临床试验术语英汉对照
临床试验中英对照词汇表englishvocabularyofclinicaltrials
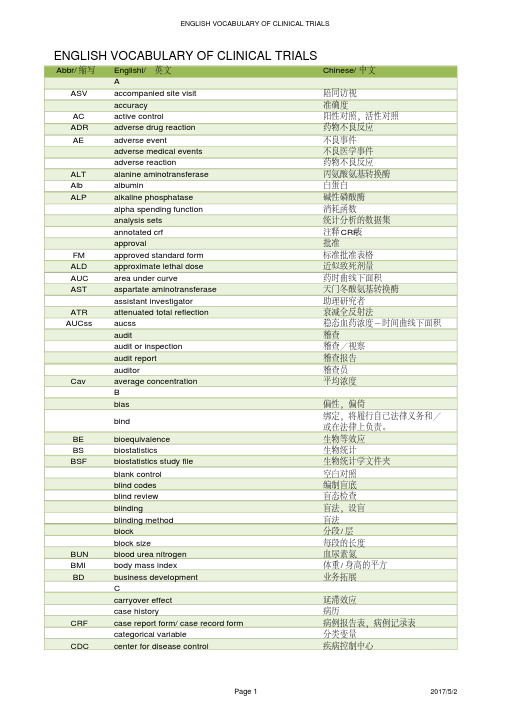
Abbr/缩写Englishi/英文Chinese/中文AASV accompanied site visit陪同访视accuracy准确度AC active control阳性对照,活性对照ADR adverse drug reaction药物不良反应AE adverse event不良事件adverse medical events不良医学事件adverse reaction药物不良反应ALT alanine aminotransferase丙氨酸氨基转换酶Alb albumin白蛋白ALP alkaline phosphatase碱性磷酸酶alpha spending function消耗函数analysis sets统计分析的数据集annotated crf注释CRF表approval批准FM approved standard form标准批准表格ALD approximate lethal dose近似致死剂量AUC area under curve药时曲线下面积AST aspartate aminotransferase天门冬酸氨基转换酶assistant investigator助理研究者ATR attenuated total reflection衰减全反射法AUCss aucss稳态血药浓度-时间曲线下面积audit稽查audit or inspection稽查/视察audit report稽查报告auditor稽查员Cav average concentration平均浓度Bbias偏性,偏倚bind 绑定,将履行自己法律义务和/或在法律上负责。
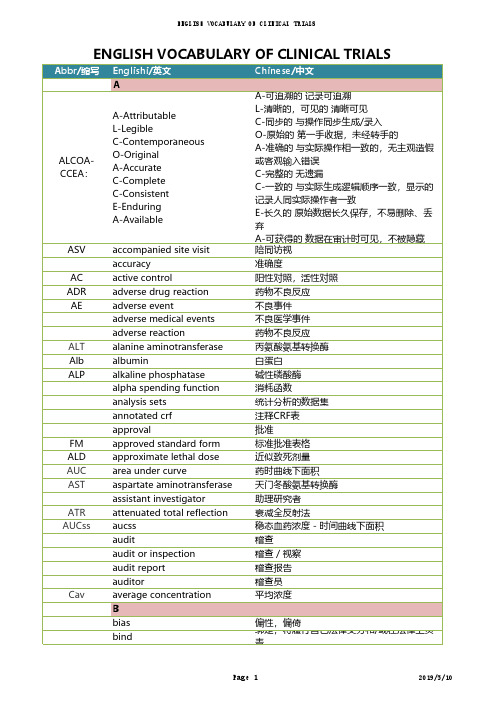
BE bioequivalence生物等效应BS biostatistics生物统计BSF biostatistics study file生物统计学文件夹blank control空白对照blind codes编制盲底blind review盲态检查blinding盲法,设盲blinding method盲法block分段/层block size每段的长度BUN blood urea nitrogen血尿素氮BMI body mass index体重/身高的平方BD business development业务拓展Ccarryover effect延滞效应case history病历CRF case report form/ case record form病例报告表,病例记录表categorical variable分类变量CDC center for disease control疾病控制中心ENGLISH VOCABULARY OF CLINICAL TRIALSCDE center for drug evaluation 国家食品药品监督管理局药品审评中心CCF central clinical file申办方临床研究文件夹CEC central ethics committee中心伦理委员会CIF central investigator's file中心研究者文件夹CDA certified data analyst数据分析师COF change order form工作变更申请表CMC chemistry manufacturing and controls临床前药学研究CMO chief media officer首席医学官CFDA china food and drug administration国家食品药品监督管理总局CD circular dichroism圆二色谱CL clearance清除率clinical auditor临床稽查员CDMS clinical data management system临床数据管理系统clinical director临床总监clinical equivalence临床等效应CMA clinical monitoring associate临床研究监查助理CM clinical monitoring/operations临床监查/运营CPA clinical project assistant临床项目助理CRA clinical research associate临床研究监察员,临床研究助理CRC clinical research coordinator临床协调员clinical study临床研究CSDs clinical study documents临床研究文件CSR clinical study report临床研究报告CTMS clinical trail management system临床试验管理系统clinical trial临床试验CTA clinical trial agreement临床试验协议CTA clinical trial application临床试验申请CTA clinical trial assistant临床研究助理CTX clinical trial exemption临床试验免责CTP clinical trial protocol临床试验方案CTS clinical trial supplies临床试验用品clinical trial/ study report临床试验报告Cmax cmax,maximum concentration峰浓度CI co-inveatigator合作研究者CTD common technical document国际药品注册申请技术文件格式comparison对照CR complete response完全缓解compliance依从性composite variable复合变量CATD computer-assisted trial design计算机辅助试验设计confidence interval可信区间confidence level置信水平CDA confidentiality disclosure agreement保密协议consistency test一致性检验CRO contract research organization合同研究组织contract/ agreement协议/合同control group对照组CD controlled documents控制文件coordinating committee协调委员会COI coordinating investigator协调研究者CAPA corrective action & preventive action纠正/预防措施CIOMS council for international organizations ofmedical sciences国际医学科学组织理事会cov close out visits关闭访视crea肌酐cross-over study交叉设计Css css, steady-state concentration稳浓度cure痊愈CV curriculum vitea履历DDCRF data clarification and resolution form数据澄清和解决表DCF data clarification form数据澄清表DCR data clarification report数据澄清报告DM data management数据管理DMP data management plan数据管理计划书DMSF data management study file数据管理研究文件夹DQF data query form数据疑问表database建立数据库database go live数据库批准启动DF degree of fluctuation波动度/系统descriptive statistical analysis描述性统计分析dichotomies二分类DSC differential scanning calorimetry示差扫描量热法DFS disease free survival无病生存期diviation偏差documentation记录/文件DLT dose limting toxicity剂量限制毒性dose-reaction relation剂量-反应关系double blinding双盲double dummy双模拟double dummy technique双盲双模拟技术double-blinding双盲dro out脱落drug-oriented药物源性Eeffectiveness疗效eCRF electronic case report form电子病历报告表EDC electronic data capture电子数据采集系统EDP electronic data processing电子数据处理系统emergency envelope应急信件EOS end of study研究结束end point终点endpoint criteria/ measurement终点指标equivalence等效性essential documentation必须文件EC ethics committee伦理委员会ESH-ESC european soceity of hypertension-europeansociety of cardilogy欧洲高血压-心脏协会EU european union欧盟EBM evidence-based medicine循证医学excellent显效exclusion criteria排除标准Ffactorial design析因设计failure无效,失败final point终点fixed-dose procedure固定剂量法FDA food and drug administration 食品药品管理局/美国食品药品管理局forced titration强制滴定FAS full analysis set全分析集GGC-FTIR gas chromatography-fourier transforminfrared spectroscopy气相色谱-傅利叶红外联用GC-MS gas chromatography-mass spectrometer气相色谱-质谱联用generic drug通用名药global assessment variable全局评价变量GLU glucose葡萄糖GAP good agricultural practice中药材种植管理规范GCP good clinical practice药物临床试验质量管理规范GLP good laboratory practice药品实验室管理规范GMP good manufacturing practice药品生产质量管理规范GLP good non-clinical laboratory practice药物非临床研究质量管理规范GSP good supply practice药品经营质量管理规范GUP good use practice药品使用质量管理规范group sequential design成组序贯设计HHCO head of clinical operations临床运营总监HEV health economic evaluation健康经济学评价HCT historial control trial历史对照研究HIM hospital intensive monitoring医院集中监测hypothesis test假设检验IIRAEs immediately reportable adverse events立即上报的不良反应improvement好转inclusion criteria入选标准IEC independent ethics committee独立伦理委员会information gathering信息收集IT information technology信息技术IC informed consent知情同意ICF informed consent form知情同意书IR infrared radiation红外吸收光谱initial