Optovin_348575-88-2_DataSheet_MedChemExpress
游离雌三醇测定试剂盒(时间分辨荧光免疫分析法)产品技术要求模板

医疗器械产品技术要求编号:游离雌三醇测定试剂盒(时间分辨荧光免疫分析法)1.产品型号/规格及划分说明1.1产品型号/规格96人份/盒(半自动)、96人份/盒(全自动)。
1.2结构组成试剂盒由uE3校准品(冻干粉)、uE3标记物(冻干粉)、uE3抗体、uE3实验缓冲液、浓缩洗液、增强液和uE3微孔反应板组成。
1.3 适用范围用于定量检测人体血清中的游离雌三醇含量。
2.性能指标2.1装量实验缓冲液、增强液、浓缩洗液的最大允许负偏差为 6.0%。
2.2外观试剂盒中校准品、标记物为冻干粉,呈白色或者淡黄色粉末或块状物,复溶后为澄清或浅黄色液体,不应含异物、混浊或摇不散的沉淀;其它液体组份试剂均为澄清透明;微孔反应板应封口良好,无破损。
2.3最低检测限试剂盒的最低检测限应不高于 0.6 nmol/L。
2.4特异性)交叉反应系数应不高于 2%;与孕酮(P)交叉反应系数应不高于与雌二醇(E22%。
2.5线性相关系数试剂(盒)的线性范围(0.6~49.2)nmol/L 内的线性应符合如下要求:a)线性相关系数 r≥0.990;b)检测浓度<4 nmol/L 的uE3 时,线性的绝对偏差的绝对值应在 0.5 nmol/L 的范围内;检测浓度≥4 nmol/L 的uE3 时,线性相对偏差的绝对值≤15%。
2.6校准品的准确性用国家标准品制备的二级线性校准品(注册检验时使用)或经国家标准品标化的企业参考品对产品校准品(零值除外)的浓度进行测定,产品校准品 B、C 点的实测浓度与标示浓度绝对偏差的绝对值应在 0.5 nmol/L 的范围内,产品校准品 D~F 点的实测浓度与标示浓度相对偏差的绝对值≤20%。
2.7测量准确度用试剂盒对检测范围内低、中两个浓度人血清样本进行回收实验,其回收率应在(85%~115%)范围内。
2.8测量精密度2.8.1 校准品不精密度(均一性)试剂盒校准品(零值除外)B 点、C 点的不精密度(CV)应不超过 20.0%;D 点、E 点、F 点的不精密度(CV)应不超过 15.0%。
Healthy Blue SC会员手册说明书
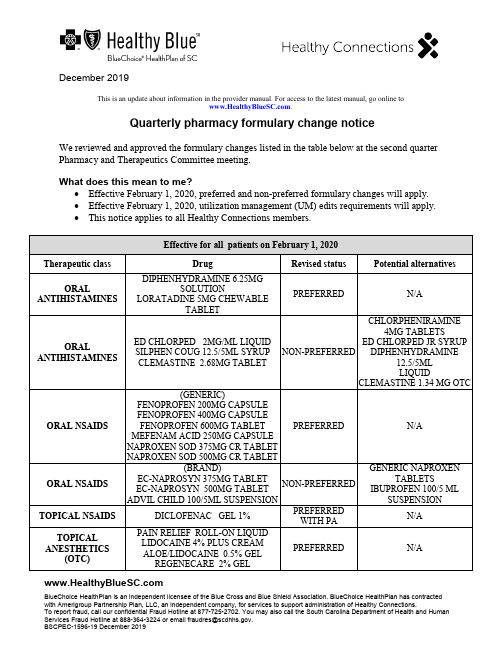
December 2019BlueChoice HealthPlan is an independent licensee of the Blue Cross and Blue Shield Association. BlueChoice HealthPlan has contracted with Amerigroup Partnership Plan, LLC, an independent company, for services to support administration of Healthy Connections.To report fraud, call our confidential Fraud Hotline at 877-725-2702. You may also call the South Carolina Department of Health and Human ************************************************************.BSCPEC-1596-19 December 2019This is an update about information in the provider manual. For access to the latest manual, go online to .Quarterly pharmacy formulary change noticeWe reviewed and approved the formulary changes listed in the table below at the second quarter Pharmacy and Therapeutics Committee meeting.What does this mean to me?• Effective February 1, 2020, preferred and non-preferred formulary changes will apply. • Effective February 1, 2020, utilization management (UM) edits requirements will apply. • This notice applies to all Healthy Connections members.Effective for all patients on February 1, 2020Therapeutic class DrugRevised status Potential alternativesORALANTIHISTAMINESDIPHENHYDRAMINE 6.25MGSOLUTIONLORATADINE 5MG CHEWABLETABLETPREFERREDN/AORALANTIHISTAMINESED CHLORPED 2MG/ML LIQUID SILPHEN COUG 12.5/5ML SYRUP CLEMASTINE 2.68MG TABLET NON-PREFERRED CHLORPHENIRAMINE4MG TABLETSED CHLORPED JR SYRUPDIPHENHYDRAMINE12.5/5MLLIQUIDCLEMASTINE 1.34 MG OTCORAL NSAIDS(GENERIC)FENOPROFEN 200MG CAPSULE FENOPROFEN 400MG CAPSULE FENOPROFEN 600MG TABLET MEFENAM ACID 250MG CAPSULE NAPROXEN SOD 375MG CR TABLET NAPROXEN SOD 500MG CR TABLETPREFERRED N/A ORAL NSAIDS(BRAND) EC-NAPROSYN 375MG TABLET EC-NAPROSYN 500MG TABLET ADVIL CHILD 100/5ML SUSPENSION NON-PREFERRED GENERIC NAPROXENTABLETSIBUPROFEN 100/5 ML SUSPENSIONTOPICAL NSAIDSDICLOFENAC GEL 1% PREFERREDWITH PAN/A TOPICALANESTHETICS(OTC)PAIN RELIEF ROLL-ON LIQUIDLIDOCAINE 4% PLUS CREAMALOE/LIDOCAINE 0.5% GELREGENECARE 2% GELPREFERRED N/ALIDODOSE 3% GELREGENECARE SPRAYALOCANE 4% GELAFTERBURN 2.5% GELXOLIDO 2% CREAM BURN RELIEF 0.5% AEROSAL ASPERCREME 4% SPRAYLIDOCAINE 3% CREAMLIDOCAINE 4% CREAMLIDOCAINE 5% CREAMAFTERSUN 0.5% GELLIDOCAINE 4% PADTOPICAL ANESTHETICS(RX)LIDOCAINE 3% CREAMLIDOCAINE 5% OINTMENT NON-PREFERREDOTC LIDOCAINEPRODUCTSRX LIDOCAINE5% PATCH(PA REQUIRED)MISCELLANEOUS ANTICONVULSANTSPREGABALIN 25MG CAPSULEPREGABALIN 50MG CAPSULEPREGABALIN 75MG CAPSULEPREGABALIN 100MG CAPSULEPREGABALIN 150MG CAPSULEPREGABALIN 200MG CAPSULEPREGABALIN 225MG CAPSULEPREGABALIN 300MG CAPSULEPREGABALIN SOL 20MG/MLPREFERREDWITH NO PRIORAUTHORIZATION(PA)N/AATOPICDERMATITIS PIMECROLIMUS 1% CREAMPREFERREDWITH STEPTHERAPY (ST)N/AFIBRATESFENOFIBRATE 130MG CAPSULEFENOFIBRATE 145MG TABLETFENOFIBRIC 35MG TABLETFENOFIBRIC 105MG TABLETFENOFIBRIC 135MG DR CAPSULENON-PREFERREDWITH