Interactions of quaternary ammonium salt-type gemini surfactants with
复合季铵盐消毒液工作温度

复合季铵盐消毒液工作温度英文回答:The working temperature of compound quaternary ammonium salt disinfectant depends on the specific formulation and purpose of use. Generally, the recommended working temperature for compound quaternary ammonium salt disinfectant is around room temperature, which is typically between 20 to 25 degrees Celsius (68 to 77 degrees Fahrenheit). This temperature range is considered optimal for the disinfection efficacy of the compound quaternary ammonium salt.Compound quaternary ammonium salt disinfectants are commonly used in various settings, such as healthcare facilities, food processing plants, and household cleaning. In healthcare settings, for example, these disinfectants may be used to clean and disinfect surfaces, medical equipment, and instruments. The working temperature is crucial to ensure the effectiveness of the disinfectant inkilling or inactivating microorganisms.In some cases, the working temperature may vary depending on the specific application. For instance, in food processing plants, compound quaternary ammonium salt disinfectants may be used to sanitize equipment and utensils. The working temperature in this case may be higher, around 45 to 50 degrees Celsius (113 to 122 degrees Fahrenheit), to effectively eliminate foodborne pathogens.It is important to follow the manufacturer's instructions and guidelines when using compound quaternary ammonium salt disinfectants. These instructions usually include information on the recommended working temperature for optimal disinfection results. Failure to adhere to the recommended temperature range may compromise the effectiveness of the disinfectant and increase the risk of microbial contamination.中文回答:复合季铵盐消毒液的工作温度取决于具体的配方和使用目的。
甲基丙烯酸缩水甘油酯的合成研究 (1)

甲基丙烯酸缩水甘油酯的合成研究高晓蕾Ξ 卫冬燕(郑州大学化工学院,河南郑州450002)摘 要:在相转移催化剂季铵盐和阻聚剂对苯二酚存在下,采用二步法由甲基丙烯酸(M AA )和环氧氯丙烷(ECH )合成了甲基丙烯酸缩水甘油酯(G M A )。
通过正交实验确定了最佳反应条件:物料配比n (甲基丙烯酸钠)∶n (环氧氯丙烷)=1∶8,反应温度105℃,反应时间4h ,催化剂选用十六烷基三甲基溴化铵。
在该工艺条件下,产品产率为94173%,纯度为96180%。
