某公司已过新版GMP认证洁净厂房验证模版(中英文)
EUGMP-中英文对照[1]
![EUGMP-中英文对照[1]](https://img.taocdn.com/s3/m/ed974fef59eef8c75ebfb3e2.png)
EU GMP ANNEX 1 MANUFACTURE OF STERILE MEDICINAL PRODUCTS (中英文对照)(a) These are average values. (一)这些都是平均值。
(b) Individual settle plates may be exposed for less than 4 hours. (二)单个沉降皿放置的时间可以少于4小时。
20. Appropriate alert and action limits should be set for the results of particulate and microbiological monitoring. If these limits are exceeded operating procedures should prescribe corrective action。
对尘埃粒子和微生物的监控结果,要设置适当的警戒限度和行动限度。
当超出这些限度时,操作规程应说明需要采取的措施。
Isolator technology 隔离技术21. The utilisation of isolator technology to minimize human interventions in processing areas may result in a significant decrease in the risk of microbiological contamination of aseptically manufactured products from the environment. There are many possible designs of isolators and transfer devices. The isolator and the background environment should be designed so that the required air quality for the respective zones can be realised. Isolators are constructed of various materials more or less prone to puncture and leakage. Transfer devices may vary from a single door to double door designs to fully sealed systems incorporating sterilization mechanisms. 在生产区采用人员方面的隔离技术,在无菌产品的生产中,会显著降低周围环境微生物污染的风险。
制药验证文件-洁净空压机验证方案(英文版)

Qualification Protocol for New Air Compressor (COMP04)1. Short Description of Project / Facility / System1.1 Project DescriptionAs part of the “Extension of Production Facility, ChangPing” Project (EPCP, No. CNPHCP200502), a new Air Compressor COMP04 will be installed in the existing utilities area for the complementary production of compressed air.For general information, reference is made to:EPCP_11_06_INFRA_URS (current version)●INFRA URS,EPCP_01_00_HLD_URS (current version)●Project URS,EPCP_01_00_HLD_QMP (current version)●Project Qualification Master Plan,EPCP_01_00_HLD_QNA (current version)●Qualification Need Assessment,●Functional Risk Assessment EPCP_11_03_CA_FRA_2.0EPCP - CR 0238●Change request (new compressor)1.2 Functional Description for the ZT compressor with IMD dryer.NB. For the complete functionality of the CA system refer to the functional specification EPCP_11_03_CA_FS in its current version.Air drawn in through air filter (AF) and the open inlet valve of the unloader assembly (UA) is compressed in the low-pressure compressor element (El) and discharged to the intercooler (Ci).The cooled air is further compressed in the high-pressure compressor element (Eh) and discharged through the pulsation damper (AS) and the after cooler (Ca).A check valve (CV) is provided downstream of the pulsation damper (AS).When the compressor switches to unload operation, the air trapped in the pulsation damper and the high-pressure element is blown off via the blow-off silencer (US).The check valve (CV) prevents compressed air downstream the check valve to be blown off.Flow diagramAF Air filter (1) IMD bypass valve M4 Gear motor, IMD AS Pulsation damper (2) IMD air inlet valve EWD ElectronicCV Check valve drain, inlet airUS Blow-off silencer (3) DemisterCa After cooler with integrated drain (4) Nozzle Car Regeneration air cooler, IMD collector (5) IMD outlet valve FN Fan, regeneration air IS Inlet silencer cooler to air outlet valve (customer’sUA Unloader assembly installation)EWDa