Observation of the New Heavy Neutron-Rich Isotope 238Th
大亚湾反应堆中微子实验进展

宇宙线事例的模拟
31
探测器模拟
42.5cm, 91%
灵敏度和靶总靶质量的关系
中子探测效率与集光层厚度的关系
4x20 吨
15cm
PMT对液闪中不同位去
• 去年以来顺利通过了一系列重要的评审 • 2007年3月, 土建初步设计评审 • 2007年4月, 核安全评审 • 2007年4月, 美国能源部CD-1评审会 • 2007年8月, 科技部对大亚湾项目初步设计报告进
• θ13目前最好的结果由Chooz给出, 而CP破坏的相位还没有任何测量。 • 大多数理论模型预言sin22q13 ~ 0.001-0.1 • θ13的精确测量 • 其结果将决定未来轻子区CP破坏的测量, 从而有可能解决宇宙中正反
物质的不对称之谜。 • 对标准模型的扩展提供更强的约束。
90%CL
At m231 = 2.5 103 eV2, sin22 < 0.15
逊分校 ,弗吉尼亚工学院和州立大学
~ 200位研究人员
中国 (18) 高能所,北师大, 成都理工大学,原子能
院,中广核,东莞理工学院,南京大 学,南开大学,山东大学,深圳大学 ,清华大学,中国科技大学,中山大 学, 香港大学,香港中文大学,台湾
大学,交通大学,联合大学
3
为什么是θ13
• 三味中微子振荡模型由三个混合角, 两个质量方差, 一个CP相位描述, θ13是混合角之一。
16
钢罐总体机械设计
尺寸重量受严格限制 足够的强度 接口复杂
电缆穿出 4m有机玻璃罐
反射板
最小元分析 应力分布
桶底与反射板
17
支撑平台与吊具
• 支撑平台 • 中心探测器在水池中的支撑平台已完成工程设计, 并招标。 • 要求支撑110吨探测器, 水平度可调至毫米量级(刻度装置要求) • 吊具 • 装配用35吨吊具, 安装用130吨吊具。 • 已完成概念设计和详细的技术要求文档, 将由专业公司设计生产 • 2008年2月底完成设计, 2008年6月底完成生产
重离子辐照引发的25nm_NAND_Flash存储器数据位翻转

摘要为研究不同注量下浮栅存储单元位翻转特点及其翻转截面变化以及重离子入射 导 致 的 多 单 元 翻 转以两款$!2. ),)<M'8T;存储器为载体开展了重离子实验研究实验结果表 明单 个 重 离 子 在 击 中存储单元灵敏体积的情况下足以引起位翻转位 翻 转 比 率 随 着 注 量 累 积 基 本 呈 线 性 变 化 由 于 处 于 编程状态阈值电压分布低压区的存储单元倾向于在相邻或靠近的地址上集中分布随 着 注 量 累 积这 类 存储单元数量迅速减少而其他大量存储单元的翻转数增长率变化不明显因此位翻转 截 面 随 注 量 累 积 呈下降趋势重离子导致两款器件出现多单元翻转多单元翻转呈现单列双列以 及 列 间 隔 等 结 构 利 用离子电离径迹模型估算得到离子电离有效径迹半 径可 以 判 定 多 单 元 翻 转 是 由 于 一 个 离 子 的 电 离 有 效径迹覆盖了多个存储单元 关 键 词 ),)< M'8T;浮 栅 重 离 子 辐 照 注 量 相 关 性 多 单 元 翻 转 中图分类号:K"$!!! 文献标志码,!!!文章编号#***DI=+#$*$+#$D$$I>D#* $%&#*("!+J6PF($*$+(6&BQ/82(*IJJ
'3?91259!@2 &4934-&/2W3T-/58-3-;3/2A'B3203&A;38W6/&2A'B3203&2T/25'33W32BST3-T 71O829-;371O04&TTDT30-/&2/2),)<M'8T; .3.&468TV3''8T-;3.B'-/D S'3D03''BST3-T HEO9B3-&;38W6/&2/4489/8-/&23QS34/.32-8'T-B9/3T V343S34D A&4.39&2-V&-6S3T&A$!2. ),)< M'8T; .3.&4693W/03T(:;3T33QS34/.32-TV343 0&29B0-398--;3R@D#+:8293. ,003'348-&48--;3E;/28@2T-/-B-3&A,-&./0123456829 -;3R38W6@&2G3T3840;M80/'/-6/2K82P;&B R@GMK(:;33QS34/.32-43TB'-T43W38'39 -;8-C/-BST3-T V3434829&.'69/T-4/CB-39804&TT89943TT3T;3439/-846/2-V&-3T-T 439B039/2T/52/A/082-'68A-34T;&4-D-34.82238'/2582943S4&548..8C'3TB553T-/25-;8-
原子核物理(修订版)习题答案 卢希庭

Sp(6,13) (5,12) (1 H ) (6,13) 13.369MeV 7.289MeV 3.125MeV 17.533MeV
对13C,Z=6,A=13取出一个中子后变为 12C , Z=N=6偶偶核中子与质子对称相处,且质子与中 子各自成对相处,有较大的稳定性,结合能 B(Z,A-1)非常大,
部分放射性活度在任何时候都是与总放射性活 度成正比。
设总的衰变常量为 且
ln 2
I I0eet I0e T1 2
ln 2 T1
2
解得 :T1 2=3.01min
2.8
解: N (235U ) N0 (235U )e5t
N (238U ) N0 (238U )e8t
两式相其比中,5,8分别为235U ,238U的衰变概率。
1.2 1012
N12 6.981014
t 1 ln N0 T1/ 2 ln N0
N ln 2 N
2.35104 a
因为测量精度
=7% 其中 为Nc总计数 所以 =2N0c 4
又 Nc=AT A为 放14C射性活度,T为测量时间
所以
T1/
2
为 为
1T4C=的的N质半/c 量衰A 数期。,Am为 Mm样品NA中 Tl含n1/22
[1.007825 1.008665 2.014102]u 931.494MeV
2.224MeV
比结合能 同理依次为:
B
A
2.224MeV
2
1.112MeV
40C:a B 342.05Me比V 结合能
B A 8.551MeV
: 197Au B 1559.363M比eV结合能
: C 197 f
2.5
地质流体稳定同位素示踪

二、同位素效应
由同位素质量差所引起的物理和化学性质上的差异。
第二节、同位素分析结果的表达和标准
正是由于同位素效应,造成了地质过程中,两相或多相共存时,不同相中同位素比值差异——使同位素示踪成为可能。
实测δ值
δA =1000 ×(RSa - RSt)/RSt (单位:‰)
第三节、同位素分馏 (isotope fractionation)
指在一系统中,某元素的同位素以不同的比值分配到两种物质或两相中的现象,是同位素效应的表现。
热力学平衡分馏 热力学平衡分馏可以有很多过程,但都达到平衡。 例如:化学反应、扩散交换
2
动力学非平衡分馏 交换时未达到平衡 后期平衡被破坏
共生顺序判别法(P117)
例如:氧同位素有:
石英>方解石> 碱性长石> 蓝晶石>多硅白云母 >钙长石 >白云母 >…….
另一种形式(书上): 由(1+10-3 δA)/(1+ 10-3δ0)=
(1 - f α)/ (1- f)
整理得:
1+10-3 δA= (1+ 10-3δ0)(1 - f α)/ (1- f) δA= (1000+ δ0)(1 - f α)/ (1- f) -1000
例子
书上P79
思考:地质应用
分离相A的δ(平均值)计算公式: 由质量平衡公式得: RB f+RA(1- f)=R0 RA =(R0 -RB f)/ (1- f) RB的计算公式代入得: RA =(R0 - R0f (αA-B-1 f)/ (1- f) =R0(1 - f α)/ (1- f) RA /R0=(1 - f α)/ (1- f) 换算成常用的δ表示:{利用 RA/R0 =(1+10-3 δA)/(1+ 10-3δ0)} ㏑[(1+10-3 δA)/(1+ 10-3δ0)]=㏑ [(1 - f α)/ (1- f)] 左边≈ 10-3 δA- 10-3δ0 δA= δ0 +103 ㏑ [(1 - f α)/ (1- f)]
