最新工程材料科学与设计原书第2版课后习题答案4—8章
《材料科学基础》第二版 (张联盟 著)课后习题答案 武汉理工大学出版社

2-30 石棉矿如透闪石Ca2Mg5[Si4O11](OH)2具有纤维状结晶习性,而滑石Mg3[Si4O10](OH)2却具 有片状结晶习性,试解释之。
2-21 (1)画出O2-作面心立方堆积时,各四面体空隙和八面体空隙的所在位置(以一个晶胞为结构基元 表示出来);(2)计算四面体空隙数、八而休空隙数与O2-数之比
2-22 根据电价规则,在下面情况下,空隙内各需填入何种价数的阳离子,并对每一种结构举出—个例子。 (1)所有四面体空隙位置均填满;(2)所有八面体空隙位置均填满;(3)填满—半四面体空隙位置; (4)填满—半八面体空隙位置。
2-3 在立方晶系晶胞中画出下列晶面指数和晶向指数:(001)与[ ],(111)与[ 与[111],( )与[236],(257)与[ ],(123)与[ ],(102),[
2-4 定性描述晶体结构的参量有哪些?定量描述晶体结构的参量又有哪些?
答: 最紧密堆积原理是建立在质点的电子云分布呈球形对称以及无方向性的基础上的,故只适用于典型的 离子晶体和金属晶体,而不能用最密堆积原理来衡量原子晶体的稳定性。另外,金刚石的单键个数为4,即 每个原子周围有4个单键(或原子),由四面体以共顶方式共价结合形成三维空间结构,所以,虽然金刚石 结构的空间利用率很低(只有34.01%),但是它也很稳定。
答: 定性:对称轴、对称中心、晶系、点阵。定量:晶胞参数。 2-5 依据结合力的本质不同,晶体中的键合作用分为哪几类?其特点是什么?
工程材料第二版习题(1-2)章答案

塑性变形的的物理本质: 塑性变形的的物理本质: 滑移和孪生共同产生的塑性变形。 滑移和孪生共同产生的塑性变形。 P24 滑移是晶体的一部分相对另一部分做整 体刚性移动。孪生是在切应力的作用下, 体刚性移动。孪生是在切应力的作用下,晶 体的一部分相对另一部分沿着一定的晶面 孪生面) (孪生面)产生一定角度的切变
2-13、晶粒大小对金属性能有何影响?细化 13、晶粒大小对金属性能有何影响? 晶粒方法有哪些? 晶粒方法有哪些? p17 答: 在一般情况下,晶粒愈小,则金属的强度. 在一般情况下,晶粒愈小,则金属的强度.塑 性和韧性愈好. 性和韧性愈好. 细化晶粒是提高金属性能的重要途径之一, 细化晶粒是提高金属性能的重要途径之一, 晶粒愈细,强度和硬度愈高, 晶粒愈细,强度和硬度愈高,同时塑性韧性 愈好。 愈好。 细化晶粒方法有: 细化晶粒方法有: 增大过冷度; 2.变质处理 变质处理; 3.附加振 增大过冷度; 2.变质处理; 3.附加振 动或搅动等方法; 动或搅动等方法;
5、晶粒 p11 晶粒---每个小晶体具有不规则的颗粒状外形。 ---每个小晶体具有不规则的颗粒状外形 晶粒---每个小晶体具有不规则的颗粒状外形。 何谓空间点阵、晶格、晶体结构和晶胞? 2-2、何谓空间点阵、晶格、晶体结构和晶胞? 常用金属的晶体结构是什么?划出其晶胞, 常用金属的晶体结构是什么?划出其晶胞, 并分别计算起原子半径、配位数和致密度? 并分别计算起原子半径、配位数和致密度? 1、空间点阵 p9 空间点阵-----为了便于分析各种晶体中的原子 空间点阵---为了便于分析各种晶体中的原子 排列及几何形状, 排列及几何形状,通常把晶体中的原子假想为 几何结点,并用直线从其中心连接起来,使之 几何结点,并用直线从其中心连接起来, 构成一个空间格子。 构成一个空间格子。
(完整版)工程材料课后习题参考答案

工程材料第一章金属的晶体结构与结晶1.解释下列名词点缺陷:原子排列不规则的区域在空间三个方向尺寸都很小,主要指空位间隙原子、置换原子等。
线缺陷:原子排列的不规则区域在空间一个方向上的尺寸很大,而在其余两个方向上的尺寸很小。
如位错。
面缺陷:原子排列不规则的区域在空间两个方向上的尺寸很大,而另一方向上的尺寸很小。
如晶界和亚晶界。
亚晶粒:在多晶体的每一个晶粒内,晶格位向也并非完全一致,而是存在着许多尺寸很小、位向差很小的小晶块,它们相互镶嵌而成晶粒,称亚晶粒。
亚晶界:两相邻亚晶粒间的边界称为亚晶界。
刃型位错:位错可认为是晶格中一部分晶体相对于另一部分晶体的局部滑移而造成。
滑移部分与未滑移部分的交界线即为位错线。
如果相对滑移的结果上半部分多出一半原子面,多余半原子面的边缘好像插入晶体中的一把刀的刃口,故称“刃型位错”。
单晶体:如果一块晶体,其内部的晶格位向完全一致,则称这块晶体为单晶体。
多晶体:由多种晶粒组成的晶体结构称为“多晶体”。
过冷度:实际结晶温度与理论结晶温度之差称为过冷度。
自发形核:在一定条件下,从液态金属中直接产生,原子呈规则排列的结晶核心。
非自发形核:是液态金属依附在一些未溶颗粒表面所形成的晶核。
变质处理:在液态金属结晶前,特意加入某些难熔固态颗粒,造成大量可以成为非自发晶核的固态质点,使结晶时的晶核数目大大增加,从而提高了形核率,细化晶粒,这种处理方法即为变质处理。
变质剂:在浇注前所加入的难熔杂质称为变质剂。
2.常见的金属晶体结构有哪几种?α-Fe 、γ- Fe 、Al 、Cu 、Ni 、Pb 、Cr 、V 、Mg、Zn 各属何种晶体结构?答:常见金属晶体结构:体心立方晶格、面心立方晶格、密排六方晶格;α-Fe、Cr、V属于体心立方晶格;γ-Fe 、Al、Cu、Ni、Pb属于面心立方晶格;Mg、Zn属于密排六方晶格;3.配位数和致密度可以用来说明哪些问题?答:用来说明晶体中原子排列的紧密程度。
材料科学基础课后习题答案(部分)_第2版_西安交通大学_石德珂主编演示教学

CV
A exp(
EV kT
)
每个原子的质量是:
107 .9 g / mol 6.02 10 23 个 / mol
1.79 10 22 g / 个
1cm 3的原子数为:
9.58 g / cm 3 1.79 10 22 g / 个
5.35 10 22 个
2.N N0 6.021023 9.58106 5.341028 / m3
M
107.9
ne exp
N
kT
kT ln ne
N
1.381023 1073ln
3.6 1023 5.34 1028
1.761019
3.自己看
4.不用看
5. (1)1点为正刃位错,2点为右螺位错, 3 点为负刃位错,4点为左螺位错。
1 3 . V m 6 .0 2 ( 1 3 0 5 2 .3 4 5 3 ( 0 .2 2 7 2 8 .9 9 2 )) 3 4 1 0 2 1 2 .2 6 g /c m 3
14-17不用看 18自己看
第四章
1.8500C:C1Aexp(EV kT1)L200C:C2Aexp(EV kT2) C C1 2 expkEV(T12T 11)exp11..3581100 1283(21 9311123) exp274
7.在两根位错线上12,34为刃位错,其余 为螺位错。
(2)OS上的各段位错都可在该滑移面内 滑移,O’S’上的12,34位错不能运动, 其余各段都可在该滑移面内滑移。
8.(1)AB和CD位错线的形状都不变, 但AB的长度缩短b2,CD的长度增加b1
(2)AB位错上形成右螺型扭折,EF上 形成左螺型扭折。
工程材料徐自立主编课后习题答案

工程材料徐自立主编课后习题答案第一章材料的性能1-1什么是金属材料的力学性能?金属材料的力学性能包含哪些方面?所谓力学性能,是指材料抵抗外力作用所显示的性能。
力学性能包括强度刚度硬度塑性韧性和疲劳强度等1-2什么是强度?在拉伸试验中衡量金属强度的主要指标有哪些?他们在工程应用上有什么意义?强度是指材料在外力作用下,抵抗变形或断裂的能力。
在拉伸试验中衡量金属强度的主要指标有屈服强度和抗拉强度。
屈服强度的意义在于:在一般机械零件在发生少量塑性变形后,零件精度降低或其它零件的相对配合受到影响而造成失效,所以屈服强度就成为零件设计时的主要依据之一。
抗拉强度的意义在于:抗拉强度是表示材料抵抗大量均匀塑性变形的能力。
脆性材料在拉伸过程中,一般不产生颈缩现象,因此,抗拉强度就是材料的断裂强度,它表示材料抵抗断裂的能力。
抗拉强度是零件设计时的重要依据之一。
1-3什么是塑性?在拉伸试验中衡量塑性的指标有哪些?塑性是指材料在载荷作用下发生永久变形而又不破坏其完整性的能力。
拉伸试验中衡量塑性的指标有延伸率和断面收缩率。
1-4什么是硬度?指出测定金属硬度的常用方法和各自的优缺点。
硬度是指材料局部抵抗硬物压入其表面的能力。
生产中测定硬度最常用的方法有是压入法,应用较多的布氏硬度洛氏硬度和维氏硬度等试验方法。
布氏硬度试验法的优点:因压痕面积较大,能反映出较大范围内被测试材料的平均硬度,股实验结果较精确,特别适用于测定灰铸铁轴承合金等具有粗大经理或组成相得金属材料的硬度;压痕较大的另一个优点是试验数据稳定,重复性强。
其缺点是对不同材料需要换不同直径的压头和改变试验力,压痕直径的测量也比较麻烦;因压痕大,不宜测试成品和薄片金属的硬度。
洛氏硬度试验法的优点是:操作循序简便,硬度值可直接读出;压痕较小,可在工件上进行试验;采用不同标尺可测定各种软硬不同的金属厚薄不一的式样的硬度,因而广泛用于热处理质量检验。
其缺点是:因压痕较小,对组织比较粗大且不均匀的材料,测得的结果不够准确;此外,用不同标尺测得的硬度值彼此没有联系,不能直接进行比较。
工程材料第四章习题答案

工程材料作业(4)答案1.解释下列现象:(1) 在相同含碳量下,除了含Ni和Mn的合金钢外,大多数合金钢的热处理加热温度都比碳钢高。
奥氏体形成分为形核、长大、残余渗碳体溶解,奥氏体均匀化4阶段。
多数合金元素减缓A形成,Cr、Mo、W、V等强碳化物形成元素与碳亲和力大,形成的合金元素的碳化物稳定、难溶解,会显著减慢碳及合金元素的扩散速度。
但为了充分发挥合金元素的作用,又必须使其更多的溶入奥氏体中,合金钢往往需要比含碳量相同的碳钢加热到更高的温度,保温更长时间。
