化学专业英语复习资料
化学专业英语培训资料

化学专业英语培训资料unite 1. Inorganic chemistry1.1 what is chemistry(1). 重点专业词汇讲解:Chemical: adj . 化学的、化学药品Transformation: 变化,化学转变,转化Dye: n. 染料染色,或者vt. 染Charcoal: ['t?ɑk??l] 木炭Cellulose :纤维素细胞的['selj?l??z; ]Fat:n. 脂肪肥肉adj . 肥大的alkalis:碱adj . 碱性的glycerin: 甘油丙三醇alkalis: n. 碱金属alloy: 合金使成合金bronze:青铜色的n. 青铜(铜和锡的合金)brass:[brɑs] n. 黄铜(铜和锌)要求学生会区别黄铜及青铜的不同翻译Poison:毒物毒药t. 毒害放毒下毒Proton:n. 质子Nulei: n. 核(nucleus的复数形式)['njukl??s]Identical : adj . 同一的Chirality n. 手性手征和Handeness的区别Amino acid :n. 氨基酸Alanine: n.丙氨酸2. 课文中重点词组(phrase)Chemical change: 化学变化physical change:物理变化Explore: 探险研究research investigate studyIsolate: 分离chemical bonds 化学键chemical reaction:化学反应Natural substance 天然物质Coke :焦炭carbon monoxide 一氧化碳Carbon Dioxide 二氧化碳Chemical bond 化学键fundamental principle 基本原理The periodic table of elements :元素周期表numbers of protons 质子数atomic number 原子序数covalent bonds 共价键positive 正阳性negative 负阴性3. 课文中重点句子The first and most important principle is that chemical substances are made up of molecules in which atoms of various elements are linked in well-defined ways. 需要着重给学生讲解第一条也是最重要的原理是化学物质是有分子组成的,分子中的不同元素的原子是以一定的方式连接在一起的。
化学专业基础英语期末复习材料

可能的电解质potential electrolyte可逆电池reversible cell可逆过程reversible process可逆过程方程reversible process equation可逆体积功reversible volume work可逆相变reversible phase change控制步骤control step库仑计coulometer扩散控制diffusion controlled雷利公式Rayleigh equation冷冻系数coefficient of refrigeration冷却曲线cooling curve离解热heat of dissociation离解压力dissociation pressure离域子系统non-localized partic le systems离子的标准摩尔生成焓standard molar formation of ion离子的电迁移率mobility of ions离子的迁移数transport number of ions离子独立运动定律law of the independent migration of ions 离子强度ionic strength理想混合物perfect mixture理想气体ideal gas理想气体的绝热指数adiabatic index of ideal gases理想气体状态方程state equation of ideal gas连串反应consecutive reactions链反应chain reactions量热熵calorimetric entropy量子统计quantum statistics量子效率quantum yield临界参数critical parameter临界常数critical constant临界温度critical temperature临界状态critical state零级反应zero order reaction流动电势streaming potential笼罩效应cage effect摩尔电导率molar conductivity摩尔反应焓molar reaction enthalpy摩尔混合熵mole entropy of mixing摩尔气体常数molar gas constant摩尔热容molar heat capacity内扩散控制internal diffusions control内能internal energy内压力internal pressure能级energy levels能级分布energy level distribution凝固点曲线freezing point curve凝聚相condensed phase浓差超电势concentration over-potential浓差极化concentration polarization浓差电池concentration cells泡点线bubble point line配分函数partition function碰撞截面collision cross section碰撞数the number of collisions平衡分布equilibrium distribution平衡态equilibrium state平衡态近似法equilibrium state approximation平衡状态图equilibrium state diagram平均活度mean activity平均活度系统mean activity coefficient平均摩尔热容mean molar heat capacity平均质量摩尔浓度mean mass molarity平均自由程mean free path平行反应parallel reactions破乳demulsification铺展spreading普遍化范德华方程universal van der Waals equation其它功the other work气化热heat of vaporization气溶胶aerosol气体常数gas constant气体分子运动论kinetic theory of gases气体分子运动论的基本方程foundamental equation of kinetic theory of gases 气溶胶aerosol气相线vapor line迁移数transport number潜热latent heat强度量intensive quantity强度性质intensive property亲液溶胶hydrophilic sol氢电极hydrogen electrodes区域熔化zone melting热heat热爆炸heat explosion热泵heat pump热功当量mechanical equivalent of heat热函heat content热化学方程thermochemical equation热机heat engine热机效率efficiency of heat engine热力学thermodynamics热力学第一定律the first law of thermodynamics 热力学基本方程fundamental equation of thermodynamics热力学能thermodynamic energy热力学温度thermodynamic temperature热熵thermal entropy热效应heat effect熔化热heat of fusion溶胶colloidal sol溶液solution溶胀swelling乳化剂emulsifier萨克尔-泰特洛德方程Sackur-Tetrode equation三相点triple point三相平衡线triple-phase line熵判据entropy criterion渗透压osmotic pressure生成反应formation reaction舒尔采-哈迪规则Schulze-Hardy rule速度常数reaction rate constant速率方程rate equations速率控制步骤rate determining step塔费尔公式Tafel equation特鲁顿规则Trouton rule体积功volume work统计热力学statistic thermodynamics外扩散控制external diffusion control完全气体perfect gas [简明英汉词典] 理想气体韦斯顿标准电池Weston standard battery维恩效应Wien effect维里方程virial equation维里系数virial coefficient稳流过程steady flow process稳态近似法stationary state approximation物理化学Physical Chemistry物理吸附physisorptions吸附等量线adsorption isostere吸附等温线adsorption isotherm吸附等压线adsorption isobar吸附剂adsorbent吸附量extent of adsorption吸附热heat of adsorption吸附质adsorbate析出电势evolution or deposition potential稀溶液的依数性colligative properties of dilute solutions 稀释焓dilution enthalpy系统点system point系统的环境environment of system相变phase change相变焓enthalpy of phase change相变化phase change相对粘度relative viscosity相律phase rule相平衡热容heat capacity in phase equilibrium循环过程cyclic process压力商pressure quotient压缩因子compressibility factor盐桥salt bridge盐析salting out阳极anode杨氏方程Y oung’s equation液相线liquid phase lines一级相变first order phase change逸度系数coefficient of fugacity阴极cathode荧光fluorescence永动机perpetual motion machine原电池primary cell原盐效应salt effect增比粘度specific viscosity支链反应branched chain reactions直链反应straight chain reactions指前因子pre-exponential factor制冷系数coefficient of refrigeration中和热heat of neutralization转化率convert ratio状态方程state equation状态分布state distribution状态函数state function准静态过程quasi-static process准一级反应pseudo first order reaction自动催化作用auto-catalysis自由焓free enthalpy自由膨胀free expansion最低恒沸点lower azeotropic point最高恒沸点upper azeotropic point。
