an134rev1_1
EMEA 注册要求

European Medicines AgencyInspectionsLondon, 27 April 2005EMEA/CVMP/134/02 Rev 2 ConsultationCPMP/QWP/227/02 Rev 2 Consultation COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE (CVMP)GUIDELINE ON ACTIVE SUBSTANCE MASTER FILE PROCEDUREDISCUSSION AT THE HMPC November 2005 – January 2006 ADOPTION BY THE HMPC 22 January 2006 DRAFT AGREED BY QUALITY WORKING PARTY February 200623 March 2006 ADOPTION BY CHMP FOR RELEASE FORCONSULTATION20 April 2006 ADOPTION BY CVMP FOR RELEASE FORCONSULTATION30 August 2006 END OF CONSULTATION (DEADLINE FORCOMMENTS)Note:From 1st November 2005, Directive 2004/24/EC1 relating to traditional herbal medicinal products came into force in all Member States in the European Union allowing the establishment of a simplified procedure for the registration of traditional herbal medicinal products for human use.In order to facilitate the use of the ASMF procedure in the area of herbal medicinal products, the Committee for Herbal Medicinal Products proposes an Annex on herbal substances/preparations (see Annex 1, table 3) to the Guideline on the Active Substance Master File procedure.It should be noted that the principles which are outlined in this guideline in relation to traditional herbal medicinal products are equally applicable to other herbal medicinal products, both for Human and Veterinary use, which do not follow the simplified registration procedure. The new table (Annex 1, table 3) takes into account the particularities of herbal substances/preparations whilst also highlighting that this procedure is/can be applied to active substances/preparations of herbal origin, whether they be for human or veterinary use.1 Directive2004/24/EC of the European Parliament and of the Council of 31 March 2004, amending, as regards traditional herbal medicinal products, Directive 2001/83/EC on the Community code relating to medicinal products for human use.Since this revision introduces clarification rather than changing principles, the publication of a concept paper was not considered necessary.The final Guideline has been adapted to the new template for Guidelines.Comments should be provided using this template to***********.int, with a copy to ************.intGUIDELINE ON ACTIVE SUBSTANCE MASTER FILE PROCEDURETABLE OF CONTENTSEXECUTIVE SUMMARY (4)1 INTRODUCTION (4)2 SCOPE (4)BASIS (4)3 LEGAL4 MAIN GUIDELINE TEXT (4)4.1 Content of the Active Substance Master File (4)4.2 Use of the Active Substance Master File Procedure (5)4.3 Content of the Ma Dossier when the Active Substance Master File Procedure is used6 4.4 Changes and updates to the Active Substance Master File (6)ANNEX 1 (8)ANNEX 2 (13)ANNEX 3 (14)ANNEX 4 (15)ANNEX 5 (16)EXECUTIVE SUMMARY1 INTRODUCTIONThe main objective of the Active Substance Master File (ASMF) procedure, commonly known as the European Drug Master File (EDMF) procedure, is to allow valuable confidential intellectual property or 'know-how' of the manufacturer of the active substance (ASM) to be protected, while at the same time allowing the Applicant or marketing authorisation (MA) holder to take full responsibility for the medicinal product and the quality and quality control of the active substance. Competent Authorities/EMEA thus have access to the complete information that is necessary for an evaluation of the suitability of the use of the active substance in the medicinal product.2 SCOPEThis Guideline is intended to assist Applicants/MA holders in the compilation of the active substance section of their dossiers for a marketing authorisation application (MAA) or a marketing authorisation variation (MAV) of a medicinal product. It is also intended to help EDMF holders in the compilation of their EDMFs. This Guideline is not intended to give instructions to the Competent Authorities/EMEA in the administrative and scientific handling of EDMFs and related MAAs and MAVs.ASMF Procedure and herbal substances/preparationsIn accordance with Directive 2004/24/EC, the quality of traditional herbal medicinal products for human use has to be documented in accordance with existing European legislative requirements. These criteria are laid down in the following guidelines (which are applicable for all Human and Veterinary Herbal Medicinal products): ‘Guideline on quality of herbal medicinal products/traditional herbal medicinal products’ (CPMP/QWP/2819/00, EMEA/CVMP/814/00, in their latest revisions) and the ‘Guideline on specifications: test procedures and acceptance criteria for herbal substances, herbal preparations and herbal medicinal products/ traditional herbal medicinal products’ (CPMP/QWP/2820//00, EMEA/CVMP/815/00, in their latest revisions).It should be noted that the principles which are outlined in table 3 of Annex 1 in relation to traditional herbal medicinal products are equally applicable to other herbal medicinal products, both for Human and Veterinary use, which do not follow the simplified registration procedure.References:1. ‘Guideline on quality of herbal medicinal products/traditional herbal medicinal products’(CPMP/QWP/2819/00, EMEA/CVMP/814/00, in their latest revisions.)2. ‘Guideline on specifications: test procedures and acceptance criteria for herbal substances,herbal preparations and herbal medicinal products/ traditional herbal medicinal products’(CPMP/QWP/2820/00, EMEA/CVMP/815/00, in their latest revisions.)3. ‘Guideline on summary of requirements for active substances in the quality part of the dossier’(CHMP/QWP/297/97, EMEA/CVMP/1069/02, in their latest revisions.)BASIS3 LEGALAnnex I to Directive 2001/83/EC Part I, 3.2 Basic principles and requirements, (8) Active Substance Master FileTEXT4 MAINGUIDELINE4.1 Content of the Active Substance Master FileThe overall content of the EDMF should contain detailed scientific information as indicated under the various headings of the relevant Notice to Applicants for Marketing Authorisations for Medicinal Products in the Member States of the European Union (NtA). EDMFs linked to human medicinal products should be presented in the format of the Common Technical Document (CTD), see Annex 1 table 1. EDMFs linked to veterinary medicinal products should be presented in accordance withAnnex 1 table 2. EDMFs for veterinary medicinal products may also be presented in CTD format after consultation with the Competent Authorities/EMEA.The scientific information in the EDMF should be physically divided into two separate parts, namely the Applicants Part (AP) and the Restricted Part (RP). The AP contains the information that the EDMF holder regards as non-confidential to the Applicant/MA holder, whereas the RP contains the information that the EDMF holder regards as confidential, see Annex 1. It is emphasized that the AP is still a confidential document that cannot be submitted by anyone to third parties without the written consent of the EDMF holder. In all cases the AP should contain sufficient information to enable the Applicant/MA holder to take full responsibility for an evaluation of the suitability of the specifications for the active substance to control the quality of this active substance for use in the manufacture of a specified medicinal product.The RP may contain the remaining information, such as detailed information on the individual steps of the manufacturing method (reaction conditions, temperature, validation and evaluation data of critical steps) and the quality control during the manufacture method of the active substance. The Competent Authorities/EMEA may not accept that particular information has not been disclosed to the Applicant/MA holder. In such cases, the Competent Authorities/EMEA may ask for an amendment to the AP.In addition to the AP and RP, the EDMF should contain a table of contents, and a separate summary for the AP and the RP. In cases where the EDMF is provided in the CTD format, both summaries should be presented as a Quality Overall Summary (QOS). In cases where the old human or current veterinary NtA format is used, each summary should be made in the form of a written and tabulated expert report (ER). The AP and RP should each have a version number. The structure of the version numbers should be unique and follow a logical order. Preferably the following structure is used.Name EDMF holder / Name active substance / AP or RP/ version number / date in yyyy-mm-dd. 4.2 Use of the Active Substance Master File ProcedureAn EDMF can only be submitted in support of an MAA or MAV. The relationship between the quality of the active substance and its use in the medicinal product needs to be justified in this MAA or MAV. Although the EDMF procedure is developed to keep intellectual property of the ASM confidential, it is also permissible to use the procedure when there is no confidentiality issue between the Applicant/MA holder and the ASM (e.g. when the Applicant/MA holder synthesises the active substance himself). It is expected that the ASM is also the holder of the EDMF.The EDMF procedure can be used for the following active substances, including herbal active substances/preparations (except biological active substances, see the CHMP procedural announcement at the last page of this Guideline), i.e.:activesubstancesA. NewB. Existing active substances not included in the European Pharmacopoeia (Ph. Eur.) or thepharmacopoeia of an EU Member StateC. Pharmacopoeial active substances included in the Ph. Eur. or in the pharmacopoeia of an EUMember StateThe EDMF holder may have an EDMF as well as a CEP for a single active substance. Generally, it is however not acceptable that the Applicant/MA holder refers to an EDMF as well as to a CEP for a single active substance of a particular MAA. In cases where the CEP contains too little information (e.g. stability) the Competent Authorities/EMEA may decide that additional information should be provided in the dossier. In such case it may be acceptable to refer both to an EDMF and a CEP.The EDMF holder should give permission to the Competent Authorities/EMEA to assess the data in the EDMF in relation to a specific MAA/MAV, in the form of a ‘Letter of Access’, see Annex 2.The EDMF holder should submit to the Applicant/MA holder:- a copy of the latest version of the AP.- a copy of the QOS/ ER on the latest version of the AP- the letter of access where this letter has not been submitted earlier for the product concerned.In addition, the EDMF holder should submit to the Competent Authorities/EMEA:- the EDMF accompanied by a covering letter, see Annex 3.