Water Desalination Reference2009-32
环保产品认证目录09-09

工业废气吸收净化装置(HJ/T 387-2007)
20
工业废气吸附净化装置(HJ/T 386-2007)
21
工业有机废气催化净化装置(HJ/T 389-2007)
22
静电式电焊烟尘净化器(CCAEPI-RG-Q-022)
23
中小型燃油、燃气锅炉(HJ/T287-2006)
24
空气净化器(GB/T 18801-2002)
5
水处理剂聚丙烯酰胺(GB 17514-1998)
6
改性天然矿物污水处理剂(CCAEPI-RG-S-030)
7
木质净水用活性炭(GB/T 13803.2-1999)
8
火力发电厂水处理用活性炭(DL/T 582-2004)
9
悬挂式填料(HJ/T245-2006)
10
悬浮填料(HJ/T246-2006)
14
转刷曝气装置(HJ/T259-2006)
15
转盘曝气装置(HJ/T280-2006)
16
射流曝气器(HJ/T263-2006)
17
散流式曝气器(HJ/T281-2006)
18
中、微孔曝气器(HJ/T252-2006)
19
鼓风式潜水曝气机(HJ/T260-2006)
20
单级高速曝气离心鼓风机(HJ/T278-2006)
34
电子式水处理器(HG/T 3133-2006)
35
水处理用活性炭吸附罐(JB/T 10193-2000)
36
电解法二氧化氯协同消毒剂发生器(HJ/T257-2006)
37
化学法二氧化氯消毒剂发生器(HJ/T272-2006)
38
电解法次氯酸钠发生器(HJ/T258-2006)
原位净化水处理技术

试验期处理效果:
分析:
TP: 浓度均无明显变化规律,原因 是因为时间太短。 PH:3种水生植物的引入有效地稳 定了水环境中的pH 值水平 CODCr:几组试验对其都有显著的 去除效果,但有水草部分的处理效 果显得更好。 TN:对TN都有一定的去除效果, 但是水葫芦和芦苇显得更好 氨氮:对氨氮的去除与TN不同,有 其自身的特点。
3、阿科蔓生态基技术 概念:阿科蔓生态基是一种用于生态性水处理的 高科技材料, 通过发展生态基上的本土微生物群 落, 使微生物种类和生物量达到最大化, 利用其代 谢作用去除水中的污染物。产品主要可分为BDF 和SDF两大类型。
SDF型阿科蔓生态基 SDF型生态基主要由上部疏松层和下部密实层组成,其中 疏松层有利于藻类生长,密实层有利于菌类生长,也可设 计成只有疏松层和密实层。其下部材料可以切割成条带状 ,或流出过水缝口,有利于水流的控制。SDF的上部带有 黑色套筒,在安装中可以把尼龙绳穿过套筒,绳子两端固 定在岸边或池壁的桩或挂钩上,从而将SDF型生态基悬挂 安装在水体或处理池表面(也可分层安装)。如果跨度较 大,在尼龙绳上可安装浮球,保持浮力。在曝气池中, SDF型生态基可以在下面也设置套筒,避免被气流吹起。
BDF型阿科蔓生态基 BDF型生态基的材料主要由编织层和泡沫层组成 。其中编织层可根据实际需要设置纤维的疏密程 度,使其表面形成藻菌共生的微生物体系。泡沫 层位于两层编织层的中间,通过特殊的处理使其 与编织层紧密贴合在一起,它可以使BDF型生态 基在水流中保持漂浮状态。BDF的上部切割成条 带状,形成仿生水草形态,有利于生物膜与水流 的接触,底部缝制成套筒,其中可以装入砂石重 物,使其稳定地放置在水体的底部。
应用实例: ”好氧塘-水葫芦湿地-苦草湿地“示范工程位于江 苏省宜兴市大浦镇召庄河,该示范工程于2004年5 月建造完毕。
堆工原理 UNIT 1

HISTORY OF NUCLEAR POWER THE FORTH GENERATION OF NUCLEAR POWER PLANT
7
UNIT 1 HISTORY OF NUCLEAR POWER
New Words and phrases
nuclear power nuclear fission prototype nuclear reactor submarine go critical start (commenced) sea trial power operation atomic bomb hydrogen bomb nuclear power plant go into operation be put into operation self-sustaining nuclear fission chain Heavy Water Research Reactor (HWRR)
2
PREFACE
The purpose of this book is to describe the fundamental scientific and engineering principles of nuclear reactor systems, especially those used for the generation of electric power. The main emphasis is on aspects of reactor design and operation that are related to the fission process and its associated radiations, rather than on engineering areas that are not unique to nuclear reactors. The treatment is such that the book can serve as a text for students and as a reference for practicineactors for the generation of electric power stem (起源于) historically from the submarine reactor. It was early realized that a compact nuclear power plant would have great advantages for submarine propulsion, since it would make possible long voyages at high speeds without the necessity for resurfacing at frequent intervals. In 1948, therefore, the Argonne National Laboratory was assigned the task of designing a reactor that would be suitable for this purpose.