21925065_榴辉岩中单斜辉石-石榴子石镁同位素地质温度计评述

1000 0569/2020/036(06) 1705 18ActaPetrologicaSinica 岩石学报doi:10 18654/1000 0569/2020 06 04榴辉岩中单斜辉石 石榴子石镁同位素地质温度计评述黄宏炜1 杜瑾雪1 柯珊2HUANGHongWei1,DUJinXue1 andKEShan21 中国地质大学地球科学与资源学院,北京 1000832 中国地质大学地质过程与矿产资源国家重点实验室,北京 1000831 SchoolofEarthSciencesandResources,ChinaUniversityofGeosciences,Beijing100083,China2 StateKeyLaboratoryofGeologicalProcessesandMineralResources,ChinaUniversityofGeosciences,Beijing100083,China2019 11 14收稿,2020 04 08改回HuangHW,DuJXandKeS 2020 Reviewontheclinopyroxene garnetmagnesiumisotopegeothermometersforeclogites ActaPetrologicaSinica,36(6):1705-1718,doi:10 18654/1000 0569/2020 06 04Abstract Theremarkableequilibriummagnesiumisotopefractionationbetweenclinopyroxeneandgarnetobservedineclogitesmakesitapotentialhigh precisiongeothermometer Therefore,thispaperselects64pairsofclinopyroxene garnetmagnesiumisotopedataofeclogitesintheChinesesouthwesternTianshanorogen,intheDabie SuluorogenandintheKaapvaalcratonintheSouthAfricafromliteratures Then,wescreened50pairsofdatathatreachtheequilibriummagnesiumisotopefractionationbytheδ26MgCpx δ26MgGrtdiagram Usingthesemagnesiumisotopeequilibriumfractionationdata,wecalculatedpeaktemperaturesofeclogitesbymagnesiumisotopegeothermometersofHuangetal (2013)throughfirst principlescalculationandWangetal (2012)andLietal (2016)throughempiricalestimation,andcomparedthemwiththepeaktemperaturesgivenbyothergeothermometers Byanalyzingthecalculationresults,itisfoundthatfororogeniceclogites,thecalculationresultsofthegeothermometerofHuangetal (2013)areconsistentwiththosepreviouslyobtainedbytraditionalgeothermometersandphaseequilibriamodeling,whilethecalculationresultsofthegeothermometersofWangetal (2012)andLietal (2016)aresignificantlylower Forthecratoneclogites,thecalculationresultsofallthethreemagnesiumisotopegeothermometersaresignificantlydifferentfromresultsoftraditionalgeothermometersbymorethan50℃,whichismostprobablycausedbyre equilibriumofmagnesiumisotopeduringearlyretrogrademetamorphismathightemperatures Thisindicatesthatthesethreemagnesiumisotopegeothermometersarenotapplicableforthecratoneclogites Basedontheabovedata,themethodofempiricalestimationisusedtocalibrateanewclinopyroxene garnetmagnesiumisotopegeothermometer,whichisΔ26MgCpx Grt=1 11×106/[T(K)]2(R2=0 92).Inaddition,thispaperalsobrieflydiscussesapplicationprospectoftheclinopyroxene garnetmagnesiumisotopegeothermometersandtheproblemsthatshouldbepaidattentiontoduringapplication Keywords Eclogites;Isotopegeothermometer;Magnesiumisotope;Clinopyroxene garnet摘 要 榴辉岩中单斜辉石和石榴子石之间显著的镁同位素平衡分馏,使其成为一种具有潜力的高精度地质温度计。
MCNP4C_ManualH
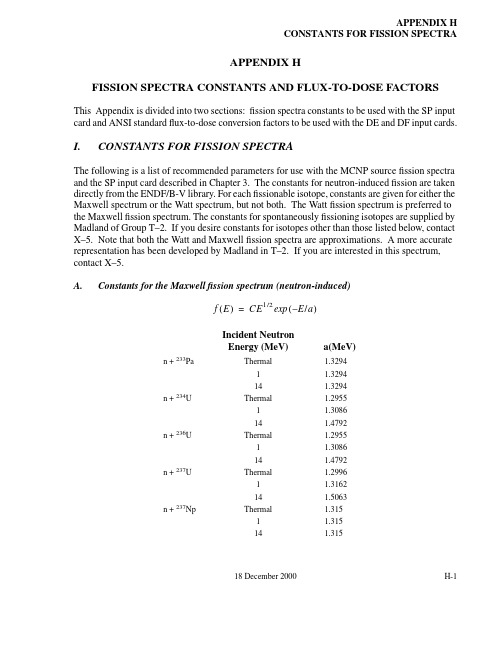
18 December 2000H-1APPENDIX HCONSTANTS FOR FISSION SPECTRAAPPENDIX HFISSION SPECTRA CONSTANTS AND FLUX-TO-DOSE FACTORSThis Appendix is divided into two sections:fission spectra constants to be used with the SP input card and ANSI standard flux-to-dose conversion factors to be used with the DE and DF input cards.I.CONSTANTS FOR FISSION SPECTRAThe following is a list of recommended parameters for use with the MCNP source fission spectra and the SP input card described in Chapter 3.The constants for neutron-induced fission are taken directly from the ENDF/B-V library.For each fissionable isotope,constants are given for either the Maxwell spectrum or the Watt spectrum, but not both. The Watt fission