meeting启动会议inspection视察/检查institution inspection机构检查IRB institutional review board机构伦理委员会ICH intennational conference on hamonization oftechnical requirements for registration ofpharmaceuticals for human use人用药品注册技术要求国际技术协调会/国际协调会议ICH-GCP intennational conference on hamonizationtripartite guideline on good clinicalpractice国际协调会议药品临床试验质量管理规范指南intention to treat意向性治疗ITT intention-to –treat意向性分析IVRS interactive voice response system互动式语音应答系统interim analysis期中分析IDB investigational drug brochure试验药物手册IND investigational new drug新药临床研究IP investigational product研究用产品investigator研究者ISF investigator site file研究者文件夹ISA investigator study agreement研究者合同IB investigator's brochure研究者手册IXRS ixsystems交互式互动服务提供商JKKPS karnofsky performance status行为状态评分LLOCF last observation carry forward最近一次观察的结转LD50lethal dose, 50%半数致死剂量LOI letter of intent意向书LOQ limit of quantitation定量限LM line manager直线经理LC-MS liquid chromatography -mass spectrometry液相色谱-质谱联用logic check逻辑检查lost of follow up失访Mmarketing approval/ authorization上市许可证masking设盲MRT mass rapid transit平均滞留时间MS mass spectrometry质谱MS-MS mass spectrometry-mass spectrometry质谱-质谱联用MSA master services agreement主服务协议matched pair匹配配对MTD maximal tolerable dose最大耐受剂量MedDRA meddra ICH国际医学用语词典medical director医学总监MW medical writing医学写作MOH ministry of health卫生部missing value缺失值mixed effect model混合效应模式monitor监查员monitoring监查monitoring report监查报告multi-center trial多中心试验NNCE new chemical entity新化学实体NDA new drug application新药上市申请NOAEL no observed adverse effect level未见不良反应剂量水平non-clinical study非临床研究non-inferiority非劣效性non-parametric statistics非参数统计方法NRCCT non-randomized concurrent controlled trial非随机同期对照试验 NA not available不可用NCS not clinically significant无临床意义ND not done未做NMR nuclear magnetic resonance核磁共振Oobedience依从性ODR odr旋光光谱offsite archive异地存档on-site monitoring现场监查on-target monitoring目标化监查open-blinding非盲open-label非盲OP operation procedure操作规程OTL opperational team lead运营团队负责人optional titration随意滴定original medical record原始医疗记录OD other documents其他文件OOS out of scope超出工作范围outcome结果outcome assessment结果指标评价outcome measurement结果指标outlier离群值OS overall survival总生存时间Pparallel group design平行组设计parameter estimation参数估计parametric statistics参数统计方法PR partial response部分缓解patient file病人档案patient history病历PR patient recruitment患者招募patient-oriented人源性PP per protocol符合方案集PMI periodic maintenance inspection定期维护检查PIN personal identification number个人确认密码PK phamacokinetics药物代谢动力学PD pharmacodynamics药效学;药代动力学PKAS pharmacokinetics analysis set药代动力学分析集placebo安慰剂placebo control安慰剂对照polytomies多分类PMS post-marketing study上市后临床试验PMS post-marketing surveillance上市后药物检测power检验效能PSV pre –study visit启动前访视precision精密度preclinical study临床前研究primary endpoint主要终点primary variable主要变量PI principal investigator主要研究者PL product license产品许可证PD progressive disease病情进展PMF project management file项目管理文件夹PM project maneger项目经理protocol试验方案protocol amendment方案补正PD protocol deviation方案偏离PFDA pure food and drug administration (美国)食品及药物管理局(正式名称为Food and Drug Administration,略作FDA)QQA quality assurance品质保证QAU quality assurance unit质量保证部门QC quality control质量控制query form应用疑问表query list应用疑问表Rrandomization随机化RCT randomized controlled trial随机对照试验range check范围检查rating scale量表reconciliation和解RM records management记录管理RA regulatory affairs注册事务RA regulatory authorities监督管理部门RSD relative standard deviation日内和日间相对标准差RM remote monitoring远程监查replication可重复RECIST response evaluation criteria in solid实体瘤疗效反应的评价标准RMV routine monitoring visits例行监查run in准备期SSAS safety analysis set安全性评价的数据集safety evaluation安全性评价sample size样本量scale of ordered categorical ratings有序分类指标SOW scope of work工作范围secondary variable次要变量sequence试验次序SAR serious adverse reaction严重不良反应seriousness严重性severity严重程度SAE severity adverse event严重不良事件significant level检验水准simple randomization简单随机single blinding单盲site audit试验机构稽查SCV site close-out visit中心关闭访视SIF site information form中心信息表SIV site intiation visits启动访视SMV site monitoring visit中心监查方式SSV site selection visit中心筛选访视SVR site visit report中心访视报告SD source data原始数据SD source document原始文件SDV soutce data verification原始数据核查specificity特异性sponsor申办者sponsor-investigator申办研究者SD stable disease病情稳定standard curve标准曲线SD standard deviation标准差SOP standard operating procedure标准操作规程SFDA state food and drug administration 中国国家食品药品监督管理局的旧称statistic统计量statistical analysis plan统计参数计划书statistical model统计模型statistical tables统计分析表stratified分层study aud研究稽查SC study coordinator研究协调员subgroup亚组Sub-I subinvestigator次要研究者SI sub-investigator助理研究者subject受试者subject diary受试者日记subject enrollment受试者入选subject enrollment log受试者入选表SIC subject identification code受试者识别代码subject recruitment受试者招募subject screening log受试者筛选表superiority检验survival analysis生存分析SUSAR suspected unexpected sar疑似非预期严重不良反应sxrd单晶X-射线衍射system audit系统稽查Ttarget variable目标变量TP template模板MRHD the maximum recommended human dose最高推荐人用剂量TG thermogravimetric analysis热重分析T1/2time 1/2消除半衰期TTP time-to-progression 疾病进展时间tlc、hplc制备色谱tmax达峰时间TP total protein总蛋白T-BIL total-bilirubin总胆红素T-CHO total-cholesterol总胆固醇TR training培训transformation变量变换treatment group试验组trial error试验误差TMF trial master file试验主文件夹trial objective试验目的trial site临床试验中心triple blinding三盲two one-side test双单侧检验UUV-VIS ultraviolet–visible spectroscopy紫外可见吸收光谱UADE unanticipated adverse drug effect非预期不良反应unblinding揭盲/破盲UADR unexpected adverse drug reaction非预期药物不良反应 UAE unexpected adverse event非预期不良事件Vvariability变异variable变量visual analogy scale直观类比打分法visual check人工检查vulnerable subject弱势受试者Wwash-out清洗期washout period洗脱期well-being福利,健康WI work instruction工作指南XYZ。
