23_4E(Ch10)
泉州七中09--10学年度上学期高二年期中考试

泉州七中09--10学年度上学期高二年期中考试化学试卷(实验班用)考试时间:90分钟满分:100分(可能用到的相对原子质量:H :1 O: 16 Cu :64 )第Ⅰ卷(共42分)一、选择题(本题共有21小题,每小题2分,共42分。
每小题只有一个选项符合题意。
)1.下列溶液一定呈中性的是()A.pH=7的溶液 B.c (H+)=1.0×10-7 mol/L的溶液C.c (H+)=c (OH—) D.pH=3的酸与pH=11碱等体积混合后的溶液2.常温下,下列变化过程不是自发的是() A.石灰石的分解 B.原电池产生电流 C.在密闭条件下体系从有序变为无序 D.铁在潮湿空气中生锈3.一定条件下,二氧化氮和四氧化二氮之间存在下列平衡:2NO2(g)N2O4(g) △H<0,在下列条件测定NO2的相对分子质量时,测定结果误差最小的值是()A.温度130℃,压强3.03×105Pa B.温度25℃,压强1.01×105PaC.温度130℃,压强5.05×105Pa D.温度0℃,压强5.05×105Pa 4.在pH = 1的无色溶液中,能大量共存的离子组是()A.NH4+ Mg2+ SO42- Cl- B.K+ Na+ HCO3- NO3-C.Al3+ Cu2+ SO42- Cl- D.Na+ AlO2— Cl- SO32-5.不能说明醋酸是弱酸的事实是()A.0.1 mol·L-1的CH3COONa溶液的pH约为9B.完全中和等体积等浓度的H2SO4溶液比等体积浓度的CH3COOH溶液消耗NaOH的量多C.0.1 mol·L-1CH3COOH溶液的pH约为2.9D.镁粉与一定量的稀H2SO4反应时,若向其中加入少量CH3COONa固体,能够降低反应速率但不改变产生气体的总量6.在一定温度下的恒容容器中,表明反应A(s)+2B(g)C(g)+D(g)已达平衡状态的是()A.混合气体的压强不随时间变化而变化 B.混合气体的密度不随时间变化而变化C.气体总物质的量不随时间变化而变化 D.单位时间内生成n mol C,同时消耗2n mol B7.1 g H2完全燃烧生成液态水放出142.9 kJ热量,表示该反应的热化学方程式正确的是()A.2H2 +O2 ==2H2O ΔH=-571.6kJ·mol-1B.H2(g)+1/2O2(g)==H2O(l) ΔH=-142.9 kJ·mol-1C.H2(g)+1/2O2(g)== H2O(l) ΔH=-285.8 kJ·mol-1D.2H2(g)+O2(g)==2H2O(l) ΔH=-571.6 kJ·mol-18.在pH都等于9的NaOH和CH3COONa两种溶液中,设由水电离产生的OH- 离子浓度分别为A mol/L与Bmol/L,则A和B关系为()A. A>BB. A=104 BC. B=10-4 AD. A=B9.图1为某化学反应的速率与时间的关系示意图。
浙江强基联盟2023-2024学年第一学期高三年级10月联考 化学含解析

浙江省强基联盟2023学年第一学期高三年级10月联考化学学科试题(答案在最后)考生须知:1.本试题卷分选择题和非选择题两部分,考试时间90分钟.2.答题前,在答题卷指定区域填写班级、姓名、考场号、座位号及准考证号.3.所有答案必须写在答题卷上,写在试卷上无效,考试结束后,只需上交答题卷.4.本卷可能用到的相对原子质量:H-1C-12O-16Na-23S-32Cu-64选择题部分一、选择题(本大题共16小题,每小题3分,共48分.每小题列出的四个备选项只有一个是符合题目要求的,不选、多选、错选均不得分)1.下列食品添加剂属于有机物的是A.小苏打 B.食盐C.维生素CD.二氧化硫【答案】C 【解析】【详解】A .小苏打是3NaHCO ,属于钠盐,A 错误;B .食盐是NaCl ,属于钠盐,B 错误;C .维生素C 为含碳化合物,属于有机物,C 正确;D .二氧化硫是氧化物,D 错误;故选C 。
2.下列化学用语表示正确的是A.2CO 分子的空间填充模型:B.3PCl 的价层电子对互斥模型:C.羟基的电子式:D.1,1-二溴乙烷的结构简式:22CH BrCH Br【答案】B 【解析】【详解】A .碳原子半径大于氧原子,二氧化碳的空间填充模型为,A 错误;B .根据VSEPR 模型,P 的孤电子对数为1,价层电子对数为3,价层电子对互斥模型为,B 正确;C .羟基的电子式为····O :H ,C 错误;D .1,1-二溴乙烷的结构简式:23CHBr CH ,D 错误;故答案为:B 。
3.次氯酸钠是一种重要的盐,下列说法不正确...的是A.次氯酸钠属于弱电解质B.次氯酸钠可分解生成氯化钠与氧气C.次氯酸钠可由NaOH 溶液与氯气反应制得D.次氯酸钠可用于游泳池的消毒【答案】A 【解析】【详解】A .次氯酸钠是盐类,属于强电解质,故A 错误;B .根据次氯酸分解生成氧气与氯化氢,次氯酸钠分解生成氯化钠与氧气,故B 正确;C .氢氧化钠与氯气反应生成氯化钠与次氯酸钠,故C 正确;D .次氯酸钠具有氧化性,可用于游泳池的消毒,故D 正确;故答案为A 。
2024届云南省大理州民族中学高三下学期5月月考理综试卷-高中化学(解析版)

大理州民族中学2023-2024学年下学期高三5月月考理科综合试卷(考试时间150分钟,满分300分)注意事项:1.答题前,考生务必用黑色碳素笔将自己的考号、姓名、考场、座位号、班级在答题卡上填写清楚。
2.每小题选出答案后,用2B 铅笔把答题卡上对应的题目的答案标号涂黑。
如需改动,用橡皮擦干净后,再选涂其他答案标号。
在试卷上作答无效。
可能用到的相对原子质量:Li-7C-12O-16Na-23S-32Ti48第I 卷(选择题,共126分)一、选择题(本题包括13小题,每题6分,共78分。
每小题只有一个选项符合题意。
)1.化学与科技、社会、生活密切相关,下列说法错误的是A.生活中,常利用高温下铝粉与氧化铁的反应来焊接钢轨B.NaOH 、23Na CO 、2Ba(OH)三种无色溶液,只选用一种试剂无法将它们鉴别出来C.维C 又称“抗坏血酸”,缺铁性贫血患者服用补铁剂时往往和维C 一起服用D.高铁酸钠是一种新型绿色消毒剂,生活中主要用于饮用水处理【答案】B 【解析】【详解】A .利用高温下铝粉与氧化铁的反应来焊接钢轨,发生铝热反应:2Al+Fe 2O 3高温2Fe+Al 2O 3,A正确;B .NaOH 、23Na CO 、2Ba(OH)三种无色溶液,只选用一种试剂硫酸可将它们鉴别出来,NaOH 滴入硫酸无现象、23Na CO 滴入硫酸有气泡产生、2Ba(OH)滴入硫酸有白色沉淀产生,B 错误;C .人体吸收亚铁离子,亚铁离子容易被氧化,而维生素C 具有还原性,能防止亚铁离子被氧化,则维生素C 可以促进人体铁元素的吸收,C 正确;D .高铁酸钠中铁的化合价为+6价,具有强氧化性,能杀菌消毒,被还原成Fe 3+,Fe 3+水解成氢氧化铁胶体,氢氧化铁胶体能净水,因此高铁酸钠是一种新型绿色消毒剂,可用于饮用水处理,D 正确;故选B 。
2.化合物2M 6H O 可用作洗涤粉,M 的结构如下,其中X 、Y 、Z 、W 是原子序数依次增大的短周期主族元素,下列说法错误的是A.Y 的基态原子核外电子排布中有1个未成对电子B.化合物WX 为离子化合物,在野外可用作生氢剂C.3YF 分子的空间构型是三角锥形D.2H Z 的键角大于2H S 的键角【答案】C 【解析】【分析】化合物2M 6H O 可用作洗涤粉,M 中X 、Y 、Z 、W 是原子序数依次增大的短周期主族元素,W 能形成带一个单位正电荷的W +离子,则W 为Na 元素;阴离子中X 、Y 、Z 形成共价键的数目分别为1、4、2,则X 为H 元素、Y 为B 元素、Z 为O 元素,【详解】A .硼元素的原子序数为5,基态原子的价电子排布式为2s 22p 1,则原子核外有1个未成对电子,A 项正确;B .WX 是NaH ,是离子化合物,NaH 中H 元素为-1价,能和水反应生成H 2,在野外可用作生氢剂,B 项正确;C .BF 3分子的空间构型是平面三角形,C 项错误;D .H 2O 和H 2S 有两对孤对电子,但是氧原子的电负性比硫原子大,氧原子对其携带的两对孤对电子的吸引比硫原子大,相应的孤对电子斥力增大使键角也相应比H 2S 大,D 项正确;答案选C 。
扬州市2023-2024学年高三下学期考前调研模拟预测测试化学试题+答案

第 1 页 共 6 页扬州市2023-2024学年高三考前调研测试化学2024.05注意事项:考生在答题前请认真阅读本注意事项及各题答题要求1.本试卷共6页,包含选择题[第1题~第13题,共39分]、非选择题[第14题~第17题,共61分]两部分。
本次考试时间为75分钟,满分100分。
考试结束后,请将答题卡交回。
2.答题前,请考生务必将自己的学校、姓名、准考证号用0.5毫米的黑色签字笔写在答题卡上相应的位置,在相应区域贴好条形码。
3.选择题每小题选出答案后,请用2B 铅笔在答题卡指定区域填涂,如需改动,用橡皮擦干净后,再填涂其它答案。
非选择题请用0.5毫米的黑色签字笔在答题卡指定区域作答。
在试卷或草稿纸上作答一律无效。
4.如有作图需要,可用2B 铅笔作答,并请加黑加粗,描写清楚。
可能用到的相对原子质量:H -1 C -12 N -14 O -16 Na -23 P -31 S -32 Fe -56选择题(共39分)单项选择题:本题包括13小题,每小题3分,共计39分。
每小题只有一个选项符合题意。
1.钠钾合金常温下呈液态,可用作快中子反应堆的热交换剂,这主要是因为钠钾合金A .易与O 2反应B .易与H 2O 反应C .熔点低、导热性好D .密度小、硬度小2.K 2Cr 2O 7检验酒精的反应为 2K 2Cr 2O 7+3C 2H 5OH +8H 2SO 4=3CH 3COOH +2Cr 2(SO 4)3+2K 2SO 4+11H 2O 。
下列说法正确的是A .中子数为28的铬原子:24 52 CrB .H 2O 的电子式:H H OC .K +的结构示意图:D .C 2H 5OH 中氧元素的化合价:-23.实验室制取少量C 2H 4的原理及装置均正确的是A .制取C 2H 4B .干燥C 2H 4 C .收集C 2H 4D .吸收C 2H 4 4.将Ca 3(PO 4)2、SiO 2和C 在高温下焙烧可以得到单质磷。
浙江省丽水市“五校高中发展共同体”2024-2025学年高二上学期10月联考化学试题含答案

绝密★考试结束前2024学年第一学期丽水五校高中发展共同体10月联考高二年级化学学科试题(答案在最后)考生须知:1.本卷共8页满分100分,考试时间90分钟。
2.答题前,在答题卷指定区域填写班级、姓名、考场号、座位号及准考证号并填涂相应数字。
3.所有答案必须写在答题纸上,写在试卷上无效。
4.考试结束后,只需上交答题纸。
可能用到的相对原子质量:H 1C 12N 14O 16Na 23S 32Cl 35.5选择题部分一、选择题(本大题共20小题,1-10题每小题2分,11-20题每小题3分,共50分。
每小题列出的四个备选项中只有一个是符合题目要求的,不选、多选、错选均不得分。
)1.下列物质因水解呈酸性的是A.4NH Cl B.23Na CO C.24Na SO D.32NH H O【答案】A 【解析】【详解】A .NH 4Cl 属于强酸弱碱盐,铵根水解显酸性,A 符合题意;B .Na 2CO 3是强碱弱酸盐,碳酸根水溶液呈碱性,B 不符合题意;C .Na 2SO 4是强酸强碱盐,溶液显中性,C 不符合题意;D .NH 3⋅H 2O 是弱碱,电离出OH-,溶液显碱性,铵根离子水解溶液呈酸性,D 不符合题意;故选A 。
2.下列仪器与名称相符的是A.封液漏斗B.坩埚C.三脚架D.干燥管【答案】D【解析】【详解】A .依据形状可知,该仪器的名称为球形分液漏斗,而不是封液漏斗,A 错误;B .依据形状可知,图示仪器为蒸发皿,B 错误;C .该仪器为泥三角,不是三脚架,C 错误;D .依据形状可知,图示仪器为干燥管,D 正确;故答案为:D 。
3.下列说法正确的是A.352Cl 和372Cl 互为同素异形体B.葡萄糖与蔗糖互为同分异构体C.D 和T 互为同位素D.乙烷和环己烷互为同系物【答案】C 【解析】【详解】A .352Cl 和372Cl 都是Cl 原子形成的双原子分子,不属于不同单质,两者不互为同素异形体,A 错误;B .葡萄糖(C 6H 12O 6)与蔗糖(C 12H 22O 11)分子式不同,两者不互为同分异构体,B 错误;C .D 和T 质子数都是1,中子数分别是1和2,属于质子数相同而中子数不同的同种元素的不同原子,两者互为同位素,C 正确;D .乙烷和环己烷结构不相似,且分子组成上也不是相差若干个-CH 2-原子团,两者不互为同系物,D 错误;故选C 。
浙江省湖州市长兴县龙山教育集团共同体2024-2025学年八年级上学期10月月考数学试题
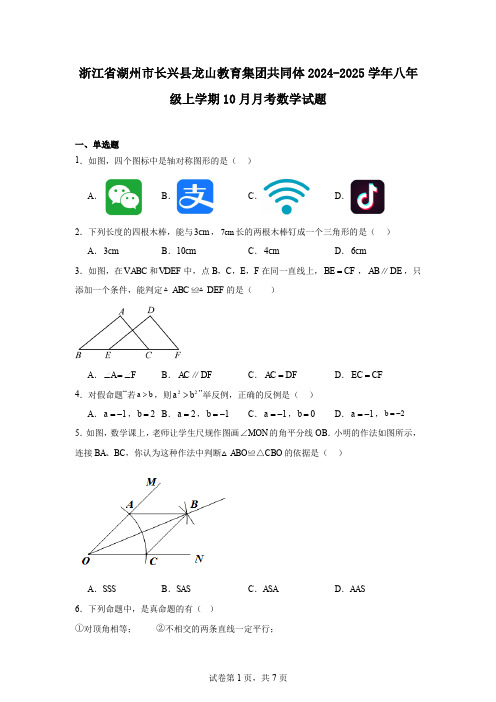
