LCL161_COA_16169_MedChemExpress
CIL56-SDS-MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-07-2019Print Date:Jan.-07-20191. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CIL56Catalog No. :HY-112063CAS No. :300802-28-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word No data availableHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effectsPrecautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor ⁄ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents ⁄ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C23H27N3O5S2Molecular Weight:489.61CAS No. :300802-28-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077 Class: 9 Packing group: III EMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077 Class: 9 Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis)reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:Acute Health Hazard16. OTHER INFORMATIONCopyright 2019 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
朗道多项生化定值质控

0843PAGE 1 OF 24LIQUID ASSAYED CHEMISTRY CONTROL PREMIUM PLUS - LEVEL 1 (LIQ CHEM ASY PREMIUM PLUS 1)Cat. No . LAL 4213 Lot No . 153UL Size : 12 x 5 ml Expiry : 2015-02INTENDED USEThis product is intended for in vitro diagnostic use, in the quality control of diagnostic assays. The Liquid Assayed Chemistry Control Premium Plus is for the control of accuracy.DEVICE DESCRIPTIONThe Liquid Assayed Chemistry Control Premium Plus is supplied at 3 levels, level 1, 2 and 3. Target values and ranges are supplied for the analytes listed in the values section at all three levels.SAFETY PRECAUTIONS AND WARNINGSFor in vitro diagnostic use only. Do not pipette by mouth. Exercise the normal precautions required for handling laboratory reagents.Human source material from which this product has been derived has been tested at donor level for the Human Immunodeficiency Virus (HIV 1, HIV 2) antibody, Hepatitis B Surface Antigen (HbsAg), and Hepatitis C Virus (HCV) antibody and found to be NON-REACTIVE. FDA approved methods have been used to conduct these tests.However, since no method can offer complete assurance as to the absence of infectious agents, this material and all patient samples should be handled as though capable of transmitting infectious diseases and disposed of accordingly.Health and Safety Data Sheets are available on request.STORAGE AND STABILITY OPENED: Store refrigerated (+2ºC to + 8ºC). Thawed serum is stable for 7 days at +2ºC to +8ºC, with the followingexceptions: Troponin T is stable for 3 days at +2ºC to +8ºC. Only the required amount of product should be removed. After use, any residual product should NOT BE RETURNED to the original vial.UNOPENED: Store frozen at -20ºC to -70ºC. Stable to expiration date printed on individual vials (see Limitations).LIMITATIONSFor Total Acid Phosphatase, the material should be stabilised by adding 1 drop (25 µl – 30 µl) of 0.7M Acetic acid solution to 1ml of the serum after thawing. After stabilisation, Total Acid Phosphatase is stable for 7 days at +2ºC to +8ºC. Bilirubin in the serum is light sensitive and it is recommended that the serum is stored in the dark.ALT, Total Acid Phosphatase, Alkaline Phosphatase, Total and Direct Bilirubin values may gradually decrease during the products shelf