临床试验英语词汇

临床试验英语词汇-CAL-FENGHAI.-(YICAI)-Company One1专业术语、缩略语中英对照表缩略语英文全称中文全称ABE Average Bioequivalence 平均生物等效性AC Active control 阳性对照ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者ALB Albumin 白蛋白ALD Approximate Lethal Dose 近似致死剂量ALP Alkaline phosphatase 碱性磷酸酶ALT Alanine aminotransferase 丙氨酸转氨酶ANDA Abbreviated New Drug Application 简化新药申请ANOVA Analysis of variance 方差分析AST Aspartate aminotransferase 天冬氨酸转氨酶ATR Attenuated total reflection 衰减全反射法BA Bioavailability 生物利用度BE Bioequivalence 生物等效性BMI Body Mass Index 体质指数BUN Blood urea nitrogen 血尿素氮CATD Computer-assisted trial design 计算机辅助试验设计CDER Center of Drug Evaluation and Research 药品评价和研究中心CFR Code of Federal Regulation 美国联邦法规CI Co-Investigator 合作研究者CI Confidence Interval 可信区间COI Coordinating Investigator 协调研究者CRC Clinical Research Coordinator 临床研究协调者CRF Case Report/Record Form 病历报告表/病例记录表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告CTX Clinical Trial Exemption 临床试验免责CHMP Committee for Medicinal 人用药委会Products for Human UseDSC Differential scanning 差示扫描热量计DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统EWP Europe Working Party 欧洲工作组FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GCP Good Laboratory Practice 药物非临床试验质量管理规范GLU Glucose 葡萄糖GMP Good Manufacturing Practice 药品生产质量管理规范HEV Health economic evaluation 健康经济学评价IB Investigator’s Brochure研究者手册IBE IndividualBioequivalence 个体生物等效性IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会ITT Intention-to –treat 意向性分析IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统LD50 Medial lethal dose 半数致死剂量LLOQ Lower Limit of quantitation 定量下限LOCF Last observation carry forward 最接近一次观察的结转LOQ Limit of Quantitation 检测限MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部MRT Mean residence time 平均滞留时间MTD Maximum Tolerated Dose 最大耐受剂量ND Not detectable 无法定量NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)NMR Nuclear Magnetic Resonance 核磁共振PD Pharmacodynamics 药效动力学PI Principal Investigator 主要研究者PK Pharmacokinetics 药物动力学PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PP Per protocol 符合方案集PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QAU Quality Assurance Unit 质量保证部门QC Quality Control 质量控制QWP Quality Working Party 质量工作组RA Regulatory Authorities 监督管理部门REV Revision 修订SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SFDA State Food and Drug Administration 国家食品药品监督管理局SI Sponsor-Investigator 申办研究者SI Sub-investigator 助理研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂T-BIL Total Bilirubin 总胆红素T-CHO Total Cholesterol 总胆固醇TG Thromboglobulin 血小板球蛋白Tmax Time of maximum concentration 达峰时间TP Total proteinum 总蛋白UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO- WHO International Conference WHO 国际药品管理当局会议ICDR A of Drug Regulatory AuthoritiesAberrant result 异常结果Absorption phase 吸收相Absorption 吸收Accuracy 准确度Accurate 精密度Administer 给药Amendment修正案Approval 批准Assess 估计Audit Report 稽查报告Audit 稽查Auditor 稽查员Analytical run/batch:分析批Benefit 获益Bias 偏性,偏倚Bioequivalence 生物等效Biosimilar /Follow-on biologics 生物仿制药Blank Control 空白对照Blind codes 编制盲底Blind review 盲态检查 /盲态审核Blinding method 盲法Blinding/masking 盲法/设盲Block size 每段的长度Block 层 /分段BCS 生物药剂学分类系统Carryover effect 延滞效应Case history 病历Clinical equivalence 临床等效性Clinical study 临床研究Clinical Trial Report 临床试验报告Comparison 对照Compensation 补偿,赔偿金Compliance 依从性Concomitant 伴随的Conduct 行为Confidence level 置信水平Consistency test 一致性检验Contract/ agreement 协议/合同Control group 对照组Coordinating Committee 协调委员会Crossover design 交叉设计Cross-over Study 交叉研究Cure 痊愈Data management 数据管理Descriptive statistical analysis 描述性统计分析Dichotomies 二分类Dispense 分布Diviation 偏差Documentation 记录/文件Dosage forms 剂型Dose dumping 剂量倾卸(药物迅速释放入血而达到危险浓度)Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模Drop out 脱落Effectiveness 疗效Elimination phase 消除相Emergency envelope 应急信件Enantiomers 对映体End point 终点Endpoint criteria/ measurement 终点指标Enterohepatic recycling 肠肝循环Essential Documentation 必需文件Ethical 伦理的Ethics committee 伦理委员会Evaluate 评估Exclusion Criteria 排除标准Excretion 排泄Expedite 促进Extrapolated 外推的Essentially similar product:基本相似药物Factorial design 析因设计Failure 无效,失败Finacing 财务,资金Final point 终点First pass metabolism 首过代谢Fixed-dose procedure 固定剂量法Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Gene mutation 基因突变Genotoxicity tests 生殖毒性试验Global assessment variable 全局评价变量Group sequential design 成组序贯设计Hypothesis test 假设检验Highly permeable:高渗透性Highly soluble:高溶解度Highly variable drug:高变异性药物Highly:Variable Drug 高变异性药物HVDP:高变异药物制剂Identification 鉴别,身份证Improvement 好转In vitro 体外In vivo 体内Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Instruction 指令,说明Integrity 完整,正直Intercurrent 中间发生的,间发的Inter-individual variability 个体间变异性Interim analysis 期中分析Investigational Product 试验药物Investigator 研究者Involve 引起,包括IR 红外吸收光谱Innovator Product:原创药Ka 吸收速率常LC-MS 液相色谱-质谱联用logarithmic transformation 对数转换Logic check 逻辑检查Lost of follow up 失访Mask 面具,掩饰Matched pair 匹配配对Metabolism 代谢Missing value 缺失值Mixed effect model 混合效应模式Modified release products 改良释放剂型Monitor 监查员Monitoring Plan 监察计划Monitoring Report 监察报告MS-MS 质谱-质谱联用Multi-center Trial 