要求医疗器械制
造商按照规定的质量管理体系进行生产和控制。
3. ISO 14971:医疗器械风险管理标准,要求制造商对医疗设
备的风险进行评估和管理。
4. ASTM D624:测试ABS材料的撕裂强度和断裂延伸率。
5. ASTM D638:测试ABS材料的拉伸强度和伸长率。
6. ASTM D256:测试ABS材料的缺口冲击强度。
7. ASTM D790:测试ABS材料的弯曲强度和弯曲模量。
8. USP Class VI:美国药典对医疗材料生物相容性的要求。
这些标准旨在确保医用级ABS材料具有良好的生物相容性、
力学性能和化学稳定性,能够满足医疗器械和设备的严格要求。
使用符合医用级ABS标准的材料可以降低医疗设备的风险,
并提供更安全可靠的治疗环境。
iso10993生物相容性

iso10993生物相容性
ISO10993的目的是建立一种特定的生物相容性检测标准,以确保医疗器械、其组件及任何附件在确定的体系中没有生物学安全风险和毒性,以及在临床使用时无害于患者。
该标准包括所有相关技术条款和测试程序,以确定医疗器械,其组件及任何附件的生物相容性。
ISO 10993的主要内容可以分为7部分:定义、测试框架、生物相容性测试、综合测试计划、术语、引用文献和临床评价。
在定义方面,标准提供了关于医疗器械安全性的基本概念,并对术语进行了解释。
测试框架方面,标准中提到任何危险性和有效性评估都必须考虑到临床环境,以及它们所涉及的生物变量和组件之间的相互影响。
根据ISO 10993的要求,制造商应对医疗器械的生物相容性进行测试,以确定生物安全风险和毒性。
生物相容性测试包括但不限于体外腐蚀性测试、动物毒性测试、自体安全性测试和可接受性测试。
检测的结果由制造商提供,可以利用来协助审查和评估注册人请求的子部件的生物安全性。
最后,ISO 10993的最佳实践要求临床使用前要进行临床评价,以确保医疗器械在临床使用过程中能够确保患者安全性。
该标准也要求在临床使用过程中仍需要定期评估,以确保产品符合标准要求,并且在未来使用过程中仍可安全使用。
ISO 10993的发布旨在确保医疗器械的生物安全性,保护患者的安全。
有效实施ISO 10993有助于确保医疗器械的持续性、安全性和有效性,为医护人员和患者提供了更好的安全保障。
PEEK PPSU材料比较
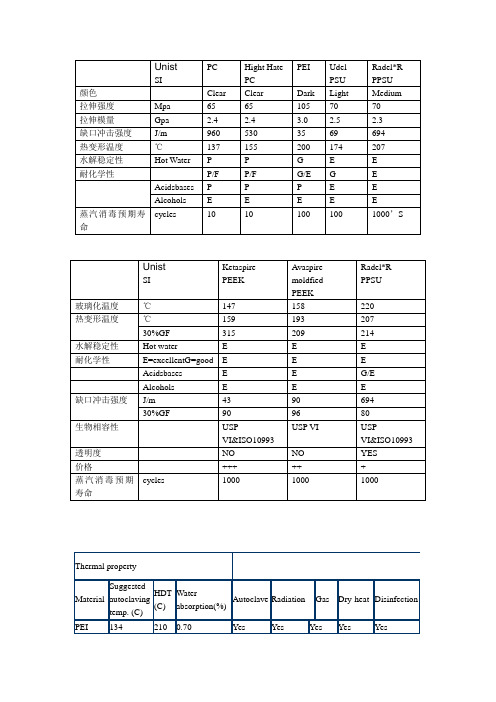
ULTEM1000在高温高压蒸气循环300次以上仍保持优良的拉伸强度
环氧乙烷消毒
•多用于一次性使用器具的消毒
•ULTEM 树脂保留>95%的机械性能
伽玛射线消毒
•离子射线可能导致塑料褪色或发黄.