印尼水处理强制标准_PerMen_008_Tahun_2009_Translated11012012_

REGULATION OF THE STATE MINISTER OF ENVIRONMENTNUMBER 08 YEAR 2009ONWASTE WATER QUALITY FOR THERMAL POWER PLANT BUSINESSAND/OR ACTIVITIESSTATE MINISTER OF ENVIRONMENT,Whereas : a. in order to conserve environment function, it is necessary totake control on business and / or activities that potentiallycause pollution and / or destruction to environment.b.business and / or thermal power plant is a business and / oractivities that potentially cause pollution and / or destruction ofenvironment, so that it is necessary to take control on disposalof waste water from business and / or thermal power plantactivities.c.based on considerations as referred to letter a and letter b, aswell as to implement regulation Chapter 21 verse (1)Government Regulation Number 82 Year 2001 on Water QualityManagement and Water Pollution Control, it is necessary toestablish Regulation of The Minister of Environment on WateWater Quality for Business and / or Thermal Power PlantActivities.Remembering:1. Laws Number 15 Year 1985 on Electricity (State Gazette of Republic of Indonesia Year 1985 Number 74, Additional StateGazette of Republic of Indonesia Number 331);ws Number 23 Year 1997 on Environment Management(State Gazette of Republic of Indonesia Year 1997 Number 68,Additional State Gazette of Republic of Indonesia Number3699);ws Number 27 Year 2003 on Geothermal (State Gazette ofRepublic of Indonesia Year 2003 Number 115, AdditionalState Gazette of Republic of Indonesia Number 4327);ws Number 32 Year 2004 regarding Local Government(State Gazette of Republic of Indonesia Year 2004 Number125, Additional State Gazette of Republic of IndonesiaNumber 4437) as last modified with Laws Number 12 Year2008 regarding Second Amendment of Laws Number 32 Year2004 regarding Local Government (State Gazette of Republicof Indonesia Year 2008 Number 59, Additional State Gazetteof Republic of Indonesia Number 4844);ernment Regulation Number 19 Year 1999 regardingPollution Control and / or Sea Destruction (State Gazette ofRepublic of Indonesia Year 1999 Number 32, AdditionnalState Gazette of Republic of Indonesia Number 3816)ernment Regulation Number 27 Year 1999 regardingEnvironmental Impact Assesment (State Gazette of Republicof Indonesia Year 1999 Number 59, Additional State Gazetteof Republic of Indonesia Number 3838);ernment Regulation Number 82 Year 2001 regardingWater Quality Management and Water Pollution Control(State Gazette of Republic of Indonesia Year 2001 Number153, Additional State Gazette of Republic of IndonesiaNumber 4161);ernment Regulation Number 38 Year 2007 regardingDivision of Government Affairs between Government,Provincial Government, and Distric / Municipal Government(State Gazette of Republic of Indonesia Year 2007 Number82, Additional State Gazette of Republic of Indonesia Number4737);9.Presidential Regulation Number 9 Year 2005 regardingposition, task, function, organization structure and workprocedures of State Ministry of Republic of Indonesia as hasbeen ammended several times, last ammended withPresidential Regulation Number 94 Year 2006RESOLVE:Stipulate : REGULATION OF STATE MINISTER OF ENVIRONMENTREGARDING WASTE WATER QUALITY FOR BUSINESSAND / OR ACTIVITIES OF THERMAL POWER PLANT.Chapter 1In this Minister Regulation, what is meant by1.Business and / or activities of thermal power plant is business and oractivity that utilize either solid, liquid, and gas fuel or mixed as well as utilizing geothermal steam to produce electricity2.Waste water is residual result of a business and / or activities in the formof liquid.3.Main Process is the process that produces waste water that sources fromwashing process (with or without chemical) of all metal equipment, blow down cooling tower, blow down boiler, laboratory, and resin regeneration of water treatment.4.Supporting activity is the activity that cover activity of cooling water facility,activity of desalination facility, activity of coal stockpile, and discharge activity of flue gas desulphurization (FGD) activity of the sea water scrubber.5.Oily water is the wastewater containing oil that sources from work floordrainage, seepage, wastewater leakage from equipment washing, spill fromoperational activity that is disposed to environment media through separator pool or oil separator or oil catcher or oil trap.6.Blowdown boiler is the effort to discharge minimum wastewater from boilerrecirculation process based on best engineering practice.7.Blowdown cooling tower is the effort to discharge wastewater as a product ofcondensation of cooling process of the cooling tower based on best engineering practice.8.Heat water is wastewater that sources from cooling process that utilize seawater as raw water and distributed once (in once through system) through condenser toward the sea.9.Desalination or reverse osmosis (RO) is water purification process thatproduce wastewater in the form of brine reject.10.Flue gas desulphurization (FGD) with sea water wet scrubber system issulphur absorption system from gas discharge emission using seawater.11.Coal stockpile is the coal stack that produce wastewater in the form ofrunoff water12.Water treatment plant (WTP) or demineralization is raw water purificationprocess for process or domestic purpose13.Wastewater quality is the limit or tolerable contaminant level and / ornumber of contaminant in the wastewater that will be discharged or disposed to water source from business and / or activity14.Maximum wastewater level is the maximum level allowable to be dischargedto environment.15.Normal condition is the operation condition in accordance with operationdesign parameter16.Abnormal condition is the operation condition beyond normal operationparameter and still controllable namely: start-up, shutdown and upset.17.Emergency condition is the operation condition outside normal operationparameter and uncontrollable.18.Setup point is one or more location as reference in monitoring wastewaterquality.19.Related institution is the institution that responsible for electricity.20.Minister is the minister that organize government affairs in the field ofenvironment management.Chapter 2Business type and / or activity that is regulated in this Minister Regulationcovers activity:a.Coal Fired Steam Power Plantb.Gas Fired Power Plantbined Cycle Power Plantd.Diesel Power Plante.Geothermal Power PlantChapter 3Waste water from business and / or activity as referred to in Chapter 2 sourcefrom:a. main processb. supporting activity; andc. other activity that produce oily water.Chapter 4Waste water quality that is regulated in this Minister Regulation covers:a.waste water quality source from main process as specified in Attachment Ias integral part of this Minister Regulation;b.waste water quality source from supporting activity as specified inAttachment II as integral part of this Minister Regulation; andc.waste water quality source from other activity that produce oily water asspecified in Attachment III as integral part of this Minister RegulationChapter 5(1)In normal condition, waste water quality as referred to in Chapter 4 at alltime shall not be exceeded by business and / or thermal power plant activitycaretaker as referred to in Chapter 2.