Atrazine removal by powdered activated carbon in floc blanket reactors
依特立生化学名称

依特立生化学名称
依特立生的化学名称为磷酰二胺吗啉代寡聚体(PMO)亚类的反义寡核苷酸,英文名为Eteplirsen,别名依特普森、AVI-4658。
分子式为C364H569N177O122P30,分子量为10305.7道尔顿,结构中包含30个碱基序列、39个吗啉环、1个哌嗪环。
依特立生是一种治疗杜氏肌营养不良症的创新疗法,通过激活DMD基因的外显子跳跃,部分修复了DMD蛋白质的缺失,能够显著减缓患者的肌肉退化,在改善肌肉力量和功能、提升生活质量等方面也具有积极的效果。
尽管依特立生疗效显著,但其成本较为昂贵,且临床数据有限,仍需更多的研究来确定其长期疗效和安全性。
艾沙康唑硫酸盐说明书

艾沙康唑硫酸盐;Isavuconazonium sulfate产品编号:MB5282质量标准:>95%,BR包装规格:20MG;100MG;产品形式:solid基本信息简介:艾沙康唑硫酸盐是艾沙康唑的前体药物,进入体内后代谢为艾沙康唑后,发挥抗真菌的作用机制。
别名:N-methyl-[2-[[[1-[1-[(2R,3R)-3-[4-(4-cyanophenyl)-2-thiazolyl]-2-(2,5-difluorophenyl)-2-hydroxybutyl]-4H-1,2,4-triazolium-4-yl]ethoxy]carbonyl]methylamino]-3-pyridinyl]methyl ester, glycine, monosulfate物理性状及指标:外观:………………白色至类白色固体溶解性:……………Soluble in DMSO;Water Insoluble含量: (95)储存条件:-20℃,避光防潮密闭干燥生物活性2015年3月6日,美国FDA优先审批批准日本安斯泰来的抗真菌新药艾沙康唑硫酸盐(Isavuconazonium sulfate),以商品名Cresemba上市。
用于治疗侵入性曲霉病和毛霉菌病,这两种真菌感染多发于血癌患者中。
艾沙康唑硫酸盐是艾沙康唑的前体药物,进入体内后代谢为艾沙康唑后,发挥抗真菌的作用机制。
艾沙康唑硫酸盐有口服和注射剂,艾沙康唑较辉瑞的伏立康唑(Vfend)更安全有效,病患死亡率更低。
艾沙呋唑是唑类抗真菌药艾沙呋唑(ISA)的水溶性前药形式。
口服伊沙呋唑铵可提高对唑敏感和耐药的烟曲霉感染大鼠模型在0.25-512毫克/千克/天剂量范围内(伊沙当量=0.12-245.8毫克/千克/天)的存活率。
以40-60 mg/kg的Isa当量剂量给药时,Isavuconazonium可减轻真菌负荷和机体介导的肺损伤,并提高实验性侵袭性肺曲霉病兔模型的存活率。
阿泰灵分子式

阿泰灵分子式
阿泰灵(Ateplase)是一种由纤维蛋白原激活剂(tPA)组成的生物制剂,其在医学领域具有重要的治疗作用。
阿泰灵的主要作用是溶解血栓,改善血流,从而帮助预防心脑血管疾病的发生。
阿泰灵的分子式为C179H234N43O73S,这是一个由多个氨基酸组成的蛋白质分子。
氨基酸是生物体内蛋白质的基石,它们通过特定的化学键连接在一起,形成多肽链,最终形成具有特定功能的蛋白质。
阿泰灵的分子式表明它是一种大分子蛋白质,具有复杂的结构和生物活性。
在医学领域,阿泰灵被广泛应用于血栓治疗。
它可以溶解血栓,恢复血流,减轻血管阻塞带来的危害。
特别是在心肌梗塞、中风等急性心脑血管事件中,阿泰灵的应用可以挽救患者的生命,降低致残率。
此外,阿泰灵还可以用于治疗深静脉血栓、肺栓塞等疾病。
尽管阿泰灵在治疗血栓方面具有显著的优点,但它的使用也存在一定的局限性。
例如,阿泰灵只能在外周血循环中使用,不能在颅内出血的情况下使用。
此外,过量使用阿泰灵可能导致出血风险增加,因此需要在专业医生的指导下使用。
总之,阿泰灵作为一种具有生物活性的蛋白质分子,其在医学领域的应用价值不容忽视。
随着科学技术的不断发展,阿泰灵在未来有望得到更广泛的应用,为更多患者带来福祉。
然而,关于阿泰灵的研究和临床应用仍需不断深入,以充分发挥其潜力,同时确保患者的安全。
维莫非尼片说明书

核准日期:2017年3月10日修改日期:2017年5月26日2017年11月29日2018年7月24日2020年2月13日2021年2月26日2022年1月17日2022年2月22日维莫非尼片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名:维莫非尼片商品名:佐博伏®,英文商品名Zelboraf®英文名:Vemurafenib film-coated tablets汉语拼音:Weimofeini Pian【成份】本品主要活性成分为维莫非尼,以维莫非尼和琥珀酸醋酸羟丙甲纤维素固体分散体存在。
化学名称:丙烷-1-磺酸{3-[5-(4-氯苯基)-1H- 吡咯并[2,3-b]吡啶-3-羰基]-2,4-二氟代苯基}- 酰胺。
化学结构式:分子式:C23H18ClF2N3O3S分子量:489.93【性状】两面凸起、粉白色至橙白色的薄膜衣片。
【适应症】佐博伏®适用于治疗经CFDA批准的检测方法确定的BRAF V600突变阳性的不可切除或转移性黑色素瘤。
【规格】240 mg【用法用量】患者必须经由CFDA批准的检测方法确定的证明肿瘤为BRAF V600突变阳性,才可使用佐博伏®治疗。
佐博伏®不能用于BRAF野生型黑色素瘤患者。
首剂药物应在上午服用,第二剂应在此后约12小时,即晚上服用。
每次服药均可随餐或空腹服用。
用一杯水送服药物,服药时整片吞下佐博伏®片剂。
不应咀嚼或碾碎佐博伏®片剂。
标准剂量佐博伏®的推荐剂量为960 mg(四片240 mg片剂),每日两次。
治疗持续时间建议佐博伏®治疗应持续至疾病进展或发生不可接受的毒性反应(参见表1和表2)。
漏服如果漏服一剂计划的药物,可在下一剂服药4小时以前补服漏服的药物,以维持每日两次的给药方案。
不应同时服用两剂药物。
呕吐如果佐博伏®服药后发生呕吐,患者不应追加剂量,而应按常规剂量继续治疗。
重庆华邦制药 他扎罗汀凝胶说明书
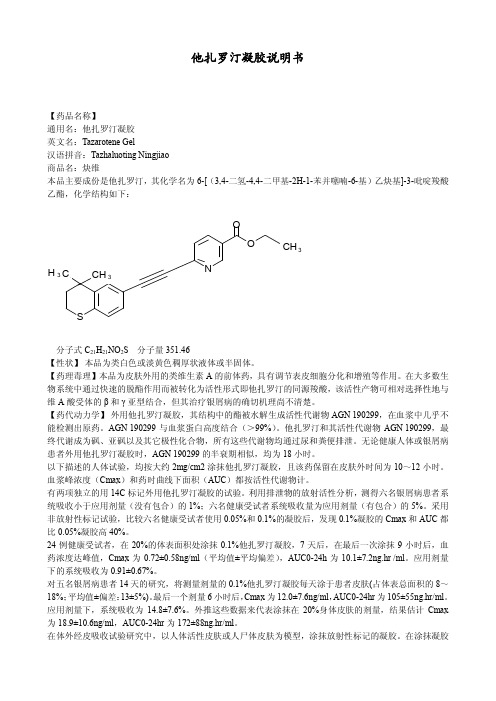
他扎罗汀凝胶说明书【药品名称】通用名:他扎罗汀凝胶英文名:Tazarotene Gel汉语拼音:Tazhaluoting Ningjiao商品名:炔维本品主要成份是他扎罗汀,其化学名为 6[(3,4二氢4,4二甲基2H1苯并噻喃6基)乙炔基]3吡啶羧酸 乙酯,化学结构如下:SNOOCH3 CH 33 CH分子式 C21H21NO2S 分子量 351.46【性状】 本品为类白色或淡黄色稠厚状液体或半固体。
【药理毒理】本品为皮肤外用的类维生素 A的前体药,具有调节表皮细胞分化和增殖等作用。
在大多数生 物系统中通过快速的脱酯作用而被转化为活性形式即他扎罗汀的同源羧酸,该活性产物可相对选择性地与 维 A酸受体的 β 和 γ 亚型结合,但其治疗银屑病的确切机理尚不清楚。
【药代动力学】 外用他扎罗汀凝胶,其结构中的酯被水解生成活性代谢物 AGN 190299,在血浆中几乎不 能检测出原药。
AGN 190299 与血浆蛋白高度结合(>99%)。
他扎罗汀和其活性代谢物 AGN 190299,最 终代谢成为砜、亚砜以及其它极性化合物,所有这些代谢物均通过尿和粪便排泄。
无论健康人体或银屑病 患者外用他扎罗汀凝胶时,AGN190299 的半衰期相似,均为 18小时。
以下描述的人体试验,均按大约 2mg/cm2 涂抹他扎罗汀凝胶,且该药保留在皮肤外时间为 10~12 小时。
血浆峰浓度(Cmax)和药时曲线下面积(AUC)都按活性代谢物计。
有两项独立的用 14C 标记外用他扎罗汀凝胶的试验。
利用排泄物的放射活性分析,测得六名银屑病患者系 统吸收小于应用剂量(没有包合)的 1%;六名健康受试者系统吸收量为应用剂量(有包合)的 5%。
采用 非放射性标记试验,比较六名健康受试者使用0.05%和 0.1%的凝胶后,发现 0.1%凝胶的 Cmax 和 AUC 都 比 0.05%凝胶高 40%。
8 心邀生物供应 阿法替尼杂质对照品
杂质对照品推荐——心邀生物阿法替尼系列★产品均有现货供应★阿法替尼是新一代口服小分子酪氨酸激酶抑制剂(TKI),是首个不可逆ErbB家族阻滞剂,可作用于包括EGFR在内的整个ErbB家族。
与第一代可逆的EGFR TKI不同的是,阿法替尼会不可逆地与EGFR结合,从而达到关闭癌细胞信号通路、抑制肿瘤生长的目的。
阿法替尼系列杂质心邀生物现货供应心邀生物的研发团队,对抗肿瘤和免疫药的杂质对照品深入研究,具有全套的杂质系列对照品和中间体,对于阿法替尼系列杂质杂质对照品,质优价好,纯度高,满足客户需求。
【阿法替尼中间体杂质3】英文名:Afatinib Intermediate IV Impurity 3CAS号:N/A货号:MDZ000489分子式:C19H17ClN4O5分子量:416.82纯度:95%以上【阿法替尼中间体杂质7】英文名:Afatinib Impurity 7CAS号:N/A货号:MDZ000490分子式:C14H7Cl2FN4O2分子量:353.14纯度:95%以上【阿法替尼中间体杂质A】英文名:Afatinib Impurity ACAS号:N/A货号:MDZ000491分子式:C18H14ClFN4O4分子量:404.78纯度:95%以上【马来酸阿法替尼中间体杂质4】英文名:Afatinib Intermediate IV Impurity 4 CAS号:N/A货号:MDZ000493分子式:C18H16Cl2N4O3分子量:407.25纯度:95%以上【马来酸阿法替尼中间体杂质5】英文名:Afatinib Intermediate IV Impurity 5 CAS号:N/A货号:MDZ000494分子式:C14H7ClF2N4O2分子量:336.68纯度:95%以上【马来酸阿法替尼中间体杂质6】英文名:Afatinib Intermediate IV Impurity 6 CAS号:N/A货号:MDZ000495分子式:C18H14ClFN4O4分子量:404.78纯度:95%以上【马来酸阿法替尼中间体杂质I】英文名:Afatinib Intermediate IV Impurity 1 CAS号:N/A货号:MDZ000492分子式:C12H11N3O5分子量:277.23纯度:95%以上以上产品仅为例,更多阿法替尼杂质详情可咨询官网,心邀生物对阿法替尼杂质系列还有许多研制优势,并且以上产品均有现货供应,欢迎来购!专业、全面为您提供所需杂质对照品、化合物合成以及详细技术资料,助您在药研或一致性评价上节约时间和成本。
8-亚甲基-甲胺-尼泊尔鸢尾异黄酮和以该化合物为活性成分的药

专利名称:8-亚甲基-甲胺-尼泊尔鸢尾异黄酮和以该化合物为活性成分的药物组合物
专利类型:发明专利
发明人:仲英,王福文,刘鲁,解砚英,王菊,牟艳玲,左春旭,胡志力,王元书,周玲
申请号:CN200610068403.5
申请日:20060818
公开号:CN1907979A
公开日:
20070207
专利内容由知识产权出版社提供
摘要:本发明涉及新颖的结构式(I)的尼泊尔鸢尾异黄酮衍生物及其药用盐。
本发明还涉及该化合物的制备方法,以该化合物为活性成份的药物组合物,以及本发明化合物和药用组合物在制备改善或治疗心、脑血管疾病和抗骨质疏松的药物中的应用。