•ULTEM 树脂保持它的物理性能, 并且在暴露于10Mrads下颜色几乎没有改变
高温蒸煮灭菌
•利用饱和水蒸汽在高压下的湿热, 来破坏细胞的主要结构, 达到灭菌的效果. 通常用于反复多次使用的医疗器械.
•根据医疗器械的不同要求, 采用不同的灭菌温度和时间
➢比如, 采用高温(134ºC)来提高较低温度下(121ºC)的灭菌效率(即: 20分钟对30分钟)
γ射线和电子辐射
采用γ射线或电子辐射进行灭菌处理, 通常用于一次使用的医疗器械. 取代EtO(环氧乙烷), 具有更清洁, 更安全, 无残留, 及灭菌时间短等优势.
生物相容性: 通过一系列的试验来判断塑料材料是否可用, 或析出物是否会导致对人体的潜在危害.
测试标准/ 方法:
-ISO 10993: 由ISO和FDA建立标准.
1995年以后成为国际通行的标准.
-USP Class VI: 由美国药典建立标准.
1995年之前主要采用的国际标准
聚砜类化合物:韧性/ 透明性-400C到2000C长期热稳定性水解和尺寸稳定性耐屈服性
重复使用的设备需要经过1,000 次以上消毒循环:
浸泡、清洗和消毒
•酶清洁剂、碱性消毒剂、酸性中和剂以及
蒸汽灭菌方法(高压灭菌)
•蒸汽(典型条件:134°C/ 4到20分钟)
•锅炉添加剂(吗啉、联胺和胺类)或者
对热敏性设备采用的低温方法
环氧乙烷 过氧化氢等离子
HLD。
USP88classVIvsISO10993
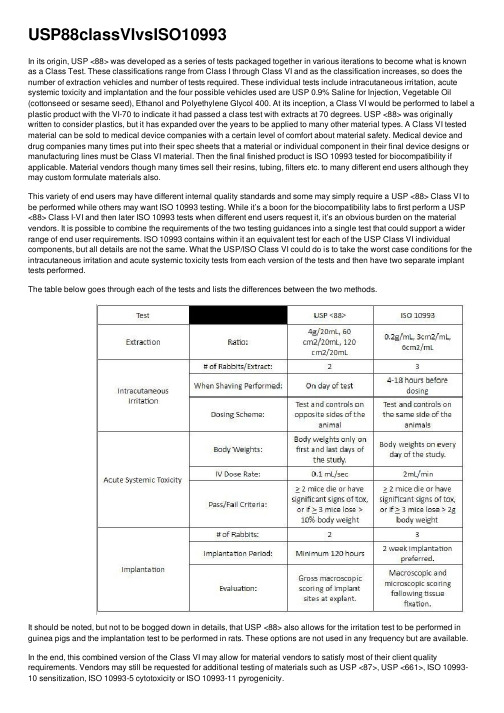
USP88classVIvsISO10993In its origin, USP <88> was developed as a series of tests packaged together in various iterations to become what is known as a Class Test. These classifications range from Class I through Class VI and as the classification increases, so does the number of extraction vehicles and number of tests required. These individual tests include intracutaneous irritation, acute systemic toxicity and implantation and the four possible vehicles used are USP 0.9% Saline for Injection, Vegetable Oil (cottonseed or sesame seed), Ethanol and Polyethylene Glycol 400. At its inception, a Class VI would be performed to label a plastic product with the VI-70 to indicate it had passed a class test with extracts at 70 degrees. USP <88> was originally written to consider plastics, but it has expanded over the years to be applied to many other material types. A Class VI tested material can be sold to medical device companies with a certain level of comfort about material safety. Medical device and drug companies many times put into their spec sheets that a material or individual component in their final device designs or manufacturing lines must be Class VI material. Then the final finished product is ISO 10993 tested for biocompatibility if applicable. Material vendors though many times sell their resins, tubing, filters etc. to many different end users although they may custom formulate materials also.This variety of end users may have different internal quality standards and some may simply require a USP <88> Class VI to be performed while others may want ISO 10993 testing. While it’s a boon for the biocompatibility labs to first perform a USP <88> Class I-VI and then later ISO 10993 tests when different end users request it, it’s an obvious burden on the material vendors. It is possible to combine the requirements of the two testing guidances into a single test that could support a wider range of end user requirements. ISO 10993 contains within it an equivalent test for each of the USP Class VI individual components, but all details are not the same. What the USP/ISO Class VI could do is to take the worst case conditions for the intracutaneous irritation and acute systemic