英文版大学物理 第八章
《大学物理》第八章 毕萨定律S
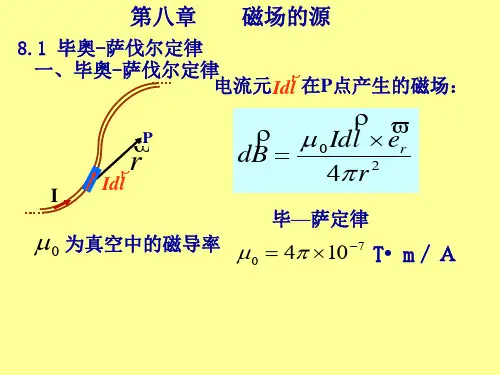
”
. 例Id载ly任流意2长一r直解点导:P根线的取据,磁任毕其其感意—电在应电萨流P强流点定强度元产理度BI生d为l的I?,磁试场d计为B 算方:导向线为旁Idl r
ol
ro
P
dB
各电流元产生的
o Idlsin 4dB 方r向2 垂直纸面向里。
I
1
B dB
B
ab
dr
其中B adbr、0cd与B板 d面r 等 距B离 d。0r
bc
cd
da
B
c
Bab Bcd 2Bab
而 o Ii o j ab
B
1 2
o
j
. . . . 与P点到平板的距离无关。
dl
dl
aB
b
B
1 2
o
j
与P点到平板的距离无关。
)
0m 2x3
r
B
xP
2)
在圆心处(x=0):
B
0 I
2R
(磁偶极子的场)
如考虑一段“圆弧形”载流线在圆心的磁场贡
献:
B 0I 2R 2
圆弧对圆心 所张的角
例 一直螺线管轴线上的磁场 B ?
已知:导线通有电流I,单位长度
B
2
oI R2
(
x2
R2
)
3 2
I
若令L 积分B回 d路r LL的L 绕B向dl 相反:0 若积分回L 路不包围电流I : B
I
dr
0
L
B
r
I
L
L
几点注意事项:
大学物理第八章磁场的源

磁场源的定义与分类
磁场源
能够产生磁场的物体或电流。
分类
天然磁场源(地球磁场、磁铁等)和人工磁场源(电流线圈、电磁铁等)。
磁场源的重要性
磁场源在物理学中具有重要地位,是研究电磁相互作用和电磁场 理论的基础。
磁场源的应用广泛,如磁力选矿、磁悬浮列车、核磁共振成像等 。
02
磁场源的基本性质
磁场强度与磁感应强度
磁场强度
描述磁场源的强弱程度,用符号H表示,单位为A/m 。
磁感应强度
描述磁场对通电导体的作用力,用符号B表示,单位为 T(特斯拉)。
磁场强度与磁感应强度之间的关系
H = B/μ0,其中μ0为真空磁导率,约等于4π×10^7H/m。
磁化强度与磁化电流
1 2
磁化强度
描述物质被磁化的程度,用符号M表示,单位为 A/m。
大学物理第八章磁场源
目
CONTENCT
录
• 磁场源概述 • 磁场源的基本性质 • 电流的磁场 • 磁场的源:永磁体 • 磁场的源:电磁铁 • 磁场源的测量与控制
01
磁场源概述
磁场与磁力
磁场
是由磁体或电流产生的空间场,对放入其中的磁体或电流产生力 的作用。
磁力
是磁场对放入其中的磁体或电流的作用力,表现为吸引或排斥。
在交通领域,永磁体被用于制造高速和高效 的交通工具,如高速列车和电动汽车等。
在医疗领域,永磁体被用于治疗疾病和 诊断,如磁共振成像和肿瘤治疗等。
05
磁场的源:电磁铁
电磁铁的工作原理
02
01
03
电磁铁由线圈和铁芯组成,当电流通过线圈时,线圈 产生磁场,磁场与铁芯相互作用产生磁力。
磁力的大小与电流强度、线圈匝数、铁芯材料等因素 有关。
大学物理8-5 静电场的能量
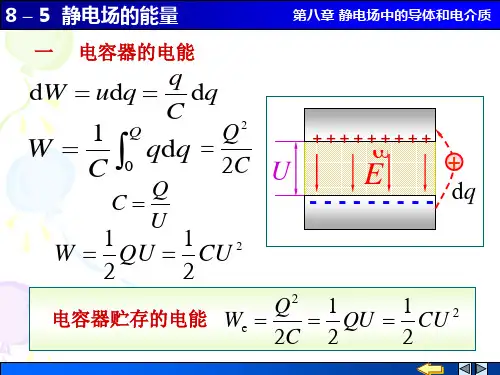
E ( R1 r R2 ) 2π 0 r
r R1
max Eb 2π 0 R1
l
max 2 0 R1 Eb
-+ - + R1 - + R2 -+
8 – 5
静电场的能量
第八章 静电场中的导体和电介 质
(2)电场的能量
E ( R1 r R2 ) 2π 0 r
( R1 r R2 )
1 1 R12 Eb2 2 wm 0 Em 0 2 2 2 r
R2
沿轴线单位长度的最大电场能量
Wm wm dV
2 1 2 b
R1
1 R E 0 2 1 2rdr 2 r
2 1
2 b
R2 4 1 0R E ln 5.76 10 J m R1
8 – 5
静电场的能量
第八章 静电场中的导体和电介 质
作业:
Q2 6 8 0 R
2
R
0
Q 2 dr 4 r dr R r 2 8 0
2 2
Q Q 3Q 40 0 R 8 0 R 20 0 R
8 – 5
静电场的能量
例8-6 如图所示,球形电容器的内、外半径分别为 R1和 所带电荷为 Q.若在两球壳间充以相对介电常数为 的电介质,求此电容器贮存的电场能量.