浙江省湖州市长兴县龙山教育集团共同体2024-2025学年八年级上学期10月月考数学试题一、单选题1.如图,四个图标中是轴对称图形的是( )A .B .C .D . 2.下列长度的四根木棒,能与3cm ,7cm 长的两根木棒钉成一个三角形的是( ) A .3cm B .10cm C .4cm D .6cm3.如图,在ABC V 和DEF V 中,点B ,C ,E ,F 在同一直线上,BE CF =,AB DE ∥,只添加一个条件,能判定ABC DEF ≌△△的是( )A .A F ∠=∠B .AC DF ∥ C .AC DF =D .EC CF = 4.对假命题“若a b >,则22a b >”举反例,正确的反例是( )A .1a =-,2b =B .2a =,1b =-C .1a =-,0b =D .1a =-,2b =- 5.如图,数学课上,老师让学生尺规作图画∠MON 的角平分线OB .小明的作法如图所示,连接BA 、BC ,你认为这种作法中判断△ABO ≌△CBO 的依据是( )A .SSSB .SASC .ASAD .AAS6.下列命题中,是真命题的有( )①对顶角相等; ②不相交的两条直线一定平行;③等角的补角相等; ④如果a b >,那么a b >A .①和②B .①和③C .②和③D .③和④7.如图,将ABC V 纸片沿DE 折叠,使点A 落在四边形BCED 内点A '的位置35A ∠=︒,则12∠+∠的度数是( )A .80︒B .70︒C .45︒D .35︒8.如图,在44⨯的正方形网格中有两个格点A 、B ,连接AB ,在网格中再找一个格点C ,使得ABC V 是等腰三角形,满足条件的格点C 的个数是( )A .5B .6C .8D .99.如图,1AP 为ABC V 的中线,2AP 为1APC V 的中线,3AP 为2V APC 的中线,…,按此规律,1n AP +为V n AP C 的中线,若1△ABP 的面积为1,则V n AP C 的面积为( )A .2nB .2n -C .12n -D .12n -10.如图,在Rt ABC △中,AC BC =,点P 是BC 上一点,BD AP ⊥交AP 延长线于点D ,连接,CD CH CD ⊥交AD 于点H ,已知16ACP PBD S S -=△△,则下列结论:①CAP CBD ∠=∠;②ACH BCD △≌△;③16CHD S =△;④4CD =,其中正确的结论有( )个.A .1个B .2个C .3个D .4个二、填空题11.请将命题“对顶角相等”改写为“如果……,那么……”的形式:.12.我们知道要使四边形木架不变形,至少要钉一根木条,如图,有一个正五边形木框,要使五边形木架不变形,至少要钉根木条.13.已知ABC V 的三个内角度数比为2:3:4,则这个三角形是三角形.14.已知a ,b 是一个等腰三角形的两边长,且a ,b 2(84)0b -=,则此等腰三角形周长为.15.如图,CA BC ⊥,垂足为C ,2cm =AC ,6cm BC =,射线BM BQ ⊥,垂足为B ,动点P 从C 点出发以1cm /s 的速度沿射线CQ 运动,点N 为射线BM 上一动点,满足PN AB =,随着P 点运动而运动,当点P 运动秒时,BCA V 与点P 、N 、B 为顶点的三角形全等(时间不等于0).16.如图,ABC V 的角平分线BD 、CE 交于点O .延长BC 至F ,CG 与BD 的延长线相交于点G ,且2A G ∠=∠,:3:4OD DG =,若DOC △的面积为6,10CG =,则线段CO 的长度为.三、解答题17.已知:如图,点A 、D 、B 、E 在同一直线上,AC EF AD BE A E ==∠=∠,,.求证:ABC EDF △≌△.18.如图,在ABC V 中,ABC ∠的平分线交AC 于点D ,过点D 作DE BC ∥交AB 于点E ,若80A ∠=︒,40C ∠=︒,求BDE ∠的度数.19.如图,在正方形网格中,每个小正方形的边长都为1,网格中有一个格点ABC V .(即三角形的顶点都在格点上)(1)在图中作出ABC V 关于直线l 对称的A B C '''V ;(要求:A 与A ',B 与B ',C 与C '相对应)(2)若有一格点P 到点A B 、的距离相等(PA PB =),则网格中满足条件的点P 共有________个;(3)在直线l 上找一点Q ,使QB QC +的值最小.20.如图①,油纸伞是中国传统工艺品之一,起源于中国的一种纸制或布制伞.油纸伞的制作工艺十分巧妙,如图②,伞圈D 沿着伞柄AP 滑动时,伞柄AP 始终平分同一平面内两条伞骨所成的BAC ∠,伞骨BD ,CD 的B ,C 点固定不动,且到点A 的距离AB AC =.(1)当D 点在伞柄AP 上滑动时,处于同一平面的两条伞骨BD 和CD 相等吗?请说明理由.(2)如图③,当油纸伞撑开时,伞的边缘M ,N 与点D 在同一直线上,若140BAC ∠=︒,120MBD ∠=︒,求CDA ∠的度数.21.如图,ABC V 和ADE V 两个大小不同的等腰直角三角形,AB AC =,AE AD =, 90BAC EAD ∠=∠=︒,B 、C 、E 在同一条直线上,连接DC ,交AE 于点F .(1)求证:ABE ACD V V ≌;(2)若3BE CE =,6CD =,求DCE △的面积.22.王强同学用10块高度都是2cm 的相同长方体小木块,垒了两堵与地面垂直的木墙,木墙之间刚好可以放进一个等腰直角三角板(AC BC =,90ACB ∠=︒),点A 和B 分别与木墙的顶端重合.(1)求证:ADC CEB △≌△;(2)求两堵木墙之间的距离.23.综合与探究:爱思考的小明在学习过程中,发现课本有一道习题,他在思考过程中,对习题做了一定变式,让我们来一起看一下吧.在ABC V 中,ABC ∠与ACB ∠的平分线相交于点P .(1)如图1,如果80A ∠=︒,那么BPC ∠=___________°(2)如图2,作ABC V 的外角MBC ∠,NCB ∠的平分线交于点Q ,试探究Q ∠与BPC ∠的数量关系.(3)如图3,在(2)的条件下,延长线段BP QC ,交于点E ,在BQE △中,若4Q E ∠=∠,求A ∠的度数.24.【初步探索】(1)如图1,在四边形ABCD 中,90AB AD B ADC ∠∠===︒,,E ,F 分别是BC CD ,上的点,且EF BE FD =+,探究图中BAE FAD EAF ∠∠∠,,之间的数量关系.小明同学探究此问题的方法是:延长FD 到点G ,使DG BE =.连接AG ,先证明ABE ADG △≌△,再证明AEF AGF V V ≌,可得出结论,则他的结论应是________.【灵活运用】(2)如图2,若在四边形ABCD 中,180AB AD B D E F =∠+∠=︒,,,分别是BC CD ,上的点,且EF BE FD =+,上述结论是否仍然成立,并说明理由;【拓展延伸】(3)如图3,已知在四边形ABCD 中,180ABC ADC AB AD ∠+∠=︒=,,若点E 在CB 的延长线上,点F 在CD 的延长线上,且仍然满足EF BE FD =+,请直接写出EAF ∠与DAB ∠的数量关系.。
长虹CH 16机芯介绍及总线调试方法及代表机型总线数-12页文档资料
长虹CH 16机芯介绍及总线调试方法及代表机型总线数-、机芯介绍长虹CH-16机芯是长虹公司2019年引进菲利浦公司超级芯片--TDA9370开发的一种电视机芯。
该机芯的电视小信号处理、CPU、TV/AV切换开关,波段切换开关等电路都集成在一块芯片TDA9370内部。
使电路结构更加流畅。
外部调试点无乎为零。
大大地提高了整机的可靠性,也使维修更加方便,广泛装配21、25、29、34英寸电视机。
二、适用机型长虹的CH-16机芯的电视机,最初都用"SF"来代表"CH-16"机芯。
主要机型有:SF2115、SF2198、SF2139、SF2598、SF2515、SF2915、SF2998、SF3498F、HY29S86、PF2198、PF2598、PF2998、SF2939等三、CH-16机芯彩电I 2C总线系统的调整方法(1)进入维修状的方法将本机音量减至最小,使用K16型遥控器(K16C、K16D、K16H)或K3型遥控器(K3A、K3D、K3E等),按住静音键不放,此时荧光屏上出现红色的"静音X"字样。
紧接着按本机键中的"菜单键",屏幕上出现红色的"S"字符,表示本机已经进入维修状态。
(注有时按本机面板控制键中的"菜单键"有时需要多停留几秒钟)。
(2)选项与调整进入"S"状态以后,按压遥控器或本机节目选择键可选择所需调整的项目。
按压遥控器或本机音量键调整总线数据。
(3)调试完毕,遥控关机,即可退出"S"状态,并自动关机。
(一)小屏幕机芯总线数据参考机型有SF2198、SF2139、PF2198等21英寸小屏幕电视机项目释义数据(十六进制)预置范围备注OPT1选项1 FC 0-FF功能选择,不能随意更改OPT2选项2 6C 0-FF同上OPT3选项3 F2 0-FF同上OPT4选项4 4E 0-FF同上AVG 20 0-3F VS场线性23 0-3F SC 15 0-3F 5VA 50Hz场幅23 0-3F5VSH 50Hz帧中心16 0-3F 5HS 50Hz行中心1A 0-3F 50V字符中心位置37 0-3F RCUT红截止1F 0-3F GCUT绿截止1F 0-3F RDRV红激励20 0-3F GDRV绿激励20 0-3F BDRV兰激励20 0-3F AGC AGC设定14 0-3F音量00音量最小25 0-3F可影响声音的变化音量25通常音量35 0-3F亮度50通常亮度1C 0-3F亮度99最高亮度设置3F 0-3F对比度50对比度适中值20 0-3F对对度99最大对比度设置3F 0-3F色饱和99最大色饱和度3F 0-3F YDEL亮度信号延迟0D 0-0F CL彩色预置09 0-0F PODE模式选择10 0-1F(二)SF3498总线数据表项目释义数据(十六进制)预置范围备注OP1选项1 FE 0-FF功能性选选择OP2选项2 48 0-FF同上OP3选项3 C6 0-FF同上AVG 28 0-3F VX00 19 0-3F VS场线性23 0-3F SC帧线性20 0-3F 5VA 50Hz场幅19 0-3F 5VSH 50Hz场中心23 0-3F 5HA行幅2A 0-3F 5HS 50Hz行中心20 0-3F 5EW枕校1F 0-3F 50V字符上下位置3F 0-3F HP行补偿2C 0-3F TC四角补偿20 0-3F VCP帧补偿30 0-3F BCP 2B 0-3F HB 20 0-3F RCUT 红截止21 0-3F GCUT绿截止24 0-3F RDRV红驱动15 0-3F GDRV绿驱动18 0-3F BDRV兰驱动20 0-3F AGC AGC设定16 0-3F VOL音量28 0-3F 音量00最小音量39 0-3F可影响声音变化音量25中间音量3C 0-3F亮度50通常亮度设置1F 0-33可影响整机亮度亮度99最高亮度设置33 0-3F对比度50通常对比度0C 0-3F对比度99最大对比度3F 0-3F彩色99色饱和度最大3F 0-3F可能引起无彩IF0 20 0-3F YDEL亮度延迟00 0-0F CL对比度预置0B 0-0F PODE模式选择10 0-1F CH-16机芯概述及检修提示CH-16机芯是在菲利浦公司研究开发的超大规模集成电路TDA9370的基础上开发的另一种电视机芯。
第23讲电解池的原理(原卷版)
第二十三讲 电解原理一、电解、电解池、放电1.电解:电流通过电解质溶液(或熔融电解质)而在阴阳两极引起氧化还原反应的过程。
2.电解池:把电能转化为化学能的装置,也叫电解槽。
3.放电:当电解质溶液中的阴或阳离子到达阳或阴极时,阴离子失去电子发生氧化反应或阳离子获得电子发生还原反应的过程。
二、电解原理构成条件 ①直流电源;②两个电极;③闭合回路; ④电解质溶液或熔融电解质电极阳极 与电源正极相连,发生氧化反应 阴极与电源负极相连,发生还原反应离子流向 阴离子移向阳极;阳离子移向阴极(阴找阳、阳找阴) 电子流向电源负极流向电解池阴极;电解池阳极流向电源正极(电子不下水、离子不上岸)1、电子流向(外电源)负极→(电解池)阴极 (电解池)阳极→(外电源)正极 2、电解池的构造和阴阳极的判断阳极:与直流电源的正极相连的电极,发生氧化反应 阴极:与直流电源的负极相连的电极,发生还原反应要点集结知识精讲3、阴、阳极的判断:三、阴、阳极的放电顺序1.阳极放电顺序(1)若是活性电极做阳极,则活性电极首先失电子,发生氧化反应;(2)若是Pt、Au、C等惰性电极作阳极,则仅是溶液中的具有还原性的离子放电。
即:S2>I>Br>Cl>OH>SO42-等(最高价的含氧酸根离子)>F。
2.阴极放电顺序(1)阴极上放电的总是溶液(或熔融电解质)中的具有氧化性的离子,与电极材料无关;(2)氧化性强的离子先放电,放电顺序如下:Ag+> Fe3+>Cu2+> H+(酸) > Fe2+>Zn2+>H+(水)>Al3+>Mg2+>Na+>Ca2+>K+注:①阴阳离子在两极上放电顺序复杂,与离子性质、溶液浓度、电流强度、电极材料等都有关,不应将放电顺序绝对化,以上仅是一般规律。
②电解过程中析出的物质的量(或析出物质的质量):在电解若干串联电解池中的溶液时,各阴极或阳极所通过的电量相等,析出物质的量取决于电路中通过的电量。
有机化学试题库