STFENOFIBRATE134MG, 160MG, 200MG,43MG, 48MG,54 MG,67 MGFENOFIBRIC ACID 45 MGALCOHOL SWABS (MANUFACTURERS) GLOBAL DIABETICRITE AID NON-PREFERREDMANUFACTURERSBD DIABETESDYNAREXHEALTH MARTULTIMEDALCOHOL SWABS (MANUFACTURERS) BD DIABETESDYNAREXHEALTH MARTULTIMEDPREFERRED N/AIRON SUPPLEMENTS (GENERIC OTC)IRON 45MG TABLETSLOW-RELEASE FE 45MG TABLETHEMAX TABLETGENTLE IRON 28MG CAPSULEHIGH POTENCY FE 27MG TABLETNU-IRON 150 150MG CAPSULEABATRON AF TABLETSLOW IRON 50MG TABLETPREFERRED N/AFERGON 27MG TABLETIRON SUPPLEMENTS(BRAND OTC)FOLITAB 500 TABLET IRON 28MG TABLETFERROUS GLUC 324MG TABLETEZFE 200MG CAPSULEFERROUS GLUC TAB 324MGFERROUS SULF 324MG EC TABLETFERRETTS 325MG TABLETFERREX 150MG CAPSULEFERREX 28 MIS FERREX 150 PLUS CAPSULE FERREX 150 FORTE PL CAPSULECHEWABLE IRONPEDIATRIC IRON CHEWABLEFERROUS SUL 220/5ML LIQUIDFERROUS SULF 300/5ML SYRUPFEOSOL 200MG TABLETSLOW RELEASE FE 143MG CRTABLETNON- PREFERRED OTC GENERIC IRONSUPPLEMENTSRX PRODUCTS:HEMATOGEN FA CAPSULEHEMETAB TABLETMULTIGEN TABLETMULTIGEN PLS TABLETMULTIGEN FOLICTABLETFERRAPLUS 90 TABLETTARON FORTE CAPSULEFOLIVANE-F CAPSULEFOLIVANE-PLS CAPSULECENTRATEX CAPSULEIRON SUPPLEMENTS(PRESCRIPTIONSTRENGTH)IFEREX 150 FORTE CAPSULE HEMATOGEN CAPSULE HEMATOGEN FORTE CAPSULE TRICON CAPSULE MYFERON 150 FORTE CAPSULE FERROCITE PLUS TABLET FEROCON CAPSULE PUREVIT DUA FE PLUS CAPSULE HEMATINIC PL VIT/MIN TABLET HEMATINIC/FA TABLET POLY-IRON 150 FORT CAPSULE CORVITA 150 TABLET TRIGELS-F FORTE CAPSULE TL ICON CAPSULE SE-TAN PLUS CAPSULE NON- PREFERRED OTC GENERIC IRON SUPPLEMENTSRX PRODUCTS:HEMATOGEN FA CAPSULE HEMETAB TABLET MULTIGEN TABLET MULTIGEN PLS TABLET MULTIGEN FOLIC TABLET FERRAPLUS 90 TABLETTARON FORTE CAPSULE FOLIVANE-F CAPSULEFOLIVANE-PLS CAPSULE CENTRATEX CAPSULEUM edits — effective for all members no later than February 1, 2020 No changes in preferred/non-preferred status revision or addition to UM edit onlyANDROGENS*JATENZO CAPSULE ADD ST WITH QUANTITY LIMITS (QL)58 MG AND 198 MG QL: 4 PER DAY 237 MG QL: 2 PER DAY ANTICONVULSANTSNAYZILAM SPRAY 5MG ADD PA WITH QLQL: 50 MG PER 30 DAYS ANTICONVULSANTSOXTELLAR XR 150 MGOXTELLAR XR 600 MGREVISED QL LIMIT:150 MG: 3 TABLETS PER DAY 600 MG: 4 TABLETS PER DAYANTINEOPLASTICAGENTSPIQRAY 200 MG TABLETSPIQRAY 250 MG TABLETSPIQRAY 300 MG TABLETSADD PA WITH QL QL: 1 CARTON PER 28 DAYS ANTINEOPLASTICAGENTSXPOVIO PAK 60MGXPOVIO PAK 80MGXPOVIO PAK 100MGADD QL 1 CARTON PER 28 DAYSANTINEOPLASTICAGENTSNUBEQA 300MG TABLET ADD QL 4 TABLETS PER DAY ANTINEOPLASTICAGENTS TURALIO CAP 200MG ADD QL 4 TABLETS PER DAY ANTINEOPLASTICAGENTS PIQRAY 200MG TAB DOSE PIQRAY 300MG TAB DOSE PIQRAY 250MG TAB DOSE REVISE QL1 CARTON PER 28 DAYS CHOLESTEROLAGENTS EZALLOR SPRINKLE 5 MG CAP EZALLOR SPRINKLE 10 MG CAP EZALLOR SPRINKLE 20 MG CAP EZALLOR SPRINKLE 40 MG CAP ADD PA AND QLQL: 1 TABLET PER DAY COPD AGENTS DUAKLIR 400/12 INHALER ADD ST AND QLQL: 1 INHALER PER 30 DAYSCYSTIC FIBROSISAGENTSKALYDECO PAK 25MG ADD QL2 PACKETS PER DAYCYSTIC FIBROSISAGENTSORKAMBI GRANULES ADD QL2 PACKETS PER DAY HIVDOVATO TABLET EDURANT 25 MG TABLET DELSTRIGO TABLET COMPLERA TABLET ODEFSEY TABLET JULUCA TABLET ADD PA FOR NEW STARTS AND ADD QLQL: 1 PER DAY HIVINTELENCE TABLET ADD PA FOR NEW STARTS AND ADD QLQL:200 MG- 2 TABLETS PER DAY 400 MG- 4 TABLETS PER DAY 25 MG – 16 TABLETS PER DAYHIVATRIPLA TABLET BIKTARVY TABLET CIMDUO TABLET DESCOVY TABLETEMTRIVA 200 MG CAPSULE EPIVIR 300 MG TABLET EPZICOM TABLET EVOTAZ TABLET GENVOYA TABLET PIFELTRO 100 MG TABLET PREZCOBIX TABLET PREZISTA 800 MG TABLET REYATAZ 300 MG CAPSULESTRIBILD TABLET SUSTIVA 600 MG TABLETSYMFI TABLET SYMFI LO TABLET SYMTUZA TABLET TRIUMEQ TABLET TRUVADA TABLET TYBOST 150 MG TABLET VIDEX EC 400 MG CAPSULE VIDEX EC 250 MG CAPSULE VIRAMUNE XR 400 MG TABLETADD QL 1 PER DAYTEMIXYS TABLETHIVREYATAZ 200 MG CAPSULE REYATAZ 150 MG CAPSULE VIDEX EC 200 MG CAPSULE ZERIT 40 MG