Lecture7.ppt
合集下载
Lecture 7_opening para_topic 2.ppt

Lecture Seven
Beginning sentences in opening paragraphs
Related topic 2: Problem and phenomenon
2016/7/10
School of Foreign Studies
1
Beginning sentence 1
Recently the rise … has drawn public attention Recently the rise … has caused wide concern Recently the rise … has arisen as controversial/as noteworthy.
the rise in phenomenon/problem/question of… has aroused popular/grave/world-wide attention has aroused general/considerable/international concern has arisen/loomed up/cropped up as controversial/as noteworthy/more distinctly for settlement.
2016/7/10 School of Foreign Studies 2
Beginning sentence 2
Recently
the issue of …has been in the limelight.
Recently the issue (problem/question) of … has been in the limelight has been brought into focus has been brought to public attention has posed among the general public.
Beginning sentences in opening paragraphs
Related topic 2: Problem and phenomenon
2016/7/10
School of Foreign Studies
1
Beginning sentence 1
Recently the rise … has drawn public attention Recently the rise … has caused wide concern Recently the rise … has arisen as controversial/as noteworthy.
the rise in phenomenon/problem/question of… has aroused popular/grave/world-wide attention has aroused general/considerable/international concern has arisen/loomed up/cropped up as controversial/as noteworthy/more distinctly for settlement.
2016/7/10 School of Foreign Studies 2
Beginning sentence 2
Recently
the issue of …has been in the limelight.
Recently the issue (problem/question) of … has been in the limelight has been brought into focus has been brought to public attention has posed among the general public.
Lecture 07 最小值原理

H (xf , λf ,u f ) = 0
若能知道λ (0)的值,就能根据协态方程解得最优控制
λ (t ) = exp(− AT t )λ0 , u * = − sgn( BT exp(− AT t )λ0 )
如果λ T b j ≠ 0, 可以确定最优控制 u * = − sgn(λ T b j ); j 如果λ T b j = 0, 则不能确定u *。 j 如果λ T b j = 0只在t的离散点上成立,则在这些点上u *作边界 j 值的切换; 如果λ T b j = 0在某一段时间间隔成立,这时无法确定u *j的值
5.1 最小值原理
系统运动方程 & x = f ( x, u, t ) 式中:x ∈ Rn − 状态变量;u ∈ Rm − 控制变量;t ∈[t0 , t f ] − 时间变量。 给定系统的初始时刻和初始状态 x(t0 ) = x0 系统的终端时刻和状态满足r个约束方程 n( x(t f ), t f ) = 0 控制变量满足若干不等式约束 g (u, t ) ≤ 0 最优控制问题的性能指标为 J = θ ( x(t f ), t f ) + ∫ φ ( x, u, t )dt
关于判别线性定常系统最优控制问题的正规性和奇异性, 有以下定理。 & 定理7-1 对于线性定常系统x=Ax+Bu 快速最优控制奇异的充要条件是,m个矩阵 Uj = [b j Ab j A2b j K An −1b j ], j = 1, 2,K , m 中,至少有一个是奇异的。 定理7-2 定理7-3ห้องสมุดไป่ตู้对于线性定常系统,快速最优控制正规的充要条件是: 对于正规快速最优控制问题,其最优控制律u*的每一个 m个矩阵Uj全部是非奇异的。
(1)快速最优控制的正规与奇异问题 定义7-1 若所有函数λ T b j 在时间间隔[0,tf ]上只存在有限个零点,则 对应的快速最优控制问题成为正规的。 定义7-2 如果对所有j=1,2, ,m,至少存在一个λ T b j函数在某一段时间 K 间隔[t1 ,t2 ]∈[0,tf ]上的取值为零,则对应的快速最优控制问题是奇异 的,并称时间间隔[t1 ,t2 ]为奇异段。 对于正规快速最优控制问题,完全能确定最优控制律u* ,即每个控制分量 u*均在边界值之间切换,且切换点就是λ T b j =0的时刻。这种控制方式成为 j “乒乒控制”。 对于奇异快速最优控制问题,在奇异段[t1 ,t2 ]上不能确定最优控制律,但 不表明最优控制u*不存在。因为在奇异段u*上,H函数与对应的u*无关,u* j j 可以取任意容许值,仍能满足最小值原理。此时快速最优控制不再具有 “乒乒控制”形式
lecture 7---NOESY
H
H
NO2
H 3C S
NO 2 H 3C S
H
7 .9 8
NO2
H 3C S
6.29
H
NO 2 H 3C S
2D NOESY
2D NOESY
8 - 7, 12 7 - 18, 18' 3 - 5, 10 5 - 11, 16, 18'
9 - 10, 17, 17'
10 - 16 11 - 18, 16, 14, 18' 18 - 13, 18' 16 - 14, 17 13 - 14, 17, 17' 13' - 17, 17' 17 - 17'
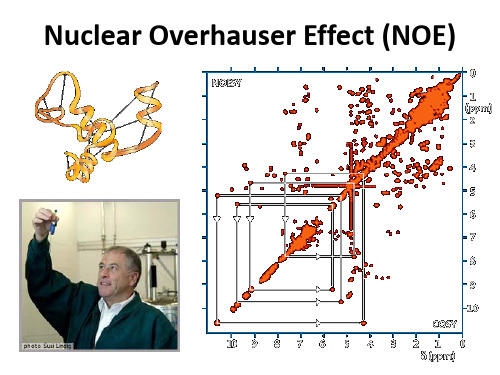
当两个核AX在空间上很接近时,可以通过偶极--偶 极作用相互影响。选择A的频率照射,会影响X的能级 分布,从而改变其信号强度。
Nuclear Overhauser Effect (NOE)
bb
ba X2 A1 单量子数驰豫W1不产生NOE效应。 A2 ab X1 双量子数驰豫W2和零量子数驰豫W0 W2
2D NOESY
2D NOESY
2D NOESY
TOCSY (solid lines) and NOESY (dotted lines)
AVANCE 900
2.0 mM Lysozyme NOESY
Nuclear Overhauser Effect (NOE)
NOE实验对环境变化非常敏感,为了得到高质量的谱图, 有如下建议
NMR
PBu3 PiBu3 -32.5 ppm -45.3 ppm
PsBu3
PtBu3
7.9 ppm
63 ppm
I=1/2 Natural abundance Reference compound 100% 85% H3PO4 in H2O = 0 ppm 6.67 × 10-3 37.7
lecture7-memory