申请人:山东省医学科学院药物研究所
地址:250062 山东省济南市历下区经十路89号
国籍:CN
更多信息请下载全文后查看。
枸杞叶多酚的超声辅助酶法提取工艺优化及抗氧化活性分析

山西农业科学 2023,51(9):1060-1068Journal of Shanxi Agricultural Sciences枸杞叶多酚的超声辅助酶法提取工艺优化及抗氧化活性分析闫帅帅,晋程妮,武颖,徐建国,张亮亮(山西师范大学 食品科学学院,山西 太原 030031)摘要:对枸杞叶中具有生物活性的化合物进行提取是提升枸杞资源利用效率的前提。
为了提高枸杞叶中多酚物质的提取效率及探究其生物活性功能,以枸杞叶为试验原料,基于超声波辅助酶法探究乙醇体积分数、料液比、超声时间和纤维素酶添加量等4个单因素对枸杞叶多酚提取效率的影响,通过响应面分析优化枸杞叶多酚的提取工艺,并探究枸杞叶多酚提取物对DPPH 和ABTS 自由基的清除能力。
结果表明,4个单因素对枸杞叶多酚提取效率均具有一定影响,枸杞叶多酚提取效率均呈先升高后降低的趋势;经过拟合优化取得最优的提取条件为:乙醇体积分数68%、料液比1∶89(g/mL )、超声时间39 min 、纤维素酶添加量2 mg/g ,在此条件下,枸杞叶提取液中多酚含量为5.09 mg/g ,与预测值(5.21 mg/g )的相对误差仅为2.3%。
抗氧化试验表明,枸杞叶多酚提取物对DPPH 和ABTS 自由基的清除率随着溶液多酚质量浓度的增加而升高,半数抑制浓度IC 50值分别为27.28、190.00 μg/mL ,表明具有较好的抗氧化能力。
综上,超声辅助酶法可提高枸杞叶多酚的提取效率,所提取的枸杞叶中多酚具有明显的体外抗氧化活性。
关键词:枸杞叶多酚;超声辅助酶法;响应面分析法;抗氧化活性中图分类号:TS209 文献标识码:A 文章编号:1002‒2481(2023)09‒1060‒09Optimization of Ultrasound-Assisted Enzymatic Extraction Technology andAntioxidant Activity of Polyphenols from Lycium barbarum LeavesYAN Shuaishuai ,JIN Chengni ,WU Ying ,XU Jianguo ,ZHANG Liangliang (College of Food Science ,Shanxi Normal University ,Taiyuan 030031,China )Abstract :The extraction of bioactive compounds from Lycium barbarum leaves is the premise to improve the utilization efficiency of Lycium barbarum resources. In order to improve the extraction efficiency and explore biological activity of polyphenols from Lycium barbarum leaves, in this study, taking Lycium barbarum leaves as the test material, the effects of four single factors including ethanol volume fraction, solid -liquid ratio, ultrasonic time, and the amount of cellulase added on the extraction efficiency of polyphenols from Lycium barbarum leaves were investigated using the ultrasonic -assisted enzymatic extraction. The response surface analysis was used to optimize the extraction technology of polyphenols from Lycium barbarum leaves and the scavenging ability of polyphenol extracts from Lycium barbarum leaves on DPPH and ABTS free radicals was studied. The results showed that the four single factors had the same trend that increased first and then decreased on the extraction efficiency of polyphenols from Lycium barbarum leaves. After design optimization, the optimal extraction conditions were defined with: 68% of ethanol volume fraction, 1∶89(g/mL) of solid -liquid ratio, 39 min of ultrasonic time, and 2 mg/g of cellulase, while the content of polyphenols from Lycium barbarum leaves obtained under this condition was 5.09 mg/g, which had a relative error with the predicted value(5.21 mg/g) was 2.3%. The results of antioxidant activities showed that the removal rate of the polyphenols from Lycium barbarum leaves on DPPH and ABTS increased with increasing mass concentration of polyphenols. The IC 50 values of half inhibitory concentration were 27.28 μg/mL and 190 μg/mL, respectively, which indicated that Lycium barbarum leaves had excellent antioxidant capacity. In conclusion, the extraction efficiency of polyphenols from Lycium barbarum leaves could be improved by ultrasonic -assisted enzymatic extraction, and the polyphenols extracted from Lycium barbarum leaves had significant antioxidant activity in vitro .Key words :polyphenols from Lycium barbarum leaves; ultrasound -assisted enzymatic extraction technology; response sur⁃face methodology; antioxidant activitydoidoi:10.3969/j.issn.1002-2481.2023.09.11收稿日期:2023-01-06基金项目:山西省自然科学基金项目(202103021223247);山西师范大学自然科学基金基础研究项目(02080183)作者简介:闫帅帅(1994-),男,山西晋城人,讲师,博士,主要从事食品营养与安全研究工作。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ATRAZINE REMOVAL BY POWDERED ACTIVATEDCARBON IN FLOC BLANKET REACTORSCARLOS CAMPOS 1*M ,VERNON L.SNOEYINK 2,BENITO MARINAS 2M ,I.BAUDIN 1and INE11CIRSEE,Lyonnaise des Eaux,38Rue du President Wilson,78230Le Pecq,France and 2University ofIllinois at Urbana-Champaign,Department of Civil and Environmental Engineering,205N.MathewsAve.,Urbana,IL 61801,USA(First received 1May 1999;accepted in revised form 26June 1999)Abstract ÐThe application of powdered activated carbon (PAC)to up¯ow ¯oc blanket reactors (FBR)is widely used to reduce the concentration of organic compounds present in drinking water sources.Long carbon retention times can be reached due to the high solids concentrations attainable in the blanket,potentially resulting in organic loadings close to the maximum adsorptive capacity predicted by the isotherm.However,some operating parameters can compromise both carbon retention time and adsorption capacity,leading to poor adsorption performance.The objective of this study was to point out these parameters and to determine whether the carbon capacity determined by the bottle point isotherm test can be used to predict the removal of organic micropollutants by PAC applied in FBRs.For this purpose,a laboratory-scale up¯ow FBR was used to evaluate the steady-state removal of atrazine spiked in a natural water.The results of this