spectrum is preferred to the Maxwell fission spectrum.The constants for spontaneously fissioning isotopes are supplied by Madland of Group T–2.If you desire constants for isotopes other than those listed below,contact X–5. Note that both the Watt and Maxwell fission spectra are approximations. A more accurate representation has been developed by Madland in T–2. If you are interested in this spectrum,contact X–5.A.Constants for the Maxwell fission spectrum (neutron-induced)Incident Neutron Energy (MeV)a(MeV)n +233PaThermal 1.32941 1.329414 1.3294n +234UThermal 1.29551 1.308614 1.4792n +236UThermal 1.29551 1.308614 1.4792n +237UThermal 1.29961 1.316214 1.5063n +237NpThermal 1.3151 1.315141.315f E ()CE1/2E /a –()exp =APPENDIX HCONSTANTS FOR FISSION SPECTRAIncident NeutronEnergy (MeV)a(MeV)n +238Pu Thermal 1.3301 1.33014 1.330n +240Pu Thermal 1.3461 1.361514 1.547n +241Pu Thermal 1.35971 1.375214 1.5323n +242Pu Thermal 1.3371 1.35414 1.552n +241Am Thermal 1.3301 1.33014 1.330n +242m Pu Thermal 1.3301 1.33014 1.330n +243Am Thermal 1.3301 1.33014 1.330n +242Cm Thermal 1.3301 1.33014 1.330n +244Cm Thermal 1.3301 1.33014 1.330n +245Cm Thermal 1.45011 1.468714 1.6844n +246Cm Thermal 1.36241 1.407514 1.6412 H-218 December 200018 December 2000H-3APPENDIX HFlUX-TO-DOSE CONVERSION FACTORSB.Constants for the Watt Fission Spectrum1.Neutron-Induced Fission2.Spontaneous FissionII.FlUX-TO-DOSE CONVERSION FACTORSThis section presents several flux-to-dose rate conversion factor sets for use on the DE and DF tally cards to convert from calculated particle flux to human biological dose equivalent rate.These sets of conversion factors are not the only ones in existence, nor are they recommended by thisIncident Neutron Energy (MeV)a(MeV)b(MeV –1)n +232ThThermal 1.0888 1.68711 1.1096 1.631614 1.1700 1.4610n +233UThermal 0.977 2.54610.977 2.54614 1.0036 2.6377n +235UThermal 0.988 2.24910.988 2.24914 1.028 2.084n +238UThermal 0.88111 3.400510.89506 3.2953140.96534 2.8330n +239PuThermal 0.966 2.84210.966 2.842141.0552.383a(MeV)b(MeV –1)240Pu 0.799 4.903242Pu 0.833668 4.431658242Cm 0.891 4.046244Cm 0.906 3.848252Cf1.0252.926f E ()C E /a –()bE ()1/2sinh exp =APPENDIX HFlUX-TO-DOSE CONVERSION FACTORSpublication.Rather,they are presented for convenience should you decide that one is appropriate for your use. The original publication cited or other sources should be consulted to determine if they are appropriate for your application.Although the various conversion factor sets differ from one another, it seems to be the consensus of the health physics community that they do not differ significantly from most health physics applications where accuracies of 20% are generally acceptable. Some of the dif f erences in the various sets are attributable to different assumptions about source directionality, phantom geometry, and depth of penetration. The neutron quality factors, derived primarily from animal experiments, are also somewhat different.Be aware that conversion factor sets are subject to change based on the actions of various national and international organizations such as the National Council on Radiation Protection and Measurements (NCRP), the International Commission on Radiological Protection (ICRP), the International Commission on Radiation Units and Measurements(ICRU),the American National Standards Institute(ANSI),and the American Nuclear Society(ANS).Changes may be based on the re-evaluation of existing