Electronic drain, after cooler (6) Rotor, IMDOP Oil pump (7) Regulating valve regeneration airCi Intercooler with integrated drain collectorGC Oil sump (gear casing)EWDi Electronic drain, intercoolerCo Oil coolerEh High-pressure compressor elementOF Oil filterEl Low-pressure compressor elementFN1 Cooling fan (ZT)1.3 Main emphasis on quality critical issues1.3.1 GMP Risk AssessmentThe air compressor installation will be in compliance with the valid Project Qualification Master Plan, Doc. No. EPCP_01_00_HLD_QMP, EPCP_11_03_CA_FRA in their current version.1.3.2 Critical measurement instrumentsInstruments for : Operating pressure, Dew point and Temperature.1.3.3 Critical items/functionsSpecification for construction material of operational parts.Air drying process.1.3.4 Qualification strategyThe air compressor qualification is covered by the following documents:URS,GMP and Functional Risk AssessmentQualification Protocol (QP)DQ, IQ & OQQualification Report (QR).Notes:1. No separate CSV Qualification Protocol and Qualification Report are needed since itis a Class 4 system.2. A 21CFR Part 11 Risk Assessment is not requested because the control system is notable to store any data.3. The air compressor and its auxiliaries will be commissioned with the support of aVendor Engineer.5. T he Qualification (DQ/IQ/OQ) will be prepared by BNP’s own Engineers, based onthis QP. The Qualification Documentation will be prepared by Luwa.6. The calibration during IQ will be executed by the vendor technician supervised byBNP R&M engineers.7. The qualification strategy and tests scope will be based on the qualification of theexisting air compressor (same supplier, same type). No new GMP and Risk Assessment will be prepared.2. Steps covered in this ProtocolFor the Qualification of the air compressor, the present QP will be followed. The qualification documents for the air compressor will be created (DL and DQ, IQ, OQ) and will be filed with this qualification.NB: This Qualification plan does not include interfacing utilities. For information about the qualification of the CA system, see EPCP_11_03_CA_QP in its current version.Design Qualification (DQ): yes: no: NA: Commissioning (GEP) 1): yes: no: NA: Installations Qualification (IQ): yes: no: NA: Operational Qualification (OQ): yes: no: NA: Operational Qualification (PQ) 2): yes: no: NA:1) Only FAT and correct installation before IQ (SAT)2) PQ will be done by checking the Pressure and microbial test at the exhaust of the new air compressor. No water content testing will be made as the dryness and oil free status is certified by the manufacturer.Copies of the certificates will be filed in the qualification binder.3. Documentation3.1 Qualification Documents List (GPE_FRM_511_0071)Refer to “Qualification Document List for the air com pressor, EPCP_11_03_CA_COMP04_DL” in its current version.3.2 Project Specific SOP’sNo project specific SOPs for the commissioning execution of this new air compressor.3.3 Administration of DocumentsThe Documents are filed according to “Document Management Syst em:Doc. No EPCP_01_00_HLD_DMS” in its current version.4. Basics4.1 Basic SOP’sGPE_SOP_508_0484: Concept for Commissioning and QualificationEPCP-SOP-001: CommissioningGPE_SOP_511_0042: Specification, Execution and Deviation Resolution of TestsGPE_SOP_511_0030 Handling of