8 – 5 一
静电场的能量 电容器的电能
第八章 静电场中的导体和电介 质
q d W udq d q C
1 W C
Q
0
1 1 W QU CU 2 2 2
Q2 1 1 电容器贮存的电能 We QU CU 2 2C 2 2
大学物理第八章静电场(答案)
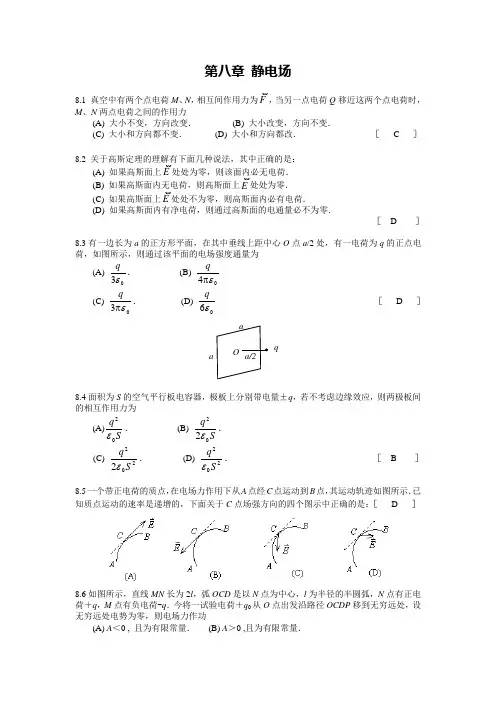
第八章 静电场8.1 真空中有两个点电荷M 、N ,相互间作用力为F,当另一点电荷Q 移近这两个点电荷时,M 、N两点电荷之间的作用力 (A) 大小不变,方向改变. (B) 大小改变,方向不变.(C) 大小和方向都不变. (D) 大小和方向都改. [ C ]8.2 关于高斯定理的理解有下面几种说法,其中正确的是:(A) 如果高斯面上E处处为零,则该面内必无电荷.(B) 如果高斯面内无电荷,则高斯面上E处处为零.(C) 如果高斯面上E处处不为零,则高斯面内必有电荷.(D) 如果高斯面内有净电荷,则通过高斯面的电通量必不为零.[ D ]8.3有一边长为a 的正方形平面,在其中垂线上距中心O 点a /2处,有一电荷为q 的正点电荷,如图所示,则通过该平面的电场强度通量为(A)03εq . (B) 04επq (C) 03επq . (D) 06εq[ D ]q8.4面积为S 的空气平行板电容器,极板上分别带电量±q ,若不考虑边缘效应,则两极板间的相互作用力为(A)Sq 02ε. (B) S q 022ε.(C) 2022S q ε. (D) 202Sq ε. [ B ]8.5一个带正电荷的质点,在电场力作用下从A 点经C 点运动到B 点,其运动轨迹如图所示.已知质点运动的速率是递增的,下面关于C 点场强方向的四个图示中正确的是:[ D ]8.6如图所示,直线MN 长为2l ,弧OCD 是以N 点为中心,l 为半径的半圆弧,N 点有正电荷+q ,M 点有负电荷-q .今将一试验电荷+q 0从O 点出发沿路径OCDP 移到无穷远处,设无穷远处电势为零,则电场力作功(A) A <0 , 且为有限常量. (B) A >0 ,且为有限常量.(C) A =∞. (D) A =0. [ D ]-8.7静电场中某点电势的数值等于 (A)试验电荷q 0置于该点时具有的电势能. (B)单位试验电荷置于该点时具有的电势能. (C)单位正电荷置于该点时具有的电势能.(D)把单位正电荷从该点移到电势零点外力所作的功. [ C ]8.8已知某电场的电场线分布情况如图所示.现观察到一负电荷从M 点移到N 点.有人根据这个图作出下列几点结论,其中哪点是正确的?(A) 电场强度E M <E N . (B) 电势U M <U N .(C) 电势能W M <W N . (D) 电场力的功A >0.[ C ]A8.9 电荷为+q 和-2q 的两个点电荷分别置于x =1 m 和x =-1 m 处.一试验电荷置于x 轴上何处,它受到的合力等于零?解:设试验电荷置于x 处所受合力为零,即该点场强为零.()()0142142020=+π-+-πx qx q εε 2分 得 x 2-6x +1=0, ()223±=x m因23-=x 点处于q 、-2q 两点电荷之间,该处场强不可能为零.故舍去.得()223+=x m3分8.10 如图所示,真空中一长为L 的均匀带电细直杆,总电荷为q ,试求在直杆延长线上距杆的一端距离为d 的P 点的电场强度.L解:设杆的左端为坐标原点O ,x 轴沿直杆方向.带电直杆的电荷线密度为λ=q / L ,在x 处取一电荷元d q = λd x = q d x / L ,它在P 点的场强:()204d d x d L q E -+π=ε()204d x d L L x q -+π=ε 2分d EO总场强为 ⎰+π=Lx d L x L q E 020)(d 4-ε()d L d q+π=04ε 3分 方向沿x 轴,即杆的延长线方向.8.11 一个细玻璃棒被弯成半径为R 的半圆形,沿其上半部分均匀分布有电荷+Q ,沿其下半部分均匀分布有电荷-Q ,如图所示.试求圆心O 处的电场强度.解:把所有电荷都当作正电荷处理. 在θ处取微小电荷 d q = λd l = 2Q d θ / π。
大学物理 第八章 热力学基础

CV
2019/5/21
P.12/42
§8.2 热力学第一定律
热力学基础
§8.2.1 热力学第一定律 本质:包括热现象在内的能量守恒和转换定律。
E2 E1 W Q (E2 E1) W E W
Q
dQ dE dW
Q
E E2 E1
W
+ 系统吸热 内能增加 系统对外界做功
系统放热 内能减少 外界对系统做功
2019/5/21
P.13/42
热力学基础
热力学第一定律适用于任何系统(气液固)的任何过 程(非准静态过程也适用),
Q E PdV
热力学第一定律的另一叙述:第一类永动机 是不可 能制成的。
第一类永动机:Q = 0, E = 0 ,A > 0的机器;
过一系列变化后又回一开始的状态,用W1表示外界对 气体做的功,W2表示气体对外界做的功,Q1表示气体 吸收的热量,Q2表示气体放出的热量,则在整个过程中 一定有( A )
A.Q1—Q2=W2—W1 ; B.Q1=Q2
C.W1=W2 ;
D.Q1>Q2
2019/5/21
P.16/42
【例8-4】如图,一个四周绝热的气缸热,力中学基间础 有 一固定的用导热材料制成的导热板C把气缸分 成 A.B 两部分,D是一绝热活塞, A中盛有 1mol He, B中盛有1mol N2, 今外界缓慢地
等压膨胀过程 V2>V1 , A>0 又T2>T1, 即E2-E1>0 ∴Q>0 。气体吸收的热量,一部分用于内能的增加,
一部分用于对外作功;
等压压缩过程 A<0 , T2<T1, 即E2-E1<0 ∴Q<0 。
(10)第八章 相变(讲义)

8.2.3 范德瓦耳斯等温线 •范德瓦耳斯方程 在理想气体模型中,忽略了分子的体积和分子间的引力。范德瓦耳斯把气体分子看作有相
互吸引作用的刚球,将理想气体的压强加以修正,从而导出了范德瓦耳斯(J.D.van der Waals, 1837 ——1923,荷兰物理学家,1910 年获诺贝尔奖)方程。
液相存在的区域,右方表示气相存在的区域,而汽化曲线上的点就是两相平衡共存的区域。所
以,汽化曲线也可以说是液态和气态的分界线。而表示气液两相存在的区域的 p − T 图就称为气
液二相图。
p DK
BC O
A T
D K
CB A
Three-dimensional sketch of the equation for the typical pure PVT system.
第八章 相变(Phase Transitions)
So far we’ve dealt mostly with gases in this course, because they’re nicБайду номын сангаас and easy. Most of the concepts are transferable to liquids and solids also, but one thing we haven’t touched on at all is how substances behave, from a thermodynamic point of view, as they change from one phase to another.