toxicity tests from each version of the tests and then have two separate implant tests performed.The table below goes through each of the tests and lists the differences between the two methods.It should be noted, but not to be bogged down in details, that USP <88> also allows for the irritation test to be performed in guinea pigs and the implantation test to be performed in rats. These options are not used in any frequency but are available. In the end, this combined version of the Class VI may allow for material vendors to satisfy most of their client quality requirements. Vendors may still be requested for additional testing of materials such as USP <87>, USP <661>, ISO 10993-10 sensitization, ISO 10993-5 cytotoxicity or ISO 10993-11 pyrogenicity.Now, I know there may be some manufacturers out there wondering if they can submit USP <88> testing in lieu of ISO 10993 testing and the answer is an emphatic no! Also, just because all of the materials used to make a device are USP <88> Class VI certified it does not mean manufacturers are exempt from 10993-1 evaluation. When different material components are combined to form finished products that are then sterilized, there are a lot of chemical interactions going on which can form byproducts. Analytical chemists and sterilization specialists can talk your ear off on this and I promise you’ll learn a few new things after talking with them. Class VI tests are an important tool in your toolbox, but you’ll need all your tools to build your framework, biological evaluation andfinal toxicological risk assessments.Moving forward, there are current discussions about USP <87> and <88> that they become more of a risk based evaluation of raw materials or manufacturing components closer to a ISO 10993-1 approach. A meeting was held at USP regarding this topic and while changes aren’t imminent, they are on the horizon.。
ISO10993测试报告

输液器用热塑性弹性体测试报告汇总实验名称测试时间测试单位实验依据概述结论遗传毒性染色体畸变实验2010.9.27国家食品药品监督管理局济南医疗器械质量监督检测中心国际标准化组织ISO10993-3《遗传毒性,致癌性以及生殖毒性实验》使用中国仓鼠肺细胞(CHL)对被测样品遗传毒性进行评价。
数据结果分析显示,样品浸提液没有抑制CHL细胞的生长。
样品未引起染色体畸变数目的显著增加。
达到了有效测试标准。
被测样品浸提液没有诱导哺乳动物体细胞产生染色体畸变,认为被测样品无遗传毒性。
热原实验2010.10.20国家食品药品监督管理局济南医疗器械质量监督检测中心国际标准化组织ISO 10993-11医疗设备器械的生物学评价第11部分:全身毒性实验使用实验用兔对样品浸提液的致热性进行评价。
3只实验用兔在3小时观察时间内最高升温值分别为0.0℃、0.3℃、0.2℃,符合《国家药典》标准。
在3小时观察时间内体温变化在《国家药典》的范围内,检测物品没有致热性。
皮内反应实验2010.10.20国家食品药品监督管理局济南医疗器械质量监督检测中心国际标准化组ISO10993-10:2002/ Amd1:2006《医疗器械的生物学评价第10部分,刺激与迟发型超敏反应实验》使用雄性和雌性家兔进行皮内刺激实验,评价样品材料产生刺激反应的潜在可能性。
所有动物在实验过程中临床观察正常。
0.9%NaCl (SC),棉籽油(CSO)浸提液家兔皮内刺激反应综合平均记分之差为0。
该产品0.9%NaCl(SC),棉籽油(CSO)浸提液家兔皮内刺激反应综合平均记分之差不大于1.0,符合实验要求。
致敏实验-最大剂量法2010.11.1国家食品药品监督管理局济南医疗器械质量监督检测中心国际标准化组ISO10993-10:2002/ Amd 1:2006《医疗器械的生物学评价第10部分,刺激与迟发型超敏反应实验》0.9%氯化钠(SC),棉籽油(CSO)样品浸提液皮内注射并封存固定于豚鼠上用以诱导致敏反应。
硅橡胶材料标准规范

硅橡胶材料标准规范硅橡胶是一种高分子材料,具有耐高温、耐磨损、耐老化、耐紫外线、耐氧化、电绝缘等优良特性。
因此,在航空、航天、电子、汽车、医疗等领域都有广泛的应用。