(2)For business and / or activity that operates after issuance of this MinisterRegulation, especially for heat water temperature parameter, analysis basedstandard quality with more stringent regulation than waste water quality asreferred in Chapter 4 shall be applied.(3)Waste water quality as referred to in verse (1) is set according to maximumlevel.Chapter 6(1) Provincial Government may set:a.waste water standard quality for business and / or thermal power plantactivity with the same or more stringent provision than standard quality as referred in Chapter 4; and / orb.additional parameter out of parameters as specified in Attachment of thisMinister Regulation after approved by Minister.(2) Minister may approve or reject additional parameter as referred in clause (1)letter b at the latest 90 (ninety) working days since acceptance of the request bypaying attention to suggestion and consideration from related technical institution.(3) if in the period as mentioned in clause (2) Minister does not give decisionagainst the request as referred in clause (1) letter b, the request is deemed approved.(4) Rejection of the request as referred in clause (2) is accompanied by reasons.(5) Waste water quality as referred in clause (1) is set with provincial regulation.Chapter 7In case the result of Environmental Impact Analysis / AMDAL of the business and/ or thermal power plant activity requires stringent waste water quality than wastewater quality as referred in Chapter 4 or Chapter 6, then waste water quality forbusiness and / or thermal power plant activity as required by AMDAL is appliedChapter 8In case the result of the analysis of waste water discharge for business and / orthermal power plant activity requires stringent waste water quality than waste water quality as referred in Chapter 4, Chapter 6, or Chapter 7, then waste water quality according to analysis result is applied.5Chapter 9Caretaker of the business and / or thermal power plant activity is obliged to:a.identify waste water sources, including putting name code and thequantity;b.determine waste water source coordinate, arrangement point, and wastewater discharge point;c.perform waste water channel documentation;d.perform waste water treatment so that the quality of waste water disposalwill not exceed waste water quality standard as regulated in this MinisterRegulation;e.apply waterproof waste water channel system so that waste water seepagewill not occur;f.separate waste water disposal channel with rain water runoff channel;g.install measuring instrument for waste water flow and perform daily wastewater flow recording;h.perform recording of real production monthly;i.not performing waste water dilution, including mixing coolant disposal towaste water discharge flow;j.perform calibration or function check of the waste water measuring instrument;k.create log book system or electronic enterprise system for waste water management;l.arrange and set up procedure for handling abnormal condition and emergency condition;m.check the content of waste water standard quality parameter as listed in the attachment of this Minister Regulation periodically at least once (1) inone (1) month and once (1) in three (3) months performed in accredited laboratory;n.check the content of waste water standard quality parameter special for Diesel Power Plant in accredited laboratory at least once (1) in six (6) months;o.perform monitoring of daily waste water flow from waste water of main process and hot water;p.calculate waste water pollution load by multiply waste water flow with waste water standard quality parameter concentration;q.submit report about real monthly production recording, laboratory analysis result, daily wastewater flow, and wastewater pollution load as referred in letter h, letter m, letter o, and letter p, once (1) in three (3) months and laboratory analysis result as referred in letter n, once (1) in six (6) months to Mayor with copy to Governor, Minister, and technical institutions;r.notify abnormal events and emergency conditions within 1 x 24 hours to Mayor with copy to Governor, Minister and technical institutions; ands.report any counter measures for abnormal events and emergency conditions at the latest 7 x 24 hours to Mayor with copy to Governor, Minister and technical institutions.Chapter 10(1)Waste water standard quality for business and / or thermal power plantactivity which has been set less stringent before this Minister Regulation isset, is obliged to adjust with this Minister Regulation in at least one (1) year.(2)Waste water disposal permit for business and / or thermal power plantactivity which has been existed before this Minister Regulation become effective, remains valid until the period of validity over.Chapter 11At the time this Minister Regulation becomes effective, all regulations related with waste water standard quality for business and / or thermal power plant activity which has been existed, remain valid as long as they do not conflict withthis Minister Regulation.Chapter 12This Minister Regulation becomes effective on the date of signingSet in JakartaOn : 7 April 2009MINISTER OFENVIRONMENTRACHMAT WITOELARAttachment IRegulation of Minister of EnvironmentNumber : 08 Year 2009Date : 7 April 2009WASTE WATER STANDARD QUALITY FOR THERMAL POWER PLANT BUSINESSAND / OR ACTIVITY SOURCE FROM MAIN PROCESSA Source Main ProcessNo. Parameter Unit Maximum Content1.Ph - 6 - 92.TSS mg/L 1003.Oil and Fat mg/L 104.Free Chlorine (Cl2)* mg/L 0,55.Total Chromium (Cr)mg/L 0,56.Copper (Cu) mg/L 17.Ferro (Fe) mg/L 38.Zinc (Zn) mg/L 19.Phosphat (PO4-) **mg/L 10Note: * If cooling tower blowdown is flown to Waste Water Treatment Installation (IPAL)** If performing Phosphat injectionB. Source Blowdown BoilerNo. Parameter Unit Maximum Content1.pH - 6 – 92.Copper (Cu) mg/L 13.Ferro (Fe) mg/L 3Note : If blow down boiler maximum waste water is not flown to IPAL (Waste Water Treatment InstallationC. Source Blowdown Cooling TowerNo. Parameter Unit Maximum Content1 pH - 6-92Free Chlorine (Cl2)mg/L 13 Zinc (Zn)mg/L 14 Photyhat (PO4-) mg/L 10Note : If source waste water blowdown cooling tower is not flown to Waste Water Treatmet Installation (IPAL)D. Source Demineralization /WTPNo. Parameter Unit Maximum Content1.pH - 6 - 92.TSS mg/L 100Note : If waste water demineralization / WTP is not flown to Waste Water TreatmentInstallation (IPAL).STATE MINISTER OF ENVIRONMENT,RACHMAT WITOELAR9Attachment IIRegulation of State Minister ofEnvironmentNumber : 08 Year 2009Date : 7 April 2009WASTE WATER STANDARD QUALITY FOR THERMAL POWER PLANT BUSINESSAND / OR ACTIVITY SOURCE FROM SUPPORTING ACTIVITYA. Source Cooler (Heat Water)No. Parameter Unit Maximum ContentI. Temperature o C 40*2. Free Chlorine (Cl2)mg/L 0,5Note: If heat water source is not flown to Waste Water Treatment Installation (IPAL)* Monthly average measurement result at