controlled_living radical polymerization part 2
活性可控自由基聚合

活性可控⾃由基聚合活性/可控⾃由基聚合在20世纪50、60年代,⾃由基聚合达到了它的⿍盛时期。
但由于存在链转移和链终⽌反应,传统⾃由基聚合不能较好地控制分⼦量及⼤分⼦结构[1]。
1956年美国科学家Szwarc等提出了活性聚合的概念[2],活性聚合具有⽆终⽌、⽆转移、引发速率远远⼤于链增长速率等特点,与传统⾃由基聚合相⽐能更好地实现对分⼦结构的控制,是实现分⼦设计、合成具有特定结构和性能聚合物的重要⼿段。
但离⼦型活性聚合反应条件⽐较苛刻、适⽤单体较少,且只能在⾮⽔介质中进⾏,导致⼯业化成本居⾼不下,较难⼴泛实现⼯业化。
鉴于活性聚合和⾃由基聚合各⾃的优缺点,⾼分⼦合成化学家们联想到将⼆者结合,即可控活性⾃由基聚合(CRP)或活性可控⾃由基聚合。
CRP可以合成具有新型拓扑结构的聚合物、不同成分的聚合物以及在⾼分⼦或各种化合物的不同部分链接官能团,适⽤单体较多,产物的应⽤较⼴,⼯业化成本较低。
⽬前实现“活性”/可控⾃由基聚合可分以下⼏种途径: (1) 稳定“活性”⾃由基聚合(SFRP);(2) 原⼦转移⾃由基聚合(ATRP);(3)可逆加成-断裂链转移聚合(RAFT)。
⼀、稳定“活性”⾃由基聚合(SFRP)SFRP属于⾮催化性体系,是利⽤稳定⾃由基来控制⾃由基聚合。
其机理是按照下⾯的可逆反应进⾏:外加的稳定⾃由基X·可与活性⾃由基P·迅速进⾏失活反应,⽣成“休眠种”P-X,P-X能可逆分解,⼜形成X·及活性种⾃由基P·⽽链增长。
有研究表明,使⽤烷氧胺作引发剂效果好[3]。
反应体系中的⾃由基活性种P·可抑制在较低的浓度,这样就可以减少⾃由基活性种之间的不可逆终⽌作⽤,从⽽聚合反应得到控制。
稳定⾃由基X·,主要有TEMPO(2,2,6,6-四甲基-1-哌啶氮氧⾃由基)和CoⅡ·,TEMPO属于稳定的有机⾃由基;CoⅡ·属于稳定的有机⾦属⾃由基。
植物生理学专业英语地中英文对照

植物生理学专业英语的中英文对照(按汉字笔画排序)一画乙醛酸体glyoxysome乙醛酸循环glyoxylate cycle ,GAC乙醇酸glycolate ,glycolic acid乙醇酸氧化酶glycolate oxidase乙醇酸氧化途径glycolic acid oxidati on pathway 乙醇脱氢酶alcohol dehydrogenase乙烯ethylene乙烯利ethrel二画二型性别dimorphism二苯脲diphenylurea二酰甘油diacylglycerol ,DG,DAG二硝基酚dinitrophenol ,DNP 11,3-二磷酸甘油酸1,3-bisphosphoglycerate二氧化碳猝发CO2 outburst二氧化碳饱和点CO2 saturatio n poi nt二氧化碳补偿点CO2 compe nsati on point二氨丙烷diaminopropane2,4-二氯苯氧乙酸2,4-dichlorophenoxyacetic acid,2,4-D二氢玉米素dihydrozeatin二氢吡咯pyrroline二氢红花菜豆酸dihydrophaseic acid二羟丙酮磷酸dihydroxyacet one phosphate , DHAP人工种子artificial seeds儿茶酚氧化酶catechol oxidase三画三十烷醇1-triaco ntan ol三碘苯甲酸2,3,5-triiodobenzoic acid ,TIBA三氯苯氧乙酸trichlorophenoxyacetic acid三重反应triple response 293三羧酸循环tricarboxylic acid cycle ,TCAC干旱drought干旱胁迫drought stress土壤一植物一大气连续体soil-pla nt-atmosphere con ti nuum,SPAC 下调作用down regulation大纤丝macrofibril大量元素major element ,微量元素macroelement小孔扩散律small pore diffusion law尸胺cadaverine 299己糖磷酸途径hexose mo nophosphate pathway , HMP 己糖激酶hexokinase马来酰肼maleic hydrazide,MH四画开放体系open system天然单性结实n atural parthe no carpy天冬氨酸aspartate,Asp天冬氨酸转氨酶aspartate amino tran sferase天线色素antenna pigment无土栽培soilless culture无融合生殖apomixis无辐射退激radiationless deexcitation无氧呼吸an aerobic respirati on 1无氧呼吸消失点an aerobic respiratio n ext in cti on point 无籽果实seedless fruit无限生长in determi nate growth无孢子生殖apospory无丝分裂amitosis木酮糖 5 磷酸xylulose-5-phosphate木质素lignin支链淀粉amylopectin区域化compartmentation瓦伯格效应Warburg effect日中性植物day neutral plant中日照植物in termediate day len gth pla nt中央液泡central vacuoleC 3 —C 4 中间植物C 3 —C 4 in termediate pla nt 中间丝in termediate filame nt中层middle lamella水培hydroponics水培法water culture method水势water potential水杨基氧肟酸salicylhydroxamate ,SHAM水杨酸salicylic acid,SA水生植物hydrophytes水氧化钟water oxidizing clock水合补偿点hydration compensation point水分亏缺water deficit水分平衡water balanee水分临界期critical period of water水分代谢water bolism水溶清蛋白albumin水通道蛋白water channel protein水孑L蛋白aquaporins内吞endocytosis内聚力cohesion内聚力学说cohesion theory 内在蛋白intrinsic protein 内酯酶lactonase内转换internal conversion内向K + 通道in ward K + channel 内质网endoplasmic reticulum,ER 内膜endomembrane 内能internal energy贝壳杉烯kaurene气培法aerop on ics气相色谱gas chromatography气腔网络air space netwotk气穴现象cavitation气孔开度stomatal aperture气孔运动stomatal movement 气孑L蒸腾stomatal transpiration 气孑L下腔substomatal cavities 气孑L频度stomatal frequency 毛管水capillary water 毛细作用capillarity 长日植物long day plant长短日植物long short day plant片层lamellae化学势chemical potential化学信号chemical signal化学渗透学说chemiosmotic theory反向传递antiport反馈抑制feedback inhibition反馈调节feedback regulation反应中心reaction center ,反应中心色素分子reacti on cen ter pigme nt介电常数dielectric constant分支酶branching enzyme分化differentiation分泌囊泡secretory vesicles比热容specific heat比集运转速率specific mass transfer rate ,SMTR比久B9,二甲胺基琥珀酰胺酸dimethyl aminosuccinamic acid 巴斯德效应Pasteur effect双"S” 形生长曲线double sigmoid growth curve双光系统two photosystem双向运输bidirectional transport双受精double fertilization五画末端氧化酶terminal oxidase玉米素zeatin , ZT玉米素核苷zeatin riboside玉米素顺反异构酶zeat in cistra ns isomerase玉米赤霉烯酮zearaienone正常性种子orthodox seed正化学信号positive chemical signal正向重性positive gravitropism正效应物positive effector去春化作用devernalization去极化depolarization去镁叶绿素pheophytin ,Pheo甘氨酸甜菜碱glycine betaine甘油三酯triacylglycerols,TAG甘油 3 磷酸glycerol-3-phosphate甘油 3 磷酸脱氢酶glycerol-3-phosphate dehydrogenase甘油醛 3 磷酸glyceraldehyde-3-phosphate ,GAP甘油醛 3 磷酸脱氢酶ghyceraldehyde-3-phosphate dehydrogenase 甘油酸激酶glycerate kinase可溶性氧化酶soluble oxidase可溶性淀粉合成酶soluble starch syn thase丙酮酸pyruvate丙酮酸磷酸二激酶pyruvate phosphate diki nase PPPk丙酮酸脱氢酶复合体pyruvic acid dehydroge nase complex丙酮酸脱羧酶pyruvic acid decarboxylase丙酮酸激酶pyruvate kinase丙氨酸甜菜碱alaninebetaine丙糖磷酸异构酶triose phosphate isomerase戊糖磷酸途径pentose phosphate pathway ,PPP 平衡压力balanee pressure平衡石statolith平衡溶液balanee solution平衡细胞statocyles灭光信号light off signal卡尔文循环Calvin cycle叶黄素xanthophyll叶面营养foliar nutrition叶面积系数leaf area index ,LAI叶肉细胞mesophyll cell叶绿素chlorophyll,Chl叶绿体chloroplast叶绿体被膜chloroplast envelope5 -甲硫基核苷5 -methylthioribose甲硫氨酸methionine甲瓦龙酸甲羟戊酸mevalo nic acid电中性electro neutrality电化学势electrochemical potential电负性electro negative田间持水量field capacity四氢吡喃苄基腺嘌呤tetrahydropyra nyl ben zylade nine生理碱性盐physiologically alkaline salt生理酸性盐physiologically acid salt生理中性盐physiologically neutral salt生理钟physiological clock生理休眠physiological dormancy生殖生长reproductive growth生殖纟田胞无孢子生殖gen erat ine apospory生物大分子biomacromolecule生物固氮biological nitrogen fixation生物钟biological clock生物氧化biological oxidation生物分子biomolecule生物膜biomembrane生长growth生长素auxin生长素梯度学说aux in gradie nt theory 生长素赖氨酸合成酶IAA lysinesynthetase 生长素结合蛋白aux in bi ndi ng protein生长抑制剂growth inhibitor生长大周期grand period of growth生长呼吸growth respiration生长延缓剂growth retardant生长的周期性growth periodicity生长效率growth efficiency生长发育growth and development 生命周期life cycle生色团chromophore代谢bolism代谢库bolic sink代谢源bolic source夕卜排exocytosis外植体expla nt夕卜在蛋白extrinsic protein夕卜连丝ectodesmata外向K + 通道outward K + channel 夕卜膜outer membrane饥饿基因famine gene主动转运active transport主动吸水active absorption of water 主动吸收active absorption主宰酶master enzyme主导库dominant sink半醌semi quinone半支莲醛potulai半透膜semipermeable membrane半自主性纟田胞器semiaut onom ous organ elle半月苔酸lunlaric acid半纤维素hemicellulose它感化合物allelochemical必需元素essential element永久萎蔫permanent wilting永久萎蔫系数permanent wilting coefficient皮孑L蒸腾lenticular transpiration发酵fermentation发育development对氯汞苯磺酸parachloro-mercuribenzene sulfonate幼年期juvenile phase丝状亚基fibrous subunits丝氨酸serine丝氨酸羟甲基转移酶seri ne glyoxylate aminotran sferase 六画动作电位action potential , AP动力蛋白dynamin老化aging共振传递resonance transfer共价键covale nt