有机化学试题库00(总79页)--本页仅作为文档封面,使用时请直接删除即可----内页可以根据需求调整合适字体及大小--有机化学试题库一、命名下列各化合物:CH 3-CH(CH 3)-CH(Cl)-CH 3C 2H 5CH(NH 2)CH 33CH2CH2-NH2NO 2CHO CO-NH2CH 3NO 2CH 3CH 3NH 2Br C H 2CH-CCH(CH 3)3CCH 2CH 2C(CH 3)3CH 3CH(C 2H 5)CH 2CH(CH 3)22H 5CH 332H 52H 5HClClCH3C 2H5C3C H3HCH 3CH 3(CH 3)2CHCH 2C(CH 3)2CH 2OH CH 3CHCHCH 2CHCH 3OH CH 3C 2H 5OC 2H 5C 6H 5OCH 3CH 3OCH 2CH 3CH 3CH 3Cl CH 3HHCHOCH2OHH H Cl HOCH 3CH 2-CO-C 2H 5C 2H 5CHCH 32CHO 3-CO-CH 2-CO-CH 3O OO CH 2COOCH 3CO-N(CH 3)2COOHCOOHH H NH 2CH 3O CH 2OHHHOHH OH OHHOH H OCH 2OH H OH HH O H OH CH 2OHO CH 2OHH H OHH OH OHHH OHNSOCOOHC 2H 5CH 3O NC 17H 35COOHCH 3C 2H 5ClH ClHCH 3C 2H 5HH H HCH 3CH 32CHCH 2CH2CHOOH3CH 3(CH 3)3CCH 2CH(CH 3)2CH 2Cl NH 2(CH 3)3NC 6H 5OHCH 3CH 2CHCH 2CH 2CHCH 3N(CH 3)2CH 3NO2CH 2COOHNCOOHCOOHO OH OHH HOH 2C CH 2OH OH O OH OH HOC 2H 5OHCH 2OHCH 2CH=CH2N CH3CH 2CH3OOOHOOHC 6H 52H 333BrCH 3OCHOClNH 2Cl NH 2CH 3C CH CH COOH ONHCOCH(CH 3)2CH 3CH2COC 2H5OH OH H CH 3CH 3CH 2CH 2CH 3NO 2ClH 32H 53CH 3COClSO 2NHCH 3H 33NHOCH 3CH 2ON(CH 3)2C CH 3CC CCHO O CH 3COClOMeHCOOHHCOOHC 6H5CHCHCOOHCH 2COOHC 2H 5CHCH 2CH 2CO OCH 3CH 2CH COOHOHCH 3CH 2CH 2COOCH 3O C CH 3C CH 3COOC 2H5O C 6H 5CH 2CCH 2COOC 2H 5O2H5OOCCHCOOC2H 5CH 3C COOC 2H 5CH 2C 6H 5CH 2OH 5C 2OOCCHCOOC 2H 5CH 2CHCH 2CH 2CH 3CH 2CH 3CH 2CH 3CH 3CH 2CH 3CHCH 2CHCH 2CHCH 3CH CH 3CH 2CH 3CH 33CH 3CH 3CH 3CHCH 23CH 33CH2CHCH 2CCH 2CHCH 2CH 3CH 3CH 3CH 2CH 3CH 3CH 3CH 3CHCH 2CH 2CHCH 3CH 2CH 3CH 2CH 3CH 3C H 3C H C HCH 2CH 3CH3C2H 5CH 2CH 3CH 2CH(CH 3)2CH 2H CH 3CH 2CH 2CH 3CH 2CH 3CH 3CH 2CH 3CH 2CH 2CH 2CH 3CH 2CH3(CH 3)2CHCH 3H CH 3C H 3HHCH 2CH 3C H2ClCH2CH 2CHCH 2CH2CH3HH CH 3CHCH 3HCH 3HCH=CH 332CH3ClCH 3CH 2CH 3CH 3CH 3CH 3NO 2CH=CH 2CH 2ClClSO 3H CH 3BrBr3)2CHCH SO 3HNO 2CH 3CH 3HO 3SSO 3H(C 6H 5)3CHC 6H 5H OH 3CH(CH 3)CH 2CH 3HCl CH 3C H 3HHCH 2ClBrCH 3SO 3HBrNO 2CH3CH 3CH 3CH(CH 3)2C3CH(CH 3)2SO 3HCl二、写出下列各化合物的结构式:1、2,3,3,4-四甲基戊烷2、2-甲基-3-乙基己烷3、2,6-二甲基-3,6-二乙基辛烷4、(Z)-2-戊烯5、2-乙基-1-戊烯6、(E)-6,7,11-三甲基-3-十四碳烯7、(2Z ,4E)-2,4-己二烯 8、顺-1,4-二甲基环己烷 9、反-1,2-二乙基环戊烷 10、二环[2,1,0]戊烷 11、2,4-二硝基甲苯 12、对甲苯乙炔13、邻苯二乙烯 14、4-羟基-3-溴苯甲酸 15、2,6-二甲基-4-溴苯甲醛 16、对甲基苯甲醇 17、5-己烯-3-醇 18、4-异丙基-2,6-二溴苯酚 19、3-硝基-4-羟基苯磺酸 20、乙酰乙酸乙酯 21、4-苯基-3-丁烯-2-酮 22、3-甲基环己酮 23、(Z)-2-丁烯醛 24、2,2-二甲基丙醛 25、α-D-吡喃葡萄糖 26、β-D-吡喃葡萄糖 27、α-D-呋喃果糖 28、β-D-呋喃果糖 29、(2R,3S)-酒石酸 30、N-甲基苯甲酰胺 31、N-苯基对苯二胺 32、N-苯基甲酰胺 33、噻吩 34、N,N-二乙基苯甲酰胺35、油酸 36、2-氨基乙酰胺37、缩二脲 38、茚三酮39、(R)-氟氯溴甲烷 40、糠醛41、丙酸酐 42、4-甲基-2-氨基戊酸43、甲基乙基异丙基胺 44、α-D-葡萄糖45、α-麦芽糖(构象式) 46、2-甲基-4-甲硫基丁酸47、(S)-3-对羟基苯基-2-氨基丙酸 48、四氢噻吩49、4-甲酰氨基乙酰氯 50、7,7-二甲基二环[2,2,1]-2,5-庚二烯51、α-呋喃甲醛 52、α-甲基葡萄糖苷53、(Z,Z)–2–溴-2,4–辛二烯 54、(2R,3R)–3–氯-2–溴戊烷55、(1R,3R)–3–甲基环己醇 56、N,N –二乙基间烯丙基苯甲酰胺57、2–甲基–3,3–二氯戊酸钠 58、二环[壬酮59、异丙基烯丙基酮 60、反–1–甲基–4–溴环己烷61、2,3-二甲基-2-戊烯 62、(Z)-2-已烯63、(E)-4-环己基-2-戊烯 64、2,5-二甲基-顺-3-已烯65、2,2-二甲基-3-已炔 66、(Z,E) -2,4-庚二烯67、3-甲基-1,5-已烯 68、2,4-二硝基氟苯69、六溴代苯 70、氯化苄71、2-甲基-2,3-二碘丁烷 72、2-氯-2-丁烯73、3-苯基-1-溴-2-丁烯 74、3-甲氧基-2-戊醇75、5-溴-1-己炔-3-醇 76、3-苯基-1,2-戊二醇77、1-甲基环己醇 78、3,5-二甲基苯酚79、4-环戊烯-1,3-二醇 80、4-戊烯-2-酮81、3-甲基-2-丁烯酮 82、3-羟基丁醛83、3-苯基丙烯醛 84、4-甲基环己基甲醛85、4-甲酰基苯甲酸 86、2,4-环戊二烯甲酸87、甲酸异丙酯 88、对硝基乙酰苯胺 89、乙酸正丁酯 90、氯乙酸异戊酯 91、N,N-二甲基乙酰胺 92、间二甲苯 93、均三甲苯 94、对溴硝基苯95、2,4-二氯基硝基苯 96、3-溴-4-硝基甲苯 97、间硝基甲苯 98、4-氯-2,3-二硝基甲苯 99、2-溴-4-硝基异丙苯 100、5-硝基萘-2-磺酸 101、1,8-二硝基萘 102、对联三苯103、对二氯苯 104、(R )-2-溴丁烷 105、间碘甲苯 106、4-硝基-1,2-二溴苯三、判断选择:1.)。
testbank_4e_CH03
Chapter 3 Amino Acids, Peptides, and ProteinsMultiple Choice Questions1.Amino acidsPage: 76Difficulty: 1Ans: CThe chirality of an amino acid results from the fact that its α carbon:A)has no net charge.B)is a carboxylic acid.C)is bonded to four different chemical groups.D)is in the L absolute configuration in naturally occurring proteins.E)is symmetric.2.Amino acidsPage: 76Difficulty: 2Ans: BOf the 20 standard amino acids, only ___________ is not optically active. The reason is that its side chain ___________.A)alanine; is a simple methyl groupB)glycine; is a hydrogen atomC)glycine; is unbranchedD)lysine; contains only nitrogenE)proline; forms a covalent bond with the amino group3.Amino acidsPage: 79Difficulty: 1Ans: CTwo amino acids of the standard 20 contain sulfur atoms. They are:A)cysteine and serine.B)cysteine and threonine.C)methionine and cysteineD)methionine and serineE)threonine and serine.4.Amino acidsPage: 79Difficulty: 1Ans: AAll of the amino acids that are found in proteins, except for proline, contain a(n):A)amino group.B)carbonyl group.C)carboxyl group.D)ester group.E)thiol group.5.Amino acidsPages: 79–80Difficulty: 3Ans: CWhich of the following statements about aromatic amino acids is correct?A)All are strongly hydrophilic.B)Histidine’s ring structure results in its being categorized as aromatic or basic, depending on pH.C)On a molar basis, tryptophan absorbs more ultraviolet light than tyrosine.D)The major contribution to the characteristic absorption of light at 280 nm by proteins is thephenylalanine R group.E)The presence of a ring structure in its R group determines whether or not an amino acid isaromatic.6.Amino acidsPage: 80Difficulty: 2Ans: AWhich of the following statements about cystine is correct?A)Cystine forms when the —CH2—SH R group is oxidized to form a —CH2—S—S—CH2—disulfide bridge between two cysteines.B)Cystine is an example of a nonstandard amino acid, derived by linking two standard amino acids.C)Cystine is formed by the oxidation of the carboxylic acid group on cysteine.D)Cystine is formed through a peptide linkage between two cysteines.E)Two cystines are released when a —CH2—S—S—CH2— disulfide bridge is reduced to —CH2—SH.7.Amino acidsPage: 80Difficulty: 2Ans: AThe uncommon amino acid selenocysteine has an R group with the structure —CH2—SeH (p K a≈ 5).In an aqueous solution, pH = 7.0, selenocysteine would:A)be a fully ionized zwitterion with no net charge.B)be found in proteins as D-selenocysteine.C)never be found in a protein.D)be nonionic.E)not be optically active.8.Amino acidsPage: 81Difficulty: 1Ans: AAmino acids are ampholytes because they can function as either a(n):A)acid or a base.B)neutral molecule or an ion.C)polar or a nonpolar molecule.D)standard or a nonstandard monomer in proteins.E)transparent