Changes (Engineering Projects)4.2 Project Specific AgreementsTwo types of training activities will be conducted one training for qualification execution and an other training for operation and maintenance.Qualification execution training is mandatory for all persons taking part in the qualification work. The training will be conducted by Luwa, and each test person shall complete the training before the empowerment to execute qualification and commissioning activities. Operation and maintenance training will be conducted by the EPCP or the vendor. Luwa operators, engineers or technicians should complete the training based on the functional requirements.Luwa Persoamal Training Record (FRM_000037) and Training Record (FRM_000039) will be used to document the training.5. Qualification Activities (Action Plan)5.1 Project Time ScheduleRefer to project time schedule in its current version.5.2 Qualification Activities (included in Project Time Schedule)All qualification activities are summarized in “Section 1.3 Main emphasis on quality critical issues”, “Section 3.1 Qualification Documents List”.6. Organization / Responsibility6.1 Qualification OrganizationRefer to “Qualification Master Plan, EPCP_01_00_HLD_QMP” for responsibilities matrix. 6.2 Project OrganizationThe organization is within the current EPCP organization chart in PPM.7. Attachments to ProtocolAttachment 1 Qualification Document List for air new compressor,EPCP_11_03_CA_COMP04_DL in its current version.。
欧盟GMP附录15确认和验证中英文新版

欧盟GMP附录15确认和验证欧盟GMP附录15确认和验证ANNEX 15 附件15Qualification and Validation确认和验证Table of Contents 目录1. Qualification and Validation 确认和验证2. Planning for Validation 验证计划3. Documentation 文件4. Qualification 确认5. Process Validation 工艺验证6. Cleaning Validation 清洁验证7. Change Control 变更控制8. Revalidation 再验证9. Glossary 术语表Qualification and Validation 确认和验证Principle 原理1.This Annex describes the principles of qualification and validation which are applicable to the manufacture of medicinal products. It is a requirement of GMP that manufacturers identify what validation work is needed to prove control of the critical aspects of their particular operations. Significant changes to the facilities, the equipment and the processes, which may affect the quality of the product, should be validated. A risk assessment approach should be used to determine the scope and extent of validation.1.本附件描述了确认和验证的原理,适用于医药产品的生产者。
GMP规范中英文对照

GMP规范中英文对照Chapter 1: General Provisions第一章总则Article 1: This Regulation is enacted in accordance with the "Drug Administration Law of The People's Republic of China".第一条根据《中华人民共和国药品管理法》规定,制定本规范。
Article 2: This Regulation is promulgated as the basic guideline for manufacturing and quality control of pharmaceutical products. This Regulation shall be applicable to all the manufacturing processes of drug preparations and to the key manufacturing processes of raw materials which may cause variation in the quality of finished products.第二条本规范是药品生产和质量管理的基本准则。
适用于药品制剂生产的全过程、原料药生产中影响成品质量的关键工序。
Chapter 2: Organization and PersonnelArticle 3: A pharmaceutical enterprise shall establish production and quality control departments. The responsibilities of departments at all levels and personnel shall be clarified, and each department shall be staffed by an appropriate number of management and technical personnel with expert knowledge, manufacturing experience and organization ability. 第三条药品生产企业应建立生产和质量管理机构。