关键词:甲基丙烯酸;甲基丙烯酸缩水甘油酯;相转移催化剂R esearch on Synthesis of G lycidyl MethacrylateG AO Xiao 2lei ,WEI Dong 2yan(C ollege of Chemical Engineering ,Zhengzhou University ,Zhengzhou 450002,China )Abstract :G lycidyl methacrylate (G M A )is synthesized in tw o steps based on the reaction of methyl methacrylate (M M A )and epichlorhydrin (ECH )in presence of quaternary amm onium salt as phase trans fer catalyst and 1,42dihydroxy 2benzene as poly 2merization inhibitor.The optimum reaction conditions are obtained by orthog onal experimental design :n (s odium methacrylate )∶n (ECH )=1∶8,reaction temperature is 105℃,reaction time is 4h ,and taking hexadecyltrimethylamm onium bromide as phase trans fer catalyst.Under these conditions the yield of product can be reached 94173%with purity of 96180%.K ey w ords :methyl methacrylate ;glycidyl methacrylates ;phase trans fer catalyst 甲基丙烯酸缩水甘油酯(G M A )又称甲基丙烯酸环氧丙酯,是无色透明的液体,密度11074,折光率114480,沸点189℃,熔点-50℃,闪点84℃,色相(APH A )<75,不溶于水,易溶于有机溶剂,对皮肤和粘膜有刺激性,几乎无毒。
有机硅季铵盐—纳米银复合织物抗菌整理剂制备及应用的研究

有机硅季铵盐一纳米银复合织物抗菌整理剂的制备及应用研究摘要有机硅季铵盐类和银系抗菌剂是目前应用最普遍、效果最好的织物抗菌整理剂。
银具有极强的杀菌能力、杀菌耐久性良好、用量小,无毒、无刺激;而有机硅季铵盐不仅赋予织物优良抗菌性,同时还具备良好的吸水性、柔软性、平滑性及回弹性。
这两类抗菌整理剂各具特色,可以互为补充,克服目前纺织品抗菌卫生整理中存在的问题,在纺织行业有较大的应用潜力。
本文在详细分析国内外织物抗菌整理剂特别是有机硅季铵盐及银系抗菌剂的合成、分类、性能及应用等方面最新研究进展的基础上,针对目前国内使用的有机硅季铵盐类抗菌整理剂大多数从国外进口、成本较高,以及银易变色的现状,研制开发了一种新型的有机硅季铵盐ASQA和复合型抗菌整理剂Ag—ASQA。
在100℃用油酸甲酯对氨乙基氨丙基二甲氧基硅烷fDL.602)进行酰胺化反应6h后,再经硫酸二甲酯季铵化反应制备了一种新型的油酰胺乙基二甲基氨丙基硅烷季铵盐(ASQA),并对其结构进行了表征。
用质量分数为1.0%的ASQA溶液整理过的坯布,对大肠杆菌及金黄色葡萄球菌的抑菌率分别为99.55%和99.82%,且具有优良的抗菌耐洗性,洗涤30次后抑菌率仍大于80%。
通过化学还原法以过氧化氢为还原荆、在分散剂聚乙烯吡咯烷酮(PVP)及自制多羧酸聚合物(PMAA)的保护下分别制备了60~90nm和90~150nm的银粉;并通过X.射线衍射仪(XRD)及透射电镜(TEM)观察了银粉的结构及粒径分布;为了避免银变色.影响织物外观,将纳米银粉通过分散剂和粘合剂包覆在纺织纤维上,达到,抗菌持久的良好效果。
抑菌圈实验结果表明:用PVP分散的银粉质量浓度为0.5g·L-1时,对金黄色葡萄球菌的抑菌圈直径为13.1mm,对大肠杆菌的抑菌圈直径为13.7mm。
将抑菌幽试验的培养皿放置6个l月后抑菌环仍然清晰未受细菌感染,具有较好的抗菌耐久性。
为了克服单一抗菌剂的抗菌局限,本文采用无机一有机复配的方式,将有机硅季铵盐ASQA溶液与纳米银粉分散液复配,制备了抗菌整理液Ag-ASQA。
四丁基氟化铵溶解度曲线_概述说明

四丁基氟化铵溶解度曲线概述说明1. 引言1.1 概述本文旨在探讨四丁基氟化铵(Tetra-n-butylammonium fluoride)的溶解度曲线,并对其进行深入分析和讨论。
四丁基氟化铵是一种重要的有机金属试剂,其在化学研究和工业生产中具有广泛的应用。
了解其溶解度特性对于优化催化反应、合成有机材料以及设计高效分离方法等领域至关重要。
1.2 文章结构本文共包括五个部分。
首先,引言部分将介绍文章的背景和目的。
然后,正文部分将详细阐述四丁基氟化铵溶解度的定义、影响因素以及测定方法。
接下来,结果与讨论部分将对实验结果进行分析,并探讨可能的影响因素,并与已有数据进行比较和分析。
最后,结论部分将总结实验结果和主要发现。
1.3 目的本文旨在提供一个全面而准确的关于四丁基氟化铵溶解度曲线的概述,以便研究者能更好地理解该物质在不同条件下的溶解行为。
通过对影响溶解度的因素和测定方法的详细介绍,本文将为相关领域的研究提供理论基础和实验指导,同时也为进一步探索四丁基氟化铵溶液性质的应用提供参考。
最终,通过本文内容的阐述与讨论,希望能够为相关研究领域的发展做出一定的贡献。
2. 正文:2.1 四丁基氟化铵溶解度的定义四丁基氟化铵是一种常用的阳离子表面活性剂,其溶解度是指在给定温度条件下,在水或其他溶剂中能够溶解的最大量四丁基氟化铵。