多中心试验Negative 阴性,否定的Non-clinical Study 非临床研究Non-inferiority 非劣效性Non-Linear Pharmacokinetics 非线性药代动力学Non-parametric statistics 非参数统计方法NTID:窄治疗指数制剂Obedience 依从性Open-blinding 非盲Open-label 非盲Original Medical Record 原始医疗记录Outcome Assessment 结果评价Outcome measurement 结果指标Outlier 离群值OIP 经口服吸收药物Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient History 病历Per protocol, PP 符合方案集Permeability 渗透性Pharmacodynamic characteristics 药效学特征Pharmacokinetic characteristics 药代学特征Placebo Control 安慰剂对照Placebo 安慰剂Polytomies 多分类Post-dosing postures 给药后坐姿Potential 潜在的Power 检验效能Precision 精密度Preclinical Study 临床前研究Precursor 母体前体Premature 过早的,早发Primary endpoint 主要终点Primary variable 主要变量Prodrug 药物前体Protocol amendment 方案补正Protocol Amendments 修正案Protocol 试验方案Quality Control Sample:质控样品Rapidly dissolving:快速溶出Racemates 外消旋物Randomization 随机 /随机化Range check 范围检Rating scale 量表Recruit 招募,新会员Replication 可重复Retrieval 取回,补修Revise 修正Risk 风险Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample Size 样本量、样本大小Sampling schedules 采血计划Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single Blinding 单盲Site audit 试验机构稽查Solubility 溶解度Specificity 特异性Specify 叙述,说明Sponsor-investigator 申办研究者Standard curve 标准曲线Statistical model 统计模型Statistical tables 统计分析表Steady state 稳态Storage 储存Stratified 分层Study Audit 研究稽查Study Site 研究中心Subgroup 亚组Sub-investigator 助理研究者Subject Enrollment Log 受试者入选表Subject Enrollment 受试者入选Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Subject Screening Log 受试者筛选表Subject 受试者Submit 交付,委托Superiority 检验Supplemental 增补的Supra-bioavailability 超生物利用度(试验药的生物利用度大于对照药)Survival analysis 生存分析System Audit 系统稽查SmPC:药品说明书Standard Sample:标准样品Target variable 目标变量Treatment group 试验组Trial error 试验误差Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Trial site 试验场所Triple Blinding 三盲Two one-side test 双单侧检验Therapeutic equivalence:治疗等效性Un-blinding 破盲/揭盲Verify 查证、核实Visual analogy scale 直观类比打分法Vulnerable subject 弱势受试者Wash-out Period 洗脱期Well-being 福利,健康Withdraw 撤回,取消药代动力学参数Ae(0-t):给药到t时尿中排泄的累计原形药。
临床试验常用英文缩写及注释参考

临床试验常用英文缩写及注释参考1. IRB:Institutional Review Board,研究机构审评委员会。
负责审核和批准医学研究,确保受试者的权益得到保障。
2. CRA:Clinical Research Associate,临床研究助理。
负责临床试验的执行和监督,协助研究者完成各项任务。
3. SAE:Serious Adverse Event,严重不良事件。
临床试验期间发生的任何导致死亡、危及生命、重要器官功能或功能衰竭等严重后果的事件。
4. SPC:Summary Safety Profile,汇总安全性摘要。
临床试验结束后,对试验期间发生的不良事件进行汇总和分析,以评估试验的安全性。
5. ECG:Electrocardiogram,心电图。
用于监测心血管系统状况,评估药物对心脏的影响。
6. TDM:Therapeutic Drug Monitoring,治疗药物监测。
用于评估药物在患者体内的浓度,以指导临床用药。
7. IRN:Interim Analysis,中期分析。
在临床试验中期对数据进行分析,评估试验的效果和可行性。
8. RCT:Randomized Controlled Trial,随机对照试验。
一种常见的临床试验设计方法,将受试者随机分为试验组和对照组,比较试验药物和安慰剂或常规治疗的效果。
9. MTD:Maximum Tolerated Dose,最大耐受剂量。
在临床试验中,找到能够使大多数受试者耐受的最大药物剂量。
10. DLT:Dose-Limiting Toxicity,剂量限制毒性。
在临床试验中,观察到任何可能导致严重不适或威胁生命的副作用,被认为是继续增加剂量的限制条件。
11. PP population:入选患者群体。
在临床试验中,指符合入选标准并完成试验的患者群体。
12. OMP:Observational Medical Outcomes Project,观察性医学项目。
临床试验英语词汇

专业术语、缩略语中英对照表缩略语英文全称中文全称ABE Average Bioequivalence 平均生物等效性AC Active control 阳性对照ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者ALB Albumin 白蛋白ALD Approximate Lethal Dose 近似致死剂量ALP Alkaline phosphatase 碱性磷酸酶ALT Alanine aminotransferase 丙氨酸转氨酶ANDA Abbreviated New Drug Application 简化新药申请ANOV A Analysis of variance 方差分析AST Aspartate aminotransferase 天冬氨酸转氨酶ATR Attenuated total reflection 衰减全反射法BA Bioavailability 生物利用度BE Bioequivalence 生物等效性BMI Body Mass Index 体质指数BUN Blood urea nitrogen 血尿素氮CATD Computer-assisted trial design 计算机辅助试验设计CDER Center of Drug Evaluation and Research 药品评价和研究中心CFR Code of Federal Regulation 美国联邦法规CI Co-Investigator 合作研究者CI Confidence Interval 可信区间COI Coordinating Investigator 协调研究者CRC Clinical Research Coordinator 临床研究协调者CRF Case Report/Record Form 病历报告表/病例记录表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告CTX Clinical Trial Exemption 临床试验免责CHMP Committee for Medicinal 人用药委会Products for Human UseDSC Differential scanning 差示扫描热量计DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统EWP Europe Working Party 欧洲工作组FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GCP Good Laboratory Practice 药物非临床试验质量管理规范GLU Glucose 葡萄糖GMP Good Manufacturing Practice 药品生产质量管理规范HEV Health economic evaluation 健康经济学评价IB Investigator’s Brochure研究者手册IBE IndividualBioequivalence 个体生物等效性IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会ITT Intention-to –treat 意向性分析IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统LD50 Medial lethal dose 半数致死剂量LLOQ Lower Limit of quantitation 定量下限LOCF Last observation carry forward 最接近一次观察的结转LOQ Limit of Quantitation 检测限MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部MRT Mean residence time 平均滞留时间MTD Maximum Tolerated Dose 最大耐受剂量ND Not detectable 无法定量NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)NMR Nuclear Magnetic Resonance 核磁共振PD Pharmacodynamics 药效动力学PI Principal Investigator 主要研究者PK Pharmacokinetics 药物动力学PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PP Per protocol 符合方案集PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QAU Quality Assurance Unit 质量保证部门QC Quality Control 质量控制QWP Quality Working Party 质量工作组RA Regulatory Authorities 监督管理部门REV Revision 修订SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SFDA State Food and Drug Administration 国家食品药品监督管理局SI Sponsor-Investigator 申办研究者SI Sub-investigator 助理研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂T-BIL