化学与化工专业英语

ClOIO-
Hypochlorite
Hypoiodite
PO23- Hypophosphite
(4). Acid radicals for persalts (高酸根Per -ate ) Anion’s name = Per-central Element’s root -ate
for example: ClO4IO4MnO4Perchlorate Periodate Permanganate
Naming metal ions (cations) for metal oxides, bases and salts
1. Single valence ions
Cation’s name = Element for example: Na+ Sodium K+ Potassium Al3+ Aluminum Ca2+ Calcium
• • • • • • • • • • • •
Mad doctor 精神病科医生(不是发疯的医生) Personal remark 人身攻击(并非个人评论) Service station 加油站(不是服务站) Rest room 厕所(不是休息室) In ones birthday suit 赤身裸体(不是穿着生日礼服) An apple of love 西红柿(不是爱情之果)
5. Letter Symbolizing
(字母象形法)
6. Other Regular Words
掌握构词法、利用前后缀、根据上下文 • -----提高猜测生词的能力! • 研究生考试中,专业英语笔试主要考察同学 们在化学专业英语词汇方面的积累,要求对 常用专业词汇比较熟悉。考试内容一般会从 最新的专业杂志的优秀论文中摘选。
化学专业英语复习.docx

REVIEWL Choose the best answer (20 points, 2 points for each)1.What is amino benzene most commonly known as? ( D )A・ quinoline B. toluene C. Indole D. aniline2.Sulfur in the form of SO2 obtained in the roasting of B ores is recovered and converted to sulfuric acid?A・ sulfate B. sulfide C.sulfane D. sulfiteII. NomenclaturePart A: Name the following compounds in English (16 points, 2 points for each)⑴一氧化碳(2))氢氧化铜(3)氯化钠Part B: Translate the following compounds into Chinese. (14 points, 1 point for each) (1)silver fluoride (2) mercury nitrate Hg(NC)3)2(3) Nickel sulfate (4) potassium hydroxideIIL Translate the following sentences into Chinese (5% for each answer) Pick the size of your separatory (分液漏斗)funnel. You will usually use 125 or 250-mL, large scale reactions (大型反应) (1-10 g) can require 500-mL or 1-L sizes. Remember that your sep. funnel will contain the solvent and wash liquid which must be thoroughly (彻底)mixed.IV.Write a chemical equation representing each of the following reaction and calculate (10% for all the answers)Write a chemical equation representing each of the following:(a)Solid aluminum sulfide reacts with liquid water to give solid aluminum hydroxide and gaseous hydrogen sulfide.(b)Gaseous ozone reacts with gaseous nitrogen monoxide to produce gaseous nitrogen dioxide and gaseous oxygem(c)Gaseous nitrogen reacts with gaseous hydrogen at 400°C and 250 atm pressure in the presence of FeO as a catalyst to yield gaseous ammonia.V.Read the Abstract and then answer the questions: (20% for all the answers )Title: Reductive Adsorption of Gold(III) by Crosslinked LignophenolAuthor: PARAJULI D, INOUE K, KURIYAMA M, FUNAOKAM, MAK1N0 K*To whom correspondence should be addressed. E-mail: km@Abstract: The adsorption behavior of acrosslinked lignophenol (木质素酚)gel for various metal ions from chloride medium was investigated. The gel (凝胶) was found to be only effective (有效)for the adsorption (吸附作用)of Au (III) from weak to strong acidic medium (酸性媒介).The analysis of XRD-specrum and SEM-images of the adsorbent after adsorption revealed the formation of elemental gold by the reduction of the Au (III) ion. Such a result is promising for the development of a recovery system for gold from various industrial wastes.(1)What do the words ''Reductive adsorptiorT (还原吸附),“adsorbent"(吸附剂)and "recovery" (回收)mean in this abstract? (6% for the answer)(2)What are the applications for the adsorption of acrosslinked lignophenol (木质素酚) gel in this paper? (4% for the answer)(3)What are the two analytical methods mentioned in this abstract? (6% for the answer)(4)Who are the first author and corresponding author in this paper and how can you contact with them? (4% for the answer)Forty of the most common chemical elementshttp://\v\vw・Quia・Com/ifb/65539・htmlPrefixper-/hypo-高/次mono- (monocycle)一 di- (bicycle)二tri- (tricycle/triangle)Htetra- (tetragon/quadrangle) 29penta- (pentagon)E hexa ・(hexagon)六 hepta- (heptagon)七 octa- (octopus/octagon))\ nona ・(nonagon)九 deca- (decade/decagon)+Suffix -ide ■化. -ite 亚盐 -ate 正盐 -ous 亚酸 -ic 正酸Mono=l Di=2 Tri=3 Tetra=4 Penta=5Hexa=6 Hepta=7 Octa=8 Nona=9 Deca=10 N2O5 dinitrogen pentoxideN2O4 dinitrogen tetroxideN2O3 dinitrogen trioxideN2O2 dinitrogen dioxideN2O dinitrogen oxideNO2 nitrogen dioxideNO nitrogen oxideExceptions that use the ide endingshydroxide (OH ), cyanide (CN~), ammonium (NH/)NH4I ammonium iodide (NH3H2O ammonia)KCN potassium