DNA细胞转染试剂(Entranster)使用手册
转染试剂使用说明书

210102
1ml
1. 2. 3. 4. 5. 6.
适用范围及特点: 适应于众多原代培养细胞和转化细胞株的基因转染 适用于瞬时转染和稳定转染 适应于贴壁细胞和悬浮细胞转染 转染效率高且稳定,在有无血清存在的细胞培养基中均能获得高效率转染 细胞毒性低 转染程序简单,转染实验可以在半小时内完成
产品储存: GenFectinTM (1.0mg/ml) 在室温下运输,试剂到时请即存放于 4℃,在 4℃可存放
2. 3).
配制转染工作液: ( 6 孔板或 35 mm 平皿, 2 ml 培养液) 取 5~8μ g DNA (起始用量 5μ g) ,加入稀释液中至总体积为 100μ l,轻轻混匀,室 温放置。
4).
先将 GenFectin TM 涡旋振荡混匀。取 GenFectinTM 1~4μ0μl,轻轻混匀,室温放置 5 分钟。
8).
稳定转染时,于转染后 24~48 小时消化细胞分至 3~5 个培养皿中,加适当浓度的 相应抗生素(如 G418)筛选。
建议的起始转染条件 : 培养容器
96 孔板 24 孔板 6 孔板 35mm 培养皿 60mm 培养皿
转染前一天 接种细胞数
1-1.510 个 0.5-1 10 个 2-4 10 个 2-4 10 个 4-6 105 个
3.
GenFectinTM 在转染中不受血清影响,所以 GenFectinTM / DNA 复合物能直接加到 含血清的培养基中,但稀释 GenFectinTM 和 DNA 的缓冲液不能混有血清,因为 GenFectinTM 在制备 GenFectinTM / DNA 复合物之前可能会与血清中的蛋白质反 应,影响转染效率。
-5-
英格恩entranster转染试剂说明书

英格恩entranster转染试剂说明书一、产品概述英格恩 Entanster 转染试剂是一种用于转染外源 DNA/RNA 到细胞内的试剂。
它是经过优化的化学试剂组合,可以将外源 DNA/RNA 高效地传递到各种细胞中,并促进其定向表达。
本试剂适用于体外转染实验和基因工程研究,具有高转染效率、低细胞毒性、简单易用等优点。
二、试剂成分英格恩 Entanster 转染试剂主要成分包括转染缓冲液和转染增强剂。
转染缓冲液中包含有机溶剂、非离子表面活性剂等,用于稳定 DNA/RNA与转染剂的结合。
转染增强剂含有具有阴离子表面活性剂、脂质、蛋白质等物质,可以提高细胞膜通透性和转染效率。
三、使用说明1.储存和稀释:试剂应储存于-20℃的冰箱中,保持干燥和避光。
使用前需要将试剂溶解在适当体积的转染缓冲液中,最佳稀释比例为1:9、溶液需充分混匀,离心并去除任何沉淀物。
2.样品准备:在转染前,将目标DNA/RNA溶解在适当的缓冲液中,浓度通常在0.1-1μg/μL最佳。
确保样品充分溶解并无明显沉淀。
3.转染操作:对于已经培养至适当倍数的细胞,将细胞用预先暖和的转染缓冲液洗涤一次,并去除缓冲液。
将转染缓冲液和溶解好的目标DNA/RNA混合,稍微搅拌均匀。
与此同时,将适量的转染增强剂加入到混合物中,充分混合。
然后将混合物直接滴加到细胞上,并轻轻摇晃培养板以确保混合物均匀分布。
4.培养和检测:将含有转染混合物的培养板放回恒温培养箱中,保持恒定的温度和CO2浓度。
培养时间和温度根据需要进行调整,通常在24-48小时后可以进行下一步实验或观察。
四、注意事项1.试剂需保存在干燥、阴凉、避光的环境中,避免结冰或暴露在高温环境中。
2.操作中需佩戴手套并遵循生物安全实验操作规范。
3.使用前需充分摇匀试剂瓶,确保所有试剂成分充分混合均匀。
4.转染缓冲液中的有机溶剂可能对一些细胞有毒性,因此在选择试剂时应参考相关文献或尝试不同的浓度和时间,以确定最佳条件。
Thermo turbofect 转染试剂说明书

PRODUCT INFORMATIONThermo ScientificTurboFect Transfection Reagent#R0531 1 mLfor _in vitro transfectionsLot XXXXXXXX Expiry Date MM.YYYYFFFFR0531FFFFXXXXXXXXwww. /onebioStorage: Upon receipt store at 4°C.Product shipped at ambient temperature.hMade in LithuaniaIntroductionThe Thermo Scientific™ TurboFect™ Transfection Reagent is a solution of cationic polymer in water. The polymers and deoxyribonucleic acid (DNA) form compact, stable, positively charged complexes, which protect DNA from degradation and facilitate gene delivery into eukaryotic cells. The transfection reagent is ideal for transfection of primary cells, difficult-to-transfect cells and other types of cells with transfection being possible in the presence or absence of serum. The transfection reagent demonstrates superior transfection efficiency and minimal toxicity when compared to lipid-based or other polymer-based transfection reagents.Rev.11 c Important Product Information• DNA quality is critical for successful transfection.For excitation/emission absorbance at 260/280nm, a ratio of 1.8 or higher is recommended.