data and calculations or on the availability of new information. Currently, a revision of the 1977 ANSI/ANS1 conversion factors is under way and the ICRP and NCRP are considering an increase in the neutron quality factors by a factor of 2 to 2.5.In addition to biological dose factors, a reference is given for silicon displacement kerma factors for potential use in radiation effects assessment of electronic semiconductor devices. The use of these factors is subject to the same caveats stated above for biological dose rates.A.Biological Dose Equivalent Rate FactorsIn the following discussions,dose rate will be used interchangeably with biological dose equivalent rate.In all cases the conversion factors will contain the quality factors used to convert the absorbed dose in rads to rem.The neutron quality factors implicit in the conversion factors are also tabulated for information. For consistency, all conversion factors are given in units of rem/h per unit flux (particles/cm2-s)rather than in the units given by the original publication.The interpolation mode chosen should correspond to that recommended by the reference. For example, the ANSI/ANS publication recommends log-log interpolation;significant differences at interpolated energies can result if a different interpolation scheme is used.1.NeutronsThe NCRP-38 (Ref. 2) and ICRP-21 (Ref. 3) neutron flux-to-dose rate conversion factors and quality factors are listed in Table H.1. Note that the 1977 ANSI/ANS factors referred to earlier were taken from NCRP-38 and therefore are not listed separately.2.PhotonsH-418 December 200018 December 2000H-5APPENDIX HFlUX-TO-DOSE CONVERSION FACTORSThe 1977 ANSI/ANS 1 and the ICRP-21 (Ref. 3) photon flux-to-dose rate conversion factors are given inTable H.2.No tabulated set of photon conversion factors have been provided by the NCRP as far as can be determined.Note that the 1977ANSI/ANS and the ICRP-21conversion factor sets differ significantly (>20%)below approximately 0.7MeV with maximum disagreement occuring at ~0.06 MeV , where the ANSI/ANS value is about 2.3 times larger than the ICRP value.B.Silicon Displacement Kerma FactorsRadiation damage to or effects on electronic components are often of interest in radiation fields.Of particular interest are the absorbed dose in rads and silicon displacement kerma factors. The absorbed dose may be calculated for a specific material by using the FM tally card discussed in Chapter 3 with an appropriate constant C to convert from the MCNP default units to rads. The silicon displacement kermas, however, are given as a function of energy, similar to the biological conversion factors.Therefore,they may be implemented on the DE and DF cards.One source of these kerma factors and a discussion of their significance and use can be found in Reference 4.TABLE H-1:Neutron Flux-to-Dose Rate Conversion Factors and Quality FactorsNCRP-38,ANSI/ANS-6.1.1-1977**Extracted from American National Standard ANSI/ANS-6.1.1-1977with permission of the publisher,the American Nuclear Society.ICRP-21Energy, E(MeV)DF(E)(rem/hr)/(n/cm 2-s)Quality FactorDF(E)(rem/hr)/(n/cm 2-s)Quality Factor2.5E–083.67E–06 2.0 3.85E–06 2.31.0E–07 3.67E–06 2.04.17E–06 2.01.0E–06 4.46E–06 2.0 4.55E–06 2.01.0E–05 4.54E–06 2.0 4.35E–06 2.01.0E–04 4.18E–06 2.0 4.17E–06 2.01.0E–03 3.76E–06 2.0 3.70E–06 2.01.0E–02 3.56E–06 2.5 3.57E–06 2.01.0E–01 2.17E–057.5 2.08E–057.45.0E–019.26E–0511.07.14E–0511.01.0 