or a light-absorbing compound.9.Amino acidsPages: 82–83Difficulty: 2Ans: DTitration of valine by a strong base, for example NaOH, reveals two p K’s. The titration reaction occurring at p K2 (p K2 = 9.62) is:A) —COOH + OH−→—COO− + H2O.B) —COOH + —NH2→—COO− + —NH2+.C) —COO− + —NH2+→—COOH + —NH2.D) —NH3+ + OH−→—NH2 + H2O.E) —NH2 + OH−→—NH− + H2O.10.Amino acidsPages: 82–83Difficulty: 1Ans: CIn a highly basic solution, pH = 13, the dominant form of glycine is:A)NH2—CH2—COOH.B)NH2—CH2—COO−.C)NH2—CH3+—COO−.D)NH3+—CH2—COOH.E)NH3+—CH2—COO−.11.Amino acidsPage: 84Difficulty: 2Ans: BFor amino acids with neutral R groups, at any pH below the pI of the amino acid, the population of amino acids in solution will have:A) a net negative charge.B) a net positive charge.C)no charged groups.D)no net charge.E)positive and negative charges in equal concentration.12.Amino acidsPage: 84Difficulty: 3Ans: BWhat is the approximate charge difference between glutamic acid and α-ketoglutarate at pH 9.5?A)0B)½C)1D)1½E)213.Peptides and proteinsPage: 85Difficulty: 1Ans: BThe formation of a peptide bond between two amino acids is an example of a(n) ______________ reaction.A)cleavageB)condensationC)group transferD)isomerizationE)oxidation reduction14.Peptides and proteinsPage: 86Difficulty: 1Ans: CThe peptide alanylglutamylglycylalanylleucine has:A) a disulfide bridge.B)five peptide bonds.C)four peptide bonds.D)no free carboxyl group.E)two free amino groups.15.Peptides and proteinsPage: 86Difficulty: 1Ans: CAn octapeptide composed of four repeating glycylalanyl units has:A)one free amino group on an alanyl residue.B)one free amino group on an alanyl residue and one free carboxyl group on a glycyl residue.C)one free amino group on a glycyl residue and one free carboxyl group on an alanyl residue.D)two free amino and two free carboxyl groups.E)two free carboxyl groups, both on glycyl residues.16.Peptides and proteinsPage: 86Difficulty: 1Ans: CAt the isoelectric pH of a tetrapeptide:A)only the amino and carboxyl termini contribute charge.B)the amino and carboxyl termini are not charged.C)the total net charge is zero.D)there are four ionic charges.E)two internal amino acids of the tetrapeptide cannot have ionizable R groups.17.Peptides and proteinsPages: 87–88Difficulty: 2Ans: CWhich of the following is correct with respect to the amino acid composition of proteins?A)Larger proteins have a more uniform distribution of amino acids than smaller proteins.B)Proteins contain at least one each of the 20 different standard amino acids.C)Proteins with different functions usually differ significantly in their amino acid composition.D)Proteins with the same molecular weight have the same amino acid composition.E)The average molecular weight of an amino acid in a protein increases with the size of the protein.18.Peptides and proteinsPage: 87Difficulty: 2Ans: BThe average molecular weight of the 20 standard amino acids is 138, but biochemists use 110 when estimating the number of amino acids in a protein of known molecular weight. Why?A)The number 110 is based on the fact that the average molecular weight of a protein is 110,000with an average of 1,000 amino acids.B)The number 110 reflects the higher proportion of small amino acids in proteins, as well as theloss of water when the peptide bond forms.C)The number 110 reflects the number of amino acids found in the typical small protein, and onlysmall proteins have their molecular weight estimated this way.D)The number 110 takes into account the relatively small size of nonstandard amino acids.E)The number 138 represents the molecular weight of conjugated amino acids.19.Peptides and proteinsPage: 88Difficulty: 1Ans: CIn a conjugated protein, a prosthetic group is:A) a fibrous region of a globular protein.B) a nonidentical subunit of a protein with many identical subunits.C) a part of the protein that is not composed of amino acids.D) a subunit of an oligomeric protein.E)synonymous with “protomer.”20.Peptides and proteinsPage: 88Difficulty: 1Ans: AProsthetic groups in the class of proteins known as glycoproteins are composed of:A)carbohydrates.B)flavin nucleotides.C)lipids.D)metals .E)phosphates.21.Peptides and proteinsPage: 88Difficulty: 1Ans: BWhich of the following refers to particularly stable arrangements of amino acid residues in a protein that give rise to recurring patterns?A)Primary structureB)Secondary structureC)Tertiary structureD)Quaternary structureE)None of the above22.Peptides and proteinsPage: 88Difficulty: 1Ans: DWhich of the following describes the overall three-dimensional folding of a polypeptide?A)Primary structureB)Secondary structureC)Tertiary structureD)Quaternary structureE)None of the above23.Working with proteinsPage: 89Difficulty: 1Ans: EFor the study of a protein in detail, an effort is usually made to first:A)conjugate the protein to a known molecule.B)determine its amino acid composition.C)determine its amino acid sequence.D)determine its molecular weight.E)purify the protein.24.Working with proteinsPage: 91Difficulty: 2Ans: BIn a mixture of the five proteins listed below, which should elute second in size-exclusion (gel-filtration) chromatography?A)cytochrome c M r = 13,000B)immunoglobulin G M r = 145,000C)ribonuclease A M r = 13,700D)RNA polymerase M r = 450,000E)serum albumin M r = 68,50025.Working with proteinsPage: 92Difficulty: 2Ans: EBy adding SDS (sodium dodecyl sulfate) during the electrophoresis of proteins, it is possible to:A)determine a protein’s isoelectric point.B)determine an enzyme’s specific activity.C)determine the amino acid composition of the protein.D)preserve a protein’s native structure and biological activity.E)separate proteins exclusively on the basis of molecular weight.26.Working with proteinsPage: 93Difficulty: 2Ans: BTo