study showed that the PAC was not used at its maximum capacity predicted by batch isotherm experiments,which cannot be attributable to the fact that carbon retention times were lower than 20h.It is hypothesized that carbon capacity for micropollutants in continuous-¯ow systems,where the carbon retention time is higher than the hydraulic retention time,is a function of the DOC throughput.This study also showed that carbon retention time decreases with increasing carbon dose,or hydraulic loading rate.72000Elsevier Science Ltd.All rights reservedKey words Ðadsorption,atrazine,carbon retention time,dissolved organic carbon,¯oc blanket reactor,powdered activated carbonINTRODUCTIONRecent concern over the presence of organic sub-stances of public health signi®cance in drinking water has led to the development of innovative water treatment technologies for their removal.Or-ganic compounds in ®nished water can cause aes-thetic problems,such as taste and odors,and adverse health e ects.Naturally occurring organic matter (NOM)is also a problem because it can cause color and can be the precursor material for the formation of organic disinfection by-products (James M.Montgomery,Consulting Engineers,Inc.(JMM),1985).Adsorption onto powdered activated carbon (PAC)is one process that is widely used to remove organic compounds from drinking water.Appli-cation of PAC in a conventional treatment plant can be performed at di erent locations within thetreatment train.However,other processes,such as coagulation,sedimentation,or oxidation may inter-fere with adsorption and reduce its e ciency (Najm et al .,1990;Snoeyink and Summers,1999).When PAC is added to conventional treatment units,such as coagulation or sedimentation,the carbon resi-dence time (CRT)can be very close to the hydraulic residence time,typically from 0.5to 2h.Such CRTs are too short to allow the PAC to reach its maximum adsorption capacity for most target or-ganic compounds.One reactor con®guration of interest for PAC ap-plications is the up¯ow solids contact clari®er,also known as the ¯oc blanket reactor (FBR).These reactors rely on a high-density blanket of solids to enhance ¯occulation of the coagulated suspended matter.Similarly,this blanket of solids can also entrap PAC particles that would otherwise be washed out of the reactor with the e uent due to their low density.Consequently,a high PAC con-centration can be maintained in the ¯uidized blan-ket,and retention times on the order of days can be achieved (Hoehn et al .,1987;Kassam et al .,1991).Wat.Res.Vol.34,No.16,pp.4070±4080,200072000Elsevier Science Ltd.All rights reservedPrinted in Great Britain0043-1354/00/$-see front matter4070/locate/watresPII:S0043-1354(00)00169-X*Author to whom all correspondence should be addressed.Tel.:+33-1-34-802-383;fax:+33-1-305-36209;e-mail:carlos.campos@lyonnaise-des-eaux.frHowever,some operating parameters can compro-mise both carbon retention time and adsorption ca-pacity,leading to poor adsorption performance.A few studies have attempted to evaluate adsorp-tion e ciencies when PAC is added to FBRs.Najm et al.(1993)evaluated the removal of2,4,6-trichlor-ophenol(TCP)from natural water by PAC in a laboratory-scale FBR.Since steady-state removals of TCP in the FBR were very close to those pre-dicted by the isotherm,it was concluded that the PAC was used at its maximum capacity in the blan-ket.Najm et al.(1993)also proposed a method to evaluate the extent of adsorption of organic com-pounds onto PAC in continuous-¯ow processes. However,no®eld data were used to verify the accu-racy of the predictions presented for various carbon retention times.A later study on TCP removal from the same natural water pointed out the possi-bility of heterogeneous reactions of TCP with dis-solved oxygen on the surface of the carbon particles (Adham et al.,1991).If this reaction occurred,TCP removals observed in the FBR e uent would have been not entirely attributable to adsorption onto PAC since surface reaction with oxygen may have consumed the TCP.Thus,further experimentation is needed to assess whether full isotherm capacity can be achieved in FBRs.Carbon accumulation in the blanket of a slurry recirculating clari®er was evaluated in both pilot and full-scale systems by Kassam et al.