life.Bacterial contamination of the thawed serum will cause reductions in the stability of many components. The control should not be used as a calibration material.PREPARATION1. Allow the frozen control to thaw at room temperature (+15ºC to +25ºC) until completely thawed. Swirl the contents to ensurehomogeneity.2. Refer to the Control section of the individual analyser application.3. Refrigerate any unused material. Prior to reuse, mix contents thoroughly.MATERIALS PROVIDEDLiquid Assayed Chemistry Control Premium Plus - Level 1 12 x 5 mlMATERIALS REQUIRED BUT NOT PROVIDED NoneASSIGNED VALUESEach lot of serum is submitted to a number of external laboratories. Values are assigned from a consensus of results obtained by these laboratories and internal testing conducted at Randox Laboratories Ltd. With each batch, a control range is provided for individual parameters and each parameter method.If an instrument specific value is not available, refer to the Mean of all Instruments section. If necessary, contact Randox Laboratories – Customer Technical Services, Northern Ireland, Tel: +44 (0) 28 9445 1070 or email Technical.Services@29 Nov 13 rwPage 2 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 3 of 24 29/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 4 of 24 29/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 5 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 6 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 7 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 8 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 9 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 10 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 11 of 2429/11/2013___________________________________________________________________________________________________Page 12 of 2429/11/2013___________________________________________________________________________________________________Page 13 of 2429/11/2013___________________________________________________________________________________________________Page 14 of 2429/11/2013___________________________________________________________________________________________________Page 15 of 2429/11/2013___________________________________________________________________________________________________Page 16 of 2429/11/2013___________________________________________________________________________________________________Page 17 of 2429/11/2013___________________________________________________________________________________________________Page 18 of 2429/11/2013___________________________________________________________________________________________________Page 19 of 2429/11/2013___________________________________________________________________________________________________Page 20 of 2429/11/2013___________________________________________________________________________________________________Page 21 of 2429/11/2013___________________________________________________________________________________________________Page 22 of 2429/11/2013___________________________________________________________________________________________________Page 23 of 2429/11/2013___________________________________________________________________________________________________Page 24 of 2429/11/2013___________________________________________________________________________________________________。
