有机化学双语综合测试题
大学有机化学英文版答案
6-methyl-1,4-heptadiyne
4.26 Draw structural corresponding to the following IUPAC names (a) 3-ethyl-1-heptyne (b) 3,5-dimethyl-4-hexen-1-yne 4.32 Predict the products of the following reactions. Indicate regioselectivity where relevant. (The aromatic ring is inert to all the indicated reagents)
2.43 Draw structural formulas for each of the following substances a) 2-methylheptane b) 4-ethyl-2-methylhexane c) 4-ethyl-3,4-dimethyloctane d) 2,4,4-trimethylheptane
(d) 2-butanone(CH3CH2COCH3)
2 2 4 4
5.26 Give IUPAC names for the following compounds:
CH3
COOH (b ) Br
(a )
C H 2C H 2C H 2C H C H 3
4-methyl-1-phenylpentane
(b )
HOC C Cl C
H
Z
H
Z
OCH3
3.47 Name the following cycloalkenes : Cl (c) C l cis-3,5-dichlorocyclopentene
化学英语考试题及答案

化学英语考试题及答案一、选择题(每题2分,共20分)1. What is the chemical symbol for oxygen?A. OB. OHC. H2OD. O22. Which of the following is not a basic unit of a chemical element?A. AtomB. MoleculeC. ProtonD. Electron3. What is the law of conservation of mass?A. Mass is neither created nor destroyed in a chemical reaction.B. Mass is always created in a chemical reaction.C. Mass is always destroyed in a chemical reaction.D. None of the above.4. What is the correct chemical formula for water?A. H2OB. H2O2C. O2HD. OH-5. Which of the following is a noble gas?A. Oxygen (O)B. Helium (He)C. Carbon (C)D. Nitrogen (N)6. What is the valency of hydrogen in the compound HCl?A. +1B. -1C. +2D. -27. Which of the following is a compound?A. Oxygen gas (O2)B. Carbon dioxide (CO2)C. Hydrogen gas (H2)D. Nitrogen gas (N2)8. What is the common name for the chemical NaCl?A. SodiumB. ChlorineC. Table saltD. Baking soda9. What is the atomic number of carbon?A. 6B. 16C. 12D. 810. What is the process of a substance changing from a solidto a liquid state called?A. SublimationB. VaporizationC. MeltingD. Condensation二、填空题(每空1分,共10分)11. The chemical equation for the combustion of methane is__________.Answer: CH4 + 2O2 → CO2 + 2H2O12. The symbol for the element with atomic number 17 is__________.Answer: Cl13. The process of a substance changing from a liquid to agas is known as __________.Answer: Vaporization14. The chemical formula for calcium carbonate is __________. Answer: CaCO315. The valency of chlorine in the compound KCl is __________. Answer: -116. The noble gas that is used in balloons is __________.Answer: Helium (He)17. The atomic number of oxygen is __________.Answer: 818. The process of a substance changing from a gas to aliquid is known as __________.Answer: Condensation19. The chemical symbol for the element with the highest atomic mass is __________.Answer: Og (Oganesson)20. The law that states the total mass of the reactants equals the total mass of the products in a chemical reaction is known as the __________.Answer: Law of Conservation of Mass三、简答题(每题5分,共20分)21. Explain the difference between a pure substance and a mixture.Answer: A pure substance consists of a single type of particle, while a mixture is composed of two or moredifferent substances that are not chemically combined.22. What is an acid and what are its properties?Answer: An acid is a substance that donates hydrogen ions (H+) when dissolved in water. Properties of acids include a sour taste, the ability to change the color of certain indicators, and the ability to react with bases to form salts and water.23. Describe the process of photosynthesis.Answer: Photosynthesis is the process by which green plants and some other organisms use sunlight to synthesize foods with the help of chlorophyll pigments. It involves theconversion of carbon dioxide and water into glucose and oxygen in the presence of light energy.24. What is a chemical equilibrium and how is it represented? Answer: Chemical equilibrium is the state in a reversible reaction where the rate of the forward reaction equals the rate of the reverse reaction, resulting in no net change in the concentrations of reactants and products. It is represented by the equilibrium constant expression, Kc, which is the ratio of the concentrations of products to reactants raised to their respective stoichiometric coefficients.四、计算题(每题5分,共10分)25. If 10 grams of sodium bicarbonate (NaHCO3) are dissolved in water and reacted with an excess of hydrochloric acid (HCl), calculate the volume。
化学专业外语练习题