cyanideCa(OH)2 calcium hydroxideNaOH sodium hydroxideFe(OH)2 iron(H) hydroxide/ ferrous hydroxideFe(OH)3 iron(III) hydroxide/ferric hydroxideCu (OH)2 copper(II) hydroxide/cupric hydroxideCu (OH) copper(I) hydroxide/cuprous hydroxideWrite for the following compounds Sulfur trioxide SO3Cupric chloride CuCI2Ferrous sulfideBarium peroxide BaO2Silicon iodide Sil4Sodium hydroxideAmmonium chloride NH4CIPotassium KCNTungsten carbide WCCalcium bromide anhydrous CaBr2FeSC)3 iron(II)/ferrous sulfite Fe2(SC)3)3 iron(III)/ferric sulfiteFeS€)4 iron(II)/fenous sulfate Fe2(SC)4)3 iron(III)/ferric sulfateGive the formulas of thefollowing compoundsperchloric acidammonium paratungstate (偏卡乌酸纟安) hydroiodic acid (氢碘酸 HI )divanadium pentoxide (五氧化二磷) carbon tetrafluoride tetraphosphorus hexaoxidemercurous chloridepotassium fluorosilicate (氟硅酸钾) hydrogen peroxide calcium hypochloriteammonium ferric sulfate (硫酸铁辛安)potassium sulfite calcium nitrate barium phosphate potassium dichromate sodium chlorite sulfate sodium ferric chlorate cuprous nitriteK 2so 3 Ca (NO 3)2 Ba 3(PO 4)2K 2Cr 2O 7 NaCIO 2 PbSO 4 Na 3PO 3 Fe (CIO 3)HCl hydrochloric acidNaClOsodium hypochlorite HC10 hypochlorous acid NaC102 sodium chlorite HC1O 2 chlorous acid NaClO 3 sodium chlorate HCIO3chloric acidNaClO 4sodium perchlorateHCIO4 perchloric acidpotassium bromatesodium hydrogen phosphate, anhydrous (无水)sodium thiosulfate ferric hydroxide rare earth oxalate (RE 草cuprous sulfidesodium tetraboratemercuric sulfocyanide sodium bicarbonateName the following compoundshydrochloric acid (氢氯酸,盐酸,HC1) ferrous oxidemagnesium chloride potassium permanganate (高猛酸钾,KMnO4)ammonium nitritesilver sulfidehydrogen dioxide lead(II) sufate chromium hydroxide calcium phosphate sodium bicarbonate hypochlorous acidisooctane异辛烷CH3-C(CH3)2-CH2-CH(CH3)-CH3(2,2,4-trimethylpentane)C primary (伯)C secondary (仲)C tertiary (叔)C quarternary (季)neobuane 正丁烷Examples of Simple GydoalkaJftfeSNote: sydWQpan貝is isomeric with 陂C6H4(CH3)2 dimethylbenzene/xylene (o-,m-,p-)o- (ortho)令B 1,2-dimethylbenzene/o-xylenem- (meta) 间1,3-dimethylbenzene/m-xyleneX'i 1,4-dimethylbenzene/p-xylene P- (para)Note: C6H5- phenylC6H5CH2- benzyl1 .Common Functional GroupsGive the formulas (or Chinese names) of the following compounds (1)potassium hydrogen carbonate(3) sodium chlorous (亚氯酸钠)(5) 1,3-pentadiene(7) methoxybenzene(9) polytetrafluoroethylene■graph描绘器,记录器photograph, chromatograph, telegraph■graphy⑴图示法,画法,写法chromatography, photography, calligraphy, telegraphy ⑵记,志biography, geography, bibliography■scope观察的仪器,…镜microscope, specroscope (spectromicroscope), telescope ■scopy观察,检验spectroscopy, microscopy, telescopy■meteT计量器,计,表thermometer, spectrometer, gravimeter, colorimeter, telemeter metry测量(学),度量(学)thermometry, gravimetry, colorimetry, volumetry, iodimetry(2) cupric sulfide(4) periodic acid(6) butyl pentanoate(8) propanaltitrimetric analysis i商定卜析instrumental analysis 仪器分析(1) AAS: atomic absorption spectroscopy (原子吸收光谱法)(2) AES: atomic emission spectroscopy (原子发射光谱法) (3) AFS: atomic fluorescence spectroscopy (原子荧光光谱法) (4) FTIR: Fourier-transform infrared spectroscopy (红外光谱) (5) NMR: nuclear magnetic resonance spectroscopy (核磁共振光谱) (6) GC: gas chromatography (气相色谱法)(7) HPLC:high-performance liquid chromatography (高效液和色谱法)(8) ICP-MS: (9) UV-VIS: inductively coupled plasma mass spectroscopy (电感耦合等 离子体质谱) ultraviolet-visible spectroscopy (紫外一可见光谱) (10) TEM: transmission electron microscopy (透身寸 电子显微镜) (ll)SEM: scanning electron microscopy (扌H 描电子显微镜) (12) XRD:X-ray diffraction (X 射线衍射)Practice(1)二氧化碳(5)二甲醛(2)氢氧化铜(3)氯酸(4)高猛酸银(AgMnO4)(6)乙酸(7)淚苯⑻甲酸甲酯(1 )carbon dioxide (2)copper( II) hydroxide (3)chloric acid (氯酸) (4)silver permanganate (5)dimethyl(methyl) ether (6)ethanoic acid(7)bromobenzene (8)methyl methanoate (formate)(2) mercury nitrate (4) calcium phosphide (6) magnesium nitride (8) ethylcyclohexane (10) nitrobenzene(12) 4-hydroxydiphenyl ether (14) 3-ethyl-6-methyl-2,6-octadiene (2) HgNO3⑷ Ca3P2(6) Mg3N2(1) iron oxide (3) Nickel sulfide (5) zinc oxide (7) hydrogen peroxide (9) 1,4-pentadiene(11) 1,2,3-propanetriol (13) o-dibromobenzene (l)Fe2O3 (3) NiS (5) ZnO(10)硝基苯(12) 4■梵棊二苯瞇(14) 6■甲基・3■乙基・2,6■辛二烯Translate the following sentences into Chinese (5% for each answer)Unsaturated unbranched acyclic hydrocarbons having one double bond are named by replacing the ending "ane^ of the name of the corresponding saturated hydrocarbon with the ending "ene M . If there are two or more double bonds, the ending will be "adiene^, "atriene ,\ etc.含一个双键的不饱和直连脂肪怪命名时用“ene”作为结尾替代相应的饱和烧结尾字 母“ane”,如有两个或多个双键,则以二烯“adien 尹和三烯“atrience"结尾。
(完整版)化学专业英语
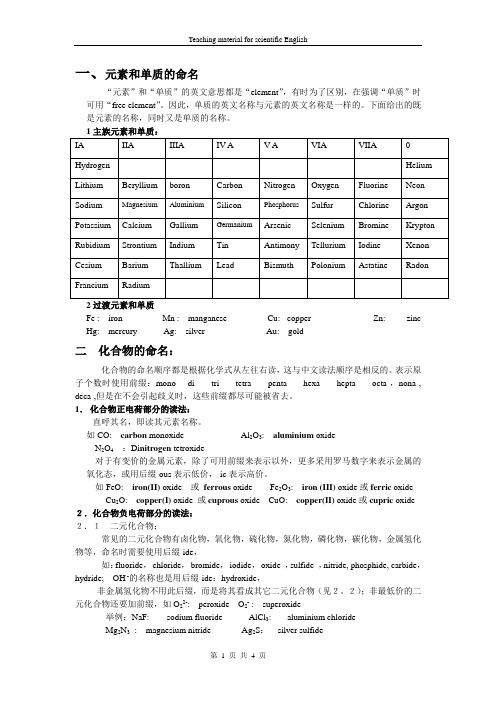