为了保证硅橡胶产品的质量、可靠性和相互兼容性,需要制定硅橡胶材料标准规范。
本文将从材料性能、试验方法、应用标准等方面探讨硅橡胶材料标准规范。
一、材料性能硅橡胶的材料性能是制定标准规范的重要依据。
为了满足不同领域应用的要求,可将硅橡胶材料性能分为物理性能、机械性能、热学性能、化学性能、电学性能等多个方面。
1.物理性能硅橡胶的物理性能包括密度、硬度、弹性模量、伸长率、拉伸强度、抗撕裂性等。
这些性能对于硅橡胶的加工、成型、使用都有重要的影响,其中硬度和弹性模量是最为重要的指标之一。
2.机械性能硅橡胶的机械性能分为静态和动态两种。
静态机械性能包括压缩强度、剪切强度、抗张强度、抗扭强度、压缩模量等。
动态机械性能包括动态拉伸强度、动态剪切强度、动态蠕变强度等。
3.热学性能硅橡胶的热学性能包括热膨胀系数、热导率、热传导系数、热容量等。
这些性能在高温环境下尤为重要。
4.化学性能硅橡胶的化学性能包括耐酸碱、耐油、耐溶剂、耐氧化、耐紫外线等指标。
这些性能对于硅橡胶的应用环境选择和使用寿命有决定性的影响。
5.电学性能硅橡胶的电学性能包括介电常数、介电强度、体电阻率、表面电阻率等。
这些性能在电气领域的应用尤为重要。
二、试验方法制定硅橡胶材料标准规范需要考虑到试验方法的标准化,以保证试验结果的可靠性和准确性。
1.物理试验硅橡胶的物理试验包括密度测定、硬度测定、弹性模量测定、伸长率测定、拉伸强度测定、抗撕裂性测定等。
2.机械试验硅橡胶的机械试验包括压缩强度测定、剪切强度测定、抗张强度测定、抗扭强度测定、压缩模量测定等。
3.热学试验硅橡胶的热学试验包括热膨胀系数测定、热导率测定、热传导系数测定、热容量测定等。
4.化学试验硅橡胶的化学试验包括酸碱耐性测定、油耐性测定、溶剂耐性测定、氧化稳定性测定、紫外线稳定性测定等。
硅橡胶

硅橡胶|硅橡胶性能|硅橡胶物性简介硅橡胶作为常用橡胶的一种,其属于合成橡胶中特殊橡胶类。
其具有许多优良性能,如无毒,与人体相容性好,耐高低温,耐臭氧,耐紫外线等。
随着人们环保意识的增强与硅橡胶价格的走低,硅橡胶也应用越来越广泛。
本文将对硅橡胶物理性能作简要介绍:1、机械性能:体现机械性能的参数有硬度,拉伸强度,抗撕强度,扯断伸长率,回弹性,压缩户久变形率等。
硅橡胶的硬度现在可以做到SHOREA 0-90度,常用硬度为30-70度,30度以下为低硬度硅胶,70度以上为高硬度硅胶。
硅橡胶的拉伸强度一般在10MPa以下,做到11较难,12很难做到。
一般来说气相硅橡胶要比沉淀硅橡胶拉伸强度要高。
抗撕强度:常用测度抗撕强度的方法有直角法和半圆法,两者测试值不同,用直角法测试高抗撕硅胶一般能达到将近50Kgf/cm,用半圆法测试可达35Kgf/cm以上。
高抗撕硅胶都是气相法硅橡胶。
硅橡胶的扯断伸长率一般随着硬度提高而减小,因而低硬度硅橡胶的扯断伸长率要更高,有的可达1000%以上,气相法硅橡胶的扯断伸长率经同硬度的沉定法硅橡胶要高。
硅橡胶的压缩永久变形率最低的可作到5%以下,这一参数与回弹性有关,但又不尽相同,其是作硅橡胶胶辊,密封件等产品关注的一个重要参数。
回弹性硅橡胶重要机械性能之一,高回弹硅橡胶的回弹性可做到70以上,有的甚至可达802、硅橡胶的热性能:硅橡胶是在现在橡胶品种中最耐高低温的品种,低温可耐零下90摄氏度,高温可达300摄氏度以上。
普通硅橡胶的工作温度范围一般零下40度到零上180度之间。
事实上很多时候选用硅橡胶都是因为其热性能的优势才采用的,如在寒冷地区的冬天,一些橡胶件就必须使用硅橡胶才行。
3、硅橡胶电性能:一般硅橡胶是绝缘的,特制硅橡胶才是导电的,判断硅橡胶是否导电的参数是体积电阻率,其单位是Ω*m,体积电阻率在109数值以上为绝缘硅橡胶,数值在106-109之间的,为抗静电硅橡胶,106以下的为导电硅橡胶。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
In its origin, USP <88> was developed as a series of tests packaged together in various iterations to become what is known as a Class Test. These classifications range from Class I through Class VI and as the classification increases, so does the number of extraction vehicles and number of tests required. These individual tests include intracutaneous irritation, acute systemic toxicity and implantation and the four possible vehicles used are USP 0.9% Saline for Injection, Vegetable Oil (cottonseed or sesame seed), Ethanol and Polyethylene Glycol 400. At its inception, a Class VI would be performed to label a plastic product with the VI-70 to indicate it had passed a class test with extracts at 70 degrees. USP <88> was originally written to consider plastics, but it has expanded over the years to be applied to many other material types. A Class VI tested material can be sold to medical device companies with a certain level of comfort about material safety. Medical device and drug companies many times put into their spec sheets that a material or individual component in their final device designs or manufacturing lines must be Class VI material. Then the final finished product is ISO 10993 tested for biocompatibility if applicable. Material vendors though many times sell their resins, tubing, filters etc. to many different end users although they may custom formulate materials also.