GMP洁净厂房验收合规性验证方案(依洁净厂房设计规范编制)

× × × × × X 有限公司No.GMP-3-20220301洁净厂房设施验证方案(依照GB50457/GB50591标准编制)另附1篇洁净厂房和设施的验证(第26页)编制人: ***日期: 2022-3-28审核人: ***日期: 2022-3-28批准人: ***日期: 2022-3-28工程部发布验证小组人员名单1. 主题内容 (4)2.验证目的 (4)3. 验证的范围 (4)4.概述 (4)5.职责 (4)6.验证内容 (5)6.1厂房设计的确认 (5)6.1.1..................................................................................................... 厂房周边环境的确认 (5)6.1.2..................................................................................................... 厂房内一般生产区、洁净室(区)的设计确认 (6)6.1.3..................................................................................................... 资料档案 (9)6.2厂房设施的安装确认 (11)6.2.1..................................................................................................... 厂房的结构确认 (11)6.2.2..................................................................................................... 装修材料的确认 (12)6.2.3..................................................................................................... 设施的确认 (14)6.2.4..................................................................................................... 空气净化系统的确认 (15)6.2.5..................................................................................................... 公用工程的确认 (16)6.2.6..................................................................................................... 给、排水系统的确认 (18)6.3厂房设施的运行确认 (19)6.3.1..................................................................................................... 检测用仪器仪表的确认 (19)6.3.2..................................................................................................... 厂房设施运行确认 (19)6.3.3..................................................................................................... 厂房设施的性能确认 (20)7.再验证 (22)8.验证结论及评价 (22)9.验证证书 (22)10.附录 (23)附录1:厂区总平面布置图(图略,请根据企业实际自行添加,下同) (24)附录2:厂区常年风向图 (24)附录3:厂区人流、物流图 (24)附录4:生产区工艺设备平面布置图 (24)附录5:洁净区工艺设备平面布置图 (24)附录6:设备平面布置图 (25)附录7:洁净区送审图 (25)附录8:施工完成后实际平面布置图 (25)附录9:洁净区施工图 (25)洁净厂房设施验证方案1.主题内容本方案规定了X X X公司TU-5车间洁净厂房与设施验证的具体要求。
洁净厂房及空调验证报告(模板)

XXXXXX有限公司验证报告编号:VD-001洁净厂房及空调验证方案起草人:检验员日期:审核人:质量部经理日期:批准人:质量部经理日期:目录1. 目的 (3)2. 描述 (3)2.1 确认背景及车间基本情况 (3)2.2 确认依据 (3)2.3 确认计划 (3)3. 范围 (3)4. 确认小组及职责 (4)5. 确认内容及标准 (4)5.1 安装再确认 (4)5.2 运行再确认 (9)5.3 性能再确认 (10)6. 确认结论 (10)7. 再确认周期 (10)8. 验证报告 (11)1. 目的对洁净厂房及空调进行确认,对其安装、运行、性能技术参数进行测试,确认厂房设施性能的可靠性。
确认洁净厂房及空调符合设计要求,符合医疗器械生产质量管理规范及YY0033-2000的要求,能满足产品的生产需要。
2. 描述2.1 再确认背景及车间基本情况本公司主要生产医用一次性口罩,洁净厂房由东莞市德长发净化设备有限公司设计、施工。
十万级洁净室面积1663.5m2,分别有更衣室、缓冲间、生产车间、內包间、洁具间、洗衣房、工位器具房等功能间,由空气净化系统对各功能间进行空气净化。
车间平面图见附件1。
纯化水采用二级反渗透制备,通过不锈钢304管道送至各使用水点。
排水采用不锈钢洁净地漏排放,避免污染。
动力管线穿越吊顶,采用硅胶密封。
围护结构、隔断和吊顶采用彩钢板,门、窗、墙角均采用优质铝合金材料。
联接处用圆弧型,易于清洁。
根据需要,有关功能间装有微压差计和温湿度仪,便于日常运行监控。
2.2 再确认依据2.2.1 GB 50243 通风与空调施工及验收规范2.2.2 GB 50073 洁净厂房设计规范2.2.3 GB 50457 医药工业洁净厂房设计规范2.2.4 GB 50591 洁净室施工及验收规范2.2.5 YY 0033 无菌医疗器具生产管理规范2.2.6 GB/T 16292 医药工业洁净室(区)悬浮粒子的测试方法2.2.7 GB/T 16293 医药工业洁净室(区)浮游菌的测试方法2.2.8 GB/T 16294 医药工业洁净室(区)沉降菌的测试方法2.2.9 医疗器械生产质量管理规范2.3 确认计划2020年03月01日至2020年03月23日3. 范围洁净厂房及有关设施。
洁净空调操作验证-功能测试(双语版)
Main Functions test
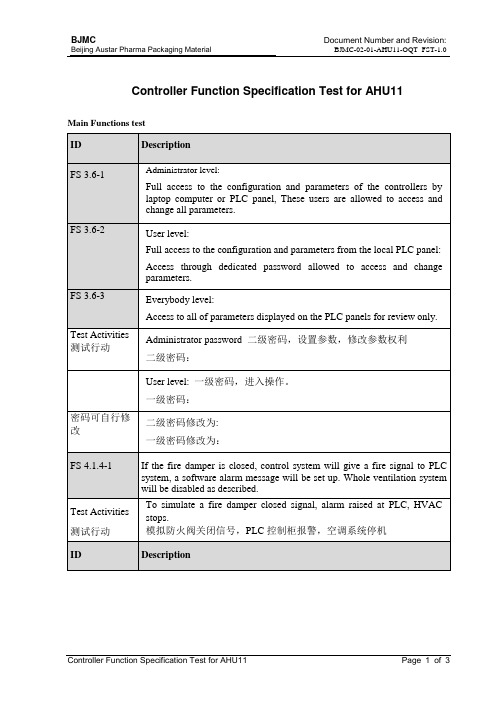
ID
Description
FS 3.6-1
Administrator level:
Full access to the configuration and parameters of the controllers by laptop computer orPLCpanel,Thess andchangeall parameters.