CAPSULE ZERIT 30 MG CAPSULE COMBIVIR TABLET DUTREBIS TABLET EPIVIR 150 MG TABLET ISENTRESS HD 600 MG TABLET PREZISTA 600 MG TABLET RETROVIR 300 MG TABLET SELZENTRY 75 MG TABLET TIVICAY 10 MG, 25 MG AND 50 MGTABLETTRIZIVIR TABLETVIRAMUNE 200 MG TABLET ZIAGEN 300 MG TABLET ADD QL 2 PER DAYHIV ISENTRESS 100 MG GRANULE PACKET FOR SUSPENSION ADD QL2 PACKETS PER DAYHIVVIDEX EC 125 MG CAPSULE VIRAMUNE XR 100MG TABLET ADD QL 3 PER DAYHIVAPTIVUS 250 MG CAPSULE INVIRASE 500 MG TABLET ISENTRESS 400 MG TABLET KALETRA 200 MG-50 MG TABLETLEXIVA 700 MG TABLET SELZENTRY 300 MG TABLET SELZENTRY 150 MG TABLET SUSTIVA 200 MG CAPSULE VIRACEPT 625 MG TABLET ZERIT 20 MG CAPSULE ZERIT 15 MG CAPSULEADD QL 4 PER DAYHIVREYATAZ 50 MG POWDER FORSUSPENSIONADD QL5 PACKETS PER DAYHIVCRIXIVAN 400 MG CAPSULE PREZISTA 150 MG TABLET RESCRIPTOR 200 MG TABLET RETROVIR 100 MG CAPSULE ISENTRESS 100 MG CHEWABLEADD QL 6 PER DAY HIV SELZENTRY 25 MG TABLET ADD QL 8 PER DAY HIV TROGARZO 150MG/ML VIAL ADD QL8 VIALS PER 28 DAYSHIVINVIRASE 200 MG CAPSULE KALETRA 100 MG-25 MG TABLETPREZISTA 75 MG TABLET VIRACEPT 250 MG TABLET ADD QL 10 PER DAY HIVCRIXIVAN 200 MG CAPSULE NORVIR 100 MG TABLET NORVIR 100 MG CAPSULEADD QL 12 PER DAYNORVIR 100 MG ORAL POWDERPACKETRESCRIPTOR 100 MG TABLET SUSTIVA 50 MG CAPSULEHIV APTIVUS 100 MG/ML SOLUTION ADD QL 13 ML PER DAYHIV PREZISTA 100 MG/ML SUSPENSION ADD QL 14 ML PER DAY HIV KALETRA 400 MG-100 MG/5 MLORAL SOLUTIONNORVIR 80 MG/ML ORAL SOLUTION ADD QL 16 ML PER DAY HIV ISENTRESS 25 MG CHEWABLE ADD QL24 TABLETS PER DAYHIV EMTRIVA 10 MG/ML SOLUTION ADD QL 29 ML PER DAYHIVEPIVIR 10 MG/ML ORAL SOLUTION ZIAGEN 20 MG/ML SOLUTION ADD QL 32 ML PER DAY HIVVIDEX 4 GM PEDIATRIC ORALSOLUTIONVIDEX 2 GM PEDIATRIC ORALSOLUTIONVIRAMUNE 50 MG/5 MLSUSPENSION ADD QL 40 ML PER DAY HIV VIRACEPT 50 MG/G POWDERADD QL 53 GM PER DAYHIV FUZEON 90 MG VIAL ADD QL60 VIALS PER 30 DAYSHIV LEXIVA 50 MG/ML SUSPENSION ADD QL 60 ML PER DAYHIV SELZENTRY 20 MG/ML ORALSOLUTION ADD QL 62 ML PER DAYHIV RETROVIR 10 MG/ML SYRUP ADD QL 64 ML PER DAYHIVZERIT 1 MG/ML SOLUTION ADD QL 80 ML PER DAY IRRITABLE BOWEL SYNDROME (IBS)AGENTSZELNORM 6MG TABLET ADD PA AND QL QL 2 TABLETS PER DAY LAMBERT-EATON MYASTHENIC SYNDROME AGENTSRUZURGI 10MG TABLET ADD PA AND QL QL 10 TABLETS PER DAYNARCOTIC ANTAGONISTS SUBLOCADE 100/0.5 INJECTION SUBLOCADE 300/1.5 INJECTION REMOVE PANARCOTIC ANTAGONISTS VIVITROL 380MG INEJCTION REMOVE PA AND ADD QL QL 1 VIAL PER 28 DAYSNARCOTIC ANTAGONISTS ZUBSOLV 2.9-0.71 SUB REVISE QL QL 5 PER DAY ORAL DIABETICAGENTS*QTERNMET XR TABLETADD ST AND QLQL:5 MG/5 MG/1000 MG, 10 MG/5 MG/1000 MG:1 TABLET PER DAY2.5 MG/2.5 MG/1000 MG, 5 MG/2.5 MG/10000MG: 2 TABLETS PER DAYORAL DIABETICAGENTS QTERN 5-5MG TABLET ADD QL1 TABLET 28 DAYSINJECTABLE DIABETIC AGENTSOZEMPIC 2/1.5ML INJECTION ADD QL 1 PER 28 DAYSPRENATAL VITAMINS DUET DHADUET DHA BALANCEDNESTABS ABC NESTABS DHA OBTREX DHA SELECT-OB+DHATHERANATAL COMPLETEVITAFOL FE+ VITAFOL-OB+DHABAL-CARE DHA ESSENTIAL ADD QL 2 PER DAYPRENATAL VITAMINS CITRANATAL B-CALMADD QL 3 PER DAYTOPICAL ANTIPRURITICS DOXEPIN HCL 5% CREAM,ZONALON 5% CREAM, PRUDOXIN5% CREAM ADD PA AND QLQL 1 TUBE PER FILL; 1 FILL PER 3 MONTHSTOPICAL ANESTHETIC COMBINATIONSLIDOCAINE/PRILOCAINE CREAMREVISE QL30 GM PER 30 DAYS* Clinical edits will be put in place as these new drugs to come market.What action do I need to take?Please review these changes and work with your Healthy Connections members to transition them to formulary alternatives. If you determine preferred formulary alternatives are notclinically appropriate for specific members, you will need to obtain prior authorization (PA) to continue coverage beyond the applicable effective date.What if I need assistance?We recognize the unique aspects of member cases. If your Healthy Connections member cannot be converted to a formulary alternative for medical reasons, call our Pharmacy department at 866-902-1689 and follow the voice prompts for pharmacy PA.You can find the Preferred Drug List on our website at > Providers > Pharmacy Information. If you need assistance with any other item, contact the Customer Care Center at 866-757-8286.。