一般以摩尔浓度或质量浓度表示。
2.2 影响四丁基氟化铵溶解度的因素四丁基氟化铵的溶解度受到多种因素的影响,包括温度、溶剂性质、离子强度等。
以下为主要因素的说明:2.2.1 温度:温度是影响四丁基氟化铵溶解度最重要的因素之一。
通常情况下,随着温度升高,四丁基氟化铵在水中的溶解度会增加。
这是由于温度升高会增加分子间距和分子热运动速率,促进离子势垒突破和分子间相互作用降低,从而增加了四丁基氟化铵分子在水中的扩散速率和相对稳定性。
2.2.2 溶剂性质:不同溶剂对四丁基氟化铵的溶解度有所差异。
一般来说,极性溶剂如水会更好地溶解四丁基氟化铵,而非极性溶剂如石油醚等对其溶解度较低。
相转移

14
Permanganate Oxidation
COMPOUND QUANTITY
1-癸烯 苯 高锰酸钾 三辛酰基甲基氯化铵
乙醚
28g 50mL 125g 5g 100mL
过量的高锰通过添加亚硫酸钠溶液破坏。将反应混合物过滤 以除去的MnO2并用稀HCl酸化。MnOI用100毫升苯洗涤,将其也 用于洗涤滤液中的水相。将合并的苯溶液用100毫升10%NaOH溶 液混合并振荡。含水碱性相用乙醚洗涤,然后用盐酸酸化。加入 100ml 乙醚用来分离羧酸,并在醚溶液干燥(硫酸钠)。乙醚蒸 发留下29g(91%)的壬酸(98%纯度通过GLC测定的)。
这些通过制备气液色谱分离。主要成分(93%)是预期的2-己 基 -1 , 1- 氯代异戊烯,通过 IR 和 NMR 谱,这些用 Weinberg.1 方法 制备的样品的气液色谱保留时间都可以证明。次要组分(7%), 用核磁和质谱鉴定为2-戊基-3-甲基-1,1-氯代异戊烯。这两种产品的 分离的产率分别为60和4%,基于未回收的氯仿。
COMPOUND QUANTITY
氯仿 1-辛烯 NaOH溶液 三辛酰基甲基氯化铵
NaCl
37.5g 75g 150g 5g 20mL
400mL
H2O
用 GLC 分析有机层的 组成,结果表明它含有 59 %的 1- 辛烯, 9.6 %氯仿, 还有 31 个产品。通过一个 12 英 寸 真 空 夹 套 Vigreux 柱蒸馏,得到 28 克馏分产 物 , 沸 点 49℃ ( 0.20.5mm )的,通过 GLC 分 析出包含两种组分。
铜_季铵盐复配木材防腐剂的防腐性能

铜2季铵盐复配木材防腐剂的防腐性能ΞFAN G G Z 方桂珍,任世学(东北林业大学林产化工学院,黑龙江哈尔滨150040)摘 要: 在实验室内采用常规的真空2加压法浸注试件,土壤木块法进行防腐实验,检验了铜2季铵盐类防腐剂(FFJ 21、FFJ 22和FFJ 23)对白腐采绒革盖菌[Coriolus versicolor (L.ex Fr.)Quel.]和褐腐绵腐卧孔菌[Poria placenta (Fr.)Cooke.]的防腐性能,结果表明:在较低的保持量下,都有较好的防腐效果。
与百菌清(可湿性粉剂)、五氯酚钠、三唑酮相比较,它们对白腐菌的防腐性能与百菌清相近,比五氯酚钠和三唑酮好。
关键词: 季铵盐;木材防腐剂中图分类号:S782.33 文献标识码:A 文章编号:025322417(2002)0120071203与铜复配是木材防腐剂发展的一种重要趋势。
一般认为,铜对真菌有较好的抑制作用,再加上它价格适中,对环境柔和,对人畜无害,故被广泛应用于木材防腐剂中。
美国木材保护协会(AWPA )标准中水溶性防腐剂绝大多数都使用了铜,如ACC (酸性铬酸铜)、ACA (氨溶砷酸铜)、ACZA (氨溶砷锌)、CCA 2A (铜铬砷2A )、CCA 2B (铜铬砷2B )、CCA 2C (铜铬砷2C )、ACQ 2A (氨溶季铵铜2A )、ACQ 2B (氨溶季铵铜2B )、ACQ 2D (氨溶季铵铜2D )、CDDC (二甲基二硫代氨基甲酸铜)、CC (柠檬酸铜)、CBA 2A (硼唑铜)等[1]。
关于季铵盐类与其它药剂复合用作防腐剂、防变色剂和防霉剂的研究较多。
如DDAC (二甲基二癸基氯化铵)和IPBC (32碘代222丙炔基甲氨酸丁酯)混合使用具有广泛的杀菌性,并提高了抗流失性;将DDAC 和百菌清复合使用作为防霉剂等[2~3]。
目前,国外研究趋势是将季铵盐与铜盐复配制成ACQ 作水溶性防腐剂[4]。
高效液相色谱法测定延胡索药材中7种异喹啉类生物碱的含量

高效液相色谱法测定延胡索药材中7种异喹啉类生物碱的含量赵新娟;沈梅;石俊敏;韩伟立【摘要】本文建立了同时测定延胡索中巴马汀、小檗碱、去氢紫堇碱、四氢巴马汀、异紫堇球碱、紫堇碱和四氢黄连碱7种主要异喹啉生物碱含量的高效液相色谱方法,并考察了不同来源延胡索中异喹啉生物碱的含量.采用Agilent SB C18柱色谱柱(4.6×250 mm,5μm),流动相为乙腈-0.1%的醋酸水溶液(三乙胺调pH至5.0),梯度洗脱,流速为1.0 mL/min,检测波长为280nm.巴马汀、小檗碱、去氢紫堇碱、四氢巴马汀、异紫堇球碱、紫堇碱和四氢黄连碱在2.0~40.1、2.0~39.5、5.1~101.3、5.0~99.8、2.1 ~41.2、5.0~100.1 μg/mL和2.0 ~39.7μg/mL浓度范围内线性良好,平均加样回收率分别为95.6%、96.1%、96.5%、101.4%、101.9%、97.3%和102.3%,RSD分别为2.77%、2.50%、3.33%、4.18%、2.93%、2.86%和2.60%.