缩,压缩过程中压强和体积的关系曲线称为等温线,见图。在液化过程 BC 中,压强保持 p0 不 变,气液两相的总体积则由于气体数量的减小而减小。
大学物理第八章课后习题答案
大学物理第八章课后习题答案-CAL-FENGHAI-(2020YEAR-YICAI)_JINGBIAN第八章电磁感应电磁场8 -1一根无限长平行直导线载有电流I,一矩形线圈位于导线平面内沿垂直于载流导线方向以恒定速率运动(如图所示),则()(A)线圈中无感应电流(B)线圈中感应电流为顺时针方向(C)线圈中感应电流为逆时针方向(D)线圈中感应电流方向无法确定分析与解由右手定则可以判断,在矩形线圈附近磁场垂直纸面朝里,磁场是非均匀场,距离长直载流导线越远,磁场越弱.因而当矩形线圈朝下运动时,在线圈中产生感应电流,感应电流方向由法拉第电磁感应定律可以判定.因而正确答案为(B).8 -2将形状完全相同的铜环和木环静止放置在交变磁场中,并假设通过两环面的磁通量随时间的变化率相等,不计自感时则()(A)铜环中有感应电流,木环中无感应电流(B)铜环中有感应电流,木环中有感应电流(C)铜环中感应电动势大,木环中感应电动势小(D)铜环中感应电动势小,木环中感应电动势大23分析与解 根据法拉第电磁感应定律,铜环、木环中的感应电场大小相等,但在木环中不会形成电流.因而正确答案为(A ).8 -3 有两个线圈,线圈1 对线圈2 的互感系数为M 21 ,而线圈2 对线圈1的互感系数为M 12 .若它们分别流过i 1 和i 2 的变化电流且ti t i d d d d 21<,并设由i 2变化在线圈1 中产生的互感电动势为ε12 ,由i 1 变化在线圈2 中产生的互感电动势为ε21 ,下述论断正确的是( ).(A )2112M M = ,1221εε=(B )2112M M ≠ ,1221εε≠(C )2112M M =, 1221εε<(D )2112M M = ,1221εε<分析与解 教材中已经证明M21 =M12 ,电磁感应定律t i M εd d 12121=;ti M εd d 21212=.因而正确答案为(D ). 8 -4 对位移电流,下述四种说法中哪一种说法是正确的是( )(A ) 位移电流的实质是变化的电场(B ) 位移电流和传导电流一样是定向运动的电荷(C ) 位移电流服从传导电流遵循的所有定律(D ) 位移电流的磁效应不服从安培环路定理分析与解 位移电流的实质是变化的电场.变化的电场激发磁场,在这一点位移电流等效于传导电流,但是位移电流不是走向运动的电荷,也就不服从焦耳热效应、安培力等定律.因而正确答案为(A ).48 -5 下列概念正确的是( )(A ) 感应电场是保守场(B ) 感应电场的电场线是一组闭合曲线(C ) LI Φm =,因而线圈的自感系数与回路的电流成反比(D ) LI Φm =,回路的磁通量越大,回路的自感系数也一定大 分析与解 对照感应电场的性质,感应电场的电场线是一组闭合曲线.因而正确答案为(B ).8 -6 一铁心上绕有线圈100匝,已知铁心中磁通量与时间的关系为()Wb π100sin 100.85t Φ⨯=,求在s 100.12-⨯=t 时,线圈中的感应电动势.分析 由于线圈有N 匝相同回路,线圈中的感应电动势等于各匝回路的感应电动势的代数和,在此情况下,法拉第电磁感应定律通常写成tψt ΦN ξd d d d -=-=,其中ΦN ψ=称为磁链. 解 线圈中总的感应电动势()()t tΦNξπ100cos 51.2d d =-= 当s 100.12-⨯=t 时,V 51.2=ξ. 8 -7 有两根相距为d 的无限长平行直导线,它们通以大小相等流向相反的电流,且电流均以tI d d 的变化率增长.若有一边长为d 的正方形线圈与两导线处于同一平面内,如图所示.求线圈中的感应电动势.5分析 本题仍可用法拉第电磁感应定律tΦξd d -=来求解.由于回路处在非均匀磁场中,磁通量就需用⎰⋅=SΦS B d 来计算(其中B 为两无限长直电流单独存在时产生的磁感强度B 1 与B 2 之和). 为了积分的需要,建立如图所示的坐标系.由于B 仅与x 有关,即()B B x =,故取一个平行于长直导线的宽为dx 、长为d 的面元dS ,如图中阴影部分所示,则x d S d d =,所以,总磁通量可通过线积分求得(若取面元y x S d d d =,则上述积分实际上为二重积分).本题在工程技术中又称为互感现象,也可用公式tl M E M d d -=求解. 解1 穿过面元dS 的磁通量为()x d xI μx d d x I μΦd π2d π2d d d d 0021-+=⋅+⋅=⋅=S B S B S B 因此穿过线圈的磁通量为()43ln π2d π2d π2d 02020Id μx x Id μx d x Id μΦΦd d dd =-+==⎰⎰⎰ 再由法拉第电磁感应定律,有6tI d μt ΦE d d 43ln π2d d 0⎪⎭⎫ ⎝⎛=-= 解2 当两长直导线有电流I 通过时,穿过线圈的磁通量为 43ln π20dI μΦ=线圈与两长直导线间的互感为 43ln π20d μI ΦM == 当电流以tl d d 变化时,线圈中的互感电动势为 tI d μt I M E d d 43ln π2d d 0⎪⎭⎫ ⎝⎛=-= 试想:如线圈又以速率v 沿水平向右运动,如何用法拉第电磁感应定律求图示位置的电动势呢此时线圈中既有动生电动势,又有感生电动势.设时刻t ,线圈左端距右侧直导线的距离为ξ,则穿过回路的磁通量()ξf ΦS,1d =⋅=⎰S B ,它表现为变量I 和ξ的二元函数,将Φ代入t ΦE d d -= 即可求解,求解时应按复合函数求导,注意,其中v =tξd d ,再令ξ=d 即可求得图示位置处回路中的总电动势.最终结果为两项,其中一项为动生电动势,另一项为感生电动势.8 -8 有一测量磁感强度的线圈,其截面积S =4.0 cm 2 、匝数N =160 匝、电阻R =50Ω.线圈与一内阻R i =30Ω的冲击电流计相连.若开始时,线圈的平面与均匀磁场的磁感强度B 相垂直,然后线圈的平面很快地转到与B 的方向平行.此时从冲击电流计中测得电荷值54.010C q -=⨯.问此均匀磁场的磁感强度B 的值为多少7分析 在电磁感应现象中,闭合回路中的感应电动势和感应电流与磁通量变化的快慢有关,而在一段时间内,通过导体截面的感应电量只与磁通量变化的大小有关,与磁通量变化的快慢无关.工程中常通过感应电量的测定来确定磁场的强弱. 