Co、Ni等部分非碳化物形成元素,因增大碳的扩散速度,使奥氏体的形成速度加快。
而Al、Si、Mn等合金元素对奥氏体形成速度的影响不大。
阻碍晶粒长大,合金钢需要更高的加热温度,更长的保温时间,才能保证奥氏体均匀化。
(加热温度升高了,但一般不会引起晶粒粗大:大多数合金元素都有阻碍奥氏体晶粒长大的作用。
碳化物形成元素的作用最明显,因其形成的碳化物高温下稳定性高,很难完全溶入奥氏体,未溶的细小碳化物颗粒,分布在奥氏体晶界上,有效的阻止晶粒长大,起到细化晶粒的作用。
所以,合金钢虽然热处理加热温度高,但一般不用担心晶粒粗大。
强烈阻碍晶粒长大的元素:V、Ti、Nb、Zr;中等阻碍的:W、Mo、Cr;影响不大的:Si、Ni、Cu;促进晶粒长大的:Mn、P、B)(2) 在相同含碳量下,含碳化物形成元素的合金钢比碳钢具有较高的回火稳定性。
回火过程一般分为:马氏体分解、残余奥氏体转变、碳化物类型转变和碳化物长大。
合金元素在回火过程中,推迟马氏体的分解和残余奥氏体的转变(即在较高温度才出现分解和转变),提高铁素体的再结晶温度,使碳化物难以聚集长大而保持较大的弥散度。
因此,提高了钢对回火软化的抗力,即提高了钢的回火稳定性。
使得合金钢在相同温度下回火时,比同样质量分数的碳钢具有更高的硬度和强度(对工具钢,耐热钢更重要),或在保证相同强度的条件下,可在更高的温度下回火,而韧性更好(对结构钢更重要。
材料科学基础习题第四章答案与翻译
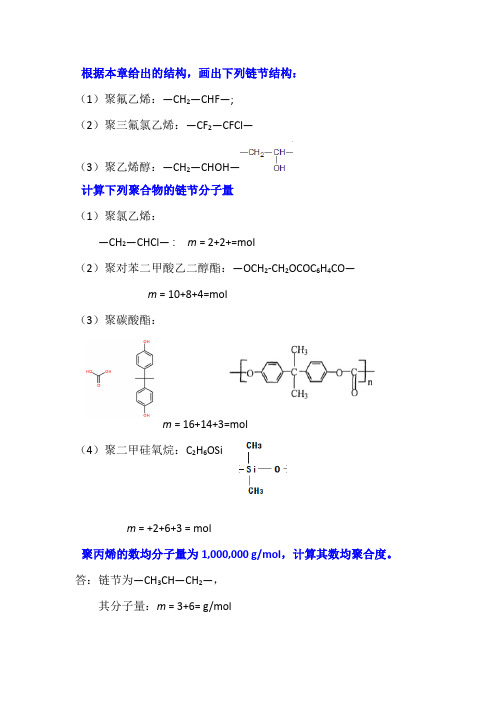
根据本章给出的结构,画出下列链节结构:(1)聚氟乙烯:—CH2—CHF—;(2)聚三氟氯乙烯:—CF2—CFCl—(3)聚乙烯醇:—CH2—CHOH—计算下列聚合物的链节分子量(1)聚氯乙烯:—CH2—CHCl— : m = 2+2+=mol(2)聚对苯二甲酸乙二醇酯:—OCH2-CH2OCOC6H4CO—m = 10+8+4=mol(3)聚碳酸酯:m = 16+14+3=mol(4)聚二甲硅氧烷:C2H6OSim = +2+6+3 = mol聚丙烯的数均分子量为1,000,000 g/mol,计算其数均聚合度。
答:链节为—CH3CH—CH2—,其分子量:m = 3+6= g/mol(a) 计算聚苯乙烯链节的分子量答:链节为CHC6H5CH2,分子量:m = 8+8=(b) 计算重均聚合度为25000的聚苯乙烯的重均分子量答:= 25000 g/mol = 2603800 g/mol下表列出了聚丙烯的分子量,计算(a) 数均分子量(b) 重均分子量(c) 数均聚合度(d) 重均聚合度x i w i 分子量分布(g/mol)8,00016,00016,00024,00024,00032,00032,00040,00040,00048,00048,00056,000答:(a)= 12000+20000+28000+36000+44000+52000 = 600+3200+6720+10080+8800+3640 = 33040 (g/mol)(b)= 12000+20000+28000+36000+44000+52000 = 240+2000+5600+10800+11880+10920 = 41440 (g/mol)(c)聚丙烯链节的分子量:m = g/mol(d)下表列出了某聚合物的分子量分布。
计算(a) 数均分子量(b) 重均分子量(c) 如果已知这一聚合物的重均聚合度为780,指出此聚合物为表所列聚合物中的哪一个为什么(d) 这一材料的数均聚合度为多少分子量分布(g/mol)x i w i15,00030,00030,00045,00045,00060,00060,00075,00075,00090,00090,000105,000105,000120,000120,000135,000答:(a)= 22500+37500+52500+67500+82500+97500+112500+127500 = 900+2625+8400+17550+19800+11700+9000+3825 = 73800 (g/mol)(b)= 22500+37500+52500+67500+82500+97500+112500+127500 = 225+1500+5775+16200+22275+15600+13500+ 6375 = 81450 (g/mol)(c)此聚合物为聚苯乙烯根据下面的分子量分布和重均聚合度为585的条件,判断是否为聚甲基丙烯酸甲酯均聚物分子量分布(g/mol)x i w i8,00020,00020,00032,00032,00044,00044,00056,00056,00068,00068,00080,00080,00092,000答:聚甲基丙烯酸甲酯链节分子式为:C5H8O2(—CH2CH3COOCH3C—);其分子量m = 5+8+2=mol重均分子量为:=14000+26000+38000+50000+62000+74000+86000=140+1300+4560+12500+16740+15540+7740=58520与条件相符,能形成均聚物高密度聚乙烯通过诱导氯原子随机取代氢而被氯化。
最新土木工程材料第二版(湖南大学、天津大学、同济大学、东南大学_合编)课后习题答案

土木工程材料第二版课后习题答案土木工程材料的基本性第一章(1)当某一建筑材料的孔隙率增大时,材料的密度、表观密度、强度、吸水率、搞冻性及导热性是下降、上生还是不变?(2)材料的密度、近似密度、表观密度、零积密度有何差别?答:(3)材料的孔隙率和空隙率的含义如何?如何测定?了解它们有何意义?答:P指材料体积内,孔隙体积所占的百分比:P′指材料在散粒堆积体积中,颗粒之间的空隙体积所占的百分比:了解它们的意义为:在土木工程设计、施工中,正确地使用材料,掌握工程质量。
(4)亲水性材料与憎水性材料是怎样区分的?举例说明怎样改变材料的变水性与憎水性?答:材料与水接触时能被水润湿的性质称为亲水性材料;材料与水接触时不能被水润湿的性质称为憎水性材料。
例如:塑料可制成有许多小而连通的孔隙,使其具有亲水性。
例如:钢筋混凝土屋面可涂抹、覆盖、粘贴憎水性材料,使其具有憎水性。
(5)普通粘土砖进行搞压实验,浸水饱和后的破坏荷载为183KN,干燥状态的破坏荷载为207KN(受压面积为115mmX120mm),问此砖是否宜用于建筑物中常与水接触的部位?答:(6)塑性材料和塑性材料在外国作用下,其变形性能有何改变?答:塑性材料在外力作用下,能产生变形,并保持变形后的尺寸且不产生裂缝;脆性材料在外力作用下,当外力达到一定限度后,突然破坏,无明显的塑性变形。
(7)材料的耐久性应包括哪些内容?答:材料在满足力学性能的基础上,还包括具有抵抗物理、化学、生物和老化的作用,以保证建筑物经久耐用和减少维修费用。
(8)建筑物的屋面、外墙、甚而所使用的材料各应具备哪些性质?答:建筑物的屋面材料应具有良好的防水性及隔热性能;外墙材料应具有良好的耐外性、抗风化性及一定的装饰性;而基础所用材料应具有足够的强度及良好的耐水性。
第1章天然石材(1)岩石按成因可分为哪几类?举例说明。
答:可分为三大类:1)岩浆岩,也称火成岩,是由地壳内的岩浆冷凝而成,具有结晶构造而没有层理。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
工程材料科学与设计原书第2版课后习题答案4—8章仅供学习与交流,如有侵权请联系网站删除 谢谢166Solutions to Chapter 41.FIND: What material has a property that is hugely affected by a small impurity level? SOLUTION: Electrical conductivity spans a wide range. Incorporation of a few parts per million impurities can change electrical conductivity orders of magnitude. Small cracks in brittle materials decrease their tensile strength by orders of magnitude. Small additions of impurity can change the color of gems.COMMENTS: These are but a few examples.2.COMPUTE: The temperature at which the vacancy concentration is one half that of 25o C.GIVEN: C 2 = C C 25v C 35vo oEQUATION:⎪⎪⎭⎫⎝⎛RT Q - = C fv v expwhere C v = vacancy concentrationQ fv = activation energy for vacancy information R = gas constant 8.314 J/mole-KT = absolute temperatureIn the present problem C)25(C = C C);35(C = C o v 2v o v 1vand T 1 = 35 + 273 = 308KT 2 = 25 + 273 = 298Kalso C v(35o C) = 2C v(25o C) 仅供学习与交流,如有侵权请联系网站删除谢谢166仅供学习与交流,如有侵权请联系网站删除 谢谢166Thus, Solving for Q fv we get Q fv = 52893.5 J/mole.Using this value of Q fv , the C v (25o C) can be calculatedThe problem requires us to calculate the temperature at which the vacancy concentration is ½ C v (25o C).