材料科学基础英文课件3
材料科学基础第三章-孪生微观方面
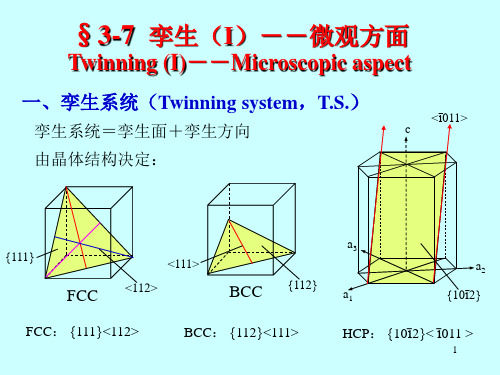
[1 2 1 0]
• •[01 10] •[1120]
HCP取向胞
26
HCP取向胞的应用:
• 不管哪一组的K1 & K2,只要落在K1 & K2之间,均为锐角区, 落在K1 & K2之外均为钝角区
• 不管哪一组的K1 & K2,只要落在A 区,均为锐角区
• 与A区共边的,称为B区(B1~B6) • 与A区共顶的,称为C区(C1~C6) • 与A区不接触的,称为D区(D1~
• 挛晶和基体要成映像关系,对称面为孪生面 • Janson规则:走近路原理(原子移动的位移最小) (1ī1)
FCC 2
BACBACBA
[ī12]
(1ī1)
FCC
3
C B AC B (B) (C) (A) (B) (C) A C B A
[ī12]
(1ī1)
FCC
[ī12]
[ī12]
FCC晶体孪生时原子的运动
23
二、不同方面
7) 变形的条件
高对称(晶体结构)、高温、低应变速率的条件下,有利于滑移 低对称(晶体结构)、低温、高应变速率的条件下,有利于孪生
24
作业: 3-15 自学“滑移和孪生的比较”
25
问题2: Zn单晶任意的晶向[uvtw]方向在孪生后长度的变化情况
c
a3 a2
a1
K1
K2
I、 II:(10 1 2)、(1 012 )
D6),均为钝角区 • [uvtw]位于哪个区,通过判断是锐角
还是钝角来判断其长度的变化。
D6
D5
C6
B6 C1
D1
C BB A • BB C 5
5 VI
材料科学基础英文版课件()

Principles of Fracture Mechanics (1)
Brittle fracture of normally ductile materials requires us to have a good understanding of the mechanisms of fracture. To do this, we need to know the knowledge of fracture mechanics Concerned with the relationship between material properties, stress level, crackproducing flaws, and crack propagation mechanisms
Ductile Fracture (1)
Features of ductile fracture
Moderately ductile Some deformation
Macroscopically
Highly ductile Considerable deformation
Necking down to Some necking a point
Kt
m o
2( a )1 2
t
Principles of Fracture Mechanics (3)
It is seen from the above that stress concentration occurs
Not only at microscopic flaws or cracks
Brittle Fracture (2)
Fracture surface markings for brittle fracture
【大学】材料科学基础(中英版)

Materials science Materials engineering Relations between Materials science and engineering Four components of discipline of materials science and engineering
第9页
Course book
Textbook
《无机材料科学基础》,宋晓岚,黄学辉,化学工业出版社,2006 Fundamentals of Ceramics, Michel Barsoum, Institute of Physics, 2003
References
《材料导论》,师昌绪,化学工业出版社,2002 《材料科学基础》,陆佩文,武汉理工大学出版社, 1996 《材料科学基础》,潘金生,清华大学出版社,1998 《材料科学与工程基础》,(美)史密斯等,机械工业出版社,2006 Fundamentals of materials science and engineering. (fifth edition) William D.
第20页
材料科学与工程
什么是材料科学?
材料科学是一门以固体材料为研究对象,以固体物理、热力学、动力学、 量子力学、冶金、化学为基础的应用学科,主要探讨材料的组成、结构与 性能之间的规律的一门基础应用学科,是研究材料共性的一门学科。
什么是材料工程
材料工程是对材料的结构进行设计和处理,并制备出具有某些特定性质的 工艺即工程技术问题。
Instructor: Times Location: Purpose:
review lecture concepts discuss homework, quizzes exams
材料科学基础英文版课件

Classification of grain boundary segregation
• Equilibrium segregation
– Thermodynamic process – Driving force: solute-boundary binding energy (the
difference in energy caused by a solute atom between staying in the grain interior and on the grain boundary
– One dislocation just has one b
– For metals, b normally points in a close-packed crystal direction and its magnitude is the interatomic spacing because the slip direction is normally in the close-packed direction
• To reduce the surface energy, the materials tend to minimize the total surface area
Grain Boundaries
In polycrystalline materials, a grain boundary is the boundary between two adjacent grains which have different orientations
Linear Defect – Dislocations
Features: one dimensional
3_《材料科学基础》第三章_晶体结构缺陷((上)