Total Bilirubin 总胆红素T-CHO Total Cholesterol 总胆固醇TG Thromboglobulin 血小板球蛋白Tmax Time of maximum concentration 达峰时间TP Total proteinum 总蛋白UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO- WHO International Conference WHO 国际药品管理当局会议ICDR A of Drug Regulatory AuthoritiesAberrant result 异常结果Absorption phase 吸收相Absorption 吸收Accuracy 准确度Accurate 精密度Administer 给药Amendment修正案Approval 批准Assess 估计Audit Report 稽查报告Audit 稽查Auditor 稽查员Analytical run/batch:分析批Benefit 获益Bias 偏性,偏倚Bioequivalence 生物等效Biosimilar /Follow-on biologics 生物仿制药Blank Control 空白对照Blind codes 编制盲底Blind review 盲态检查/盲态审核Blinding method 盲法Blinding/masking 盲法/设盲Block size 每段的长度Block 层/分段BCS 生物药剂学分类系统Carryover effect 延滞效应Case history 病历Clinical equivalence 临床等效性Clinical study 临床研究Clinical Trial Report 临床试验报告Comparison 对照Compensation 补偿,赔偿金Compliance 依从性Concomitant 伴随的Conduct 行为Confidence level 置信水平Consistency test 一致性检验Contract/ agreement 协议/合同Control group 对照组Coordinating Committee 协调委员会Crossover design 交叉设计Cross-over Study 交叉研究Cure 痊愈Data management 数据管理Descriptive statistical analysis 描述性统计分析Dichotomies 二分类Dispense 分布Diviation 偏差Documentation 记录/文件Dosage forms 剂型Dose dumping 剂量倾卸(药物迅速释放入血而达到危险浓度)Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模Drop out 脱落Effectiveness 疗效Elimination phase 消除相Emergency envelope 应急信件Enantiomers 对映体End point 终点Endpoint criteria/ measurement 终点指标Enterohepatic recycling 肠肝循环Essential Documentation 必需文件Ethical 伦理的Ethics committee 伦理委员会Evaluate 评估Exclusion Criteria 排除标准Excretion 排泄Expedite 促进Extrapolated 外推的Essentially similar product:基本相似药物Factorial design 析因设计Failure 无效,失败Finacing 财务,资金Final point 终点First pass metabolism 首过代谢Fixed-dose procedure 固定剂量法Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Gene mutation 基因突变Genotoxicity tests 生殖毒性试验Global assessment variable 全局评价变量Group sequential design 成组序贯设计Hypothesis test 假设检验Highly permeable:高渗透性Highly soluble:高溶解度Highly variable drug:高变异性药物Highly:Variable Drug 高变异性药物HVDP:高变异药物制剂Identification 鉴别,身份证Improvement 好转In vitro 体外In vivo 体内Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Instruction 指令,说明Integrity 完整,正直Intercurrent 中间发生的,间发的Inter-individual variability 个体间变异性Interim analysis 期中分析Investigational Product 试验药物Investigator 研究者Involve 引起,包括IR 红外吸收光谱Innovator Product:原创药Ka 吸收速率常LC-MS 液相色谱-质谱联用logarithmic transformation 对数转换Logic check 逻辑检查Lost of follow up 失访Mask 面具,掩饰Matched pair 匹配配对Metabolism 代谢Missing value 缺失值Mixed effect model 混合效应模式Modified release products 改良释放剂型Monitor 监查员Monitoring Plan 监察计划Monitoring Report 监察报告MS-MS 质谱-质谱联用Multi-center Trial 多中心试验Negative 阴性,否定的Non-clinical Study 非临床研究Non-inferiority 非劣效性Non-Linear Pharmacokinetics 非线性药代动力学Non-parametric statistics 非参数统计方法NTID:窄治疗指数制剂Obedience 依从性Open-blinding 非盲Open-label 非盲Original Medical Record 原始医疗记录Outcome Assessment 结果评价Outcome measurement 结果指标Outlier 离群值OIP 经口服吸收药物Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient History 病历Per protocol,PP 符合方案集Permeability 渗透性Pharmacodynamic characteristics 药效学特征Pharmacokinetic characteristics 药代学特征Placebo Control 安慰剂对照Placebo 安慰剂Polytomies 多分类Post-dosing postures 给药后坐姿Potential 潜在的Power 检验效能Precision 精密度Preclinical Study 临床前研究Precursor 母体前体Premature 过早的,早发Primary endpoint 主要终点Primary variable 主要变量Prodrug 药物前体Protocol amendment 方案补正Protocol Amendments 修正案Protocol 试验方案Quality Control Sample:质控样品Rapidly dissolving:快速溶出Racemates 外消旋物Randomization 随机/随机化Range check 范围检Rating scale 量表Recruit 招募,新会员Replication 可重复Retrieval 取回,补修Revise 修正Risk 风险Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample Size 样本量、样本大小Sampling schedules 采血计划Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single Blinding 单盲Site audit 试验机构稽查Solubility 溶解度Specificity 特异性Specify 叙述,说明Sponsor-investigator 申办研究者Standard curve 标准曲线Statistical model 统计模型Statistical tables 统计分析表Steady state 稳态Storage 储存Stratified 分层Study Audit 研究稽查Study Site 研究中心Subgroup 亚组Sub-investigator 助理研究者Subject Enrollment Log 受试者入选表Subject Enrollment 受试者入选Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Subject Screening Log 受试者筛选表Subject 受试者Submit 交付,委托Superiority 检验Supplemental 增补的Supra-bioavailability 超生物利用度(试验药的生物利用度大于对照药)Survival analysis 生存分析System Audit 系统稽查SmPC:药品说明书Standard Sample:标准样品Target variable 目标变量Treatment group 试验组Trial error 试验误差Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Trial site 试验场所Triple Blinding 三盲Two one-side test 双单侧检验Therapeutic equivalence:治疗等效性Un-blinding 破盲/揭盲Verify 查证、核实Visual analogy scale 直观类比打分法Vulnerable subject 弱势受试者Wash-out Period 洗脱期Well-being 福利,健康Withdraw 撤回,取消药代动力学参数Ae(0-t):给药到t时尿中排泄的累计原形药。
临床试验中英对照词汇表english vocabulary of clinical trials-yrn2019051011
首席医学官 首席营销官
国家食品药品监督管理总局
圆二色谱 清除率 临床稽查员 临床数据管理系统 临床总监 临床等效应 临床研究监查助理 临床监查/运营 临床项目助理 临床研究监察员,临床研究助理 临床协调员 临床研究
Page 2
2019/5/10
ENGLISH VOCABULARY OF CLINICAL TRIALS
resolution
数据澄清和解决表
Page 3
2019/5/10
ENGLISH VOCABULARY OF CLINICAL TRIALS
DCF DCR DM DMP DMSF DQF DVP
DF
DSC DFS
DLT
eCRF EDC EDP EOS
EC ESH-ESC
EU EBM
data clarification form
标准批准表格
ALD
approximate lethal dose
近似致死剂量
AUC
area under curve
药时曲线下面积
AST
aspartate aminotransferase 天门冬酸氨基转换酶
assistant investigator
助理研究者
ATR
attenuated total reflection
国际医学科学组织理事会
sciences
close out visits
关闭访视
crea
肌酐
cross-over study
交叉设计
css, steady-state concentration 稳浓度