解 在线圈转过90°角时,通过线圈平面磁通量的变化量为NBS NBS ΦΦΦ=-=-=0Δ12 因此,流过导体截面的电量为ii R RNBS R R Φq +=+=Δ 则 ()T 050.0=+=NSR R q B i 8 -9 如图所示,一长直导线中通有I =5.0 A 的电流,在距导线9.0 cm 处,放一面积为0.10 cm 2 ,10 匝的小圆线圈,线圈中的磁场可看作是均匀的.今在1.0 ×10-2 s 内把此线圈移至距长直导线10.0 cm 处.求:(1) 线圈中平均感应电动势;(2) 设线圈的电阻为1.0×10-2Ω,求通过线圈横截面的感应电荷.8分析 虽然线圈处于非均匀磁场中,但由于线圈的面积很小,可近似认为穿过线圈平面的磁场是均匀的,因而可近似用NBS ψ=来计算线圈在始、末两个位置的磁链.解 (1) 在始、末状态,通过线圈的磁链分别为1011π2r ISμN S NB ψ==,2022π2r IS μN S NB ψ== 则线圈中的平均感应电动势为 V 1011.111πΔ2ΔΔ8210-⨯=⎪⎪⎭⎫ ⎝⎛-==r r t IS μN t ΦE 电动势的指向为顺时针方向.(2) 通过线圈导线横截面的感应电荷为tΦE d d -= 8 -10 如图(a)所示,把一半径为R 的半圆形导线OP 置于磁感强度为B 的均匀磁场中,当导线以速率v 水平向右平动时,求导线中感应电动势E 的大小,哪一端电势较高9分析 本题及后面几题中的电动势均为动生电动势,除仍可由tΦE d d -=求解外(必须设法构造一个闭合回路),还可直接用公式()l B d ⋅⨯=⎰l E v 求解.在用后一种方法求解时,应注意导体上任一导线元dl 上的动生电动势()l B d d ⋅⨯=v E .在一般情况下,上述各量可能是dl 所在位置的函数.矢量(v ×B )的方向就是导线中电势升高的方向. 解1 如图(b)所示,假想半圆形导线O P 在宽为2R 的静止形导轨上滑动,两者之间形成一个闭合回路.设顺时针方向为回路正向,任一时刻端点O 或端点P 距 形导轨左侧距离为x ,则B R Rx Φ⎪⎭⎫ ⎝⎛+=2π212 即B R tx RB t ΦE v 2d d 2d d -=-=-= 由于静止的 形导轨上的电动势为零,则E =-2R v B .式中负号表示电动势的方向为逆时针,对OP 段来说端点P 的电势较高. 解2 建立如图(c )所示的坐标系,在导体上任意处取导体元dl ,则()θR θB l θB E o d cos d cos 90sin d d v v ==⋅⨯=l B vB R θθBR E v v 2d cos d E π/2π/2===⎰⎰- 由矢量(v ×B )的指向可知,端点P 的电势较高.10 解3 连接OP 使导线构成一个闭合回路.由于磁场是均匀的,在任意时刻,穿过回路的磁通量==BS Φ常数.由法拉第电磁感应定律tΦE d d -=可知,E =0 又因 E =E OP +E PO即 E OP =-E PO =2R v B由上述结果可知,在均匀磁场中,任意闭合导体回路平动所产生的动生电动势为零;而任意曲线形导体上的动生电动势就等于其两端所连直线形导体上的动生电动势.上述求解方法是叠加思想的逆运用,即补偿的方法.8 -11 长为L 的铜棒,以距端点r 处为支点,以角速率ω绕通过支点且垂直于铜棒的轴转动.设磁感强度为B 的均匀磁场与轴平行,求棒两端的电势差.分析 应该注意棒两端的电势差与棒上的动生电动势是两个不同的概念,如同电源的端电压与电源电动势的不同.在开路时,两者大小相等,方向相反(电动势的方向是电势升高的方向,而电势差的正方向是电势降落的方向).本题可直接用积分法求解棒上的电动势,亦可以将整个棒的电动势看作是O A 棒与O B 棒上电动势的代数和,如图(b)所示.而E O A 和E O B 则可以直接利用第8 -2 节例1 给出的结果.解1 如图(a)所示,在棒上距点O 为l 处取导体元dl ,则()()r L lB ωl lB ωE L-r r AB AB 221d d --=-=⋅⨯=⎰⎰-l B v 因此棒两端的电势差为()r L lB ωE U AB AB 221--== 当L >2r 时,端点A 处的电势较高解2 将AB 棒上的电动势看作是O A 棒和O B 棒上电动势的代数和,如图(b)所示.其中221r ωB E OA =,()221r L B ωE OB -= 则()r L BL ωE E E OB OA AB 221--=-= 8 -12 如图所示,长为L 的导体棒OP ,处于均匀磁场中,并绕OO ′轴以角速度ω旋转,棒与转轴间夹角恒为θ,磁感强度B 与转轴平行.求OP 棒在图示位置处的电动势.分析 如前所述,本题既可以用法拉第电磁感应定律t ΦE d d -= 计算(此时必须构造一个包含OP 导体在内的闭合回路, 如直角三角形导体回路OPQO ),也可用()l B d ⋅⨯=⎰lE v 来计算.由于对称性,导体OP 旋转至任何位置时产生的电动势与图示位置是相同的.解1 由上分析,得()l B d ⋅⨯=⎰OP OP E v l αB l o d cos 90sin ⎰=v()()l θB θωl o d 90cos sin ⎰-=l()⎰==L θL B ωl l θB ω022sin 21d sin 由矢量B ⨯v 的方向可知端点P 的电势较高.解2 设想导体OP 为直角三角形导体回路OPQO 中的一部分,任一时刻穿过回路的磁通量Φ为零,则回路的总电动势QO PQ OP E E E t ΦE ++==-=0d d 显然,E QO =0,所以()221PQ B ωE E E QO PQ OP ==-= 由上可知,导体棒OP 旋转时,在单位时间内切割的磁感线数与导体棒QP 等效.后者是垂直切割的情况.8 -13 如图(a)所示,金属杆AB 以匀速12.0m s -=⋅v 平行于一长直导线移动,此导线通有电流I =40A .求杆中的感应电动势,杆的哪一端电势较高分析 本题可用两种方法求解.(1) 用公式()l B d ⋅⨯=⎰lE v 求解,建立图(a )所示的坐标系,所取导体元x l d d =,该处的磁感强度xI μB π20=.(2) 用法拉第电磁感应定律求解,需构造一个包含杆AB 在内的闭合回路.为此可设想杆AB 在一个静止的形导轨上滑动,如图(b)所示.设时刻t ,杆AB 距导轨下端CD 的距离为y ,先用公式⎰⋅=SΦS B d 求得穿过该回路的磁通量,再代入公式tΦE d d -=,即可求得回路的电动势,亦即本题杆中的电动势. 解1 根据分析,杆中的感应电动势为()V 1084.311ln 2πd 2πd d 50m 1.1m 1.00-⨯-=-=-==⋅⨯=⎰⎰v v v I μx x μxl E AB AB l B 式中负号表示电动势方向由B 指向A ,故点A 电势较高. 