bond共向传递体symport共质体symplast共质体运输symplastic transport共质体装载symplastic phloem loading共质体途径symplast pathway亚硝酸还原酶nitrite reductase ,NiR亚胺环已酮cycloheximide亚麻酸linolenic acid亚精胺spermidine亚油酸linoleic acid过氧化氢酶catalase ,CAT过氧化物酶peroxidase ,POD过氧化物体peroxisome过敏反应hypersensitive reaction再春化现象revernalization再生阶段regeneration phase再分酉己redistribution 再分化redifferentiation 扩散作用diffusion ,协助扩散facilitated diffusion 西罗血红素sirohaem 压力探针pressure probe 压力势pressure potential 压力流学说pressure flow hypothesis 压力室法pressure chamber 有氧呼吸aerobic respiration 有益元素beneficial elements 有限生长determinate growth 有丝分裂reductionmitosis 灰分ash灰分元素ash element死亡激素death hormone成花素florigen成花决定态floral determinated state 成花启动floral evocation 成花诱导floral induction 成膜体phragmoplast成熟maturation光形态建成photomorphogenesis光呼吸photorespiration光呼吸碳氧化循环photorespirati on carb on oxidati on cycle光敏色素phytochrome , PHY光保护作用photoprotection光化学烟雾photochemical smog光反应light reaction光合速率photosynthetic rate光合有效辐射photosynthetically active radiation ,PAR光合碳还原循环photos yn thetic carb on reducti on cycle光合磷酸化photophosphorylation光合链photosynthetic chain光合午睡现象midday depression of photosynthesis光合作用photosynthesis光合作用的光抑制photoi nhibitio n of photo syn thesis 光合膜photosynthetic membrane光合产物photosynthetic yield光合单位photosynthetic unit光合滞后期lag phase of photosynthesis光合纟田菌photosynthetic bacteria光受体photoreceptor光周期photoperiod光周期现象photoperiodism光周期诱导photoperiodic induction光饱和点light saturation point光系统I photosystem I, PS I光系统n photosystem n, PS n光亲和标记photoaffinity labling光滑性内质网smooth en doplasmic reticulum光补偿点light compensation point光调节因子light regulated element光能利用率efficiency for solar energy utilization早熟发芽precocious germ in atio n早前期带preprophase band ,PPB ,吐水guttation同型二聚体homodimer同化物assimilate同化物运输assimilate transportation同化作用assimilation同化力assimilatory power同源异型基因homeotic gene同源异型突变homeotic mutation吸胀吸水imbibing absorption of water吸胀作用imbibition ,吸收光谱absorption spectrum吸附作用absorption回补机制replenishing mechanism 传递体transporter 休眠dormancy 休眠素dormin伤呼吸wound respiration 伤流bleeding 伤流液bleeding sap 自动催化作用autocatalysis 自花授粉self pollination 自由基free radical 自由水free water 自由空间free space 自由能free energy 自交不亲和性self in compatibility 自交不育self in fertility 自养性autotropism 自溶作用autolysis 向光性phototropism 向重性gravitropism向化性chemotropism向触性thigmotropism向性运动tropic movement后熟作用after ripening近似昼夜节奏circadian rhythm杀粉蝶菌素 A piericidin A合子zygote肌动蛋白acti n肌醇三磷酸inositol-1,4,5-triphophate ,IP3肌醇磷脂lipositol负化学信号negative chemical signal负向重性negative gravitropism负效应物negative effector多元酚氧化酶polyphenol oxidase多聚核糖体polyribosome多聚化截留机理polymerizati on trap mecha nism多聚半乳糖醛酸酶polygalacturo nase多克隆抗体polyclonal antibody多胺ployamines,PA色素pigment色素蛋白复合体pigme nt protein complex色氨酸tryptophan色氨酸单加氧酶thyphopha n mono oxyge nase 色胺tryptamine交替氧化酶alter native oxidase 交替途径alter native pathway 交叉适应现象cross adaptation 交叉忍耐cross tolerances 次生壁sec on dary wall 次级共运转secondary cotransport 次级电子供体secondaryelectron donor 次级电子受体secondary electron acceptor 次级库subord in ate sinks 异花授粉allogamy 异构酶isomerase 异柠檬酸裂解酶isocitratelyase 异柠檬酸脱氢酶isocitric acid dehydroge nase 异戊烯基腺苷isope ntenyl ade nosin e,iPA 异戊烯基腺嘌呤isope nteny lade nin e,ip 异戊烯转移酶isope ntenyl tran sferase 异戊烯焦磷酸isope ntenyl pyrophosphate 异化作用disassimilation 阳生植物sun plant 阴生植物shade plant红花菜豆酸phaseic acid红光red light 红降red drop 纤维素cellulose 纤维素酶cellulase 七画麦芽糖酶maltase形态发生morphogenesis远红光far red light运动反应motor response 运转器translocator韧皮部装载phloem loading 韧皮部卸出phloem unioading 韧皮蛋白P 蛋白phloem protein 坏死性死亡necrosis death 赤藓糖 4 磷酸erythrose-4-phosphate 赤霉素gibberellin ,GA 赤霉烷gibberellane 抑制剂depressant 抗坏血酸氧化酶ascorbate oxidase 抗盐性salt resista nee 抗热性heat resista nee 抗蒸腾剂antitranspirant 抗旱性drought resista nee 抗虫性pest resista nee 抗氰呼吸cyanide resista nt respirati on 抗氰氧化酶cyanide resista nt oxidase 抗冻性freez ing resista nee 抗冷性chilli ng resista nee 抗病性disease resista nee 抗性resista nce,hard in ess 抗涝性flood resista nee 抗张强度tensile strength 壳梭孢菌素fusieoeein ,FC 拟核体nucleoid 拟脂体lipid body 芽休眠bud dormancy花芽分化flower bud differentiation花的发端initiation of flower花熟状态ripeness to flower state花粉pollen花粉粒pollen grain6-苄基腺嘌呤6-benzyl adenine ,BA克隆clone极性polarity极性运输polar transport 极性分子polar molecule 束缚型赤霉素conjugated gibberellin 束缚型生长素bou nd auxin 束缚水bound water两极光周期植物amphophotoperiodism pla nt 还原阶段reduction phase 旱害drought injury旱生植物xerophytes吲哚丁酸indole-3-butyric acid ,IBA吲哚丙酮酸in dole pyruvic acid 吲哚丙酸in dole propio nic acid 吲哚乙醛in dole acetaldehyde 吲哚乙酰天冬氨酸in dole acetyl aspartic acid吲哚乙酰葡萄糖in dole acetyl glucose 吲哚乙酰肌醇in dole acetyl in ositol 吲哚乙酸indole-3-acetic acid ,IAA 吲哚乙酸氧化酶IAA oxidase 吲哚乙腈in dole aceton itrile 另U藻蓝蛋白allophycocyanin 延胡索酸酶fumarase体细胞无孢子生殖somatic apospory体细胞胚somatic embryo伸展蛋白extensin低温诱导蛋白low temperature in duced protein希尔氧化剂Hill oxidant希尔反应Hill reaction谷氨酰胺合成酶glutamine synthetase ,GS谷氨酸合酶glutamate synthase,GOGAT谷氨酸脱氢酶glutamate dehydrogenase ,GDH谷氨酸乙醛酸转氨酶glutamate glyoxylate aminotran sferase 谷胱甘肽glutathione谷胱甘肽过氧化物酶glutathione peroxidase ,GPX谷胱甘肽还原酶glutathione reductase ,GR令邻香豆酸ocoumaric acid令邻近纟田胞neighbouring cell免疫immune角质蒸腾cuticular transpiration系统获得性抗性systemic acquired resista nee 系统肽systemin冻害freezing injury库sink库强sink strength应变素allergens冷击蛋白cold shock protein冷响应蛋白cold responsive protein冷害chilli ng injury间质stroma泛醌ubiquinone,UQ泛醌氧化还原酶ubiq uinone oxidoreductase 完熟ripening初生壁primary wall初级共运转primary cotransport识另U recognition层积处理stratification张力tension阿拉伯半乳糖蛋白arab ino galacta n prote in 阿斯匹林aspirin驱动蛋白kinesin纺锤体spindle八画环式光合磷酸化cyclic photophosphorylati on环式电子传递cyclic electron transport环境污染environmental pollution环腺苷酸cyclic adenosine monophosphate,cAMP 环脂肪酸cyclic fatty acid环割试验girdling experiment表面张力surface tension ,表异构酶epimerase表观光合速率apparent photosynthetic rate表观库强apparent sink strength顶端优势apical dominance茉莉酸jasmonic acid ,JA茉莉酸甲酯methyl jasmonate ,MeJA茉莉酸类jasmonates苯丙氨酸phenylalanine苯丙氨酸解氨酶phe ny lala nine ammonia lyase 苯乙酸phenylactic acid苹果酸malate,Mal苹果酸酶malic enzyme苹果酸代谢学说malate bolism theory苹果酸合成酶malate synthetase苹果酸脱氢酶malic acid dehydrogenase直链淀粉amylose板块镶嵌模型plate mosaic model刺激感受stimulus perception刺激性单性结实stimulative parthe nocarpy矿质元素mineral element矿质营养mineral nutrition转运肽transit peptide转基因植物transgenic plant转醛醇酶transaldolase转酮酶transketolase转移细胞transfer cell,TC转化酶invertase ,转录因子tranion factors非环式光合磷酸化non cyclic photophosphorylati on 非环式电子传递non cyclic electr on tran sport非堆叠区nonappressed region果胶pectin果胶物质pectic substances果胶酶pectinase果胶酸pectic