condenser outletB. Source DesalinationNo. Parameter Unit Maximum Content1.pH - 6 – 92.Salinity °/00In the radius of 30 mfrom waste waterdisposal location to thesea, waste watersalinity must havealready the same withnatural salinity content.Note : If the waste water source from desalination is not flown to Waste Water TreatmentInstallation (IPAL)C. Source FGD System Sea Water Wet ScrubberNo. Parameter Unit Maximum Content1.pH 6 - 92.SO4 (2-)% Maximum contentincrement of Sulphate is4% compared withsulphate content of seawater inlet point.Note: If waste water source from FGD of Sea Water Wet Scrubber System is not flown toWaste Water Treatment Installation (IPAL)D. Source Coal StockpileNo. Parameter Unit Maximum Content1.pH - 6 – 92.TSS mg/L 2003.Fe mg/L 54.Mn mg/L 2Note: If waste water source from Coal Stockpile is not flown to WasteWater Treatment Installation (IPAL)MINISTER OFENVIRONMENT,RACHMAT WITOELAR.Attachment IIIRegulation of Minister ofEnvironmentNumber : 08 Year 2009Date : 7 April 2009WASTE WATER STANDARD QUALITY FOR THERMAL POWER PLANT BUSINESSAND OR ACTIVITYOILY WATERNO Parameter Unit Maximum Content1 COD* mg/ L 3002 TOG**mg/ L 1103 Oil and Fat mg/ L 15Note: If oily waste water is not flown to Waste Water Treatment Installation (IPAL)* Parameter COD is valid until 31 December 2009** Parameter Total Organic Carbon (TOC) comes into force on 1 January 2010STATE MINISTER OFENVIRONMENT,RAHMAT WITOELAR。
Parker Hannifin 全球空气准备系统-P32系列过滤器 压力减压器说明书
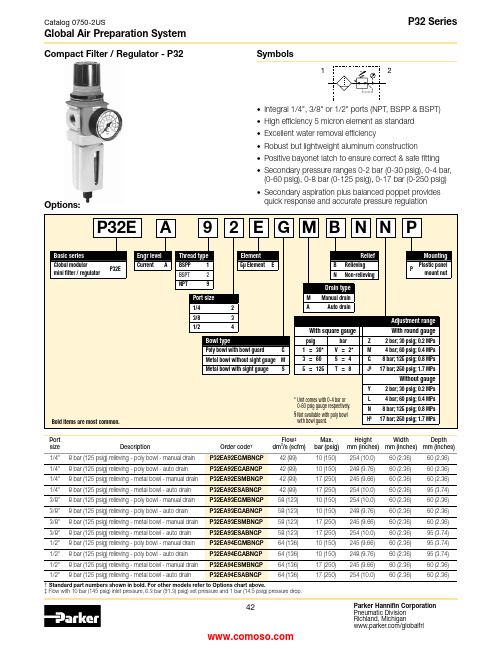
Global Air Preparation SystemCompact Filter / Regulator - P32SymbolsPort†Flow ‡3Max.Height Width Depth • Integral 1/4", 3/8" or 1/2" ports (NPT, BSPP & BSPT)• High efficiency 5 micron element as standard • Excellent water removal efficiency• Robust but lightweight aluminum construction • Positive bayonet latch to ensure correct & safe fitting • Secondary pressure ranges 0-2 bar (0-30 psig), 0-4 bar, (0-60 psig), 0-8 bar (0-125 psig), 0-17 bar (0-250 psig)• Secondary aspiration plus balanced poppet provides quick response and accurate pressure regulation‡ Flow with 10 bar (145 psig) inlet pressure, 6.3 bar (91.3) psig) set pressure and 1 bar (14.5 psig) pressure drop.1Options:Global Air Preparation SystemS e c o n d a r y P r e s s u r e - b a r S e c o n d a r y P r e s s u r e - (p s i g )0204060801001201400Flow - dm 3/s Flow - (scfm)40802060120180160100140S e c o n d a r y P r e s s u r e - b a r S e c o n d a r y P r e s s u r e - (p s i g )204060801001201400Flow - dm 3/s Flow - (scfm)20406080100120140S e c o n d a r y P r e s s u r e - b a r S e c o n d a r y P r e s s u r e - (p s i g )0204060801001201400Flow - dm 3/s Flow - (SCFM)20406080100120140160For best performance, regulated pressure should always be set by increasing the pressure up to the desired setting.Flow ChartsSpecificationsFlow capacity* 1/4 42 dm 3/s (89 scfm) 3/8 58 dm 3/s (123 scfm) 1/2 64 dm 3/s (136 scfm)Operating Plastic bowl -25°C to 52°C (-13°F to 125°F) temperature Metal bowl -25°C to 65.5°C (-13°F to 150°F)Max. supply Plastic bowl 10 bar (150 psig) pressure Metal bowl 17 bar (250 psig)Standard filtration 5 micronUseful retention † 51 cm 3(1.7 US oz.)Adjusting range pressure 0-2 bar (30 psig) 0-4 bar (60 psig) 0-8 bar (125 psig) 0-17 bar (250 psig)Port size BSPP / BSPT / NPT 1/4, 3/8, 1/2Gauge port (2 ea.) BSPP / BSPT / NPT 1/4Weight0.53 kg (1.17 lbs)* Inlet pressure 10 bar (145 psig). Secondary pressure 6.3 bar (91.3 psig).†Useful retention refers to volume below the quiet zone baffle.Air quality :Within ISO 8573-1: 1991 Class 3 (Particulates) Within ISO 8573-1: 2001 Class 6 (Particulates)Material SpecificationsBody Aluminum Adjustment knob AcetalBody capABS Element retainer / Baffle Acetal Bowl Plastic bowl Polycarbonate Metal bowl Zinc Bowl guard NylonFilter elementSintered polyethylene Seals NitrileSpringsMain regulating / valve Steel / S.S.Valve assembly Brass / Nitrile Diaphragm assembly Nitrile / ZincPanel nut Acetal Sight gaugeMetal bowl Polycarbonate Repair and Service KitsPlastic bowl / Bowl guard manual drain P32KA00BGM Metal bowl / Sight gauge manual drain P32KA00BSM Auto drainP32KA00DA 5µ particle filter elementP32KA00ESE Regulator repair kit - relieving P32KA00RB Regulator repair kit - non-relieving P32KA00RC Panel mount nut - aluminum P32KA00MM Panel mount nut - plasticP32KA00MP Angle bracket (fits to panel mount threads) P32KA00MR T -bracket (fits to body connector) P32KA00MB T -bracket with body connector P32KA00MT Body connectorP32KA00CB1/4 Filter / Regulator3/8 Filter/Regulator1/2 Filter/RegulatorWARNINGProduct rupture can cause serious injury.Do not connect regulator to bottled gas.Do not exceed Maximum primary pressure rating.!Gauges50mm (2") Round 1/4" center back mount0-30 psig / 0-2 bar K4520N140300-60 psig / 0-4 bar K4520N140600-160 psig / 0-11 bar K4520N141600-300 psig / 0-20 bar K4520N14300Manual DrainAutomatic DrainDimensions mm (inches)。
东丽海水淡化反渗透膜及其应用

高脱硼海水淡化膜可以降低SWRO后续流程负荷。
1.2 反渗透脱硼技术
单级SWRO产水硼浓度(计算值)
SWRO产水硼浓度 (mg/L) 海水 (温度、TDS、硼浓度) 日本 (25ºC, 3.5%, 5mg/L) 膜元件硼脱除率 90% 1.5 1.6 3.0 95% 0.9 TM820R/M系列 1.0 2.0 0.5 1.1 0.2 0.4 97% 0.4 99% 0.2
100
RO膜
支持膜 基材
硼酸 (分子直径0.4nm)
硼 去 95 除 率 90
(%)
改良膜
传统膜
0.5 0.6 0.7 0.8
孔
85 0.4
制水量(m3/m2・日)
新技术的要点: 亲水性改性设计保证水通量
TBMC正采用上述技术制造所有反渗透膜元件
1.2 反渗透脱硼技术
海水淡化系统中高脱硼反渗透膜的应用 海水淡化系统中高
超高压脱盐层 交联芳香族聚酰胺 0.3μm 支撑层 聚砜 45μm 基层 无纺布 100μm
既抗高压 又保通量
RO膜的放大图 (UHR-FE-SEM) x 50,000
产品水
2.4 BCS的优势
两段法海水淡化的水量平衡图
( ) : 水的流率
常规海水淡化系统(回收率 40%)
(250) (150)
(100)
Features/特征
99.80 99.80 99.75
8,500 (32.2) 7,000 (26.5) 6,000 (22.7)
95 95 93 93
High Boron Rejection/高脱硼率 91 High Water Productivity/高产水量 92 High Boron Rejection/高脱硼率 High Water Productivity/高产水量 Alkaline Tolerance/高耐碱(pH 10) High Boron Rejection/高脱硼率
离子膜烧碱翻译
离子膜处理氯碱过程中饱和盐水伊朗,克尔曼沙阿,拉齐大学化学工程系S.S. Madaeni , V. Kazemi摘要氯碱厂的饱和卤水含有一些杂质包括Mg2+, Ca2+, Fe2+和SO42−对氯碱装置性能产生影响。
膜可结合其它处理方法来减少杂质到一个理想的水平。
在这项工作中,总共有七个聚合物膜(FT30,PVD,DOW-PS,TFC - SR,BW30,37100和NF45)可用来处理饱和盐水。
聚对苯二甲酸乙二醇脂PVD膜在8 个大气压和40摄氏度下显示性最佳性能,包括流量适中,适当的去除杂质能力和对氯化钠的最小排斥反应。
亲水的聚砜37100膜表现出最高的光通量(300 l/m2 H),没有足够去除杂质的能力。
纳滤聚对苯二甲酸乙二醇酯NF45膜表现出对Fe2 +的去除(98%)能力与对NaCl的最大排斥能力(21%)。
DOW-PS膜表现出对SO42-的最大去除能力。
离子排斥效应取决于的水合半径大小和离子浓度的高低。
越大的水化半径和越低浓度的离子,表现出最高的排斥反应。
关键词:膜;盐水;处理;氯碱;反渗透1、简介有三种不同的氯碱处理系统,包括汞电池,隔膜电解槽,离子膜槽。
这些氯碱处理系统是基于电解氯化钠或氯化钾形成的产品氯气,氢气和氢氧化钠或氢氧化钾。
在产品的质量和操作上的技术上,三者之间有着显著的技术差异[1]。
这些技术之间的重要区别之一是卤水净化步骤。
汞电池和隔膜电解槽的技术不需要高纯度盐水。
但是膜细胞过程,必须具有高纯度的盐水。
隔膜和汞电解槽过程的沉淀和过滤,使得卤水净化足够充分,但对于离子膜槽,还要进行盐水的二级处理过程。
影响离子交换膜性能最主要的杂质是镁离子和钙离子。
这些杂质结合氢氧化钠,沉淀在其表面对离子交换膜产生影响[2]。
水的含盐量很重要,因此分离单价多价离子是工业应用中的一个重大问题。
一个典型的例子是饮用水的生产。
二价钙,镁等应(部分)除去而得到软水[3]。
近年来,膜分离过程已经被用来集中或分馏的悬浮颗粒和溶解物质。
纯水系统技术手册上
〄 3. 鹼度(Alkalinity)〆
〄 鹼度是指水中可以和酸中和之成分的含量,主要成分為氫氧根(OH-),碳酸 〄 根(CO3,)及碳酸氫根(HCO3-),由於碳酸鈣是最主要的結垢來源,因此分析 〄 水中的鹼度對結垢預測是非常重要的。
〄 4. 硬度(Hardness)〆
〄 〄 〄 〄 〄 硬度最主要來源為鈣和鎂,因此在水質分析上鈣硬度和鎂硬度兩者的總和稱 為總硬度,常用的硬度單位為ppm as CaCO3,硬度成分依能否以加熱方式去 除而被分為永久硬度和暫時硬度,永久硬度是指鈣,鎂的氯化物、矽酸鹽和硫酸 鹽等,硬度成分在水被加熱的過程中不會沉澱下來,暫時硬度主要係指碳酸 鈣,這些成分在加熱時會結晶析出形成沉澱物。
〄 2.導電度與比電阻值(Conductivity and Resistivity)〆
〄 〄 〄 〄 〄 導電度與電阻值均用來表示水中導電物質的多寡。導電度的單位是 Microsiemens / cm,比電阻值的表示單位是ohms-cm。導電度與比電阻兩 者互為倒數。一般處理水的導電度較高,因此水質可以用導電度來表示々 至於純水,尤其是超純水,由於導電度極低,因此以比電阻值來表示較方便。