化学专业外语练习题1、c Which of the following belongs to phosphate?( sodium phosphite)(a) PH3 (b) Na3PO3(c) Na3PO4 (d) P2、b 1H and 2H are ____ and occupy the same position in periodic Table.(a) isomers (b) isotopes (c) redox bodies (d) amphoteric compounds3、d The ____ of a carbon is 12(a) the weight of molecule (b) molecular number(c) the weight of atom (d) atomic weight4、Which of the following belong to nitrate? C(a) NH3 (b) KNO3 (c) KNO2 (d) NO5、Which of the following belongs to secondary amine? B4、The number of outmost electron in carbon atom is _C_.(a) 1 (b) 2 (c) 4 (d) 65、In reaction H2 + CuO — H2O + Cu, __B__ is reduced.(a) H2 (b) CuO (c) Cu (d) H2O6、Which of the following is charged? _C_(a) atom (b) molecule (c) proton (d) neutron7、Who first presented Periodic Table of elements? C(a) Democritus (b) Boyle (c) Mendeleev (d) Dalton8、Which of the following is classified into amine? B(a) NH3 (b) CH3NH2 (c) NaNH2 (d) N29、The substances on the left side of the chemical equation are known as __d_ .(a) reactant (b) reactor (c) reductant (d) reaction10、Who first present the model of an atom in 1900s? b(a) Plato (b) Dalton (c) Mendeleev (d) Boyle11、Which of the following belongs to nitrite? b(a) HNO2 (b) KNO2 (c) Mg3N2 (d) NH312、Which of the following belongs to metal? b(a) selenium (b) sodium (c) Tellurium (d) Helium13、d The elementary particle of ____ is uncharged. d(a) proton (b) electron (c) ion (d) neutron14、d Bromine has ____ electrons in its outermost energy level. d(a) 2 (b) 3 (c) 8 (d) 715、a Which of the following is non-metal?(a) chlorine (b) lead (c) copper (d) mercury16、a The atomic number of helium is ____.(a) 2 (b) 4 (c) 3 (d) 017、d The horizontal rows of the periodic table are called ____.(a) energy level (b) groups (c) electron configuration (d) periods18、a The symbol for element silver is ____.(a) Ag (b) Sn (c) Hg (d) Au19、c The atomic number of carbon is ____.(a) 13 (b) 6 (c) 12 (d) 120、b Which of the following is inert element?(a) hydrogen (b) helium (c) tellurium (d) potassium21、c Which of the following belongs to halogen?(a) carbon (b) lithium (c) fluorine (d) neon22、a Which of the following belongs to noble gas?(a) neon (b) potassium (c) iodine (d) lead23、a Which of the following belongs to alkali metal?(a) silver (b) sodium (c) tellurium (d) bromine24、b Which of the following belongs to metal halide?(a) KClO3 (b) KCl (c) HCl (d) Cl225、a The ____ of carbon is is 1s22s22p2(a) electron configuration (b) periodicity (c) general property (d) inertness26、b The general property of metal is ____.(a) soft (b) lustrous (c) flammable (d) toxic27、c Fluorine, chlorine, bromine and iodine belong to ____ group.(a) alkaline metal (b) transition metal (c) halogen (d) alkaline earth metal28、b Few reactions occur on helium and argon, so the two elements are chemically ____.(a) reactive (b) inert (c) mild (d) strong29、a The standard enthalpy for normal oxygen is ____.(a) 0 (b) >0 (c) <0 (d) ≠030、c The maximum electronegative element in Periodic Table is ____.(a) H (b) Na (c) F (d) He31、b Which of the following belongs to the complex?(a) FeF3 (b) Na3[FeF6] (c) Fe3O4 (d) Fe32、d When solid NaCl is put into water, the solid ―disappears‖. The whole system is called ____.(a) soluble (b) solvent (c) solubility (d) solution33、d Mn2+ can be ____ to MNO4- by (NH4)2S2O8 in acidic solution.(a) reduced (b) exchanged (c) substituted (d) oxidized34、a Charged atoms are called ____.(a) ions (b) protons (c) electrons (d) molecules35、b Which of the following is classified into oxo-anion ?(a) O2- (b) SO42- (c) S2- (d) S2-36、a Reaction CH4 +Cl2→ CH3Cl + HCl is called ____ reaction.(a) substitution (b) oxidation (c) reduction (d) exchange37、c Benzene is often used as ____ both in laboratory and in chemical industry.(a) solution (b) solvation (c) solvent (d) solute38、c Standard entropy of any species is ____.(a) 0 (b) ≤ 0 (c) > 0 (d) both (b) and (c)39、b If aqueous NaCl is wanted to separate, ____ is good choice.(a) substitution (b) distillation (c) coordination (d) bond40、b Alcohol is readily ____ and we smell it everywhere in air.(a) soluble (b) volatile (c) reactive (d) precipitated41、b As a general rule, the coordination compounds(the complex) with coordination number of six are observed _____ structure(a) tetrahedral (b) octahedral (c) octagonal (d) bipyridyl42、d The demanded energy that a gaseous neutral atom loses one electron at thermodynamic standard condition is called the first ____.(a) energy level (b) electron configuration (c) activation energy (d) ionization energy43、c NaCl is ____ in water.(a) solution (b) solvent (c) soluble (d) precipitated44、d When AgNO3 is mixed with NaCl, AgCl ____ is developed.(a) solution (b) solvent (c) soluble (d) precipitated45、b Which of the following belongs to hydrocarbon?(a) C11H22O11 (b) C8H18 (c) CH3COOH (d) H2SO446、a In K4[FeF6], the charge of the complex part is ____.(a) -4 (b) +2 (c) +1 (d) –147、b Which of the following belongs to substitution reaction?(a) H2 + Cl2— HCl (b) C6H6 + Cl2— C6H5Cl + HCl (c) H2O — H2 + O2 (d) S+ O2— SO248、a In K4[FeF6], the oxidation number(or state) of Fe is ____.