determine the isoelectric point of a protein, first establish that a gel:A)contains a denaturing detergent that can distribute uniform negative charges over the protein’s surface.B)exhibits a stable pH gradient when ampholytes become distributed in an electric field.C)is washed with an antibody specific to the protein of interest.D)neutralizes all ionic groups on a protein by titrating them with strong bases.E)relates the unknown protein to a series of protein markers with known molecular weights, M r.27.Working with proteinsPages: 93–94Difficulty: 3Ans: AThe first step in two-dimensional gel electrophoresis generates a series of protein bands by isoelectric focusing. In a second step, a strip of this gel is turned 90 degrees, placed on another gel containing SDS, and electric current is again applied. In this second step:A)proteins with similar isoelectric points become further separated according to their molecularweights.B)the individual bands become stained so that the isoelectric focus pattern can be visualized.C)the individual bands become visualized by interacting with protein-specific antibodies in thesecond gel.D)the individual bands undergo a second, more intense isoelectric focusing.E)the proteins in the bands separate more completely because the second electric current is in theopposite polarity to the first current.28.Working with proteinsPages: 94–95Difficulty: 1Ans: BThe term specific activity differs from the term activity in that specific activity:A)is measured only under optimal conditions.B)is the activity (enzyme units) in a milligram of protein.C)is the activity (enzyme units) of a specific protein.D)refers only to a purified protein.E)refers to proteins other than enzymes.29.The covalent structure of proteinsPage: 96Difficulty: 1Ans: BThe functional differences, as well as differences in three-dimensional structures, between twodifferent enzymes from E.coli result directly from their different:A)affinities for ATP.B)amino acid sequences.C)roles in DNA metabolism.D)roles in the metabolism of E.coli.E)secondary structures.30.The covalent structure of proteinsPage: 99Difficulty: 2Ans: COne method used to prevent disulfide bond interference with protein sequencing procedures is:A)cleaving proteins with proteases that specifically recognize disulfide bonds.B)protecting the disulfide bridge against spontaneous reduction to cysteinyl sulfhydryl groups.C)reducing disulfide bridges and preventing their re-formation by further modifying the —SHgroups.D)removing cystines from protein sequences by proteolytic cleavage.E)sequencing proteins that do not contain cysteinyl residues.31.The covalent structure of proteinsPage: 100Difficulty: 3Ans: CA nonapeptide was determined to have the following amino acid composition: (Lys)2, (Gly) 2, (Phe) 2,His, Leu, Met. The native peptide was incubated with 1-fluoro-2,4-dinitrobenzene (FDNB) and then hydrolyzed; 2,4-dinitrophenylhistidine was identified by HPLC. When the native peptide wasexposed to cyanogen bromide (CNBr), an octapeptide and free glycine were recovered. Incubation of the native peptide with trypsin gave a pentapeptide, a tripeptide, and free Lys. 2,4-Dinitrophenyl-histidine was recovered from the pentapeptide, and 2,4-dinitrophenylphenylalanine was recovered from the tripeptide. Digestion with the enzyme pepsin produced a dipeptide, a tripeptide, and atetrapeptide. The tetrapeptide was composed of (Lys) 2, Phe, and Gly. The native sequence was determined to be:A)Gly–Phe–Lys–Lys–Gly–Leu–Met–Phe–His.B)His–Leu–Gly–Lys–Lys–Phe–Phe–Gly–Met.C)His–Leu–Phe–Gly–Lys–Lys–Phe–Met–Gly.D)His–Phe–Leu–Gly–Lys–Lys–Phe–Met–Gly.E)Met–Leu–Phe–Lys–Phe–Gly–Gly–Lys–His.32.The covalent structure of proteinsPage: 100Difficulty: 1Ans: CEven when a gene is available and its sequence of nucleotides is known, chemical studies of the protein are still required to determine:A)molecular weight of the protein.B)the amino-terminal amino acid.C)the location of disulfide bonds.D)the number of amino acids in the protein.E)whether the protein has the amino acid methionine in its sequence.33.The covalent structure of proteinsPage: 101Difficulty: 1Ans: CThe term “proteome” has been used to describe:A)regions (domains) within proteins.B)regularities in protein structures.C)the complement of proteins encoded by an organism’s DNA.D)the structure of a protein-synthesizing ribosome.E)the tertiary structure of a protein.34.The covalent structure of proteinsPages: 102–103Difficulty: 2Ans: CA major advance in the application of mass spectrometry to macromolecules came with thedevelopment of techniques to overcome which of the following problems?A)Macromolecules were insoluble in the solvents used in mass spectrometry.B)Mass spectrometric analyses of macromolecules were too complex to interpret.C)Mass spectrometric analysis involved molecules in the gas phase.D)Most macromolecules could not be purified to the degree required for mass spectrometricanalysis.E)The specialized instruments required were prohibitively expensive.35.Protein sequences and evolutionPages: 107–109Difficulty: 3Ans: ACompare the following sequences taken from four different proteins, and select the answer that best characterizes their relationships.A B C1 DVEKGKKIDIMKCS HTVEKGGKHKTGPNLH GLFGRKTGQAPGYSYT2 DVQRALKIDNNLGQ HTVEKGAKHKTAPNVH GLADRIAYQAKATNEE3 LVTRPLYIFPNEGQ HTLEKAAKHKTGPNLH ALKSSKDLMFTVINDD4 FFMNEDALVARSSN HQFAASSIHKNAPQFH NLKDSKTYLKPVISETA)Based only on sequences in column B, protein 4 reveals the greatest evolutionary divergence.B)Comparing proteins 1 and 2 in column A reveals that these two proteins have diverged the mostthroughout evolution.C)Protein 4 is the protein that shows the greatest overall homology to protein 1.D)Proteins 2 and 3 show a greater evolutionary distance than