This variety of end users may have different internal quality standards and some may simply require a USP <88> Class VI to be performed while others may want ISO 10993 testing. While it’s a boon for the biocompatibility labs to first perform a USP <88> Class I-VI and then later ISO 10993 tests when different end users request it, it’s an obvious burden on the material vendors. It is possible to combine the requirements of the two testing guidances into a single test that could support a wider range of end user requirements. ISO 10993 contains within it an equivalent test for each of the USP Class VI individual components, but all details are not the same. What the USP/ISO Class VI could do is to take the worst case conditions for the intracutaneous irritation and acute systemic toxicity tests from each version of the tests and then have two separate implant tests performed.
The table below goes through each of the tests and lists the differences between the two methods.
It should be noted, but not to be bogged down in details, that USP <88> also allows for the irritation test to be performed in guinea pigs and the implantation test to be performed in rats. These options are not used in any frequency but are available.
In the end, this combined version of the Class VI may allow for material vendors to satisfy most of their client quality requirements. Vendors may still be requested for additional testing of materials such as USP <87>, USP <661>, ISO 10993-10 sensitization, ISO 10993-5 cytotoxicity or ISO 10993-11 pyrogenicity.
Now, I know there may be some manufacturers out there wondering if they can submit USP <88> testing in lieu of ISO 10993 testing and the answer is an emphatic no! Also, just because all of the materials used to make a device are USP <88> Class VI certified it does not mean manufacturers are exempt from 10993-1 evaluation. When different material components are combined to form finished products that are then sterilized, there are a lot of chemical interactions going on which can form byproducts. Analytical chemists and sterilization specialists can talk your ear off on this and I promise you’ll learn a few new things after talking with them. Class VI tests are an important tool in your toolbox, but you’ll need all your tools to build your framework, biological evaluation and
final toxicological risk assessments.
Moving forward, there are current discussions about USP <87> and <88> that they become more of a risk based evaluation of raw materials or manufacturing components closer to a ISO 10993-1 approach. A meeting was held at USP regarding this topic and while changes aren’t imminent, they are on the horizon.。