• Endotoxin-contaminated DNA results in inefficient transfection and can cause high cellular toxicity. • At the time of transfection, the optimal confluency for adherent cells is 70-90%.• Plate suspension cells at an optimal density to ensure logarithmic growth at the time of transfection. • Transient transgene expression occurs within 2-72 hours after transfection.• The optimal incubation time must be empirically determined and depends on the cell type, promoterstrength and expression product.• High transfection efficiency depends on thetransgene promoter and the cell line used.• Cytomegalovirus (CMV) promoter is commonly used for high gene expression in a variety of cell lines;however, other promoters such as simian virus(SV40) and Rous sarcoma virus (RSV) can also beused.• The volume of transfection reagent used depends on the amount of DNA, transgene and cells to betransfected. The ratios presented in the protocolsbelow are starting amounts and may be optimized. • Antibiotics do not interfere with transfectionreagent/DNA complex formation, cell transfection ortransfection efficiency.CERTIFICATE OF ANALYSISTransfection efficiency was tested on HeLa cells using1 µg of eGFP expressing plasmid and2 µL of TurboFect per 5 × 104 cells in 24-well plate. Transfection efficiency, i.e. the percentage of transfected cells, is90±10% as estimated by flow cytometry.Quality authorized by:Jurgita Zilinskiene Protocol for Transfection of CellsA. Material RequiredGrowth medium: Serum-free DMEM, RPMI orother growth mediumB. General Protocol for Transfection of Adherentand Suspension Cells in a 24-well PlateNote: The protocol is optimized for transfection in a24-well plate format. At the time of transfection, theoptimal confluency for adherent cells is 70-90%.Suspension cells should be in logarithmic growth phaseat the time of transfection. For best results, start with 1µgof DNA and 2µL of transfection reagent per well in a24-well plate (See Table 1). Subsequent optimization canfurther increase transfection efficiency, depending on thecell line and transgene used.1. In each well, seed ~5 × 104 adherent cells or~5 × 105 suspension cells in 1mL of growth medium24 hours before transfection.Note: Prepare transfection reagent immediatelybefore transfection.2. Dilute 1µg of DNA in 100µL of serum-free DMEM orother serum-free growth medium.3. Briefly vortex the transfection reagent and add 2µLto the diluted DNA. Mix immediately by pipetting orvortexing.4. Incubate 15-20 minutes at room temperature.5. Add 100µL of the