Extraction and stripping kinetics of copper (Ⅱ) by N902 using single drop technique
化学工程与工艺专业英语课后习题参考答案
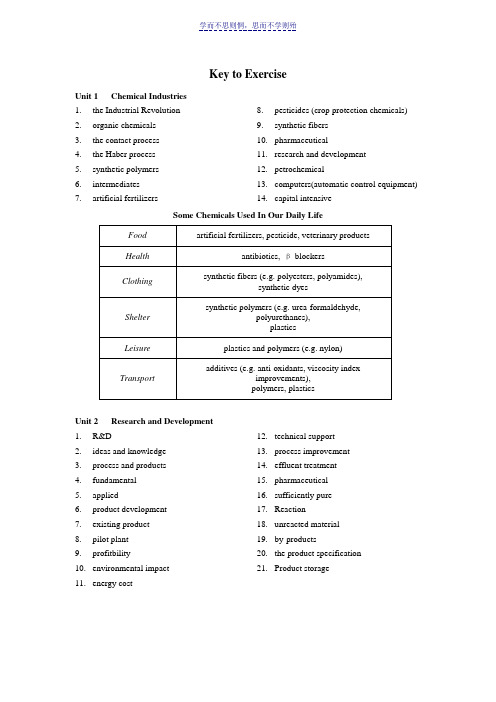
学而不思则惘,思而不学则殆Key to Exercise Unit 1 Chemical Industries1.the Industrial Revolutionanic chemicals3.the contact process4.the Haber process5.synthetic polymers6.intermediates7.artificial fertilizers 8.pesticides (crop protection chemicals)9.synthetic fibers10.pharmaceutical11.research and development12.petrochemicalputers(automatic control equipment)14.capital intensiveSome Chemicals Used In Our Daily LifeUnit 2 Research and Development1.R&D2.ideas and knowledge3.process and products4.fundamental5.applied6.product development7.existing product8.pilot plant9.profitbility10.environmental impact11.energy cost 12.technical support13.process improvement14.effluent treatment15.pharmaceutical16.sufficiently pure17.Reaction18.unreacted material19.by-products20.the product specification21.Product storageUnit 3 Typical Activities of Chemical Engineers1.Mechanical2.electrical3.civil4.scale-upmercial-size6.reactors7.distillation columns8.pumps9.control and instrumentation10.mathematics11.industry12.academia13.steam 14.cooling water15.an economical16.to improve17.P&I Drawings18.Equipment Specification Sheets19.Construction20.capacity and performance21.bottlenecks22.Technical Sales23.new or improved24.engineering methods25.configurationsUnit 4 Sources of Chemicals1.inorganic chemicals2.derive from (originate from)3.petrochemical processes4.Metallic ores5.extraction process6.non-renewable resource7.renewable sources8.energy source9.fermentation process10.selective 11.raw material12.separation and purification13.food industry14.to be wetted15.Key to success16.Crushing and grinding17.Sieving18.Stirring and bubbling19.Surface active agents20.OverflowingUnit 5 Basic Chemicals 1. Ethylene 2. acetic acid 3.4. Polyvinyl acetate5. Emulsion paintUnit 6 Chlor-Alkali and Related Processes 1. Ammonia 2. ammonia absorber 3. NaCl & NH 4OH 4.5. NH 4Cl6. Rotary drier7. Light Na 2CO 3Unit 7 Ammonia, Nitric Acid and Urea 1. kinetically inert 2. some iron compounds 3. exothermic 4. conversion 5. a reasonable speed 6. lower pressures 7. higher temperatures 8.9. energy 10. steam reforming 11. carbon monoxide 12. secondary reformer 13. the shift reaction 14. methane 15. 3:1Unit 8 Petroleum Processing 1. organic chemicals 2. H:C ratios3. high temperature carbonization4. crude tar5. pyrolysis6. poor selectivity7. consumption of hydrogen8. the pilot stage9. surface and underground 10.fluidized bed 11. Biotechnology 12. sulfur speciesUnit 9 PolymersUnit 10 What Is Chemical EngineeringMicroscale (≤10-3m)●Atomic and molecular studies of catalysts●Chemical processing in the manufacture of integrated circuits●Studies of the dynamics of suspensions and microstructured fluidsMesoscale (10-3-102m)●Improving the rate and capacity of separations equipment●Design of injection molding equipment to produce car bumpers madefrom polymers●Designing feedback control systems for bioreactorsMacroscale (>10m)●Operability analysis and control system synthesis for an entire chemicalplant●Mathematical modeling of transport and chemical reactions ofcombustion-generated air pollutants●Manipulating a petroleum reservoir during enhanced oil recoverythrough remote sensing of process data, development and use of dynamicmodels of underground interactions, and selective injection of chemicalsto improve efficiency of recoveryUnit 12 What Do We Mean by Transport Phenomena?1.density2.viscosity3.tube diameter4.Reynolds5.eddiesminar flow7.turbulent flow 8.velocity fluctuations9.solid surface10.ideal fluids11.viscosity12.Prandtl13.fluid dynamicsUnit 13 Unit Operations in Chemical Engineering 1. physical 2. unit operations 3. identical 4. A. D. Little 5. fluid flow6. membrane separation7. crystallization8. filtration9. material balance 10. equilibrium stage model 11. Hydrocyclones 12. Filtration 13. Gravity 14. VaccumUnit 14 Distillation Operations 1. relative volatilities 2. contacting trays 3. reboiler4. an overhead condenser5. reflux6. plates7. packing8.9. rectifying section 10. energy-input requirement 11. overall thermodynamic efficiency 12. tray efficiencies 13. Batch operation 14. composition 15. a rectifying batch 1 < 2 < 3Unit 15 Solvent Extraction, Leaching and Adsorption 1. a liquid solvent 2. solubilities 3. leaching 4. distillation 5. extract 6. raffinate 7. countercurrent 8. a fluid 9. adsorbed phase 10. 400,000 11. original condition 12. total pressure 13. equivalent numbers 14. H + or OH –15. regenerant 16. process flow rates17. deterioration of performance 18. closely similar 19. stationary phase 20. mobile phase21. distribution coefficients 22. selective membranes 23. synthetic24. ambient temperature 25. ultrafiltration26. reverse osmosis (RO).Unit 16 Evaporation, Crystallization and Drying 1. concentrate solutions 2. solids 3. circulation 4. viscosity 5. heat sensitivity 6. heat transfer surfaces 7. the long tube8. multiple-effect evaporators 9.10. condensers 11. supersaturation 12. circulation pump 13. heat exchanger 14. swirl breaker 15. circulating pipe 16. Product17. non-condensable gasUnit 17 Chemical Reaction Engineering1.design2.optimization3.control4.unit operations (UO)5.many disciplines6.kinetics7.thermodynamics,8.fluid mechanics9.microscopic10.chemical reactions 11.more valuable products12.harmless products13.serves the needs14.the chemical reactors15.flowchart16.necessarily17.tail18.each reaction19.temperature and concentrations20.linearUnit 18 Chemical Engineering Modeling1.optimization2.mathematical equations3.time4.experiments5.greater understanding6.empirical approach7.experimental