(a) 2 (b) 0 (c) 6 (d) 449、__c__ is defined as required energy to break chemical bond into neutral species at thermodynamic standard condition. For example breaking H-H bond into two H· atom requires 104kJ/mol.(a) Lattice energy (b) Hydration energy (c) Bond energy (d) Activation energy50、a When gaseous HCl dissolves into water, ____ solution forms.(a) acidic (b) basic (c) salty (d) no51、b When ionic compounds dissolve in water, the ions interact with water molecules. Such process is called ____.(a) coprecipitation (b) ionization (c) hydration (d) coordination52、d When ions dissolve in water, they are surrounded by water molecules. Such process is called ____.(a) sublimation (b) substitution (c) ionization (d) solvation53、c When benzene is added in aqueous solution containing iodine, most of iodine then transfers into benzene because of the more solubility of iodine in benzene. Such process is called ___.(a) refinement (b) titration (c) extraction (d) diffraction54、d Which of the following is classified into transition element?(a) silicon (b) tin (c) helium (d) copper55、__b__ H2SO4 is often used in chemical experiment.(a) Volatile (b) Concentrated (c) Flammable (d) Inert56、a Electrolysis of aqueous solution will produce____.(a) H2 (b) H (c) water (d) OH-57、d Zinc reacts with ____ H2SO4 to generate hydrogen gas at room temperature.(a) concentrated (b) cold (c) hot (d) dilute58、a Which of the following belongs to oxoacid?(a) H2SO4 (b) H2S (c) H2O (d) HS-59、c Which of the following belongs to diatomic molecule?(a) HCN (b) KClO3 (c) HCl (d) C8H1060、c If solid NaCl is added into its saturation solution , ____ is developed.(a) transparent solution (b) colloidal (c) precipitate (d) dissolution61、c Which of the following belongs to alkali earth metal?(a) potassium (b) lead (c) calcium (d) boron62、b Which of the following belongs to hydride?(a) CuSO4·5H2O (b) H2S (c) C6H6 (d) H2SO463、a Which of the following belongs to carbohydrate?(a) C11H22O11 (b) C10H8 (c) HCHO (d) CO264、a Which of the following belongs to representative element?(a) carbon (b) iron (c) copper (d) zinc65、d Which of the following belongs to alkaline earth element?(a) potassium (b) mercury (c) boron (d) magnesium66、a Benzene usually carries out ____ reaction, as described belowC6H6 +Cl2---------C6H5Cl + HCl(a) substituent (b) substitution (c) displacement (d) alternative67、a The general formula of saturation alkane is ____(a) C n H2n+2 (b) C n H2n (c) C n H2n-2 (d) C n H2n-668、c There are ____ carbon atoms in parent chain of 2,3-dimethylbutane.(a) 2 (b) 5 (c) 4 (d) 669、c Which of the following is trans-2-butene?70、d The vinyl group has the structure of ____.(a) CH3CH2— (b) C6H5— (c) CH≡C— (d) CH2=CH—71、b Compounds that containing both hydroxyls and carbon-carbon double bonds are called ____(a) polyols (b) unsaturated alcohols (c) cyclols (d) thiol72、c Dienes contain ____ carbon-carbon double bonds.(a) di- (b) eth- (c) two (d) secondary73、a Which of the following is classified into alkenol?(a) CH2=CHCH2OH (b) CH3CH2CH2OH (c) CH≡CCH2OH (d) HOCH2CH2CH2OH74、b Vinylcyclopropane is classified into ____(a) alkane (b) alkene (c) alkyne (d) alkenyne75、c The functional group of alcohol is ____.(a) hydrate (b) hydroxide (c) hydroxyl (d) hydride76、a Alkynes contain carbon-carbon ____ bond(a) triple (b) three (c) tertiary (d) prop77、b Methylenecyclopentane is classified into ____(a) alkane (b) alkene (c) alkyne (d) alkenyne78、c Which of the following is the formula or general formula of ammonia?(a) C6H5OH (b) C6H5NH2 (c) NH3 (d) RNH279、a A primary amine contains ____ hydrogens attached to its nitrogen atom.(a) 2 (b) 3 (c) 4 (d) no80、d Reaction between methanamine and hydrochloric acid produces ___ salt.(a) imide (b) imine (c) amide (d) ammonium81、b The functional group of amine is ____.(a) ammonia (b) amino (c) ammonium (d) ammine82、a Alkanes contain carbon-carbon ____ bonds.(a) single (b) double (c) triple (d) both (b) and (c)83、d (+)-Glucose (C6H12O6) is ___ aldehyde, which contains hydroxyls and distinguishesfrom common aldehydes or alcohols in properties.(a) polyhydrate (b) many-hydrooxy (c) multihydroxyl (d) polyhydroxy84、b Compounds containing —OH and C=C groups are called ____ alcohols, because of the presence of carbon-carbon double bonds.(a) primary (b) unsaturated (c) monohydroxyl (d) polyolic85、b Oxacyclopropane contains ____ atom(a) nitrogen (b) oxygen (c) sulfur (d) phosphorus86、a azacyclopropane contains ____ atom(a) nitrogen (b) oxygen (c) sulfur (d) phosphorus87、 azacyclopropane is classified into ___?_(a) amine (b) ether (c) alkane (d) alkene88、a Oxacyclopropane contains ____ carbons(a) 2 (b) 3 (c) 4 (d) 589、d Which of the following belongs to tertiary amine?