(1991). Although equilibrium capacities appeared to decrease for CRTs longer than100h,the results were not conclusive.Previous studies point out important parameters a ecting the adsorption e ciency of PAC in solids contact clari®ers,but do not present a valid approach to predict adsorption performance.The objective of this study is to verify the hypothesis that the steady-state removal of the micropollutant atrazine by PAC in a FBR can be predicted by the isotherm.The e ects of carbon dose and hydraulic loading rate on carbon concentration and carbon retention time in the blanket are also evaluated.MATERIALSWater sourceCentral Illinois groundwater with a pH of7.8,a dis-solved organic carbon(DOC)concentration of2.1mg/l, and an alkalinity of290mg/l as CaCO3was used in this study.An in¯uent suspended solids concentration of 10mg/l was achieved by adding clay(kaolinite)to the low turbidity groundwater.CoagulantsThe coagulant was reagent grade aluminum sulfate (Mallinckrodt,Inc.Paris,KC)dosed at a concentration of 40mg/l as alum together with1mg/l of a cationic poly-electrolyte(Calgon Corporation,Pittsburgh,PA).These concentrations were kept constant for all the experiments performed with the FBR.AdsorbentWPH PAC(Calgon Corporation,Pittsburgh,PA)with an iodine number of1200,6.7%ash,3%moisture,appar-ent density of0.54g/cm3,and geometric mean diameter of 10m m was used in this study.AtrazineAdsorption equilibrium and kinetics parameters were determined in batch experiments using14C-labeled atrazine (Ciba±Geigy,Greensboro,NC)with a speci®c activity of 8.3mCi/mmole.Atrazine samples were analyzed by mix-ing2.5ml aliquots of each sample with18ml of scintil-lation cocktail(Ecoscint,National Diagnostics,Manville, NJ).The emitted radiation of the mixture was measured in a liquid scintillation analyzer(Tri-Carb Model1600CA, Packard Instrument Company,Downers Grove,IL)for 30min.The detection limit of the method was0.1m g/l without need for sample concentration.Non-labeled atrazine(Chem Service,West Chester,PA), added as a step-input,was used for the experiments with the FBR.The enzyme linked immunoassay(RAPIDAssay, Strategic Diagnostics Inc.,Newtown,PA)with a detection limit of0.05m g/l was used to analyze samples containing non-labeled atrazine.All reagents involved in the analysis were supplied with the test kits provided by the manufac-turer.A series of standards prepared using14C-labeled atrazine were also analyzed using the RAPIDAssay to con®rm that atrazine results from both methods were comparable in the concentration range used in the FBR experiments.Triplicate samples at concentrations ranging from0.3to3.7m g/l analyzed by both methods were found to correlate well r2 0X995).Floc blanket reactorThe FBR consisted of a Plexiglas cylinder with an in-ternal diameter of19cm and a depth of51cm,and a con-ical bottom piece with a depth of17cm.The total volume of the blanket and the water above it was15.5l.An in-ternal tube located axially fed the in¯uent water into the bottom conical section.The water¯owed upwards through the solids blanket,and the e uent was discharged through a circular weir located at the top of the cylindrical section. Excess solids formed during the coagulation±¯occulation process as well as excess PAC particles were removed from four symmetrically distributed tubes located in the cylinder at the level corresponding to a blanket volume of 7.25l,38cm from the bottom of the reactor.Three sampling ports located at depths corresponding to1,4 and6l(15,26and34cm from the bottom,respectively) were installed to facilitate sampling for PAC density deter-mination at di erent depths in the blanket.A schematic of the FBR system is presented in Fig.1.A 208-l continuously stirred stainless steel barrel was usedasFig.1.Schematic of the laboratory-scale FBR.Atrazine removal by PAC/FBR4071an equalization basin to ensure constant in¯uent water quality.The in¯uent water was pumped from the equaliza-tion basin at a¯ow rate of0.6l/min and dosed with atra-zine,clay,alum,and polyelectrolyte from stock solutions by means of peristaltic pumps(Cole±Parmer,Chicago, IL).The in¯uent was mixed with these solids and chemi-cals in an airtight rapid mix basin.Water from the rapid mix basin¯owed into an open basin which had an outlet designed to serve as a siphon to dispense periodic pulses of water to the FBR.Fresh PAC was applied directly into this siphon basin from a stock slurry with a peristaltic pump.EXPERIMENTAL METHODS Determination of equilibrium parametersSeven-day isotherm tests were conducted in raw and coagulated groundwater for both atrazine and DOC,following the bottle point isotherm technique as outlined by Randtke and Snoeyink(1983). Duplicate samples were taken from each bottle,®l-tered through a0.45m m membrane®lter(Micron Separations,Westboro,MA)and analyzed for DOC using a Phoenix8000TOC Analyzer(Tek-mar-Dohrmann,Cincinnati,OH).Samples contain-ing14C-labeled atrazine were analyzed using the procedure outlined previously.The amount adsorbed onto the PAC was calculated by using a mass balance.Solid and liquid phase concentrations were plotted on a log±log scale graph and the data were®tted with the Freundlich equation.The pro-cedure to evaluate equilibrium parameters for atra-zine in the presence of NOM is discussed in the section on mathematical modeling. Determination of kinetic parametersBatch kinetic tests were run with both raw and coagulated groundwater in order to determine the homogeneous surface