过表达慢病毒载体构建和包装手册 version1
捷尼科比亚 慢病毒表达包装试剂盒 使用手册说明书

Lenti-Pac ™ HIV Expression Packaging KitFor optimized production of recombinant lentivirusCat. No. HPK-LvTR-20 (20 transfections) Cat. No. HPK-LvTR-40 (40 transfections)User Manual Version VGeneCopoeia, Inc.9620 Medical Center Drive, #101 Rockville, MD 20850 USA301-762-0888 or 866-360-9531***********************© 2009 GeneCopoeia, Inc.G C n p o eiae e o TMExpressway to DiscoveryUSER MANUALLenti-Pac™ HIV Expression Packaging KitI. IntroductionII. Kit Contents and StorageIII. Additional Materials Required or RecommendedIV. Getting StartedV. Lentivirus ProductionVI. Lentivirus Titer Estimation by TransductionVII. Transduction of Target Cells with LentivirusesVIII. Limited Use License and WarrantyI. IntroductionGeneCopoeia has multiple sets of over 40,000 human and mouse ORF expression clones as well as multiple sets of small hairpin RNAi (shRNA) clones against genome-wide target genes from human, mouse, rat, and other mammals in HIV-based lentiviral vector systems. HIV- (human immunodeficiency virus) based vectors are currently the most popular lentiviral-based expression systems and are very effective at transducing genes into a wide variety of dividing and non-dividing mammalian cells, both in vitro and in vivo. The lentiviral expression vectors can integrate into the genome of the target cells, resulting in the stable expression of transgenes. The GeneCopoeia third generation HIV-based lentivector systems meet Biosafety Level 2 (BSL-2) requirements based on the criteria published by the Centers for Disease Control.The GeneCopoeia Lenti-Pac™ HIV Expression Packaging System includes an optimized lentiviral packaging plasmid mix, an eGFP positive control plasmid, a new transfection reagent, EndoFectin™optimized for virus production and TiterBoost™ reagent that further increases the titers 5-10 fold. When combined with GeneCopoeia HIV-based lentiviral constructs the results are high titers and robust expression levels. The Lenti-Pac HIV Expression Packaging System safely ensures efficient expression of recombinant transcripts in mammalian cells.A dvantages of OmicsLink™ Lentiviral ORF Expression Clones and shRNA Clones∙High efficiency of gene delivery to virtually all mammalian cell types in vitro as well in vivo∙High expression levels of delivered genes (ORF expression clones)∙High knockdown efficiency against target mRNA transcripts (shRNA clones)∙Self-inactivation and no unwanted viral replicationII. Contents and Shipping/ StorageContents and storage recommendations for the Lenti-Pac HIV Expression Packaging Kits(Cat. No. HPK-LvTR-20/HPK-LvTR-40) are provided in the following table.III. Additional Materials Required or Recommended1. GeneCopoeia GCI-L3 chemically competent cells (GeneCopoeia Cat No. STK300-10). Alternatively, Stbl3™chemically competent cells (Invitrogen Cat No. C7373-03) may be used.2. GeneCopoeia 293Ta Lentiviral packaging cell line(GeneCopoeia Cat No. Clv-PK-01). Alternatively, theHEK 293T/17 cell line (ATCC Cat No. CRL-11268) can also be used.3. H1299 cell line (ATCC Cat No.CRL-5803), or HT-1080 cell line (ATCC Cat No. CCL-121) for lentivirus titerestimation. H1299 cells are preferred over HT-1080 cells for lentivirus titration.4. DMEM with glucose, L-glutamine and sodium pyruvate (Mediatech Cat No. 10-013-CV)5. Fetal bovine serum (Thermo Scientific Cat No. SH300700.02)6. Opti-MEM® I Reduced-Serum Medium (Invitrogen Cat No. 31985-062/31985-070).7. Polybrene(Sigma-Aldrich Cat No. H9268): 10 mg/ml solution dissolved in 150 mM NaCl and sterile-filtered.Store usable aliquots at -20o C. Working stock can be stored at 4o C for up to two months.8. Crystal Violet (Sigma-Aldrich Cat No. C3886): 0.5% (W/V) solution dissolved in 25% methanol.9. Penicillin-Streptomycin for mammalian cell culture (Sigma-Aldrich Cat No. P4333)10. Antibiotics for selecting stably transduced cells: puromycin (Invivogen Cat No. ant-pr-1/ant-pr-5), hygromycinB (Invivogen Cat No. ant-hm-1/ant-hm-5), Neomycin (G-418) (Invivogen Cat