½ C v (25o C) = 2.675 x 10-10Thusfor solving T, we get: T = 288.63K or 15.63o C. 3.COMPUTE: C)80( C 3 = (T) C o v vGIVEN: C) 80( C 41 = C) 25( C o v ovEQUATION:⎪⎭⎫⎝⎛298.R Q - C) 25( C Sv o v expDividing (1) by (2) we get:仅供学习与交流,如有侵权请联系网站删除 谢谢166Solving for Q, we get: Q = 22033.56 J/mole= exp(-7.511) = 5.46 x 10-4The problem requires computing a temperature at which C v = 3C v (80o C).3C v (80o C) = 3 x 5.46 x 10-4= 1.63 x 10-3⎪⎭⎫⎝⎛T x 8.3122033.56- = 10 x 1.633-ex psolving for T, we get: T = 413.05K or 140.05o C4. 5. FIND: Are Al and Zn completely soluble in solid solution?If Al-Zn system obeys all the Hume-Rothery rules. Then it is expected to show complete solubility.(i) The atomic radii of Al and Zn are 0.143nm and 0.133 nm respectively. The difference in their radii is 7.5% which is less than 15%.(ii) The electronegativities of Al and Zn are 1.61 an 1.65 respectively which are also very similar.(iii) The most common valence of Al is +3 and +2 for Zn.(iv) Al has an FCC structure where Zn has a HCP structure.It appears that Al-Zn system obeys 3 out of 4 Hume-Rothery rules. In this case they are not expected to be completely soluble.6. SHOW: The extent of solid solution formation in the following systems using Hume-Rothery Rules.(a) Al in NiSize: r(Ni) = 0.125nm; r(Al) = 0.143nm difference = 14.4%Electronegativity: Al = 1.61; Ni = 1.91Most Common Valence: Al3+; Ni2+Crystal Structure: Al: FCC; Ni:FCCThe crystal structure of Al and Ni are the same and the most common valencies are also comparable. However, the size difference is close to 15% and the difference iselectronegativities is rather significant.Based on this, it appears that Ni and Al would not form a solid solution over the entirecompositional range.(b) Ti in NiSize: r(Ti) = 0.147 nm, r(Ni) = 0.125nm difference = 17.6%Electronegativity: Ti: 1.54; Ni: 1.91Valence: Ti4+; Ni2+Crystal Structure: Ti:HCP; Ni FCCTi in Ni would not exhibit extensive solid solubility(c) Zn in FeSize r(Zn) = 0.133nm; r(Fe) - 0.124nm difference = 7.25%Electronegativity: Zn = 1.65; Fe = 1.83Most Common Valence: Zn2+; Fe2+Crystal Structure: An: HCP; Fe: BCCSince electronegativities and crystal structures are very different, Zn - Fe will not exhibit extensive solid solubility.(d) Si in AlSize r(Si) = 0.117 nm; r(Al) = 0.143nm; difference = 22.2%Electronegativity: (Si) = 1.90; Al = 1.61Valence: Si4+; A;3+Crystal Structure: Si: Diamond Cubic; Al: FCCSince the size difference is greater than 15%, and the crystal structures are different, Si-Al would not exhibit extensive solid solubility.仅供学习与交流,如有侵权请联系网站删除谢谢166(e) Li in AlSize r(li): 0.152, r(Al): 0.143; difference - 6.29%Electronegativity: Li: 0.98; Al: 1.61Most Common Valence: Li1+; Al3+Crystal Structure: Li:BCC; Al: FCCSince electronegativity and crystal structures are very different, Li-Al will not exhibitextensive solid solubility.(f) Cu in AuSize r(Cu) = 0.125nm; r(au) = 0.144nm; difference = 12.5%Electronegativity: Cu = 1.90; Au = 1.93Most Common Valence: Cu+; Au+Crystal Structure: Cu:FCC; Au:FCCCu-Au will exhibit extensive solid solubility.(g) Mn in FeSize r(Mn) = 0.112, r(Fe) = 0.124 difference = 10.71%Electronegativity: Mn 1.55; Fe 1.83Most Common Valence: Mn2+; Fe2+Crystal Structure: Mn:BCC; Fe BCCThe difference in electronegativity is high but Mn-Fe does obey the other 3 Hume-Rothery rules. Therefore, it will form solid solutions but not over the entirecompositional range.(h) Cr in FeSize r(Cr) = 0.125nm, Fe = 0.144nm difference = 12.5%Electronegativity: Cr = 1.66; Fe = 1.83Most Common Valence: Cr3+; Fe2+Crystal Structure: Cr:BCC; Fe:BCCCr in Fe will exhibit extensive solid solubility but not over the entire compositional range since it obeys only 3 of 4 Hume-Rothery rules.(i) Ni in FeSize r(Ni) = 0.125nm, r(Fe) = 0.124nm difference = 0.8%Electronegativity: Ni: 1.91; Fe 1.83Most Common Valence: Ni3+; Fe3+仅供学习与交流,如有侵权请联系网站删除谢谢166Crystal Structure: Ni:FCC; Fe: BCCNi and Fe obeys 3 of the 4 Hume-Rothery rules therefore, extensive solid solution will be exhibited but not over the entire compositional range.7. (a) When one attempts to add a small amount of Ni to Cu, Ni is the solute and Cu is thesolvent.