(Ionic Exchange) (Removed >1 µm size particle)
RO Tank
To Polishing System
Note: U.V. : Ultraviolet Lamp SF : Safety Filter(10µm pore size) PF : Post Filter(1µm pore size) RO : Reversed Osmosis
〄 7. 淤泥污堵指數(Silt Density Index, SDI)〆
International Journal of Electrical Power & Energy Systems
Integrating agroecology and landscape multifunctionality in Vermont: An evolving86Agricultural Systems, Volume 103, Issue 5, June 2010, Pages 327-341Sarah Taylor Lovell, S’ra DeSantis, Chloe A. Nathan, Meryl Breton Olson, V.Ernesto Méndez, Hisashi C. Kominami, Daniel L. Erickson, Katlyn S. Morris,William B. MorrisClose preview | Purchase PDF (1589 K) | Related articles | Related reference work articlesAbstract | Figures/Tables | ReferencesAbstractAgroecosystems cover vast areas of land worldwide and are known to have a large impacton the environment, yet these highly modified landscapes are rarely considered ascandidates for landscape design. While intentionally-designed agricultural landscapes couldserve many different functions, few resources exist for evaluating the design of thesecomplex landscapes, particularly at the scale of the whole-farm. The objective of this paper isto introduce an evolving framework for evaluating the design of agroecosystems based on acritical review of the literature on landscape multifunctionality and agroecology. We considerhow agroecosystems might be designed to incorporate additional functions while adhering toagroecology principles for managing the landscape. The framework includes an assessmenttool for evaluating farm design based on the extent of fine-scale land use features and theirspecific functions, to consider the present state of the farm, to plan for future conditions, or tocompare alternative futures for the design of the farm. We apply this framework to two farmsin Vermont that are recognized locally as successful, multifunctional landscapes. TheIntervale Center, an agricultural landscape located within the city limits, serves as anincubator for new farm startups and provides unique cultural functions that benefit the localcommunity. Butterworks Farm, a private operation producing organic yogurt and other foodproducts, achieves important ecological functions through an integrated crop-livestocksystem. These farms and many others in Vermont serve as models of a framework that integrates landscape multifunctionality and agroecology in the design of the landscape. In the discussion section, we draw from the literature and our work to propose a set of important themes that might be considered for future research.Article Outline1. Introduction2. Literature review2.1. Agroecology2.2. Landscape multifunctionality3. Framework for designing agroecosystems4. Methods4.1. Study site4.2. Semi-structured interviews4.3. Agroecosystem design assessment tool5. Results5.1. Regional characterization and context of Vermont agricultural landscape5.2. Case study 1: the Intervale5.2.1. Overview and site history5.2.2. Production functions5.2.3. Ecological functions5.2.4. Cultural functions5.2.5. Multifunctional landscape assessment5.3. Case study 2: Butterworks Farm5.3.1. Overview and site history5.3.2. Production functions5.3.3. Ecological functions5.3.4. Cultural functions5.3.5. Multifunctional landscape assessment6. Discussion6.1. Study limitations6.2. Future research6.3. Emerging themes6.3.1. Theme 1: policy-driven versus grass-roots initiatives6.3.2. Theme 2: assessment methods and tools6.3.3. Theme 3: synergies and integration of functions6.3.4. Theme 4: geography of agricultural systems6.3.5. Theme 5: alternative farm types7. ConclusionAcknowledgementsReferencesPlanning production on a single processor with sequence-dependent setups part 1:87Computers & Chemical Engineering, Volume 25, Issues 7-8, 15 August 2001,Pages 1021-1030Hong-Choon Oh, I. A. KarimiClose preview | Purchase PDF (154 K) | Related articles | Related reference work articlesAbstractAbstractProduction planning of processors located within in a facility or distributed across facilities isa routine and crucial industrial activity. So far, most attempts at this have treated planninghorizon as a decision variable, and have limited their scope to sequence-independentsetups. In this two-part paper, we present a new and improved methodology for solving thesingle machine economic lot scheduling problem (ELSP) with sequence-dependent setupsand a given planning horizon. We decompose the entire complex problem into twosubproblems; one involving lot sizing and the other involving lot sequencing and scheduling.In this part, we present a novel mixed integer nonlinear programming (MINLP) formulation forthe lot-sizing problem. Using a multi-segment separable programming approach, wetransform this MINLP into a MILP and propose one rigorous and two heuristic algorithms forthe latter. Based on a thorough numerical evaluation using randomly simulated largeproblems, we find that our best heuristic gives solutions within 0.01% of the optimal on anaverage and in much less time than the optimal algorithm. Furthermore, it works equally wellon problems with sequence-independent setups. Overall, our methodology is well suited forreal-life large-scale industrial problems.Advances in genetically engineered (transgenic) plants in pest management—an88Crop Protection, Volume 22, Issue 9, November 2003, Pages 1071-1086R. Mohan Babu, A. Sajeena, K. Seetharaman, M. S. ReddyClose preview | Purchase PDF (369 K) | Related articles | Related reference work articlesAbstract | Figures/Tables | ReferencesAbstractTransgenic plants are produced via Agrobacterium mediated transformation and other directDNA transfer methods. A number of transgenes conferring resistance to insects, diseasesand herbicide tolerance have been transferred into crop plants from a wide range of plantand bacterial systems. In the majority of the cases, the genes showing expression intransgenic plants are stably inherited into the progeny without detrimental effects on therecipient plant. More interestingly, transgenic plants under field conditions have alsomaintained increased levels of insect resistance. Now, transgenic crops occupy 44.2 millionhectares on global basis. During the last 15 years, transformations have been produced inmore than 100 plant species; notable examples include maize, wheat, soybean, tomato,potato, cotton, rice, etc. Amongst these herbicide tolerant and insect tolerant cotton, maizeand soybean carrying Bacillus thuringiensis(Bt) genes are grown on a commercial scale.Genetic transformation and gene transfer are routine in many laboratories. However,isolation of useful genes and their expression to the desired level to control insect pests stillinvolves considerable experimentation and resources. Developing pest resistant varieties byinsertion of a few or single specific gene(s) is becoming an important component of breeding. Use of endotoxin genes such as Bt and plant derived genes (proteinase inhibitors) to the desired levels offers new opportunities to control insects and