ID
Description
FS 4.2.1.1-1
The AHU start-up manually. And all system air dampers are controlled by hand.
FS 4.2.1.1-2
The exhaust fan start-up manually. And it only cans start-up at rest.The supply fan should be started firstly when the AHU system start.
二级密码:
User level:一级密码,进入操作。
一级密码:
密码可自行修改
二级密码修改为:
一级密码修改为:
FS 4.1.4-1
If thefiredamper is closed,controlsystem will give a fire signal toPLCsystem, a software alarm message will be set up. Whole ventilation system will be disabled as described.
洁净空调系统设计验证文件清单(英文版)

HVAC/AHU11
Document Code
BJMC_02_01_AHU11_DQT
Revision
1.0
Approval of Test Scope (Continueon the next page)
Name
Signature Reason
Department / Function
The alarm list is approved by discipline engineers in correct version.
Version No.:______
Approval of Test Results
Name
Signature Reason
Department / Function
Version No.:______
1.4
Sensor risk assessment check;
BJMC_02_01_AHU11_SENRA
Check that the sensor risk assessment is approved by discipline engineers, ensure the correct version.
Check that the function specification is approved by discipline engineers, ensure the correct version
The function Specification is approved by discipline engineers in correct version.
Date
Signature
Mark Hu
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Clean Room
Installation Qualification Protocol
洁净室
安装确认方案
System No. 系统编号: CLR-01
Index 目录
1. PURPOSE目的 (3)
2. SCOPE范围 (3)
3. RESPONSIBILITY职责 (3)
4. REGULATION AND GUIDANCE 法规和指南 (3)
5. ABBREVIATIONS缩略语 (3)
6. SYSTEM DESCRIPTION 系统描述 (3)
7. GOOD DOCUMENTATION PRACTICE文件管理规范 (5)
8. TEST LIST测试列表 (6)
9. PERSONNEL IDENTIFICATION人员确认 (6)
10. PROCEDURE过程 (7)
10.1 先决条件 (7)
10.2 文件确认 (9)
10.3 图确认 (10)
10.4 房间组件检查 (13)
10.5 仪器仪表校验 (15)
10.6 洁净室建造装修检查 (17)
10.7 电器安装检查 (23)
11. DEVIATION REPORT偏差报告 (30)
1. Purpose目的
本安装确认方案的目的是测试、检查和洁净室是按照相应设计要求和供应商的建议进行安装的。
安装确认的测试和检查的结果将按照该验证方案进行记录。
安装确认将确定直接影响系统的关键部件被正确地安装,并符合设计文件需求;确定支持文件、质量文件在现场。
测试和检查的结果将按照该验证方案进行记录。
2. Scope范围
本方案确定了***********公司口服固体项目车间的洁净室(位号:***********)的安装确认。
3. Responsibility职责