不同来源延胡索样品中7种异喹啉类生物碱含量差异较大,研究表明该方法准确、可靠,可用于延胡索原药材质量控制.【期刊名称】《天然产物研究与开发》【年(卷),期】2015(027)012【总页数】5页(P2074-2078)【关键词】高效液相色谱法;异喹啉类生物碱;延胡索;含量测定【作者】赵新娟;沈梅;石俊敏;韩伟立【作者单位】山西省晋中市第一人民医院药剂科,晋中030600;南方医科大学公共卫生与热带医学学院卫生检测中心,广州510515;华南师范大学药物研究院,广州510632;南方医科大学公共卫生与热带医学学院卫生检测中心,广州510515【正文语种】中文【中图分类】R917延胡索为罂粟科紫堇属植物延胡索(Corydalis yanhusuo W.T.Wang)的干燥块茎,其具有活血、利气和止痛的功效。
化学基础英文30阴离子重排_anionic_rearrangements
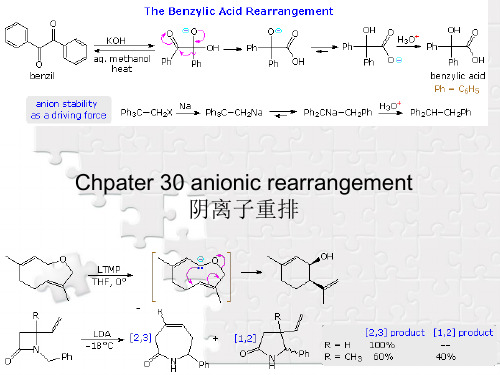
nucleophilic bases often leads to a skeletal rearrangement known as the Favorskii rearrangement. As depicted in the following diagram, this reaction is believed to proceed by way of a cyclopropanone intermediate. Facile conversion of cyclopropanones to hydrates and hemiacetals (relief of angle strain) occurs, and the cyclopropoxide conjugate base undergoes ring opening and solvent protonation.
30-2 Brook Rearrangement
The rearrangement of silicon groups from carbon to oxygen is called the Brook rearrangement. An important driving force for this shift is the increased bond strength of the Si–O bond (110 Kcal/mol) compared with the Si–C bond (76 Kcal/mol). The example given in the following equation is catalyzed by base, and a cyclic transition state is indicated by the high entropy of activation.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Department of Applied Chemistry and Institute of Colloid and Interface Science, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan Received 22 September 2003; accepted 4 March 2004 Available online 10 April 2004
T. Yoshimura et al. / Journal of Colloid and Interface Science 275 (2004) 618–622
619
2.3. Measurements The surface tension of aqueous solutions of surfactants/PSS mixed systems was measured with a Krüss K100 tensiometer by using the Wilhelmy plate technique. Steadystate fluorescence spectra of pyrene in the mixed systems were obtained using a Hitachi 650-10S spectrophotometer in the range 360–400 nm at an excitation wavelength of 335 nm, where the concentration of pyrene was 1 × 10−5 mol dm−3 . Dynamic light scattering was also performed with a DLS-7000, Otsuka Electronics Company, Ltd., spectrophotometer using an argon laser of 488 nm. All solutions were filtered with a 0.2-µm filter of mixed cellulose acetate. All measurements were carried out at 25 ◦ C.