一、元素和单质的命名“元素”和“单质”的英文意思都是“element”,有时为了区别,在强调“单质”时可用“free element”。
因此,单质的英文名称与元素的英文名称是一样的。
下面给出的既是元素的名称,同时又是单质的名称。
2过渡元素和单质Fe : iron Mn : manganese Cu: copper Zn: zinc Hg: mercury Ag: silver Au: gold二化合物的命名:化合物的命名顺序都是根据化学式从左往右读,这与中文读法顺序是相反的。
表示原子个数时使用前缀:mono-di -tri- tetra -penta- hexa-hepta- octa-,nona-, deca-,但是在不会引起歧义时,这些前缀都尽可能被省去。
1.化合物正电荷部分的读法:直呼其名,即读其元素名称。
如CO: carbon monoxide Al2O3: aluminium oxideN2O4:Di nitrogen tetroxide对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous表示低价,-ic表示高价。
如FeO: iron(II) oxide 或ferrous oxide Fe2O3: iron (III) oxide或ferric oxide Cu2O: copper(I) oxide 或cuprous oxide CuO: copper(II) oxide或cupric oxide 2.化合物负电荷部分的读法:2.1二元化合物:常见的二元化合物有卤化物,氧化物,硫化物,氮化物,磷化物,碳化物,金属氢化物等,命名时需要使用后缀-ide,如:fluoride,chloride,bromide,iodide,oxide ,sulfide ,nitride, phosphide, carbide,hydride; OH -的名称也是用后缀-ide:hydroxide,非金属氢化物不用此后缀,而是将其看成其它二元化合物(见2。
化学专业英语总结

化学专业英语资料总结\基础化学常用英语词汇基础化学常用英语词汇1. The Ideal-Gas Equation 理想气体状态方程2. Partial Pressures 分压3. Real Gases: Deviation from Ideal Behavior 真实气体:对理想气体行为的偏离4. The van der Waals Equation 范德华方程5. System and Surroundings 系统与环境6. State and State Functions 状态与状态函数7. Process 过程8. Phase 相9. The First Law of Thermodynamics 热力学第一定律10. Heat and Work 热与功11. Endothermic and Exothermic Processes 吸热与发热过程12. Enthalpies of Reactions 反应热13. Hess’s Law 盖斯定律14. Enthalpies of Formation 生成焓15. Reaction Rates 反应速率16. Reaction Order 反应级数17. Rate Constants 速率常数18. Activation Energy 活化能19. The Arrhenius Equation 阿累尼乌斯方程20. Reaction Mechanisms 反应机理21. Homogeneous Catalysis 均相催化剂22. Heterogeneous Catalysis 非均相催化剂23. Enzymes 酶24. The Equilibrium Constant 平衡常数25. the Direction of Reaction 反应方向26. Le Chatelier’s Principle 列•沙特列原理27. Effects of Volume, Pressure, Temperature Changes and Catalystsi. 体积,压力,温度变化以及催化剂的影响28. Spontaneous Processes 自发过程29. Entropy (Standard Entropy) 熵(标准熵)30. The Second Law of Thermodynamics 热力学第二定律31. Entropy Changes 熵变32. Standard Free-Energy Changes 标准自由能变33. Acid-Bases 酸碱34. The Dissociation of Water 水离解35. The Proton in Water 水合质子36. The pH Scales pH值37. Bronsted-Lowry Acids and Bases Bronsted-Lowry 酸和碱38. Proton-Transfer Reactions 质子转移反应39. Conjugate Acid-Base Pairs 共轭酸碱对40. Relative Strength of Acids and Bases 酸碱的相对强度41. Lewis Acids and Bases 路易斯酸碱42. Hydrolysis of Metal Ions 金属离子的水解43. Buffer Solutions 缓冲溶液44. The Common-Ion Effects 同离子效应45. Buffer Capacity 缓冲容量46. Formation of Complex Ions 配离子的形成47. Solubility 溶解度48. The Solubility-Product Constant Ksp溶度积常数49. Precipitation and separation of Ions 离子的沉淀与分离50. Selective Precipitation of Ions 离子的选择沉淀51. Oxidation-Reduction Reactions 氧化还原反应52. Oxidation Number 氧化数53. Balancing Oxidation-Reduction Equations 氧化还原反应方程的配平54. Half-Reaction 半反应55. Galvani Cell 原电池56. Voltaic Cell 伏特电池57. Cell EMF 电池电动势58. Standard Electrode Potentials 标准电极电势59. Oxidizing and Reducing Agents 氧化剂和还原剂60. The Nernst Equation 能斯特方程61. Electrolysis 电解62. The Wave Behavior of Electrons 电子的波动性63. Bohr’s Model of The Hydrogen Atom 氢原子的波尔模型64. Line Spectra 线光谱65. Quantum Numbers 量子数66. Electron Spin 电子自旋67. Atomic Orbital 原子轨道68. The s (p, d, f) Orbital s(p,d,f)轨道69. Many-Electron Atoms 多电子原子70. Energies of Orbital 轨道能量71. The Pauli Exclusion Principle 泡林不相容原理72. Electron Configurations 电子构型73. The Periodic Table 周期表74. Row 行75. Group 族76. Isotopes, Atomic Numbers, and Mass Numbers 同位素,原子数,质量数77. Periodic Properties of the Elements 元素的周期律78. Radius of Atoms 原子半径79. Ionization Energy 电离能80. Electronegativity 电负性81. Effective Nuclear Charge 有效核电荷82. Electron Affinities 亲电性83. Metals 金属84. Nonmetals 非金属85. Valence Bond Theory 价键理论86. Covalence Bond 共价键87. Orbital Overlap 轨道重叠88. Multiple Bonds 重键89. Hybrid Orbital 杂化轨道90. The VSEPR Model 价层电子对互斥理论91. Molecular Geometries 分子空间构型92. Molecular Orbital 分子轨道93. Diatomic Molecules 双原子分子94. Bond Length 键长95. Bond Order 键级96. Bond Angles 键角97. Bond Enthalpies 键能98. Bond Polarity 键矩99. Dipole Moments 偶极矩100. Polarity Molecules 极性分子101. Polyatomic Molecules 多原子分子102. Crystal Structure 晶体结构103. Non-Crystal 非晶体104. Close Packing of Spheres 球密堆积105. Metallic Solids 金属晶体106. Metallic Bond 金属键107. Alloys 合金108. Ionic Solids 离子晶体109. Ion-Dipole Forces 离子偶极力110. Molecular Forces 分子间力111. Intermolecular Forces 分子间作用力112. Hydrogen Bonding 氢键113. Covalent-Network Solids 原子晶体114. Compounds 化合物115. The Nomenclature, Composition and Structure of Complexes 配合物的命名,组成和结构116. Charges, Coordination Numbers, and Geometries 电荷数、配位数、及几何构型117. Chelates 螯合物118. Isomerism 异构现象119. Structural Isomerism 结构异构120. Stereoisomerism 立体异构121. Magnetism 磁性122. Electron Configurations in Octahedral Complexes 八面体构型配合物的电子分布123. Tetrahedral and Square-planar Complexes 四面体和平面四边形配合物124. General Characteristics 共性125. s-Block Elements