- the Letter of Access where this letter has not been submitted earlier for the product concerned. The EDMF holder should submit the EDMF to the Competent Authority/EMEA either for each MAA and each MAV or only once according to national requirements. The submission of the relevant documentation by the EDMF holder to the Competent Authority/EMEA must be synchronised to arrive at approximately the same time as the MAA or the MAV.Where the EDMF procedure is used, the Applicant/MA holder should submit the MAA or MAV to the Competent Authorities/EMEA together with the Letter of Access where this Letter has not been submitted earlier by the MA holder/Applicant himself or by the EDMF holder for the product concerned.Where the same active substance is used in a number of applications for different products in one or more Member States, the EDMF holder should submit identical documentation to every Competent Authority/EMEA. Consequently, the Competent Authorities/EMEA may require that any EDMF updates made in relation to one MA should apply to all. It is the EDMF holder’s responsibility to notify the MA holders and Competent Authorities/EMEA concerned about any changes to the AP and/or RP, so that the MA holders can update all affected MAs accordingly.4.3 Content of the Ma Dossier when the Active Substance Master File Procedure is usedThe Applicant/MA holder is responsible for ensuring that he has access to all relevant information concerning the current manufacture of the active substance.The specifications used by the Applicant/MA holder to control the correct quality of the active substance should be laid down unambiguously in the MA dossier (NtA CTD format section 3.2.S.4.1 and 3.2.S.4.2 or old human/veterinary NtA format part IIC1). The Applicant/MA holder should include a copy of the AP in the MA dossier (NtA CTD format section 3.2.S or NtA old human/veterinary format part IIC1). The version of the AP in the MA dossier should be the most recent and it should be identical to the AP as supplied by the EDMF holder to the Competent Authority/EMEA as part of the EDMF. The Applicant/MA holder should include all relevant details from the AP in the QOS/ER of the MA dossier. Issues of the EDMF that are specifically relevant to the product under consideration should be highlighted in the QOS/ER of the MA dossier.In the case of a single supplier and where the EDMF procedure or CEP procedure is used, the specifications of the Applicant/MA holder in the MA dossier should in principle be identical to those of the EDMF holder or the CEP holder. The Applicant/MA holder does however not need to accept redundant specifications, unnecessarily tight specification limits or outdated analytical methods. In cases where the Applicant/MA holder uses a different analytical method than that described in the EDMF, both methods should be validated. Technical specifications relevant for the medicinal product, which are normally not part of the specifications in the EDMF (e.g. particle size), should be part of the specifications of the Applicant/MA holder.In cases where there is more than one supplier, there should be one single compiled specification that is identical for each supplier. It is acceptable to lay down in the specification more than one acceptance criterion and/or analytical method for a single parameter with the statement ‘if tested’ (e.g. in case of residual solvents).4.4 Changes and updates to the Active Substance Master FileAs for medicinal products, EDMF holders should keep the content of their EDMFs updated with respect to the actual synthesis/manufacturing process. The quality control methods should be kept inline with the current regulatory and scientific requirements. EDMF holders shall not modify the contents of their EDMF (e.g. manufacturing process or specifications) without informing each Applicant/MA holder and each Competent Authority/EMEA. Before implementation, any change to the EDMF should be reported by every MA holder to the relevant Competent Authority/EMEA by means of an appropriate variation procedure. A covering letter should be provided. In cases where the contents of the EDMF cannot be changed for a certain period of time because of other procedural provisions (i.e. mainly because of ongoing MRP procedures), the EDMF holder should still provide the aforementioned data to the MA holder and Competent Authorities/EMEA making reference to this reason and requesting a later date of implementation.The EDMF holders’ covering letter to the Competent Authorities/EMEA should contain the following information (if available):- A tabular list summarising the changes carried out since the first compilation of the EDMF.- An overview comparing the old and new content of the EDMF.- Information as to whether the change has already been accepted, rejected or withdrawn by another Member State.- The names of the relevant Applicants, MA holders and MAs.- The new AP and/or RP with each the new version number.- An updated QOS/ER if relevant.At the occasion of the 5-yearly renewal of a medicinal product, MA holders are required to declare that the quality of the product, in respect of the methods of preparation and control, has been regularly updated by variation procedure to take account of technical and scientific progress, and that the product conforms with current CPMP/CVMP quality guidelines. They will also declare that no changes have been made to the product particulars other than those approved by the Competent Authority/EMEA.MA holders should therefore verify with their EDMF holders whether the above declaration can be met in respect to the active substance particulars. In case changes have not been notified to the MA holder and Competent Authority/EMEA, the necessary variation procedure should be initiated without delay.ANNEX 1 OVERVIEW EDMF CONTENTSTable 1 NtA CTD format ApplicantsPart Restricted Part3.2.S.1 Generalinformation x3.2.S.1.1 Nomenclature x3.2.S.1.2 Structure x3.2.S.1.3 Generalproperties x3.2.S.2 Manufacture x X3.2.S.2.1 Manufacturer(s) x3.2.S.2.2 Description of Manufacturing Process and Processcontrols1) 2) 3.2.S.2.3 Control of Materials X3.2.S.2.4 Control of critical steps and intermediates 3) 4)3.2.S.2.5 Process validation and/or Evaluation X3.2.S.2.6 Manufacturing Process Development X3.2.S.3 Characterisation x3.2.S.3.1 Elucidation of Structure and other Characteristics x3.2.S.3.2 Impurities x 5)3.2.S.4 Control of Drug Substance x3.2.S.4.1 Specification x3.2.S.4.2 Analyticalprocedures x3.2.S.4.3 Validation of analytical procedures x3.2.S.4.4 Batchanalysis x3.2.S.4.5 Justification of specification x 6)3.2.S.5 Reference standards or materials x3.2.S.6 Container Closure System x3.2.S.7 Stability x3.2.S.7.1 Stability summary and conclusion x3.2.S.7.2 Post-approval Stability Protocol and StabilityCommitmentx 3.2.S.7.3 Stabilitydata xTable 2 NtA veterinary format / old human format ApplicantsPart Restricted PartIIC.1 Name(s) and site(s) of ASM x XIIC.1.1 Specifications and routine tests xIIC.1.2.1 Nomenclature xIIC.1.2.2 Description xIIC.1.2.3 Brief outline of the manufacturing route (flow chart) xIIC.1.2.3 Detailed description manufacturing method XIIC.1.2.4 QC during manufacture 3) 4) Process validation and evaluation of data XIIC.1.2.5 DevelopmentChemistry xEvidence of structure xPotentialIsomerism xPhysiochemicalcharacterisation xAnalyticalvalidation xIIC.1.2.6 Impurities x 5) IIC.1.2.7 Batchanalysis xIIF1 Stability xFlow chart and short description is regarded as sufficient, if detailed information is presented in the Restricted Part. However, full validation data on the sterilisation process may be requested in the Applicants Part (in cases where there is no further sterilisation of the final product).detailed information.In so far as the information is also relevant for the Applicant/MA holder.In so far as the information is related to the detailed description of the manufacturing process and in so far as this information is not relevant for the Applicant/MA holder.In so far as the information is related to the detailed description of the manufacturing process and in so far as the EDMF holder sufficiently justifies that there is no need to control these impurities in the final active substance.In so far as the information is related to the detailed description of the manufacturing process, control of materials and process validation.Table 3 NtA CTD format 2Herbal Active Substances/ PreparationsApplicants Part Restricted Part 3.2.S.1 Generalinformation X3.2.S.1.1 Nomenclature XFor herbal substance:Binomial scientific name of plant (genus, species, varietyand author), and chemotype (where applicable)Parts of the plantsDefinition of the herbal substanceOther names (synonyms mentioned in otherPharmacopoeias)Laboratory codeFor herbal preparationsBinomial scientific name of plant (genus, species, varietyand author), and chemotype (where applicable)Parts of the plantsDefinition of the herbal preparationRatio of the herbal substance to the herbal preparationExtraction solvent(s)Other names (synonyms mentioned in otherPharmacopoeias)Laboratory code3.2.S.1.2 Structure X- Physicalform- Description of the constituents withknown therapeutic activity or markers (molecularformula, relative molecular mass, structural formula,including relative and absolute stereochemistry, themolecular formula, and the relative molecular mass).- Otherconstituent(s)3.2.S.1.3 General properties X3.2.S.2 Manufacturer(s)For herbal substancesThe name, address, and responsibility of each supplier,including contractorseach proposed site or facility involved inproduction/collection and testing of the herbal substanceshould be provided, where appropriate.For herbal preparationsThe name, address, and responsibility of each manufacturer, including contractors, and each proposed manufacturing site or facility involved in manufacturing and testing of the herbal preparation XX2 EDMFs for Veterinary herbal medicinal products should be presented in the Veterinary NtA format (see table 2) unless prior authorisation has been received from the Competent Authorities/EMEA. (A ‘Correlation Table’ for the CTD and NtA formats is available at /F2/eudralex/vol-2/B/ctd_06-2004.pdf)should be provided, where appropriate.3.2.S.2.2 Description of critical steps and intermediates Flow chart Detailedinformation For herbal substancesInformation should be provided to adequately describethe plant productionand plant collection, including:Geographical source of medicinal plantCultivation, harvesting, drying and storage conditionsFor herbal preparationsInformation should be provided to adequately describethe manufacturingprocess of the herbal preparation, including:Description of processingSolvents, reagentsPurification stagesStandardisation3.2.S.2.3 Control of materials X3.2.S.2.4 Control of critical steps and intermediates If also relevant forXthe MAholder/applicant3.2.S.2.5 Process validation and/or evaluation X X3.2.S.2.6 Manufacturing Process Development XA brief summary describing the development of theherbal substance(s) and herbal preparation(s) whereapplicable should be provided, taking into considerationthe proposed route of administration and usage. Resultscomparing the phytochemical composition of the herbalsubstance(s) and herbal preparation(s) where applicableused in supporting bibliographic data and the herbalsubstance(s) and herbal preparation(s) where applicabledescribed in S1 should be discussed, where appropriate.3.2.S.3 Characterisation X3.2.S.3.1 Elucidation of structure and other characteristics XFor herbal substancesInformation on the botanical, macroscopical,microscopical, phytochemical characterisation, andbiological activity if necessary, should be provided:For herbal preparationsInformation on the phyto- and physicochemicalcharacterisation, and biological activity if necessary,should be provided:3.2.S.3.2 Impurities X3.2.S.4 Control of drug substance X3.2.S.4.1 Specification X3.2.S.4.2 Analytical procedure X3.2.S.4.3 Validation of analytical procedure X3.2.S.4.4 Batch analysis X3.2.S.4.5 Justification of specification X X 3.2.S.5 Reference standards of materials X3.2.S.6 Container closure system X3.2.S.7 Stability X3.2.S.7.1 Stability summary and conclusion X3.2.S.7.2 Post-approval stability protocol and stability commitment X3.2.S.7.3 Stability data XTEMPLATE LETTER OF ACCESS[Address of Competent Authority/EMEA][Date and place]LETTER OF ACCESSNumber of Active Substance Master File: [if known, or to be given by the Competent Authority/EMEA or procedure reference number/community reference number in Centralised Procedure ]Manufacturing site: [name and address]Active Substance Master File holder: [name and address]The aforementioned Active Substance Master File holder hereby authorises the [name of Competent Authority/EMEA including all CPMP or CVMP Members and their experts] to refer to and review the above mentioned Active Substance Master File in support of the following Marketing Authorisation Application(s) or Marketing Authorisation Variation(s)3 submitted by [name /Marketing Authorisation holder/Applicant] on [planned date of submission]:[Name of product and Marketing Authorisation number, if known][Name of Applicant or Marketing Authorisation holder]The aforementioned Active Substance Master File holder commits to ensure batch to batch consistency and to inform [name of Marketing Authorisation holder/Applicant] and Competent Authority/EMEA of any change in the Active Substance Master File.Signature for the Active Substance Master File holder[Name and address][Signature]3 i.e. to introduce a new EDMF from a new AS