di usion coe cient,D s,of DOC and atrazine.These kinetic tests were per-formed for a minimum of4h.Identical mixing con-ditions were applied to all batch reactors tested simultaneously by means of an overhead stirrer (Phipps&Bird,Richmond,VA)that rotated at a minimum of240rpm.Prior to its use in batch tests, PAC was soaked overnight in organic-free deio-nized distilled water(DOC<0.1mg/l)to allow for complete wetting of the pores.The di usion coe cient for DOC adsorption was estimated using a search routine based on the Homogeneous Surface Di usion Model(HSDM) developed for single-solute systems,which mini-mizes the di erences between the predicted kinetic curve and the experimental data(Hand et al., 1983).The atrazine apparent di usion coe cient in natural water was evaluated by using the pseudo single-solute approach of Qi et al.(1994),which is discussed in the section on mathematical modeling. PAC concentration in the blanketWhen the concentration of PAC in the FBR e uent is negligible compared to the PAC concen-tration in the blanket,the average residence time of the PAC particles at steady-state can be calculated by dividing the mass of carbon present in the blan-ket by the PAC dosage rate.Thus,the CRT can be evaluated with the following expression(Najm et al.,1993):CRTr b V bC c Q l1where r b is the average PAC concentration in the ¯oc blanket,V b is the volume of the blanket,Q l is the in¯uent water¯owrate,and C c is the carbon dose.The PAC concentration(r b)was evaluated at three depths of the blanket in order to determine whether the pulsed feed provided a homogeneous ¯uidization for the range of PAC doses tested.One hundred milliliter samples were withdrawn at sample ports corresponding to1,4and6l volumes of the blanket from the bottom of the reactor. Average PAC densities were calculated by account-ing for the fractions of the blanket volumes rep-resented by each sampling port.The sampling sequence was from top to bottom in order to pre-vent disturbances of blanket structure in subsequent samples.For all FBR experiments,a second batch of samples was collected12h after taking the®rst batch of samples,in order to check whether steady-state conditions were fully developed for each car-bon dose.Total solids and PAC mass were deter-mined using the procedure outlined by Najm et al. (1993).MATHEMATICAL MODELINGNajm et al.(1993),Qi et al.(1994),and Najm (1996)proposed models to describe the steady-state removal of organic micropollutants from natural waters when PAC is applied in various continuous-¯ow systems.In their systems,it was assumed that the PAC particles had an exponential residence time distribution,and that all particles were exposed to the same adsorbate concentration in sol-ution.Under these assumptions in a PAC/UF sys-tem operated as a completely mixed tank reactor (CSTR),Qi et al.(1994)successfully veri®ed atra-zine removals predictions for various CRTs using the expression proposed by Nakhla et al.(1989):q FBR q I,FBRHd1À6p2Ii 11i2 1 i2p2D sR2CRTIe2 with:q I,FBR KC1a n FBR 3 where K and1/n are the Freundlich equilibrium parameters,D s is the surface di usion coe cient,Carlos Campos et al. 4072CRT is the carbon retention time in the reactor, q FBR is the amount adsorbed per mass of carbon, and q I,FBR is the amount of organic compound adsorbed per mass of carbon at equilibrium with the concentration exiting the reactor,C FBR.The concentration of the organic compound in solution exiting the FBR can be evaluated by using a mass balance in the reactor:C inÀC FBRÀq FBR C c 0 4 where C in and C FBR are the atrazine concentrations in the FBR in¯uent and e uent,respectively,and C c is the carbon dose.The application of equation(3)requires the prior determination of the maximum carbon capacity for the given initial concentration of the trace organic compound in the presence of NOM.For such mul-tisolute systems,adsorption capacity dependence on initial concentration of the trace compound has been characterized for a variety of trace organic compounds(Najm et al.,1991;Qi et al.,1994;Gil-logly et al.,1998;Knappe et al.1998).Using the Ideal Adsorbed Solution Theory coupled with the Equivalent Background Compound Method, Knappe et al.(1998)developed a simple method to predict adsorption capacities of trace organic com-pounds in the presence of NOM.This method reduces signi®cantly the amount of experimental data to be collected and eliminates the need for computer modeling.For a given carbon dose,the percentage of trace compound remaining in solution at equilibrium is independent of the initial concen-tration of the trace compound.For atrazine,this method is valid as long as the initial concentration is less than50m g/l.Once the plot of percent atra-zine remaining vs PAC dose is established,carbon capacity can be calculated for each carbon dose for any initial concentration(Knappe et al.1998).In this study,the simpli®ed approach proposed by Knappe et al.