transfection reagent/DNA mixturedrop-wise to each well. Do not remove the growthmedium from the cells before adding thetransfection reagent/DNA mixture.6. Gently rock the plate to achieve even distribution ofthe complexes immediately after adding thetransfection reagent.7. Incubate at 37°C in a CO2 incubator.8. Analyze transgene expression after 24-48 hours.For stable transfection, grow cells in selectivemedium for 10-15 days.C. Protocol for Reverse Transfection of Adherentand Suspension Cells in a 24-well PlateNote: The protocol is optimized for transfection in a24-well plate format. Scale-up quantities and volumesaccording to the number of cells/wells to be transfected(See Table 1). For best results, start with 1µg of DNAand 2µL of transfection reagent per well in a 24-wellplate. Subsequent optimization can further increasetransfection efficiency, depending on the cell line andtransgene used.Note: Prepare transfection reagent immediately beforetransfection.1. Dilute 1µg of DNA in 100µL of serum-free DMEMor other serum-free growth medium.2. Briefly vortex the transfection reagent and add 2µLto the diluted DNA. Mix immediately by pipetting orvortexing.3. Incubate 15-20 minutes at room temperature.4. Evenly distribute 100µL of the transfection reagent/DNA mixture at the bottom of the well of a 24-wellplate.5. Gently layer 1mL of ~105 adherent cells or ~106suspension cells per well on top of the transfectionreagent/DNA mixture.6. Incubate at 37°C in a CO2 incubator.7. Analyze transgene expression after 24-48 hours.Note: Plates can be centrifuged for 2-5 minutesat 200 × g to collect cells at the bottom of the plate.Table 1. Scale-up ratios for transfection of adherent and suspension cells using TurboFect Transfection Reagent.Tissue cultureplate Growth area(cm2/well)Volume ofmedium (mL)No. of adherent(suspension) cells toseed the day beforetransfection*Amount of DNAVolume of TurboFectTransfection Reagent (µL)(µg) (µL)** Recommended Range96-well plate 0.3 0.2 0.5-1.2 × 104(2.0 × 104)0.2 20 0.4 0.3-0.648-well plate 0.7 0.5 1.0-3.0 × 104(5.0 × 104)0.5 50 1.0 0.5-1.424-well plate 2.0 1.0 2.0-6.0 × 104(1.0 × 105)1.0 1002.0 1.0-2.812-well plate 4.0 2.0 0.4-1.2 × 105(2.0 × 105)2.0 200 4.0 2.6-6.06-well plate 9.5 4.0 0.8-2.4 × 105(4.0 × 105)4.0 400 6.0 4.0-8.060mm plate20 6.0 2.0-6.3 × 105(1.0 × 106)6.0 600 12.0 8.0-16.0*Values for suspension cells are in parentheses.