acid果糖1, 6 二磷酸fructose-1,6-bisphosphate,FBP果糖1, 6 二磷酸酯酶fructose-1,6-bispho sphate phosphatase 果糖-6-磷酸fructose-6-phosphate,F6P果糖激酶fructoki nase固氮酶nitrogenase固定化细胞immobilized cells呼吸速率respiratory rate呼吸电子传递链respiratory electr on tran sport cha in呼吸跃变respiratory climacteric呼吸链respiratory chain呼吸作用respiration呼吸系数respiratory coefficient呼吸底物respiratory substrate吸呼效率respiratory ratio呼吸商respiratory quotient ,RQ岩棉栽培rockwool culture罗汉松内酯podolactone物理信号physical signal物候期phe no logical period质蓝素plastocyanin ,PC质体plastid质体醌plastoquinone ,PQ ,质体小球plastoglobulus质壁分离plasmolysis ,质壁分离复原deplasmolysis质子动力proton motive force ,pmf质子泵proton pump质外体apoplast质外体运输apoplastic transport质外体装载apoplasmic phloem loading质外体途径即oplast pathway受体receptor ,,受精作用fertilization孚L酸脱氢酶lactic acid dehydrogenase乳酸发酵lactate fermentation胁迫stress胁迫激素stress hormone胁变strain周期性growth periodicity鱼藤酮rote none饱和蒸气压saturation vapor pressure夜间断night break底物水平磷酸化substrate level phosphorylation 放热呼吸thermogenic respiration放氧复合体oxygen evolving complex ,放射免疫检测法radioimmu no assay放线菌素actinomycin D净光合速率net photosynthetic rate净同化率net assimilation rate,NAR ,育性转化fertility change性另U表达sex expression单"S” 形生长曲线single sigmoid growth curve 单盐毒害toxiciy of single salt单克隆抗体monoclonal antibody单酚氧化酶monophenol oxidase单倍体无配子生殖haploid apogamr单倍体孤雄生殖haploid an droge nesis单倍体孤雌生殖haploid parthe nogen esis单向传递体uniport单性结实parthenocarpy法呢基焦磷酸farnesyl pyrophosphate ,油菜素brassin油菜素内酯brass in olide ,BR油菜素甾体类化合物brassi nosteroids油酸oleic acid油体oil body油体蛋白oleosins泥炭培养peat culture空种皮技术empty seed coat technique衬质matrix衬质势matrix potential衬质水势matrix water potential降解breakdown线性期linear phase 线粒体mitochondria ,细菌叶绿素bacteriochlorophyll 细胞克隆cell clone 细胞板cell plate 细胞松弛素 B cytochalasin B 细胞融合cell fusion 细胞浆cytosol细胞器cell organelle细胞骨架cytoskeleton细胞繁殖cell reproduction细胞质环流cyclosis纟田胞质基质cytoplasmic matrix 纟田胞质膜plasma membrane 细胞全能性totipotency 细胞分裂素cytokinin , CTK 细胞分裂素氧化酶cytoki nin oxidase细胞分化cell differentiation 纟田胞膜cell membrane 细胞周期cell cycle细胞色素cytochrome , Cyt纟田胞色素氧化酶cytochrome oxidase纟田胞衰老cellular aging细胞液cell sap细胞学说cell theory细胞壁cell wall孤立体系isolated system孢粉素pollenin孢子体型不亲和sporophyric self in compatibility,SSI九画春化素vernalin春化作用vernalization圭寸闭体系closed system指数期logarithmic phase草酰乙酸oxaloacetic acid , OAA荧光fluoresce nee荧光猝灭剂fluoresce nee que ncher ,胡萝卜素carotene枯斑necrotic spot相互竞争allelospoly相生相克它感作用allelopathy相关性correlation相对生长速率relative growth rate , RGR 相对自由空间relative free space,RFS 柠檬酸循环citric acid cycle 柠檬酸合成酶citrate synthase 砂培sand culture砂基培养法sand culture method砂砾栽培gravel culture临界日长critical daylength临界暗期critical dark period钙调蛋白钙调素calmoduli n,CaM 氢醌hydro quinone氢化酶hydrogenase选择吸收selective absorption种子劣变seed deterioration种子生活力seed viability种子休眠seed dormancy种子的寿命seed Iongevity种子活力seed vigor秋水仙素colchicine ,重力势gravitational potential重力水gravitational water复种指数multiple crop index复合脂类complex lipids顺乌头酸酶aconitase保卫细胞guard cell信号转导signal transduction ,信息传递message transportation 胚芽鞘coleoptile胚柄suspensor胚状体embryoid胚胎萌发viviparous germ in atio n ,vivipary胚胎晚期丰富蛋白late embryoge nesis abundant prote in 丄EA 胚胎发生embryogenesis胞间连丝plasmodesma胞间层intercellular layer亲和性compatibility亲和力afinity类胡萝卜素carotenoid类胡萝卜素途径carote noid pathway类囊体thylakoid前质体proplastid ,前馈活化feedforward activation逆境environmental stress逆境逃避stress avoidanee逆境乙烯stress ethylene逆境蛋白stress protein逆境忍耐stress toleranee总光合速率gross photosynthetie rate活化酶aetivase活性氧active oxygen ,染色体chromosome染色质ehromati n染色单体chromatid穿梭运动shuttle streaming诱导酶indueed enzyme ,诱导性单性结实in dueed parthe no earpy昼夜节律eireadian rhythm昼夜周期性daily periodieity结构酶constitutive enzyme结合态淀粉合成酶granule bound starch syn thase 结合蛋白binding protein 绝对生长速率absolute growth rate ,AGR十画顽拗性种子reealeitrant seed载体carrier载色体ehromatophore盐碱土saline and alkaline soil盐逆境蛋白salt stress protein盐溶清蛋白globulin盐害salt injury热电偶thermocouple热休克蛋白热激蛋白heat shock prote in s,HSPs热害heat injury热力学thermodynamics莽草酸shikimic acid真核生物eukaryote真核细胞eukaryotic cell真光合速率true photosynthetic rate核基质nuclear matrix核酮糖1, 5 二磷酸ribulose BF-1,5-bisphosphate, BFQ RuBP核酮糖1, 5 二磷酸羧化酶/ 加氧酶BFQ ribulose-1,5-bisphosphate carboxyla se/oxygenase ,Rubisco核酮糖-5-磷酸ribulose-5-phosphate,Ru5P核酮糖-5-磷酸表异构酶ribulose-5-phosphate epimerase核酮糖-5-磷酸激酶ribulose-5-phosphate kinase,Ru5PK核小体nucleosome核仁nucleolus核质nucleoplasm核膜nuclear membrane核糖=5-磷酸ribose-5-phosphate,R5P核糖-5-磷酸异构酶ribose-5-phosphate isomerase 核糖体ribosome 核液karyolymph核孑L nuclear pore根压root pressure根冠比root top ratio,R/T砾培gravel culture原核生物prokaryote原核细胞prokaryotic cell原果胶protopectin原生质protoplasm原生质体protoplast原初电子供体primary electr on donor原初电子受体primary electr on acceptor 1原初反应primary reaction原初主动运转primary active tran sport原发优势primigenic dominance配子体型不亲和gametophytic self in compatibility,GSI致电泵electrogenic pump圆球体spherosome铁硫黄素蛋白iron sulfur flavoprote in铁硫蛋白iron sulfur protein铁氧还蛋白ferredoxin,Fd铁氧还蛋白-NADP+ 还原酶ferredoxin-NADP+reductase , FNR氧化磷酸化oxidative phosphorylation氧自由基oxygen free radical氧饱和点oxygen saturation point1-氨基环丙烷-1-羧酸1-aminocyclopropane-1-carboxylic acid,ACC氨基酮戊酸aminolevulinic acid氨基氧乙酸amino oxyacetic acid,AOA氨基乙氧基乙烯基甘氨酸ami noethoxyvi nyl glyci ne,AVG缺绿症chlorosis敌草隆Diuron,DCMU爱默生增益效应Emers on enhan ceme nt effect脂氧合酶lipoxygenase ,脂肪酸fatty acid胶体colloid胼胝质callose衰老senescenee衰老相关基因sen esce nee associated gene衰老特定基因sen esce nee specific gene衰减期senescenee phase高尔基体Golgi body高效液相层析high performa nee liquid chromatography 高温胁迫hightemperature stress病原菌disease produeing germ病原相关蛋白pathoge nesis related prote in s,PRs 病原物causal organism病害disease离区abseission zone离层abseission layer离子载体抑制剂ion ophore depressa nt离子颉颃ion antagonism离子交换ion exchange离子通道ion ehannel凋亡apoptosis凋亡小体apoptotie body涝害flood injury酒精发酵aleohol fermentation流动镶嵌模型fluid mosaie model被动运输passive transport被动吸水passive absorption of water被动吸收passive absorption能荷energy eharge能量梯度energy gradient通道channel继代培养subculture十一画堆叠区appressed region授粉pollination培养基medium接触态建成thigmomorphogenesis接触交换contact exchange基态ground state基因组genomes基质matrix基质片层stroma lamella基质类囊体stroma thylakoid基粒granum基粒片层grana lamella基粒类囊体grana thylakoid基细胞basal cell黄素腺嘌呤二核苷酸flavin ade nine din ucleotide,FAD 黄素单核苷酸flavi n mo non ucleotide,FMN黄化现象etiolation黄质醛xanthoxin ,萘基邻氨甲酰苯甲酸nap hthyphthalamic acid萘氧乙酸naphthoxyacetic acid萘乙酸naphthalene acetic acid,NAA , 萌发germ in ati on萝卜酰胺raphanusamide萝卜宁raphanusanin萎蔫wilting菊芋素heliangint营养转移nu trie nt divers ion营养生长vegetative growth营养膜技术nutrient