strategies involving combination of genes. Transgenic technology should be integrated in a total system approach for ecologically friendly and sustainable pest management. Issues related to Intellectual property rights, regulatory concerns, and public perceptions for release of transgenics need to be considered. Providing wealth of information on gene expression in higher plants by switching the gene on and off as and when required, makes gene manipulation a more direct process for genetic improvement of crops.Article Outline1. Introduction2. Methods for producing transgenic plants2.1. Agrobacterium-mediated gene transfer2.2. Direct gene transfer2.3. Polyethylene glycol (PEG) mediated gene transfer2.4. Electroporation2.5. Microinjection2.6. Microprojectile bombardment2.7. Approaches for developing genetically engineered plants resistant to insects2.8. Bacillus thruingiensis (Bt) genes2.9. Genetic manipulation of Bt2.10. Plasmid curing and conjugal transfer2.11. Recombinant DNA technology2.12. Field trial testing of Bt crops2.13. Plant derived genes2.14. Proteinase inhibitor genes2.15. α-Amylase inhibitor genes2.16. Lectin genes2.17. Other novel genes2.18. Gene pyramiding (combination of multiple gene effect)2.19. Commercialized transgenic crops3. ConclusionReferences89Master plan for water framework directive activities in Ireland leading to River Basin Management Plans Original Research ArticleDesalination, Volume 226, Issues 1-3, 25 June 2008, Pages 134-142Ray Earle, Sean BlacklockeClose preview | Purchase PDF (621 K) | Related articles | Related reference work articlesAbstractAbstractThe water framework directive master plan (WFDMP) in Ireland is being developed jointly by the Department of the Environment, Heritage and Local Government (DEHLG), the Environmental Protection Agency (EPA), the River Basin District (RBD) competent authorities (namely lead Local Authorities) and stakeholders including the relevant public authorities. The WFDMP was recently adopted by the National Technical Coordination Group (NTCG) and has been uploaded onto the website (www.wfdireland.ie) as the fundamental document coordinating WFD related activities in Ireland leading to the River Basin Management Plans (RBMP). There are eight River Basin Districts (RBD) covering the entire island of Ireland and accordingly there will be eight River Basin Management Plans (RBMP). Key elements of the new ecological approach to adaptive management of water resources are in focus namely public participation and integrated water resources planning.The WFDMP sets out the overall work plan required to meet the Republic of Ireland’s obligations under the WFD and associated national Water Policy Regulations (SI No. 722 of 2003) which transposed the WFD into Irish law and very close co-operation with the UK authorities is essential to ensure harmonization for three of the RBDs that straddle the border with Northern Ireland (NI) with one RBD being entirely within NI. Having successfullycompleted Article 3 (designate RBD areas and Competent Authorities), 5 (Characterisation)and 6 (Register of Protected Areas) requirements, the following prerequisite deadlines andmilestones lie ahead leading to the production of the first River Basin Management Plans(RBMP) in 2009: (a) classification systems (including EQS for priority substances), (b)programme of monitoring, (c) timetable and work programme for production of RBMP, (d)Overview of the significant water management issues (SWMI) in RBDs (for purpose of publicinformation and consultation), (e) draft RBMP, (f) environmental objectives, (g) programmeof measures and (h) RBMPs.The paper will address the above milestones in detail and report on and provide a snapshotof Ireland’s position in implementing the WFD.Alleviating piracy through open source strategy: An exploratory study of business90The Journal of Strategic Information Systems, Volume 18, Issue 4, December2009, Pages 165-177T. Pykäläinen, D. Yang, T. FangClose preview | Purchase PDF (222 K) | Related articles | Related reference work articlesAbstract | Figures/Tables | ReferencesAbstractThis paper advances the existing knowledge of anti-piracy strategies by proposing an opensource strategy (OS strategy) to alleviate software piracy based on a qualitative,case-based, exploratory study of eight software firms operating in China. The paper showsthat the OS strategy is conditionally adoptable, depending on how users are willing to pay forservices (market conditions); how critical and complex software is required for upgrading andmodifications (software conditions); and how firms can avoid resources overloading and/orshortage (firm conditions). The paper also identifies several new indicators to assess theeffectiveness of the OS strategy against piracy. Managerial implications about how toimprove business in piracy-ridden environment are discussed.Article Outline1. Introduction2. Strategies against piracy2.1. Existing strategies against piracy and strategic effectiveness2.2. Open source strategy (OS strategy)3. Methodology3.1. Research design and sample characteristics3.2. Interview question, pre-test, and data collection3.3. Analysis methods3.4. Reliability and validity4. Findings4.1. Firms description4.2. Feasibility of OS strategy against piracy and conditions for effectiveness4.3. Indicators of the OS strategy’s effectiveness against piracy5. Discussions and conclusions5.1. Discussions5.2. Research contribution5.3. Implications for practice5.4. Limitations and directions for future researchReferences91Merging the acquisitions and serials department at the University of New Mexico: acase study Original Research ArticleLibrary Acquisitions: Practice & Theory, Volume 22, Issue 3, Autumn 1998,Pages 259-270Sever Bordeianu, Linda K. Lewis, Frances C. WilkinsonShow preview | Purchase PDF (59 K) | Related articles | Related reference work articles92Spatial analysis of recreational boating as a first key step for marine spatial planning in Mallorca (Balearic Islands, Spain)Original Research ArticleOcean & Coastal Management, Volume 54, Issue 3, March 2011, Pages241-249P. Balaguer, A. Diedrich, R. Sardá, M. Fuster, B. Cañellas, J. TintoréShow preview | Purchase PDF (991 K) | Related articles | Related reference work articlesResearch highlights►This paper provides an approximation of the capacity of the coastal zones (seabeds available for anchoring). ► The results can be a decision tool for the proper management of the coastal zone. ► The work is based on the use of GIS (Geographic Information Systems). ► The developed method is applicable to any coastal area and is considered useful for the future management.Monitoring the commitment and child-friendliness of governments: A new approach93Child Abuse & Neglect, Volume 34, Issue 1, January 2010, Pages 34-44Assefa BequeleShow preview | Purchase PDF (1599 K) | Related articles | Related reference work articlesPrediction of global solar irradiance based on time series analysis: Application to94ArticleSolar Energy, Volume 84, Issue 10, October 2010, Pages 1772-1781Luis Martín, Luis F. Zarzalejo, Jesús Polo, Ana Navarro, Ruth Marchante,Marco ConyShow preview | Purchase PDF (702 K) | Related articles | Related reference work articles你为企业“冲锋陷阵”还是“救险解围”?2010年的南非世界杯赛渐入佳境,各支幸存的球队即将在通向大力神杯的道路上展开最后冲刺,比赛正日趋于白热化。