cure
痊愈
curriculum vitea
临床试验常用中英文词汇

SFDA Glossary:GCP,GLP,TRIAL Accuracy 准确度Active control,AC 阳性对照,活性对照Adverse drug reaction,ADR 药物不良反应Adverse event,AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反应Alb 白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP 碱性磷酸酶Alpha spending function 消耗函数ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态血药浓度-时间曲线下面积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性,偏倚Bioequivalence 生物等效应Blank control 空白对照Blind codes 编制盲底Blind review 盲态审核Blind review 盲态检查Blinding method 盲法Blinding/ masking 盲法,设盲Block 分段Block 层Block size 每段的长度BUN 尿素氮Carryover effect 延滞效应Case history 病历Case report form 病例报告表Case report form/ case record form,CRF 病例报告表,病例记录表Categorical variable 分类变量Cav 平均浓度CD 圆二色谱CL 清除率Clinical equivalence 临床等效应Crossover design 交叉设计Cross-over study 交叉研究Css 稳浓度Cure 痊愈Data management 数据管理Database 建立数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies 二分类Diviation 偏差Documentation 记录/文件Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模拟Double dummy technique 双盲双模拟技术Double-blinding 双盲Drop out 脱落DSC 差示扫描热量计Effectiveness 疗效Electronic data capture,EDC 电子数据采集系统Electronic data processing,EDP 电子数据处理系统Emergency envelope 应急信件End point 终点Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential documentation 必须文件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure 无效,失败Final point 终点Fixed-dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Global assessment variable 全局评价变量GLU 血糖Good clinical practice,GCP 药物临床试验质量管理规范Good manufacture practice,GMP 药品生产质量管理规范Good non-clinical laboratory practice,GLP 药物非临床研究质量管理规范Group sequential design 成组序贯设计Health economic evaluation,HEV 健康经济学评价Hypothesis test 假设检验Hypothesis testing 假设检验International Conference of Harmonization,ICH 人用药品注册技术要求国际技术协调会,国际协调会议Improvement 好转Inclusion criteria 入选标准Independent ethics committee,IEC 独立伦理委员会Information consent form,ICF 知情同意书Information gathering 信息收集Informed consent,IC 知情同意Initial meeting 启动会议Inspection 视察/检查Institution inspection 机构检查Institution review board,IBR 机构审查委员会Intention to treat 意向治疗(——临床领域)Intention-to –treat,ITT 意向性分析(-统计学)Interactive voice response system,IVRS 互动式语音应答系统Interim analysis 期中分析Investigator 研究者Investigator's brochure,IB 研究者手册IR 红外吸收光谱Ka 吸收速率常Last observation carry forward,LOCF 最接近一次观察的结转LC-MS 液相色谱-质谱联用LD50 板数致死剂量Logic check 逻辑检查LOQ (Limit of Quantitation)定量限LOCF,Last observation carry forward 最近一次观察的结转Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监查员Monitoring 监查Monitoring report 监查报告MRT 平均滞留时间MS 质谱MS-MS 质谱-质谱联用MTD(Maximum Tolerated Dose)最大耐受剂量Multicenter trial 多中心试验Multi-center trial 多中心试验New chemical entity,NCE 新化学实体New drug application,NDA 新药申请NMR 核磁共振谱Non-clinical study 非临床研究Non-inferiority 非劣效性Non-parametric statistics 非参数统计方法Obedience 依从性ODR 旋光光谱Open-blinding 非盲Open-label 非盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient history 病历Per protocol,PP 符合方案集Placebo 安慰剂Placebo control 安慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principal investigator 主要研究者Principle investigator,PI 主要研究者Product license,PL 产品许可证Protocol 试验方案Protocol 试验方案Protocol amendment 方案补正Quality assurance unit,QAU 质量保证部门Quality assurance,QA 质量保证Quality control,QC 质量控制Query list,query form 应用疑问表Randomization 随机化Randomization 随机Range check 范围检查Rating scale 量表Regulatory authorities,RA 监督管理部门Replication 可重复RSD 日内和日间相对标准差Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample size 样本含量Sample size 样本量,样本大小Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event,SAE 严重不良事件Serious adverse reaction,SAR 严重不良反应Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single blinding 单盲Single-blinding 单盲Site audit 试验机构稽查SOP 试验室的标准操作规程Source data verification,SDV 原始数据核准Source data,SD 原始数据Source document,SD 原始文件Specificity 特异性Sponsor 申办者Sponsor-investigator 申办研究者Standard curve 标准曲线Standard operating procedure,SOP 标准操作规程Statistic 统计量Statistical analysis plan 统计分析计划Statistical analysis plan 统计参数计划书Statistical analysis plan,SAP 统计分析计划Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study audit 研究稽查Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject diary 受试者日记Subject enrollment 受试者入选Subject enrollment log 受试者入选表Subject identification code,SIC 受试者识别代码Subject recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis 生存分析SXRD 单晶X-射线衍射System audit 系统稽查T1/2 消除半衰期Target variable 目标变量T-BIL 总胆红素T-CHO 总胆固醇TG 热重分析TLC、HPLC 制备色谱Tmax 峰时间TP 总蛋白Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial master file 试验总档案Trial objective 试验目的Trial site 试验场所Triple blinding 三盲Two one-side test 双单侧检验Unblinding 揭盲Unblinding 破盲Unexpected adverse event,UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类比打分法Visual check 人工检查Vulnerable subject 弱势受试者Wash-out 清洗期Washout period 洗脱期Well-being 福利,健康。
临床试验常用中英文词汇