film technique,NFT梅勒反应Mehler's reaction副卫细胞subsidiary cell酚氧化酶phenol oxidase辅酶 A coenzyme A,CoA辅助色素accessory photosynthetic pigments悬浮培养suspension culture甜菜碱betaines第二信使second messenger敏感性sensitivity偶联因子coupling factor偶联部位coupled site偏向受精preferential fertilization假环式光合磷酸化pseudocyclic photopho sphorylati on 假环式电子传递pseudocyclic electron transport脯氨酸proline,Pro ,脯氨酸甜菜碱prol in ebeta ine脱落abscission脱落素abscisin脱落酸abscisic acid,ABA脱支酶debranching enzyme脱分化dedifferentiation脱羧作用decarboxylationI阈时presentation time羟基丙酮酸还原酶hydroxypyruvate reductase羟脯氨酸hydroxyproline , Hyp粘性plasticity粘附力adhesionJP 粗糙型内质网rough en doplasmic reticulum,RER JP 烯醇化酶enolase液晶态liquid crystalline state液泡膜tonoplast淀粉酶amylase淀粉磷酸化酶starch phosphorylase淀粉体amyloplast ,淀粉合成酶starch synthase淀粉粒starch grain渗透势osmotic potential渗透吸水osmotic absorption of water渗透作用osmosis渗透胁迫osmotic stress渗透调节osmotic adjustment寄主host寄主特异毒素host specific tox in密度density弹性胁变elastic strain蛋白磷酸酯酶protein phosphatase蛋白激酶protein kinase ,隐花植物cryptogamia隐花色素cryptochrome ,维持呼吸maintenance respiration维管束鞘细胞bundle sheath cell ,BSC 绿色荧光蛋白gree n fluoresce nt prote in 绿色硫细菌green sulfur bacteria十二画琥珀酸硫激酶succinic thiodinase琥珀酸脱氢酶succinic dehydrogenase琥珀酸:泛醌氧化还原酶succinate:ubiquinone oxidoreductase超氧化物歧化酶superoxide dismutase,SOD颉颃作用antagonism插入蛋白integral protein葡聚糖ployglucosan葡萄糖-6-磷酸glucose-6-phosphate,G6P葡萄糖 6 磷酸脱氢酶glucose-6-phosphate dehydrogenase 植醇phytol 植物生长物质plant growth substanee植物生长调节剂pla nt growth regulator植物激素plant hormone植物组织培养plant tissue culture植保素phytoalexin硝酸还原酶nitrate reductase ,NR硫氧还蛋白thioredoxin硫胺素焦磷酸thiamine pyrophosphate硫脂sulpholipid硫辛酸lipoic acid雄性素androecious line雄性生殖单位male gerem unit ,MGU暂时萎蔫temporary wilting紫黄质violaxanthin紫外线诱导蛋白UV in duced protein紫色硫细菌purple sulfur bacteria紫色非硫纟田菌purple non sulfur bacteria量子需要量quantum requirememt量子效率quantum efficiency量子产额quantum yield喷灌spray irrigation景天科酸代谢crassulacean acid bolism,CAM景天庚酮糖-1,7-二磷酸sedoheptulose-1,7-bisphosphate ,SBP景天庚酮糖-1,7-二磷酸酯酶sedoheptulose-1,7-bisphosphate phosphatase SBPase景天庚酮糖-7-磷酸sedoheptulose-7-phosphate ,S7P蛭石栽培vermiculaponics短日植物short-day plant短长日植物short-long day plant氰化物cyanide氯丁唑多效唑,PP333, paclobutrazol4-氯吲哚乙酸4-chloroindole-3-acetic acid氯氟代烃chlorofluorocarbous2-氯乙基膦酸2-chloroethyl phosphonic acid稀土元素rare earth element筛管sieve tube筛管装载sieve loadi ng筛管分子sieve eleme nt筛管分子伴胞复合体sieve element companion cell ,CC集光色素light harvest ing pigme nt集体效应group effect集流mass flow焦磷酸磷酸果糖激酶pyrophosphate phosphofructoki nase湿害waterlogging温周期现象thermoperiodicity温度补偿点temperature compensation point游离型生长素free auxin 270富含羟脯氨酸的糖蛋白hydroxyproline rich glycoprotein ,HRGP强迫休眠epistotic dorma ncy十三画蓝光效应blue light effect蒸气压梯度vapor pressure gradient蒸腾拉力tran spirati onal pull蒸腾速率tran spirati on rate蒸腾作用tran spirati on蒸腾系数tran spirati on coefficie nt蒸腾效率tran spirati on ratio蒸腾流-内聚力-张力学说transpiration-cohesion tension theory 蒸发vaporization a-酮戊二酸脱氢酶复合体a -ketoglutaric acid dehydroge nase complex 酪氨酸酶tyrosinase感震性seism on asty感受perception感受蛋白sensor protein感夜性nyctinasty感性运动nastic movement感温性therm on asty4-碘苯氧乙酸4-iodo phenoxy acetic acid雾培spray culture暗呼吸dark respiration暗反应dark reaction跨膜蛋白transmembrane protein蜂蜡醇myricylalcohol嵴cristae锯木培sawdust culture矮壮素氯化氯胆碱chlorocholine chloride ,CCC 微量元素mi no releme nt微团micell微管microtubule微管蛋白tubulin微体microbody微注射法microinject ion tech nique微梁系统microtrabecular system微纤丝microfibril微丝microfilament愈伤组织callus腺苷三磷酸酶adenosine triphosphatase , ATPase腺苷酸激酶adenylate kinase解偶联剂uncoupler新黄质neoxanthin2 羧基3 酮基阿拉伯糖醇1,5 二磷酸2-carboxy-3-ketoarabinitol-1,5-bispho sphate2 羧基D 阿拉伯糖醇1 磷酸2-carboxy-D-arabinitol1phosphate羧化效率carboxylation efficiency羧化阶段carboxylation phase塑性胁变plastic strain源source源-库单位source-sink unit源强source strength溶酶体lysosome溶质势solute potential溶胶sol溶液培养法solution culture method叠氮化物azide十四画聚光色素复合体light harvest ing pigme nt complex 蔗糖磷酸磷酸酯酶sucrose phosphate phosphatase蔗糖磷酸合成酶sucrose phosphate syn thase,SPS 蔗糖一质子共运输蛋白sucrose-H + symporter,蔗糖合成酶sucrose synthase,SS 碱土alkaline soil碳酸酐酶carbonic anhydrase,CA碳同化carb on dioxide assimilati on酶联免疫吸附检测法en zyme lin ked immunoso rbe nt assay酶复合体enzyme complex 198酶放大的免疫鉴定法en zyme amplified immuno assay酸生长理论acid growth theory酸化作用acidification需水量water requirement雌蕊pistil雌雄同株同花植物herm aphroditic pla nt雌雄同株异花植物mo noecious pla nt雌雄异株植物dioecious plant雌性生殖单位female germ unit雌性系gynoeciousline锻炼hardening膜动转运cytosis膜不饱和脂肪酸指数un saturated fatty acid in dex 膜片钳patch clamp,PC膜间空间intermembrane space腐胺putrescine精胺spermine漫灌wild flooding irrigation滴灌drip irrigation滴漏式hourglass寡霉素oligomycin寡糖素oligosaccharin缩合酶condensing enzyme十五画增效作用synergism醇溶谷蛋白prolamin潜在库强potential sink strength缬氨霉素valinomycin十六画操纵子operon燕麦试法avena test薄层层析thin layer chromatography整形素morphactin整合integration醛缩酶aldolase膨压turgor pressure膨压素turgorins膨压运动turgor movement凝聚condensation凝集素lectins凝胶gel糖酵解glycolysis糖脂glycolipid激素受体hormone receptor激动素kin etin ,KT激发态excited state激发子elicitor激子传递exciton transfer十七画磷酸运转器pi translocator ,PT3-磷酸甘油醛脱氢酶3-phosphoglyceraldehyde dehydroge nase 磷酸甘油酸phosphoglycerate ,PGA磷酸甘油酸变位酶phosphoglyceromutase磷酸甘油酸激酶phosphoglyceric kin ase,PGK磷酸葡萄糖酸phosphogluconate6-磷酸葡萄糖酸内酯6-phosphogluco nolacto ne6-磷酸葡萄糖酸脱氢酶6-phosphogluco nate dehydroge nase磷酸葡萄糖变位酶phosphoglucomutase磷酸蔗糖合成酶sucrose phosphate syn thetase,SPS磷酸丙糖运转器triose phosphate tran slocator磷酸丙糖异构酶phosphotrioseisomerase磷酸酯酶phosphatase磷酸水解酶phosphorhydrolase磷酸烯醇式丙酮酸phosphenoIpruvate ,PEP磷酸烯醇式丙酮酸羧化酶phosphoe no Ipyruvate carboxylase,PEPC 磷酸烯醇式丙酮酸羧激酶PEP carboxyki nase磷酸乙醇酸磷酸脂酶phosphoglycolate phosphatase磷酸己糖异构酶phosphohexoseisomerase磷光phosphoresce nee磷脂phospholipid磷脂酰甘油phosphatidylglycerol磷脂酰胆碱phosphatidylcholine磷脂酰肌醇phosphatidylinositol,PI磷脂酰乙醇胺phosphatidylethanolamine磷脂酰丝氨酸phosphatidylserine磷脂酶phos pholipase十九画藻蓝蛋白phycocyanin 藻胆素phycobilin 藻红蛋白phycoerythrin二十二画囊腔lumen。
Controlled Growth of Monodisperse Silica Spheres

Instruments
Electron microscopes Zeiss E M 9 and Philips E M 100 B and partiee size analyzer Zeiss TGZ 3 were used.
Procedures
Throughout the investigation, ammonia was used as the catalyst causing the formation of spherical particles. In many cases, it was applied by adding saturated alcoholic solutions of ammonia to the reaction vessel. In other cases, particularly when high ammonia concentrations were desired, saturated ammonium hydroxide solution was used and the water content was taken into account.