(1998)to estimate equilibrium ca-pacities is coupled with equations(2)±(4)to predict steady-state atrazine removals in up¯ow FBRs for a variety of carbon doses and blanket operating con-ditions.Model predictions are compared with ex-perimental data and the applicability of this modeling approach in continuous-¯ow adsorption systems is discussed.RESULTS AND DISCUSSION Evaluation of equilibrium parameters Determination of the equilibrium parameters for atrazine adsorption in the FBR required the evalu-ation of the e ect of coagulation of a fraction of the in¯uent DOC on the atrazine adsorption iso-therm.For this purpose,DOC isotherms were con-ducted in raw and coagulated groundwater using the same coagulant dose as in the continuous-¯ow experiments.Addition of40mg/l aluminum sulfate resulted in a coagulation pH of7.4.Coagulation reduced the DOC concentration from2.1to1.7mg/ l.Figure2shows the results of these isotherm tests and the®t of the experimental data using the Freundlich isotherm.The same Freundlich par-ameters,a K of39.5(mg DOC/g PAC)(l/mg)1/n and a1/n of1.47,were used to®t the experimental data for both the raw and coagulated water r2 0X9718).Data points corresponding to carbon doses higher than300mg/l were excluded from this analysis since these carbon doses are too high to be used in drinking water applications.Additional iso-therms were determined in raw water only and assumed to apply to coagulated water.Atrazine adsorption isotherms were conducted in groundwater to evaluate the dependence of atrazine capacity on its initial concentration.Three isotherm tests,one in organic-free water and two in ground-water were performed to calibrate the EBC model and to develop the plot of percent atrazine remain-ing vs PAC dose proposed by Knappe et al.(1998). The atrazine initial concentrations used for the groundwater isotherms were54.7and 2.0m g/l, whereas515.8m g/l was used in the single-solute iso-therm test.No losses of atrazine were observed in the blanks of the isotherm tests,which con®rmed that adsorption on either the Te¯on tape or the glassware did not contribute to atrazine removal. Figure3shows the experimental data used to cali-brate and verify the EBC model,and the increase in competition from NOM as atrazine initial concen-tration decreases.The single-solute isotherm par-ameters,K and1/n,were238.2(m mol/g)(l/m mol)1/n and0.44,respectively.The EBC parameters from the calibration were a K of1229.4(m mol/g)(l/ m mol)1/n,a slope1/n of0.53,and an initial concen-tration of1.07m mol/l.An additional isotherm in groundwater,using an initial concentration of 28.5m g/l,was used to verify the predictions of the EBCmodel.Fig.2.Isotherm for DOC adsorption from raw and coa-gulated groundwater onto WPH PAC.Atrazine removal by PAC/FBR4073The applicability of the Knappe et al .(1998)approach is illustrated in Fig.4,where the equili-brium data and model predictions for various atra-zine initial concentrations are plotted as percent atrazine remaining vs PAC dose.Also shown in Fig.4are the percent atrazine remaining data that were obtained in the isotherm experiment using coa-gulated groundwater.The agreement of these data with the other data sets provides further evidence to show that coagulation did not a ect atrazine adsorption capacity.Reduction of adsorption capacity for atrazine due to NOM adsorption was evaluated in an iso-therm experiment where atrazine was introduced after the PAC had been in contact with the in¯uent NOM for 7days.The initial atrazine concentration used in this experiment was 40.3m g/l,and the PAC doses ranged from 2to 20mg/l.After contact with atrazine for 7days,the atrazine percent remaining in solution was higher than predicted by the EBC model for simultaneous adsorption of NOM andatrazine.As shown in Table 1,the di erences in percent atrazine remaining decreased as PAC dose increased.Although the percentage di erences are small,they translate to capacity reductions of up to 24%.Evaluation of di usion coe cientThis section presents the results of a series of kin-etic tests performed to evaluate the e ect of coagu-lation and NOM preloading on atrazine adsorption kinetics.The results of DOC adsorption kinetics can be found elsewhere (Campos,1999).Five batch reactors were run in parallel using the same carbon dose of 4mg/l and the same groundwater dosed with 10mg/l of kaolinite.The DOC and atrazine concentrations of these tests were 2.1mg/l and 9.8m g/l,respectively.In the ®rst reactor,PAC was added to a groundwater spiked with atrazine,to simulate the adsorption conditions in the absence of coagulation.In the second reactor,PAC and 40mg/l of alum were added to groundwater spiked with atrazine,to simulate simultaneous coagulation and adsorption conditions.In the third reactor,PAC was added to a solution that