design8.differing process condition9.control systems 10.feeding strategies11.training and education12.definition of problem13.mathematical model14.numerical methods15.tabulated or graphical16.experimental datarmation1.the preliminary economics2.technological changes3.pilot-plant data4.process alternatives5.trade-offs6.Off-design7.Feedstocks 8.optimize9.plant operations10.energy11.bottlenecking12.yield and throughput13.Revamping14.new catalystUnit 19 Introduction to Process Design1. a flowsheet2.control scheme3.process manuals4.profit5.sustainable industrial activities6.waste7.health8.safety9. a reactor10.tradeoffs11.optimizations12.hierarchyUnit 20 Materials Science and Chemical Engineering1.the producing species2.nutrient medium3.fermentation step4.biomass5.biomass separation6.drying agent7.product8.water9.biological purificationUnit 21 Chemical Industry and Environment1.Atmospheric chemistry2.stratospheric ozone depletion3.acid rain4.environmentally friendly products5.biodegradable6.harmful by-product7.efficiently8.power plant emissions 9.different plastics10.recycled or disposed11.acidic waste solutionsanic components13.membrane technology14.biotechnology15.microorganisms。
Tessier 提取法英文版

Talanta46(1998)449–455Extraction procedures for the determination of heavy metals incontaminated soil and sedimentGemma RauretDept.Quı´mica Analı´tica,Uni6ersitat de Barcelona,Barcelona,SpainReceived25May1997;accepted14October1997AbstractExtraction tests are commonly used to study the mobility of metals in soils and sediments by mimicking different environmental conditions or dramatic changes on them.The results obtained by determining the extractable elements are dependent on the extraction procedure applied.The paper summarises state of the art extraction procedures used for heavy metal determination in contaminated soil and sediments.Two types of extraction are considered:single and sequential.Special attention is paid to the Standard,Measurement and Testing projects from the European Commission which focused on the harmonisation of the extraction procedures and on preparing soil and sediment certified reference materials for extractable heavy metal contents.©1998Elsevier Science B.V.All rights reserved. Keywords:Extraction procedures;Heavy metals;Contaminated soil;Sediment;Certified reference materials1.IntroductionTrace metals in soils and sediments may exist in different chemical forms or ways of binding.In unpolluted soils or sediments trace metals are mainly bound to silicates and primary minerals forming relatively immobile species,whereas in polluted ones trace metals are generally more mobile and bound to other soil or sediments phases.In environmental studies the determina-tion of the different ways of binding gives more information on trace metal mobility,as well as on their availability or toxicity,in comparison with the total element content.However,the determi-nation of the different ways of binding is difficult and often impossible.Different approaches are used for soil and sediment analysis,many of them focused on pollutant desorption from the solid phase;others are focused on the pollutant adsorp-tion from a solution by the solid phase.Among those approaches based on desorption,leaching procedures are the most widely accepted and used.Extraction procedures by means of a single extractant are widely used in soil science.These procedures are designed to dissolve a phase whose element content is correlated with the availability of the element to the plants.This approach is well established for major elements and nutrients and it is commonly applied in studies of fertility and quality of crops,for predicting the uptake of essential elements,for diagnosis of deficiency or excess of one element in a soil,in studies of the physical-chemical behaviour of elements in soils0039-9140/98/$19.00©1998Elsevier Science B.V.All rights reserved. PII S0039-9140(97)00406-2G.Rauret/Talanta46(1998)449–455 450and for survey purposes.To a lesser extent they are applied to elements considered as pollutants such as heavy metals.The application of extrac-tion procedures to polluted or naturally contami-nated soils is mainly focused to ascertain the potential availability and mobility of pollutants which is related to soil-plant transfer of pollutants and to study its migration in a soil profile which is usually connected with groundwater problems[1]. For sediment analysis,extraction is used to asses long term emission potential of pollutants and to study the distribution of pollutants among the geochemical phases.As far as heavy metals are concerned sediments are usually a sink but may also become a source under certain condi-tions,especially in heavily contaminated areas or in drastically changing environments.Chemical extraction of sediments has proven to be adequate for determining the metal associated with source constituents in sedimentary deposits[2],but the general aim of many studies involving chemical extraction is the determination of element distri-bution among different phases of a sediment. Single extractants are usually chosen to evaluate a particular release controlling mechanism such as desorption by increasing salinity or complexing by competing organic agents.Generally,fractions can be isolated more specifically by using sequen-tial extraction schemes.For sediments these pro-cedures are frequently used and are designed in relation to the problems arising from disposal of dredged materials.Extraction tests,either in soils and sediments, are always restricted to a reduced group of ele-ments and as far as soil is concerned they are applied to a particular type of soil;silicious,car-bonated or organic.In