1.32E–0411.0 1.18E–0410.62.0 1.43E–049.32.5 1.25E–049.05.0 1.56E–048.0 1.47E–047.87.0 1.47E–047.010.0 1.47E–046.5 1.47E–04 6.814.0 2.08E–047.520.02.27E–048.01.54E–046.0APPENDIX HFlUX-TO-DOSE CONVERSION FACTORSTABLE H-2:Photon Flux-to-Dose Rate Conversion Factors ANSI/ANS–6.1.1–1977ICRP-21Energy, E (MeV)DF(E)(rem/hr)/(p/cm2-s)Energy, E(MeV)DF(E)(rem/hr)/(p/cm2-s)0.01 3.96E–060.01 2.78E–060.03 5.82E–070.015 1.11E–060.05 2.90E–070.02 5.88E–070.07 2.58E–070.03 2.56E–070.1 2.83E–070.04 1.56E–070.15 3.79E–070.05 1.20E–070.2 5.01E–070.06 1.11E–070.25 6.31E–070.08 1.20E–070.37.59E–070.1 1.47E–070.358.78E–070.15 2.38E–070.49.85E–070.2 3.45E–070.45 1.08E–060.3 5.56E–070.5 1.17E–060.47.69E–070.55 1.27E–060.59.09E–070.6 1.36E–060.6 1.14E–060.65 1.44E–060.8 1.47E–060.7 1.52E–06 1. 1.79E–060.8 1.68E–06 1.5 2.44E–061.0 1.98E–062.3.03E–061.42.51E–063.4.00E–061.82.99E–06 4. 4.76E–062.23.42E–06 5. 5.56E–062.63.82E–06 6. 6.25E–062.8 4.01E–068.7.69E–063.254.41E–0610.9.09E–063.754.83E–064.255.23E–064.755.60E–065.0 5.80E–065.256.01E–065.756.37E–066.25 6.74E–066.757.11E–06H-618 December 200018 December 2000H-7APPENDIX H REFERENCESIII.REFERENCES1.ANS-6.1.1Working Group,M.E.Battat (Chairman),‘‘American National Standard Neutron and Gamma-Ray Flux-to-Dose Rate Factors,’’ ANSI/ANS-6.1.1-1977 (N666), American Nuclear Society, LaGrange Park, Illinois (1977).2.NCRP Scientific Committee 4 on Heavy Particles, H. H. Rossi, chairman, ‘‘Protection Against Neutron Radiation,’’ NCRP-38, National Council on Radiation Protection and Measurements (January 1971).3.ICRP Committee 3 Task Group, P. Grande and M. C. O’Riordan, chairmen, ‘‘Data for Protection Against Ionizing Radiation from External Sources: Supplement to ICRPPublication 15,’’ICRP-21,International Commission on Radiological Protection,Pergamon Press (April 1971).4.ASTM Committee E-10on Nuclear Technology and Applications,‘‘Characterizing Neutron Energy Fluence Spectra in Terms of an Equivalent Monoenergetic Neutron Fluence for Radiation-Hardness Testing of Electronics,’’ American Society for Testing and Materials Standard E722-80, Annual Book of ASTM Standards (1980).7.57.66E–069.08.77E–0611.0 1.03E–0513.0 1.18E–0515.01.33E–05TABLE H-2: (Cont.)Photon Flux-to-Dose Rate Conversion FactorsANSI/ANS–6.1.1–1977ICRP-21Energy, E (MeV)DF(E)(rem/hr)/(p/cm 2-s)Energy, E (MeV)DF(E)(rem/hr)/(p/cm 2-s)CHAPTER 2INP FileH-818 December 2000。
反应堆物理题库
西安交通大学——核反应堆物理分析(共470题)从反应堆物理的角度看,良好的慢化剂材料应具有什么样的性能?答案:慢化剂是快中子与它的核发生碰撞后能减速成热中子的材料,这与它的三种中子物理性能有关:δ-平均对数能量缩减;Σs-宏观散射截面;Σa-宏观吸收截面。
综合评价应是δ和Σs都比较大而Σa又较小的材料才是较好的慢化材料,定量地用慢化能力δΣs和慢化比δ和Σs/Σa来比较。
试列出常用慢化剂的慢化能力和慢化比。
核力所具有的特点是什么?答案:基本特点是:核力是短程力,作用范围大约是1~2×10-13cm;核力是吸引力,中子与中子,质子与中子,质子与质子之间均是强吸引力。
核力与电荷无关。
核力具有饱和性,每一核子只与其邻近的数目有限的几个核子发生相互作用。
4. 定性地说明:为什么燃料温度Tf越高逃脱共振吸收几率P越小?答案:逃脱共振吸收几率P是快中子慢化成热中子过程中逃脱238U共振吸收峰的几率,在燃料温度低的时候,ζa共振峰又高又窄,如图所示,当燃料温度升高后,238U的ζa的共振峰高度下降了,然而却变宽了,因而不仅原来共振峰处能量的中子被吸收,而且该能量左右的中子也会被吸收。
温度越高共振峰变得越宽,能被该共振峰吸收的中子越多,逃脱共振吸收几率P就越小,这种效应也称为多谱勒展宽。
试定性地解释燃料芯块的自屏效应。
答案:中子在燃料中穿行一定距离时的吸收几率,可表示为:P(a)=1-e-X/λ其中λ为吸收平均自由程,X为中子穿行距离。
一般认为X=5λ时,中子几乎都被吸收了[P(a)→1]。
对于压水堆,燃料用富集度为3.0%的UO2,中子能量为6.7ev,穿行距离在5λa=0.0315cm内被吸收的几率为99.3%,所以很难有6.7ev的中子能进入到燃料芯块中心,这种现象称为自屏效应。
6. 什么是过渡周期?什么是渐近周期?答案:在零功率时,当阶跃输入-正反应性ρ0(ρ0<β)后,反应堆功率的上升速率(或周期)是随ρ0输入后的时间t而改变的(如图所示)。
edexcel as chemistry vocabulary