s区元素126. Alkali Metals 碱金属127. Alkaline Earth Metals 碱土金属128. Hydrides 氢化物129. Oxides 氧化物130. Peroxides and Superoxides过氧化物和超氧化物131. Hydroxides 氢氧化物132. Salts 盐133. p-Block Elements p区元素134. Boron Group (Boron, Aluminium, Gallium, Indium, Thallium) 硼族(硼,铝,镓,铟,铊)135. Borane硼烷136. Carbon Group (Carbon, Silicon, Germanium, Tin, Lead) 碳族(碳,硅,锗,锡,铅)137. Graphite, Carbon Monoxide, Carbon Dioxide 石墨,一氧化碳,二氧化碳138. Carbonic Acid, Carbonates and Carbides 碳酸,碳酸盐,碳化物139. Occurrence and Preparation of Silicon 硅的存在和制备140. Silicic Acid,Silicates 硅酸,硅酸盐141. Nitrogen Group (Phosphorus, Arsenic, Antimony, and Bismuth) 氮族(磷,砷,锑,铋)142. Ammonia, Nitric Acid, Phosphoric Acid 氨,硝酸,磷酸143. Phosphorates, phosphorus Halides 磷酸盐,卤化磷144. Oxygen Group (Oxygen, Sulfur, Selenium, and Tellurium) 氧族元素(氧,硫,硒,碲)145. Ozone, Hydrogen Peroxide 臭氧,过氧化氢146. Sulfides 硫化物147. Halogens (Fluorine, Chlorine, Bromine, Iodine) 卤素(氟,氯,溴,碘)148. Halides, Chloride 卤化物,氯化物149. The Noble Gases 稀有气体150. Noble-Gas Compounds 稀有气体化合物151. d-Block elements d区元素152. Transition Metals 过渡金属153. Potassium Dichromate 重铬酸钾154. Potassium Permanganate 高锰酸钾155. Iron Copper Zinc Mercury 铁,铜,锌,汞156. f-Block Elements f区元素157. Lanthanides 镧系元素158. Radioactivity 放射性159. Nuclear Chemistry 核化学160. Nuclear Fission 核裂变161. Nuclear Fusion 核聚变162. analytical chemistry 分析化学163. qualitative analysis 定性分析164. quantitative analysis 定量分析165. chemical analysis 化学分析166. instrumental analysis 仪器分析167. titrimetry滴定分析168. gravimetric analysis 重量分析法169. regent 试剂170. chromatographic analysis 色谱分析171. product 产物172. electrochemical analysis 电化学分析173. on-line analysis 在线分析174. macro analysis 常量分析175. characteristic 表征176. micro analysis 微量分析177. deformation analysis 形态分析178. semimicro analysis 半微量分析179. systematical error 系统误差180. routine analysis 常规分析181. random error 偶然误差182. arbitration analysis 仲裁分析183. gross error 过失误差184. normal distribution 正态分布185. accuracy 准确度186. deviation偏差187. precision 精密度188. relative standard deviation 相对标准偏差(RSD)189. coefficient variation 变异系数(CV)190. confidence level 置信水平191. confidence interval 置信区间192. significant test 显著性检验193. significant figure 有效数字194. standard solution 标准溶液195. titration 滴定196. stoichiometric point 化学计量点197. end point滴定终点。
化学专业英语

unite 1. Inorganic chemistry1.1 what is chemistry(1). 重点专业词汇讲解:Chemical: adj . 化学的、化学药品Transformation: 变化,化学转变,转化Dye: n. 染料染色,或者vt. 染Charcoal: ['t ? ck??l] 木炭Cellulose : 纤维素细胞的['selj?l??z; ] Fat:n. 脂肪肥肉adj . 肥大的alkalis :碱adj . 碱性的glycerin: 甘油丙三醇alkalis: n. 碱金属alloy: 合金使成合金bronze:青铜色的n. 青铜(铜和锡的合金)brass:[br a s] n.黄铜(铜和锌)要求学生会区别黄铜及青铜的不同翻译Poison:毒物毒药t.毒害放毒下毒Proton:n. 质子Nulei: n.核(nucleus 的复数形式)['njukl ??s]Identical : adj . 同一的Chirality n.手性手征和Handeness的区别Amino acid :n. 氨基酸Ala nine: n.丙氨酸2. 课文中重点词组(phrase)Chemical change:化学变化physical change:物理变化Explore: 探险研究research investigate studyIsolate: 分离chemical bonds 化学键chemical reaction :化学反应Natural substance 天然物质Coke : 焦炭carbon monoxide 一氧化碳Carbon Dioxide 二氧化碳Chemical bond 化学键fundamental principle 基本原理The periodic table of elements :元素周期表numbers of protons 质子数atomic number 原子序数covalent bonds 共价键positive 正阳性negative 负阴性3. 课文中重点句子The first and most important principle is that chemical substances are made up of molecules in which atoms of various elements are linked in well-defined ways. 需要着重给学生讲解第一条也是最重要的原理是化学物质是有分子组成的,分子中的不同元素的原子是以一定的方式连接在一起的。
化学专业英语

化学专业英语1、化学专业英语:一、无机化学术语1、periodic table 元素周期表2、electronic structure电子构型3、wavelength波长4、frequency频率5、wave number波数6、diffraction衍射7、quantum量子8、quantized量子化9、quantum theory量子理论10、photoelectric effect光电效应11、photon光子12、quantum mechanics量子力学13、Heisenberg uncertainty principle海森堡测不准原理14、momentum动量15、angular momentum角动量16、ground state基态17、excited states激发态18、quantum number量子数19、atomic orbital原子轨道20、the four quantum numbers四个量子数21、electron configuration电子构型22、Pauli exclusion principle泡利不相容原理23、Hund’s principle洪特规则24、paramagnetism顺磁性25、diamagnetism反磁性26、period周期27、noble gas惰性气体28、Representative elements代表性元素29、Transition elements过渡元素30、Metals金属31、nonmetals非金属32、semiconducting elements半导体元素33、chemical bond化学键34、valence electrons价电子35、Lewis symbol路易斯符号36、Chemical stability化学稳定性37、octet rule八隅体规则38、chemical reactivity化学反应性39、metallic bonding金属键40、ionic bonding 离子键41、Lewis structures路易斯结构42、nonbonding electron pairs(lone pairs)非成键电子对43、covalent bonding共价键44、single单键45、multiple(double,triple) and coordinate(donor atom and acceptor atom) covalent bond配位键46、resonance共振47、resonance hybrid共振杂化48、nonpolar and polar covalent bond非极性和极性共价键49、dipole偶极50、network covalent substances51、bond dissociation energy键解离能52、lattice energy点阵能,晶格能53、atomic radii原子半径54、effective nuclear charge有效核电荷55、screening effect屏蔽效应56、Scanning 扫描57、Lanthanide