点缺陷(零维缺陷)--原子尺度的偏离.
按 缺
例:空位、间隙原子、杂质原子等
陷 线缺陷(一维缺陷)--原子行列的偏离.
的
例:位错等
几 何
面缺陷(二维缺陷)--表面、界面处原子排列混乱.
形
例:表面、晶界、堆积层错、镶嵌结构等
态 体缺陷(三维缺陷)--局部的三维空间偏离理想晶体的周期性
例:异相夹杂物、孔洞、亚结构等
1、 固溶体的分类
(1) 按杂质原子的位置分: 置换型固溶体—杂质原子进入晶格中正常结点位置而取代基
质中的原子。例MgO-CoO形成Mg1-xCoxO固溶体。 间隙型固溶体—杂质原子进入晶格中的间隙位置。
有时俩
(2)按杂质原子的固溶度x分: 无限(连续)固溶体—溶质和溶剂任意比例固溶(x=0~1)。
多相系统
均一单相系统
Compounds AmBn
原子间相互反应生成
均一单相系统
结构
各自有各自的结构
A structure
structure
+ B structure
结构与基质相同 A structure
结构既不同于A也不同于B New structure
化学计量 A/B
不定
固溶比例不定
m:n 整数比或接近整数比的一定范围内
四、固溶体Solid solution(杂质缺陷)
1、固溶体的分类 2、置换型固溶体 3、间隙型固溶体 4、形成固溶体后对晶体性质的影响 5、固溶体的研究方法
①固溶体:含有外来杂质原子的单一均匀的晶态固体。 例:MgO晶体中含有FeO杂质 → Mg1-xFexO
基质 溶剂 主晶相
杂质 溶质 掺杂剂
萤石CaF2(F-空位)
skja_03 Fundamentals of Crystallography 材料科学基础(英文课件)