三腾孕化剂501-CS30产品数据资料说明书

Product Data SheetTrigonox 501-CS301,2,4,5,7,8-Hexoxonane, 3,6,9-trimethyl-3,6,9-tris(Et and Pr) derivs, 30%solution in odorless mineral spiritsTrigonox® 501-CS30 is an initiator for the production of controlled rheology polypropylene (CR-PP) and high-temperature polymerization of ethylene.CAS number1613243-54-1EINECS/ELINCS No.810-295-5TSCA statuslisted on inventorySpecificationsAppearance Clear liquid at 25°CColor40 Pt-Co max.Total active oxygen 5.17-5.48 %CharacteristicsDensity, 20°C0.86 g/cm³ApplicationsTrigonox® 501-CS30 is an efficient peroxide formulation for the production of controlled rheology polypropylene (CR-PP) in an extrusion process. Trigonox® 501-CS30 allows polypropylene producers great flexibility in controlling a polymer’s Melt Flow Index (MFI). Small changes in either peroxide concentration or process temperature can produce significantly different MFI’s. An important advantage of Trigonox® 501-CS30 is that the final CR-PP contains a low content of volatiles originating from peroxide decomposition products. Trigonox® 501-CS30 forms no acetone and no tert-butanol.Half-life dataThe reactivity of an organic peroxide is usually given by its half-life (t1/2) at various temperatures. For Trigonox® 501-CS30 in chlorobenzene half-life at other temperatures can be calculated by using the equations and constants mentioned below:0.1 hr170°C (338°F)1 hr146°C (295°F)10 hr125°C (257°F)Formula 1kd = A·e-Ea/RTFormula 2t½ = (ln2)/kdEa150.60 kJ/moleA 1.09E+15 s-1R8.3142 J/mole·KT(273.15+°C) KThermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT110°C (230°F)Method The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts max.40°C (104°F)Ts min.-30°C (-22°F)Note When stored under these recommended storage conditions, Trigonox® 501-CS30 will remain within the Nouryon specifications for a period of at least 12months after delivery.Packaging and transportThe standard packaging is a 770 kg Polyethylene Intermediate Bulk Container (PE IBC). Both packaging and transport meet the international regulations. For the availability of other packed quantities consult your Nouryon representative. Trigonox® 501-CS30 is classified as Organic peroxide type F; liquid, Division 5.2; UN 3109 for land and sea transport and classified as Organic peroxide type C; liquid, Division 5.2; UN 3103 for air transport.Safety and handlingKeep away from open fire, sparks and other sources of heat or ignition. Avoid contact with reducing agents (e.g. amines), acids, alkalis and heavy metal compounds (e.g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Trigonox® 501-CS30. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsCarbon dioxide, Methane, Ethane, Propane, Methyl ethyl ketone, Methyl propyl ketone, Methyl acetate, Ethyl acetate, Propyl acetateAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® is a registered trademark of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2023-6-22© 2023Polymer production Trigonox 501-CS30。
hss-p-5.75.09 - hyaluronic acid derivatives说明书