解2 设顺时针方向为回路AB CD 的正向,根据分析,在距直导线x 处,取宽为dx 、长为y 的面元dS ,则穿过面元的磁通量为x y xI μΦd 2πd d 0=⋅=S B 穿过回路的磁通量为11ln 2πd 2πd 0m1.1m 1.00⎰⎰-===S Iy μx y x I μΦΦ 回路的电动势为V 1084.32πd d 11ln 2πd d 500-⨯-=-=-=-=Iy μt y x I μt ΦE 由于静止的形导轨上电动势为零,所以 V 1084.35-⨯-==E E AB式中负号说明回路电动势方向为逆时针,对AB 导体来说,电动势方向应由B 指向A ,故点A 电势较高.8 -14 如图(a)所示,在“无限长”直载流导线的近旁,放置一个矩形导体线框,该线框在垂直于导线方向上以匀速率v 向右移动,求在图示位置处,线框中感应电动势的大小和方向.分析 本题亦可用两种方法求解.其中应注意下列两点:1.当闭合导体线框在磁场中运动时,线框中的总电动势就等于框上各段导体中的动生电动势的代数和.如图(a)所示,导体eh 段和fg 段上的电动势为零[此两段导体上处处满足()0l B =⋅⨯d v ],因而线框中的总电动势为()()()()hg ef hgef gh ef E E E -=⋅⨯-⋅⨯=⋅⨯+⋅⨯=⎰⎰⎰⎰l B l B l B l B d d d d v v v v 其等效电路如图(b)所示.2.用公式tΦE d d -=求解,式中Φ是线框运动至任意位置处时,穿过线框的磁通量.为此设时刻t 时,线框左边距导线的距离为ξ,如图(c )所示,显然ξ是时间t 的函数,且有v =tξd d .在求得线框在任意位置处的电动势E (ξ)后,再令ξ=d ,即可得线框在题目所给位置处的电动势.解1 根据分析,线框中的电动势为hg ef E E E -=()()⎰⎰⋅⨯-⋅⨯=hgef l B l B d d v v ()⎰⎰+-=2201000d 2πd 2πl l l l d I μl d I μv v ()1202πl d I I μ+=1vI 由E ef >E hg 可知,线框中的电动势方向为efgh .解2 设顺时针方向为线框回路的正向.根据分析,在任意位置处,穿过线框的磁通量为()()ξl ξξx Il μdx ξx Il μΦl 120020ln π2π21++=+=⎰ 相应电动势为()()1120π2d d l ξξl l I μt ΦξE +=-=v 令ξ=d ,得线框在图示位置处的电动势为 ()1120π2l d d l l I μE +=v 由E >0 可知,线框中电动势方向为顺时针方向.*8 -15 有一长为l ,宽为b 的矩形导线框架,其质量为m ,电阻为R .在t =0时,框架从距水平面y =0 的上方h 处由静止自由下落,如图所示.磁场的分布为:在y =0 的水平面上方没有磁场;在y =0 的水平面下方有磁感强度为B 的均匀磁场,B 的方向垂直纸面向里.已知框架在时刻t 1 和t 2 的位置如图中所示.求在下述时间内,框架的速度与时间的关系:(1) t 1 ≥t >0,即框架进入磁场前;(2) t 2 ≥t ≥t 1 ,即框架进入磁场, 但尚未全部进入磁场;(3)t >t 2 ,即框架全部进入磁场后.分析 设线框刚进入磁场(t 1 时刻)和全部进入磁场(t 2 时刻)的瞬间,其速度分别为v 10 和v 20 .在情况(1)和(3)中,线框中无感应电流,线框仅在重力作用下作落体运动,其速度与时间的关系分别为v =gt (t <t 1)和v =v 20 +g (t -t 2 )(t >t 2 ).而在t 1<t <t 2这段时间内,线框运动较为复杂,由于穿过线框回路的磁通量变化,使得回路中有感应电流存在,从而使线框除受重力外,还受到一个向上的安培力F A ,其大小与速度有关,即()A A F F =v .根据牛顿运动定律,此时线框的运动微分方程为()tv v d d m F mg A =-,解此微分方程可得t 1<t <t 2 时间内线框的速度与时间的关系式.解 (1) 根据分析,在1t t ≤时间内,线框为自由落体运动,于是()11t t gt ≤=v 其中1t t =时,gh 2101==v v(2) 线框进入磁场后,受到向上的安培力为v Rl B IlB F A 22== 根据牛顿运动定律,可得线框运动的微分方程tv m v d d 22=-R l B mg 令mRl B K 22=,整理上式并分离变量积分,有 ⎰⎰=-t t t g 110d d vv Kv v 积分后将gh 210=v 代入,可得()()[]1212t t K e gh K g g K----=v (3) 线框全部进入磁场后(t >t 2),作初速为v 20 的落体运动,故有()()()[]()222031221t t g e gh K g g Kt t g t t K -+--=-+=--v v 8 -16 有一磁感强度为B 的均匀磁场,以恒定的变化率t d d B 在变化.把一块质量为m 的铜,拉成截面半径为r 的导线,并用它做成一个半径为R 的圆形回路.圆形回路的平面与磁感强度B 垂直.试证:这回路中的感应电流为td d π4B d ρm I =式中ρ 为铜的电阻率,d 为铜的密度. 解 圆形回路导线长为πR 2,导线截面积为2πr ,其电阻R ′为22rR ρS l ρR ==' 在均匀磁场中,穿过该回路的磁通量为BS Φ=,由法拉第电磁感应定律可得回路中的感应电流为t t t d d 2πd d π1d d 122B ρRr B R R ΦR R E I ='='='= 而2ππ2r R d m =,即dm Rr π2π2=,代入上式可得 td d π4B d ρm I = 8 -17 半径为R =2.0 cm 的无限长直载流密绕螺线管,管内磁场可视为均匀磁场,管外磁场可近似看作零.若通电电流均匀变化,使得磁感强度B 随时间的变化率td d B 为常量,且为正值,试求:(1) 管内外由磁场变化激发的感生电场分布;(2) 如1s T 010.0d d -⋅=tB ,求距螺线管中心轴r =5.0 cm 处感生电场的大小和方向.分析 变化磁场可以在空间激发感生电场,感生电场的空间分布与场源———变化的磁场(包括磁场的空间分布以及磁场的变化率td d B 等)密切相关,即S B l E d d ⋅∂∂-=⎰⎰S S k t .在一般情况下,求解感生电场的分布是困难的.但对于本题这种特殊情况,则可以利用场的对称性进行求解.可以设想,无限长直螺线管内磁场具有柱对称性,其横截面的磁场分布如图所示.