a regulatory context,two applications for leaching tests can be recognised: the assessment or prediction of the environmental effects of a pollutant concentration in the environ-ment and the promulgation of guidelines or objec-tives for soil quality as for example for land application of sewage sludge or dredge sediments. The data obtained when applying these tests are used for decision makers in topics such as land use of soil or in countermeasures monly used extraction procedures in soils During the last decades several extraction pro-cedures for extractable heavy metals in soils have been developed and modified.In this respect,two groups of tests must be considered:the single reagent extraction test,one extraction solution and one soil sample,and in the sequential extrac-tion procedures,several extraction solutions are used sequentially to the same sample although this last type of extraction is still in development for soils.Both types of extraction are applied using not only different extracting schemes but also different laboratory conditions.This leads to the use of a great deal of extraction procedures. In Table1a summary of the most common leaching test are given.Table1Most common single extraction testsType and solution strength Reference Group[3]HNO30.43–2mol l−1Acid extractionAqua regia[4]HCl0.1–1mol l−1[3]CH3COOH0.1mol l−1[5]Melich1:[6]HCl0.05mol l−1+H2SO40.0125mol l−1EDTA0.01–0.05mols l−1[3] Chelatingagents at different pH[7]DTPA0.005mol l−1+TEA0.1mol l−1CaCl20.01mol l−1Melich3:[8]CH3COOH0.02mol l−1NH4F0.015mol l−1HNO30.013mol l−1EDTA0.001mol l−1NH4–acetate,acetic acidBuffered salt[9]buffer pH=7;1mol l−1solution[3]NH4–acetate,acetic acidbuffer pH=4.8;1mol l−1Unbuffered salt CaCl20.1mol l−1[3]solutionCaCl20.05mol l−1[3][3]CaCl20.01mol l−1NaNO30.1mol l−1[10]NH4NO31mol l−1[3]AlCl30.3mol l−1[11]BaCl20.1mol l−1[12]G.Rauret/Talanta46(1998)449–455451 Table2Extraction methods proposed for standardisation or standardised in some European countriesMethod MethodCountry Reference[15]Mobile trace element determination1mol l−1NH4NO3Germany[16]Available Cu,Zn and Mn evaluation for fer-France0.01mol l−1Na2–EDTA+1mol l−1tilisation purposesCH3COONH4at pH=7DTPA0.005mol l−1+TEA0.1mol l−1+CaCl20.01mol l−1at pH=7.3Available Cu,Zn,Fe and Mn evaluation inItaly0.02mol l−1EDTA+0.5mol l−1[17]acidic soilsCH3COONH4at pH=4.6DTPA0.005mol l−1+TEA0.1mol l−1+CaCl20.01mol l−1at pH=7.3[18]Availability and mobility of heavy metals inCaCl20.1mol l−1Netherlandspolluted soils evaluationSoluble heavy metal(Cu,Zn,Cd,Pb and[19] Switzerland NaNO30.1mol l−1Ni)determination and ecotoxicity risk evalu-ationUnited Kingdom EDTA0.05mol l−1at pH=4[20]Cu availability evaluationFrom Table1it can be observed that a single extraction including a large spectra of extractants are used.It ranges from very strong acids,such as aqua regia,nitric acid or hydrochloric acid,to neutral unbuffered salt solutions,mainly CaCl2or NaNO3.Other extractants such as buffered salt solutions or complexing agents are frequently ap-plied,because of their ability to form very stable water soluble complexes with a wide range of cations.Hot water is also used for the extraction of boron.Basic extraction by using sodium hy-droxide is used to assess the influence of the dissolved organic carbon in the release of heavy metals from soils.A large number of extractants are reviewed by Pickering[13]and Lebourg[14]. The increasing performance of the analytical techniques used for element determination in an extract,together with the increasing evidence that exchangeable metals better correlate with plant uptake,has lead extraction methods to evolve towards the use of less and less aggressive solu-tions[10].These solutions are sometimes called soft extractants and are based on non buffered salt solutions although diluted acids and complex-ant agents are also included in the group.Neutral salts dissolve mainly the cation exchangeable frac-tion although in some cases the complexing ability of the anion can play a certain role.Diluted acids dissolve partially trace elements associated to dif-ferent fractions such as exchangeable,carbonates, iron and manganese oxides and organic matter. Complexing agents dissolve not only exchange-able element fraction but also the element fraction forming organic matter complexes and the ele-ment fractionfixed on the soil hydroxides.Nowa-days it is generally accepted that extractants are not selective and that minor variations in analyti-cal procedures have significant effects on the re-sults.Some leaching procedures for soils have been adopted officially or its adoption is under study in different countries with different objectives[14]. An account of these methods are given on Table 2.monly used extraction procedures in sedimentsAs for soils,exchangeable metal in sediments are selectively displaced by soft extractants.Other extractants used are less selective and they co-ex-tract the exchangeable fraction together with metals bound to different sediment phases moreG.Rauret/Talanta46(1998)449–455 452or less extensively.The phases considered relevant in heavy metals adsorption in sediments are ox-ides,sulphides and organic matter.Fractionation is usually performed by using sequential extrac-tion schemes.The fractions obtained,when apply-ing these schemes,are related to exchangeable metals,metals mainly bound to carbonates, metals released in reducible conditions such as those bound to hydrous oxides of Fe and Mn, metals bonded to oxidable components such as organic matter and sulphides and residual frac-tion.The extractants more commonly used in sequential extraction schemes are generally ap-plied according to the following