contained groundwater previously spiked with atrazine and alum (2h),to simulate coagulation and adsorption occurring sequentially.No atrazine removal was observed by alum coagulation or by adsorption onto kaolinite.In the fourth unit,atrazine was spiked to a solution in which groundwater,alum,and PAC were allowed to react for 2h prior to the atrazine ad-dition.This was done to investigate the e ect of prior PAC exposure to NOM and alum on atrazine adsorption kinetics.A ®fth reactor reproduced the conditions tested with the fourth unit test but with-out alum,to isolate the preloading e ect of NOM from any fouling associated with the formation of aluminum colloids in the pores of the activated car-bonparticles.Fig.3.Atrazine isotherms in organic-free water and inCentral Illinoisgroundwater.Fig.4.Percent remaining vs carbon dose for various atra-zine initialconcentrations.Fig.5.E ect of coagulation on atrazine adsorption kin-etics.Carlos Campos et al.4074The results of these tests (Fig.5)are consistent with the equilibrium experiments.Neither simul-taneous nor prior NOM coagulation a ected atra-zine adsorption kinetics.An apparent di usion coe cient of 2X 0Â10À11cm 2/min,comparable to the value obtained by other authors (Qi et al .,1994),was obtained by ®tting the pseudo-single solute HSDM predictions using the equilibrium ca-pacity with the kinetic data corresponding to simul-taneous adsorption of NOM and atrazine in the batch reactor.However,a signi®cant reduction in atrazine adsorption rate was observed when the PAC was pre-exposed to NOM for 2h,and this e ect was not changed by alum coagulation.This result shows that NOM can hinder atrazine adsorp-tion.An HSDM model prediction,using the di u-sion coe cient obtained in the case of simultaneous adsorption of NOM and atrazine,and a capacity reduced by 24%in accordance with the NOM pre-loaded isotherm,did not ®t the experimental data well.Consequently,a reduction in equilibrium ca-pacity alone could not explain the reduction in the adsorption rate.The HSDM ®t of this set of kinetic data using the isotherm capacity reduced by 24%resulted in a di usion coe cient of 7X 0Â10À12cm 2/min.Therefore,prior adsorption of NOM appeared to reduce both di usivity and capacity.The reduction of adsorption rate might be caused by a reduction of surface di usion coe cient due to pore blockage of the adsorbed NOM molecules,and/or by the reduction in adsorption capacity for atrazine due to irreversible adsorption of NOM molecules in sites potentially available for atrazine adsorption.This observation agrees with those of Pelekani (1999)who showed that the rate of adsorption decreases as pore blockage from com-peting adsorbate occurs.Also,this author showed that capacity decreases as the adsorbed NOM increases.Furthermore,according to the HSDM model,a decrease in capacity will result in a decrease in the rate of adsorption (Crittenden and Weber,1978).The observation that the rate of atrazine adsorp-tion was the same when the water was coagulated as it was when no coagulant was applied is consist-ent with the isotherm experiments.It shows that co-agulation did not a ect atrazine concentration.In addition,this observation shows that alum did notremove the NOM fraction that competes directly with atrazine for adsorption sites.E ect of carbon dose on carbon retention time (CRT)When analyzing the factors a ecting the CRT in FBRs,it should be noted that the parameters in equation (1)are not independent.For the same in¯uent quality characteristics,the concentration of carbon in the blanket (r b )is dependent not only on the carbon dose (C c )but also on the hydraulic load-ing rate.The dependence of PAC density in ¯oc blankets on PAC dose has been reported in pre-vious studies (Kassam et al .,1991;Najm et al .,1993).This dependence was also observed for the range of PAC doses tested in this study:PAC den-sity in the blanket increased as PAC dose increased (2±15mg/l)for a constant in¯uent water quality (in¯uent turbidity),coagulating conditions,and hydraulic loading rate.However,the CRT decreased as PAC increased because PAC density did not increase proportionally with PAC dose.The observed relationship is depicted in Fig.6.A similar e ect may be expected if the in¯uent turbidity increases since the solids removal rate would increase to maintain steady-state conditions in the blanket (Kassam et al .,1991).E ect of blanket depth on carbon retention time According to equation (1),CRT should increaseTable 1.Isotherm experiment for atrazine adsorption after the PAC had been contacted with NOM for 7daysPAC dose (mg/l)C eq (m g/l)C /C 0measured (%)C /C 0predicted a (%)Di erence (%)218.746.335.011.447.4218.411.9 6.56.1 3.097.7 5.1 2.59.1 1.42 3.5 2.2 1.312.10.88 2.2 1.2 1.020.70.320.80.40.4aFrom C /C 0vs PAC dose plot in rawwater.Fig.6.E ect of PAC dose on carbon retention time in the