1 Formulae, equations and amounts of substance1.1 The foundations of chemistry1.FormulaeA chemical formula is a way of expressing information about the proportions ofatoms that constitute a particular chemical compound, using a single line of chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, and plus (+) and minus (−) signs.2.EquationA chemical equation is the symbolic representation of a chemical reactionwherein the reactant entities are given on the left-hand side and the product entities on the right-hand side3.Amount4.SubstanceA chemical substance is a form of matter that has constant chemical compositionand characteristic properties. It cannot be separated into components by physical separation methods, i.e. without breaking chemical bonds. It can be solid, liquid, gas, or plasma.5.AtomThe atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons.6.ObservationObservation is the active acquisition of information from a primary source.7.ExperimentAn experiment is an orderly procedure carried out with the goal of verifying, refuting, or establishing the validity of a hypothesis.8.Periodic TableThe periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations, and recurring chemical properties.9.NucleusThe nucleus is the very dense region consisting of protons and neutrons at the center of an atom.10.ProtonThe proton is a subatomic particle with the symbol p or p+ and a positive electric charge of 1 elementary charge.11.NeutronThe neutron is a subatomic particle that has the symbol n or n0. Neutrons have no net electric charge and a mass slightly larger than that of a proton12.ElectronThe electron (symbol: e−) is a subatomic particle with a negative elementaryelectric charge.13.ElementA chemical element is a pure chemical substance consisting of a single type ofatom distinguished by its atomic number, which is the number of protons in its atomic nucleus. Elements are divided into metals, metalloids, and nonmetals. 14.Atomic numberThe atomic number of a chemical element (also known as its proton number) is the number of protons found in the nucleus of an atom of that element, and therefore identical to the charge number of the nucleus.15.Mass numberThe mass number (A), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.16.SymbolA chemical symbol is a 1-, 2-, or 3-letter internationally agreed code for achemical element, usually derived from the name of the element, often in Latin.Only the first letter is capitalized.17.IsotopeIsotopes are variants of a particular chemical element such that, while all isotopes of a given element have the same number of protons in each atom, they differ in neutron number.18.PropertyProperty is that which belongs to or with something, whether as an attribute or as a component of said thing.19.StableStable means when a system is in its lowest energy state, or chemical equilibrium with its environment.1.2 Formulae and equations 09.03.2014 20.Ionic bonding (bond)Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions.In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be of a more complex nature, e.g. molecular ions like NH4+ or SO42-21.Covalent bonding (bond)A covalent bond is a chemical bond that involves the sharing of electron pairs betweenatoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.22.IonAn ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving the atom a net positive or negative electrical charge.23.Balanced equationOne balances a chemical equation by changing the scalar number for each chemical formula.24.Spectator ions旁观离子25.Overall ionic equation Or Net ionic equation净离子方程式26.Elements:Zn: ZincH: HydrogenAl: AluminumLi: LithiumSi: SiliconCu: CooperAg: SilverU: UraniumNa: SodiumCl: ChlorineO: OxygenMg: MagnesiumN: Nitrogen1.3 How big and heavy are atoms? 