(b) Based on the relative sizes of Ni and Cu, radius of Ni = 0.128nm, radius of Cu =0.125nm, these two are expected to form substitutional solid solutions.(c) Ni and Cu will be completely soluble in each other because they obey all four Hume-Rothery rules.8. FIND: Predict how Cu dissolves in Al.DATA: Cu Alatomic radius (A) 1.28 1.43electronegativity 1.90 1.61valence 1+,2+ 3+crystal structure FCC FCCSOLUTION: All of Hume-Rothery's rules must be followed for a substitutional solution.In this case, the valences do not match. Cu will not go into substitutional positions in Al toa large extent.COMMENTS: This principle is often used to precipitation harden Al using Cu.9. What type of solid solution is expected to form when C is added to Fe?The radius of carbon atom is 0.077nm and that of an Fe atom is 0.124nm. The sizedifference between these two is ~61% which is much grater than ~15%. Thus, these two are not expected to form substitutional solid solution.If we compare the size ratio of C to Fe atoms with the size of tetrahedral and octahedral interstitial sites in BCC iron, we find that C does not easily fit into either type ofinterstitial position. C, however, forms an interstitial solid solution with Fe but thesolubility is limited.10. FIND: Calculate the activation for vacancy formation in Fe.GIVEN: The vacancy concentration at 727 C = 1000K is 0.00022.SOLUTION: We use equation 4.2-2 to solve this problem:C v = exp (-Q fv/RT)Solving for Q fv:Q fv = -RT ln C v = -(8.31 J/mole-K)(1000K) ln 0.00022 = 7.0 x 104 J/mole仅供学习与交流,如有侵权请联系网站删除谢谢16611. SHOW: A Schottky and Frenkel defect in MgF2 structuresA 2-D representation of the MgF2 structure containing a Schottky defect and a Frenkeldefect is shown below.12. Explain why the following statement is incorrect: In ionic solids the number of cationvacancies is equal to the number of anion vacancies.In ionic crystals, even in the presence of vacancies, the charge neutrality must bemaintained. Therefore, single vacancies do not occur in ionic crystals since removal of a single ion would lead to charge imbalance. Instead the vacancies occur in a manner such that the anion: cation vacancy ratio render the solid electrically neutral. This, however, does not mean that the anion vacancies are equal to cation vacancies. For example, aSchottky defect in MgCl2 or MgF2 involves two Cl- or F- cation vacancies for every Mg2+ anion vacancy to maintain electrical neutrality.The number of cation vacancies equals the number of anion vacancies only for thelimiting case where the chemical formula of the compound is MX.13. Calculate the number of defects created when 2 moles of NiO are added to 98 moles ofSiO2. Also, determine the type of defect created.GIVEN: Neglect interstial vacanciesWe have 2 moles of NiO and 98 moles of SiO2. Since NiO is a 1:1 compound there are 2 moles of Ni2+ ions and 2 moles of O2- ions present. SiO2 on the other hand is a 1:2compound; therefore, there are 98 moles of Si4+ and 196 moles of O2-. The total number of each type of ion isN Ni = 2 molesN Si = 98 molesN O2 = 196 molesThe total number of moles of ions in the system isN T = N Ni + N Si + N O = 2 + 98 + 196 = 196 molesEach substitution of an Ni2+ for Si4+ results in a loss of 2 positive charges. If nointerstitials are created, this loss of positive charge is balanced by the creation of anion 仅供学习与交流,如有侵权请联系网站删除谢谢166vacancies. Charge neutrality requires one oxygen vacancy created for every Ni2+ ion.Therefore, the number of oxygen vacancies isN Ov = N Ni = 2 molesThere are 2 moles of oxygen ion vacancies created with the addition of 2 moles of NiO to98 moles of SiO2.14. Calculate the number of defects created when 1 mole of MgO is added to 99 moles ofAl2O3.MgO is a 1:1 compound, therefore there is 1 mole of Mg2+ ions and 1 mole of O2- ions in the system.From Al2O3, there are 198 moles of Al3+ ions and 297 moles of O2- ions in the system.Each substitution of an Mg2+ ion for Al3+ ion results in a loss of one positive charge. This loss of positive charge is balanced by oxygen vacancy. Charge neutrality requires oneoxygen vacancy to be created for every two Mg2+ ion3. Therefore the number of oxygenion vacancies created is0.5 moles of oxygen ion vacancies are created by the addition of 1 mole of MgO to 99moles of Al2O3.15. COMPUTE: Relative concentration of cation vacancies, anion vacancies and cationinterstitials.GIVEN:Q Cv = 20kJ/moleQ Av = 40kJ/moleQ CI = 30kJ/moleASSUMPTION: assume room temperatureT = 298KConcentration of cation vacancies, C Cv is given by仅供学习与交流,如有侵权请联系网站删除谢谢166仅供学习与交流,如有侵权请联系网站删除 谢谢166Similarly for anion vacancies and for cation interstitials 16.