proteins 1 and 4.E)The portions of amino acid sequence shown suggest that these proteins are completely unrelated.Short Answer Questions36.Amino acidsPage: 76Difficulty: 1What are the structural characteristics common to all amino acids found in naturally occurringproteins?Ans: All amino acids found in naturally occurring proteins have an α carbon to which are attached a carboxylic acid, an amine, a hydrogen, and a variable side chain. All the amino acids are also in the L configuration.37.Amino acidsPage: 79Difficulty: 1Only one of the common amino acids has no free α-amino group. Name this amino acid and draw its structure.Ans: The amino acid L-proline has no free α-amino group, but rather has an imino group formed by cyclization of the R-group aliphatic chain with the amino group (see Fig. 3-5, p. 79).38.Amino acidsPages: 78–79Difficulty: 2Briefly describe the five major groupings of amino acids.Ans: Amino acids may be categorized by the chemistry of their R groups: (1) nonpolar aliphatics; (2) polar, uncharged; (3) aromatic; (4) positively charged; (5) negatively charged. (See Fig. 3-5, p. 79.)39.Amino acidsPages: 78–79Difficulty: 2A B C D E__________________________________________________________________Tyr-Lys-Met Gly-Pro-Arg Asp-Trp-Tyr Asp-His-Glu Leu-Val-PheWhich one of the above tripeptides:____(a) is most negatively charged at pH 7?____(b) will yield DNP-tyrosine when reacted with l-fluoro-2,4-dinitrobenzene andhydrolyzed in acid?____(c) contains the largest number of nonpolar R groups?____(d) contains sulfur?____(e) will have the greatest light absorbance at 280 nm?Ans: (a) D; (b) A; (c) E; (d) A; (e) C40.Amino acidsPage: 79Difficulty: 2Draw the structures of the amino acids phenylalanine and aspartate in the ionization state you would expect at pH 7.0. Why is aspartate very soluble in water, whereas phenylalanine is much less soluble?Ans: Aspartate has a polar (hydrophilic) side chain, which forms hydrogen bonds with water. In contrast, phenylalanine has a nonpolar (hydrophobic) side chain. (See Fig. 3-5, p. 79 for structures.)41.Amino acidsPage: 80Difficulty: 3Name two uncommon amino acids that occur in proteins. By what route do they get into proteins?Ans: Some examples are 4-hydroxyproline, 5-hydroxylysine, γ-carboxyglutamate, N-methyllysine, desmosine, and selenocysteine. Uncommon amino acids in proteins (other than selenocysteine)usually result from chemical modifications of standard amino acid R groups after a protein has been synthesized.42.Amino acidsPage: 81Difficulty: 1Why do amino acids, when dissolved in water, become zwitterions?Ans: Near pH = 7, the carboxylic acid group (—COOH) will dissociate to become a negativelycharged —COO– group, and the —NH2 amino group will attract a proton to become a positively charged —NH3+ group.43.Amino acidsPage: 82Difficulty: 1As more OH– equivalents (base) are added to an amino acid solution, what titration reaction will occur around pH = 9.5?Ans: Around pH = 9.5, the —NH3+ group will be titrated according to the reaction: —NH3+ + OH–→ —NH2 + H2O.44.Amino acidsPage: 83Difficulty: 3In the amino acid glycine, what effect does the positively charged —NH3+ group have on the p K a of an amino acid’s —COOH group?Ans: The positively charged amino group stabilizes the negatively charged ionized form of thecarboxyl group, —COO–, and repels the departing H+ thereby promoting deprotonation. The effect is to lower the p K a of the carboxyl group (see Fig. 3-11, p. 83).45.Amino acidsPage: 83Difficulty: 3How does the shape of a titration curve confirm the fact that the pH region of greatest buffering power for an amino acid solution is around its p K’s?Ans: In a certain range around the p K a’s of an amino acid, the titration curve levels off. Thisindicates that for a solution with pH ≈ p K, any given addition of base or acid equivalents will result in the smallest change in pH—which is the definition of a buffer.46.Amino acidsPage: 83Difficulty: 2Leucine has two dissociable protons: one with a p K a of 2.3, the other with a p K a of 9.7. Sketch a properly labeled titration curve for leucine titrated with NaOH; indicate where the pH = p K and the region(s) in which buffering occurs.Ans: See the titration curve for glycine in Fig. 3-10, p. 83.47.Amino acidsPage: 84Difficulty: 2What is the pI, and how is it determined for amino acids that have nonionizable R groups?Ans: The pI is the isoelectric point. It occurs at a characteristic pH when a molecule has an equal number of positive and negative charges, or no net charge. For amino acids with nonionizable R groups, pI is the arithmetic mean of a molecule’s two p K a values:pI = 1/2 (p K1 + p K2)48. Amino acidsPage: 84Difficulty: 2The amino acid histidine has a side chain for which the p K a is 6.0. Calculate what fraction of the histidine side chains will carry a positive charge at pH 5.4. Be sure to show your work.Ans: pH = p K a + log [acid]base][conjugate p K a – pH = log base][conjugate [acid]antilog (p K a – pH) =base][conjugate [acid]antilog (6.0 – 5.4) =base] [conjugate [acid]4 = [acid]/[conjugate base], or4[conjugate base] = [acid]Therefore, at pH 5.4, 4/5 (80%) of the histidine will be in the protonated form.49. Amino acidsPage: 84Difficulty: 2The amino acid histidine has three ionizable groups, with p K a values of 1.8, 6.0, and 9.2. (a) Which p K a corresponds to the histidine side chain? (b) In a solution at pH 5.4, what percentage of thehistidine side chains will carry a positive charge?Ans: (a) 6.0; (b) 80%. (See the previous problem for expanded solution to this problem.)50. Amino acidsPage: 85Difficulty: 2What is the uniquely important acid-base characteristic of the histidine R group?Ans: Only the imidazole ring of the histidine R group has a p K a near physiological