线粒体呼吸链复合体Ⅳ 细胞色素 C 氧化酶活性检测试剂盒说明书

线粒体呼吸链复合体Ⅳ/细胞色素C 氧化酶活性检测试剂盒说明书微量法注意:本产品试剂有所变动,请注意并严格按照该说明书操作。
货号:BC0945规格:100T/96S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系索莱宝工作人员。
试剂名称规格 保存条件 提取液液体75 mL×2瓶 2-8℃保存 试剂一液体33mL×1瓶 2-8℃保存 试剂二粉剂×2瓶 -20℃保存 试剂三粉剂×2支 2-8℃保存溶液的配制:1、 试剂二:试剂放于试剂瓶内玻璃瓶中。
临用前取1支加入13.5mL 试剂一溶解,用不完的试剂-20℃分装保存2周,避免反复冻融;2、 试剂三:试剂置于试剂瓶内EP 管中;临用前取1支加入2mL 试剂一溶解,用不完的试剂-20℃保存2周,避免反复冻融;3、 工作液的配制:临用前取0.5mL 试剂三加入到溶解好的4.5mL 试剂二中混合备用(约25T ),或者按比例现用现配。
产品说明:线粒体复合体Ⅳ又称细胞色素C 氧化酶,也是线粒体呼吸电子传递链主路和支路的共有成分,负责催化还原型细胞色素C 的氧化,并最终把电子传递给氧生成水。
还原型细胞色素C 在550nm 有特征光吸收,线粒体复合体Ⅳ催化还原型细胞色素C 生成氧化型细胞色素C ,因此550nm 光吸收下降速率能够反映线粒体复合体Ⅳ酶活性。
Reduced Cytochrome C (550nm ) Oxidized Cytochrome C注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:可见分光光度计/酶标仪、台式离心机、水浴锅/恒温培养箱、可调式移液器、微量玻璃比色皿/96孔板、研钵/匀浆器/细胞超声破碎仪、冰和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1. 称取约0.1g 组织或收集500万细胞,加入1.0 mL 提取液,用冰浴匀浆器或研钵匀浆。
EPZ015666_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:EPZ015666 is an orally available inhibitor of PRMT5 enzymatic activity in biochemical assays with IC 50 of 22 nM and broad selectivity against a panel of other histone methyltransferases.IC50 & Target: IC50: 22 nM (PRMT5)[1]In Vitro: Treatment of MCL cell lines with EPZ015666 leads to inhibition of SmD3 methylation and cell death, with IC 50 values in the nanomolar range [1]. EPZ015666, a potent peptide–competitive and SAM–cooperative inhibitor with >10,000–fold specificity againstPRMT5 relative to other methyltransferases [2].In Vivo: EPZ015666 is orally bioavailable and amenable to in vivo studies. We performed 21–d efficacy studies in severe combined immunodeficiency (SCID) mice bearing subcutaneous Z–138 and Maver–1 xenografts, with twice–daily (BID) oral dosing on four dose groups: 25, 50, 100 and 200 mg per kilogram of body weight (mg/kg). After 21 d of continuous dosing, animals areeuthanized, and blood and tissues are analyzed to determine the relationship between methyl–mark pharmacodynamics andtumor–growth inhibition (TGI). EPZ015666 showed dose–dependent exposure and TGI after 21 d in both MCL models. Tumors in all EPZ015666 dose groups measured on day 21 showed statistically significant differences in weight, volume and tumor growth compared to vehicle–treated tumors. Dosing at 200 mg/kg BID induced tumor stasis in Z–138 cells, with >93% TGI after 21 d,whereas Maver–1 cells showed >70% TGI. Additionally, a third MCL xenograft is tested using the Granta–519 cell line, afast–growing model that reached endpoint on day 18 and showed dose–dependent efficacy with 45% TGI in the 200 mg/kg group.EPZ015666 is well tolerated in all three models, with minimal bodyweight loss in the 200 mg/kg dose group and no other clinical observations [1].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]EPZ015666 is serially diluted threefold from 1,000 to 0.051 nM and spotted into a 384–well polypropyleneV–bottom microplate. 