部分报告法实验
• 实验结果表明,在全部报告法实验中,被 试看到的或能记住的字母确实要比被被试 报告出来的多。
• Sperling(1960)证实了感觉记忆的存在, 这样两种记忆说得以发展,出现了多存贮 模型,或说信息三级加工模型。
2、证明图像记忆的特性实验(了解)
• 1、sperling(1960)改变声音信号延迟的 时距p76 • 2、Erikson&Collins(1967)点模式实验 p77
• 3、动物实验——老鼠电休克
2、自由回忆实验(Free recall )
• Murdock(1962)让被试听一个由30个词构成的 词表,每次呈现一个词,每次1秒,然后进行自由 回忆,根据结果得到一条系列位置曲线。
•
结论
• 系列位置曲线(the serial position curve)。 • 系列位置曲线可以分为3 个部分: – 近因效应(recenty effect) – 首因效应(primary effect) – 渐进线(asymptote)
Cognitive Psychology Memory
ZHOU HENG
记忆关注的问题
• • • • • 我们的记忆是怎么发生的? 记忆的不同阶段分别是什么,有什么作用? 哪些因素影响我们成功创建新的记忆? 记忆的遗忘是如何发生的? 记忆为什么会说谎?
本章关键名词
• • • • • 两种记忆说 多贮存说 全部报告法和部分报告法 系列位置效应 感觉记忆
思考题
• 1、 两种记忆说的基本内容是什么?
• 2、 你认为应如何解释系列位置效应? • 3、 感觉记忆有什么特性(特点)?
• 4、 多存贮模型的基本思想是什么?
总结
Lecture7 嵌期权债券

因此, 可赎回债券的价格=不可赎回债券的价格― 赎回期权的价格。 在任意给定的收益率水平下,不可赎回债 券与可赎回债券之间的价格差就是嵌入式 期权的价格。 同样, 可回售债券的价格=不可赎回债券的价格+ 回售期权的价格。
7.3 定价模型
当存在嵌入式期权时,需要计算该期权的价 格,而期权的价格与利率的波动性密切相关, 因而, 当存在嵌入式期权时,就必须考虑利率的 波动性。 常用的利率模型是根据短期利率如何随时 间变化而建立的无套利模型。
r1H : 从现在起 1年后较低的 1年期远期利率 ; r1L : 从现在起 1年后较高的 1年期远期利率 ; r1H r1L e 2
确定节点处债券的价值:
Value of Two-Period Option-Free Bond: C = 8 and F =100
Buu 108
Bu
.5[97.297 8].5[98.630 8] 110 . B0 96.330 B0
第一种债券相当于拥有一只20年期不可赎回债券, 且债券持有人给债券发行人一项赎回期权,使发行 人拥有5年后可以104元的价格赎回15年的现金流权 利。 第二种债券相当于拥有一支10年期不可赎回债券, 且债券持有人出售给债券发行人一项赎回期权,使 发行人拥有以100元的价格赎回合约中规定的现金流, 或债券被赎回时所有剩余现金流的权利。 因此,债券持有人购买可赎回债券相当于进行了两 笔独立的交易:以某个价格从债券发行人那里购买 了不可赎回债券,同时,又向债券发行人出售了一 项赎回期权。
Lecture 7 嵌期权债券
7.1 债券嵌期权概述
常见的债权嵌期权主要有: 赎回权、回售权、 转股权、提前偿付权、本息截留权、利率 上下限选择权等。 期权的嵌入可能影响债券现金流的大小、 方向及时间。利率上下限选择权则将影响 债券适用的利率。
Lecture_7