manufacturer.PART OF COVERING LETTERThis Active Substance Master File is submitted in relation to the Marketing Authorisation Application/Marketing Authorisation Variation:[Number of national, centralised or mutual recognition procedure][Name of product in national, centralised or mutual recognition procedure][Name of Applicant/Marketing Authorisation holder for the application concerned][Concerned Member States in mutual recognition]And describes <changes to> the manufacturing process and specifications of the (or one of the) active substance(s) of this Marketing Authorisation Application or Marketing Authorisation Variation. [Name active substance]The version number of this Active Substance Master File isApplicants part: version [version number]Restricted part: version [version number]This Active Substance Master File has previously been submitted for assessment in combination with a Marketing Authorisation Application/ Marketing Authorisation Variation for a medicinal product within the European Union:NoYes, within the following National, Centralised or Mutual recognition procedure:[Number of National, Centralised or Mutual Recognition Procedure][Name of product in National, Centralised or Mutual Recognition Procedure] [Authorisation number and date of approval of the products concerned][Rapporteur or Reference Member State][Concerned Member States in Mutual Recognition][Version number Applicants Part][Version number Restricted Part]Note:Information in italic font can be left blank if not known.Information in normal font is always required.LIST OF ABBREVIATIONSAbbreviation Full text AP Applicants Part (of EDMF) ASM Active Substance Manufacturer ASMF Active Substance Master File CEP European procedure for a certificate of suitability of monographs of theEuropean pharmacopoeia (here on chemical purity)CTD Common Technical Document EDMF European Drug Master File EDQM European Directorate for the Quality of Medicines EMEA European Medicines Evaluation Agency ER Written and tabulated expert report (refers to MA dossiers in old human or existingNtA veterinary format)ICH International Conference on HarmonisationMA Marketing Authorisation MAA Marketing Authorisation Application (including line extensions) MAV Marketing Authorisation Variation NtA Notice to Applicants Ph. Eur. European Pharmacopoeia RP Restricted Part (of EDMF) QOS Quality Overall Summary (refers to MA dossiers in NtA CTD format)GLOSSARYItem DefinitionActive Substance Manufacturer A party involved in the manufacturing chain of the activesubstance, including agents, brokers, traders, distributors,repackers or relabellers.Active Substance Master File holder This is the company that has the ultimate responsibility forthe Active Substance Master File.Applicant This is the company requesting a Marketing Authorisationfor a medicinal product.European Drug Master File The old name of the Active Substance Master File Marketing Authorisation holder This is the company that is responsible for the medicinalproduct on the marketManufacturing chain A clear flow chart or written text explaining themanufacturing and distribution route of the active substancefrom the first starting materials to the final active substanceas delivered to the Applicant/Marketing Authorisationholder.New active substance According to ICH Q6A new drug substance that isThe designated therapeutic moiety, which has not previouslybeen registered in a region or Member State (also referred toas a new molecular entity or new chemical entity). It may bea complex, simple ester, or salt of a previously approved drugsubstance.Quality According to ICH Q6A that isThe suitability of either a drug substance or drug product forits intended use. This term includes such attributes as theidentity, strength and purity.Specification According to ICH Q6A that isA list of test, references to analytical procedures, andappropriate acceptance criteria which are numerical limits,ranges or other criteria to which a drug substance or drugproduct should conform to be considered acceptable for itsintended use. Conformance to specifications means that thedrug substance and/or drug product, when tested according tothe listed analytical procedures will meet the listedacceptance criteria. Specifications are critical qualitystandards that are proposed and justified by the manufacturerand approved by regulatory authorities.。
ViewFlex Xtra ICE Catheter 重新处理指南说明书

Page 1 of 7 Reprocessed byInstructions for useReprocessed ViewFlex Xtra ICE CatheterReprocessed device for single useCaution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.▪STERILE▪Exposed to Ethylene Oxide (EO) gasExplanation of symbolsSymbolRules/StandardReferenceISO 7000RegistrationNumberSymbol Title DescriptionRx Only 21CFR801 N/A Prescription only Indicates Federal (USA) law restricting device to saleby or on order of a physician.ISO 15223-1Clause 5.1.1 3082 Manufacturer Indicates the medical device manufacturer.ISO 15223-1Clause 5.1.3 2497 Date of manufactureIndicates the date when the medical device wasmanufactured.ISO 15223-1Clause 5.2.3 2501Sterilized usingethylene oxideIndicates a medical device that has been sterilizedusing ethylene oxide.ISO 15223-1Clause 5.1.4 2607 Use-by dateIndicates the date after which the medical device is notto be used.ISO 15223-1Clause 5.1.5 2492 Batch codeIndicates the manufacturer’s batch code so that thebatch or lot can be identified.ISO 15223-1Clause 5.1.6 2493 Catalogue numberIndicates the manufacturer’s catalogue number so thatthe medical device can be identified.ISO 15223-1Clause 5.1.7 2498 Serial numberIndicates the manufacturer’s serial number so that aspecific medical device can be identified.ISO 15223-1Clause 5.4.3 1641Consult instructionsfor useIndicates the need for the user to consult theinstructions for use.ISO 15223-1Clause 5.4.2 1051 Do not re-useIndicates a medical device that is intended for one use,or for use on a single patient during a single procedure.ISO 15223-1Clause 5.2.6 2608 Do not resterilize Indicates a medical device that is not to be resterilized.ISO 15223-1Clause 5.2.8 2606Do not use ifpackage is damagedIndicates a medical device that should not be used ifthe package has been damaged or opened.ISO 15223-1Clause 5.3.2 0624Keep away fromsunlightIndicates a medical device that needs protection fromlight sources.ISO 15223-1Clause 5.3.3 0615Protect from heatand radioactivesourcesIndicates a medical device that needs to be protectionfrom heat and radioactive sourcesISO 15223-1Clause 5.3.4 0626 Keep dryIndicates a medical device that needs to be protectedfrom moisture.Stryker’s Sustainability Solutions ©20231810 W Drake Dr.Tempe AZ, 85283888 888 3433Reprocessed ViewFlex Xtra ICE Catheter DescriptionThe Reprocessed ViewFlex Xtra ICE Catheter is a temporary intracardiac ultrasound catheter intended for use in patients to accurately visualize cardiac structures, blood flow and other devices within the heart when connected to compatible intracardiac ultrasound console via the compatible ViewFlex™ Catheter Interface Module. Examples of the types of devices that can be visualized include, and are not limited to, intracardiac catheters, septal occluders, delivery wires, delivery sheaths, sizing balloons and transseptal needles. The use of these images is limited to visualization with no direct or indirect diagnostic use.The Reprocessed ViewFlex Xtra ICE Catheter has a useable length of 90 cm, with a 9 French (F) shaft with an ultrasound transducer. A 10F introducer is recommended for use with this catheter for insertion into the femoral or jugular veins. The catheter tip has four-directional deflection allowing for Left-Right and Posterior-Anterior deflection, with an angle of at least 120 degrees in each direction.The Reprocessed ViewFlex Xtra ICE Catheter is compatible with the ultrasound consoles listed in the table below. See table below for specifics on each ultrasound consoles.H701375 H700296 Compatible ViewFlex Catheter Interface Module 100038191 H701374100043720Maximum Viewing Depth 18 cm 18 cm 18 cm*All consoles are not available in all countries.a CX50 is a trademark of Koninklijke Philips Electronics.N.V.Indications for useThe Reprocessed ViewFlex Xtra ICE Catheter is indicated for use in adult and adolescent pediatric patients to visualize cardiac structures, blood flow and other devices within the heart.Contraindications for useThe Reprocessed ViewFlex Xtra Ice Catheter is contraindicated:▪If there is an occurrence of conditions that create unacceptable risk during catheterization.▪If the patient that has a mechanical tricuspid valve (a prosthetic tissue valve is permissible).▪If the patient has ongoing sepsis or known hypercoagulable state where the catheter could serve as a focal point for septic or bland thrombus formation.▪If the patient has any condition that, in the opinion of the investigator, contraindicates the placement and use of the cardiac catheter or internal ultrasound.Warnings▪The Reprocessed ViewFlex Xtra ICE catheter and system should be used only by or under the direct supervision of a physician thoroughly trained in sonography and ultrasound technology, or with the assistance of asonographer or physician trained in ultrasound technology.▪The Reprocessed ViewFlex Xtra ICE catheter and system should be used only by or under the direct supervision of a physician thoroughly trained in the techniques of cardiac placement during interventional andelectrophysiology procedures.▪The Reprocessed ViewFlex Xtra ICE Catheter is to be used only with the ViewFlex Catheter Interface Module, the ViewMate and the Phillips CX50 ultrasound consoles. Any other use or inappropriate electrical connection may pose a serious risk to patient safety.▪The Reprocessed ViewFlex Xtra ICE Catheter includes a 9F shaft. The physician should consider anatomical size restrictions if considering use of the ViewFlex Xtra ICE catheter on pediatric patients.▪The Reprocessed ViewFlex Xtra ICE catheter is to be used for ultrasound imaging only.▪Do not immerse the proximal handle or cable connector in fluid. Electrical performance may be affected.▪Do not use the Reprocessed ViewFlex Xtra ICE catheter if the packaging is opened or damaged.▪Do not use the Reprocessed ViewFlex Xtra ICE catheter if it is damaged.▪Tactile feedback of reprocessed devices may vary during use.Precautions▪Do not attempt to use the Reprocessed ViewFlex Xtra ICE Catheter prior to completely reading and understanding the Directions for Use.▪The Reprocessed ViewFlex Xtra Ice catheters are supplied sterile only if packaging is not damaged or open.▪Inspect the packaging and catheter for damage or defects prior to use.▪The Reprocessed ViewFlex Xtra ICE Catheters have been sterilized using EtO. Do not attempt to sterilize the catheters by autoclave, gamma or ultraviolet radiation, or liquid sterilizing solutions.▪Do not bend, kink, stretch, or forcefully wipe the catheter. These actions may damage the catheter.▪Do not use mechanical tools or forceps to grip the catheter.▪Have antiarrhythmic drugs, an external defibrillator, and respiratory assist equipment available in case of complications during the use of this device.