(a) Describe a Schottky defect in U 2(b) Would you expect to find more cation or anion Frenkel defects in this compound? Why?UO 2 has a fluorite structure with U 4+ ions occupying FCC lattice sites and O 2- occupying tetrahedral interstitial sites.(a) A Schottky defect in UO 2 will involve one U 4+ cation vacancy and 2 O 2- anion vacancies.(b) In general cation Frenkel defects are more common than anion Frenkel defects because cations are usually smaller. In this case, the radii of U 4+ is 0.106nm and that of O 2- is 0.132nm. The U 4+ cation is smaller than the O 2- anion. However, the size difference is not very high. Still, cation Frenkel defects are expected to be more. 17.Ionic compound Li2O(a) Describe a Schottky defect (b) Describe a Frenkel defectLi 2O has an antifluorite structure. O 2- ions occupy FCC lattice sites and Li + occupies tetrahedral interstitial sites.(a) A Schottky defect in Li2O involves 2 Li2+ cation vacancies and one O2- anion vacancy(b) The ionic radii of Li+ and O2- are 0.078nm and 0.132nm respectively. This materialis most likely to exhibit cation Frenkel defect since the size of the cation is much smaller than the anion.18. DETERMINE:(a) Interstitial Na+ ions(b) Interstitial O2- ions(c) Vacant Na+ sites(d) Vacant O2- sites in Na2OGIVEN: r(Na+) = 0.098nmr(O2-) = 0.132nmNa2O structure is similar to antifluorite structure. Na+ ions occupy tetrahedral interstitial sites and O2- ions occupy FCC lattice sites.Since the ratio of Na:0 is 2:1 for this materials, a Schottky defect results in 2 cationvacancies for every one anion vacancy.no. of vacant Na+ sites = 2 x no. of vacant O2- sitesA cation Frenkel defect is more likely to occur in this material(a) Interstitial Na+ ions = 1(b) Interstitial O2- ions = 0(c) Vacant Na+ sites = 2(d) Vacant O2- sites = 119. SOLVENT: AuSOLUTE: N, Ag or CsDETERMINE: (a) which element is most likely to form an interstitial solid solution.(b) which element is most likely to form a substitutional solid solution.r(Au) = 0.144nmr(N) = 0.071nmr(Ag) = 0.144nmr(Cs) = 0.265nm(a) Based on atomic radii N is most likely to form are interstitial solid solution with Auas solvent.仅供学习与交流,如有侵权请联系网站删除谢谢166仅供学习与交流,如有侵权请联系网站删除 谢谢166(b) Ag is most likely to form a substitutional solid solution because the size difference between Au & N and Au & Cs is more than 15%.In addition, Au and Ag have similar valence, and crystal structure. Theelectronegativities are not quite similar, but since Ag-Au system obeys 3 out of 4 of the Hume-Rothery rules, Ag is the most likely element with which Au forms a substitutional solid solution. Section 4.4 Diffusion20. Under what condition can Fick’s first law be used to solve diffusion problems. The Fick’s first law can be used to solve diffusion problems provided the concentration gradient does not change with time.21.GIVEN: 1 wt% B is added to Fe.FIND: (a) if B would be present as an interstitial impurity or substitutional impurity, (b) fraction of sites occupied by B atoms, (c) if Fe containing B were to be gas carburized, would the process be faster or slower than for Fe which has no B? Explain.r(B) = 0.097nm r(Fe) = 0.124nm(a) Based on the atomic radii B would be present as an interstitial impurity(b) amount of B present = 1 wt%As a basis of calculation assume 100gms of material.Determine the no. of moles of Fe and B present.Total no. of moles of Fe and B = 1.773 + 0.092 = 1.865 moles.Fraction of sites occupied by B atoms = 1.8650.092= mole fraction of B = 0.049Thus, B roughly occupies 5% of the sites.