CONTROLLED GROWTH OF MONODISPERSE SILICA SPHERES particles. In an attempt to duplicate these findings, many of our experiments resulted in gel formation and only in a few cases did the electron mierographs show particles of ellipsoidal shape and a size range near 0.08 t~. Then, a systematic study of the reaction parameters was made and after some drastic changes of the experimental conditions, quasi-monodisperse suspensions of silica spheres of sizes up to 2 ~ were finally obtained within less than an hour and the reaction no longer required extremely pure reactants. EXPERIMENTAL
醋酸乙烯酯的_活性_可控自由基聚合研究进展_蒋波

第25卷第4期高分子材料科学与工程Vol .25,No .4 2009年4月POLYMER MA TERIALS SCIENCE AND ENGINEERINGApr .2009醋酸乙烯酯的“活性”/可控自由基聚合研究进展蒋 波,易玲敏,詹晓力,陈 碧,陈丰秋(浙江大学化学工程与生物工程学系,浙江杭州310027)摘要:醋酸乙烯酯在聚合中容易发生链转移和链终止反应,所以实现醋酸乙烯酯的“活性”/可控自由基聚合是一个巨大的挑战。
文中从不同的活性自由基聚合方法角度对醋酸乙烯酯的“活性”/可控自由基聚合研究进行了综述。
在众多活性自由基聚合方法中以黄原酸酯、二硫代胺基甲酸酯为链转移剂的RA FT 聚合和以乙酰丙酮钴络合物为调控剂的钴调控自由基聚合真正实现了它的“活性”/可控自由基聚合。
关键词:醋酸乙烯酯;“活性”/可控自由基聚合;RAF T ;黄原酸酯中图分类号:T Q 325.5 文献标识码:A 文章编号:1000-7555(2009)04-0163-04收稿日期:2008-02-06基金项目:国家自然科学基金资助项目(20606029)通讯联系人:詹晓力,主要从事化学反应工程、聚合物与聚合反应工程的研究, E -mail :xlzhan @zj u .edu .cn 醋酸乙烯酯(VAc )是一种常见的单体,其聚合物聚醋酸乙烯酯(PVAc )在生物医药领域有广泛的应用[1,2],实现VAc 的“活性”/可控聚合有很重要的意义。
由于VAc 只能用自由基方法聚合,而且VAc 的自由基异常活泼,所以实现VAc 的“活性”/可控自由基聚合是一个挑战。
尽管许多研究者用不同的活性自由基聚合方法进行了VAc 的“活性”/可控聚合,但是真正实现VAc “活性”/可控聚合的报道并不多[3~6]。
本文针对VAc 的“活性”/可控自由基聚合,从不同的活性自由基聚合方法角度综述了国内外的研究进展。
1 引发转移终止剂法(Iniferter )1982年,日本学者大津隆行等人[3]提出了Iniferter 聚合方法。
controlled-current techniques
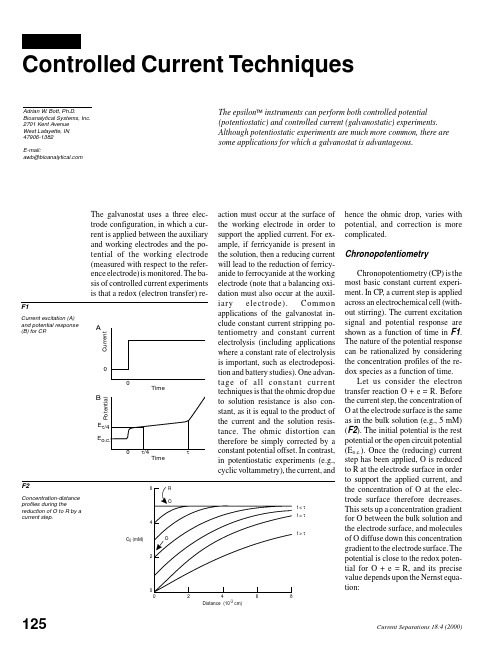
Potential
B
action must occur at the surface of the working electrode in order to support the applied current. For example, if ferricyanide is present in the solution, then a reducing current will lead to the reduction of ferricyanide to ferrocyanide at the working electrode (note that a balancing oxidation must also occur at the auxiliar y electrode). Common applications of the galvanostat include constant current stripping potentiometry and constant current electrolysis (including applications where a constant rate of electrolysis is important, such as electrodeposition and battery studies). One advantag e of all con stan t cu rrent techniques is that the ohmic drop due to solution resistance is also constant, as it is equal to the product of the current and the solution resistance. The ohmic distortion can therefore be simply corrected by a constant potential offset. In contrast, in potentiostatic experiments (e.g., cyclic voltammetry), the current, and
聚合与高分子化学反应专业基本词汇

齐聚反应 (曾用名) oligomerization
22 调聚反应 telomerization
23 自发聚合 spontaneous polymerization
52 等离子体聚合 plasma polymerization
53 易位聚合 metathesis polymerization
54 开环易位聚合 ring opening metathesis polymerization,ROMP
40 自由基异构化聚合 free radical isomerization polymerization
41 自由基开环聚合 radical ring opening polymerization
42 氧化还原聚合 redox polymerization
17 环状单体 cyclic monomer
18 共聚单体 comonomer
19 聚合[反应] polymerization
20 均聚反应 homopolymerization
64 假正离子聚合 pseudo cationic polymerization
65 假正离子活[性]聚合 pseudo cationic living polymerization
66 活性正离子聚合 living cationic polymerization
24 预聚合 prepolymerization
25 后聚合 post polymerization
26 再聚合 repolymerization
27 铸塑聚合, 浇铸聚合 cast polymerization
5.2 聚合与高分子化学反应
国产盐酸奥洛他定口服制剂质量评价

doi:10.3969/j.issn.1009⁃6469.2020.11.007◇药物分析◇国产盐酸奥洛他定口服制剂质量评价王立峰1,王新亮1,张春鸿2,刘尧2,马郑2作者单位:1辽宁中医药大学附属医院药学部,辽宁沈阳110032;2大连市药品检验所化学室,辽宁大连116000通信作者:马郑,男,副主任药师,研究方向为药品质量评价及质量标准的提升研究,E⁃mail:*******************摘要:目的评价国产盐酸奥洛他定口服制剂的质量。
方法采用高效液相色谱法(HPLC)测定奥洛他定制剂中的有关物质,控制4个特定杂质,并与原研制剂的杂质谱对比研究。
结果国产盐酸奥洛他定片的杂质种类、数量均不大于原研制剂。
但胶囊剂杂质种类、数量均大于原研制剂。
结论基于杂质谱等方面研究,国产盐酸奥洛他定片质量好。
关键词:盐酸奥洛他定/分析;药物污染;口服制剂;质量评价;对比研究Quality evaluation of domestic olopatadinehydrochloride oral preparationsWANG Lifeng1,WANG Xinliang1,ZHANG Chunhong2,LIU Yao2,MA Zheng2Author Affiliations:1Department of Pharmacy,The Affiliated Hospital of Liaoning University of TraditionalChinese Medicine,Shenyang,Liaoning110032,China;2Chemistry Room,Dalian Institute ofDrug Control,Dalian,Liaoning116000,ChinaAbstract:Objective To investigate the quality of domestic olopatadine hydrochloride oral preparations.Methods The HPLC method was established to determine the related substances in the olopatadine hydrochloride oral preparations and control four known parative study on impurity profile was performed between generic drugs and reference listed drug(RLD).Re⁃sults The types and quantities of impurities in domestic olopatadine hydrochloride tablets were not greater than those in the RLD. While the types and quantities of impurities in domestic olopatadinehydrochloride capsules were greater than those in the RLD. Conclusion Based on the investigation of impurity profile,the quailty of domestic olopatadine hydrochloride tablets is good.Key words:Olopatadine hydrochloride/analysis;Drug contamination;Oral preparations;Quality evaluation;Comparative study盐酸奥洛他定为新型组胺受体阻断剂。
Synthesis and characterization of Ce_0.8Sm_0.2O_1.9 nanopowders using an acrylamide polymerizati

J OURNAL OF RARE EARTHS,Vol.28,No.1,Feb.2010,p.92F j y S f T D f (B ),N S F f(BK 3)N B R f (B 363)SUN Y (y z_@y ;T +6556)DOI 6S ()65Synthesis and characterization of Ce 0.8Sm 0.2O 1.9nanopowders using an acrylamide polymer ization processZHENG Yingping (郑颖平)1,WANG Shaorong (王绍荣)2,WANG Zhenrong (王振荣)2,WU Liwei (邬理伟)1,SUN Yueming (孙岳明)1(1.School of Chemistry and Chemical Engineering,S outheast Univers ity,Nanjing 211189,C hina; 2.S hanghai Institute of Ceramics,Chinese Academy of Sciences,S hanghai 200050,China)Received 11May 2009;revised 10July 2009Abstract:Ce 0.8Sm 0.2O 1.9(SDC)nanopowders were synthesized by an acrylamide polymerization process.The XRD results showed that SDC powders prepared at 700°C possessed a cubic fluorite structure.Transmission electron microscopy (TEM)indicated that the particle sizes of powders were in the range of 10–15nm.A 98.3%of theoretical density was obtained when the SDC pellets were sintered at 1350°C for 5h,indicating that the powders had good sinterability.The conductivity of the sintered SDC ceramics was 0.019S/cm at 600°C and the activa-tion energy was only 0.697eV.Furthermore,a unit cell was fabricated from the powders and the maximum power density of 0.169W/cm 2was achieved at 700°C with humidified hydrogen as the fuel and air as the oxidant.Keywords:acrylamide polymerization;doped ceria;solid oxide fuel cell;sintering;electrical conductivity;rare earthsCerium oxide-based materials have attracted increasing interest as the electrolyte for solid oxide fuel cells (SOFCs),especially for intermediate temperature SOFCs (ITSOFCs,600–800°C),due to their high ionic conductivity [1–8].For example,Ce 0.8Sm 0.2O 1.9shows high ionic conductivity of around 0.1S/cm,which is three times higher than that of the conventional 8YSZ (8mol.