(a) CH3NH2 (b) (CH3)2NH (c) (NH3)3N (d) N(CH3)4+90、a Which of the following contains carbonyl group?(a) CH3COOH (b) CH3OCH3 (c) CH3CH2OH (d) CH3CH2Cl91/c Which of the following contains hydroxyl group?(a) CH3CHO (b) CH3OCH3 (c) CH3CH2OH (d) CH3CH2Cl92、c Which of the following belongs to saturated ketone?(a) CH3CHO (b) CH2=CHCOCH3 (c) CH3CH2COCH3 (d) CH3COOH93、c Carboxylic acids react with alcohols to form ____.(a) acid halides (b) acid anhydrides (c) esters (d) amides94、c The general formula of organic amides is ____.(a) RNH2 (b) RNH3+X- (c) RCONH2 (d) NaNH295、b Decarboxylation, elimination of the ___ as CO2is of importance for β-keto acid.(a) —OR (b) —COOH (c) —CHO (d) —CONH296、a Reaction of acid chloride with water forms ____.(a) carboxylic acid (b) chlorocarboxylic acid (c) ester (d) phenol97、b We can deduce that thiophene contains ___ element though its detailed structure is unknown.(a) O (b) S (c) N (d) C98、a Which of the following is neutralization reaction?(a) acid and base (b) salt and salt (c) salt and base (d) acid salt99、b Reaction of carboxylic acid with alcohol is defined as ____.(a) etherification (b) esterification (c) epoxide (d) elimination100、b Reaction of acid anhydrides with ammonia produce____(a) ammonium (b) amide (c) amine (d) amino101、d In K4[FeF6], the Fe is called ____.(a) dipole moment (b) coordination number (c) ligand (d) centre ion or atom102、a Concentration of concentrated sulfuric acid is ______(a) 18M (b) 12M (c) 6M (d) 2M103、a The detailed, step-by-step description of a chemical reaction is called ____, e.g., hydrogen reacts with oxygen to generate water. Such reaction involves the following step:H2→ H·H· +O2→ HO· + O·H· + HO·→ H2O(a) mechanism (b) dynamic equation (c) activation energy (d) process104、a Hydrolysis of FeCl3 shows ____(a) acidic (b) neutral (c) alkaline (d) amphoteric105、c Carbocation carries ____ charge.(a) no (b) neutral (c) positive (d) varying106、a A large excess of alcohol is added in the following reaction, CH3COOH +CH3CH2CH2OH------CH3COOC3H7 + H2O, the equilibrium shifts ____.(a) forwards (b) backwards (c) nothing (d) uncertainy107、c The reaction CH3COOH+ C2H5OH ----- CH3COOC2H5 is a ____ reaction because it proceeds in both direction.(a) forwards (b) backwards c) reversible d) reverse108、a If an entity combines with a proton, it is called ____.(a) protonation (b) protolysis (c) protophile (d) non-protic109、c The key raw material for chlor-alkali is ____.(a) lime (b) brine (c) soda ash (d) wood pulp110、a Soda ash is composed of ____.(a) sodium carbonate (b) sodium bicarbonate (c) ammonium bicarbonate (d) mixture between and sodium carbonate and sodium bicarbonate111、c According to the reaction 2NaCl +2 H2O ---------2NaOH + H2 +Cl2, the ration of caustic soda to chlorine is ____ (a) 1:2 (b )1:1 (c)2:1 (d)1:0112、c Sodium hydroxide, called ____ because of its corrosion, will burn or, even corrode our tissue and skin if it spots on them.(a) soda ash (b) caustic sodium (c) caustic soda (d) lime113、d Quicklime is manufactured by the thermal _______ (1200℃~1500℃) of limestone according to the equation: CaCO3 ------- CaO + CO2(a) combination (b) transfer (c) hydrolysis (d) decomposition114、b Which of the product derived from chlor-alkali industry?(a) electricity (b) chlorine (c) limestone (d) refractory115、c When an ammoniated solution of salt is bubbled by carbon dioxide, the process is called ____.(a) decomposition (b) calcine (c) carbonation (d) oxidation116、c In Solvay Process, separation between NaHCO3 precipitates and liquor should be carried out through ____. (a) drying (b) distillation (c) filtering (d) concentration117、c To remove some water from wet CaCO3, ____ is available.(a) electrolysis (b) metathesis (c) drying (d) solubility product118、c If you want to clear water from aqueous NaCl, the reasonable approach is ____.(a) cracking (b) humidification (c) distillation (d) agitation119、c If a solid NaCl is available from its aqueous solution, the reasonable method is ____ (a) lixiviation (b) sedimentation (c) crystallization (d)centrifugation120、d Which of the following is classified into metathesis?(a) H2 + O2— H2O (b) HCl + NaCl — NaCl + H2O(c) Zn + HCl — ZnCl2 + H2 (d) Na2SO4 + BaCl2— BaSO4 + NaCl121、d Silicate-gel is often used as ____ in chemical laboratory.(a) oxidant (b) packing material (c) initiator (d) drying agent122、b If we want to obtain solid NaCl from its aqueous solution, the reasonable operation is ____.(a) pyrolysis (b) crystallization (c) adsorption (d) adsorption123、d Which one of the following belongs to chloride?(a) Cl2 (b) NaClO3 (c) HClO3 (d) NaCl124、b All detergents, such as soap, contain ____.