09.10.201427.MoleculeA molecule is an electrically neutral group of two or more atoms held together by chemical bonds28.Relative atomic massThe relative atomic mass of an element is defined as the average mass of its isotopes compared with the mass of an atom of the carbon-12 isotopes29.Relative atomic mass scaleThe scale by which chemists compare the mass of all atoms to the mass of a carbon-12 isotopes.30.Weighted mean 加权平均值The weighted mean is similar to an arithmetic mean (算术平均值) (the most common type of average), where instead of each of the data points contributing equally to the final average, some data points contribute more than others.31.Elements:Br: bromineC: carbonFe: iron1.4 The mole 09.12.201432.MoleA mole of any substance is defined as the amount of substance that contains as many particles (atoms, ions or molecules) as there are atoms in exactly 12g of carbon-12.33.Avogadro constantThe Avogadro constant has the unit particles per mole (mol-1). L or NA34.Molar massMolar mass is just the numerical value of the relative atomic mass, g/mol35.SI unitThe International System of Units (abbreviated SI from French: Le Système international d'unités) is the modern form of the metric system and is the world's most widely used system of measurement, used in both everyday commerce and science.36.SI base unitThe base SI units are the metre (m), the kilogeam (kg), the second (s), the ampere (A), the Kelvin (K), the candela (cd), and the mole (mol).37.SI derived unitDerived units are formed by powers, products or quotients of the base units and are unlimited in number.1.5 using moles36.Relative formula massThe sum of the relative atomic masses of all the atoms in the chemical formula.37.Relative molecular mass (M r)Relative formula mass in covalent compounds.38.Molar massMolar mass is the relative molecular formula mass in grams per mole39.Avogadro’s lawEqual volumes of all gases contain equal numbers of molecules, provided that they are at the same temperature and pressure. And when the gas is in the standard temperature and pressure (STP), I mol of any gas is 24 dm3 or 24L.40.Molar volumeOne mole of any gas must occupy the same volume under the same conditions, Vm. For example, in STP conditions.1.6 Calculating formulae using moles 09.17.2014 41.Empirical formulaSimplest formula of a compound showing the whole number ratios of the atoms42.Molecular formulaFormula of a compound showing how many of each atom there are1.7 Measuring concentration43.SolutionA solution is a homogeneous mixture composed of only one phase. In such a mixture, asolute is a substance dissolved in another substance, known as a solvent.44.ConcentrationMeasure of the amount of a solute dissolved in a solvent to form a solution.45.Molar solutionA solution of concentration 1 molar (1 mol/dm3)46.Volumetric flask (量瓶)47.Methodology(方法论)48.Creatinine肌酐(creatinine,Cre)是肌肉在人体内代谢的产物,每20g肌肉代谢可产生1mg肌酐。
“壳-幔”混合成因高Ba-Sr花岗质岩浆演化——以华北克拉通中部带老山和狐偃山岩体为例
3874
ActaPetrologicaSinica 岩石学报 2017,33(12)