order:unbuffered salts,weak acids,reducing agents,oxidising agents and strong acids.In Table3the extractants most commonly used to isolate each fraction are given[21].The water soluble fraction may be obtained by two ways,by sampling sediment pore solution using in situ filtration,dialysis tubes or bags,or by a leaching procedure in the laboratory.When this procedure is used the pH may be indeterminate because of the low buffering capacity of the extractant and problems with readsorption occurs.Exchangeable fraction uses an electrolyte such as salts of strong acids and bases or salts of weak acids and bases at pH7to prevent oxyhydroxy phases precipitation. The carbonate bound fraction generally uses an acid such as acetic or a buffer solution acetic acid-sodium acetate at pH5.These reagents are not able to attack all the carbonate content,as for example dolomitic carbonates,neither to attack carbonate selectively as they also remove partially organically bound trace metals.The fraction ob-tained when a reducing solution is used as extrac-tant is mainly related to metals bound to iron and manganese oxides.Hydroxylamine in acid solu-tion is the reducing agent most widely used to solubilise these oxides although iron oxide is not completely dissolved.Ammonium oxalate seems to be most effective when used in the dark,al-though some problems in heavy metals oxalate phase precipitation may occur even at low pH. The sodium dithionite/citrate/carbonate reagent dissolves the oxide and hydroxyoxides but can attack iron rich silicates.So reducing extractants are neither selective nor completely effective for iron and manganese oxides.Other group of ex-tractants used sequentially includes oxidising reagents which destroy organic matter and also oxidises sulphides to sulphates.The extractants most widely used in this group are H2O2and NaOCl.Hydrogen peroxide seems to be more efficient if used after the oxide extraction step. The most widely used extraction scheme is the one proposed by Tessier[22]which has been modified by several authors[23–25].Many of these modifications make more specific the isola-tion of the iron and manganese oxide and hydrox-ide phases.The Tessier procedure is schematised in Table4together with the modified procedures of Fo¨rstner[26]and of Meguelatti[24].4.Harmonisation and method validationOwing to the need of establishing common schemes in Europe for extractable trace metals in soils and sediments the EC Standards,Measure-ment and Testing Programme,formerly BCR(Bu-Table3Most common extractants used in sequential extraction schemesType and solution strenght GroupH2OWater soluble fractionExchangeable and weakly NaNO30.1mol l−1 adsorbed fractionKNO30.1mol l−1MgCl21mol l−1CaCl20.05mol l−1Ca(NO3)20.1mol l−1NH4OAc1mol l−1pH=7 Carbonate bound fraction HOAc0.5mol l−1HOAc/NaOAc1mol l−1pH=5Fractions bound to hydrous NH2OH.HCl0.04mol l−1 oxides of Fe and Mn in acetic or nitric acidNH4OxSodium ditionite,sodiumcitrate,sodium bicarbonate(DCB)Organically bound fraction H2O2NaOClG.Rauret/Talanta46(1998)449–455453 Table4Sequential extraction schemes2345Method1HF/HClO4NaOAc1mol l−1H2O28.8mol l−1Tessier et al.NH2OH.HCl0.04molMgCl2mol l−1l−1HNO3/NH4OAc residual pH7pH525%HOAcsilicate phaseorganic matter+sul-exchangeable carbonate Fe/Mn oxidesphideHNO3NH4Ox/HOx0.1mol H2O28.8mol l−1NH2OH.HCl0.1molFo¨rstner NaOAc1moll−1l−1l−1residualpH7NH4OAcpH5easily reducible pH3in darksilicate phasemoderately reducible organic matter+sul-exchan+carbphideNaOAc1mol l−1NH2OH.HCl0.1molMeguellati BaCl21mol l−1H2O28.8mol l−1+ashingl−1HNO3+HF/HClresidualpH525%HOAcorganic matter+sul-pH7phidesilicate phaseFe/Mn oxidesexchangeable carbonatereau Community of Reference),has sponsored from1987several projects focused on single ex-traction for soils and sequential extraction for soils and sediments.The project started with the intercomparison of existing procedures tested in an interlaboratory exercise[27].The next step was to adopt common procedures for single extraction of trace metals from mineral soils.The second step was to adopt a common procedure for se-quential extraction of sediment.As a conclusion of thefirst step,single extraction procedures using acetic acid,0.43mol l−1,and EDTA,0.005mol l−1for mineral soils and a mixture of DTPA, 0.005mol l−1diethylenetriamine pentaacetic acid, 0.01mol l−1CaCl2and0.1mol l−1tri-ethanolamine for calcareous soils were adopted for extractable Cd,Cr,Cu,Ni,Pb and Zn.In order to improve the quality of the determination of extractable metal content in different types of soil using the procedures previously adopted,the extraction procedures were validated by means of intercomparison exercises[28,29].Moreover the lack of suitable certified reference materials for this type of studies did not enable the quality of the measurements to be controlled.With the pur-pose to overcome this problem three certified reference materials:a terra rossa soil,a sewage amended soil and a calcareous soil have been prepared and their extractable trace metal con-tents were certified(CRM483,CRM484and CRM600)[30,31].The second step of the EC,Standards,Mea-surement and Testing was focused on a feasibility study on the adoption and validation of a sequen-tial extraction scheme for sediment samples.In a workshop held in1992in Sitges(Spain)a sequen-tial extraction scheme was proposed which in-cludes three steps:acetic acid,hydroxylamine hydrochloride or a reducing reagent and hydrogen peroxide or an oxidising reagent.This procedure is schematised in Table5.Moreover in this work-shop the main analytical limitations in sequential extraction of trace metals in sediments were thor-oughly discussed and practical recommendations