**The volume of DNA should be 1/10 the volume of the culture medium used for dilution of the DNA.Note: These numbers were determined using HeLa and Jurkat cells. Actual values depend on the cell type. The amount of DNA and TurboFect Transfection Reagent used may require optimization.Additional InformationA. Cells successfully transfected with TurboFect Transfection Reagent.Permanently growing cell lines Primary cell culturesCos-7 African green monkey kidney cellsHeLa Human cervix adenocarcinoma cellsCHO Chinese hamster ovary cellsHEK293 Human embryonic kidney cellsB50 Rat nervous tissue neuronal cellsCalu1 Human lung epidermoid carcinoma cells RAW264 Mouse leukaemic monocyte-macrophage cells WEHI Mouse B cell lymphoma cellsMDCK Madin Darby Canine Kidney cellsRaji Human Burkitt’s lymphoma cellsCOLO Human colon adenocarcinoma cellsJurkat Human leukaemic T cellsSp2/Ag14 Mouse myeloma cellsHeLa S3 Human cervix carcinoma cellsHep2C Human larynx carcinoma cellsL929 Mouse connective tissue fibroblastsNIH3T3 Mouse embryo fibroblasts Rat fibroblastsMouse bone marrow-deriveddendritic cellsMouse bone marrow-derivedmacrophagesHuman lung fibroblasts (HLF)TroubleshootingProblem Possible Cause SolutionLow transfectionefficiencySuboptimal transfection reagent/DNAratioOptimize the amount of transfection reagent added tothe fixed amount of DNASuboptimal quantity of DNAOptimize the amount of DNA used for transfectionKeep the amount of transfection reagent constantPoor DNA qualityUse high-quality DNA with an absorbance ratio greaterthan 1.8 at 260/280nmSuboptimal cell confluencyOptimize cell plating conditionsEnsure adhered cells are 70-90% confluent at the timeof transfectionEnsure that suspension cells are in logarithmic growthphase at the time of transfectionMycoplasma contamination Regularly check cells for mycoplasma infectionHigh cellulartoxicityToxic transgene Verify if the expressed transgene is toxicSuboptimal incubation conditionsReduce incubation time of the polyplexes with the cellsReplace the transfection mixture 3-4 hours later withnew growth mediumExcess amount of DNA Reduce the quantity of DNA used for transfectionCell density was too lowIncrease the plating density of cells used fortransfectionEndotoxin or other toxic materials werepresent with transgeneEnsure transgene is free of toxic substancesRepeat insertion of gene into new toxin-free plasmidpreparationRelated Thermo Scientific Products16146-89Pierce® Luciferase Assay Kits and Reagents88273 High Capacity Endotoxin Removal Spin Columns, 0.1mL, 5/pkgNote:This product or the use of this product is covered by US patent application US20100041739A1 and corresponding counterparts. The purchase of thisproduct includes a non-transferable license to use this product for the purchaser's internal research. All other commercial uses of this product, includingwithout limitation product use for