blanket.V b 7X 25l,hydraulic loading rate=1.3m/h.Atrazine removal by PAC/FBR4075in direct proportion to blanket depth.In this study,the e ect of blanket depth on CRT was evaluated for two di erent PAC doses (4and 8mg/l)and a hydraulic loading rate of 1.3m/h (see Table 2).For a PAC dose of 4mg/l,increasing the blanket volume from 7.25to 14l did not greatly a ect the PAC concentration in the blanket,while the CRT increased by a factor of 1.7,from 17to 29h.Simi-larly,for a PAC dose of 8mg/l,increasing the volume of the blanket by a factor of 1.9resulted in a CRT increase by a factor of 1.5,from 15to 23h.In this case,both solids and PAC densities were 20±30%lower for the larger blanket volume than those obtained for a blanket of 7.25l,which suggests that coagulating conditions and resulting ¯oc structure may have been slightly di erent.In full-scale applications of the FBR,increasing CRT by increasing blanket depths may not be a feasible alternative.These types of clari®ers usually operate with blanket depths of 2±3m,and oper-ators may have no control over the blanket depth.Under such conditions,increasing the CRT would have to be accomplished by increasing the blanket density,and there is a limit to the maximum density that can be achieved.For example,with a PAC dose of 10mg/l,a hydraulic loading rate of 4m/h and a blanket depth of 2m,a PAC concentration in the blanket higher than 500mg/l would be necessary to provide a CRT greater than one day.Achieving such PAC concentration may not be possible under this hydraulic loading rate.E ect of hydraulic loading rate on carbon retention timeHydraulic loading rate plays an important role in determining the PAC density in the ¯uidized blan-ket and consequently,in determining CRT.In this study,the blanket ¯uidization conditions and the resulting PAC concentrations were evaluated when the hydraulic loading rate was reduced from 1.3m/h to 0.65m/h,for two di erent PAC doses (4and 8mg/l)and a blanket volume of 14l.PAC concen-trations and total solids resulting from these runs are summarized in Table 2.As hydraulic loading rate decreased,the ¯ow velocity decreased,resultingin denser blankets.For a carbon dose of 4mg/l,the decrease in hydraulic loading rate resulted in an increase in PAC concentration from 300to 625mg/l,and an increase in CRT by a factor of 4,from 29to 122h.Similarly,for a carbon dose of 8mg/l,PAC densities in the blanket increased from 480to 1365mg/l,which resulted in an increase of CRT from 23to 133h.These observations were consistent with the ex-perimental data reported by Najm et al .(1993)for which in¯uent water quality and coagulant doses were very similar to those used in this study.These authors reported that an increase in the hydraulic loading rate from 1to 1.5m/h resulted in a re-duction in the CRT from 25to 10h,for a PAC dose of 10mg/l.This observation has important en-gineering implications since a decrease in CRT may reduce PAC adsorption e ciency.Hydraulic load-ing rates typically used in the design of these type of clari®ers range from 1.3to 3m/h (Kawamura,1991),to as high as 4±8m/h (Degre mont,1989).Fluidization conditions in the blanketFluidization conditions in the blanket were alsoevaluated in this study in order to validate the assumption of a completely mixed blanket.Previous studies on slurry recirculating clari®ers (Kassam et al .,1991)reported that the PAC was evenly distrib-uted throughout the blanket.However,when ¯uidi-zation of the blanket in an FBR is accomplished by using hydraulic pulses,higher PAC densities may result at the bottom of the reactor.In this study,PAC concentrations measured at the bottom of the blanket were higher than those near the top of the blanket.This observation was more pronounced with increasing PAC dose,as illustrated in Fig.7.Consequently,some combination of operating con-ditions,such as low hydraulic loading rate and high carbon doses,may result in PAC density gradients in the blanket,and therefore,the assumption of a completely mixed ¯uidized bed would not be entirely applicable.Another important aspect for validating the assumption of a homogeneous blanket,was to determine whether segregation of the PAC particlesTable 2.Summary of FBR operating conditions and PAC/solids concentrations in blanketPAC dose (mg/l)Hyd.load (m/h)Blanket vol.(l)PAC/Solids (mg/l/mg/l)PAC (%)CRT hours1-l Port4-l Port 6-l Port Average 2 1.37.25206/1089177/928157/820183/96119184 1.37.25366/1376329/1192315/1155340/125727174 1.314312/1667296/1231296/1190300/1298232940.6514714/2877610/2457598/2400625/2510251228 1.37.25654/1970565/1641529/1476591/172634158 1.314565/1439473/1257454/1218480/1271382380.65142302/53821151/27001100/25701365/31914313315 1.37.25977/2307713/1602599/1289787/1797441130a1.3a 7.25a 1750/5750a 1500/3250a1400/2500a1500/4052aNC 11aaFrom Schimmoller et al .(1995).NC:not constant.Carlos Campos et al.4076。