5.75.09Section:Prescription DrugsEffective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject:Hyaluronic Acid DerivativesPage:1 of 7Last Review Date:March 13, 2020Hyaluronic Acid DerivativesDescriptionDurolane, Euflexxa, GelSyn-3, GenVisc 850, Hyalgan , SodiumHyaluronate, Supartz , Synojoynt*, Triluron, TriVisc, Visco-3 (sodium hyaluronate)Gel-ONE , Hymovis, Monovisc, Orthovisc (hyaluronan)Synvisc, Synvisc-One (hylan G-F 20)Bolded medications are the preferred products*These medications are included in this policy but are not available in the market as of yetBackgroundOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint . The goal of therapy is torestore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1).The American College of Rheumatology (ACR) updated its guidelines for the treatment of osteoarthritis (OA) of the knee in 2012. In mild symptomatic OA, treatment may be limited toFederal Employee Program® 1310 G Street, N.W.Washington, D.C. 20005 202.942.1000Fax 202.942.1125Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 2 of 7patient education, physical and occupational therapy and other non-pharmacologic modalities. Nonpharmacologic modalities strongly recommended for the management of knee OA were aerobic, aquatic, and/or resistance exercises as well as weight loss for overweight patients. Nonpharmacologic modalities conditionally recommended for knee OA included medial wedge insoles for valgus knee OA, subtalar strapped lateral insoles for varus knee OA, medially directed patellar taping, manual therapy, walking aids, thermal agents, tai chi, self-management programs, and psychosocial interventions. Pharmacologic modalities conditionally recommended for the initial management of patients with knee OA included acetaminophen, oral and topical NSAIDs, tramadol, and intraarticular corticosteroid injections (1).Regulatory StatusFDA-approved indication: Hyaluronic acid derivatives are indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy, simple analgesics (e.g., acetaminophen), NSAIDs, tramadol, or intra-articular steroid injections (2-18).The hyaluronic acid derivatives are contraindicated for use in patients with known hypersensitivity to hyaluronan (sodium hyaluronate) preparations. Orthovisc lists hypersensitivity to gram positive bacterial proteins as an additional contraindication (4). Caution should be exercised when Gel-One, Hyalgan, Visco-3, Synvisc, Synvisc-One, Supartz, and Triluron are administered to patients with allergies to avian proteins, feathers, and egg products (3-8, 18).Hyaluronic acid derivatives are contraindicated to treat patients with knee joint infections, infections or skin diseases in the area of the injection site (2-17).A treatment cycle for most of the hyaluronan derivatives typically involves multiple weekly injections. Euflexxa, GelSyn-3, Sodium Hyaluronate, Synvisc, Triluron, TriVisc, and Visco-3 are given for a total of three injections. Orthovisc is given for three or four injections. GenVisc 850, Supartz and Hyalgan are given for a total of three or five injections. Durolane, Gel-One, Synojoynt, and Synvisc-One differ from the other hyaluronan derivatives in that it only requires one injection. Repeat courses of hyaluronan derivatives may be administered if symptoms return (2-18).Upon the basis of high quality supporting evidence, the American Academy of Orthopedic Surgeons cannot recommend using hyaluronic acid for patients with symptomatic osteoarthritis of the knee (19).Related policiesSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 3 of 7Hyaluronate PowderPolicyThis policy statement applies to clinical review performed for pre-service (Prior Approval, Precertification, Advanced Benefit Determination, etc.) and/or post-service claims.Hyaluronic acid derivatives may be considered medically necessary for the treatment of osteoarthritis of the knee and if the conditions indicated below are met.Hyaluronic acid derivatives may be considered investigational for all other indications.Prior-Approval RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Inadequate response to TWO or more of the following conservative non-pharmacologic therapy:a. Cardiovascular (aerobic) activity, such as: walking, biking, stationarybike, aquatic exerciseb. Resistance exercisec. Weight reduction (for persons who are