3H–SAM is serially diluted twofold in assay buffer for a seven–point dilution series with a top concentration of 700 nM (final assay concentration). Reactions are initiated by the addition of 4 nM enzyme and 40 nM peptide (final assayconcentrations for both). Reactions are incubated for 60 min and quenched by the addition of 10 μL per well of 600 μM unlabeled SAM in assay buffer (final assay concentration). For the peptide competition, EPZ015666 is serially diluted threefold from 1,000 to 0.051 nM and spotted into a 384–well polypropylene V–bottom microplate. Peptide is serially diluted twofold in assay buffer for a seven–point dilution series with a top concentration of 480 nM (final assay concentration). Reactions are initiated by the addition of 4 nM enzyme and 75 nM 3H–SAM (final assay concentrations for both). Reactions are incubated for 60 min, and the reactions are quenched by the addition of 10 μL per well of 600 μM unlabeled SAM in assay buffer (final assay concentration)[1].Cell Assay: EPZ015666 is dissolved in DMSO and stored, and then diluted with appropriate medium (final DMSO 0.2%)before use [1].[1]Cultured cells in linear/log–phase growth are split to a seeding density of 2×105 cells/mL in 2–20 mL of media,depending on the yield required at the end of the growth period. Compound is diluted in DMSO and added to each culture vesselProduct Name:EPZ015666Cat. No.:HY-12727CAS No.:1616391-65-1Molecular Formula:C 20H 25N 5O 3Molecular Weight:383.44Target:Histone Methyltransferase Pathway:Epigenetics Solubility:DMSOwith a final DMSO concentration of 0.2%. Cells are allowed to grow for 96 h undisturbed. At the conclusion of each treatment period, cells are harvested by centrifugation (5 min, 1,200 rpm), and cell pellets are rinsed once with PBS before being frozen on dry ice pending further processing. Long–term proliferation assays are performed on all MCL lines, with slight adjustments to initial seeding densities, depending on growth characteristics for each cell line. All assays are carried out for 12 d[1].Animal Administration: EPZ015666 is formulated in 20% N–N–dimethylacetamide in water (Mice)[1].[1]Mice[1]Male CD–1 mice (25–40 g; n=6, with 3 per time point) are treated with a single dose of EPZ015666 at 2 mg/kg by intravenoustail–vein injection and 10 mg/kg by oral gavage administration, with both doses formulated in 20% N–N–dimethylacetamide in water. Animals are fasted overnight and weighed before dose administration on the day of dosing. Approximately 30 μL ofblood are taken from animals by submandibular or retro–orbital bleeding at pre–specified time intervals (seven time points). For the last time point (24 h), samples are collected via cardiac puncture while the animals are under anesthesia (70% CO2:30% O2). Blood samples are transferred into K2–EDTA tubes and placed on wet ice before centrifugation at 4°C (3,000g, 15 min) to obtain plasma within 30 min after sample collection. Plasma samples are stored at -70±10°C before protein precipitation and LC–MS/MS analysis. We constructed standard calibration curves by analyzing a series of control plasma aliquots containing 100 ng/mL labetalol as an internal standard and 1–3,000 ng/mL EPZ015666. Four levels of quality control are also included in the analysis (3–2,400 ng/mL EPZ015666). Data are analyzed using Phoenix WinNonlin 6.2.1.References:[1]. Chan–Penebre E, et al. A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat Chem Biol. 2015 Jun;11(6):432–7.[2]. Kryukov GV, et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science. 2016 Mar 11;351(6278):1214–8.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