▪The device should only be used in patients that have received anticoagulation prior or during the procedure. Adverse reactionsAlthough temporary intracardiac catheter sonography procedures have been proven to be safe, the physician should also be aware that complications can occur with the use of any cardiac catheter.Risks that may be associated with the use of the Reprocessed ViewFlex Xtra ICE catheter are those that may be encountered with the introduction and placement of temporary cardiac catheter or pacing lead. As a result of the delivery of electrical energy during internal defibrillation additional risk may result.Adverse events related to cardiac catheterization have been documented and include, but are not limited to:▪Bleeding, hematoma or thrombus at the catheter introduction site▪Cardiac irritability▪Catheter kinking or excessive bending▪Infection/sepsis▪Intercostal or phrenic nerve stimulation▪Mechanical induction of arrhythmias or asystole▪Perforation causing cardiac tamponade▪Perforation of the chamber or vessel wall▪Pneumothorax▪Pulmonary infarction▪Thrombophlebitis▪Tricuspid valve injury▪VasospasmImportant AdviceAny alleged malfunctions, deficiencies, or deterioration in the characteristics and/or performance of this device, along with any alleged inadequacy in the labeling or Instruction for Use, which might lead or have led to a serious injury or death must be brought to the attention of Stryker Sustainability Solutions.Directions for usePreparationIt is recommended practice to have on hand a duplicate of each sterilized item when introducing a catheter. In case the aseptic technique is compromised the procedure can continue.Image Quality Interference (noise)If severe RF interference is experienced during ablation procedures, relocate and/or shield the Reprocessed ViewFlex Xtra ICE catheter electrical extension and Catheter Interface Module.Catheter Insertion and Positioning1.Follow a suitable surgery protocol. The instruction are provided as a general guide and are intended forinformation purposes only, the physician may alter the catheter insertion techniques based on standard clinical practice.2.The Reprocessed ViewFlex Xtra ICE catheter is intended for use during single patient procedure. Do notattempt to resterilize. Stryker will not accept Reprocessed ViewFlex Xtra ICE Catheters for reprocessing that have been reprocessed and sterilized by other facilities.3.The package label is detachable and may be affixed to the medical record of the patient.4.Before beginning the procedure, verify overall compatibility of all instruments and accessories.5.Connect the patient to a vital signs monitor. Track patient vital signs throughout the procedure.6.Inspect packaging before opening. The contents of the package are sterile if the package has not beencompromised.7.Do not use the Reprocessed ViewFlex Xtra ICE Catheter if the sterility has been compromised. If the packageis damaged or if it was opened and the instrument not used, return the Reprocessed ViewFlex Xtra ICECatheter and the package to Stryker.8.Prepare the insertion site using cutdown or percutaneous entry technique. Use a 10F or larger introducersheath.NOTE: It is possible to transfix the femoral artery during percutaneous entry into the femoral vein. Follow proper femoral vein puncture technique.ing proper sterile technique, remove the Reprocessed ViewFlex Xtra ICE Catheter from the package andplace i t in a sterile work area.10.Carefully inspect the catheter for tip integrity and catheter condition. Do not use the catheter if any damage isnoted. Return the Reprocessed ViewFlex Xtra ICE Catheter and packaging to Stryker if it is not in acceptable condition for the procedure.11.Connect the Reprocessed ViewFlex Xtra ICE Catheter connector edge to the ViewFlex Catheter InterfaceModule. Refer to the ViewFlex Catheter Interface Module Instructions for Use for additionalinstructions, precautions, and information on catheter connection.12.Prior to insertion, test that the catheter is imaging by placing the tip in sterile fluid. Movement willappear on the ultrasound console monitor.13.Hold the catheter 1 to 2 cm from the introducer valve and feed it into the introducer slowly to preventbuckling of t he catheter tip.14.Gently insert the catheter into the selected vein and advance the catheter into the heart. Confirm catheterposition with the use of fluoroscopy, if needed. Do not remove and re-insert the catheter into the introducer more than two (2) times during the procedure.15.The Reprocessed ViewFlex Xtra ICE Catheter tip may be deflected as desired during the procedure:▪For Posterior – Anterior deflection, rotate the gray deflection knob labeled P/A clockwise or counterclockwise▪For Left – Right deflection, rotate the green deflection knob labeled L/R clockwise or counterclockwise16.The catheter handle should be secure at all times during the procedure. Do not allow the catheter handle orconnection cable to fall or tug on the catheter body.NOTE: Do not leave the catheter in the patient longer than 12 hours. Transducer performance or incidence of insertion site complications increase significantly with catheters which remain in dwelling longer than this specified time.17.Return both knobs to the neutral position to straighten the distal tip of the catheter before removing thecatheter from the heart. Using fluoroscopy, verify that the distal tip of the catheter is straightened beforeremoving the catheter f rom the heart.18.Refer to the ultrasound console Users’ Manual for additional sonography instructions, precautions, andinformation on catheter connection.Storage and handling▪Room Temperature: 18°C to +26°C (64°F to 79°F)▪Use product on a first-in, first-out basis prior to expiration or use by date on the labelTransport▪Temperature: -20°C to +50°C (-4°F to 122°F)▪Relative Humidity: 25% to 90%Standards and IEC ClassificationsThe Reprocessed ViewFlex Xtra ICE Catheter meets all pertinent clauses of IEC 60601-1 Edition 3+A1;A2, IEC 60601-1-2 Edition 4.0, and IEC 60601-2-37 Edition 2.1.If the Reprocessed ViewFlex Xtra ICE Catheter experiences loss or degradation of the essential performance described in these instructions as a result of EMC disturbances, there would be no effect to intended use.The medical device is suitable to be used in the Professional Healthcare Facility Environment. WarrantyReprocessed productsStryker warrants all reprocessed products, subject to the exceptions provided herein, to be free from defects in reprocessing and to substantially conform to the product specifications contained in the documentation provided by Stryker with the products for one use in accordance with the instructions for use of such product.Products for which Stryker is the original manufacturerStryker warrants all products for which it is the original manufacturer, subject to the exceptions provided herein, to be free from defects in design, materials and workmanship and to substantially conform to the product specifications contained in the documentation provided by Stryker with the products for a period of one year from the date of purchase.General warranty terms applicable to all productsTo the fullest extent permitted by law, the express warranty set forth herein is the only warranty applicable to the products and is expressly in lieu of any other warranty by Stryker, expressed or implied, including, but not limited to, any implied warranty or merchantability or fitness for a particular purpose. In no event will Stryker’s liability arising in connection with the sale of the product (whether under the theories of breach of contract, tort, misrepresentation, fraud, warranty, negligence, strict liability or any other theory of law) exceed the purchase price, current market value or residual value of the products, whichever is less. Stryker shall not be liable for indirect, special, incidental, punitive, or consequential damages resulting from any breach of warranty or under any other legal theory.This warranty shall apply only to the original end-user purchaser of products directly from Stryker or a Stryker authorized distributor. This warranty may not be transferred or assigned without the express written consent of Stryker.This warranty does not apply to: (1) products that have been misused, neglected, modified, altered, adjusted, tampered with, improperly installed or refurbished; (2) products that have been repaired by any person other than Stryker personnel without the prior written consent of Stryker; (3) products that have been subjected to unusual stress or have not been maintained in accordance with the instructions in the user manual or as demonstrated by a Stryker representative; (4) products on which any original serial numbers or other identification marks have been removed or destroyed; or (5) products that have been repaired with any unauthorized or non-Stryker components.If a valid warranty claim is received within thirty (30) days of the expiration of the applicable warranty period, Stryker will, in its sole discretion: (1) replace the product at no charge with a product that is at least functionally equivalent to the original product or (2) refund the purchase price of the product. If a refund is provided by Stryker, the product for which the refund is provided must be returned to Stryker and will become Stryker’s property. In any event, Stryker’s liability for breach of warranty shall be limited to the replacement value of the defective or non-conforming part or component.If Stryker determines in its reasonable discretion that the claimed defect or non-conformance in the product is excluded from warranty coverage as described hereunder, it will notify the customer of such determination and will provide an estimate of the cost of repair of the product. In such an event, any repair would be performed at Stryker’s standard rates.Products and product components repaired or replaced under this warranty continue to be warranted as described herein during the initial applicable warranty period or, if the initial warranty period has expired by the time the product is repaired or replaced, for thirty (30) days after delivery of the repaired or replaced product. When a product or component is replaced, the item provided in replacement will be the customer’s property and the replaced item will be Stryker’s property. If a refund is provided by Stryker, the product for which the refund is provided must be returned to Stryker and will become Stryker’s property.ViewFlex and ViewMate are trademarks of St. Jude Medical, Inc.ICE EL10105 Rev. E 07/2023 RM705008。
各种字母代号LAPRIL-C003 Rev

PROJECT STANDARD 项目标准PROCESS 程序LAPRIL-C003Rev. 01FLOW SUBSTANCES AND MATERIAL SELECTION GUIDE 流体物质与材料选择准则CONTENTS目录APPENDIX 附件1234561a)b)c)d)e)f)g)l)o)p)s)w)General 概述Flow substance code 流体物质编号Design and Operation Pressure and Temperature设计、工作压力和温度Valve codes 阀门编号Pipe, valve and gasket materials 管道、阀门和垫片材料Valve selection guidelines 阀门选择准则Flow Substance, Piping and Valve Material Recommendations流体物质管道和阀门材料建议Pressurized air or vacuum 加压空气或真空Additional materials 其它材料Condensates 冷凝物Chemicals 化工品Effluent 污水Feed water 进水Gases 气体Liquors 液体Oils 油Pulp stock 浆料Solid materialsr) 固体材料)Steam 蒸汽Waters 水DISTRIBUTION分发:Project Director, Project Area Mgrs, Project AreaCoordinators, Procurement MgrEngineering Mgr, Engineering Discipline Mgrs,Signed PDF to Documentation Superviser将项目负责人、项目区域经理、项目区域协调员、采购经理、工程经理、工程纪律经理签字的PDF分发至文件编制主管。
BT134-800 4Q Triac 27 April 2018 产品数据手册说明书
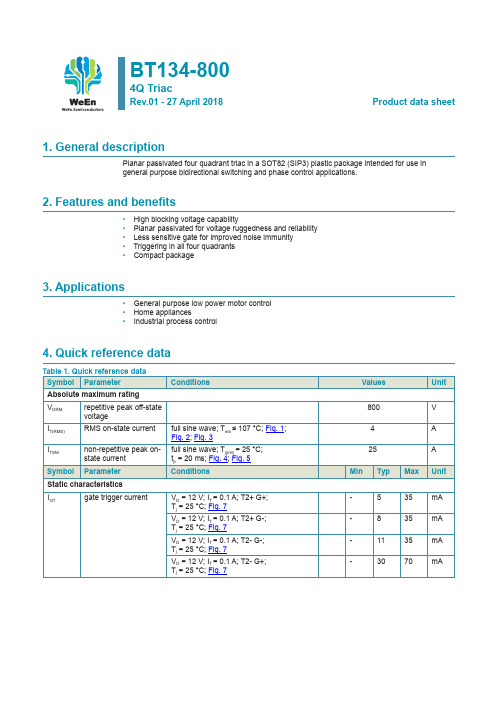