pH (p K a = 6.0),which suggests that histidine may provide buffering power in intercellular and intracellular fluids.51. Peptides and proteinsPage: 86Difficulty: 1How can a polypeptide have only one free amino group and one free carboxyl group?Ans: This is possible only if the peptide has no side chains with carboxyl or amino groups. Then,with the exception of the single amino-terminal amino acid and the single carboxyl-terminal amino acid, all the other α-amino and carboxyl groups are covalently condensed into peptide bonds.52.Peptides and proteinsPage: 86Difficulty: 1Hydrolysis of peptide bonds is an exergonic reaction. Why, then, are peptide bonds quite stable?Ans: Peptide bonds are stable because hydrolysis of peptide (or amide) bonds has a high activation energy and as a result occurs very slowly.53.Peptides and proteinsPage: 86Difficulty: 2Draw the structure of Gly–Ala–Glu in the ionic form that predominates at pH 7.Ans: The peptide must have an amino-terminal Gly residue, a carboxyl-terminal Glu residue, and ionized amino and carboxyl groups.54.Peptides and proteinsPage: 86Difficulty: 2The artificial sweetener NutraSweet®, also called aspartame, is a simple dipeptide,aspartylphenylalanine methyl ester, on which the free carboxyl of the dipeptide is esterified to methyl alcohol. Draw the structure of aspartame, showing the ionizable groups in the form they have at pH 7.(The ionizable group in the side chain of aspartate has a p K a of 3.96.)Ans: See the structure on p. 86.55.Peptides and proteinsPage: 87Difficulty: 1If the average molecular weight of the 20 standard amino acids is 138, why do biochemists divide a protein’s molecular weight by 110 to estimate its number of amino acid residues?Ans: For each peptide bond formed, a molecule of water is lost, bringing the average molecular weight down to 120. To reflect the preponderance of low-molecular-weight amino acids, the average molecular weight is lowered further to 110.56.Peptides and proteinsPage: 87Difficulty: 2Lys residues make up 10.5% of the weight of ribonuclease. The ribonuclease molecule contains 10 Lys residues. Calculate the molecular weight of ribonuclease.Ans: From the structure of lysine, we can calculate its molecular weight (146); when it condenses (loses H2O, M r = 18) to form a peptide bond, the resulting residue contributes 146 – 18 = 128 to the protein’s molecular weight. If 10 Lys residues contribute 10.5% of the protein’s molecular weight, each Lys residue is 1.05%. To calculate the total molecular weight, divide 128 by 1.05% (0.0105);the result is 12,190. (The actual value is 13,700.)57.Peptides and proteinsPage: 88Difficulty: 1Define the primary structure of a protein.Ans: The primary structure of a protein is its unique sequence of amino acids and any disulfidebridges present in the native structure, that is, its covalent bond structure.58.Working with proteinsPages: 90-91Difficulty: 2Why do smaller molecules elute after large molecules when a mixture of proteins is passed through a size-exclusion (gel filtration) column?Ans: The column matrix is composed of cross-linked polymers with pores of selected sizes. Smaller molecules can enter pores in the polymer beads from which larger molecules would be excluded.Smaller molecules therefore have a larger three-dimensional space in which to diffuse, making their path through the column longer. Larger molecules migrate faster because they pass directly through the column, unhindered by the bead pores.59.Working with proteinsPages: 90-91Difficulty: 2For each of these methods of separating proteins, describe the principle of the method, and tell what property of proteins allows their separation by this technique.(a) ion-exchange chromatography(b) size-exclusion (gel filtration) chromatography(c)affinity chromatographyAns: (a) Ion-exchange chromatography separates proteins on the basis of their charges. (b) Size-exclusion or gel filtration chromatography separates on the basis of size (c) Affinity chromatography separates proteins with specific, high affinity for some ligand (attached to an inert support) from other proteins with no such affinity. (See Fig. 3-18, p. 91.)60.Working with proteinsPages: 90-93Difficulty: 2A biochemist is attempting to separate a DNA-binding protein (protein X) from other proteins in asolution. Only three other proteins (A, B, and C) are present. The proteins have the followingproperties:pI(isoelectric Size Bind topoint)M r DNA?––––––––––––––––––––––––––––––––––––––––––protein A7.482,000yesprotein B 3.821,500yesprotein C7.923,000noprotein X7.822,000yes––––––––––––––––––––––––––––––––––––––––––What type of protein separation techniques might she use to separate(a) protein X from protein A?(b) protein X from protein B?(c) protein X from protein C?Ans: (a) size-exclusion (gel filtration) chromatography to separate on the basis of size; (b) ion-exchange chromatography or isoelectric focusing to separate on the basis of charge; (c) specific affinity chromatography, using immobilized DNA.61.Working with proteinsPage: 92Difficulty: 2What factors would make it difficult to interpret the results of a gel electrophoresis of proteins in the absence of sodium dodecyl sulfate (SDS)?Ans: Without SDS, protein migration through a gel would be influenced by the protein’s intrinsic net charge—which could be positive or negative—and its unique three-dimensional shape, in addition to its molecular weight. Thus, it would be difficult to ascertain the difference between proteins based upon a comparison of their mobilities in gel electrophoresis.62.Working with proteinsPages: 93-95Difficulty: 2How can isoelectric focusing be used in conjunction with SDS gel electrophoresis?Ans: Isoelectric focusing can separate proteins of the same molecular weight on