2
Introduction
• The monetary or fiscal policy may be expansionary or
contractionary, as judged from the effect on the short-run equilibrium output level
It follows that monetary policy has no impact on either the interest rate or the level of income, i.e. the monetary policy becomes powerless. Possibility of a liquidity trap is mentioned by John Maynard Keynes. That possibility had some times become real in some countries.
3
Monetary Policy
• The monetary policy is specifically the government’s
policy regarding the money supply.
The policy may be seen alternatively as to achieve a target interest rate (recall from IS-LM model how interest rate is affected by the money supply)
disequilibrium
– At the prevailing interest rate and level of income, people are holding more money than they want – Portfolio holders attempt to reduce their money holdings by buying other assets → changes asset prices and yields – The change in money supply changes interest rates 2. A change in interest rates affects aggregate demand, e.g.
lecture 7 布里渊区.ppt

Homework
1.
考虑一个ABAB…AB原子线,A-B键长为
a 2
A,B原子的散射因子分别为 f A 和 f B
,入射X射线垂直于原子线。
(1) 给出θ方向(θ是衍射光束与原子线之间的夹角)衍射加
强条件;
(2)计算衍射强度;
(3)讨论
f A f B 情况。
A
B
A
B
a/2
a/2
2. 用波长为λ 的X射线,照射晶格常数为a 的金刚石结构晶体, 问要得到衍射面指数为(220)的衍射斑点,对应的布拉格 角应是多少?
如图2.7所示是一个十二面体。
第一布里渊区种典型 对称点的坐标为:
: 2 (0, 0, 0)
a
H : 2 (1, 0, 0)
a
N : 2 (1 , 1 , 0)
a 22
P : 2 (1 , 1 , 1)
a 222
图2.7 体心立方正格子的第一布里渊区
(5)面心立方结构晶体点阵的布里渊区 取面心立方的原胞基矢为:
布里渊散射条件和布里渊区(Brillouin zone)
1、布里渊散射条件(Brillouin’s diffraction condition )
如图2.4所示是倒空间的二维格子。
r k
r G
1
r G
2ቤተ መጻሕፍቲ ባይዱ
图2.4 倒空间的二维格子
O 点是到空间的原点,考虑连接原点和任意一个倒格点的倒格 矢。作垂直平分线(三维情形将是垂直平分面),如果入射波 矢满足(2.3.2)式,将(2.3.2)式两边同除以4,散射条件 则可写成
对于三维简立方结构晶格点阵来说,其正格子基矢为
r a1
r ai
Lecture 7