由其激发的感生电场也一定有相应的对称性,考虑到感生电场的电场线为闭合曲线,因而本题中感生电场的电场线一定是一系列以螺线管中心轴为圆心的同心圆.同一圆周上各点的电场强度E k 的大小相等,方向沿圆周的切线方向.图中虚线表示r <R 和r >R 两个区域的电场线.电场线绕向取决于磁场的变化情况,由楞次定律可知,当0d d <t B 时,电场线绕向与B 方向满足右螺旋关系;当0d d >t B 时,电场线绕向与前者相反.解 如图所示,分别在r <R 和r >R 的两个区域内任取一电场线为闭合回路l (半径为r 的圆),依照右手定则,不妨设顺时针方向为回路正向.(1) r <R , tB r t r E E k l k d d πd d d π2d 2-=⋅-=⋅=⋅=⎰⎰S B l E tB r E k d d 2-= r >R , t B R t r E E k lk d d πd d d π2d 2-=⋅-=⋅=⋅=⎰⎰S B l E tB r R E k d d 22-= 由于0d d >tB ,故电场线的绕向为逆时针. (2) 由于r >R ,所求点在螺线管外,因此tB r R E k d d 22-= 将r 、R 、tB d d 的数值代入,可得15m V 100.4--⋅⨯-=k E ,式中负号表示E k 的方向是逆时针的.8 -18 在半径为R 的圆柱形空间中存在着均匀磁场,B 的方向与柱的轴线平行.如图(a)所示,有一长为l 的金属棒放在磁场中,设B 随时间的变化率tB d d 为常量.试证:棒上感应电动势的大小为分析 变化磁场在其周围激发感生电场,把导体置于感生电场中,导体中的自由电子就会在电场力的作用下移动,在棒内两端形成正负电荷的积累,从而产生感生电动势.由于本题的感生电场分布与上题所述情况完全相同,故可利用上题结果,由⎰⋅=lk E l E d 计算棒上感生电动势.此外,还可连接OP 、OQ ,设想PQOP 构成一个闭合导体回路,用法拉第电磁感应定律求解,由于OP 、OQ 沿半径方向,与通过该处的感生电场强度E k 处处垂直,故0d =⋅l E k ,OP 、OQ 两段均无电动势,这样,由法拉第电磁感应定律求出的闭合回路的总电动势,就是导体棒PQ 上的电动势.证1 由法拉第电磁感应定律,有 22Δ22d d d d d d ⎪⎭⎫ ⎝⎛-==-==l R l t B t B S t ΦE E PQ 证2 由题8 -17可知,在r <R 区域,感生电场强度的大小tB r E k d d 2= 设PQ 上线元dx 处,E k 的方向如图(b )所示,则金属杆PQ 上的电动势为()()222202/2d d d 2/d d 2d cos d l R l t B x r l R t B r x θE E l k k PQ -=-==⋅=⎰⎰x E 讨论 假如金属棒PQ 有一段在圆外,则圆外一段导体上有无电动势 该如何求解8 -19 截面积为长方形的环形均匀密绕螺绕环,其尺寸如图(a)所示,共有N 匝(图中仅画出少量几匝),求该螺绕环的自感L .分析 如同电容一样,自感和互感都是与回路系统自身性质(如形状、匝数、介质等)有关的量.求自感L 的方法有两种:1.设有电流I 通过线圈,计算磁场穿过自身回路的总磁通量,再用公式IΦL =计算L .2.让回路中通以变化率已知的电流,测出回路中的感应电动势E L ,由公式t I E L L d /d =计算L .式中E L 和tI d d 都较容易通过实验测定,所以此方法一般适合于工程中.此外,还可通过计算能量的方法求解.解 用方法1 求解,设有电流I 通过线圈,线圈回路呈长方形,如图(b)所示,由安培环路定理可求得在R 1 <r <R 2 范围内的磁场分布为xNI μB π20=由于线圈由N 匝相同的回路构成,所以穿过自身回路的磁链为 12200ln π2d π2d 21R R hI N μx h x NI μN N ψS R R ==⋅=⎰⎰S B 则1220ln π2R R h N μI ψL = 若管中充满均匀同种磁介质,其相对磁导率为μr ,则自感将增大μr 倍.8 -20 如图所示,螺线管的管心是两个套在一起的同轴圆柱体,其截面积分别为S 1 和S 2 ,磁导率分别为μ1 和μ2 ,管长为l ,匝数为N ,求螺线管的自感.(设管的截面很小)分析 本题求解时应注意磁介质的存在对磁场的影响.在无介质时,通电螺线管内的磁场是均匀的,磁感强度为B 0 ,由于磁介质的存在,在不同磁介质中磁感强度分别为μ1 B 0 和μ2 B 0 .通过线圈横截面的总磁通量是截面积分别为S 1 和S 2 的两部分磁通量之和.由自感的定义可解得结果.解 设有电流I 通过螺线管,则管中两介质中磁感强度分别为I L N μnl μB 111==,I LN μnl μB 222== 通过N 匝回路的磁链为221121S NB S NB ΨΨΨ+=+=则自感2211221S μS μlN I ψL L L +==+= 8 -21 有两根半径均为a 的平行长直导线,它们中心距离为d .试求长为l的一对导线的自感(导线内部的磁通量可略去不计).分析 两平行长直导线可以看成无限长但宽为d 的矩形回路的一部分.设在矩形回路中通有逆时针方向电流I ,然后计算图中阴影部分(宽为d 、长为l )的磁通量.该区域内磁场可以看成两无限长直载流导线分别在该区域产生的磁场的叠加.解 在如图所示的坐标中,当两导线中通有图示的电流I 时,两平行导线间的磁感强度为()r d I μr I μB -+=π2π200 穿过图中阴影部分的磁通量为 aa d l μr Bl ΦS a d a -==⋅=⎰⎰-ln πd d 0S B 则长为l 的一对导线的自感为aa d l μI ΦL -==ln π0 如导线内部磁通量不能忽略,则一对导线的自感为212L L L +=.L 1 称为外自感,即本题已求出的L ,L 2 称为一根导线的内自感.长为l 的导线的内自感8π02l μL =,有兴趣的读者可自行求解. 8 -22 如图所示,在一柱形纸筒上绕有两组相同线圈AB 和A ′B ′,每个线圈的自感均为L ,求:(1) A 和A ′相接时,B 和B ′间的自感L 1 ;(2) A ′和B 相接时,A 和B ′间的自感L 2 .分析 无论线圈AB 和A ′B ′作哪种方式连接,均可看成一个大线圈回路的两个部分,故仍可从自感系数的定义出发求解.求解过程中可利用磁通量叠加的方法,如每一组载流线圈单独存在时穿过自身回路的磁通量为Φ,则穿过两线圈回路的磁通量为2Φ;而当两组线圈按(1)或(2)方式连接后,则穿过大线圈回路的总磁通量为2Φ±2Φ,“ ±”取决于电流在两组线圈中的流向是相同或是相反.