were given[32,33].These recommendations deal with sampling and sample pre-treatment,practical experiences with reagents and matrices and ana-lytical problems after extraction.Once the scheme was designed,it was tested through two round robin exercises using two dif-ferent type of sediment,silicious and calcareous [34].In these exercises some critical parameters in the protocol were identified such as the type and the speed of the shaking and the need of an optimal separation of the liquid–solid phases af-ter the extraction.It was stated that the sedimentG.Rauret/Talanta46(1998)449–455 454should be continually in suspension during the extraction.In these intercomparison exercises an important decrease was noted on the acceptable set of values for concentration in the extract lower than10m g l−1,which illustrates the difficulties experienced by a number of laboratories in the determination of such concentration levels in these matrices.It was concluded that when elec-trothermal atomic absorption spectrometry is used for thefinal determination,the method of standard additions is strongly recommended for calibration.The results obtained in the round robin exercises encouraged to proceed with the organisation of a certification campaign in order to produce a sediment reference material follow-ing the sequential extraction scheme adopted.So the next step of the project was the preparation of a sediment certified reference material for the extractable contents of Cd,Cr,Cu,Ni.Pb and Zn,following the three-step sequential extraction procedure.A silicious type sediment with rather high trace metal content was chosen for this pur-pose.This material has been recently certified for five metals,Cd,Cr,Ni,Pb and Zn in thefirst step,Cd,Ni and Zn in the second step and Cd,Ni and Pb in the third step[35].Not all the elements were certified because the lack of reproducibility atributable to non adherence to the protocol,in the acceptance of too large tolerances in the con-ditions specified in it or in the existence of critical aspects in the procedure referred mainly to the second step.These aspects were mainly pH,redox conditions and possible losses of sediment in the transfer.The results obtained in the certification exercise recommended to continue the develop-ment of the extraction protocol in order to in-crease reproducibility.Consequently the causes of non reproducibility are now under study in a new SMT project.5.ConclusionsThe advantages of a differential analysis over investigations of total metal contents and about the usefulness of single and sequential chemical extraction for predicting long-term adverse effects of heavy metals from polluted solid material,soils and sediments,is beyond any doubt.The ad-vances in thisfield,especially to make available soil and sediment certified reference materials for extractable element contents by using harmonised procedures,is going to increase the quality of the results due to the possibility of verifying the ana-lytical quality control.Nevertheless some problems need to be solved with these procedures for example:(1)reactions are not selective and are influenced by the experi-mental conditions so it is necessary to identify the main variables which involves a lack of reproduci-bility when applying a procedure,to write very well defined protocols and to validate them;(2) labile fractions could be transformed during sam-ple preparation and during sequential extraction schemes application so problems encountered when preparing certified reference materials are not representing all the problems to be found when working with environmental samples such as wet sediments,some work in this area is needed;(3)analytical problems due to the low level of metals to be measured in the different fractions especially when using soft extractants; and(4)the procedures need to be optimised and validated for different type of soils,including organic soils and sediments.Table5EC Standard,Measurements and Testing procedureConditionsStep10.11mol l−1HOAc,V m−140ml g−1temp.20o C,shaking overnight20.1mol l−1NH2OH.HCl(pH=2with HNO3)V m−140ml.g−1temp.20o Cshaking overnight8.8mol l−1H2O2(pH=2–3with HNO3)3V m−1=10ml g−1room temperature1h.New addition10ml g−185o C for1h.reduce volume to few ml.1mol l−1NH4Oac(pH=2with HNO3)V m−1=50ml g−120o Cshaking overnightG.Rauret/Talanta46(1998)449–455455References[1]H.A.van der Sloot,L.Heasman,Ph.Quevauviller(Eds.),Harmonization of leaching/extraction test,Chap.3, 1997,41–56.[2]H.A.van der Sloot,L.Heasman,Ph.Quevauviller(Eds.),Harmonization of leaching/extraction test,Chap.5, 1997,pp.75–99.[3]I.Novozamski,Th.M.Lexmon,V.J.G.Houba,Int.J.Environ.Anal.Chem.51(1993)47–58.[4]E.Colinet,H.Gonska,B.Griepink,H.Muntau,EURReport8833EN,1983,p57.[5]A.M.Ure,Ph.Quevauviller,H.Muntau,B.Griepink,Int.J.Environ.Anal.Chem.51(1993)135–151.[6]C.L.Mulchi,C.A.Adamu,P.F.Bell,R.L.Chaney,Com-mon.Soil Sci.Plant Anal.23(1992)1053–1059.[7]W.L.Lindsay,W.A.Norvell,Soil Sci.Soc.Am.J.42(1978)421–428.[8]A.Melich,Common.Soil Sci.Plant Anal.15(1984)1409–1416.[9]A.M.Ure,R.Thomas, D.Litlejohn,Int.J.Environ.Anal.Chem.51(1993)65–84.[10]S.K.Gupta,C Aten,Int.J.Environ.Anal.Chem.51(1993)25–46.[11]J.C.Hughes,A.D.Noble,Common.Soil Sci.Plant Anal.22(1991)1753–1766.[12]C.Juste,P.Solda,Agronomie8(1988)897–904.[13]W.P.Pickering,Ore Geol.Rev.1(1986)83–146.[14]A.Lebourg,T.Sterckeman,H.Cielsielki,N.Proix,Agronomie16(1996)201–215.[15]DIN(Deutches Institut fu¨r Normung)(Ed.)Bo-denbeschaffenheit.Vornorm DIN V19730,in:Boden -Chemische Bodenuntersuchungsverfahren,DIN,Berlin, 1993,p.4.[16]AFNOR(Association Francaisede Normalization),AFNOR,Paris,1994,p.250.[17]UNICHIM(Ente Nazionale Italiano di Unificazione),UNICHIM,Milan1991.