overweight)d. Participation in self-management programse. Wear of medially directed patellar tapingf. Wear of wedged insolesg. Thermal agentsh. Walking aidsi. Physical therapyj. Occupational therapy2. Inadequate response, intolerance, or contraindication to TWO or more of thefollowing:Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 4 of 7a. Acetaminophenb. Oral NSAIDsc. Topical NSAIDs3. Inadequate response, intolerance, or contraindication to intra-articularsteroid injections in which efficacy lasted less than 8 weeks4. Radiologic confirmation of Kellgren-Lawrence Scale score of grade 2 orgreater5. NO dual therapy with another hyaluronic acid injectable6. Non-preferred medications only: Patient MUST have tried at least TWO ofthe preferred products unless the patient has a valid medical exception (e.g.inadequate treatment response, intolerance, contraindication)Prior – Approval Renewal RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Documentation of improvement in pain with previous course of treatment2. At least 12 months has elapsed since last injection of the prior treatmentcycle3. Documentation of reduction of dosing of NSAIDs or other analgesicsduring the 12 month period following the last injection of the prior treatmentcycle4. NO dual therapy with another hyaluronic acid injectable5. Non-preferred medications only: Patient MUST have tried at least TWOof the preferred products unless the patient has a valid medical exception(e.g. inadequate treatment response, intolerance, contraindication) Policy GuidelinesPre - PA AllowanceNoneSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 5 of 7Prior - Approval LimitsDuration12 monthsQuantity One course of therapy for each kneePrior – Approval Renewal LimitsSame as aboveRationaleSummaryOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint. The goal of therapy is to restore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1-18).Prior approval is required to ensure the safe, clinically appropriate and cost effective use of the hyaluronic acid derivatives while maintaining optimal therapeutic outcomes.References1. American College of Rheumatology, Subcommittee on Osteoarthritis Guidelines.Recommendations for the medical management of osteoarthritis of the hip and knee:2012 update. Arthritis Care & Research 2012; 64(4):465-474.2. Euflexxa [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc.; July 2016.3. Hyalgan [package insert]. Parsippany, NJ: Fidia Pharma USA Inc.; May 2014.4. Orthovisc [package insert]. Woburn, MA: Anika Therapeutics; June 2005.5. Supartz [package insert]. Durham, NC: Bioventus LLC; April 2015.6. Synvisc [package insert]. Ridgefield, NJ: Genzyme Corp.; December 2014.7. Synvisc-One [package insert]. Ridgefield, NJ: Genzyme Corp.; September 2014;8. Gel-One [package insert]. Warsaw, IN: Zimmer Inc.; May 2011.9. Monovisc [package insert]. Bedford, MA: Anika Therapeutics; December 2013.10. Hymovis [package insert]. Parsippany, NJ: O Fidia Pharma USA Inc.; October 2015.Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 6 of 711. GenVisc 850 [package insert]. Doylestown, PA: OrthogenRx Inc.; January 2015.12. GelSyn-3 [package insert]. Durham, NC: Bioventus LLC; January 2016.13. Durolane [package insert]. Durham, NC: Bioventus LLC; November 2017.14. Visco-3 [package insert]. Warsaw, IN: Zimmer, Inc.; May 2017.15. Sodium Hyaluronate [package insert]. North Wales, PA: Teva Pharmaceuticals USA,Inc.; March 2019.16. Synojoynt [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.;September 2019.17. TriVisc [package insert]. Doylestown, PA: OrthogenRx, Inc.; September 2018.18. Triluron [package insert]. Florham Park, NJ: Fidia Pharma USA Inc.; March 2019.19. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee.Evidence-based guideline 2nd edition. May 2013.Policy HistoryDate Action ReasonJanuary 2012 Added minimum age - only approved for adultsDecember 2012 Annual editorial review and reference updateDecember 2013 Annual editorial review and reference updateMarch 2014 Annual editorial reviewAddition of examples of non-pharmacological agents and agents of priorfailure medications.April 2014 Line-Addition of Monovisc to PAMarch 2015 Annual criteria review and reference updateMarch 2016 Change from one tried and failed to two tried and failed non-pharmacologic and pharmacologic therapies and addition of the tried and failed of intra-articular steroid and radiologic confirmation of Kellgren-Lawrence Scalescore of grade 2 or greaterAddition of HymovisPolicy # change from 5.11.04 to 5.75.09May 2016 Addition of GelSyn-3 and GenVisc 850December 2016 Annual editorial review and reference updateAdded: no dual therapy with another hyaluronic acid injectableMarch 2017 Bolded preferred products in the title pageJuly 2017 GelSyn-3 has been changed to preferredSeptember 2017 Annual reviewDecember 2017 Addition of Durolane and Visco-3March 2018 Annual editorial reviewRemoval of Tramadol from the T/F listSeptember 2019 Annual review and reference update. Addition of Sodium Hyaluronate,Synojoynt, and TriViscSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 7 of 7December 2019 Annual review. Addition of requirement to trial preferred products January 2020 Addition of TriluronMarch 2020 Annual reviewKeywordsThis policy was approved by the FEP® Pharmacy and Medical Policy Committee on March 13, 2020 and is effective on April 1, 2020.。
人促肾上皮质激素释放激素(CRH)ELISA试剂盒说明书
人促肾上皮质激素释放激素(CRH)酶联免疫分析试剂盒使用说明书厦门慧嘉生物科技有限公司本试剂仅供研究使用目的:本试剂盒用于测定人血清,血浆及相关液体样本中促肾上皮质激素释放激素(CRH)的含量。
实验原理:本试剂盒应用双抗体夹心法测定标本中人促肾上皮质激素释放激素(CRH)水平。
用纯化的人促肾上皮质激素释放激素(CRH)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入促肾上皮质激素释放激素(CRH),再与HRP标记的羊抗人抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB显色。
TMB在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的促肾上皮质激素释放激素(CRH)呈正相关。
用酶标仪在450nm波长下测定吸光度(OD值),通过标准曲线计算样品中人促肾上皮质激素释放激素(CRH)浓度。
试剂盒组成:样本处理及要求:1. 血清:室温血液自然凝固10-20分钟,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如出现沉淀,应再次离心。
2. 血浆:应根据标本的要求选择EDTA或柠檬酸钠作为抗凝剂,混合10-20分钟后,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如有沉淀形成,应该再次离心。
3. 尿液:用无菌管收集,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如有沉淀形成,应再次离心。
胸腹水、脑脊液参照实行。
4. 细胞培养上清:检测分泌性的成份时,用无菌管收集。
离心20分钟左右(2000-3000转/分)。
仔细收集上清。
检测细胞内的成份时,用PBS(PH7.2-7.4)稀释细胞悬液,细胞浓度达到100万/ml左右。
通过反复冻融,以使细胞破坏并放出细胞内成份。
离心20分钟左右(2000-3000转/分)。
仔细收集上清。
保存过程中如有沉淀形成,应再次离心。
5. 组织标本:切割标本后,称取重量。
FDA批准的精准医疗诊断体外器械一览表List of Cleared or Approved Companion Diagnostic Devices
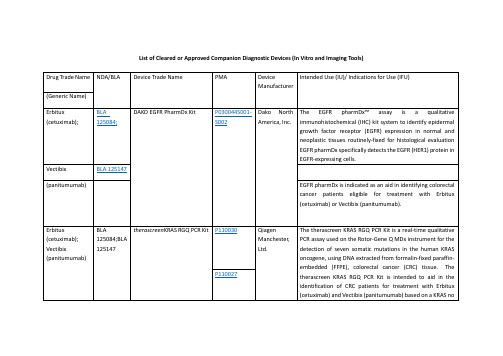
Drug Trade Name
NDA/BLA
Device Trade Name
PMA
Device Manufacturer
Intended Use (IU)/ Indications for Use (IFU)
(imatinibmesylate)
NDA 021588
The c-KitpharmDxis indicated as an aid in the differential diagnosis of gastrointestinal stromal tumors (GIST). After diagnosis of GIST, results from c-KitpharmDxmay be used as an aid in identifying those patients eligible for treatment withGleevec/Glivec(imatinibmesylate).
(deferasirox)
Gilotrif
NDA 201292
therascreenEGFR RGQ PCR Kit
P120022
QiagenManchester, Ltd.
ThetherascreenEGFR RGQ PCR Kit is a real-time PCR test for the qualitative detection of exon 19 deletions and exon 21 (L858R) substitution mutations of the epidermal growth factor receptor (EGFR) gene in DNA derived from formalin-fixed paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC) tumor tissue. The test is intended to be used to select patients with NSCLC for whom GILOTRIF (afatinib), an EGFR tyrosine kinase inhibitor (TKI), is indicated. Safety and efficacy of GILOTRIF (afatinib) have not been established in patients whose tumors have L861Q, G719X, S768I, exon 20 insertions, and T790M mutations, which are also detected by thetherascreenEGFR RGQ PCR Kit.