(a) chromatograph (b) surfactants (c) bleaching powder (d) catalysts125、Look at the following two electrode reaction(1) or (2):(1) Cl- -2e → Cl2(2) 2H2O +2 e → H2 +2OH-a Electrode (1) is ____(a) cathode (b) anode (c) negative electrode (d) electron127、c Bleaching powder is often used as ____ in public water supplies.(a) infection (b) alkalisource (c) disinfectant (d) raw material128、a The raw material to produce sulfuric acid is____.(a) sulfide (b) sulfur (c) sulfate (d) sulfonate129、c Electrolysis of aqueous HCl will generate __?__.(a) hydride (b) hydrogen ion (c) hydrogen (d) hydrate130、b Which of the following shows alkaline?(a) chlorine (b) caustic soda (c) sulfuric acid (d) hydrogen131、a The method to produce industrially sulfuric acid is ____.(a) contact process (b) Solovy Process (c) electrolysis (d) lead chamber132、c Which of the following belongs to sulfide?(a) Na2S2O3 (b) Na2SO3 (c) Na2S (d) Na2SO4133、c If excess sulfur trioxide is dissolved into pure sulfuric acid, ____ forms.(a) concentrated sulfuric acid (b) dilute sulfuric acid(c) oleum (d) divanadium pentoxide134、b The method to synthesize ammonia is ____ .(a) Solvay Process (b) Habor Process(c) Catalytic Reforming (d) methanation135、d Prior to reforming reaction, the syngas requires _______ process, in which sulfur-containing compounds must be removed as they poison both the reforming catalysts and Harber catalysts.(a) sulfonation (b) sulfurization (c) sulfates (d) desulfurization136、d If a steam is required to be converted into liquid, _____ is available.(a) absorption tower (b) reformer (c) membrane cell (d) condenser137、c Benzene is ____ with concentrated nitric acid to form nitrobenzene, as described below: C6H6 + HNO3— C6H5NO2 + H2O(a) nitrogenated (b) nitrized (c) nitrated (d) nitricated138、b The following reaction is exothermic:4NH3 + 5O2— 4NO + 6H2O, therefore, low temperature favors the reaction ____.(a) backwards (b) forwards (c) no shift (d) uncertain139、a In the following reaction, Cu + 2H2SO4— CuSO4 + SO2 + 2H2O____ is oxidized.(a) Cu (b) H2SO4 (c) SO2 (d) H2O140、b The raw material to produce industrially nitric acid is ____.(a) nitrogen (b) ammonia (c) amine (d) ammine141、a Dehydration of pure nitric acid will give ____.(a) H2O (b) N2O5 (c) NO2 (d) NO142、c About 65% nitric acid reacts with ammonia to give ____.(a) nitrogen (b) ammonium nitrate (c) nitrogen dioxide (d) dinitrogen pentoxide.143、b Which of the following is an important precursor for petrochemicals?(a) ethane (b) ethylene (c) ethanol (d) ethanoic anhydride144、a Demethylation of methylcyclohexane gives ____.(a) cyclohexane (b) cyclohexyl (c) cyclohexene (d) benzene145、Dehydrogenation of cyclohexane gives __?__.(a) cyclohexene (b) cyclohexanone (c) cyclohexyl (d) cyclohexanecarboxylic acid146、a Precisely, the molecular weight of polymer, often greater than 107, equals to that of____ multiplied by degree of polymerization.(a) monomer (b) mole (c) covalent bond (d) radical147、b Vulcanization of rubber refers to addition of some ____ in the framework of rubber body.(a) vanadium (b) sulfur (c) chlorine (d) nitrogen148、c When the degree of polymerization reaches less than 20, we say the polymer is ____.(a) the intermediate (d) the distillate (c) oligomer (d) monomer149、c At the top of the distillation tower, there is a ____ to cool the vapor into reflux liquid and to recycle into the tower.(a) heat exchanger (b) furnace (c) working tank (c) condenser150、bIn sulfuric acid industry, elemental sulfur is combusted in ____ to general sulfur dioxide.(a) fractionating tower (b) furnace (c) steam stripper (d) working tank151、c At the bottom of the distillation tower, there is a(an) ____ to boil the liquid into vapor and to recycle into the tower.(a) still (b) auxiliary (c) tank (c) boiler152、d If a new bond between polymeric chains is developed, the process is called ____.(a) covalence (b) side-chain (c) fabrication (d) cross-linking153、a Which of following belongs to synthetic polymer?(a) plastic film (b) silk (c) cellulose (d) starch154、a Copolymers are derived from polymerization of ____ monomers, such as C6H5OH and HCHO.(a) different (b) usual (c) the same (d) conventional155、d Any commodity should have a technical ____ if they go into public commerce.(a) specific (b) specify (c) specialty (d) specification156、c The ____ of Zn-Cu alloy(solid solution) is as follow: 30%Zn and 70%Cu.(a) composite (b) component (c) composition (d) complex157、d As a convention, [Cu(NH3)4]SO4 is never considered a salt, but a ____.(a) composite (b) component (c) composition (d) complex158、b After synthesis of a product, the product must be ___ because the mixture contains the desirable products as well as the remaining reactants, catalysts, by-products.(a) pre-treated (b) post-treated (c) blended (d) eliminated159、a When ethanol is dissolved into water, a ____ solution is developed.