the basis of differing isoelectric points. SDS gel electrophoresis can then separate proteins with the same isoelectric points on the basis of differing molecular weights. When combined in two-dimensional electrophoresis, a great resolution of large numbers of proteins can be achieved.63.Working with proteinsPages: 94-95Difficulty: 3You are given a solution containing an enzyme that converts B into A. Describe what you would do to determine the specific activity of this enzyme solution.Ans: First, add a known volume of the enzyme solution (say, 0.01 mL) to a solution of its substrate B and measure the initial rate at which product A is formed, expressed as µmol/mL of enzymesolution/min. Then measure the total protein concentration, expressed as mg/mL. Finally, divide the enzyme activity (µmol/min/mL) by the protein concentration (mg/mL); the quotient is the specific activity.64.Working with proteinsPages: 94-95Difficulty: 2As a protein is purified, both the amount of total protein and the activity of the purified proteindecrease. Why, then, does the specific activity of the purified protein increase?Ans: Specific activity is the units of enzyme activity (µmol of product/min) divided by the amount of protein (mg). As the protein is purified, some of it is lost in each step, resulting in a drop in activity.However, other contaminating proteins are lost to a much greater extent. Therefore, with eachpurification step, the purified protein constitutes a greater proportion of the total, resulting in an increase in specific activity. (See also Table 3-5, p. 92.)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Measuring a Nation’s Income
Microeconomics is the study of how individual households and firms make decisions and how they interact with one another in markets. Macroeconomics is the study of the economy as a whole. Its goal is to explain the economic changes that affect many households, firms, and markets at once.
© 2007 Thomson South-Western
THE COMPONENTS OF GDP
GDP (Y) is the sum of the following:
Consumption (C) Investment (I) Government Purchases (G) Net Exports出口 (NX)
• “ . . . Within a Country . . .”
– It measures the value of production within the geographic confines of a country.
• “. . . In a Given Period of Time.”
Y = C + I + G + NX
© 2007 Thomson South-Western
THE COMPONENTS OF GDP
• Consumption (C):
• The spending by households on goods and services, with the exception of purchases of new housing.
© 2007 Thomson South-Western
REAL VERSUS NOMINAL GDP
• An accurate view of the economy requires adjusting nominal to real GDP by using the GDP deflator.
Factors of production
Wages, rent, and profit
MARKETS FOR FACTORS OF PRODUCTION •Households sell •Firms buy
Labor, land,
and capita资本l
Income = Flow of inputs and outputs
© 2007 Thomson South-Western
The GDP Deflator
• The GDP deflator is a measure of the price level calculated as the ratio of nominal GDP to real GDP times 100. • It tells us what portion of the rise in nominal GDP that is attributable to a rise in prices rather than a rise in the quantities produced.
© 2007 Thomson South-Western
Figure 1 The Circular-Flow Diagram
MARKETS FOR GOODS AND SERVICES •Firms sell Goods •Households buy and services sold Revenue Spending Goods and services bought
= Flow of dollars
© 2007ቤተ መጻሕፍቲ ባይዱThomson South-Western
THE MEASUREMENT OF GROSS DOMESTIC PRODUCT
• Gross 总的domestic国内 product (GDP) is a measure of the income and expenditures of an economy. • GDP is the total market value of all final goods and services produced within a country in a given period of time.
© 2007 Thomson South-Western
Table 2 Real and Nominal GDP
© 2007 Thomson South-Western
Table 2 Real and Nominal GDP
© 2007 Thomson South-Western
Table 2 Real and Nominal GDP
• “. . . Goods and Services . . .”
– It includes both tangible goods (food, clothing, cars) and 有 形的intangible services (haircuts, housecleaning, doctor
• Investment (I):
• The spending on capital equipment, inventories, and structures, including new housing.
© 2007 Thomson South-Western
THE COMPONENTS OF GDP
© 2007 Thomson South-Western
THE MEASUREMENT OF GROSS DOMESTIC PRODUCT
• The equality of income and expenditure can be illustrated with the circular-flow diagram.
© 2007 Thomson South-Western
THE ECONOMY’S INCOME AND EXPENDITURE
• When judging whether the economy is doing well or poorly, it is natural to look at the total income that everyone in the economy is earning.
– Includes all items produced in the economy and legally sold in markets
• “. . . Final . . .”
– It records only the value of final goods, not intermediate 中 间的goods (the value is counted only once).
FIRMS •Produce and sell goods and services •Hire and use factors of production
HOUSEHOLDS •Buy and consume goods and services •Own and sell factors of production
© 2007 Thomson South-Western
Measuring a Nation’s Income
Macroeconomics answers questions like the following:
Why is average income high in some countries and low in others? Why do prices rise rapidly in some time periods while they are more stable in others? Why do production and employment expand in some years and contract in others?
© 2007 Thomson South-Western
THE MEASUREMENT OF GROSS DOMESTIC PRODUCT
• “. . . Produced . . .”
– It includes goods and services currently produced, not transactions involving goods produced in the past.
THE COMPONENTS OF GDP
• GDP includes all items produced in the economy and sold legally in markets. • What Is Not Counted in GDP?
– GDP excludes most items that are produced and consumed at home and that never enter the marketplace. – It excludes items produced and sold illicitly违法的, such as illegal drugs.
– It measures the value of production that takes place within a specific interval of time, usually a year or a quarter (three months).