(c) If Fe containing B were to be gas carburized the process would be slower than for Fewhich has no B simply because the presence of B atoms already in interstitial sites leave fewer sites for interstitial C to diffuse through.22. Determine which type of diffusion would be easier(a) C in HCP Ti(b) N in BCC Ti(c) Ti in BCC Tir(C) << r(Ti) so we can predict that diffusion occurs via an interstitial mechanismr(N)<<r(Ti). In this case the diffusion also occurs via interstitial mechanism.Ti in BCC Ti is a case of self-diffusion and self-diffusion occurs via a vacancymechanism. In general the activation energy for self diffusion is higher than interstitialmechanism because vacancy mechanism involves two steps. One is to create a vacancyand second is to promote a vacancy/atom exchange. Thus Ti in BCC Ti will be theslowest.The activation energy for diffusion via interstitial mechanism is just the energy necessary to move an atom into a neighboring interstitial site. An open crystal structure, as opposed to a dense structure, should have a lower activation energy. Between BCC Ti and HCPTi, BCC Ti has a more open structure (lower APF) than HCP Ti.Thus, N in BCC Ti diffusion would be the easiest by virtue of its lowest activation energy.23. GIVEN: C1 = 0.19 at % at surfaceC2 = 0.18 at % at 1.2mm below the surfaceD = 4 x 10-14 m2/seca o = 4.049 A oCOMPUTE: Flux of copper atoms from surface to interior.We must first calculate the concentration gradient in terms of [copper atoms/cm3/cm]. Itcan be calculated as follows:The concentration gradient is then仅供学习与交流,如有侵权请联系网站删除谢谢16624. FIND: Predict whether diffusion is faster in vitreous or crystalline silica.GIVEN: Diffusion is the movement of atoms through the material one step at a time.The ease of movement is in part determined by the amount of space that surrounds each atom. In more open or less dense structures, atoms have an increased chance of beingable to squeeze past a neighbor into a new position.SOLUTION: Diffusion can be thought of as an Arrhenius process. The activation energy is that required to move an atom from one position to another, as shown in Fig. 2.3-2. In a crystal the activation energy will be greater than in a glass, since the density is higher and there is less free, or unoccupied, volume. Thus, we expect diffusion to be slower in crystal than in glasses at the same temperature.COMMENTS: When a noncrystalline material is raised to a temperature above the glass transition temperature, diffusion increases enormously. In metals this brings about rapid crystallization. In some ceramic and polymer systems, crystallization may be slow or absent.25. FIND: Do textile dyes more readily penetrate crystalline or noncrystalline regions?GIVEN: Most textile fibers are semicrystalline, containing both crystalline andnoncrystalline regions. The density of the noncrystalline regions is less than that of the crystalline regions. Often dyeing is conducted at a temperature at which thenoncrystalline regions are above their glass transition temperature.SOLUTION: Dye penetration through the glass will be greater than that through thecrystal; however, the rate of dyeing is not sufficiently high to be commercially feasible.The temperature must be raised so that the noncrystalline polymer is in the rubber state.Diffusion becomes rapid (radially inward) into the small fibers.COMMENTS: One of the key lessons that dye houses learn is that a sufficient amount of noncrystalline poorly oriented polymer must be present in the fiber. The temperature of the dye bath needs to be above the glass transition temperature. Sometimes water and carriers 仅供学习与交流,如有侵权请联系网站删除谢谢166仅供学习与交流,如有侵权请联系网站删除 谢谢166are used to swell the noncrystalline regions to get yet a greater diffusion rate. The dyes may attach to the polymer using ionic bonds or covalent bonds. Unattached dye may wash out later. 