(a) homogeneous (b) heterogeneous (c) viscose (d) latent160、a The reaction H2 + O2 -----H2O is ____.(a) exothermic (b) endothermic (c) isothermal (d) thermodynamic161、c Which of the follow is used as the semi-conductor material?(a) Fe (b) N2 (c) Si (d) He162、c Homopolymers are derived from polymerization of ____ monomers, such as polyethylene.(a) different (b) usual (c) the same (d) conventional163、a Activate carbon is often used as adsorbent in laboratory due to its large ____ surface.(a) specific (b) specify (c) specialty (d) specification164、b To obtain pure substance from the mixture, ____ is a good choice.(a) catalysis (b) purification (c) reactor design (d) solubility165、d If a reaction is carried out at a very high pressure, _____ is a good choice.(a) furnace (b) fractionating tower (c) heat exchanger (d) autoclave166、d Which of the following is classified into polymer?(a) formaldehyde (b) silica (c) sulfur trioxide (d) epoxy resin 167、a Which of the following is classified into synthetic polymer?(a) resin (b) vinyl chloride (c) epoxide (d) silica。
西北农林科技大学全英有机化学课程试卷2013

西北农林科技大学本科课程考试参考答案2012—2013学年第2学期《有机化学》(双语)课程A卷专业年级:生技基、生工基地等7个班命题教师:王俊儒,陶虎,汤江江考试时间:2013-07- 10 8:30-10:30 a.m.Part I - (3 points each, 24 points in all)Give the correct IUPAC name (BOTH IN CHINESE AND ENGLISH) or molecular structure for each of the compounds BELOW.(Note the configuration if there any).按系统命名法写出下列化合物的英文和中文名称(每小题3分,计24分)1 (3R,4S)-3-氯-4-溴庚烷(3R,4S)-4-bromo-3-chloroheptane2 7,7-二甲基二环[2.2.1]庚烷7,7-dimethylbicyclo[2.2.1]heptane3 (R,Z)-4-甲基-2-己烯(R,Z)-4-methylhex-2-ene4 (2S,3S)-2,3-二羟基丁醛(2S,3S)-2,3-dihydroxybutanal5 N,N-二甲基苯甲酰胺N,N-dimethylbenzamide678Part II -MULTIPLE CHOICE (2 point each, 40 points in all)多选题(每小题2分,计40分)9. D; 10. B; 11. C; 12. D; 13. B;14. D; 15. D; 16. D; 17. BE; 18. C;19. E; 20. B; 21. C; 22. A; 23. D;24. A(印刷上结构末端少了一个甲基(—),应为.建议不管答什么都给分);25. AB; 26. C; 27. A; 28. C;Part III- REACTIONS and SYNTHESIS (2 points for 29-35, 3 pointsfor 36-39, 26 points)反应和合成题(29-35每小题2分,36-39每小题3分,计26分)29 30 3132 33 343536;37;;3839Part IV –MECHANISMS (5 points for each, 10 POINTS)机理题(每小题5分,计10分)40.41.。
华南理工有机化学双语综合测试题(英文版)

2011 年有机化学自测题(英文版-中英对照)Ⅲ. Choose the best answers for each of the following questions. 1. Single choice (only one choice is correct) for 1~70 For CH3CH=C=CH2, point out the hybridization of each carbon(from left to right)? 对于有机物 CH3CH=C=CH2, 请指出每个碳的杂化方式(从左至右). A. sp3 sp2 sp2 sp2 B. sp 3 sp2 sp sp 2 C. sp 3 sp 2 sp sp D. sp3 sp sp sp 2. Which of the following is electrophilic reagent? 属于亲电试剂的是: A. HNO3 B. NaHSO3 C. H2N-NH2 D. HCN 3. Which of the following is nucleophilic reagent? 属于亲核试剂的是: A. Br2 B. NaHSO3 C. H2SO4 D. HCl 4. Which of the following substituents activates an aromatic nucleus? 下列哪个取代基可以活化芳香环? A. —COOH B. —NO2 C. —OCH3 D. —SO3H 5. Which of the following does not give the iodoform test? 不能发生碘仿反应的是:A. C6H5CHO B. CH3 C O CH3 C. CH3CH2OH D. CH3C-C6H5 O6. Which of the following structural formulas has no geometrical isomers? 不存在几何异构(顺反异构)的是:A. CH3CH=CHCH3 B. C6H5CH=CHBr C. Cl Cl Cl D. Cl Cl7. Which of the following is aromatic? 具有芳香性的化合物是:A. O B. C. N H D.8. Which of the following lettered carbon-carbon bonds is the longest? 用字母标记的碳碳键中,键长最长的是:a A. B. CH3 CH b c C. CH3 C CH D. CH3 d CH3CH29. Which of the following carbocations is most stable? 最稳定的碳正离子是:+ A. CH3 C CH3 + B. CH3 CH CH3 + C. CH3 CH2 + D. CH3CH=CH210. Which of the following compounds yields a yellow oil when treated with nitrous acid? 能与亚硝酸作用生成黄色油状物的物质是:A. CH3CH2NH2 B. (CH3CH2)2NH C. (CH3CH2)3N D. NH211. Which of the following is easiest water-soluble? 最易溶于水的是:A. CH2-CH-CH2 OH OH OH B. OH C. E. CH3CHCH2CH2OH CH312. Which of the following cannot react with silver nitrate to produce silver chloride at ordinary conditions? 1通常情况下不能与硝酸银反应生成氯化银的物质是:A. CH3C-Cl O B. (CH3)3C-Cl C. CH2=CHCHCH3 Cl D. Cl13. Which one cannot be converted into amides by nucleophilic acyl substitution reaction with acid chlorides, anhydrides, or esters? 下列哪种物质不能和酰氯、酸酐、酯类通过亲核的酰基取代反应形成酰胺? A. aniline (苯胺) B. (C2H5 )3N C. C2H5NHCH3 D. (C2H5 )3C-NH2 14. Which of the following reagents cannot react with 2,4-pentadione 下列哪种试剂不能与 2,4-戊二酮(乙酰丙酮)反应? (A) Na (B) Br2 (C) NaHSO3 (D) NaHCO3 15. Which of the following shows π-π conjugate system? 具有 π-π 共轭体系的是: A. 1,3-butadiene B. ClCH=CHCH2CH3 C. +CH2CH=CH2 D.CH2=CH-CH2CH=CH2 16. Which of the following shows p-π conjugate system? 具有 p-π 共轭体系的是: A. benzaldehyde B. 1,3-cyclohexadiene C. ClCH=CH2 D. ClCH2CH=CH2 17. Which substituent on an aromatic ring is ortho-para director? 下列芳香环上的取代基,属于邻-对位定位基的是: A. -CHO 碱性最强的是: A. NH3 碱性最弱的是A.N HB. -SO3HC. -CH=CH2D. -CN18. Which of the following shows the strongest basicity? B.