contraction镧系收缩58、isoelectronic ions等电子离子59、ionization energy电离能60、noble gas configuration惰性气体构型61、electron affinity电子亲和能62、pseudo-noble gas configuration稀有气体原子实63、polarization of an ion离子极化64、electronegativity电负性65、electronegative atom电正性原子66、electropositive atom电负性原子67、Oxidation numbers氧化值68、Oxidation state氧化态69、molecular geometry分子几何70、bond axis键轴71、valence bond theory价键理论72、hybridization杂化73、isomers异构体74、structural isomers结构异构75、delocalized electrons离域电子76、dipole moment偶极矩77、London bond色散力78、nuclide核素79、nucleons核子80、mass defect质量缺陷81、nuclear binding energy核结合能82、nuclear fusion核聚变83、nuclear fission核裂变84、radioactivity放射性85、radionuclides放射性核素86、magic number幻数87、bombardment reaction轰击反应88、antineutrino反中微子89、neutrino中微子90、positron正电子(阳电子)91、electron capture电子捕获92、chain reaction链式反应93、crtical mass临界质量94、nuclear reaction 核反应95、thermonuclear reactions热核反应96、breeder reactor增殖反应97、hydration水合98、solvation溶剂化99、chemical equilibrium化学平衡100、hydrolysis水解101、hydrates水合物102、efflorescence风化物103、hygroscopic 吸湿104、deliquescence潮解105、electrolytes电解质106、strong(weak)electrolytes强电解质107、nonelectrolytes非电解质108、acidic(alkaline)aqueous solution109、polyprotic acids多元酸110、neutralization中和反应111、complex ion络合离子112、ligands配体113、hard water 硬水114、carbonate hardness碳酸盐硬度115、water softening水软化116、permanent hardness永久硬度117、ion exchange离子交换118、fossil fuels化石燃料119、oxidation氧化120、reduction还原121、oxidation-reduction(redox)reactions氧化还原反应122、oxidizing agent氧化剂123、heavy water重水124、absorption吸附125、acidic anhydride(oxide)酸性酸酐126、basic anhydride(oxide)碱性酸酐127、amphoteric两性128、allotropes同素异形体129、acid salt酸式盐130、oxidizing anion氧化性阴离子131、disproportionation reaction歧化反应132、oxidizing acids氧化性酸。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Naming Inorganic CompoundsIntroduction:1.10 million known chemical substances.Need to establish a set of rules leading to informative, systematic name for each substance.2.Nomenclature: basic rules for naming simple compounds (organic compounds,inorganic compounds)Contents of current section:1.Preparatory materials(names of common elements in the periodic table);2.Ionic compounds (cations, anions,compounds);3.Acids;4.Molecular compounds Common Elements:Ac-Actinium锕, Ag-Silver, Al-Aluminum, Ar-Argon, As-Arsenic, Au-Gold, B-Boron, Ba-Barium, Be-Beryllium, Bi-Bismuth, Br-Bromine, C-Carbon, Ca-Calcium, Cd-Cadmium, Ce-Cerium铈, Cl-Chlorine, Co-Cobalt, Cr-Chromium, Cs-Cesium铯, Cu-Copper, F-Fluorine, Fe-Iron,Ga-Gallium镓, Ge-Germanium锗, H-Hydrogen, He-Helium, Hg-Mercury, I-Iodine, In-Indium, Ir-Iridium铱, K-Potassium, Kr-Krypton, La-Lanthanum镧, Li-Lithium, Mg-Magnesium, Mn-Manganese, Mo-Molybdenum钼, N-Nitrogen, Na-Sodium, Nb-Niobium铌, Nd-Neodymium钕, Ne-Neon, Ni-Nickel, O-Oxygen, Os-Osmium锇, P-Phosphorus, Pb- Lead, Pd-Palladium钯, Po-Polonium钋,Pt-Platinum, Pu-Plutonium钚, Ra-Radium, Rb-Rubidium铷, Re-Rhenium铼, Rn-Radon氡, Ru-Ruthenium钌, S-Sulfur, Sb-Antimony锑, Sc-Scandium钪, Se-Selenium硒, Si-Silicon, Sm-Samarium钐, Sn-Tin,Sr-Strontium锶, Ta-Tantalum钽, Te-Tellurium, Ti-Titanium, Tl-Thallium, U-Uranium, V-Vanadium钒,W-Tungsten, Xe-Xenon, Y-Yttrium钇, Zn-Zinc, Zr-Zirconium锆Ionic compoundsGeneral rule :The names of ionic compounds are based on the names of the ions of which they are composed. The positive ion (cation) is always named first and listed first in writing the formula for the compound. The negative ion (anion) is named and written last.Eg.:NaCl (sodium chloride)Naming cationsMonatomic ions (take the name of the element itself)Zn2+ (zinc ion), Al3+ (aluminum ion)Note: for an element (especially transition metals) with more than 1 positive ion, the positive charge of the ion is indicated by a Roman numeral in parentheses following the name of the metal: Fe2+ --- iron (II) ion, Cu+ ---copper (I) ionIf unsure, use the Roman numeral designation of charges as part of the name.Naming cationsNote: A widely used older method to distinguish between two differently charged ions of a metal is to apply the ending –ous for the lower charged ions or -ic for the higher charged ions, respectively. They are added to the root of the Latin name of the element.Eg.:Fe2+ (ferrous ion), Cu+ (cuprous ion)Fe3+ (ferric ions), Cu2+ (cupric ion)Naming cationsPolyatomic cations: Groups of atoms with a positive charge.NH4+ --- ammonium ion Hg22+ ---mercury (I) ion or mercurous ionNote: Hg2+ ---mercury (II) ion, or mercuric ionCommon ions:Cations: ammonium, cesium, copper(I) or cuprous, hydrogen, lithium, potassium, silver,sodium.(+1 ions); barium, cadmium, calcium, cobalt(II) or cobaltous, copper(II) or cupric,iron(II) or ferrous, lead(II) or plumbous,magnesium, manganese(II) ormanganous,mercury(I) or mercurous, mercury(II) or mercuric, nickel, strontium, tin(II) or stannous, zinc.