[18]V.J.G.Houba,I.Novozamski,T.X.Lexmon,J.J.van derLee,Common.Soil Sci.Plant Anal.21(1990)2281–2291.[19]VSBo(Veordnung u¨ber Schadstoffgehalt im Boden)Nr.814.12,Publ.eidg.Drucksachen und Materialzentrale, Bern,1986,pp.1–4.[20]MAFF(Ministry of Agriculture,Fisheries and Food),Reference Book427MAFF,London1981.[21]A.Ure,Ph.Quevauviller,H.Muntau,B.Griepink,Re-port EUR14763EN.1993.[22]A.Tessier,P.G.X.Campbell,M.Bisson,Anal.Chem.51(1979)844.[23]W.Salomons,U.Fo¨rstner,Environ.Lett1(1980)506.[24]M.Meguellati,D.Robbe,P.Marchandise,M.Astruc,Proc.Int.Conf.on Heavy Metals in the Environment, Heidelberg,CEP Consultants,Edinburgh,1983,p.1090.[25]G.Rauret,R.Rubio,J.F.Lopez-Sanchez,Int.J.Envi-ron.Anal.Chem.36(1989)69–83.[26]U.Fo¨rstner,in:R.Lechsber,R.A.Davis,P.L’Hermitte(Eds.),Chemical Methods for Assessing Bioavailable Metals in Sludges,Elsevier,London,1985.[27]A Ure,Ph.Quevauviller,H.Muntau,B.Griepink,Int.J.Environ.Anal.Chem.51(1993)135–151.[28]Ph.Quevauviller,chica,E.Barahona,G.Rauret,A.Ure,A Gomez,H.Muntau,Sci.Total Environ.178(1996)127–132.[29]Ph.Quevauviller,G.Rauret, A.Ure,R.Rubio,J-FLo´pez-Sa´nchez,H.Fiedler,H.Muntau,Mikrochim.Acta 120(1995)289–300.[30]Ph.Quevauviller,G.Rauret,A.Ure,J.Bacon,H.Mun-tau,Report EUR17127EN,1997.[31]Ph.Quevauviller,chica,E.Barahona,G.Rauret,A.Ure,A.Gomez,H.Muntau,Report EUR17555EN,1997.[32]Ph.Quevauviller,G.Rauret,B.Griepink,Int.J.Environ.Anal.Chem.51(1993)231–235.[33]B.Griepink,Int.J.Environ.Anal.Chem.51(1993)123–128.[34]Ph.Quevauviller,G.Rauret,H.Muntau,A.M.Ure,R.Rubio,J-F Lo´pez-Sanchez,H.D.Fiedler, B.Griepink, Fres.J.Anal.Chem.349(1994)808–814.[35]Ph.Quevauviller,G.Rauret,J-F.Lo´pez-Sanchez,R.Rubio, A.Ure,H.Muntau,Report EUR17554EN, 1997.. .。
溶剂萃取-树脂吸附联合脱除盐湖老卤中的微量硼

2017年第36卷第10期 CHEMICAL INDUSTRY AND ENGINEERING PROGRESS·3625·化 工 进展溶剂萃取-树脂吸附联合脱除盐湖老卤中的微量硼王雄1,刘明言1,2,宋军超1(1 天津大学化工学院,天津化学化工协同创新中心(天津),天津 300350;2 化学工程联合国家重点实验室(天津大学),天津 300350)摘要:采用溶剂萃取-树脂吸附联合的方法对青海盐湖卤水中的硼进行了提取和脱除。
研究了pH 、相比和萃取级数对萃取过程的影响,讨论了萃取后卤水的pH 和树脂用量等对吸附过程的影响,考察了D564树脂的吸附等温线和吸附动力学特性,并对反萃和洗脱过程进行了优化。
研究结果表明:萃取过程中卤水pH 为2时,萃取效果最佳,相比和萃取级数增加至3后继续增加相比和萃取级数对进一步降低卤水中硼浓度的作用并不明显;吸附过程中卤水的pH 最佳值为7,吸附动力学数据符合准二级动力学模型,吸附等温线数据符合Langmuir 等温吸附模型;当树脂用量为6g/(100mL 卤水)时,可直接将一级萃取后卤水中的硼浓度降低至1.0mg/L 以下;建议使用0.1mol/L 的NaOH 溶液作反萃剂,0.5mol/L 的HCl 溶液作洗脱剂。
关键词:溶剂萃取;吸附;硼酸;盐湖卤水中图分类号:TQ128+.54 文献标志码:A 文章编号:1000–6613(2017)10–3625–08 DOI :10.16085/j.issn.1000-6613.2017-0106Removal of micro-amount boron from salt lake brine by solventextraction-resin adsorption combined methodWANG Xiong 1,LIU Mingyan 1,2,SONG Junchao 1(1Collaborative Innovation Center of Chemical Science and Engineering (Tianjin ),School of Chemical Engineering andTechnology ,Tianjin University ,Tianjin 300072,China ;2China State Key Laboratory of Chemical Engineering (TianjinUniversity ),Tianjin 300072,China )Abstract :A solvent extraction-resin adsorption combined method was developed to recover and remove micro-amount boron from brine of salt lake in Qinghai. The effects of pH ,phase ratio, extraction stages on extraction process, and the effect of pH of brine after first stage extraction, resin dosage on boron adsorption were investigated. The adsorption isotherm and kinetics were studied. The stripping and elution process were also optimized. The results showed that best extraction result could be obtained when pH was 2. The reduction of boron concentration was not obvious when the phase ratio and extraction stage value were above 3. The best pH for boron adsorption from brine was 7. The adsorption kinetics and adsorption isotherm data were fitted well with pseudo-second-order rate model and Langmuir model ,respectively. The concentration of boron could be reduced to 1.0mg/L or less when resin dosage is 6g/(100mL brine) using the extraction-adsorption combined method. The proper stripping agent and eluent were 0.1mol/L NaOH and 0.5mol/L HCl, respectively. Key words : solvent extraction ;adsorption ;boric acid ;salt lake brine 硼及其化合物由于其特殊的物理化学性能而成为轻工、冶金、机械和医药等工业领域的重要原料,具有“工业味精”的美誉[1]。
蕨菜多糖的提取、纯化、结构表征与抗氧化活性研究

刘恒,张秀玲,李坤,等. 蕨菜多糖的提取、纯化、结构表征与抗氧化活性研究[J]. 食品工业科技,2023,44(17):51−58. doi:10.13386/j.issn1002-0306.2022090327LIU Heng, ZHANG Xiuling, LI Kun, et al. Extraction, Purification, Structural Characterization and Antioxidant Activity of Polysaccharides from Bracken[J]. Science and Technology of Food Industry, 2023, 44(17): 51−58. (in Chinese with English abstract).doi: 10.13386/j.issn1002-0306.2022090327· 研究与探讨 ·蕨菜多糖的提取、纯化、结构表征与抗氧化活性研究刘 恒,张秀玲*,李 坤,薄艳秋*,李佳旭(东北农业大学食品学院,黑龙江哈尔滨 150030)摘 要:本试验以蕨菜干为原料,采用超声辅助法提取蕨菜粗多糖,计算提取率。
再用Sevage 法、大孔树脂、透析法进行纯化,得到纯化蕨菜多糖。
测定了蕨菜多糖的化学成分和理化性质。
采用红外光谱、刚果红试验、高效液相色谱、高碘酸氧化、碘-碘化钾反应等方法对蕨菜多糖的结构进行了表征,通过测定蕨菜多糖对DPPH 自由基、ABTS +自由基的清除能力和FRAP 能力评价了蕨菜多糖的体外抗氧化能力。
试验结果表明,蕨菜粗多糖的提取率为8.91%,纯化后蕨菜多糖含量为76.95%,总酚含量为1.03%,黄酮含量为1.90%,硫酸根含量为21.38%,糖醛酸含量为29.26%。
红外光谱结果表明,蕨菜多糖具有多糖类物质的典型特征吸收峰,以及含有糖醛酸与部分吡喃糖。
Solvent Extraction and Separation Science