water purified-EP
EUROPEAN PHARMACOPOEIA 6.0Water,purifieding Table 1927.-2,determine the conductivity limit at the measured pH value in step 6.If the measured conductivity in step 4under stage 2is not greater than the conductivity requirements for the pH determined,the water to be examined meets the requirements of the test for conductivity.If either the measured conductivity is greater than this value or the pH is outside the range of 5.0-7.0,the water to be examined does not meet the requirements of the test for conductivity.In order to ensure the appropriate quality of the water,validated procedures and in-process monitoring of theelectrical conductivity and regular microbial monitoring are applied.Highly purified water is stored in bulk and distributed in conditions designed to prevent growth of micro-organisms and to avoid any other contamination.Table 1927.-2.–Stage 3-pH and conductivity requirements(for atmosphere and temperature equilibrated samples)pH Conductivity(µS·cm −1)5.0 4.75.1 4.15.2 3.65.3 3.35.4 3.05.5 2.85.6 2.65.7 2.55.8 2.45.9 2.46.0 2.46.1 2.46.2 2.56.3 2.46.4 2.36.5 2.26.6 2.16.7 2.66.8 3.16.9 3.87.04.6CHARACTERSAppearance :clear and colourless liquid.TESTSNitrates :maximum 0.2ppm.Place 5ml in a test-tube immersed in iced water,add 0.4ml of a 100g/l solution of potassium chloride R ,0.1ml of diphenylamine solution R and,dropwise with shaking,5ml of nitrogen-free sulphuric acid R .Transfer the tube to a water-bath at 50°C.After 15min,any blue colour in the solution is not more intense than that in a reference solution prepared at the same time in the same manner using a mixture of 4.5ml of nitrate-free water R and 0.5ml of nitrate standard solution (2ppm NO 3)R .Aluminium (2.4.17):maximum 10ppb,if intended for use in the manufacture of dialysis solutions.Prescribed solution .To 400ml of the water to be examined add 10ml of acetate buffer solution pH 6.0R and 100ml of distilled water R .Reference solution .Mix 2ml of aluminium standard solution (2ppm Al)R ,10ml of acetate buffer solution pH 6.0R and 98ml of distilled water R .Blank solution .Mix 10ml of acetate buffer solution pH 6.0R and 100ml of distilled water R .Heavy metals (2.4.8):maximum 0.1ppm.Heat 200ml in a glass evaporating dish on a water-bath until the volume is reduced to 20ml.12ml of the concentrated solution complies with limit test A.Prepare the standard using 10ml of lead standard solution (1ppm Pb)R .Bacterial endotoxins (2.6.14):less than 0.25IU/ml.LABELLING The label states,where applicable,that the substance is suitable for use in the manufacture of dialysis solutions.01/2008:0008WATER,PURIFIED Aqua purificataH 2OM r 18.02[7732-18-5]DEFINITIONWater for the preparation of medicines other than those that are required to be both sterile and apyrogenic,unless otherwise justified and authorised.Purified water in bulkPRODUCTIONPurified water in bulk is prepared by distillation,by ion exchange,by reverse osmosis or by any other suitable method from water that complies with the regulations on water intended for human consumption laid down by the competent authority.During production and subsequent storage,appropriate measures are taken to ensure that the total viable aerobic count is adequately controlled and monitored.Appropriate alert and action limits are set so as to detect adverse trends.Under normal conditions,an appropriate action limit is a total viable aerobic count (2.6.12)of 100micro-organisms per millilitre,determined by membrane filtration,using agar medium S and incubating at 30-35°C for 5days.The size of the sample is to be chosen in relation to the expected result.In addition,the test for total organic carbon (2.2.44)with a limit of 0.5mg/l or alternatively the following test for oxidisable substances is carried out:to 100ml add 10ml of dilute sulphuric acid R and 0.1ml of 0.02M potassium permanganate and boil for 5min;the solution remains faintly pink.Conductivity .Determine the conductivity off-line or in-line under the following conditions.EQUIPMENT Conductivity cell :—electrodes of a suitable material such as stainless steel;General Notices (1)apply to all monographs and other texts3213Water,purified EUROPEAN PHARMACOPOEIA6.0—cell constant:within2per cent of the given value determined using a certified reference solution with aconductivity less than1500µS·cm−1. Conductometer:resolution0.1µS·cm−1on the lowest range. System calibration(conductivity cell and conductometer):—against one or more suitable certified standard solutions;—accuracy:within3per cent of the measured conductivity plus0.1µS·cm−1.Conductometer calibration:by means of precision resistors or equivalent devices,after disconnecting the conductivity cell,for all ranges used for conductivity measurement and cell calibration(with an accuracy within0.1per cent of the stated value,traceable to the official standard).If in-line conductivity cells cannot be dismantled,system calibration may be performed against a calibrated conductivity cell placed close to the cell to be calibrated in the water flow.PROCEDUREMeasure the conductivity without temperature compensation,recording simultaneously the temperature. Temperature-compensated measurement may be performed after suitable validation.The water to be examined meets the requirements if the measured conductivity at the recorded temperature is not greater than the value in Table0008.-1.Table0008.-1.–Temperature and conductivityrequirementsTemperature(°C)Conductivity (µS·cm−1)0 2.410 3.620 4.325 5.130 5.440 6.5507.1608.1709.1759.7809.7909.710010.2For temperatures not listed in Table0008.-1,calculate the maximal permitted conductivity by interpolation between the next lower and next higher data points in the table. Purified water in bulk is stored and distributed in conditions designed to prevent growth of micro-organisms and to avoid any other contamination.CHARACTERSAppearance:clear and colourless liquid.TESTSNitrates:maximum0.2ppm.Place5ml in a test-tube immersed in iced water,add0.4ml of a100g/l solution of potassium chloride R,0.1ml of diphenylamine solution R and,dropwise with shaking,5ml of nitrogen-free sulphuric acid R.Transfer the tubeto a water-bath at50°C.After15min,any blue colour in the solution is not more intense than that in a reference solution prepared at the same time in the same manner using a mixture of4.5ml of nitrate-free water R and0.5ml of nitrate standard solution(2ppm NO3)R.Aluminium(2.4.17):maximum10ppb,if intended for use in the manufacture of dialysis solutions.Prescribed solution.To400ml of the water to be examined add10ml of acetate buffer solution pH6.0R and100ml of distilled water R.Reference solution.Mix2ml of aluminium standard solution(2ppm Al)R,10ml of acetate buffer solutionpH6.0R and98ml of distilled water R.Blank solution.Mix10ml of