(2+ ions); aluminum, chromium(III) or chromic, iron(III) or ferric.(3+ ions) Naming anionsMonatomic anions (named by dropping the ending of the name of the element and adding the ending -ide ):Naming anionsPolyatomic anionsNote: only a few polyatomic anions end in -ide:OH- hydroxide ion, CN- cyanide ion O22- peroxide ion, N3- azide ionNaming anionsOxyanions (polyatomic and oxygen-containing):when an element forms two oxyanions, the name of the one containing more oxygen ends in-ate; the name of the one with less oxygen ends in -ite: Eg.:NO2- nitrite ion, SO32- sulfite ion ,NO3- nitrate ion, SO42- sulfate ionNaming anionsNote: when the series of anions of a given element extends to three or four members,prefixes are also employed. The prefix hypo-indicates less oxygen, and per- more oxygen:Eg: ClO- hypochlorite ion, ClO2- chlorite ion ClO3- chlorate ion, ClO4- perchlorate ion chlor---root of chlorineNaming anionsPractice: selenate ion (?); selenite ion (?) perbromate (?) , hypobromite (?)Note: exceptions to rules: permanganate ion is MnO4-, manganate ion is MnO42-. ferrate-(or perferrate) FeO4-,chromate CrO42-, dichromate Cr2O72-Naming anionsPolyatomic anions with hydrogen ionsThese ions are named by prefixing the word hydrogen or dihydrogen, as appropriate,to the name of the hydrogen-free anion.Alternative way is to use the prefix bi-:Eg.:HCO3- hydrogen carbonate (or bicarbonate ) ion; HSO4- hydrogen sulfate ( or bisulfate) ion; H2PO4- dihydrogen phosphate ionCommon ionsAnions: acetate, azide, bromide, chlorate,chloride, cyanide, dihydrogen phosphate,fluoride, hydride, hydrogen carbonate or bicarbonate, hydrogen sulfate or bisulfate, hydroxide, iodide, nitrate, nitrite, perchlorate, permanganate, thiocyanate, cyanate. (1- ions);carbonate, chromate, dichromate, ferrate,hydrogen phosphate, oxide, peroxide, sulfate,sulfide, sulfite, thiosulfate.(2- ions); nitride,phosphate, phosphide. (3- ions).Naming ionic compoundsWrite the formulas for ionic compounds by combining the names of cations and anions:barium bromide- BaBr2copper(II) nitrate or cupric nitrate- Cu(NO3)2aluminum oxide-Al2O3mercury(I) chloride or mercurous chlorideHg2Cl2ferric oxide, Fe2O3Practice : Name the following compounds:(a)K2SO4;(b) Ba(OH)2;(c) FeCl3 (d) NH4Cl;(e) Cr2O3;(f)Co(NO3)2Write the chemical formulas for the following compounds:(a)calcium carbonate; (b)sodium fluoride; (c) iron(II) perchlorate; (d)magnesium sulfate; (e) silver sulfide; (f) lead nitrate.Naming AcidsAn acid here is defined as a substance whose molecules yield hydrogen ions (H+) when dissolved in water.Rule : The name of an non-oxyacid is related to the name of the anion. Anions with the ending -ide associate with acids having hydro- prefix and an -ic ending:Eg:Chloride (Cl-) to hydrochloric acid (HCl) sulfide (S2- ) to hydrosulfuric acid (H2S)Note: only water solution of HCl is called hydrochloric acid, the pure compound is called hydrogen chloride.Naming the acidsFor acids derived from oxyanions (oxyacids)Rule: If the anion has an –ate (-ite) ending,the corresponding acid is given an –ic (-ous) ending. Prefixes are retained:Naming the acidsExercisesName the following acids:(a) HCN, HSCN; (b) HNO3, HNO2 (c) H2SO4 (d) H2SO3Give the chemical formulas for(a)hydrobromic acid; (b) phosphoric acid.Naming molecular compoundsRule:The procedures for naming binary (two-element) molecular compounds are similar to those for naming ionic compounds. The element with the positive nature is named first and also appears first in the chemical formula. The second element is named with an–ide ending.Eg.:HCl hydrogen chlorideNaming molecular compoundsPrefixes are used in differentiating several binary compounds formed between nonmetals.Eg:CO -carbon monoxide CO2-carbon dioxideMeaning of the Greek prefixes:mono- (1); di- (2); tri-(3); tetra-(4); penta-(5);hexa-(6); hepta-(7); octa-(8); nona-(9); deca-(10)Naming molecular compoundsNote: when the prefix ends in a or o and the name of the anion begins with a vowel (such as oxide), the a or o is often dropped. The prefix mono- is usually omitted for the first-named element.Eg.:Cl2O - dichlorine monoxide; NF3- nitrogen trifluoride; N2O4- dinitrogen tetroxide; P4S10- tetraphosphorus decasulfide;ExercisesName the following compounds:(a) SO2; (b)PCl5; (c)N2O3Give the chemical formula for(a) silicon tetrabromide (b) disulfur dichlorideExercises for ReviewSodium fluoride, magnesium bromide,hydrogen iodide, sodium azide , calcium phosphide,copper(I) chloride, potassium azide,manganese (IV) oxideK2SO3, Ca(MnO4)2 , Ba3(PO4)2, H3PO4 ,H2SO4 ,HNO3 , ZnO , BaO2 ,FeO ,CuSO4•5H2O , Mn3(PO4)2Metaphosphoric acid, phosphoric acid,hypophosphorous acid, phosphorous acid,(hypo)phosphite, (meta)phosphateammonium acetate, perbromic acid , potassium nitrite, sodium peroxide , ammonium dichromate ,sodium carbonate , silver nitrate , aluminum acetate, hydrosulfuric acid, sulfurous acid, perferric acid, perferrate ion, hypoiodite ion,iodic acid , chlorous acid, hydrochloric acidB2O3, SiO2, PCl3,SiCl4, BrF3, IBr,N2S5, PCl3, SiS, S4N2Exercise: learning for useWhen ammonium thiocyanate and barium hydroxide octahydrate are mixed at room temperature,an endothermic reaction occurs.