E-mail address: yoshimura@ch.kagu.tus.ac.jp (T. Yoshimura). 0021-9797/$ – see front matter 2004 Elsevier Inc. All rights reserved. doi:10.1016/j.jcis.2004.03.002
Abstract The interactions of cationic gemini surfactants, 1,2-bis(alkyldimethylammonio)ethane dibromide (m-2-m: m is hydrocarbon chain length, m = 10 and 12), and an anionic polymer, sodium poly(styrene sulfonate) (PSS), have been characterized by several techniques such as tensiometry, fluorescence spectroscopy, and dynamic light scattering. The surface tension of gemini surfactant/PSS mixed systems decreases with surfactant concentration, reaching break points, which are taken as critical aggregation concentrations (cac). The surface tension at the cac of mixtures is higher than that of single surfactants, and it is found that at concentrations above the cac, the surfactant molecules are associated with the polymer in the bulk. The 12-2-12/PSS mixed system shows higher surface activity than both 10-2-10/PSS and the monomeric surfactant of dodecyltrimethylammonium bromide/PSS systems. Fluorescence measurements of these mixed systems suggest the formation of a complex with a highly hydrophobic environment in the bulk of the solution. Additionally, dynamic light scattering measurements show that the hydrodynamic diameter of the 12-2-12/PSS mixed system is smaller than that of PSS only at low concentration, indicating interactions between surfactant and polymer. These result from the electrostatic attraction between ammonium and sulfate headgroups as well as the hydrophobic interaction between their hydrocarbon chains. 2004 Elsevier Inc. All rights reserved.
tant/uncharged polymer mixed systems have been reported, there have been few reports about those of gemini surfactant/polymer mixed systems [14,15]. Gemini surfactants, which have two hydrocarbon chains and two hydrophilic groups in a molecule, reportedly possess unique properties, such as better solubilization, lower Krafft temperatures, lower critical micelle concentration (cmc), greater efficiency in lowering the surface tension, and foaming properties than the conventional monomeric surfactants [16–19]. The purpose of this study is to investigate whether any interaction occurs between cationic gemini surfactant and anionic polymer at air/water interface and in solution. Gemini surfactant is used to investigate the effect of chain numbers of these compounds on interactions with anionic polymer. In this work, we investigate the interactions of cationic gemini surfactant, 1,2-bis(alkyldimethylammonio)ethane dibromide (m-2-m, where m is the hydrocarbon chain length, m = 10 and 12), and an anionic polymer, sodium poly(styrene sulfonate) (PSS), by measuring surface tension, fluorescence spectroscopy of pyrene, and dynamic light scattering. In
Journal of Colloid and Interface Science 275 (2004) 618–622 /locate/jcis
Interactions of quaternary ammonium salt-type gemini surfactants with sodium poly(styrene sulfonate)
Keywords: Cationic gemini surfactant; Anionic polyelectrolyte; Interaction; Aggregate; Surface tension; Fluorescence; Hydrodynamic diameter
1. Introduction The study of interactions between surfactants and polymers is an active field of interest in colloid science [1]. The mixtures of surfactants and polymers are used in the fields such as paints and drilling muds, etc. Important information on surfactant and polymer interathe bulk is currently being provided by techniques such as surface tension, neutron and X-ray reflection, ellipsometry, and surface rheology [2]. The mixtures of oppositely charged surfactants and polymers are very sensitive indicators of interaction [3–5]. The interaction between the cationic surfactant alkyltrimethylammonium bromide or chloride and anionic polymer such as polyacrylamide sulfonate, polystyrene sulfonate (PSS), DNA and xanthan has been investigated [6–13]. Although many studies of the properties of the conventional monomeric surfac* Corresponding author. Fax: +81-3-32352214.