acetate buffer solutionpH6.0R and100ml of distilled water R.Heavy metals(2.4.8):maximum0.1ppm.Heat200ml in a glass evaporating dish on a water-bath until the volume is reduced to20ml.12ml of the concentrated solution complies with limit test A.Prepare the standard using10ml of lead standard solution(1ppm Pb)R. Bacterial endotoxins(2.6.14):less than0.25IU/ml,if intended for use in the manufacture of dialysis solutions without a further appropriate procedure for removal of bacterial endotoxins.LABELLINGThe label states,where applicable,that the substance is suitable for use in the manufacture of dialysis solutions.Purified water in containers DEFINITIONPurified water in bulk that has been filled and stored in conditions designed to assure the required microbiological quality.It is free from any added substances. CHARACTERSAppearance:clear and colourless liquid.TESTSIt complies with the tests prescribed in the section on Purified water in bulk and with the following additional tests. Acidity or alkalinity.To10ml,freshly boiled and cooled in a borosilicate glass flask,add0.05ml of methyl red solution R. The solution is not coloured red.To10ml add0.1ml of bromothymol blue solution R1.The solution is not coloured blue.Oxidisable substances.To100ml add10ml of dilute sulphuric acid R and0.1ml of0.02M potassium permanganate and boil for5min.The solution remains faintly pink.Chlorides.To10ml add1ml of dilute nitric acid R and 0.2ml of silver nitrate solution R2.The solution shows no change in appearance for at least15min.Sulphates.To10ml add0.1ml of dilute hydrochloric acid R and0.1ml of barium chloride solution R1.The solution shows no change in appearance for at least1h. Ammonium:maximum0.2ppm.To20ml add1ml of alkaline potassium tetraiodomercurate solution R.After5min,examine the solution down the vertical axis of the tube.The solution is not more intensely coloured than a standard prepared at the same time by adding1ml of alkaline potassium tetraiodomercurate solution R to a mixture of4ml of ammonium standard solution(1ppm NH4)R and16ml of ammonium-free water R.3214See the information section on general monographs(cover pages)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Water Desalination ReporTTechnologynew Nanotube makes media splash
Researchers at Australian National University (ANU) in Canberra, Australia have published a paper that describes promising results using boron nitride nanotubes to desalt seawater. The work, done on computers using molecular dynamic simulations, shows that boron nitride nanotubes (BNT) embedded in a silicon nitride membrane have superior desalination properties over conventional polymeric membranes and carbon nanotubes (CNT).
Tom Pankratz, Editor, P.O. Box 75064, Houston, Texas 77234-5064 USATelephone: +1-281-857-6571, www.waterdesalreport.com, email: tp@globalwaterintel.com© 2009 Media Analytics. Published in cooperation with Global Water Intelligence.
31 August 2009Volume 45, Number 32
The international weekly for desalination and advanced water treatment since 1965
The first, an academic involved in new membrane technologies, said, “Interesting. Elegant. However, it seems unlikely that 100 percent salt rejection could hold up over time, and fouling limitations could impact short-term performance stability at a high flux. Surface modifications resulting from acid, base and chlorinated flushes could pose challenges for both CNT and BNT materials.”
WDR also asked Mark Shannon, director of the WaterCAMPWS at the University of Illinois. Dr Shannon said that he likes the overall idea of BNTs because the high dipole between boron and nitrogen means that the interior pore should be more selective, but he also wonders if surrounding the BNT with silicon nitride might change the electronic structure.
“The molecules at the entrance and termination of the nanotubes are absolutely vital and can dramatically change the screening potential, so much that it can switch which ion passes. The ANU modeling was done without termination of the BNT ends, so it will be interesting to see the effects of attaching different functional groups,” he said, noting that it will also be very difficult to make the BNT’s active layer long and thin enough without introducing extrinsic processing defects such as pinholes. “Going to larger diameter and longer BNTs will help, but it comes at the expense of selectivity. It is hard to have it both ways.”
FloridaStimulus package helps fund RO project
Two of four contracts, valued at almost $3.25 million, have been awarded as the City of Oldsmar moves forward with an RO project slated to receive $3 million under the American Recovery and Reinvestment Act (ARRA). The funding includes a ‘buy American’ provision and is part of the federal economic stimulus package. Florida also attached a provision that the money be committed by the first of October so that it would have an immediate impact on the local economy.
According to Lisa Rhea, Oldsmar’s utilities administrator, the project was broken down into four separate contracts: the production wells, which were awarded to Advanced Drilling for approximately $750,000, an RO equipment supply contract awarded to Harn R/O Systems for $2.49 million, the concentrate injection well, which will be awarded in early September, and the general construction contract, which is to be bid in December and awarded by mid-February.
But before utilities plan to retrofit existing RO elements with their nanotube counterparts, they should note that the performance predictions are based solely on computer simulations. These simulations may prove accurate, but they are impossible to confirm without experimental validation; then comes the daunting task of figuring out how to make the BNTs at a competitive price.
The researchers themselves are much more reasonable and cautious. Tamsyn Hilder, a postdoctoral fellow in ANU’s Research School of Biology, told WDR that while the results of the proof-of-principle computational studies are promising, there is much experimental research ahead. “We need to explore, for example, how the system we designed can be fabricated effectively and test if the engineered nano-material does indeed prevent charged particles from entering the pore,” she said.
WDR asked two other membrane scientists not affiliated with the project for their thoughts on the study results – and the science supporting it – based on a paper published in the July issue of the journal Small. Both were impressed.
Enthusiastic university press releases, and the stories they generated, claim that BNTs can operate at a flux more than four times higher than conventional membranes while achieving 100 percent salt rejection at concentrations twice that of seawater, all at operating pressures comparable to existing RO processes. Media responses are reminiscent of the wildly optimistic mid-2006 announcement of the desalting possibilities of CNT, and they are fueling buoyant news stories in the international press, predicting that BNTs “are set to transform desalination.”Water Molecules