26.CALCULATE: The factor by which the diffusion coefficient of Al in Al 2O 3 change when temperature is increased from 1800o C to 2000o C GIVEN: T 1 = 1800o C = 2073KT 2 = 2000o C = 2273KEQUATION: ⎪⎭⎫⎝⎛RT Q - D = D o ex pdividing (1) by (2), we getfrom table 4.4-1 of the text Q = 477kJ/mole and R = 8.31 J/mole-K仅供学习与交流,如有侵权请联系网站删除 谢谢166Thus, the diffusion coefficient of Al in Al 2O 3 changes by a factor of 11.43 when the temperature is increased from 1800o C to 2000o C. 27.FIND: Temperature at which a specimen of Fe must be carburized for two hours to achieve the same diffusion result as at 900o C for 15 hrs. GIVEN: T 1 = 900o C = 1173K; Q = 84000 J/molet 1 = 15 hrs; D o = 2.00 x 10-6 m 2/sec.t 2 = 2 hrs; R = 8.31 J/mole-KThe value of flux J is in units of cm 2 per sec.Flux per cm 2 J f = Jx time 3600 x 15 x dxdcD - = J 11f (1)We need the same result in 2 hours. ,J = J 2f 1f dividing (1) by (2).28. GIVEN: D = 4 x 10-4 m2/s @ 20o CC1 = 2.2 x 10-3 k mol/m3wall thickness = 3mm, diameter = 50cmheight = 10cmCOMPUTE: Initial rate of mass loss through cylinder.Initially the concentration of He outside the cylinder, C2, is zero.First, we need to convert the concentration of He from kmol/m3 into (atoms/cm3)/cm.C1 = 2.2 x 10-3 kmol/m3 = 2.2mol/m3 = 2.2 x 10-6 mol/cm3 = 2.2 x 10-6 mole/cm3In terms of (atoms/cm3)/cm仅供学习与交流,如有侵权请联系网站删除谢谢166仅供学习与交流,如有侵权请联系网站删除 谢谢166The concentration gradient isThe flux of atoms per second per cm 2 isobtained by using Fick’s first law of diffusionThe rate of mass loss is 1.766 x 1019 atoms/cm 2 sec. The total surface area of the cylinder is 2πr(r+h) where r = radius and h= height.Total surface area = 2π x 25 (25 + 10) = 5497.79 cm 2The rate of mass loss per secondNote:(i ) The steady state mass loss is calculated because the initial rate of mass loss (i.e., rate of mass loss at time t = 0) is 0. (ii ) It is assumed that the curvature of the cylinder is large enough to calculate J using the expression for plate geometry. 29. Diffusion across a polymer membrane depends not only on size of the diffusing species but also the polarity of the diffusing species. A polar membrane may pass nonpolar species but serve as a barrier to polar species.Saran wrap contains highly polar atoms making it a polar membrane which serves as a barrier to water which is a polar compound. thus, there is no diffusion of water through the package unlike polyethylene, which is a nonpolar membrane and allows diffusion of water molecules which form ice. 30.COMPUTE: Temperature required to yield a carbon content of 0.5% at a depth of 0.4mn below the surface of the rod in 48 hours. GIVEN: Carbon concentration the interior = 0.2w/oCarbon concentration in the furnace = 1.0w/o仅供学习与交流,如有侵权请联系网站删除 谢谢166Base material: HCP TiEQUATION: In this problem c(x, t) = 0.5wt%c o = 0.2 wt%c s = 1.0 wt%From figure 4.4-11, whenFrom Metals Handbook, Desk Edition, Pg. 28.66 for C diffusion in Ti, D o = 3.02 x 10-3 cm 2/sec, Q = 20,000 cal/mole = 83682 J/mole.31. The diffusion process through vacancy-interchange mechanism depends on creation ofvacancies and vacancy/atom interchange.At comparable homologous temperatures, for Ge and Cu the diffusion coefficient for that material which has a higher vacancy concentration would be higher.A covalent bond as opposed to a metallic bond is stronger and directional. It is alsodifficult to create vacancies in a covalently bonded material due to its strong bonding.Therefore, the activation energy for vacancy creation in a covalently bonded materialsuch as Ge is larger than Cu which has a weak metallic bond.The directional nature of a covalent bond places geometrical restrictions on the vacancy atom interchange which again results in an increase in the activation energy.Therefore, at comparable temperatures the diffusion coefficient for Ge will be larger. 32. FIND: Describe the energy and entropy in Fig. 4.4-5a, b, and c.SOLUTION: The order in part a is high. The materials is perfect. There is only one way to arrange the atoms in such a system. The entropy is low. In part b there is less order,仅供学习与交流,如有侵权请联系网站删除谢谢166仅供学习与交流,如有侵权请联系网站删除 谢谢166more disorder, and the entropy has increased. Part c is nearly random. It has low order andhigh entropy. Energy contains a contribution from entropy: E = H -TS, where E is energy,T is absolute temperature, and S is entropy. Assuming all other contributions to energychange negligibly (T and H), the energy of part c is the low, part a is high and part b isintermediate.COMMENTS: What is shown in going from a to c is the entropy of mixing.33. GIVEN: After 10 hrs at 550o C an oxide layer of thickness 8 μm is formed.COMPUTE: Thickness after 100 hrs.Using the definition of effective penetration distance and equation 4.4-11 of text, with γ = 2 we have Dt 2 x eff ≈.In this case34. GIVEN: D w = 1.0 x 10-12 m 2/s (water)D dc = 1.0 x 10 (dye carrier)D d = 1.0 x 10-14 m 2/s (dye)COMPUTE:(a) Times required for the water, dye and carrier to penetrate to the center of the fiber.