Solvent Extraction and SeparationScienceIntroductionSolvent extraction and separation science is a branch of chemistry that deals with the extraction of specific substances from a mixture using a liquid solvent. This process involves the use of different solvents and techniques to separate substances based on their physical and chemical properties. Solvent extraction has applications in various fields such as mining, pharmaceuticals, and the environment. In this article, we will look at the principles of solvent extraction and its applications.Principles of Solvent ExtractionThe principle of solvent extraction is based on the concept of like dissolves like. This means that substances that have similar chemical properties will dissolve in each other. The process of solvent extraction involves the use of chemical agents known as solvents to dissolve and separate a substance from a mixture. The solvent must be chosen carefully, based on the physical and chemical properties of the substance being extracted. Solvents can be polar or non-polar, and the choice of solvent plays a critical role in the efficiency of the extraction process.Application in MiningSolvent extraction has a wide range of applications in the mining industry. It is commonly used to extract metals such as gold, copper, and silver from ores and concentrates. In gold mining, cyanide is often used as a solvent to extract gold from its ore. The process involves dissolving the gold in a cyanide solution, which is then separated from the ore.Solvent extraction is also used to extract impurities from metals during the refining process. For instance, copper refining involves electrorefining, which is a process used to remove impurities from copper. The process involves placing the impure copper in asolution and passing an electric current through the solution. This causes the pure copper to collect on an electrode, while the impurities settle at the bottom. Solvent extraction is then used to extract the impurities from the solution.Application in PharmaceuticalsSolvent extraction has significant applications in the pharmaceutical industry. It is used to extract active ingredients from plant and animal tissues, which are then used to make drugs and medicines. For example, morphine, which is a painkiller, is extracted from the opium poppy using solvent extraction. Similarly, quinine, which is used to treat malaria, is obtained from the bark of the cinchona tree using solvent extraction.Solvent extraction is also used in the purification of drugs. For instance, penicillin, an antibiotic, is initially extracted from the mould using a solvent. However, the extract contains impurities and other unwanted substances. Solvent extraction is then used to isolate the pure drug from the impurities.Application in the EnvironmentSolvent extraction has significant applications in the environment. It is used to remove toxic substances from polluted soil and water. For example, organic compounds such as benzene, toluene, and xylene, which are known to be toxic to humans, can be removed from contaminated water using solvent extraction. The process involves using an organic solvent to dissolve the toxic compounds. The solvent is then separated from the water, leaving behind a purified sample.ConclusionSolvent extraction and separation science is an essential branch of chemistry that has various applications in different fields. The process involves the use of chemical agents known as solvents to dissolve and separate substances from a mixture. Solvent extraction has applications in mining, pharmaceuticals, and the environment. It plays a critical role in the extraction of metals from ores, the extraction and purification of drugs, and the removal of toxic substances from polluted water. Solvent extraction is a crucial process that helps to improve the quality of life for people in different parts of the world.。
马齿苋中抗炎活性物质的提取、分离及结构鉴定

马齿苋中抗炎活性物质的提取、分离及结构鉴定张会敏1,邢岩2,仇润慷1,张丽梅2,倪贺3,赵雷1*(1.华南农业大学食品学院,广东广州 510642)(2.国珍健康科技(北京)有限公司,北京 100000)(3.华南师范大学生命科学学院,广东广州 510640)摘要:以活性物质示踪为导向,建立脂多糖诱导的RAW264.7巨噬细胞炎症模型对马齿苋中的抗炎物质进行跟踪,采用柱层析提取法、硅胶柱色谱分离法、制备液相色谱法及气相色谱-质谱联用技术对抗炎物质进行提取分离和结构鉴定。
结果表明,石油醚-乙醇、无水乙醇和纯水溶剂依次对马齿苋样品进行提取,三种粗提物将细胞中一氧化氮(Nitric Oxide,NO)的分泌量分别减少至33.13、25.83和20.53 μmol/L,其中石油醚相粗提物的抑制效果最强(P<0.05)。
对石油醚相进一步分离得到四个组分,Fr.1、Fr.2和Fr.3组分具有较强的抗炎效果,但Fr.1和Fr.2组分含有潜在的毒性成分,选择Fr.3组分继续分离。
Fr.3组分经硅胶柱分离得到三个组分,Fr.3.1组分表现出最强的抑制NO的分泌量效果(11.80 μmol/L)。
经制备液相色谱进一步纯化及气质分析,确定Fr.3.1组分的主要成分为硬脂酸(47.09%)、邻苯二甲酸二(2-乙基己)酯(13.21%)和其他成分。
该研究建立了一种从马齿苋中分离纯化出抗炎物质方法,为马齿苋的开发利用提供理论参考。
关键词:马齿苋;抗炎活性;提取分离;鉴定文章编号:1673-9078(2024)03-191-199 DOI: 10.13982/j.mfst.1673-9078.2024.3.0324Extraction, Separation and Structural Identification of Anti-inflammatory Active Substances from Purslane (Portulaca oleracea L.)ZHANG Huimin1, XING Y an2, QIU Runkang1, ZHAGN Limei2, NI He3, ZHAO Lei1*(1.College of Food Science, South China Agricultural University, Guangzhou 510642, China)(2.Guozhen Health Technology (Beijing) Co. Ltd., Beijing 100000, China)(3.College of Life Sciences, South China Normal University, Guangzhou 510640, China)Abstract: To track the anti-inflammatory substances in purslane, the lipopolysaccharide-induced RAW264.7 macrophage inflammation model was established, which was guided by the tracer of active substances. The extraction, separation and structural identification of anti-inflammatory substances in purslane were performed by column chromatography (for extraction), silica gel column chromatography (for separation), and preparative high performance liquid chromatography and gas chromatography-mass spectrometry (for analyses). The results showed that the three crude extracts obtained from purslane through sequential extractions with petroleum ether-ethanol, anhydrous ethanol and pure引文格式:张会敏,邢岩,仇润慷,等.马齿苋中抗炎活性物质的提取、分离及结构鉴定[J] .现代食品科技,2024,40(3):191-199.ZHANG Huimin, XING Yan, QIU Runkang, et al. Extraction, separation and structural identification of anti-inflammatory active substances from purslane (Portulaca oleracea L.) [J] . Modern Food Science and Technology, 2024, 40(3): 191-199.