(CH3)2NH C. C6H5NH2 D. CH3CONH219. Which of the following shows the weakest basicity?NH2B.NC.N HD.20. Which of the following shows the strongest acidity? 酸性最强的是COOH NO2 OH C. D. H3C COOHA.COOHB.21. Which of the following can be used as Lewis base? 能用作路易斯碱的是: A. BF3 B. H2SO4 C. Br+ D. CN22. Which of the following compounds contains 1°, 2°, 3°and 4°carbon atoms? 下列哪个化合物分子中同时包含有 1°, 2°, 3° 和 4° 碳原子? A. 2, 2, 3-trimethylbutane C. 2, 3, 4-trimethylpentane 2,2,3-三甲基丁烷 2,3,4-三甲基戊烷 B. 2, 2, 3-trimethylpentane 2,3,3-三甲基戊烷 D. 3, 3-dimethylpentane 3,3-二甲基戊烷23. Which of the following carbohydrates is capable of being oxidized by bromine water? 下列哪种碳水化合物能被溴水氧化? A. fructose 果糖 B. sucrose 蔗糖 C. glucose 葡萄糖 D. cellulose 纤维素24. Which one is the most stable? 下列哪个构象最稳定?CH3 H H CH3 A. Anti H H H H H CH3 H3C H H H CH3 CH3 H H H3C CH3B. EclipsedH H C. GaucheH HD. Eclipsed225. Which one of the stability order of the following is correct? 稳定性大小排序正确的是:CH(CH3)2 H3C (a) (b)CH(CH3)2 CH3 (c) (d) H 3C CH(CH3)2H3CCH(CH3)2A. a>b>c>dB. d>a>b>cC. d>b>c>aD. d>c>a>b26. Which of the following shows the highest activity toward SN1 reaction? 在单分子亲核取代反应中活性最高的是: A.methyl bromide 甲基溴 B. ethyl bromide 乙基溴 C. 3-bromo-2-methylbutane 3-溴-2-甲基丁烷 在双分子单核取代反应中活性最高的是: A.methyl bromide 甲基溴 B. ethyl bromide 乙基溴 C. 3-bromo-2-methylbutane 3-溴-2-甲基丁烷 is reasonable? 学生们在使用泰利(Thiele)毛细管法测定萘的熔点实验中,记录了如下测定结果。
成都双语实验学校高中化学必修二第七章《有机化合物》经典测试卷(答案解析)

一、选择题1.假期,小明随父母外出旅游,不仅饱览了祖国美丽的大好河山,而且经质了收获满满的化学之旅。
乘坐汽车前往景点,小明发现火车就像一座化学材料的“陈列馆”。
下列关于汽车家零部件所属材料的说法正确的是A.前挡风玻璃属于硅酸盐制品B.安全带属于金属复合材料制品C.橡胶轮胎属于无机高分子材料制品D.座椅表面的真皮属于合成高分子材料制品2.等物质的量的下列有机物完全燃烧,消耗O2最多的是A.C6H6B.CH3CH2OH C.C2H4D.CH3COOH3.已知自动脱水−−−−→R—CHO。
现有A、B、C、D、E、F六种有机物有如下转化关系,其中A的分子式为C4H8O3。
下列判断正确的是A.反应①属于氧化反应B.有机物B、D的最简式相同C.A的同分异构体中,与A具有相同官能团的有9种D.等物质的量的D、E与足量钠反应,产生H2的量相同4.通过测定血液或尿液中某物质的含量可诊断糖尿病患者的病情,该物质为()A.蛋白质B.葡萄糖C.淀粉D.油脂5.利用下列反应不能制得括号中纯净物的是A.等物质的量的氯气与乙烷在光照条件下反应(氯乙烷)B.乙烯与水加成(乙醇)C.乙烯与氯气加成(1,2-二氯乙烷)D.氯气与苯用氯化铁作催化剂反应(氯苯)6.下列有关化学用语表示不正确的是A.蔗糖的分子式:C12H22O11B.乙炔的结构式:CH≡CHC.氯化钠的电子式:D.二硫化碳分子的比例模型:7.下列说法正确的是A.CH2=CH2、三种物质中都有碳碳双键,都可发生加成反应B.1 mol 与过量的NaOH溶液加热充分反应,能消耗3mol NaOHC.将溴水加入苯中,溴水的颜色变浅,这是由于发生了取代反应D.用溴水即可鉴别苯酚溶液、2,4一己二烯和甲苯8.下列物质的检验、分离和提纯方法,不正确的是A.用分液漏斗分离四氯化碳与水B.用硝酸银溶液检验自来水中的氯离子C.用溴水区别乙烯与甲烷D.用浓硫酸干燥NH39.2020 年春节前后,世界各地爆发了新型冠状病毒疫情。
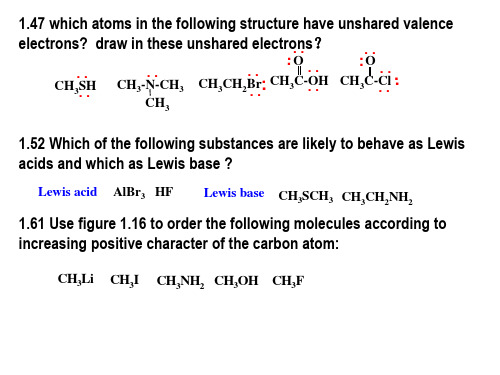
有机化学双语综合测试题

CH3CHCH2CH2CHCH3
(1)
(2)
O CH=CHCOOH CH3CH2CHCHCH2 C OH CH3 Br
(3)
NH2 COOH
(4)
(5)
(6)
NO2
S: (1) p-ethylbenzoic acid; (2) 2-ethylcyclohexanecarboxylic acid;
(3) 2,5-dimethylhexanedioic acid; (4) 3-phenylpropenoic acid (5) 4-bromo-3-methylhexanoic acid (6) 2-amino-5-nitrobenzoic acid
2-butanone acid
2-丁酮酸, β-丁酮酸
OH O CH3CH C OH
HO-CH-COOH CH2COOH
2-hydroxypropanoic acid
2-羟基丙酸, 乳酸(lactic acid) COOH
2-hydroxybutanedioic acid
2-羟基丁二酸, 苹果酸 (malic acid) 3,4,5-三羟基苯甲酸, 没食子酸(gallic acid)
S:
LiAlH4 COOH CH OH HCl 3 SOCl2 CH3 NaOH CH3I H3C H3C H3C H3C CH2OH O C-O-CH3 + H2O O C-Cl + HCl +SO2 O C-O-CH3
7
9-7 Preparation of the following compounds by using ethanoic acid with appropriate regents: (1) Ethanoyl chloride; (2) Ethanoic anhydride (3) Ethanamide
华中师范大学有机化学双语试卷

OH CH3 H3C CH3
O H O S O H O
OH2 CH3 H3C H CH3 CH3 CH3
CH3 H3C CH3 CH3 H3C
O O S O H O
H3C
CH3
第 2 页(共 3
页)
得分
------------------------------------------------- 密 ---------------------------------- 封 ----------------------------- 线 ---------------------------------------------------------
评阅人 六,propose the following reactions or the structures for compounds
1. Compound A (C4H6) reacts with hydrogen and a platinum catalyst to yield butane. Compound A reacts with Br2 in CCl4 and aqueous KMnO4. The IR spectrum of A does not have an absorption in the 2200-2300 cm-1 region. On treatment with hydrogen and Ni2B (P2 catalyst), A is converted to B (C4H8). When B is treated with OsO4 and then with NaHSO3, B is converted to C (C4H10O2). Compound C cannot be resolved. Provide structures for A-C and reactions involved. (6 points)
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Furan 呋喃
Thiophene 噻吩
Pyrrole 吡咯
Thiazole 噻唑
N O
Imidazole 咪唑
Pyrazole 吡唑
编著与制作: 南华大学 邓健
Oxazole 噁唑
4
Pyridine
吡啶
Pyrimidine
嘧啶
Pyridazine
哒嗪
Quinoline 喹啉
Isoquinoline 异喹啉
Pyridine.SO3
ClCH2CH2Cl/25℃
(CH3CO) 2O, 0 C SnCl 4 or Et2O·BF3
o
O
SO3H
2-furansulfonic acid
O
O C- CH3 + CH3CO2H
Br2 HAc/25 o C
S
Br
Cl2 50℃
CH3CO2NO2
S
Cl
30%
Cl
+13% Cl Addition S product 14%
Naphthalene
4 1 7
OH 8-Hydroxy quinoline
5 6 4 1 7 3 2
Isoquinoline
NH2
1 2 3
6
5 6
3 2
CO2H
5 4 9
7 8
Br
Indene(茚) 6-Bromoindole -3-carboxylic acid
编著与制作: 南华大学 邓健
6-Amino purine
Indole
吲哚
Purine
嘌呤
编著与制作: 南华大学 邓健
5
(1) In the numbering system of ring , hetero atoms are generally given the lowest possibly numbers. you can also number the atoms beside the hetero atom with α,β,γ,δ, and so on.