4. Regulation and Guidance 法规和指南
(SFDA) GMP 2010版
中国药典 2010版
现行版ISPE指南5“调试和确认”
洁净厂房设计规范GB13554-92
5. Abbreviations缩略语
6. System Description 系统描述
口服固体制剂的洁净室包括以下级别
D级区
非关键生产步骤的洁净区
对产品无影响的房间无需验证。
7. Good Documentation Practice文件管理规范
记录用笔:
- 使用不消退的墨水笔和记号笔,推荐使用蓝色笔记录
签名:
- 被授权的人员才能签署文件
- 应签全名,除非文件另有规定
- 签名应该是可辨认的
- 签名应始终一致
填写栏目:
- 所有栏目必须填写
- 填写内容与上面栏目相同应重新填写
- 若有单个栏目不需要填入内容,则在空白处填写英文字母“不适用”的简写“N/A”,以表示无此项内容。
- 填写记录时,若有多个栏目不需要填入内容,应用斜线划掉,斜线上方填写“N/A,下方签名和注明日期。
签名及日期应尽量沿斜线同侧填写。
更改错误:
文件刚完成,立即更改的
在错误处划线,填入正确的,签名和注明更改日期,确保原先信息仍清晰可识别
如:2010年01月01日签字,日期
事后更改的,除非立即更改的要求外,还应注明更改的原因,检查和注释可能的
影响。
记录日期:
- 年用4位数表示,日和月用2位数表示
如:2013-10-08日
- 使用缩略语:
- 在术语全称后的括号内注明缩写,然后才可以使用缩写。
- 书面语及名称:
- 使用规范的书面语及名称
- 文件前后名称要一致
8. Test List测试列表
在下面的表格列出了本方案将要执行的测试。
9. Personnel Identification人员确认
在安装确认开始前,本方案涉及的所有人员必须在下表签字。
10. Procedure过程
10.1 先决条件
目的
为了保证验证活动的连续和一致性,安装确认之前必须检查验证条件是否满足
确认参与此方案的人员都已经过培训,熟悉此方案的内容。
可接受标准
开始执行本测试之前,所有的先决条件必须得到满足。
程序
2.人员培训
在IQ开始前,对所有参与测试的人员进行IQ方案的培训。
培训应该有记录。
培训要确保测试人员熟悉本方案,能够准确地执行本方案而不会产生错误。
结论/备注
10.2 文件确认
目的
核实用于系统安装、运行、维修所需文件的有效性、可读性和完整性。
可接受标准
所有的供应商文件都是有效的、完整的,并且都是可读的。
供应商文件的语言符合业主的要求。
所有检查的图纸都应该是最终版,标有“竣工”标记。
程序
检查文件是有效、完整,且可读。
结论/备注
10.3 图确认
目的
确认房间布局与设计要求一致,且是竣工版。
可接受标准
房间布局与竣工图纸一致。
程序
检查图纸是否是竣工版。
复制一份P&ID图,按照图纸检查设备安装。
洁净灯、开关、电话、门禁和地漏均按照P&ID图纸安装;用绿色笔标注与设备相符的部分;
用红色笔标注与设备不相符合的部分并记录偏差;
附上检查过的P&ID图,并签上姓名和日期。
结论/备注
10.4 房间组件检查
目的
确认房间的关键部件与已批准的设计文件一致。
可接受标准
对着本方案、材料清单、报价单或其它可以索引的的文件(P&ID等),检查现场真实的安装情况程序
检查设备(竣工)符合要求。
任何符合竣工要求的部件都要标上“ok”,并签上名字日期。
结论/备注
10.5 仪器仪表校验
目的
确认所有关键仪表的有效性。
可接受标准
所有的关键仪表均已校验,且在有效期内。
程序
在下表中列出所有系统和验证时使用的仪器,并附上校验证书。
如果需要,可以加页。
结论/备注
10.6 洁净室建造装修检查
目的
本次测试主要检查洁净室建造和装修是否满足生产的需要。
可接受标准
施工规范都已完成。
天花板:
表面平整易清洁
硅胶气密
能载人
风口安装:
位置准确,与板材密封
易清洁
地面
平整易清洁
地漏易清洁
墙面,门窗
平整易清洁
程序
在观察情况栏中记录设备或系统相关测试结果
结论/备注
10.7 电器安装检查
目的
本次测试主要检查洁净室的电器设备是否正确安装。
可接受标准
施工规范都已完成。
开关:
易清洁
位置与竣工图一致
电话:
表面平整易清洁
位置与竣工图一致
门禁
平整易清洁
位置与竣工图一致
洁净灯
平整易清洁
位置与竣工图一致
程序
获取一份电器竣工平面图
在观察情况栏中记录设备或系统相关测试结果
结论/备注
11. Deviation Report偏差报告
测试过程中发生的任何偏差都将依据XXXXX的验证偏差管理的SOP(编号:***********)处理。
每个偏差都要给予一个唯一的编号并记录在偏差报告里。
在偏差清单中,汇总所有的偏差。
如有需要可复印偏差报告。