收稿日期:2023-03-16基金项目:国家自然科学基金资助项目(31771980);广东省自然科学基金(2023A1515012599)作者简介:张会敏(1996-),女,硕士研究生,研究方向:活性物质分离提取,E-mail:;共同第一作者:邢岩(1981-),女,博士,助理研究员,研究方向:抗氧化与抗衰老,E-mail:通讯作者:赵雷(1982-),男,博士,教授,研究方向:天然产物绿色修饰及热带水果加工,E-mail:191water solvents reduced the secretion of nitric oxide (NO) in the cells to 33.13, 25.83 and 20.53 μmol/L, respectively, with the crude petroleum ether extract exhibiting the strongest inhibitory effect (P<0.05). The petroleum ether phase was further separated into four fractions, with the Fr.1, Fr.2 and Fr.3 fractions had stronger anti-inflammatory effects, though the Fr.1 and Fr.2 fractions contained potential toxic components. Therefore, the Fr.3 fraction was selected for further separation. The Fr.3 fraction was separated through a silica gel column to obtain three fractions. The Fr.3.1 subfraction exhibited the strongest inhibitory effect against the NO secretion (11.80 μmol/L). The Fr.3.1 subfraction was further purified by the preparative liquid chromatography and GC-MS analysis, and the main components of the Fr.3.1 subfraction were identified as stearic acid (47.09%), di(2-ethylhexyl)phthalate (13.21%) and other components. This study established a method for separating and purifying anti-inflammatory substances from purslane, and provides a theoretical reference for the development and utilization of purslane.Key words: Portulaca oleracea L.; anti-inflammatory activity; extraction and isolation; identification炎症是机体受到外部刺激时做出的一种保护性生理反应,能够及时清除体内受损或死亡的细胞,帮助机体恢复内部平衡[1] 。
Solvent extraction of metals

Solvent extraction of metalsis an important technique used in the mining and metallurgical industries to separate and purify metal ions from their aqueous solutions. The process involves the transfer of metal ions from an aqueous phase to an organic phase by using a solvent that is selective for the metal ions of interest. This technique is highly effective for the separation of metals with similar chemical properties, and it is widely used for the production of precious metals, rare earth metals, and other high-value metals.The solvent extraction process is based on the principle that different metal ions have different affinities for different types of solvents. The goal of the process is to selectively extract the metal ions of interest while leaving behind other metal ions and impurities. This is achieved by using a solvent that is designed to extract the metal ions of interest and not the impurities.The solvent extraction process typically involves several stages, including leaching, solvent extraction, and stripping. During the leaching stage, a solution containing metal ions is contacted with a leaching agent, such as an acid or a base, to dissolve the metal ions into solution. The resulting solution is then subjected to a series of solvent extraction steps, during which the metal ions are selectively extracted into an organic phase by using a solvent that is designed to selectively bind with the metal ions of interest.Once the metal ions have been extracted into the organic phase, they are separated from the organic phase by a process known as stripping. During stripping, the metal ions are stripped out of the organic phase and back into an aqueous phase, where they can be further purified or used for downstream applications.One of the key advantages of solvent extraction is its ability to selectively extract metal ions from solutions containing a wide range of metal ions. This makes it ideal for the production of high-value metals such as gold, platinum, and palladium, which are commonly found in low concentrations in complex ores and minerals. Additionally, the solvent extraction process can be used to recover metals from industrial wastewaters and other byproducts, reducing the overall environmental impact of these industries.Despite its many benefits, solvent extraction also has some drawbacks. For example, the process can be relatively expensive and complex, requiring specialized equipment and operators. Additionally, some solvents used in the process can be toxic, and proper safety precautions must be taken to ensure the safety of workers and the environment.In conclusion, solvent extraction is a powerful method for the separation and purification of metal ions from aqueous solutions. It has become a widely used technology in the mining and metallurgical industries, and is essential for the production of high-value metals. While the technique has some drawbacks, its benefits far outweigh its limitations, making it an indispensable tool in the pursuit of high-quality, sustainable metal production.。
超声波提取北五味子果实及茎叶总三萜的研究_崔福顺

2013年4月第34卷第7期超声波提取北五味子果实及茎叶总三萜的研究崔福顺,李官浩,金清(延边大学农学院食品科学系,吉林延吉133000)摘要:对北五味子果实及茎叶中总三萜超声波提取工艺进行了研究。
通过单因素和正交试验考察了提取剂浓度、超声提取时间、提取温度、超声功率对总三萜提取的影响。
结果表明,当提取剂浓度为95%、超声提取时间30min 、提取温度55℃、超声功率300W 时果实中总三萜得率最高为4.35%;当提取剂浓度为90%、超声提取时间30min 、提取温度75℃、超声功率200W 时茎叶中总三萜得率最高为3.06%。
关键词:北五味子;总三萜;超声波;提取Extraction of Total Triterpenoids from Fruit ,Stems and Leaves ofSchisandra Chinensis with Ultrasonic WaveCUI Fu-shun ,LI Guan-hao ,JIN Qing(Department of Food Science of Agricultural College ,Yanbian University ,Yanji 133000,Jilin ,China )Abstract :The ultrasonic wave extraction of total triterpenoids from fruit ,stems and leave of Schisandra chinensis was investigated.The effect factors :which included concentration of alcohol ,ultrasonic wave time ,ultrasonic wave temperature ,ultrasonic wave power were investigated.The extraction process was optimized through orthogonal test.The results showed that optimum condition of fruit were as followed :concentration of alcohol 95%,ultrasonic wave time 30min ,ultrasonic wave temperature 55℃,ultrasonic wave power 300W ,the extraction rate of total triterpenoids could up to 4.35%.The optimum condition of stems and leave were as followed :concentration of alcohol 90%,ultrasonic wave time 30min ,ultrasonic wave temperature 75℃,ultrasonic wave power 200W ,the extraction rate of total triterpenoids could up to 3.06%.Key words :Schisandra chinensis ;total triterpenoids ;ultrasonic wave ;extraction作者简介:崔福顺(1966—),女(朝鲜),副教授,硕士,研究方向:食品科学。