• Things unattempted yet in prose or rhyme. --Paradise Lost, Book I, l 16
•
• I may assert eternal Providence, And justify the ways of God to men. • --Paradise Lost, Book I, l 18
•
•
Bending to look on me, I started back, It started back, but pleas'd I soon returnd, Pleas'd it returnd as soon with answering looks Of sympathie and love; there I had fixt Mine eyes till now, and pin'd with vain desire, Had not a voice thus warnd me, What thou seest, What there thou seest fair Creature is thy self, With thee it came and goes: but follow me, And I will bring thee where no shadow staies Thy coming, and thy soft imbraces, hee Whose image thou art, him thou shalt enjoy Inseparablie thine, to him shalt beare Multitudes like thy self, and thence be call'd Mother of human Race
•
• I may assert eternal Providence, And justify the ways of God to men. • --Paradise Lost, Book I, l 18
•
•
Bending to look on me, I started back, It started back, but pleas'd I soon returnd, Pleas'd it returnd as soon with answering looks Of sympathie and love; there I had fixt Mine eyes till now, and pin'd with vain desire, Had not a voice thus warnd me, What thou seest, What there thou seest fair Creature is thy self, With thee it came and goes: but follow me, And I will bring thee where no shadow staies Thy coming, and thy soft imbraces, hee Whose image thou art, him thou shalt enjoy Inseparablie thine, to him shalt beare Multitudes like thy self, and thence be call'd Mother of human Race
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
IO- + ClTS = {IOClH-}
d[IO# ] [I # ][OCl# ] = k' = k''[I # ][HOCl] dt [OH # ]
OCl- + H2O
!
HOCl + OHHOI + ClH2O + IOIO- + ClSlow (rds)
I- + HOCl OH- + HOl IO- + OCl-
Rate decreases with [H+] !
H: Similar to A: Again Preq of the form (but now X- is low [ ] reactive species): All of these ideas can also HX = X- + H+ I: Similar to B: has [HX] and [X-] in separate rds
10. A sum of n terms in the denominator indicates a succession of n steps with the first n-1 reversible Non integral order REQUIRES intermediates
VERY USEFUL PAPER!
Rate law
Mechanism
You have collected and plotted the data and have the rate law: Now what?
rate = k[A]x [B]y + ......
Some “rules” for interpreting rate laws (i.e. converting the rate ! law to a mechanism) If it is of simple form (x = 1 or 2) you are essentially done:
Some rate law interpretation “rules” Chapter 6 of Espenson and selected papers
1a. The rate law usually gives the composition of the transition state. (Especially for preequilibrium cases; Exception: for chain mechanisms) Tl3+ + Hg22+
5. The first intermediate after the TS (of the rds) contains no atoms not present in the TS (but can contain less)
!
!
2
6. Alternative mechanisms that lead to the same formulation of the TS are not kinetically distinguishable
Cl2 + H2C2O4 Observed rate law " = Cl2 + H2O H2C2O4 !
2 CO2 + 2 Cl- + 2 H+
d[CO2 ] [Cl ][H C O ] = k' 2 + 2 2 2 4 dt [H ] [Cl# ]
TS = {ClC2O4-}
HOCl + Cl- + H+ H+ + HC2O4TS = {(H2O)ClC2O4-}
4. Inverse orders are due to rapid preequilibria (usually the reagent of
inverse order complexes the reactive species). The species in the denominator are produced before the rds.
k1[H + ] K + [H + ]
k 2K + k1[H + ] K + [H + ]
both forms reactive (separate rds; k1 for HX and k2 for X-), protonated more reactive. Collapses to A, B, C in various limits! A: K>> [H+], only HX reactive kobs = k1[H+]/K B: K>> [H+], both reactive kobs = k1[H+]/K + k2 C: K≈ [H+], only HX reactive kobs = k1[H+]/(K &[H + ][Cl" ] [Cl2 ]
" [H + ][HC2O4 ] [H 2C2O4 ]
d[CO2 ] " = k[HOCl][HC2O4 ] dt
[HOCl] = K1 [Cl2 ] [H + ][Cl" ]
K a1 = !
" [HC2O4 ] =
!
K a1[H 2C2O4 ] ! + [H ]
kobs = k a [H + ] + kb [H + ]2 kobs = k + k a [H + ] + kb [H + ]2
G
OMG Study something simpler!
!
VERY USEFUL PAPER!
H kobs = k1 /[H + ]
I
!
kobs = k1 + k 2 /[H + ]
Also: Read 6.5 and 6.6 of Espenson for different version of same information be extended to other types of rapid preequilibria: See section 6.7 for examples
5
3
9. Mechanisms that have all species at equilibrium prior to the rds cannot be catalyzed or inhibited. (TS is at equilibrium with the all the reactants: can’t catalyze such a reaction)
1
2. The number of positive terms in the rate law is the number of independent, parallel pathways to product. (microscopic reversibility). Negative terms represent the reverse reaction. 3 I - + H 2 O2 + 2 H + I3- + 2 H2O
" = k1[I # ][H 2O2 ] + k2 [I # ][H 2O2 ][H + ]
Reaction occurs by 2 separate pathways
!
TS1 is {H2O2I-} and TS2 is {H3O2I}
3. A reaction order greater than 3 means the mechanism has one or more rapid preequilibria (and intermediates) before the rds Elementary steps are either unimolecular or bimolecular
!
8. The sum of all the mechanistic steps must add up to the net reaction (If a reactant stoichiometry coefficient is larger than the order of that reactant, a fast reaction that uses that reactant occurs after the rds) I- + OCl"=
Cl2 + H2C2O4 Observed rate law " = Cl2 Cl+ + Cl-
2 CO2 + 2 Cl- + 2 H+
d[CO2 ] [Cl ][H C O ] = k' 2 + 2 2 2 4 dt [H ] [Cl# ]
TS = {ClC2O4-}
K1 = K a1K a 2 =
"=k
Tl+ + 2 Hg2+ TS is {TlHg3+}
2+ [Tl 3+ ][Hg2 ] [Hg 2+ ]
1b. Remember: If you didn’t vary the concentration of something that actually mattered (the solvent, oxygen, ! water in the solvent, etc.) AND IT DIDN’T CHANGE! then its concentration is included in the rate constant.