diagnostic purposes, resale of product in the original or any modified form or product use in providing commercial servicesrequire a separate license. For further information on obtaining licenses please contact @PRODUCT USE LIMITATIONThis product is developed, designed and sold exclusively for research purposes and in vitro use only. The product was not tested for use in diagnostics orfor drug development, nor is it suitable for administration to humans. Please refer to /onebio for Material Safety Data Sheet of theproduct.Current product instructions are available at /onebio.© 2012 Thermo Fisher Scientific, Inc. All rights reserved. Unless otherwise indicated, all trademarks are property of Thermo Fisher Scientific Inc. and itssubsidiaries. Printed in the Lithuania.。
Hela细胞DNA转染试剂使用说明书
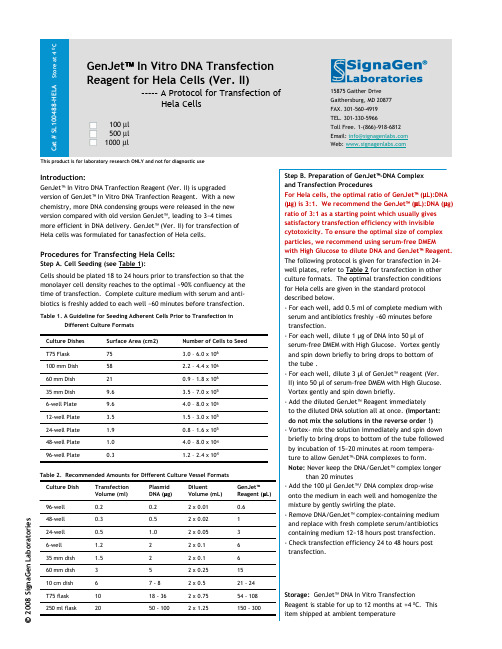
100488 4 0GenJet In Vitro DNA Transfection Reagent for Hela Cells (Ver. II)-----A Protocol for Transfection ofHela Cells100 µl 500 µl 1000 µl15875 Gaither Drive Gaithersburg, MD 20877FAX. 301-560-4919TEL. 301-330-5966Toll Free. 1-(866)-918-6812Email: *********************Web: Introduction:GenJet™In Vitro DNA Tranfection Reagent (Ver. II) is upgraded version of GenJet™In Vitro DNA Tranfection Reagent. With a new chemistry, more DNA condensing groups were released in the new version compared with old version GenJet™, leading to 3~4 times more efficient in DNA delivery. GenJet™(Ver. II) for transfection of Hela cells was formulated for tanasfection of Hela cells.Procedures for Transfecting Hela Cells:Step A. Cell Seeding (see Table 1):Cells should be plated 18 to 24 hours prior to transfection so that the monolayer cell density reaches to the optimal ~90% confluency at the time of transfection. Complete culture medium with serum and anti-biotics is freshly added to each well ~60 minutes before transfection.Table 1. A Guideline for Seeding Adherent Cells Prior to Transfection inDifferent Culture Formats This product is for laboratory research ONLY and not for diagnostic use2008 S i g n a G e n L a b o r a t o r i e sStep B. Preparation of GenJet™-DNA Complex and Transfection ProceduresFor Hela cells, the optimal ratio of GenJet™(µL):DNA (µg) is 3:1. We recommend the GenJet™(µL):DNA (µg) ratio of 3:1 as a starting point which usually gives satisfactory transfection efficiency with invisible cytotoxicity. To ensure the optimal size of complexparticles, we recommend using serum-free DMEMwith High Glucose to dilute DNA and GenJet™Reagent.The following protocol is given for transfectionin 24-well plates, refer to Table 2for transfection in other culture formats. The optimal transfection conditions for Hela cells are given in the standard protocol described below.