解 (1) 当A 和A ′连接时,AB 和A ′B ′线圈中电流流向相反,通过回路的磁通量亦相反,故总通量为0221=-=ΦΦΦ,故L 1 =0.(2) 当A ′和B 连接时,AB 和A ′B ′线圈中电流流向相同,通过回路的磁通量亦相同,故总通量为ΦΦΦΦ4222=+=, 故L I ΦI ΦL 4422===. 本题结果在工程实际中有实用意义,如按题(1)方式连接,则可构造出一个无自感的线圈.8 -23 如图所示,一面积为4.0 cm 2 共50 匝的小圆形线圈A ,放在半径为20 cm 共100 匝的大圆形线圈B 的正中央,此两线圈同心且同平面.设线圈A 内各点的磁感强度可看作是相同的.求:(1) 两线圈的互感;(2) 当线圈B 中电流的变化率为-50 A·s-1 时,线圈A 中感应电动势的大小和方向.分析 设回路Ⅰ中通有电流I 1 ,穿过回路Ⅱ的磁通量为Φ21 ,则互感M =M 21 =Φ21I 1 ;也可设回路Ⅱ通有电流I 2 ,穿过回路Ⅰ的磁通量为Φ12 ,则21212I ΦM M == . 虽然两种途径所得结果相同,但在很多情况下,不同途径所涉及的计算难易程度会有很大的不同.以本题为例,如设线圈B 中有电流I 通过,则在线圈A 中心处的磁感强度很易求得,由于线圈A 很小,其所在处的磁场可视为均匀的,因而穿过线圈A 的磁通量Φ≈BS .反之,如设线圈A 通有电流I ,其周围的磁场分布是变化的,且难以计算,因而穿过线圈B 的磁通量也就很难求得,由此可见,计算互感一定要善于选择方便的途径.解 (1) 设线圈B 有电流I 通过,它在圆心处产生的磁感强度R I μN B B 200=穿过小线圈A 的磁链近似为 A B A A A A S RI μN N S B N ψ200== 则两线圈的互感为H 1028.6260-⨯===RS μN N I ψM A B A A (2)V 1014.3d d 4-⨯=-=tI M E A 互感电动势的方向和线圈B 中的电流方向相同.8 -24 如图所示,两同轴单匝线圈A 、C 的半径分别为R 和r ,两线圈相距为d .若r 很小,可认为线圈A 在线圈C 处所产生的磁场是均匀的.求两线圈的互感.若线圈C 的匝数为N 匝,则互感又为多少解 设线圈A 中有电流I 通过,它在线圈C 所包围的平面内各点产生的磁感强度近似为()2/322202d R IR μB +=穿过线圈C 的磁通为 ()22/32220π2r d R IR μBS ψC +==则两线圈的互感为 ()2/3222202πdR R r μI ψM +== 若线圈C 的匝数为N 匝,则互感为上述值的N 倍. 8 -25 如图所示,螺绕环A 中充满了铁磁质,管的截面积S 为2.0 cm 2 ,沿环每厘米绕有100 匝线圈,通有电流I 1 =4.0 ×10 -2 A ,在环上再绕一线圈C ,共10 匝,其电阻为0.10 Ω,今将开关S 突然开启,测得线圈C 中的感应电荷为2.0 ×10 -3C .求:当螺绕环中通有电流I 1 时,铁磁质中的B 和铁磁质的相对磁导率μr .分析 本题与题8 -8 相似,均是利用冲击电流计测量电磁感应现象中通过回路的电荷的方法来计算磁场的磁感强度.线圈C 的磁通变化是与环形螺线管中的电流变化相联系的. 解 当螺绕环中通以电流I 1 时,在环内产生的磁感强度110I n μμB r =则通过线圈C 的磁链为S I n μμN BS N ψr c 11022==设断开电源过程中,通过C 的感应电荷为q C ,则有()RS I n μμN ψR ψR qc r c c 110201Δ1=--=-= 由此得 T 10.02110===S N Rqc I n μμB r 相对磁导率1991102==I n μS N Rqc μr8 -26 一个直径为0.01 m ,长为0.10 m 的长直密绕螺线管,共1 000 匝线圈,总电阻为7.76 Ω.求:(1) 如把线圈接到电动势E =2.0 V 的电池上,电流稳定后,线圈中所储存的磁能有多少 磁能密度是多少*(2) 从接通电路时算起,要使线圈储存磁能为最大储存磁能的一半,需经过多少时间分析 单一载流回路所具有的磁能,通常可用两种方法计算:(1) 如回路自感为L (已知或很容易求得),则该回路通有电流I 时所储存的磁能221LI W m =,通常称为自感磁能.(2) 由于载流回路可在空间激发磁场,磁能实际是储存于磁场之中,因而载流回路所具有的能量又可看作磁场能量,即V w W V m m d ⎰=,式中m w 为磁场能量密度,积分遍及磁场存在的空间.由于μB w m 22=,因而采用这种方法时应首先求载流回路在空间产生的磁感强度B 的分布.上述两种方法还为我们提供了计算自感的另一种途径,即运用V w LI V m d 212⎰=求解L . 解 (1) 密绕长直螺线管在忽略端部效应时,其自感l S N L 2=,电流稳定后,线圈中电流RE I =,则线圈中所储存的磁能为J 1028.3221522202-⨯===lRSE N μLI W m 在忽略端部效应时,该电流回路所产生的磁场可近似认为仅存在于螺线管。
《大学物理》第八章毕萨定律S
B 0 B B
x
都适用。
例半径为R的无限长圆柱载流直导线,电流I沿轴线 方向流动,并且载面上电流是均匀分布。计算任 意点P的B=? I 解:先分析P点的B方向 由电流对称分布可知: B oP . 取过P点半径为 r =op 的圆周L, P L上各点B大小相等,方向沿切线 r >R时 由安培环路定理得: L d B ds dB dB B dr Bdr cos 0o B 2 r I 0 . 又 B O B d r I 0 2 r P ds 与毕萨 定理结 果一致
L
若r<R 同理:
r
R
例求通电螺绕环的磁场分布。已知环管轴线的半径 为R,环上均匀密绕N匝线圈,设通有电流I。 解: 由于电流对称分布,与环共轴 的圆周上,各点B大小相等, R R1 方向沿圆周切线方向。 取以o为中心,半径为r的圆周为L R2 当R1< r <R2 I B dr Bdr cos 0o B 2 r 0 NI × × × B ×× ×× × 而 I NI × 2 r 0 i 0 × ×
4)载流回路的磁场 电流元 在空间P点产生的磁场: 0 Idl er Idl I dB
二. 磁场的高斯定理
4 r o Idl r B dB 3 4 r
2
(磁通连续原理)
定理的内容:穿过任一闭合曲面(高斯面)的总磁通量总为0
S
B
o q v r B 4 r 3
例8.4
对低速运动的 带电粒子成立!
8.3 安培环路定理
一、安培环路定理 静电场理论中,有“静电场的环路定理”L : 对于稳恒磁场,相应的“稳恒磁场的环路定理”应如何 ? B dr ?