SFDA Glossary:GCP,GLP,TRIAL Accuracy 准确度Active control,AC 阳性对照,活性对照Adverse drug reaction,ADR 药物不良反应Adverse event,AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反应Alb 白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP 碱性磷酸酶Alpha spending function 消耗函数ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态血药浓度-时间曲线下面积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性,偏倚Bioequivalence 生物等效应Blank control 空白对照Blind codes 编制盲底Blind review 盲态审核Blind review 盲态检查Blinding method 盲法Blinding/ masking 盲法,设盲Block 分段Block 层Block size 每段的长度BUN 尿素氮Carryover effect 延滞效应Case history 病历Case report form 病例报告表Case report form/ case record form,CRF 病例报告表,病例记录表Categorical variable 分类变量Cav 平均浓度CD 圆二色谱CL 清除率Clinical equivalence 临床等效应Clinical study 临床研究Clinical study report 临床试验的总结报告Clinical trial 临床试验Clinical trial application,CTA 临床试验申请Clinical trial exemption,CTX 临床试验免责Clinical trial protocol,CTP 临床试验方案Clinical trial/ study report 临床试验报告Cmax 峰浓度Co-investigator 合作研究者Comparison 对照Compliance 依从性Composite variable 复合变量Computer-assisted trial design,CATD 计算机辅助试验设计Confidence interval 可信区间Confidence level 置信水平Consistency test 一致性检验Contract research organization,CRO 合同研究组织Contract/ agreement 协议/合同Control group 对照组Coordinating committee 协调委员会Crea 肌酐CRF(case report form)病例报告表Crossover design 交叉设计Cross-over study 交叉研究Css 稳浓度Cure 痊愈Data management 数据管理Database 建立数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies 二分类Diviation 偏差Documentation 记录/文件Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模拟Double dummy technique 双盲双模拟技术Double-blinding 双盲Drop out 脱落DSC 差示扫描热量计Effectiveness 疗效Electronic data capture,EDC 电子数据采集系统Electronic data processing,EDP 电子数据处理系统Emergency envelope 应急信件End point 终点Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential documentation 必须文件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure 无效,失败Final point 终点Fixed-dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Global assessment variable 全局评价变量GLU 血糖Good clinical practice,GCP 药物临床试验质量管理规范Good manufacture practice,GMP 药品生产质量管理规范Good non-clinical laboratory practice,GLP 药物非临床研究质量管理规范Group sequential design 成组序贯设计Health economic evaluation,HEV 健康经济学评价Hypothesis test 假设检验Hypothesis testing 假设检验International Conference of Harmonization,ICH 人用药品注册技术要求国际技术协调会,国际协调会议Improvement 好转Inclusion criteria 入选标准Independent ethics committee,IEC 独立伦理委员会Information consent form,ICF 知情同意书Information gathering 信息收集Informed consent,IC 知情同意Initial meeting 启动会议Inspection 视察/检查Institution inspection 机构检查Institution review board,IBR 机构审查委员会Intention to treat 意向治疗(——临床领域)Intention-to –treat,ITT 意向性分析(-统计学)Interactive voice response system,IVRS 互动式语音应答系统Interim analysis 期中分析Investigator 研究者Investigator's brochure,IB 研究者手册IR 红外吸收光谱Ka 吸收速率常Last observation carry forward,LOCF 最接近一次观察的结转LC-MS 液相色谱-质谱联用LD50 板数致死剂量Logic check 逻辑检查LOQ (Limit of Quantitation)定量限LOCF,Last observation carry forward 最近一次观察的结转Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监查员Monitoring 监查Monitoring report 监查报告MRT 平均滞留时间MS 质谱MS-MS 质谱-质谱联用MTD(Maximum Tolerated Dose)最大耐受剂量Multicenter trial 多中心试验Multi-center trial 多中心试验New chemical entity,NCE 新化学实体New drug application,NDA 新药申请NMR 核磁共振谱Non-clinical study 非临床研究Non-inferiority 非劣效性Non-parametric statistics 非参数统计方法Obedience 依从性ODR 旋光光谱Open-blinding 非盲Open-label 非盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient history 病历Per protocol,PP 符合方案集Placebo 安慰剂Placebo control 安慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principal investigator 主要研究者Principle investigator,PI 主要研究者Product license,PL 产品许可证Protocol 试验方案Protocol 试验方案Protocol amendment 方案补正Quality assurance unit,QAU 质量保证部门Quality assurance,QA 质量保证Quality control,QC 质量控制Query list,query form 应用疑问表Randomization 随机化Randomization 随机Range check 范围检查Rating scale 量表Regulatory authorities,RA 监督管理部门Replication 可重复RSD 日内和日间相对标准差Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample size 样本含量Sample size 样本量,样本大小Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event,SAE 严重不良事件Serious adverse reaction,SAR 严重不良反应Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single blinding 单盲Single-blinding 单盲Site audit 试验机构稽查SOP 试验室的标准操作规程Source data verification,SDV 原始数据核准Source data,SD 原始数据Source document,SD 原始文件Specificity 特异性Sponsor 申办者Sponsor-investigator 申办研究者Standard curve 标准曲线Standard operating procedure,SOP 标准操作规程Statistic 统计量Statistical analysis plan 统计分析计划Statistical analysis plan 统计参数计划书Statistical analysis plan,SAP 统计分析计划Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study audit 研究稽查Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject diary 受试者日记Subject enrollment 受试者入选Subject enrollment log 受试者入选表Subject identification code,SIC 受试者识别代码Subject recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis 生存分析SXRD 单晶X-射线衍射System audit 系统稽查T1/2 消除半衰期Target variable 目标变量T-BIL 总胆红素T-CHO 总胆固醇TG 热重分析TLC、HPLC 制备色谱Tmax 峰时间TP 总蛋白Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial master file 试验总档案Trial objective 试验目的Trial site 试验场所Triple blinding 三盲Two one-side test 双单侧检验Unblinding 揭盲Unblinding 破盲Unexpected adverse event,UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类比打分法Visual check 人工检查Vulnerable subject 弱势受试者Wash-out 清洗期Washout period 洗脱期Well-being 福利,健康。
临床研究专业术语缩略语中英对照表

临床研究专业术语缩略语中英对照表为了方便专业人士在临床研究领域的交流和理解,本文提供了临床研究中常见的专业术语的缩略语中英对照表。
这些缩略语旨在简化长句和复杂术语,使他们更易于书写和快速交流。
以下是常见临床研究专业术语及其相应的缩略语及中英文对照:1. Adverse Event(AE)-- 不良事件2. Serious Adverse Event(SAE)-- 严重不良事件3. Placebo(PBO)-- 安慰剂4. Randomized Controlled Trial(RCT)-- 随机对照试验5. Informed Consent(IC)--知情同意6. Institutional Review Board(IRB)-- 伦理审查委员会7. Case Report Form(CRF)-- 病例报告表8. Data Monitoring Committee(DMC)-- 数据监测委员会9. Risk-Benefit Ratio(RBR)-- 风险-效益比10. Investigational New Drug(IND)-- 新药研究11. Good Clinical Practice(GCP)-- 良好临床实践12. Adverse Drug Reaction(ADR)-- 不良药物反应13. Intent-to-Treat(ITT)-- 治疗意向分析14. Protocol Violation(PV)-- 方案违规15. Sponsor-Investigator(SI)-- 研究发起人16. Standard Operating Procedures(SOPs)-- 标准操作规程17. Confidentiality Agreement(CA)-- 保密协议18. Data and Safety Monitoring Board(DSMB)-- 数据和安全监测委员会19. Clinical Research Coordinator(CRC)-- 临床研究协调员20. Declaration of Helsinki(DoH)--《赫尔辛基宣言》以上是临床研究专业术语的缩略语中英对照表。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