-For each well, add 0.5 ml of complete medium with serum and antibiotics freshly ~60 minutes before transfection.-For each well, dilute 1 µg of DNA into 50 µl ofserum-free DMEM with High Glucose. Vortex gently and spin down briefly to bring drops to bottom of the tube .-For each well, dilute 3 µl of GenJet™reagent (Ver. II) into 50 µl of serum-free DMEM with High Glucose.Vortex gently and spin down briefly.-Add the diluted GenJet™Reagent immediatelyto the diluted DNA solution all at once. (Important: do not mix the solutions in the reverse order !)-Vortex-mix the solution immediately and spin down briefly to bring drops to bottom of the tube followed by incubation of 15~20 minutes at room tempera-ture to allow GenJet™-DNA complexes to form.Note:Never keep the DNA/GenJet™complex longerthan 20 minutes-Add the 100 µl GenJet™/ DNA complex drop-wise onto the medium in each well and homogenize the mixture by gently swirling the plate.-Remove DNA/GenJet™complex-containing medium and replace with fresh complete serum/antibiotics containing medium 12~18 hours post transfection. -Check transfection efficiency 24 to 48 hours post transfection.Storage:GenJet™DNA In Vitro TransfectionReagent is stable for up to 12 months at +4 0C. This item shipped at ambient temperature1.2 –2.4 x 1040.396-well Plate4.0 –8.0 x 1041.048-well Plate 0.8 –1.6 x 1051.924-well Plate 1.5 –3.0 x 1053.512-well Plate 4.0 –8.0 x 1059.66-well Plate 3.5 –7.0 x 1059.635 mm Dish 0.9 –1.8 x 1062160 mm Dish 2.2 –4.4 x 10658100 mm Dish 3.0 –6.0 x 10675T75 Flask Number of Cells to Seed Surface Area (cm2)Culture Dishes 0.62 x 0.010.20.296-well32 x 0.051.00.524-well 12 x 0.020.50.348-well 150 -3002 x 1.2550 -10020250 ml flask54 -1082 x 0.7518 -3610T75 flask 21 -242 x 0.57 -8610 cm dish 152 x 0.255360 mm dish 62 x 0.121.535 mm dish 62 x 0.121.26-well GenJet™Reagent (µL)DiluentVolume (mL)Plasmid DNA (µg)Transfection Volume (ml)Culture Dish Table 2. Recommended Amounts for Different Culture Vessel Formats。
X-tremeGENE HP DNA Transfection Reagent中文说明书

高实验重复性
取用 X-tremeGENE HP DNA Transfection Reagent 前,将试剂 瓶放置于+15 至+25℃平衡,漩涡混匀 1s 混合均匀。
请勿分装 X-tremeGENE HP DNA Transfection Reagent,将试剂 保存于原始玻璃瓶中。
尽量避免将未稀释的 X-tremeGENE HP DNA Transfection Reagent 与塑料表面接触。
每次转染实验中制备的 X-tremeGENE HP DNA Transfection Reagent:DNA 复合物的最小体积是 100μl。减少复合物制备体 系的体积将显著降低转染效率。
注意,此产品运输温度与保存温度不同。不同的运输温度不会 影响产品性能或产品稳定性。
操作注意事项
取出所需用量后,立即重新盖紧试剂瓶。
标准实验室设备 - 标准细胞培养设备(例如:生物安全柜、培养箱) - 标准移液器与微量移液器 - 涡旋混匀器 质粒制备 - 纯化质粒储液(0.1-2.0μg /μl),溶于无菌 TE(10mM Tris,
仅供生命科学研究使用。 不可用于诊断程序。
X-tremeGENE HP DNA Transfection Reagent