BT134-8004Q TriacRev.01 - 27 April 2018Product data sheet1. General descriptionPlanar passivated four quadrant triac in a SOT82 (SIP3) plastic package intended for use ingeneral purpose bidirectional switching and phase control applications.2. Features and benefits• High blocking voltage capability• Planar passivated for voltage ruggedness and reliability• Less sensitive gate for improved noise immunity• Triggering in all four quadrants• Compact package3. Applications• General purpose low power motor control• Home appliances• Industrial process control4. Quick reference data5. Pinning information6. Ordering information7. Marking8. Limiting values Table 5. Limiting values9. Thermal characteristics10. Characteristics11. Package outline12. Legal informationData sheet status[1lease consult the most recently issued document before initiating or completing a design.[2]The term 'short data sheet' is explained in section "Definitions".[3]The product status of device(s) described in this document may havechanged since this document was published and may differ in case ofmultiple devices. The latest product status information is available onthe Internet at URL h ttp://.DefinitionsDraft — The document is a draft version only. The content is still under internal review and subject to formal approval, which may result in modifications or additions. WeEn Semiconductors does not give any representations or warranties as to the accuracy or completeness of information included herein and shall have no liability for the consequences of use of such information.Short data sheet — A short data sheet is an extract from a full data sheet with the same product type number(s) and title. A short data sheet is intended for quick reference only and should not be relied upon to contain detailed and full information. For detailed and full information see the relevant full data sheet, which is available on request via the local WeEn Semiconductors sales office. In case of any inconsistency or conflict with the short data sheet, the full data sheet shall prevail.Product specification — The information and data provided in a Product data sheet shall define the specification of the product as agreed between WeEn Semiconductors and its customer, unless WeEn Semiconductors and customer have explicitly agreed otherwise in writing. In no event however, shall an agreement be valid in which the WeEn Semiconductors productis deemed to offer functions and qualities beyond those described in the Product data sheet.DisclaimersLimited warranty and liability — Information in this document is believedto be accurate and reliable. However, WeEn Semiconductors does notgive any representations or warranties, expressed or implied, as to the accuracy or completeness of such information and shall have no liability for the consequences of use of such information. WeEn Semiconductors takes no responsibility for the content in this document if provided by an information source outside of WeEn Semiconductors.In no event shall WeEn Semiconductors be liable for any indirect, incidental, punitive, special or consequential damages (including - without limitation -lost profits, lost savings, business interruption, costs related to the removal or replacement of any products or rework charges) whether or not such damages are based on tort (including negligence), warranty, breach of contract or any other legal theory.Notwithstanding any damages that customer might incur for any reason whatsoever, WeEn Semiconductors’ aggregate and cumulative liability towards customer for the products described herein shall be limited in accordance with the Terms and conditions of commercial sale of WeEn Semiconductors.Right to make changes — WeEn Semiconductors reserves the right to make changes to information published in this document, including without limitation specifications and product descriptions, at any time and without notice. This document supersedes and replaces all information supplied prior to the publication hereof.Suitability for use — WeEn Semiconductors products are not designed, authorized or warranted to be suitable for use in life support, life-criticalor safety-critical systems or equipment, nor in applications where failureor malfunction of an WeEn Semiconductors product can reasonablybe expected to result in personal injury, death or severe property or environmental damage. WeEn Semiconductors and its suppliers accept no liability for inclusion and/or use of WeEn Semiconductors products in such equipment or applications and therefore such inclusion and/or use is at the customer’s own risk.Quick reference data — The Quick reference data is an extract of the product data given in the Limiting values and Characteristics sections of this document, and as such is not complete, exhaustive or legally binding. Applications — Applications that are described herein for any of these products are for illustrative purposes only. WeEn Semiconductors makesno representation or warranty that such applications will be suitable for the specified use without further testing or modification.Customers are responsible for the design and operation of their applications and products using WeEn Semiconductors products, and WeEn Semiconductors accepts no liability for any assistance with applications or customer product design. It is customer’s sole responsibility to determine whether the WeEn Semiconductors product is suitable and fit for the customer’s applications and products planned, as well as for the planned application and use of customer’s third party customer(s). Customers should provide appropriate design and operating safeguards to minimize the risks associated with their applications and products.WeEn Semiconductors does not accept any liability related to any default, damage, costs or problem which is based on any weakness or defaultin the customer’s applications or products, or the application or use by customer’s third party customer(s). Customer is responsible for doing all necessary testing for the customer’s applications and products using WeEn Semiconductors products in order to avoid a default of the applicationsand the products or of the application or use by customer’s third party customer(s). WeEn does not accept any liability in this respect.Limiting values — Stress above one or more limiting values (as defined in the Absolute Maximum Ratings System of IEC 60134) will cause permanent damage to the device. Limiting values are stress ratings only and (proper) operation of the device at these or any other conditions above thosegiven in the Recommended operating conditions section (if present) or the Characteristics sections of this document is not warranted. Constant or repeated exposure to limiting values will permanently and irreversibly affect the quality and reliability of the device.No offer to sell or license — Nothing in this document may be interprete d or construed as an offer to sell products that is open for acceptance or the grant, conveyance or implication of any license under any copyrights, patents or other industrial or intellectual property rights.Export control — This document as well as the item(s) described herein may be subject to export control regulations. Export might require a prior authorization from competent authorities.Non-automotive qualified products — Unless this data sheet expressly states that this specific WeEn Semiconductors product is automotive qualified, the product is not suitable for automotive use. It is neither qualified nor tested in accordance with automotive testing or application requirements. WeEn Semiconductors accepts no liability for inclusion and/or use of non-automotive qualified products in automotive equipment or applications.In the event that customer uses the product for design-in and use in automotive applications to automotive specifications and standards, customer (a) shall use the product without WeEn Semiconductors’ warranty of the product for such automotive applications, use and specifications, and (b) whenever customer uses the product for automotive applications beyond WeEn Semiconductors’ specifications such use shall be solely at customer’s own risk, and (c) customer fully indemnifies WeEn Semiconductors forany liability, damages or failed product claims resulting from customer design and use of the product for automotive applications beyond WeEn Semiconductors’ standard warranty and WeEn Semiconductors’ product specifications.]PTranslations — A non-English (translated) version of a document is for reference only. The English version shall prevail in case of any discrepancy between the translated and English versions.TrademarksNotice: All referenced brands, product names, service names and trademarks are the property of their respective owners.13. Contents1. General description (1)2. Features and benefits (1)3. Applications (1)4. Quick reference data (1)5. Pinning information (2)6. Ordering information (2)7. Marking (2)8. Limiting values (3)9. Thermal characteristics (6)10. Characteristics (7)11. Package outline (10)12. Legal information (11)13. Contents (13)© WeEn Semiconductors Co., Ltd. 2018. All rights reservedFor more information, please visit: Forsalesofficeaddresses,pleasesendanemailto:**************************** Date of release: 27 April 2018。
欧盟ASMF递交法规及相关模板

31 May 2013Committee of Human Medicinal Products CHMP/QWP/227/02 Rev 3/Corr *Committee of Veterinary Medicinal Products EMEA/CVMP/134/02 Rev 3/Corr *Guideline on Active Substance Master File Procedure FinalDiscussion at the HMPC November 2005 – January 2006 Adoption by the HMPC 22 January 2006 Draft agreed by Quality Working Party 9 February 2006 Adoption by CHMP for release for consultation 23 March 2006 Adoption by CVMP for release for consultation 20 April 2006 End of consultation (deadline for comments) 30 August 2006 Agreed by Quality Working Party 1 December 2011 Adoption by CHMP for release for consultation 15 December 2011 Adoption by CVMP for release for consultation 12 January 2012 End of consultation (deadline for comments) 12 March 2012 Rev. 03 Agreed by Quality Working Party 04 May 2012 Rev. 03 Adoption by CVMP 14 June 2012 Rev. 03 Adoption by CHMP 21 June 2012 Rev. 03 Date for coming into effect 1 October 2012 This guideline replaces guideline CPMP/QWP/227/02 Rev 2 (EMEA/CVMP/134/02 Rev.2).Keywords Active substance master file, ASMF, letter of access, submission letter 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United KingdomNote:The corrections introduced to this guideline aim to support the Working Group on Active Substance Master File Procedures in their initiatives to improve the ASMF procedure across the European Regulatory Network. To this end, the annexes to the guideline have been revised, and one new annex introduced. Some minor textual changes in the main part of the guideline have been introduced as a consequence of the revised annexes.* The correction concerns a clarification in:•Annex 1, detailing which manufacturing sites are to be declared in section 3.2.S.2.1;•Annex 3, detailing the need to declare the salt form, water content and grade of the active substance, as applicable.Table of contentsExecutive summary (4)1. Introduction (background) (4)2. Scope (4)3. Legal basis (4)4. Content of the Active Substance Master File (5)5. Use of the Active Substance Master File Procedure (5)6. Content of the MA-dossier when the Active Substance Master File Procedure is used (7)7. Changes and updates to the Active Substance Master File (7)ANNEX 1 (9)ANNEX 2 (14)ANNEX 3 (15)ANNEX 4 (19)ANNEX 5 (20)ANNEX 6 (21)ANNEX 7 (22)Executive summary1. Introduction (background)The main objective of the Active Substance Master File (ASMF) procedure, formerly known as the European Drug Master File (EDMF) procedure, is to allow valuable confidential intellectual property or 'know-how' of the manufacturer of the active substance (ASM) to be protected, while at the same time allowing the Applicant or Marketing Authorisation (MA) holder to take full responsibility for the medicinal product and the quality and quality control of the active substance. National Competent Authorities/EMA thus have access to the complete information that is necessary for an evaluation of the suitability of the use of the active substance in the medicinal product.2. ScopeThis Guideline is intended to assist Applicants/MA holders in the compilation of the active substance section of their dossiers for a Marketing Authorisation Application (MAA) or a Marketing Authorisation Variation (MAV) of a medicinal product. It is also intended to help ASMF holders in the compilation of their ASMFs.ASMF Procedure and herbal substances/preparationsIn accordance with Directive 2001/83/EC as amended, the quality of traditional herbal medicinal products for human use has to be documented in accordance with existing European legislative requirements. These criteria are laid down in the following guidelines (which are applicable for all Human and Veterinary Herbal Medicinal products): ‘Guideline on quality of herbal medicinalproducts/traditional herbal medicinal products’ (CPMP/QWP/2819/00, EMEA/CVMP/814/00, in their latest revisions) and the ‘Guideline on specifications: test procedures and acceptance criteria for herbal substances, herbal preparations and herbal medicinal products/ traditional herbal medicinal products’ (CPMP/QWP/2820/00, EMEA/CVMP/815/00, in their latest revisions).It should be noted