%yttria stabilized zirconia,3×10–2S/cm)at 800°C [3,5,9].In general,nano-sized powders possess high sintering ability,and the particle size of powders greatly depends on the synthesis route.Many methods are available for the preparation of ultrafine homogeneous doped ceria powders,for instance,glycine-nitrate process [10,11],citrate-nitrate gel synthesis [12,13],carbonate coprecipitation method [14],oxalate coprecipitation route [11,15,16],homogeneous precipitation process [17],and hydrothermal process [l8].In this study,we investigated the synthesis and properties of Ce 0.8Sm 0.2O 1.9(SDC)nanocrystalline powders via an acrylamide sol-gel process.In this process,a stable precursor solution of strongly chelated cations was obtained by con-trolling the amount of ligand and the pH at first.Then the solution was easily gelled by in situ formation of poly-acrylamide gel.Fine and nano-sized powders were obtained by directly decomposing this hydrous gel through thermal treatment.Furthermore,the property of a unit cell fabricated from as-prepared powders was also studied.1Experimental1.1PreparationThe starting materials were commercial CeO 2powder and Sm 2O 3powder (purity:99.9%;Sinopharm Chemical Re-agent Co.,Ltd.,China).They were weighed according to a molar ratio of 8:2,dissolved in dilute nitrate acid separately,and then mixed with 10equivalents of EDTA.A clear solu-tion was made by slowly adding dilute ammonia under stir-ring,and pH of the solution was around 8.Then the mono-mers,acrylamide and N,N -methylenediacrylamide (6g and 1g per 100ml of solution,respectively)were added to the above solution,and then the mixture was heated at 80–90°C to produce the polyacrylamide gel.The gel was dried at 120°C overnight in an oven,and cal-cined at 700°C for 4h after being pulverized in an agate mortar to prepare crystalline SDC powders.The SDC pow-ders were pressed into pellets and sintered in air on an alu-mina board at 1350°C for 5h.The sintered pellets were ap-proximately 25mm in diameter and 0.35mm in thickness.1.2Char acter izat ionThe crystal structure of the powders was investigated with X-ray diffraction (Shimadzu XD-3A)using Cu K αradiation.The data were recorded at a scanning rate of 5(°)/min with a scanning step size of 0.02°.The morphology of the SDC powder was studied with a transmission electron microscope (TEM,JEM-2000EX).The sintering shrinkage was meas-ured with a dilatometer (NETZSCH DIL 402C)from room temperature to 1500°C.The microstructure analysis of theound at ion it em:Pro ect supporte d b the c ienti ic a nd ec hnological e ve lopment Plan o Jiangsu Province E2007014the atural cience oundat ion o Jia ngsu Province 200929and ational a si c esea rc h Program o China 2007C 900Corre sponding a uthor :ueming E-ma il:p 99ahoo.c el.:8-2-209019:10.101/1002-072109008-2ZHENG Y ingping et al.,Synthesis and characterization of Ce 0.8Sm 0.2O 1.9n anopowders using an acrylamide polymerization (93)sintered pellets was carried out using a scanning electron microscope (SEM,Hitachi X-650).The relative density of the sintered pellets was determined by standard Archimedes ’method.The ionic conductivity was measured using two-probe impedance spectroscopy.Platinum paste was applied to both sides of the sintered pellets and heated at 800°C for 2h.Measurements were performed in air using an electrochemi-cal workstation (IM6eX,Zahner)in the temperature range 600–900°C.The values of conductivity at different tem-peratures were calculated with Eq.(1):L RS=σ(1)where L is the thickness of a pellet,S the area of a pellet(S=1/4πD 2,D is the diameter),and R the resistance of a pel-let at different temperatures.The electrochemical characterization of a planar single cell was performed with humidified hydrogen as the fuel and air as the oxidant at 600–700°C using the electrochemical workstation.The anode slurry consisting of 50wt.%NiO-50wt.%SDC and cathode slurry consisting of 50wt.%La 0.8Sr 0.2MnO 3(LSM)-50wt.%SDC were deposited by a screen-printing technique onto the separate sides of SDC pellet,which was air-dried and then fired at 1000°C for 2h in air.Platinum mesh was placed on top of the anode and the cathode to act as current collectors.2Results and discussion2.1Powder characterist icsFig.1shows XRD pattern of calcined SDC powder at 700°C for 4h with an acrylamide polymerization route.The powder has a fluorite structure,and its pattern is indexed on a cubic cell,space group F23with lattice parameters of a=b=c=0.5430nm,a=β=γ=90°.TEM image of the SDC nanoparticles is shown in Fig.2.It can be seen that the nanoparticle is well-crystallized with average grain size of 12nm;and the particles are slightly agglomerated,which may be due to the partial sintering while the exothermic reactions occur.Fig.3shows the sintering curve of the compact powder sample.It can be seen that the linear shrinkage begins tode-F XRD f SD scend after 700°C.A maximum shrinkage of the sampleoccurs at near 1400°C.With further increasing of the tem-perature,the sintering curve begins to rise.Therefore,the sintering temperature of the SDC was chosen between 1350–1400°C.2.2Char acter izat ion of sint ered SDC pelletsA typical SEM image of the SDC pellet sintered at 1350°C for 5h (Fig.4(a))reveals a dense and homogeneous micro-structure,and the average grain size is 1–2μm.Fig.4(b)pre-sents the microstructure of a fractured section of the pellet.The pellet is basically dense although there are closed pores of submicron-size at the grain boundaries.The relative den-sity of SDC pellet was found to be 98.3%by the standard Archimedes ’method.The ionic conductivity was measured using two-probe impedance spectroscopy.The conductivity data were fitted with the Arrhenius Eq.