(b) Same as (a) but fiber diameter doubles (c) If thermal diffusivity of PET is secm 10 x 828-how long will it take for the heat to penetrate to the center of a 50μm diameter fiber.(a) using equation 4.4-11 of text with γ = 2.for water,for dye carriersimilarly for dye t = 6.25secs.(b) If the diameter fiber is doubled x eff = 50 x 10-6 mfor water,仅供学习与交流,如有侵权请联系网站删除谢谢166仅供学习与交流,如有侵权请联系网站删除 谢谢166similarly for dye carriert = 6250 secsand for dyet = 6.25 x 104 secs(c) Substituting D with D th , we can use the same equation to calculate the time requiredfor heat to penetrate the center of fiber diameter = 50μm.Note: The units of thermal diffusivity is m 2/sec and notK - m Watt as printed in text35. FIND: How long will it take to case carburize a steel chain to a depth of 1/16 inch?GIVEN: It requires 4 hours to carburize a plate of similar composition to a depth of1/16 inch.ASSUMPTIONS: All carburization conditions are the same in both treatments.SOLUTION: Equation 4.4-11 is used to solve the problem:仅供学习与交流,如有侵权请联系网站删除 谢谢166x eff = γ(Dt)1/2.In this situation we set up a ratio for the plate (1) and the chain (2):x x Dt Dt 121122=γγ, where γ is 1 for the plate and 2 for the chain. Subscripts were omitted for the D's, since diffusion is the same in the two cases. Similarly, x 1 = x 2 = 1/16 inch. Reducing the equation gives:112411122212212=⇒= ☺= ☺=γγγγt t t t hrs hr36. GIVEN: D m = 1 x 10-11m 2/s for moisture through wool or cottond w = 25μmd c = 2μmusing equation 4.4-11 of textwe need to find t required to reach equilibriumWool Cotton仅供学习与交流,如有侵权请联系网站删除 谢谢166(b) for a tightly packedcubic bale of fiber with side length of 1 meter.A cube has six sides and diffusion is expected to occur through all six sides. The time required to reach equilibrium Dx = t 2eff Here x eff = 0.5mNote: This solution overestimates time. A more precise solution can be obtained bysetting up the problem in 3D and solving the Fick’s second law for a cube geometry.37.EXPLAIN: Why fizz reduction is higher in plastic bottles compared to glass bottles and metal cans.Plastic bottles are soft and can be squeezed. Upon squeezing the air inside the bottle isdriven out and to maintain the equilibrium pressure over the soda, CO 2 comes out of theliquid.Such is not the case for bottles and metal cans. It is for this reason, the fizz reduction ishigh in plastic bottles.38. Compare the diffusion coefficient of methane in rubber at 239K to the diffusion of Cu inAg at the same temperature.The diffusion coefficient of methane in rubber at 293K is 1.515 x 10-6m2/sec whereas that of Cu in Ag is 1.20 x 10-4m2/sec. The difference is expected because the diffusionprocess in polymers such as rubber depends on the size of the diffusing species such asCH4 in this example. Compared to the diffusion process of Cu in Ag, which occurs due to vacancy-interchange, the size of the methane molecule is large, also, the individualmers in the polymer chains are not free to move independently.39. FIND: Make a schematic plot of D vs. T in natural rubber.GIVEN: Natural rubber has a T g of about 0 C.SKETCH:TemperatureSOLUTION: The sketch above is diagrammatic. We know that before and after the glass transition temperature the diffusion coefficient behaves as an Arrhenius function:D = D o exp (-Q/RT),so that ln D is linearly proportional to 1/T. Q is large below T g.40. EXPLAIN: Will PTFE work as a membrane to separate water vapor from benzene?PTFE is Teflon.Based on the structure of PTFE (Teflon), it appears to be a non-polar membrane.Non-polar membranes allow passage of polar molecules such as water. Furthermore, the size difference between water vapor molecule and benzene also plays an important role.A water vapor molecule is much smaller than a benzene molecule and can easily passthrough a Teflon membrane whereas benzene being a large molecule will not pass.Thus, PTFE will work as an effective membrane to separate water vapor and benzene. 41.The solution to Fick’s seco nd law for a thick plate is仅供学习与交流,如有侵权请联系网站删除谢谢166。