that the principles which are outlined in table 3 of Annex 1 in relation to traditional herbal medicinal products are equally applicable to other herbal medicinal products, both for Human and Veterinary use, which do not follow the simplified registration procedure.References:1. ‘Guideline on quality of herbal medicinal products/traditional herbal medicinal products’(CPMP/QWP/2819/00, EMEA/CVMP/814/00, in their latest revisions);2. ‘Guideline on specifications: test procedures and acceptance criteria for herbal substances, herbalpreparations and herbal medicinal products/ traditional herbal medicinal products’(CPMP/QWP/2820/00, EMEA/CVMP/815/00, in their latest revisions);3. ‘Guideline on summary of requirements for active substances in the quality part of the dossier’(CHMP/QWP/297/97, EMEA/CVMP/1069/02, in their latest revisions).3. Legal basisAnnex I to Directive 2001/83/EC as amended Part I, 3.2 Basic principles and requirements, (8) Active Substance Master File (for Human medicinal products) and Annex I to Directive 2001/82/EC asamended, Part 2.C.1 General Requirements, 1.1. Active Substances (for Veterinary medicinal products).4. Content of the Active Substance Master FileThe overall content of the ASMF should contain detailed scientific information as indicated under the various headings of the relevant Notice to Applicants for Marketing Authorisations for Medicinal Products in the Member States of the European Union (NtA).ASMFs linked to human medicinal products should be presented in the format of the Common Technical Document (CTD), see Annex 1 table 1.ASMFs linked to veterinary medicinal products should normally be presented in accordance with the format given in Annex 1 table 2, however in accordance with Parts 1.C and 2 of Directive 2001/82/EC as amended, all parts of such ASMFs (AP, RP, and their summaries) may be presented in the CTD format in the following circumstances1:•Where the active substance has been included in a medicinal product for human use authorised in accordance with the requirements of Annex I to Directive 2001/83/EC as amended;•In the case of any application for an animal species or for indications representing smaller market sectors;•Where the competent authority has publicly announced this possibility.The scientific information in the ASMF should be physically divided into two separate parts, namely the Applicant’s Part (AP) and the Restricted Part (RP). The AP contains the information that the ASMF holder regards as non-confidential to the Applicant/MA holder, whereas the RP contains the information that the ASMF holder regards as confidential, see Annex 1. It is emphasized that the AP is still a confidential document that cannot be submitted by anyone to third parties without the written consent of the ASMF holder. In all cases the AP should contain sufficient information to enable the Applicant/MA holder to take full responsibility for an evaluation of the suitability of the specification for the active substance to control the quality of this active substance for use in the manufacture of a specified medicinal product.The RP may contain the remaining information, such as detailed information on the individual steps of the manufacturing method (reaction conditions, temperature, validation and evaluation data of critical steps) and the quality control during the manufacture of the active substance. The National Competent Authorities/EMA may not accept that particular information has not been disclosed to the Applicant/MA holder. In such cases, the National Competent Authorities/EMA may ask for an amendment to the AP. In addition to the AP and RP, the ASMF should contain a table of contents, and separate summaries for both the AP and the RP. In cases where the ASMF is provided in the CTD format, both summaries should be presented as a Quality Overall Summary (QOS). In cases where the veterinary NtA format is used, they should be detailed and critical summaries. Each version of the AP and RP should have unique and independent version control numbers.5. Use of the Active Substance Master File ProcedureAn ASMF can only be submitted in support of an MAA or MAV. The relationship between the quality of the active substance and its use in the medicinal product needs to be justified in this MAA or MAV.1A correlation table should also be provided for ASMFs for Veterinary applications presented in the CTD format.Although the ASMF procedure is developed to keep intellectual property of the ASM confidential, it is also permissible to use the procedure when there is no confidentiality issue between the Applicant/MA holder and the ASM (e.g. when the Applicant/MA holder synthesises the active substance himself). It is expected that the ASM is also the holder of the ASMF.The ASMF procedure can be used for the following active substances, including herbal active substances/preparations. i.e.:A. New active substances;B. Existing active substances not included in the European Pharmacopoeia (Ph. Eur.) or thepharmacopoeia of an EU Member State;C. Pharmacopeial active substances included in the Ph. Eur. or in the pharmacopoeia of an EU MemberState.The ASMF procedure cannot be used for biological active substances, see Annex 5.The ASMF holder may have an ASMF as well as a Certificate of Suitability (CEP) issued by EDQM for a single active substance. Generally, it is however not acceptable that the Applicant/MA holder refers to an ASMF as well as to a CEP for a single active substance of a particular MAA/MAV. In cases where the CEP contains too little information (e.g. stability) the National Competent Authorities/EMA may decide that additional information should be provided in the dossier. In such case it may be acceptable to refer both to an ASMF and a CEP.The ASMF holder should give permission to the National Competent Authorities/EMA to assess the data in the ASMF in relation to a specific MAA/MAV, in the form of a ‘Letter of Access’, see Annex 2.The ASMF holder should submit to the Applicant/MA holder:• a copy of the latest version of the AP (and, if applicable, responses to deficiency letters on the AP from a NCA/EMA if not already incorporated into the AP);• a copy of the QOS or detailed and critical summary, as appropriate, on the latest version of the AP;• a copy of the Letter of Access where this letter has not been submitted earlier for the product concerned.In addition, it is an essential requirement that the ASMF holder should submit to all National Competent Authorities/EMA involved in the MAA/MAV procedure:•the ASMF (and, if applicable, responses to deficiency letters from a NCA/EMA if not already incorporated into the ASMF), accompanied by a Submission Letter and Administrative Details, see Annex 3. This also applies to the ASMF holder's responses to deficiency letters from a NCA/EMA; •the Letter of Access where this letter has not been submitted earlier for the product concerned. The ASMF holder should submit the ASMF to the National Competent Authority/EMA either for each MAA and each MAV or only once according to national requirements. The submission of the relevant documentation by the ASMF holder to the National Competent Authority/EMA must be synchronised to arrive at approximately the same time as the MAA or the MAV i.e. not more than one month before and not after the intended MAA/MAV submission date.Where the ASMF procedure is used, the Applicant/MA holder should submit the MAA or MAV to the National Competent Authorities/EMA together with the Letter of Access where this Letter has not been submitted earlier by the MA holder/Applicant himself or by the ASMF holder for the product concerned.Where the same active substance is used in a number of applications for different products in one or more Member States, the ASMF holder should submit identical documentation to every National Competent Authority/EMA. Consequently, the National Competent Authorities/EMA may require that any ASMF updates made in relation to one MA should apply to all. It is the ASMF holder’s responsibility to notify the MA holders and National Competent Authorities/EMA concerned about any changes to the AP and/or RP, so that the MA holders can update all affected MAs accordingly.6. Content of the MA-dossier when the Active Substance Master File Procedure is usedThe Applicant/MA holder is responsible for ensuring that he has access to all relevant information concerning the current manufacture of the active substance.The specification used by the Applicant/MA holder to control the correct quality of the active substance should be laid down unambiguously in the MA dossier (CTD format section 3.2.S.4.1 and 3.2.S.4.2 or old human/veterinary NtA format part 2.C.1). The Applicant/MA holder should include a copy of the AP in the MA dossier (CTD format section 3.2.S or veterinary NtA format part 2.C.1). The version of the AP in the MA dossier should be the most recent and it should be identical to the AP as supplied by the ASMF holder to the National Competent Authority/EMA as part of the ASMF. The Applicant/MA holder should include all relevant details from the AP in the QOS/detailed and critical summary of the MA dossier. Issues of the ASMF that are specifically relevant to the product under consideration should be highlighted in the QOS/detailed and critical summary of the MA dossier.In the case of a single supplier and where the ASMF procedure or CEP procedure is used, the specification for the active substance provided by the Applicant/MA holder in the MA dossier should in principle be identical to that of the ASMF holder or the CEP holder. However, the Applicant/MA holder does not need to accept redundant tests in the specification, unnecessarily tight specification limits or outdated analytical methods.In cases where the Applicant/MA holder uses a different analytical method than that described in the ASMF, both methods should be validated. Technical tests in the specification that are relevant for the medicinal product, but which are normally not part of the specification in the ASMF (e.g. particle size), should be part of the specification of the Applicant/MA holder.In cases where there is more than one supplier, the Applicant/MA holder should have one single compiled specification that is identical for each supplier. It is acceptable to lay down in the specification more than one acceptance criterion and/or analytical method for a single parameter with the statement ‘if tested’ (e.g. in case of residual solvents).7. Changes and updates to the Active Substance Master FileAs for medicinal products, ASMF holders should keep the content of their ASMFs updated with respect to the actual synthesis/manufacturing process. The quality control methods should be kept in line with the current regulatory and scientific requirements.ASMF holders shall not modify the contents of their ASMF (e.g. manufacturing process or specifications) without informing each Applicant/MA holder and each National CompetentAuthority/EMA. This obligation remains valid until the Letter of Access has been withdrawn by the ASMF holder, see Annex 4. ASMF holders should provide the updated ASMF to all interested parties with reference to the revised version number.Any change to the ASMF should be reported by every MA holder to the relevant National Competent Authority/EMA by means of an appropriate variation procedure. A Submission Letter should be provided (Annex 3).In cases where the contents of the ASMF cannot be changed for a certain period of time because of other procedural provisions (i.e. mainly because of on-going MRP procedures), the ASMF holder should still provide the aforementioned data to the MA holder and National Competent Authorities/EMA making reference to this reason and requesting a later date of implementation.At the occasion of the 5-year renewal of a medicinal product, MA holders are required to declare that the quality of the product, in respect of the methods of preparation and control, has been regularly updated by variation procedure to take account of technical