(2):0expa E T kT=σσ(2)where σ,σ0,E a ,k and T are the conductivity,pre-exponential factor,activation energy,Boltzmann constant and absolute temperature,respectively.Fig.5presents the Arrhenius plots for the sintered SDC pellet prepared by different methods.The ionic conductivity of the pellet prepared by acrylamide polymerization method is 0.019S/cm at 600°C,and the ac-tivation energy is 0.697eV.As comparison,the data of pel-lets prepared by glycine-nitrate method and citrate-nitrate method are also shown in Fig.5.Fig.2TEM image of SDCpowderF 3S f y z ig.1pattern o C powder ig.intering curve o the s nthesi ed powder compact sample94JOURNAL OF RARE EARTHS,Vol.28,No.1,Feb.2010Fig.4SEM micrographs of SDC sintered at 1350°C for 5h(a)Surface;(b)FractureFig.5Arrhenius plots for SDC pellets sintered at 1350°C for 5hfrom powders prepared by different methodsFrom Fig.5,it can be found that pellets prepared by acrylamide polymerization method have a lower activation energy and higher conductivity.This result shows that the acrylamide polymerization synthesis is an effective method to prepare doped ceria powders with an excellent electrical performance.Acrylamide gel consists of long polymeric chains,crosslinked to create an organic 3D tangled network where a solution of the respective cations is soaked.Polym-erization of the gel proceeds with the way of a chain reaction,the first step of which is the combination of an initiator with the acrylamide,which is thereby activated.As the chain of polyacrylamide grows,the active site shifts to its free end.N,N ’-methylenediacrylamide,which contains two acryla-mide units joined through –CONH 2group via a methylene group,can link two growing chains.Hence,diacrylamide enables the formation of cross-linked chains,resulting in a x y ,,[]M x f y DT x f and avoids the occurrence of unwanted precipitation.So this method allows preparing uniformly doped ceria powders.2.3Cell test result sThe cell structure consists of a porous NiO-SDC anode,a dense SDC electrolyte and a porous LSM-SDC composite cathode.The electrochemical performance of as-prepared unit cell was characterized.I-V curves and power densities are shown in Fig.6(a),and its impedance spectra measured at an open circuit condition for the cell are shown in Fig.6(b).The measured results are also listed in Table 1.From Fig.6(a),it can be found that I-V curves are non-linear,which indicates the presence of a significant polariza-tion at the electrode/electrolyte interface.As the temperature rises,the current density and power density rise.A maxi-mum power density of 0.169W/cm 2is achieved at 700°C.This value is a little low,but it must be noticed that the thickness of the SDC electrolyte is 350μm.From Table 1,it is very clear that the values of electrolyte resistance,R el ,and electrode polarization resistance,R p,a+c ,have increased significantly along with the increase in the OCV values.Meanwhile,the values of R el ,R p ,a+c ,and OCV decrease with the increase of temperature.The electrolyte resistance (R el )have decreased from 1.247to 0.556Ωcm 2as temperature increases from 600to 700°C.Fig.6I-V curves of a single cell at different temperatures (a),andimpedance spectra measured at an open circuit condition for the single cell (b)Table 1Cell performance and cell resistances *Temperature/°C OCV/V MPD/(W/cm 2)R el /(cm 2)R p ,a+c /(cm 2)R cel l /(cm 2)6000.8560.054 1.247 3.210 4.4576500.8350.0940.891 1.561 2.4527000.8050.1690.5561.1881.744*OCV:open circuit voltage,MPD:maximum power dens ity,R el :electrolyte oh-f IS,R ,+z f IS,R f IS (R =R +R ,+)com ple topo log with loops branches and interconnec-tions 19.eanwhile the comple ation o cations in solu tion b E A perm its the mi ing o species at a molecular levelmic resistance rom E p a c :electrode polari ation resistance rom E cell :cell res istance rom E c ell el p a cZHENG Y ingping et al.,Synthesis and characterization of Ce0.8Sm0.2O1.9n anopowders using an acrylamide polymerization (95)In comparison with R el of cells with thin SDC electrolytes (10μm)[20],the relative high value of R el may be related to the thicker electrolyte pellet.As shown in Table1,the elec-trode polarization resistance(R p,a+c)is dominant in the total resistance of the cell(R cel l),which is decreased from3.210 to1.188Ωcm2as temperature increases from600to700°C. Therefore,a better performance of the unit cell can be achieved at700°C.In order to further improve the cell per-formance,it is necessary to decrease the thickness of the electrolyte pellets and enhance catalytic activity of the elec-trode materials to lower the R c ell of unit cells.3ConclusionsCe0.8Sm0.2O1.9powder of12nm in average grain size was successfully synthesized by an acrylamide polymerization process.The SDC powder exhibited high sinterability,high conductivity,and low conduction activation energy.With an electrolyte pellet of350μm thick,a fuel cell with humidified hydrogen as the fuel and air as the oxidant was assembled and a maximum power density of0.169W/cm2was obtained at700°C.It is believed that SDC powders synthesized by this method would be a promising electrolyte material for intermediate temperature SOFCs.References:[1]Eguchi K.Ceramic materials containing rare earth oxides forsolid oxide fuel cell.Journal of A lloy s and Compounds,1997, 250:486.[2]Inoue T,Setoguchi T,Eguchi K,Arai H.Study of a solid oxidefuel cell with a ceria-based solid electrolyte.Solid State Ionics, 1989,35:285.[3]Yahiro H,Baba Y,Eguchi K,Arai H.High temperature fuelcell with ceria-yttria solid electrolyte.Journal of the Electro-chemical Society,1988,135:2077.[4]Tompsett G A,Sammes N M,Yamamoto O.Ceria-yttria-sta-bilized zirconia composite ceramic systems for applications as low-temperature electrolytes.Journal of the A merican Ce-ramic Society,1997,80:3181.[5]Mogensen M,Sammes N M,Tompsett G A.Physical,chemi-cal and electrochemical properties of pure and doped ceria.Solid State Ionics,2000,129:63.[6]Eguchi K,Setoguchi T,Inoue T,Arai H.Electrical propertiesof ceria-based oxides and their application to solid oxide fuelcells.Solid State Ionics,1992,52:165.[7]Hibino T,Hashimoto A,Inoue T,Tokuno J I,Yoshida S I,Sano M.A low-operating-temperature solid oxide fuel cell in hydrocarbon-air mixtures.Science,2000,288:2031.[8]Sahibzada M,Steele B C H,Zheng K,Rudkin R A,Metcalfe IS.Development of solid oxide fuel cells based on a Ce(Gd)O2x electrolyte film for intermediate temperature op-eration.Catalysis Today,1997,38:459.