(write the chemical equation). As a result of this reaction, the temperature of the system drops from about 20°C to -9 °C.The reaction of powdered aluminum with ferric oxide (known as the thermite reaction) is highly exothermic. Once started, the reaction proceeds vigorously to form aluminum oxide and molten iron. (write the chemical equation)Nomenclature for Organic Compounds and GroupsWhy Do We Need a Separate Set of Rules?Examine some typical organic compounds (Name these using typical covalent rules)CH4:Carbon tetrahydride C2H6:Dicarbon hexahydrideThat wasn’t so bad, right?How about these:C4H10:Tetracarbon decahydride C5H12:Pentacarbon hydrideSee my point?Memorizing too many prefixes for large numbersIsomers:If that’s not enough, how about this one:Rules•Identify the longest unbranched chain of carbons•Name it as normal•Identify the branch•Name it but give it a “–yl”suffix•Put the names of all branches first, then put name of longest chain•Put the number of the carbon the branch is on (start numbering from the closest single end) Nomenclature for saturated hydrocarbonsa.Alkanes(CnH2n+2烷烃)---+ anefor n<=4:methane (甲烷), ethane(乙烷),propane (丙烷), butane (丁烷).Alkanefor n>4 , for normal alkanesA Greek prefix + ane suffix (if “-aa-”, drop one “a”)5 pent(a)-,6 hex(a)-,7 hept(a)-,8 oct(a)-,9 non(a)-,10 dec(a)-, 11 undec(a)-, 12 dodec(a)-, 13 tridec(a)-,14 tetradec(a)-, 15 pentadec(a)- 16 hexadec(a)-,17 heptadec(a), 18 octadec(a)-, 19 nonadec(a)-,20 eicos(a)-, 22 docos(a)-, 24 tetracos(a)-, 30triacot(a)-, 36 hexatriacot(a)-Eg:nonadecane; C19H40 decanedithiol; HS-C10H22-SHAlkanesn >4 , alkanes’main chain with branchesThe position and name of branch groups are added as prefixes to the name of the longest hydrocarbon chain. Use Greek prefixes to indicate the number of repeated branch group.2-chloropentane, 2-methylbutane2,2,4-trimethylpentane (or isooctane)1-chloro-3,3-dimethylpentane2-methylpropane (or isobutane)Cyclo’alkanes, CnH2n (naming rule similar to the above-mentioned) + add cycloStart numbering from the most “important”branch in the ringSupplementsPrimary, secondary, tertiary, quaternary carbon atom.Normal hydrocarbon: n-hexane or s of some groups derived from alkanes by replacing “ane”with “yl”:Eg.:methyl, ethyl, n-propyl, isopropyl, n-butyl, n-pentyl, cyclopropyl, cyclobutyl, cyclohexylPracticeDraw the structural formula for each of the following compounds(1) 3-hexyne(2)1,3-pentadiene(3)cyclcobutene(4) 3, 4-diethylhexane(5) 1-butyneNomenclature of aromatic hydrocarbonsThe name of a single substituent is added to benzene as a prefix. Three structurally isomeric forms are designated ortho (o-), meta (m-), and para (p-).Eg:ethylbenzene, p-nitrobenzoic acid, hexachlorobenzene, toluene, m-xylene.group: phenyl-diphenylmethane.Nomenclature for polycyclic aromatic hydrocarbonsPolycyclic aromatic hydrocarbonsEg:Nomenclature for functional groupsHalo (-X), hydroxyl (-OH), amino (-NR3. primary: RNH2,secondary: R2NH, tertiary: R3N), formyl (-CHO),carbonyl (-CO-), carboxyl (-COOH), a’mido (-CONH2),carbonyl halide (-COX), an’hydride (-COOCOR),ester(-COOR), nitro(-NO2), nitroalkane (R-NO2),sulfonic acid (-SO3H), cyano (-CN)Functional groups with covalent single bondsAlkyl (烷基)and aryl (芳基)halides (RX)Eg:Methyl bromide (bromomethane),methyl iodide (iodomethane), ethyl bromide(bromoethane), propyl bromide (1-bromopropane),propylene dibromide (1,2-dibromopropane), vinyl chloride (chloroethene), chlorobenzene.Functional groups with covalent single bondscommercial name:methyl alcohol ( 甲醇) , ethyl alcohol (乙醇), propyl alcohol (1-propanol), ethylene glycol (乙二醇),glycerol (甘油), isopropyl alcohol (异丙醇)Functional groups with covalent single bondsNomenclature for functional group with double bondsEsters (RCOOR’)in the two-word name for an ester, the first word is the name of the R’group (methyl, ethyl, …), the second word is the name of the carboxylic acid with the final -c ic replaced by –ate (回忆无机盐的命名)methyl formate (methanoate), methyl acetate (ethanoate), ethyl benzoateNomenclature for functional group with double bonds。