and scientific progress, and that the product conforms with current CHMP/CVMP quality guidelines. They will also declare that no changes have been made to the product particulars other than those approved by the CompetentAuthority/EMA.MA holders should therefore verify with their ASMF holders whether the above declaration can be met in respect to the active substance particulars. In case changes have not been notified to the MA holder and National Competent Authority/EMA, the necessary variation procedure should be initiated without delay.ANNEX 1OVERVIEW ASMF CONTENTSTable 1CTD format Applicant’s Part Restricted Part 3.2.S.1 General information x 3.2.S.1.1 Nomenclature x 3.2.S.1.2 Structure x 3.2.S.1.3 General properties x 3.2.S.2 Manufacture x X 3.2.S.2.1 Manufacturer(s)2 x 3.2.S.2.2Description of Manufacturing Process and Process controls a) b) 3.2.S.2.3 Control of Materials X 3.2.S.2.4 Control of critical steps and intermediates c) d) 3.2.S.2.5 Process validation and/or Evaluation X 3.2.S.2.6 Manufacturing Process Development X 3.2.S.3 Characterisation x 3.2.S.3.1 Elucidation of Structure and other Characteristics x 3.2.S.3.2 Impurities x e) 3.2.S.4 Control of Drug Substance x 3.2.S.4.1 Specification x 3.2.S.4.2 Analytical procedures x 3.2.S.4.3 Validation of analytical procedures x 3.2.S.4.4 Batch analysis x 3.2.S.4.5 Justification of specification x f) 3.2.S.5 Reference standards or materials x 3.2.S.6 Container Closure System x 3.2.S.7 Stability x 3.2.S.7.1 Stability summary and conclusion x 3.2.S.7.2Post-approval Stability Protocol and Stability Commitment x 3.2.S.7.3 Stability data x 2 Including all companies involved in the manufacture of the active substance, including quality control/ in process testing sites, intermediate manufacturers, milling and sterilisation sites.Table 2 NtA veterinary format Applicant’sPart RestrictedPart2.C.1 Name(s) and site(s) of ASM x X2.C.1.1 Specifications and routine tests x2.C.1.2.1 Nomenclature x2.C.1.2.2 Description x2.C.1.2.3 Brief outline of the manufacturing route (flow chart) x2.C.1.2.3 Detailed description manufacturing method X2.C.1.2.4 QC during manufacture c) d)Process validation and evaluation of data X2.C.1.2.5 Development Chemistry xEvidence of structure xPotential Isomerism xPhysiochemical characterisation xAnalytical validation x2.C.1.2.6 Impurities x e)2.C.1.2.7 Batch analysis x2.F.1 Stability xa) Flow chart and short description is regarded as sufficient, if detailed information is presented in theRestricted Part. However, full validation data on the sterilisation process may be requested in the Applicant’s Part (in cases where there is no further sterilisation of the final product).b) Detailed information.c) As far as the information is also relevant for the Applicant/MA holder.d) As far as the information is related to the detailed description of the manufacturing process and asfar as this information is not relevant for the Applicant/MA holder.e) In so far as the information is related to the detailed description of the manufacturing process andin so far as the ASMF holder sufficiently justifies that there is no need to control these impurities in the final active substance.f) As far as the information is related to the detailed description of the manufacturing process, controlof materials and process validation.Table 3 NtA CTD format3Herbal Active Substances/ Preparations Applicant’sPartRestricted Part3.2.S.1 General information X3.2.S.1.1 Nomenclature XFor herbal substance:Binomial scientific name of plant (genus, species,variety and author), and chemotype (whereapplicable)Parts of the plantsDefinition of the herbal substanceOther names (synonyms mentioned in otherPharmacopoeias)Laboratory codeFor herbal preparationsBinomial scientific name of plant (genus, species,variety and author), and chemotype (whereapplicable)Parts of the plantsDefinition of the herbal preparationRatio of the herbal substance to the herbalpreparationExtraction solvent(s)Other names (synonyms mentioned in otherPharmacopoeias)Laboratory code3.2.S.1.2 Structure X- Physical form- Description of the constituents with knowntherapeutic activity or markers (molecular formula,relative molecular mass, structural formula, includingrelative and absolute stereochemistry, the molecularformula, and the relative molecular mass).- Other constituent(s)3.2.S.1.3 General properties X3.2.S.2 Manufacturer(s)For herbal substancesThe name, address, and responsibility of eachsupplier, including contractorseach proposed site or facility involved inproduction/collection and testing of the herbalsubstance should be provided, where appropriate.For herbal preparationsThe name, address, and responsibility of each manufacturer, including contractors, and each proposed manufacturing site or facility involved in manufacturing and testing of the herbal preparation should be provided, where appropriate. XX3.2.S.2.2 Description of critical steps and intermediates Flow chart Detailedinformation 3ASMFs for Veterinary herbal medicinal products should be presented in accordance with section 4.1 above.For herbal substancesInformation should be provided to adequatelydescribe the plant productionand plant collection, including:Geographical source of medicinal plantCultivation, harvesting, drying and storage conditionsFor herbal preparationsInformation should be provided to adequatelydescribe the manufacturingprocess of the herbal preparation, including:Description of processingSolvents, reagentsPurification stagesStandardisation3.2.S.2.3 Control of materials XX 3.2.S.2.4 Control of critical steps and intermediates If also relevantfor the MAholder/applicant3.2.S.2.5 Process validation and/or evaluation X X3.2.S.2.6 Manufacturing Process Development XA brief summary describing the development of theherbal substance(s) and herbal preparation(s) whereapplicable should be provided, taking intoconsideration the proposed route of administrationand usage. Results comparing the phytochemicalcomposition of the herbal substance(s) and herbalpreparation(s) where applicable used in supportingbibliographic data and the herbal substance(s) andherbal preparation(s) where applicable described inS1 should be discussed, where appropriate.3.2.S.3 Characterisation X3.2.S.3.1 Elucidation of structure and other characteristics XFor herbal substancesInformation on the botanical, macroscopical,microscopical, phytochemical characterisation, andbiological activity if necessary, should be provided:For herbal preparationsInformation on the phyto- and physicochemicalcharacterisation, and biological activity if necessary,should be provided:3.2.S.3.2 Impurities X3.2.S.4 Control of drug substance X3.2.S.4.1 Specification X3.2.S.4.2 Analytical procedure X3.2.S.4.3 Validation of analytical procedure X3.2.S.4.4 Batch analysis X3.2.S.4.5 Justification of specification X X 3.2.S.5 Reference standards of materials X3.2.S.6 Container closure system X3.2.S.7 Stability X3.2.S.7.1 Stability summary and conclusion X3.2.S.7.2 Post-approval stability protocol and stabilitycommitment X3.2.S.7.3 Stability data XANNEX 2(< FROM ACTIVE SUBSTANCE MASTER FILE HOLDER ON HEADED PAPER>)TEMPLATE LETTER OF ACCESS[Address of Competent Authority/EMA][Date]Number of Active Substance Master File:<EU/ASMF/XXXXX4 or National ASMF reference number5>Name of Active Substance:Internal API Code (if applicable):Active Substance Master File holder: [name and address]The aforementioned Active Substance Master File holder hereby authorises the <name of National Competent Authority> <EMA including all CHMP and CVMP Members and their experts> to refer to and review the above mentioned Active Substance Master File in support of the following Marketing Authorisation Application(s) or Marketing Authorisation Variation(s)6 submitted by [Name of Marketing Authorisation Holder/Applicant] on [planned date of submission]:[Name of product7 and Marketing Authorisation number (if known)][Name of Applicant or Marketing Authorisation holder]The aforementioned Active Substance Master File holder commits to ensure batch to batch consistency and to inform [Name of Marketing Authorisation Holder/Applicant] and Competent Authority/EMA of any change in the Active Substance Master File.The aforementioned Active Substance Master File holder hereby is informed of and accepts that the EEA National Competent Authorities, the EMA including all CHMP and CVMP Members and their experts, and the Certification of Substances Division of the European Directorate for the Quality of Medicines & Healthcare may share the assessment reports of the above mentioned Active Substance Master File amongst themselves.Signature for the Active Substance Master File holder[Name and function][Signature]4 EU/ASMF/XXXXX reference number is allocated from the CTS ASMF assessment report repository (when available) by the Competent Authority/EMA5 The national ASMF reference numbers is allocated by the Competent Authority and should be used for national Marketing Authorisations only or when the EU/ASMF reference number is not allocated6 i.e. to introduce a new ASMF from a new AS manufacturer.7If no invented name has been agreed at the time of submission for this product: it should be indicated ‘INN + Marketing Authorisation Holder name’ANNEX 3(< FROM ACTIVE SUBSTANCE MASTER FILE HOLDER ON HEADED PAPER>)Template Submission Letter and Administrative Details for documents relating to an ActiveSubstance Master File (ASMF)8From:<ASMF Holder name> <ASMF Holder address><ASMF Holder address><ASMF Holder <Post code> Town><ASMF Holder Country>To: <Name and Address of Competent Authority><Date> <Reference>Subject: Submission of documents relating to an ASMFfor <Name of Active Substance> - <EU/ASMF/XXXXX 9 or national ASMF reference number> 10Dear Sir or Madam:This Active Substance Master File is submitted in relation to the following product:Yours faithfully,<Signature of authorised contact person> <Name, address and position in company>8To be submitted together with the ASMF in conjunction with every MAA/variation submission as one document9EU/ASMF/XXXXX reference number is allocated from the CTS ASMF assessment report repository (when available) by the Competent Authority/EMA 10The national ASMF reference numbers is allocated by the Competent Authority and should be used for national Marketing Authorisations only or when EU/ASMF reference number is not allocated 11If no invented name has been agreed at the time of submission for this product: it should be indicated ‘INN + Marketing Authorisation Holder name’Medicinal productAllocated procedure number (as applicable)(Intended) Submission date of the marketingauthorisation application or variation (if known) <Name of the medicinal product>11<EMEA/H/C/product reference number/procedure reference> <RMS/H/product reference number/procedure reference> <National Marketing Application/Authorisation Reference><DD/MM/YYYY>。
散发型克雅病1例并文献复习