M5 鲑鱼精 DNA 10mg ml 说明书

北京聚合美生物科技有限公司 Mei5 Biotechnology, Co., Ltd
北京市昌平区回龙观龙域北街10号院1号楼四层422-1室(创集合大楼) 热线电话:(86)************
M5 鲑鱼精DNA 10mg/ml 使用说明书
产品名称 单位 货号 M5 鲑鱼精DNA 10mg/ml 1 ml MF475-01 M5 鲑鱼精DNA 10mg/ml 5x1 ml MF475-05
【储存条件】
-20℃保存,有效期2年。
【产品简介】
鲑鱼精DNA 溶液(10mg/ml )是经过酚氯仿抽提,超声和热变性处理的短片段的单链DNA 溶液,可直接用于Southern 、Northern 等核酸杂交中。
【操作步骤】
本产品鲑鱼精DNA 的浓度为10mg/ml ,使用时按实验具体要求操作,稀释至所需工作浓度即可。
【注意事项】
1. 如果每次的使用量很小,可以适当分装后再使用,避免反复冻融。
2. 为了您的安全和健康,请穿实验服并戴手套操作。
【备注】
本产品仅供科研使用。
在确认产品质量出现问题时,本公司承诺为客户免费更换等量的质量合格产品。
美国贝克曼库尔特流式细胞分析仪

美国贝克曼库尔特流式细胞分析仪(Beckman coulter cell)产品型号:Cell Lab Quanta SC当前价格:0.00元产品数量:0新旧程度:全新有效期至:0000-00-00所在地:产品简介:仪器简介:T细胞亚群检测的CD45/CD4/CD8/CD3、CD45/CD56/CD19/CD3;阵发性血红蛋白尿(PNH)检测的CD55、CD59;血小板无力症(GT)检测的CD41、CD61等等详细信息仪器简介:T细胞亚群检测的CD45/CD4/CD8/CD3、CD45/CD56/CD19/CD3;阵发性血红蛋白尿(PNH)检测的CD55、CD59;血小板无力症(GT)检测的CD41、CD61等等。
但对于白血病/淋巴瘤免疫分型,国际上迄今为止也没有统一的抗体组合。
在2000年国际细胞分析学会(ISAC)大会上,临床血细胞计数协会组织了一次国际专家会议,以期对检测血液淋巴系统肿瘤所需最少、最有效的单抗数达成共识。
75%与会者一致认为,对于慢性淋巴系统增殖性疾病(CLD)有9种单抗:CD5,CD19,κ,λ,CD3,CD20,CD23,CD10,CD45对初诊来说是最基本的。
淋巴瘤和CLD相似,需要至少12-16种单抗。
对于急性白血病(AL),75%的与会者认为大约13-15种单抗是最基本的:CD10,CD19,CD79a,CD13,CD33,CD34,CD45,CD2,MPO,CD7,CD14,CD3,HLA-DR等,对初步鉴别白血病系列是必需的。
其他一些(CD16,CD56,CDw65,TdT,cyCD3)可能对某些病例有用。
几乎所有的投票者都认为,要对急性白血病完善分类所需单抗的恰当数量平均为20-24种。
但这些抗体之间组合也是一大难题,目前也无统一规定(如表二)。
大会多数发言者(11/13)指出,对已确诊病人的监护和分期来说,仅需较少单抗。
抗体的质量控制是实验的关键环节。
抗体的质量包括其特异性、灵敏度、精密度。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Optovin 348575-88-2 HY-12809 315.41 C15H13N3OS2 >98%
Product Name: CAS No.: Cat. No.: MWt: Formula: Purity :
Solubility:
100 mM in DMSO
Mechanisms: Pathways:Membrane Tranporter/Ion Channel; Target:TRP Channel Biological Activity: Optovin is a reversible photoactive TRPA1 activator; stimulates human TRPA1 channels in vitro and enables repeated photoactivation of motor behaviors in wild-type zebrafish (EC50 = 2 μM). IC50 value: Target: in vitro: Optovin is a rhodanine-containing small molecule with no previously annotated biological activity. Whereas DMSO-treated animals do not respond to photic stimuli, optovin-treated animals respond to light with vigorous motor excitation at an EC50 of 2 μM. Optovin-treated animals respond to 387 nm (violet) stimuli, but not to 485 nm (blue), 560 nm (green) or longer wavelengths. optovin strongly activated 33% (35/105) of DRG neurons. optovin acts on a molecular target expressed in mustard oil responsive mammalian DRG sensory neurons; perhaps on TRPA1 itself. TrpA1 is necessary for the optovin response [1]. in vivo: Optovin also elicited nociceptive behavio...
References: [1]. Kokel D, et al. Photochemical activation of TRPA1 channels in neurons and animals. Nat Chem Biol. 2013 Apr;9():257-63.
Caution: Not fully tested. For research purposes only Medchemexpress LLC
m o c . s s e r p x e m e h c d e Am S. Uw ,w 2 5w 8: 8b 0e JW Nm ,o n c o. i s t s c e nr up Jx he t m u e oh mc nd oe Mm D@ 2 0o f n 1i el : t i i a u Sm ,E e v i r D k r a P r e e D 1 1