纤维素(CLL)含量检测试剂盒说明书__微量法UPLC-MS-4117
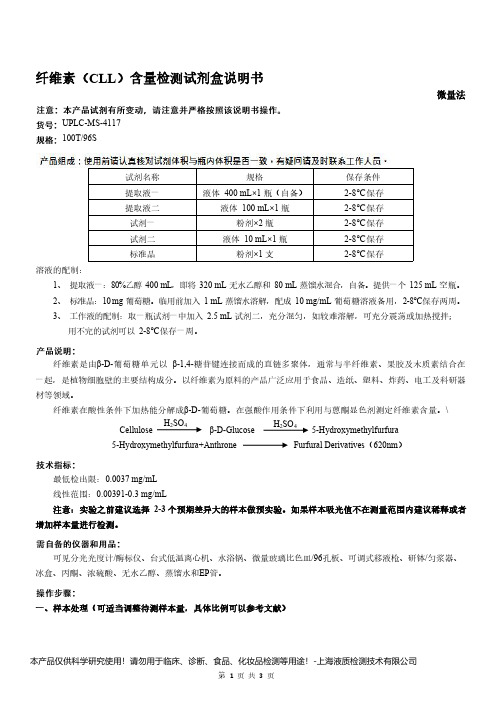
纤维素(CLL)含量检测试剂盒说明书微量法UPLC-MS-4117100T/96S试剂名称规格保存条件提取液一液体400mL×1瓶(自备)2-8℃保存提取液二液体100mL×1瓶2-8℃保存试剂一粉剂×2瓶2-8℃保存试剂二液体10mL×1瓶2-8℃保存标准品粉剂×1支2-8℃保存溶液的配制:1、提取液一:80%乙醇400mL,即将320mL无水乙醇和80mL蒸馏水混合,自备。
提供一个125mL空瓶。
2、标准品:10mg葡萄糖。
临用前加入1mL蒸馏水溶解,配成10mg/mL葡萄糖溶液备用,2-8℃保存两周。
3、工作液的配制:取一瓶试剂一中加入2.5mL试剂二,充分混匀,如较难溶解,可充分震荡或加热搅拌;用不完的试剂可以2-8℃保存一周。
纤维素是由β-D-葡萄糖单元以β-1,4-糖苷键连接而成的直链多聚体,通常与半纤维素、果胶及木质素结合在一起,是植物细胞壁的主要结构成分。
以纤维素为原料的产品广泛应用于食品、造纸、塑料、炸药、电工及科研器材等领域。
纤维素在酸性条件下加热能分解成β-D-葡萄糖。
在强酸作用条件下利用与蒽酮显色剂测定纤维素含量。
\Cellulose H2SO4β-D-Glucose H2SO45-Hydroxymethylfurfura5-Hydroxymethylfurfura+Anthrone Furfural Derivatives(620nm)最低检出限:0.0037mg/mL线性范围:0.00391-0.3mg/mL注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
可见分光光度计/酶标仪、台式低温离心机、水浴锅、微量玻璃比色皿/96孔板、可调式移液枪、研钵/匀浆器、冰盒、丙酮、浓硫酸、无水乙醇、蒸馏水和EP管。
一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1、细胞壁物质(CWM)的提取:(1)称取约0.3g(记为W1)样本,加入1mL提取液一,室温快速匀浆,90℃水浴20min,冷却至室温,6000g,25℃离心10min,弃上清。
QPCR及QRT-PCR系列产品

Invitrogen的ICFC系列产品促销1.QPCR及QRT-PCR系列产品Invitrogen公司专门为中国客户提供的定量PCR试剂盒,结合了 UDG 防止残余污染技术和SYBR® Green I 荧光染料(存在于SYBR® Green I荧光定量PCR试剂盒中),在美国接受了严格的质量监控,可提供极高灵敏度的目的序列定量检测,线性剂量低,反应浓度范围很大。
qPCR Supermix-- 即用型反应剂,专为高特异性、实时定量DNA扩增设计UDG-- 防止携带污染物,减少克隆片段假阳性结果ROX参考染料-- 适用ABI仪器的校正染料产品信息活动时间:即日起至2009年4月30日2.Gibco南美胎牛血清即日起凡优惠价¥1780购买Gibco胎牛血清500ml(目录号:C2027050)即可获赠送价值¥250现金抵用券。
您可以凭现金抵用券在英韦创津公司购买任何商品,此券有效期至2009年5月31日。
产品信息活动时间:即日起至2009年4月30日独特的采集方式:GIBCO采用无菌心脏穿刺的方式采血原装直送,避免污染:原产地采集、加工、检测、包装。
完善的质控:采集、处理、检测、运输等环节都有文件和证书。
3.Invitrogen TA Cloning克隆产品专门用于克隆Taq聚合酶扩增的PCR产物。
采用pCR载体,能产生80%以上的重组产物,90%以上重组产物都包含插入片段。
产品信息活动时间:即日起至2009年5月31日附:pCR载体优点及图谱:3’-T突出端可直接连接Taq扩增的PCR产物可选择T7或T7和Sp6启动子进行体外RNA转录和测序侧向EcoRⅠ位点的通用多接头位点方便了插入片段的切离可以选择卡那霉素或氨苄青霉素进行筛选非常简便的蓝/白克隆筛选具有M13正向和反向引物位点,方便测序4.GIBCO液体培养基系列产品创立近50年的历史,品质优秀,产品种类丰富;为了中国用户利益,特建立国内生产线;所有产品,从原材料到生产全部按照GIBCO质量标准进行,每批均送抵美国公司总部质检合格后,才在国内销售。
诺唯赞反转录试剂盒 说明书

•
•Thermo 反转录试剂盒K1622使用说明
•Thermo Scientific RevertAid First Strand cDNA Synthesis Kit是一套用于以RNA为模板高效合成第一链cDNA的完整系统,可合成长达13kb 的cDNA。
试剂盒配套提供的RiboLockRNase Inhibitor,可有效防止RNA模板的降解。
试剂盒配套提供oligo(dT)18引物和随机六聚体引物。
oligo(dT)18引物可以选择性地与mRNA中的poly (A)尾结合。
随机六聚体引物无需poly (A)尾,因此可转录mRNA 5’-端区域,也可以无poly
(A)尾的RNA (如micoRNA)为模板合成cDNA。
该试剂盒也可使用基因
特异性引物。
•Thermo 反转录试剂盒K1622使用说明优点:
•合成全长的第一链cDNA,最长可合成13kb的cDNA
•最佳反应温度为42℃
•完全一提供RT反应所需的所有组分
•应用:
•第一链cDNA合成,作为RT-PCR和实时RT-qPCR的模板
•构建全长cDNA文库
•反义RNA合成
•Thermo 反转录试剂盒K1622使用说明
•RevertAid First Strand cDNA Synthesis Kit合成全长cDNA
• 1 μg小鼠心脏总RNA作为模板,用oligo(dT)18 引物进行逆转录反应。
由此合成的cDNA,再作为Taq DNA Polymerase后续PCR扩增的模板。
末端2024 bp片段的成功扩增,表明长度为13.8 kb的全长RN***段得到成功的逆转录。
•。
一种用于从血清中检测叶酸含量的解离剂及检测方法[发明专利]
![一种用于从血清中检测叶酸含量的解离剂及检测方法[发明专利]](https://img.taocdn.com/s3/m/9f25780c172ded630a1cb6cf.png)
专利名称:一种用于从血清中检测叶酸含量的解离剂及检测方法
专利类型:发明专利
发明人:陶然
申请号:CN201911416335.0
申请日:20191231
公开号:CN111024962A
公开日:
20200417
专利内容由知识产权出版社提供
摘要:本发明涉及一种用于从血清中检测叶酸含量的解离剂及检测方法,包括蛋白变性剂、蛋白水解酶、表面活性剂和基础液,其中各组分的用量为:每100ml解离剂中含有蛋白变性剂3~8g,蛋白水解酶1000~3000U,表面活性剂0.2~0.8ml,余量为基础液。
与现有技术相比,本发明具有方法简单,灵敏度高、检测结果准确等优点。
申请人:上海复星长征医学科学有限公司
地址:200444 上海市宝山区城银路830号
国籍:CN
代理机构:上海科盛知识产权代理有限公司
代理人:蒋亮珠
更多信息请下载全文后查看。