㊃病例报告㊃散发型克雅病1例并文献复习尹阔场1,罗欣彤2,赵立明1,张海宁1,马如雪1,檀国军1(1.河北医科大学第二医院神经内科,河北石家庄050000;2.河北省人民医院神经内科,河北石家庄050000)关键词:克-亚二氏综合征;脑电描记术;脑脊液蛋白质类;磁共振成像中图分类号:R 742文献标志码:A文章编号:1004-583X (2018)01-0082-03d o i :10.3969/j .i s s n .1004-583X.2018.01.013 克雅病(C re u t zf e l d t -J a k o bd i s e a s e ,C J D )最早在1929年由C r e u t z f e l d t 和J a k o b 先后报道,是最常见的一种朊蛋白病㊂现今全世界已有50多个国家和地区发现C J D ,年发病率1~2/100万㊂此病临床较为少见,常表现为快速进展的认知障碍但不典型,容易误诊,暂无有效治疗方法,但同样以认知障碍发病的其他疾病或可有较好的预后,故临床工作中应重视认知障碍患者的诊断及鉴别诊断,避免错过可治性疾病的最佳治疗时机㊂现报道我科诊治的散发型C J D1例㊂1 临床资料1.1 病历摘要 患者男,68岁,退休医生,主因记忆力减退1个月余加重20天于2015年9月10日入院㊂患者于入院前1个月出现记忆力减退,近记忆受损较严重,表现为忘记关冰箱门㊁出门忘记拔钥匙等,当时患者及家属未予重视㊂20天前,患者于情绪波动后出现记忆力减退症状加重,表现为在家中无法独立找到厕所,不能回忆早饭内容等,期间走失1次,遂就诊于某三甲医院,查头颅磁共振成像(M R I )+磁共振弥散加权成像(DW I)(图1):双侧额顶叶皮质异常高信号,为求进一步治疗就诊于我院㊂既往高血压病史20年,最高达165/95mmH g (1mmH g =0.133k P a ),未服药;左心室肥大10年,否认糖尿病㊁冠心病病史㊂个人史㊁家族史无特殊,否认性病㊁冶游史㊂入院查体:体温36.5ħ,脉搏72次/m i n ,呼吸16次/m i n ,血压138/87mmH g ,心肺腹未见明显异常,神清语利,近记忆力减退,反应力㊁定向力尚可,双瞳孔正大等圆,直径约3mm ,直接㊁间接对光反射灵敏,眼球各方向活动充分自如,无眼震㊂双侧鼻唇沟无变浅,伸舌无偏斜,四肢肌力Ⅴ级,肌张力正常,双侧肱二三头肌腱反射㊁膝腱反射(++),双侧B a b i n s k i 征(-)㊂感觉及共济系统查体未见明显异常,颈无抵抗㊂简易智力状态检查量表(MM S E )评分25分㊂辅助检查:脑磁共振血管造影(M R A )未见明显异常,血㊁尿㊁便及凝血常规㊁生化未见明显异常㊂初步诊断为:①认知障碍待查:自身免疫性脑炎?线粒体脑肌病?克-雅脑病?②高血压2级高危㊂给予膦甲酸钠㊁醒脑静㊁甲钴胺㊁前列地尔等治疗㊂图1 患者外院所做D W I,可见脑叶皮质花边样改变通信作者:檀国军,E m a i l :T t a n g ju n @h o t m a i l .c o m 1.2 脑电图 入院后查视频脑电图(图2):重度异常,顶区可见少量8~9H z α节律,全导可见弥漫性3~7H z 慢波活动,可见3~4H z 高幅三相尖慢复合波,前头部为著㊂北京协和医院血清检测自身免疫性脑炎抗体谱均阴性㊂行小运动量试验:乳酸(运动前)2.2mm o l /L ,乳酸(运动后0分钟)7.9mm o l /L ,乳㊃28㊃‘临床荟萃“ 2018年1月5日第33卷第1期 C l i n i c a l F o c u s ,J a n u a r y 5,2018,V o l 33,N o .1Copyright ©博看网. All Rights Reserved.酸(运动后10分钟)3.3mm o l /L ㊂入院5天后患者记忆力进行性减退,间断出现不认识家人㊁大小便失禁等症状㊂图2 患者入院第5天视频脑电图图像,可见三相波 箭头所示为部分三相波1.3 脑脊液 颅压160mmH 2O (1mmH 2O =0.0098k P a ),送中国疾病预防控制中心传染病预防控制所化验14-3-3蛋白,由于患者病情进展迅速,家属对预后较为悲观,期望值较低,故要求出院回家等待化验结果,出院时查体:体温36.4ħ,脉搏72次/m i n ,呼吸18次/m i n ,血压134/78mmH g,心肺腹未见异常,患者缄默不语,精神差,反应力㊁定向力差,双瞳孔正大等圆,直径约3mm ,眼球向各方向活动充分自如,无眼震㊂双侧鼻唇沟无变浅,伸舌不偏,四肢肌力Ⅴ级,肌张力正常,四肢腱反射(++),双侧B a b i n s k i 征(-)㊂感觉及共济系统查体未见明显异常,颈抵抗(-)㊂1.4 随访 出院后随访得知患者多次出现肌阵挛表现,运动减少,运动不能,脑脊液14-3-3蛋白回报阳性,确诊为散发型C J D ,2015年12月患者离世㊂2 讨 论C JD 是一种罕见的海绵状脑病,是由朊蛋白感染所致[1]㊂朊蛋白是一种不同于细菌㊁病毒㊁真菌㊁寄生虫等病原微生物的缺乏核酸的蛋白质感染因子,它不需要核酸的复制而能自行增殖[2]㊂人类朊蛋白病的主要病理学改变为中枢神经系统星形胶质细胞增生和神经纤维的空泡样改变,其病变主要累及皮质㊁基底节及脊髓㊂C JD 的典型临床表现为精神障碍㊁痴呆㊁帕金森样表现㊁共济失调㊁肌阵挛和肌肉萎缩等[3]㊂根据发病机制可以分为散发型㊁遗传型㊁变异型和医源型4种类型[4]㊂变异型C J D 被认为是牛海绵状脑病即众所周知的疯牛病传播给人类所致,曾于1986年爆发疯牛病的英国有较多新变异型C J D 报道㊂总体而言,以散发型C J D 最为常见,占比大致为85%[5]㊂我国所发现的C J D ,绝大多数为散发型㊂脑电图是临床诊断C J D 的重要依据,但早期缺乏特征性改变,随着疾病的发展(约在发病12周),出现周期性高幅棘-慢综合波(p e r i o d i cs h a r p wa v e c o m pl e x e s ,P S W C ),部分患者在疾病晚期P S W C 会消失,代之以θ㊁δ活动㊂多次检测动态脑电图可以提高诊断阳性率㊂M a r t i n 等[6]认为DW I 诊断C J D 的特异性可达93.8%,敏感性为92.3%,典型的DW I 表现为沿双侧皮质沟回走形的花边样改变和(或)基底节区的异常高信号改变,这些改变甚至早于脑电图及脑脊液14-3-3蛋白检测[7]㊂本例患者DW I 双侧额顶叶皮质即出现花边样改变㊂脑脊液14-3-3蛋白过去被认为是诊断C J D 的重要指标,其敏感性及特异性均较高,但最近的研究表明在中枢神经系统感染㊁额颞叶痴呆㊁阿尔茨海默病㊁蛛网膜下腔出血及急性脑梗死等患者中可以出现假阳性[8]㊂目前C J D 常用的诊断标准:①临床表现:迅速进展性痴呆㊁小脑及视觉损害症状㊁锥体束及锥体外系损害㊁无动性缄默;②辅助检查证据:脑电图有典型的P S W C ㊁脑脊液14-3-3蛋白阳性㊁M R I 的DW I 或F L A I R 像上存在两个以上皮质(颞叶-顶叶-枕叶)的异常高信号或尾状核和(或)壳核异常信号;在除外其他疾病基础上,①中至少两项,加上②中的1项即为很可能的克雅病;①中任意两项,且病程不足2年为可能的克雅病[9]㊂C J D 的确诊仍依赖于脑活检㊂2010-2017年间国内文献报道我国C J D 约170例,除16例为遗传型C J D 外多为散发型C J D ,男女比例接近4ʒ3,平均发病年龄为63.75岁,首发症状以进展性大脑高级皮层功能障碍最为多见㊂目前国内诊断C J D 基本依靠病史结合脑电图㊁脑脊液与磁共振典型表现的方法进行临床诊断㊂由于C J D 发病率较低(世界范围内约为百万分之一)且死亡率为100%,所以对于C J D 的诊断应格外谨慎,需要与阿尔茨海默病㊁抑郁症㊁帕金森病等伴发精神及智力障碍的疾病及肝豆状核变性㊁多系统萎缩等伴发锥体外系损害的疾病进行鉴别[10],尤其应排除自身免疫性脑炎等可治性疾病,以免错过此类疾病的最佳治疗时机㊂本例患者入院初期曾考虑自身免疫性脑炎㊁线粒体脑肌病不除外,后依据检查化验回报及症状变化予以排除㊂C JD 的可能传播途径有医源性㊁消化道㊁遗传等,所以C J D 患者一经确诊应首先进行隔离,医务人员应避免接触患者黏膜及破损皮肤与体液,并将与患者相关的生活用品㊁医疗用品严格进行销毁㊂本病尚无有效的治疗方法,临床仅为对症处理,目前R N A 干扰㊁免疫疗法㊁药物疗法等日益受到关注㊂(下转第86页)㊃38㊃‘临床荟萃“ 2018年1月5日第33卷第1期 C l i n i c a l F o c u s ,J a n u a r y 5,2018,V o l 33,N o .1Copyright ©博看网. All Rights Reserved.美国国立综合癌症网络(N a t i o n a lC o m p r e h e n s i v e C a n c e rN e t w o r k,N C C N)指南M C D治疗建议,主要为联合化疗ʃ利妥昔单抗(R),具体为环磷酰胺+多柔比星+长春碱类+泼尼松(C HO P)㊁环磷酰胺+多柔比星+长春碱类+地塞米松(C A V D)㊁环磷酰胺+长春碱类+泼尼松(C V P)及脂质体多柔比星㊂治疗有反应者,进行临床观察,直到进展后与治疗无效者归入难治/进展组㊂最终归入复发/难治组,采用自体造血干细胞移植或其他非常用药物治疗㊂可选择的药物治疗方案有:硼替佐米(蛋白酶体抑制剂)ʃ利妥昔单抗㊁t o c i l i z u m a b(人源化抗人I L-6受体单抗)㊁a n a k i n r a(重组I L-1受体抑制剂)㊁沙利度胺ʃ利妥昔单抗㊁来那度胺㊁大剂量的齐多夫定联合缬更昔洛韦(病毒激活细胞毒药物)等单药或联合方案[8-9]㊂由于这些方案的治疗报道均为个案报道,所以它们在C D 中的治疗意义有待进一步证实㊂患者H I V-HHV-8-伴有终末器官损害[10-11],疾病活动,首先考虑R-C HO P方案,由于经济条件所限未使用美罗华,予以C HO P方案,化疗效果佳达到完全缓解㊂我们选择将本例患者临床资料加以报道,以引起临床重视㊂参考文献:[1]赖玉梅,李敏,刘翠苓,等.I L-6在C a s t l e m a n病中的表达及其临床病理学意义分析[J].中华血液学杂志,2013,34(5):404-408.[2] N i s h i m o t oN,M i v a s a k a N,Y a m a m o t o K,e ta l.L o n g-t e r ms a f e t y a n d e f f i c a c y o f t o c i l i z u m a b,a n a n t i-I L-6r e c e p t o rm o n o c l o n a l a n t i b o d y,i n m o n o t h e-r a p y,i n p a t i e n t s w i t hr h e u m a t o i da r t h r i t i s(t h eS T R E AM s t u d y):e v i d e n c eo f s a f e t ya n d e f f i c a c y i na5-y e a r e x t e n s i o ns t u d y[J].A n nR h e u m D i s,2009,68(10):1580-1584.[3] R o c aB.C a s t l e m a n sd i s e a s e:a r e v i e w[J].A I D SR e v,2009,11(1):3-7.[4] K h a s h a b MA,C a n t o M I,S i n g h V K,e ta l.A r a r ec a s eo fp e r i p a n c r e a t i cC a s t l e m a n s d i s e a s e d i a g n o s e d p r e o p e r a t i v e l y b ye n d o s c o p i c u l t r a s o u n d-g u i d e df i n e n e e d l e a s p i r a t i o n[J].E n d o s c o p y,2011,43(S u p p l e2U C T N):E128-130.[5]S h e t t y P S,P a t k a rS,S h e t T,e ta l.C a s t l e m a n sd i s e a s ep r e s e n t i n g a s p e r i p a n c r e a t i c n e o p l a s m[J].I n d i a n J S u r gO n c o l,2015,6(1):26-29.[6] M a t s u m o t oT,O k u w a k iK,K i d a M,e ta l.A p a t i e n t w i t hp a n c r e a t i c C a s t l e m a n's d i s e a s e a r i s i n g a r o u n d t h e m a i np a n c r e a t i c d u c t[J].I n t e r n M e d,2015,54(16):2007-2012.[7] L i u A Y,N a b e l C S,F i n k e l m a n B S,e t a l.I d i o p a t h i cm u l t i c e n t r i c C a s t l e m a n s d i s e a s e:a s y s t e m a t i c l i t e r a t u r e r e v i e w[J].L a n c e tH a e m a t o l,2016,3(4):163-175.[8] F a j g e n b a u m D C,U l d r i c k T S,B a g g A,e ta l.I n t e r n a t i o n a le v i d e n c e-b a s e d c o n s e n s u s d i a g n o s t i c c r i t e r i af o r HH V-8-n e g a t i v e/i d i o p a t h i cm u l t i c e n t r i cC a s t l e m a nd i s e a s e[J].B l o o d, 2017,129(12):1646-1657.[9] T a l a t N,S c h u l t e KM.C s a t l e m a n s d i s e a s e:s y s t e m a t i ca n a l y s i so f416p a t i e n t sf r o m t h el i t e r a t u r e[J].O n c o l o g i s t,2011,16(9):1316-1324.[10] C a s q u e r oA,B a r r o s oA,F e m aán d e zG u e r r r r oM L,e t a l.U s eo f r i t u x i m a b a s a s a l v a g e t h e r a p y f o r H I V-a s s o c i a t e dm u l t i c e n t r i cC a s t l e m a nd i s e a s e[J].A n nH e m a t o l,2006,5(3): 185-187.[11]S t a r k e y C R,J o s t e N E,L e e F C.N e a r-t o t a lr e s o l u t i o n o fm u l t i c e n t r i cC a s t l e m a n d i s e a s eb y p r o l o n g e dt r e a t m e n t w i t h t h a l i d o m i d e[J].A mJH e a m a t o l,2006,81(4):303-304.收稿日期:2017-08-17编辑:﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏王秋红(上接第83页)参考文献:[1]S i k o r s k aB,K n i g h tR,I r o n s i d e J W,e t a l.C r e u t z f e l d t-J a k o bd i se a s e[J].A d vE x p M e dB i o l,2012,724:76-90.[2]陈吐芬,江晓静.人类朊粒病研究进展及对人类医学的影响[J].华南国防医学杂志,2012,26(6):614-618.[3] P u o t iG,B i z z iA,F o r l o n iG,e ta l.S p o r a d i ch u m a n p r i o nd i se a s e s:m o l e c u l a r i n s i g h t s a n d d i a g n o s i s[J].L a n c e tN e u r o l o g y,2012,11(7):618-628.[4] K n i g h tR.C r e u t z f e l d t-J a k o bd i s e a s e:a r a r e c a u s eo f d e m e n t i ai ne l d e r l y p e r s o n s[J].C l i n I n f e c tD i s,2006,43(3):340-346.[5] B u c e l l i R C,A n c e sB M.D i a g n o s i sa n de v a l u a t i o no f a p a t i e n tw i t hr a p i d l yp r o g r e s s i v ed e m e n t i a[J].M o M e d,2013,110(5):422-428.[6] M a r t i nG G,Pér e z J A,C u e v a sR A.C r e u t z f e l d t-J a k o b d i s e a s e:h y p e r i n t e n s es i g n a l i nc o r t e xa n d b a s a l g a n g l i ai n m a g n e t i cr e s o n a n c e i m a g i n g[J].N e u r o l o g i a,2009,24(9):813.[7] T k i t a m o t o H.C l i n i c a l d i a g n o s i s o f MM2-t y p e s p o r a d i cC r e u t z f e l d t-J a k o bd i s e a s e[J].N e u r o l o g y,2005,64(4):643-648.[8] Z a n u s s oG,F i o r i n i M,F e r r a r iS,e ta l.C e r e b r o s p i n a l f l u i dm a r k e r s i ns p o r a d i cC r e u t z f e l d t-J a k o bd i s e a s e[J].I n tJ M o l S c i,2010,12(9):6281-6292.[9] Z e r r I,K a l l e n b e r g K,S u mm e r sD M,e ta l.U p d a t ec l i n i c a ld i a g n o s i t i c c r i te r i af o rs p o r a d i cC r e u t z f e l d t-J a k o bd i s e a s e[J].B r a i n,2009,135(4):2659-2668.[10] C h i t r a v a s N,J u n g R S,K o f s k e y D M,e t a l.T r e a t a b l en e u r o l o g i c a l d i s o r d e r s m i s d i a g n o s e d a s C r e u t z f e l d t-J a k o bd i se a s e[J].A n nN e u r o l,2011,70(3):437-444.收稿日期:2017-09-08编辑:武峪峰㊃68㊃‘临床荟萃“2018年1月5日第33卷第1期 C l i n i c a l F o c u s,J a n u a r y5,2018,V o l33,N o.1Copyright©博看网. All Rights Reserved.。
LM201U05-SLA2_CAS_1230_DELL_Rev1[1].0_Final
![LM201U05-SLA2_CAS_1230_DELL_Rev1[1].0_Final](https://img.taocdn.com/s3/m/adf21b6e58fafab069dc02e9.png)
Table 2. ELECTRICAL CHARACTERISTICS
Parameter
Values
Symbol
Units
Notes
: Power Supply Input Voltage Power Supply Input Current Power Consumption Differential Impedance Rush Current
/
/
Please return 1 copy for your confirmation with your signature and comments.
Ver 1.0
Dec. 30. 2005
1 /27
Product Specification
LM201U05 Liquid Crystal Display
The LM201U05-SLA1requires two power inputs. One is employed to power the LCD electronics and to drive the TFT array and liquid crystal. The second input which powers the CCFL, is typically generated by an inverter. The inverter is an external unit to the LCD.
1.0
Dec. 30. 2005
LM201U05 Liquid Crystal Display
Product Specification
RECORD OF REVISIONS
Page
Description
西门子SITRANS T 温度传感器操作说明说明书

2 安全说明 ........................................................................................................................................ 11
2.1 2.1.1 2.1.2 2.1.3
1.7
运输与存储.................................................................................................................... 9
1.8
保修注意事项 ................................................................................................................ 9
Siemens AG Digital Industries Postfach 48 48 90026 NÜRNBERG
德国
文件订购号: A5E03920348 Ⓟ 09/2020 本公司保留更改的权利
Copyright © Siemens AG 2020. 保留所有权利
目录
1 简介................................................................................................................................................. 7
3 安装/固定 ....................................................................................................................................... 17
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Rev. 1.1 12/03Copyright © 2003 by Silicon Laboratories
AN134-DS11AN134
M U L T I P L E -D E V I C E JTAG C O N F I G U R A T I O N I N T H E
S I L I C O N L A B S IDE
JTAG Chain Configuration
To configure the Multiple-Device JTAG Chain feature of the Silicon Labs IDE you need to provide the following information:
•The number of devices before and after the target.
•The number of bits in the IR registers of the devices before and after the target.
In the Silicon Labs IDE, press the following key combination, ctrl+shift+m , to open the Multi-device JTAG Programming dialogue box shown below. This feature is included in v1.7 and later of the IDE.
Order of Devices
Refer to the following diagram to determine the order of the devices in the chain.
•Device #0 is before Device #1 and Device #2
•Device #1 is before Device #2 and after Device #0.
•Device #2 is after Device #1 and Device #0.
AN134
2Rev. 1.1
AN134
Rev. 1.13
AN134
4
Rev. 1.1
The information in this document is believed to be accurate in all respects at the time of publication but is subject to change without notice. Silicon Laboratories assumes no responsibility for errors and omissions, and disclaims responsibility for any consequences resulting from the use of information included herein. Additionally, Silicon Laboratories assumes no responsibility for the functioning of undescribed features or parameters. Silicon Laboratories reserves the right to make changes without further notice. Silicon Laboratories makes no warranty, rep-resentation or guarantee regarding the suitability of its products for any particular purpose, nor does Silicon Laboratories assume any liability arising out of the application or use of any product or circuit, and specifically disclaims any and all liability, including without limitation conse-quential or incidental damages. Silicon Laboratories products are not designed, intended, or authorized for use in applications intended to support or sustain life, or for any other application in which the failure of the Silicon Laboratories product could create a situation where per-sonal injury or death may occur. Should Buyer purchase or use Silicon Laboratories products for any such unintended or unauthorized ap-plication, Buyer shall indemnify and hold Silicon Laboratories harmless against all claims and damages.。