临床试验常用的英文Accuracy 准确度Active control, AC 阳性对照,活性对照Adverse drug reaction, ADR 药物不良反应Adverse event, AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反应Alb 白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP 碱性磷酸酶ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态血药浓度-时间曲线下面积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性,偏倚Bioequivalence 生物等效应Blank control 空白对照Blind codes 编制盲底Blind review 盲态审核Blinding method 盲法Blinding/ masking 盲法,设盲Block 分段Block size 每段的长度BUN 尿素氮Carryover effect 延滞效应Case history 病历Case report form/ case record form, CRF 病例报告表,病例记录表Categorical variable 分类变量Cav 平均浓度CL 清除率Clinical equivalence 临床等效应Clinical study 临床研究Clinical study report 临床试验的总结报告Clinical trial 临床试验Clinical trial application, CTA 临床试验申请Clinical trial exemption, CTX 临床试验免责Clinical trial protocol, CTP 临床试验方案Clinical trial/ study report 临床试验报告C max峰浓度Co-investigator 合作研究者Comparison 对照Compliance 依从性Composite variable 复合变量Computer-assisted trial design, CATD 计算机辅助试验设计Confidence interval 可信区间Confidence level 置信水平Consistency test 一致性检验Contract research organization, CRO 合同研究组织Contract/ agreement 协议/合同Control group 对照组Coordinating committee 协调委员会Crea 肌酐CRF(case report form) 病例报告表Crossover design 交叉设计Cross-over study 交叉研究Css 稳浓度Cure 痊愈Data management 数据管理Database 建立数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies 二分类Diviation 偏差Documentation 记录/文件Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模拟Double dummy technique 双盲双模拟技术Double-blinding 双盲Drop out 脱落Effectiveness 疗效Electronic data capture, EDC 电子数据采集系统Electronic data processing, EDP 电子数据处理系统Emergency envelope 应急信件End point 终点Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential documentation 必须文件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure 无效,失败Final point 终点Fixed-dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Global assessment variable 全局评价变量GLU 血糖Good clinical practice, GCP 药物临床试验质量管理规Good manufacture practice, GMP 药品生产质量管理规Good non-clinical laboratory practice, GLP 药物非临床研究质量管理规Group sequential design 成组序贯设计Hypothesis test 假设检验International Conference of Harmonization, ICH人用药品注册技术要求国际技术协调会,国际协调会议Improvement 好转Inclusion criteria 入选标准Independent ethics committee, IEC 独立伦理委员会Information consent form, ICF 知情同意书Information gathering 信息收集Informed consent, IC 知情同意Initial meeting 启动会议Inspection 视察/检查Institution inspection 机构检查Institution review board, IBR 机构审查委员会Intention to treat 意向治疗(-- 临床领域)Intention-to –treat, ITT 意向性分析(-统计学)Interim analysis 期中分析Investigator 研究者Investigator’s brochure, IB 研究者手册IR 红外吸收光谱Ka 吸收速率常Last observation carry forward, LOCF 最接近一次观察的结转LC-MS 液相色谱-质谱联用LD50半数致死剂量Logic check 逻辑检查LOQ (Limit of Quantitation) 定量限LOCF, Last observation carry forward 最近一次观察的结转Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监查员Monitoring 监查Monitoring report 监查报告MRT 平均滞留时间MTD(Maximum Tolerated Dose)最大耐受剂量Multi-center trial 多中心试验New chemical entity, NCE 新化学实体New drug application, NDA 新药申请NMR 核磁共振谱Non-clinical study 非临床研究Non-inferiority 非劣效性Non-parametric statistics 非参数统计方法Obedience 依从性ODR 旋光光谱Open-blinding 非盲Open-label 非盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient history 病历Per protocol, PP 符合方案集Placebo 安慰剂Placebo control 安慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principal investigator 主要研究者Principle investigator, PI 主要研究者Product license, PL 产品许可证Protocol 试验方案Protocol amendment 方案补正Quality assurance unit, QAU 质量保证部门Quality assurance, QA 质量保证Quality control, QC 质量控制Query list, query form 应用疑问表Randomization 随机化Range check 围检查Rating scale 量表Regulatory authorities, RA 监督管理部门Replication 可重复RSD 日和日间相对标准差Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample size 样本量,样本大小Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event, SAE 严重不良事件Serious adverse reaction, SAR 严重不良反应Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single-blinding 单盲Site audit 试验机构稽查Source data verification, SDV 原始数据核准Source data, SD 原始数据Source document, SD 原始文件Specificity 特异性Sponsor 申办者Sponsor-investigator 申办研究者Standard curve 标准曲线Standard operating procedure, SOP 标准操作规程Statistic 统计量Statistical analysis plan, SAP 统计分析计划Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study audit 研究稽查Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject diary 受试者日记Subject enrollment 受试者入选Subject enrollment log 受试者入选表Subject identification code, SIC 受试者识别代码Subject recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis 生存分析SXRD 单晶X-射线衍射System audit 系统稽查T1/2消除半衰期Target variable 目标变量T-BIL 总胆红素T-CHO 总胆固醇TG 热重分析TLC、HPLC 制备色谱T max峰时间TP 总蛋白Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial master file 试验总档案Trial objective 试验目的Trial site 试验场所Triple blinding 三盲Two one-side test 双单侧检验Unblinding 揭盲Unexpected adverse event, UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类比打分法Visual check 人工检查Vulnerable subject 弱势受试者Wash-out period 洗脱期Well-being 福利,健康。