大学物理第八章恒定电流的磁场
Fe 2.磁性: 磁铁能吸引含有 Co 物质的性质。
Ni
3.磁极:磁铁上磁性最强的两端,分为
N S
北同 极,指向 方,
南异
斥 性相 。
吸
三.磁场
1.概念: 运动qυ电荷或电I流周围存在的物质,称为磁场。
2.对外表现
① qυ或 I 在磁场中受到力的作用。
②载流导线在磁场中移动,磁场力作功。
力的表现 功的表现
极。
然而,磁和电有很多相似之处。例如,同种电荷
互相推斥,异种电荷互相吸引;同名磁极也互相推
斥,异名磁极也互相吸引。用摩擦的方法能使物体带
上电;如果用磁铁的一极在一根钢棒上沿同一方向摩
擦几次,也能使钢棒磁化。但是,为什么正、负电荷 能够单独存在,而单个磁极却不能单独存在呢?多年 来,人们百思而不得其解。
dN B
dS
一些典型磁场的磁感线:
2.性质
①磁感线是无始无终的闭合曲线。
B
A
②任二条磁感线不相交。
B
③磁感线与电流是套合的,它们之间可用右手螺旋法 则来确定。
B
I
I
B
四.磁通量
1.定义:通过一给定曲面的磁感线的条数,称为通过该 曲面的磁通量。
电场强度通量:e S E dS
通过面元 dS的磁感线数: dN BdS BdS cos
3.电荷之间的磁相互作用与库仑相互作用的不同 ①电荷无论是静止还是运动的,它们之间都存在库仑 作用; ②只有运动的电荷之间才有磁相互作用。
四.磁感强度
电场 E 磁场 B
1.实验 在垂于电流的平面内放若干枚小磁针,发现:
①小磁针距电流远近不同,
N
受磁力大小不同。
②距电流等远处,小磁针受
2024大学物理力学第八章机械振动
动contents •简谐振动•阻尼振动与受迫振动•振动的合成与分解•振动在介质中的传播•多自由度系统的振动•非线性振动与混沌目录01简谐振动简谐振动的定义与特点定义简谐振动是最基本、最简单的振动形式,指物体在跟偏离平衡位置的位移成正比,并且总是指向平衡位置的回复力的作用下的振动。
特点简谐振动的物体所受的回复力F与物体偏离平衡位置的位移x成正比,且方向始终指向平衡位置;振动过程中,系统的机械能守恒。
动力学方程根据牛顿第二定律,简谐振动的动力学方程可以表示为F=-kx,其中F为回复力,k为比例系数,x为物体偏离平衡位置的位移。
运动学方程简谐振动的运动学方程可以表示为x=Acos(ωt+φ),其中A为振幅,ω为角频率,t为时间,φ为初相。
势能与动能在简谐振动过程中,系统的势能Ep和动能Ek都在不断变化,但它们的总和保持不变,即机械能守恒。
能量转换在振动过程中,势能和动能之间不断相互转换。
当物体向平衡位置运动时,势能减小、动能增加;当物体远离平衡位置时,势能增加、动能减小。
同方向同频率简谐振动的合成当两个同方向、同频率的简谐振动同时作用于同一物体时,它们的合振动仍然是一个简谐振动,其振幅等于两个分振动振幅的矢量和,其初相等于两个分振动初相的差。
同方向不同频率简谐振动的合成当两个同方向、不同频率的简谐振动同时作用于同一物体时,它们的合振动一般不再是简谐振动,而是比较复杂的周期性振动。
在某些特定条件下(如两个分振动的频率成简单整数比),合振动可能会呈现出一定的规律性。
相互垂直的简谐振动的合成当两个相互垂直的简谐振动同时作用于同一物体时,它们的合振动轨迹一般是一条复杂的曲线。
在某些特定条件下(如两个分振动的频率相同、相位差为90度),合振动轨迹可能会呈现出一定的规律性,如圆形、椭圆形等。
02阻尼振动与受迫振动阻尼振动的定义与分类定义阻尼振动是指振动系统在振动过程中,由于系统内部摩擦或外部介质阻力的存在,使振动幅度逐渐减小,能量逐渐耗散的振动。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
8-2 Heat and Work Done by Thermodynamic Systems Heat Heat is the energy that is transferred between a system and its environment because of a temperature difference that exists between them.
2
2
2. Constant-pressure processes An isobaric process is one in which the pressure is kept constant. p i f If the volume changes from Vi to Vf p while the pressure of the gas is held W>0 constant p, the work done by the O gas is given by Vi Vf V V W = ∫ pdV = p (Vf − Vi) V
2 1
cycle
8-3 The First Law of Thermodynamics If an amount of heat Q enters a thermodynamic system, it could manifest itself as either an increase in internal energy or a resulting quantity of work performed by the system on the surroundings, or a bit of both. Then Q = ∆Eint + W, Checkpoint 2 , where ∆Eint is the change in the system’s internal energy, W is the net work done by the system. Note that before this chapter, the term work meant that the work done on a system and is symbolized W. However, in this chapter and the next, we define the work and the symbol W as the work done by a system. If the thermodynamic system undergoes only a differential process, dQ = dEint + dW.
f i
∆Eint is independent of processes, for any process
∆Eint
From the first law of thermodynamics, heat transfer in an isobaric process is given by f Q = ∆Eint + W = ν R(T f − Ti ) + p (Vf − Vi)
Quasi-static process To maintain thermal equilibrium at each step in a thermal process, changes must be made such slowly that the time taken by the thermal process is much longer than relaxation time. Such a process is called quasi-static process, which can be represented as a curve on a p−V diagram, a p−T diagram, or a V−T diagram. Unless we state otherwise, we consider only quasistatic process. Thermodynamics is based on our ability to treat quasi-static process. There are situations (such as free expansion) in which processes occur too fast for equilibrium thermodynamics to apply.
Heat is neither released nor absorbed Q=0.
Heat is absorbed by the system from the environment Q>0.
Work Done by Thermodynamic Systems Consider a piston of area A pushed out by the pressure p of the gas contained in the cylinder. The infinitesimal work done by the gas in moving the piston ds is
A
r r dW = F ds = pAds
dW = pdV
p ds
The volume changes from Vi to Vf :
During the change in volume, the pressure and temperature may also change. There are actually many ways to take the gas from state i to state f.
8-1 Changes in Thermodynamic Systems Thermodynamic systems in equilibrium, such as a bottle of hot steam, are described by only a few thermodynamic variables. For a gas these variables are p, V, T, ν (or N). For an ideal gas, pV = νRT= NkT. Assume that ν is fixed, then it is enough to describe the (equilibrium) state on a two-dimensional plot, such as a p−V diagram, a p−T diagram, or a V−T diagram. Thermodynamic process When the surrounding is changed, a system will deviate from equilibrium. After a time τ, the system will reach a new equilibrium state (if exists). We say that the system undergoes a thermodynamic process. The time τ is called relaxation time.
8-4 Some Special Cases of the First law of Thermodynamics Q = ∆Eint + W 1. Constant-volume processes p If V = constant, W = 0. Such a process pf is called an isochoric process, f ∆Eint = Q
p Discussion W = dW = V pdV p ∫ ∫V i Process a i A system can be taken W>0 f f from a given initial state W>0 0 expanded to a given final state by V0 V p an infinite number of h p g processes. Heat may or i i may not be involved, and c c f f in general, the work W W>0 0 and the heat Q will have V 0 p V p different values for different Wnet >0 i i process. Checkpoint 1 f f W<0 0 We say that heat and work 0 V V Thermodynamic are path-dependent quantities compressed
Some quasi-static processes
p i (a) (b) (d) (c) V
(a) constant pressure (isobaric) process; (b) isothermal process; (c) isentropic (adiabatic) process; (d) constant volume (isochoric) process.
2
f f = ν R∆T = ν R(T f − Ti ) 2 2
3. Isothermal processes. Suppose that we allow an ideal gas to expand from an initial volume Vi to a final volume Vf while we keep the temperature T of the gas constant. p i pi T isothermal expansion :Vi <Vf isothermal compression : Vi >Vf pf f For an ideal gas, the internal energy W>0 is the function of temperature, thus O Vf V Vi for an isothermal process ∆Eint = 0 From the first law of thermodynamics, Q = W work done by an ideal gas during an isothermal Vf expansion V ν RT W = ∫ pdV = ∫V dV