北克拉通中部带早白垩世岩浆活动。系统的地球化学研究显示两岩体具有如下特征:(1)SiO2 含量中等(573% ~698%), 高 K2O(30% ~82%)和 Na2O(43% ~64%)含量,低 MgO(02% ~14%)、CaO、TiO2 和 P2O5 含量;(2)富集大离子亲石 元素(Ba、Sr、K)和 LREEs,亏损高场强元素(Nb、Ta、Zr、Hf)和 HREEs。全岩地球化学特征(如高 Ba、Sr含量和 K/Rb比值,低 Rb、Y含量)与高 BaSr花岗岩相符;(3)根据主量元素,相容元素(Cr、Co、Ni、V)和微量元素(Ba、Sr)特征,研究样品可分为 2 组:组 1具有高 Ba特征,Sr(87Sr/86Sr(t)=07049~07053)和 Nd(εNd(t)=-115~-97)同位素组成均一,经历了以黑云母 为主的分离结晶作用;组 2具有低 Ba特征,Sr(87Sr/86Sr(t)=07051~07070)和 Nd(εNd(t)=-187~-148)同位素组成更 加富集且分散,经历了角闪石,斜长石及磷灰石、磁铁矿和榍石等矿物分离结晶。2组样品不同的源区组分和分离结晶组合会 显著改变岩浆中特征微量元素(Ba、Sr、Rb)含量和比值(Sr/Y、Nb/Ta、Dy/Yb)。本文研究表明华北克拉通中部带岩石圈地幔 富集机制主要与元古代东、西陆块向中部带碰撞拼合时俯冲板块派生流体交代作用有关,该区中生代破坏机制为古太平洋板 块俯冲所引起的碰撞后伸展背景下富集岩石圈地幔减压熔融、底侵至下地壳并引起“壳幔”岩浆相互作用所形成的大规模深 部岩浆抽取。 关键词 高 BaSr花岗岩;岩石圈地幔交代作用;“壳幔”岩浆混合;华北克拉通破坏 中图法分类号 P588121;P5973
21431697_华北克拉通东北部新太古代晚期岩浆作用和地壳增生:锆石U-Pb-Hf同位素、微量元素
1000 0569/2020/036(04) 1076 90ActaPetrologicaSinica 岩石学报doi:10 18654/1000 0569/2020 04 07华北克拉通东北部新太古代晚期岩浆作用和地壳增生:锆石U Pb Hf同位素、微量元素和地球化学制约郝乐燃1 杨德彬1,2,3 许文良1,2 母茂松1 全籦糠1 杨浩田1 王安琪1HAOLeRan1,YANGDeBin1,2,3 ,XUWenLiang1,2,MUMaoSong1,QUANYiKang1,YANGHaoTian1andWANGAnQi11 吉林大学地球科学学院,长春 1300612 自然资源部东北亚矿产资源评价重点实验室,长春 1300613 东北亚生物演化与环境教育部重点实验室,长春 1300261 CollegeofEarthSciences,JilinUniversity,Changchun130061,China2 KeyLaboratoryofEvaluationofMineralResourcesinNortheastAsia,MinistryofNaturalResources,Changchun130061,China3 KeyLaboratoryoftheMinistryofEducationforBiologicalEvolutionandEnvironmentinNortheastAsia,Changchun130026,China2019 05 14收稿,2020 02 26改回HaoLR,YangDB,XuWL,MuMS,QuanYK,YangHTandWangAQ 2020 LateNeoarcheanmagmatismandcrustalgrowthinnortheasternNorthChinaCraton:ConstraintsfromzirconU Pb Hfisotope,traceelementsandwholerockgeochemistry ActaPetrologicaSinica,36(4):1076-1090,doi:10 18654/1000 0569/2020 04 07Abstract LA ICP MSzirconU Pbgeochronologyandtheirtraceelements,aswellaswholerockgeochemistryandzirconHfisotopestudiesonNeoarcheangraniticrocksinwesternandsouthernLiaoningregionshavebeenprovided,aimedtoconstrainthePrecambriancrustalgrowthandevolutioninthenortheasternNorthChinaCraton(NCC).TheresultsshowthatzirconsfromDiaoyutaimonzograniteinwesternLiaoning,ChengzitangneissicquartzdioriteandAnbograniticgneissinsouthernLiaoningalldevelopedmagmaticgrowthzone,andshowrelativelyhighTh/Uratios(0 24~1 75)andtypicalrareearthelementpatterns,whichindicatethattheirmagmaticorigin ZirconU PbdatingresultsshowthattheDiaoyutaimonzogranite,theoriginalrocksoftheChengzitangneissicquartzdioriteandAnbograniticgneisswereformedat2519±9Ma,2505±10Maand2519±11Ma,respectively,i e theLateNeoarchean TheNeoarcheangraniticrocksinwesternandsouthernLiaoningregionsarecharacterizedbyhighSiO2(61 85%~73 38%),lowMgO(0 36%~2 83%)andhighNa2O+K2O(7 64%~10 86%)contents,typicalofmetaluminumtoweakperaluminumandhighpotassiumcalc alkalinegranites Theywerecharacterizedbyenrichmentinlightrareearthelementsandlargeionlithophileelementsanddepletioninheavyrareearthelementsandhighfieldstrengthelements,aswellasweaklynegativeSr,P,TiandEuanomalies MagmaticzirconsallhavepositiveεHf(t)valuesrangingfrom0 4to5 9,andtheirtDM1agesvaryfrom2595to2798Mawithapeakageof2740Ma,whichisconsistentwiththemostimportantNeoarcheancrustalaccretioneventintheNCC Moreover,relativelylowTZrvaluesoftheChengzitangneissicquartzdioriteandAnbograniticgneissindicateparticipationofsubductionfluidsinthesourcearea Combinedwiththecharacteristicsofregionaltectonicevolution,wesuggestthattheNeoarcheangraniticrocksinwesternandsouthernLiaoningregionwerederivedfrompartialmeltingofthejuvenilelowercrustalmaterialsandformedinthearcenvironmentrelatedtotheplatesubduction Keywords Graniticrocks;Neoarchean;Crustalgrowth;WesternandsouthernLiaoningregions;NorthChinaCraton摘 要 辽西 辽南地区新太古代花岗质岩石的锆石LA ICP MSU Pb年代学和微量元素及全岩地球化学和锆石Hf同位素研究为探讨华北克拉通东北部前寒武纪地壳生长和演化提供了制约。