[9]Yahiro H,Baha Y,Eguchi K,Arai H.Oxygen ion conductivityof the ceria-samarium oxide system with Fluorite.Journal of Applied Electrochemistry,1988,18:527.[10]Peng R R,Xia C R,Fu Q X,Meng G Y,Peng D K.Sinteringand electrical properties of(CeO2)0.8(Sm2O3)0.1powders pre-pared by glycine-nitrate process.Materials Letters,2002,56: 1043.[11]Peng R R,Xia C R,Peng D K,Meng G Y.Effect of powderpreparation on(CeO2)0.8(Sm2O3)0.1thin film properties by screen-printing.Materials Letters,2004,58:604.[12]Peng C,Zhang Y,Cheng Z W,Cheng X,Meng J.Nitrate-citrate combustion synthesis and properties of Ce1–x Sm x O2–x/2 solid solutions.Journal of Materials Science:Materials in Electronics,2002,13:757.[13]Song X W,Peng J,Zhao Y W,Zhao W G,An S L.Synthesisand electrical conductivities of Sm2O3-CeO2systems.Journal ofRare Earths,2005,23:167.[14]Moria T,Drennanb J,Wang A Y,Aucheerlonie G,Li J,YagoA.Influence of nano-structural feature on electrolytic proper-ties in Y2O3doped CeO2system.Science and Technology of Advanced Materials,2003,4:213.[15]Gao R F,Mao Z Q.Sintering of Ce0.8Sm0.2O1.9.Journal ofRare Earths,2007,25:364.[16]Van Herle J,Horita T,KawadaT,Sakai N,Yokokana H,DokiyaM.Low temperature fabrication of(Y,Gd,Sm)-doped ceria electrolyte.Solid State Ionics,1996,86-88:1255.[17]Djuricic B,Pickering S.Nanostructured cerium oxide:prepa-ration and properties of weakly-agglomerated powders.Jour-nal ofthe European Ceramic Society,1999,19:1925.[18]Dikmen S,Shuk P,Greenblatt M,Gomez H.Hydrothermalsynthesis and properties of Ce1-x Gd x O2-δsolid solutions.Solid State Sciences,2002,4:585.[19]Sin A,Odier P.Gelation by acrylamide,a quasi-universal me-dium for the synthesis of fine oxide powders for electroce-ramic applications.Advanced Materials,2000,12:649 [20]Zhang X,Robertson M,Deces-Petit C,Qu W,Kesler O,MaricR,Ghosh D.Internal shorting and fuel loss of a low tempera-ture solid oxide fuel cell with SDC electrolyte.Journal of Power Sources,2007,164:668.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
• • •
The propagating radicals are reversibly trapped by persistent radicals that accumulate with time. Homolytic cleavage of a dormant species occurs either spontaneously or is catalyzed by a transition metal complex. The equilibria in both SFRP and ATRP are very strongly shifted towards the dormant species, preserving a concentration of growing radicals on the order of ppm.
2.7 Reversible Addition–Fragmentation Transfer (RAFT)
CRP processes based on DT do not obey the PRE. A steady state concentration of radicals is established via initiation and termination processes as in conventional RP. These processes rely on a thermodynamically neutral transfer reaction. In contrast to systems obeying the PRE, the formal equilibrium constant should be unity. A minute amount of growing radicals undergo degenerative exchange with species via a bimolecular transfer process. The exchange can proceed by atom (e.g., I) or group transfer (R–Te, R2–Sb, etc.) or by addition–fragmentation chemistry with unsaturated methacrylate oligomers or dithioesters.
Steric effects in RAFT are much more important than in ATRP. For example, the reactivity of secondary 2-bromopropionitrile in ATRP is higher than that of the tertiary 2-bromoisobutyrate. However, the opposite trend in reactivity is observed in RAFT. Similarly, t-butyl halides are inactive in ATRP but are more active than benzyl derivatives in RAFT. Additionally, acrylate derivatives are not very active in RAFT, in contrast to ATRP.
2. Structure– reactivity relationships
The leaving ability of R group
S R S C Z
R:
CH3 CH3 CH3 CH3 CH3 CH3 H CH3 CN ~ Ph > COOEt >> CN ~ Ph > CH3 > Ph > COOCH3 CH3 CH3 CH3 H H CH3 H H
1.Basic mechanism of RAFT
An end-group originating from the chain-transfer agent is reversibly exchanged between different propagating chains. The transfer reaction in RAFT is not a one-step transfer of the labile end group, but involves radical addition to the thiocarbonyl group of the dithioester to form an intermediate radical that fragments to yield a new dithioester and new radical.
The structure of the Z group is equally important. Stabilizing Z groups such as –Ph are efficient in styrene and methacrylate polymerization, but they retard polymerization of acrylates and inhibit polymerization of vinyl esters. On the other hand, very weakly stabilizing groups, such as – NR2 in dithiocarbamates or –OR in xanthates, are good for vinyl esters but inefficient for styrene.
1.Basic mechanism of RAFT
1.Basic mechanism of RAFT
The polymerization is carried out with a conventional initiator such as a peroxide or AIBN in the presence of the chain-transfer agent. The key that makes RAFT a living polymerization is the choice of the RAFT transfer agent. Living polymerization occurs with dithioesters because the transferred end group in the polymeric dithioester is as labile as the dithioester group. This results in an equilibrium between dormant polymer chains and propagating radicals (K=1), which controls the living polymerization.
2.7 Reversible Addition–Fragmentation Transfer (RAFT)
1998年,澳大利亚的Moad、Rizzardo、Thang等首次报道 Ref: Chiefari J et al., Macromolecules, 1998, 31, 5559-5562 同年,法国的Charmot等以macromolecular design via interchange of xanthate (MADIX)概念报道了类似机理的研究 Ref: Charmot D, et al., PCT patent WO 9858974, 1998 Chem Abstract 1999, 130, 82018
3. Retardation and termination in RAFT
Termination processes can in principle lead to the formation of branched (star-like) structures. Due to delocalization of spin on the aromatic ring, the growing radicals can attack the intermediate radical at several positions. Such three-arm stars have been isolated in model systems. Intermediate radicals can also in principle couple to form four-arm stars.
Processes based on degenerative transfer (DT) operate under very different principles than either SFRP or ATRP. SFRP and ATRP techniques obey the PRE:
I ki
I + CuBr2
I
2I I Br + CuBr +M I M Br + CuBr
M
I M + CuBr2
Propagation:
Pi Br + CuBr Pi +M + CuBr2
equilibrium with RX and Cu+.
kp
2.7 Reversible Addition–Fragmentation Transfer (RAFT)
5. Reverse ATRP
Reverse ATRP involves generating
Initiation:
the same ATRP system by using a combination of a conventional radical initiator such as AIBN and a transition metal in its higher oxidation state (e.g., Cu2+) . The initiator generates radicals which react with Cu2+ to establish the