Seven Crystal Systems
Triclinic
Monoclinic
Orthorhombic Tetragonal Cubic Hexagonal Rhombohedral
a≠b≠c ,α≠β≠γ≠90° a≠b≠c , α=β=90°≠γ
α=γ=90°≠β a≠b≠c ,α=β=γ=90° a=b≠c ,α=β=γ=90° a=b=c ,α=β=γ=90° a=b≠c ,α=β=90°γ=120°
5. Draw a primitive cell for BCC lattice.
Thank you !
3
2020/7/3
We identify 14 types of unit cells, or Bravais lattices, grouped in seven crystal systems.
2020/7/3
Ⅰ.Seven crystal systems
All possible structure reduce to a small number of basic unit cell geometries. ① There are only seven, unique unit cell shapes that can be stacked together to fill three-dimensional. ② We must consider how atoms can be stacked together within a given unit cell.
120o
120o 120o
c
a ba
2020/7/3
Examples and Discussions
材料科学基础(英)3-合金相图
3.1 Phases constituent of alloys (合金相结构) (types of microstructure in alloys)
•为两类: • 固溶体 • 中间相
7
3.1.1 Solid solution (固溶体)
A100 x Bx e/a 100
式中 A , B 分别为溶剂和溶质的原子价, x 为溶质的原子 数分数 ( %) 。固溶体的极限电子浓度为 1.4。超过此值时, 固溶体就不稳定而要形成另外的相
20
21
In interstitial solutions (间隙固溶体) the solute atoms fit into the spaces between the solvent atoms. These spaces are called interstice. Interstitial solid solution can form when one atom is much larger than another. Examples of atoms that can form interstitial solid solutions due to their small size are: hydrogen, carbon, nitrogen, and oxygen. Interstitial solutions are limited solid 22 solution.
间隙固溶体的溶解度与溶质原子的大小有关,还与溶剂晶 体结构中间隙的形状和大小等因素有关。
• C溶于铁形成间隙固溶体。 • C在α-Fe中的溶解度为0.02%,而在γ-Fe 中溶解度却为2.11%。 • γ-Fe为面心立方结构具有较大的八面体间 隙(ri=0.535nm) • α-Fe为体心立方结构,具有较大的四面 体间隙(ri=0.364nm)
材料科学基础双语课件
grain structure, or the presence of defects in the atom
packing) or to fabricate the material into the desired shape.
extra material, joining parts (e.g., by soldering or welding),
forming (forging, rolling, bending, etc.), or compacting particles which are then fused together (sintering, used for
gases (and most engineering materials are used in solid
form).
1.1 What is Materials Science and Engineering?
It may seem abstract and remote from real engineering to
The Science and Engineering of Materials
Aim
English atmosphere: speaking, reading, writing and lisห้องสมุดไป่ตู้ening; Specialty vocabulary; Specialty knowledge;
form. As this mixture solidifies, different structures form as a function of temperature. The phase diagrams that provide
材料科学基础英文版课件(PDF)
Law • Steady State: the concentration profile doesn't
change with time.
Steady State:
J x(left)
J x(right) J x(left) = J x(right)
x
Concentration, C, in the box doesn’t change w/time.
Non Steady State Diffusion
• Concentration profile,
dx
C(x), changes with time. J (left)
J (right)
• To conserve matter:
J (right)
− J (left)
=
dC −
dx
dt
dJ = − dC
ΔJ y
=
− ∂J y ∂y
dxdydzδt
ΔJ z
= − ∂J z ∂z
dxdydzδt
对整个元体积:
−
⎜⎜⎝⎛
∂J x ∂x
+
∂J y ∂y
+
∂J z ∂z
⎟⎟⎠⎞dxdydzδt
若 δt 时间内粒子浓度变化δc ,则在dxdydz
元体积中粒子变化为
δcdxdydz
∴ ∂c ∂t
=
−⎜⎜⎝⎛
∂J x ∂x
Fick’s Second Law
δt 时间内沿x方向扩散
元体积dxdydz
流入的粒子数: J x dydzδt
流出的粒子数:
(J x
+
∂J x ∂x
dx)dydzδt
[课件]材料科学基础 第三章晶体缺陷PPT
2018/12/13
《材料科学基础》CAI课件-李克
11
b. 螺型位错 screw dislocation
位错线bb’:已滑移区和未滑移区的边界线
特征:
1)无额外半原子面, 原子错排是轴对称的 2)分左螺旋位错,符合左手法则;右螺旋位错 ,符合右手法则 3)位错线与滑移矢量平行,且为直线,位错线的运动方向与滑移矢量垂直 4)凡是以螺型位错线为晶带轴的晶带 所有晶面都可以为滑移面。 5) 点阵畸变引起平行于位错线的切应变,无正应变。 6)螺型位错是包含几个原子宽度的线缺陷。
2018/12/13 《材料科学基础》CAI课件-李克 9
3.2.1 位错的基本类型和特征
根据几何结构特征: a. 刃型位错 edge dislocation
b. 螺型位错 screw dislocation
2018/12/13
《材料科学基础》CAI课件-李克
10
a. 刃型位错 edge dislocation
材料科学基础 第三章_晶体缺 陷
第三章 晶体缺陷
Imperfections (defects) in Crystals
It is the defects that makes materials so interesting, just like the human being.
Defects are at the heart of materials science.
1、点缺陷的形成 (production of point defects)
原因:热运动:热振动强度是温度的函数 能量起伏=〉原子脱离原来的平衡位置而迁移别处 Schottky 空位,-〉晶体表面 =〉空位(vacancy)
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1
What will it happen with temperature Nanjing University of Technology
(a)(b)(c)(d)(e)Nanjing University of Technology
Crystal
Glass
arranged in different patterns
Nanjing University of Technology
V & Q
T m
V & Q changes associated with heating and cooling in systems susceptible to glass formation.
: melting temperature
It shows linear relation
Nanjing University of Technology13
melt
Cooling
down
=
TΔ
T
Nucleation
f(x,T) = exp(-x/T) function
Nanjing University of Technology
W(r
Metastable zone of undercooling: no stable nuclei form at these
T m shown above.
Kinetic Considerations
forth across the liquid/solid interface:
Time –Temperature –Transformation Curves (TTT)
Most favorable conditions for crystallization (and so least
Note: for smaller transformation volumes, t
Nanjing University of Technology67
Nanjing University of Technology
Structural Approach to Glass Formation Hence, local structure is disordered, but there
Nanjing University of Technology
form loose network. structures.
Pauling's packing rule: satisfies Zachariasen's
•
All Rules are Satisfied: SiO
Pauling's packing rule:•violates Zachariasen's rule #2.•
Rules are Not Satisfied: Na
modifying ions
Nonbridging Oxygens
glass forming ions Al O does not form a glass.
octahedral CN preferred in Al2O3 violates Zachariasen's rule #1.
aluminophosphate
Nanjing University of Technology Nanjing University of Technology
Nanjing University of Technology Nanjing University of Technology98
Glasses
Alkalis are structural modifiers:
Every alkali ion creates one
Every alkali oxide 'molecule'
Nanjing University of Technology103
Effects of Alkaline earth oxides
Y↓, O
b ↓, O
nb
↑, structure “open”, ion easy to transfer,
TEC ↑, σ↑, η↓
•T
g (B
2
O
3
) ~260ºC (2D-network)
•T
g (SiO
2
) ~1200ºC (3D-network)
What happens when alkali oxide is added to a borate glass?
added Fewer network bridging。