139pm
0.87 1.01
14 0p m
13
9p m
1
pm 4 3
0.84 1.00
Site for nucleophilic attack
1.43
Relative electronic density
编著与制作: 南华大学 邓健 23
1. Basicity
p- conjugation
..
N
NO2 S
70%
S
Thiophene
Ac2O/- 10℃
95% H2SO4 25oC
NO2
S
5%
(69%~76%)
S
SO3H
2-Thiophenesulfonic acid
CH3COCl,SnCl 4 or (CH3CO)2O/H3PO4
S
O C-CH3 + HCl
Br2 (or I2) EtOH/0℃
N
(CH 3CO)2O,SnCl 4 100~200 oC
2-Acetylpyrrole
+ HCl
H
N2Cl
EtOH-H2O,NaAc
N
N=N
H
2. Addition
O H2 / Ni 125 ℃, 100atm
H2 / Pd-C, CH3OH, H2SO4, 3 atm
(or Na-Hg, EtOH)
C H 3 CO 2 NO 2 Ac 2 O/5 ℃
Br Br
Br Br
产物均为 2,3,4,5-Tetra四卤吡咯 bromopyrrole
NO 2
NO 2 51%
β-Nitropyrrole
13%
+
pyrrole
N SO3
+
100oC
HCl N H
O C - CH 3
SO3H (90%)
2-Pirrolesulfonic acid
> NH3 >
4.7
H
pKb: 3.7
N ..
>
9.4
NH2
>
N
no conjugation
H
13.6
8.8
Pyridine can form salt with inorganic acids.
+ HCl ——> H
编著与制作: 南华大学 邓健 24
Cl -
2. Electrophilic Substitution
4 5 1 3 2 5 4 3 1 2
4-position = 5-position
CH3
4 5 1 3 2
CH3
5 4 3 1 2
4-Methylimidazole
=
5-Methylimidazole
20
编著与制作: 南华大学 邓健
Also, 3-methylpyrazole and 5-methylpyrazole are the same compound.
Ⅱ. Chemical Properties
1. Electrophilic Substitution
They are much more reactive than benzene, and resemble the most reactive anilines and phenols toward electrophilic substitution . Reaction takes place predominantly at theα-position.
heterocycles Fivemembered ring Sixmembered ring Benzenefused Heterocyclefused
编著与制作: 南华大学 邓健 3
N O S
Fused-ring heterocycles
Ⅱ. Nomenclature:译音+“口”旁
N O S S
编著与制作: 南华大学 邓健 1
Classification & Nomenclature
Ⅰ. Classification
O
Aliphatic
Section 1
脂杂环
Aromatic
O
N H
N
O
N N N
N H
N N N H
2
芳杂环
O
N H
编著与制作: 南华大学 邓健
For aromatic heterocyclic compounds: Mono-ring
4 4 5 1 3 2
3 2 SH
Cl
5
1
CH3 5-Chloro-2-thiohydroxyimidazole 1-Methylpyrazole 2-巯基-5-氯咪唑
5 6 1 3 2
4 5
N3 S
1 2
4 5
N3 O
1 2
C2 H5
2-Ethylpyrimidine
Thiazole
Oxazole
Activity order of electrophilic aromatic substitution
NH2 NO2
≈
> H
N O
>
S
>
>
N
≈
编著与制作: 南华大学 邓健
25
Electrophilic substitution occur at 3- (or β-) position. Br2 300℃
8ห้องสมุดไป่ตู้
Sec 2 Five-membered Heterocycles
Ⅰ. Structure Ⅱ. Chemical Properties
O
S
编著与制作: 南华大学 邓健
9
Ⅰ. Structure
p orbital containing two electrons; part of aromatic cloud: π56, electron-rich aromatic heterocycle (富π芳杂环).
O Tetrahydrofuran
Conc HNO3
S
O O Sulfolane
环丁砜
S
Tetrahydrothiophene
S
H2 / Ni
High temperature,pressure
Tetrahydropyrrole
3. Acidity and Basicity of Pyrrole
Basicity:
CHAPTER 13
Heterocyclic Compounds
Sec 1 Classification & Nomenclature
Sec 2 Five-membered Heterocycles Sec 3 Six-membered Heterocycles
Sec 4 Fused Aromatic Heterocycles
p orbital containing one electron; part of aromatic system
139pm 139pm
137pm
sp2 orbital containing two electrons
Because of electron-withdrawing effect of nitrogen, the electron density of pyridine is lower than benzene, so pyridine belongs to electron-deficient aromatic heterocycles (缺π芳杂环). Site for electrophilic attack