用于真核细胞的瞬时及稳定转染
货号:06 366 244 001 货号:06 366 236 001 货号:06 366 546 001
0.4ml 1ml 5x1ml
各种转染试剂中文说明

FuGENE6(Roche)转染步骤:转染前一天将细胞分至培养板,转染当天细胞应50-80%融合。
将细胞以1-3×105/2ml接种于6孔板后孵育过夜将达到如此密度。
将FuGENE6 Reagent在室温孵育10-15分钟。
使用之前将FuGENE6颠倒混匀一下。
1.在PCR管中加入不含血清和双抗的营养液以稀释FuGENE6,直至总体积到100ul。
2.将3-6ul FuGENE6 Reagent直接加入营养液,轻弹管壁混合。
3.加入1-2ug的DNA溶液(0.02-2.0ug/ul),轻弹管壁混合。
4.室温孵育20分钟。
5.将6孔板中的旧营养液吸出,加入约1ml不含血清和双抗的营养液洗涤一次,再加入2ml不含血清和双抗的营养液。
6.将转染复合物加入细胞,混匀使之均匀分布。
7.3-8小时后,加入血清或换成含血清的营养液。
Lipofectamine 2000(Invitrogen)转染试剂转染步骤(6孔板):1.转染前一天,胰酶消化细胞并计数,细胞铺板,使其在转染日密度为90-95%。
细胞铺板在2ml含血清,不含抗生素的正常生长的培养基中。
2.对于每孔细胞,使用250ul无血清培养基(如OPTI-MEM I培养基)稀释4.0ugDNA,轻轻混匀。
3.使用前将Lipofectamine 2000转染试剂轻轻混匀,用250ul无血清培养基(如OPTI-MEM I培养基)稀释10ul Lipofectamine 2000转染试剂,轻轻混匀。
Lipofectamine 2000稀释后,在5分钟内同稀释的DNA混合(<30分钟)。
NOTE:若使用DMEM培养基,则需在5分钟内同稀释的DNA混合。
4.混合稀释的DNA(第二步)和稀释的Lipofectamine 2000(第三步)。
室温放置20分钟。
5.(optional)将6孔板中的旧营养液吸出,用无血清培养基清洗两次。
加入2ml无血清配养基。
sirna转染试剂说明书

3
1
24-well
2
500
2 x 50
6
2
12-well
4
1000
2 x 100
12
4
6-well
10
2500
2 x 250
30
10
转染实验优化: 为了提高转染效率,最好对转染条件进行优化,特别是首次使用。例如:24 孔培养板,调整 siRNA 与 RFect 试剂的用量。
siRNA 用量在 0.6-30 pmol(final concentration 1- 50 nM)之间调整,RFect 试剂用量在 1.0 - 3.0μl 之间调整。客户可按照习惯用量进行预实验,
所干扰基因本身及分析方法。可设置不同的孵育时间进行实验以确定最佳孵育时间。
转染实验要点: 转染过程不可添加抗生素,否则会导致细胞死亡; 首次实验 siRNA 的用量可稍大(一般 10 nM) ,后续实验根据实验结果修改。
RFect Transfection Reagent Formats for Various Cell Culture Vessels
传真: 0519-83382790
◎转染细胞范围广,绝大多数贴壁细胞株都能获得比较理想的转染结果
储存条件:-20℃
应用 ◎siRNA 转染 ◎antisense RNA 转染 ◎200bp 内的小分子 DNA 转染
产品介绍
RFect 小核酸转染试剂是国际知名科学家崔坤元博士领导我公司研发团队在美国西雅图实验室研发成功的一种新型的小核酸转染试剂。RFect 可用来转染 siRNA、antisense RNA、microRNA 等 200bp 以内的小分子 RNA 和 DNA,转染细胞包括绝大多数贴壁生长的细胞,如一般细胞 株、肿瘤细胞株等。目前,无论国外还是国内,转染试剂的主要成分均为脂质体或聚乙烯亚胺(Polyethylenimine, PEI),这两种成分都具有很 大的细胞毒性,并且转染效果不好。RFect 采用新型的动物源性的纳米材料,毒性很低并且拥有非常卓越的转染性能。与其它品牌的小核酸 转染试剂相比,RFect 的细胞转染阳性率一般在 90%以上,Lamin A/C 基因抑制效率在 95%以上,而其它品牌转染试剂的细胞转染阳性率一 般不超过 70%,Lamin A/C 基因抑制效率不超过 75%。RFect 细胞毒性很低,转染细胞死亡率不到 10%,而其它品牌试剂的转染细胞死亡率 一般在 30%以上。血清对转染效果没有影响,不必刻意添加或更换培养液。有关 RFect 小核酸转染试剂的材料合成和试剂配制我们已申请了 国际专利,并通过 PCT 覆盖国际上多个国家和地区。
细胞转染(Entranster)注意事项

细胞转染注意事项
1.如为贴壁生长细胞,一般要求在转染前一日,必须应用胰酶处理成单细胞悬液,重新接种于培养皿或瓶,转染当日的细胞密度以70-90%(贴壁细胞)或
2×106-4×106细胞/ml(悬浮细胞)为宜,最好在转染前4h换一次新鲜培养液。
2.用于转染的质粒DNA必须无蛋白质,无RNA和其他化学物质的污染,OD260/280比值应在1.8以上。
3.培养基中的血清
在开始准备DNA和阳离子脂质体试剂稀释液时要使用无血清的培养基,因为血清会影响复合物的形成。
其实,只要在DNA-阳离子脂质体复合物形成时不含血清,在转染过程中是可以使用血清的。
4.培养基中的抗生素
抗生素是影响转染的培养基添加物。
这些抗生素一般对于真核细胞无毒,但阳离子脂质体试剂增加了细胞的通透性,使抗生素可以进入细胞。
这降低了细胞的活性,导致转染效率降低。
这时候可以选择英格恩生物的Entranster转染试剂,非脂质体试剂,培养基可加抗生素,避免了细胞染菌。
5.设置阳性对照和阴性对照。
6.一般在转染24-48h,靶基因即在细胞内表达。
根据不同的实验目的,24-48h 后即可进行靶基因表达的检测实验。
7.如若建立稳定的细胞系,则可对靶细胞进行筛选,根据不同基因载体中所含有的抗性标志选用相应的药物,常用的真核表达基因载体的标志物有潮霉素和新霉素等。