No. ant-gn-1/ant-gn-5)11. BD Falcon® 5-ml or 14-ml Tubes (BD Falcon Cat No. 352053/352059).IV. Getting StartedUpon receipt of a new lentiviral expression plasmid, it is recommended to transform the plasmid into GeneCopoeia GCI-L3 Chemically Competent E. coli Cells (GeneCopoeia Cat No. STK300-10), or any Stbl3™-equivalent competent cell strain, and to prepare plasmid DNA with a proven plasmid DNA purification method. GeneCopoeia lentiviral ORF expression or shRNA constructs contain the ampicillin resistance gene.Quality of plasmidIt is critical to use plasmid of the highest quality. Determine the DNA concentration by reading the absorption at 260 nm. DNA purity is measured by using the 260 nm / 280 nm ratio (the ratio should be in the range of 1.8 to 2.0). Check the integrity of the plasmid by agarose gel electrophoresis.Condition of cellsAlways use healthy cells that are well maintained and passaged regularly. Make sure the culture is free from bacteria, fungi, or Mycoplasma contamination. If the cells are from a recent liquid nitrogen stock, passage the cells at least 2 times before transfection.V. Lentivirus ProductionThe GeneCopoeia HIV-Based Lentiviral Expression System is a modified version of the third generation self-inactivating (SIN) lentiviral vector system which incorporates enhanced bio-safety features and is optimized for production of high viral titers. In this system, recombinant lentiviral particles are generated by co-transfecting an HIV-based lentiviral expression plasmid together with the GeneCopoeia Lenti-Pac HIV Expression Packaging Kit into GeneCopoeia 293Ta lentiviral packaging cells (GeneCopoeia Cat No. CLv-PK-01).The lentiviral ORF/shRNA expression plasmid (lentiviral transfer vector) contains the elements required for packaging, transduction and stable integration of the viral expression construct into genomic DNA leading to expression of the ORF or shRNA hairpin. However, it lacks the elements essential for transcription and packaging of an RNA copy of the ORF/shRNA expression plasmid into recombinant pseudoviral particles. These elements are provided by the Lenti-Pac HIV packaging mix, an optimized mixture of plasmids that express the structural, regulatory, and replication genes required to produce lentivirus. See figure 1 below for schematics of this process. The lentivirus generated with this system is pseudotyped with vesicular stomatitis virus-G protein (VSV-G) which exhibits wide cell tropism and generates high titers.In addition to Lenti-Pac HIV packaging mix, this kit also includes a positive control lentiviral transfer vector that expresses the eGFP protein, EndoFectin Lenti Reagent (Cat No. EFL1001-01), and TiterBoost reagent. The EndoFectin Lenti transfection reagent is optimized for transferring plasmids into packaging cells. It guarantees higher transfection efficiency and lower cell toxicity compared to other commercially available transfection reagents. The unique TiterBoost reagent from GeneCopoeia enhances the production of lentivirus particles.Figure 1. Schematics of Lentivirus production and infection of target cellsThe following procedure provides optimized steps for lentivirus production in 293Ta packaging cells. The yield of recombinant lentiviral particles typically produced under these optimized conditions is 10 ml of 1–10 x107 infection units (ifu) per ml of un-concentrated supernatant from one 10-cm culture dish for eGFP or mCherry positive controls. This amount of pseudoviral particles is generally sufficient to infect 1–10 x108 target cells at a MOI (multiplicity of infection) equal to 1. The titers of lentivirus decrease as the size of insert increases. Actual lentivirus titers for your gene of interest will vary accordingly.Caution: Following this protocol results in the production of pseudoviral particles capable of infecting mammalian cells. The recommended guidelines for working with BSL-2 safety class must be adhered to.1. Plate packaging cellsTwo days before transfection, plate 1.3–1.5 x106of the GeneCopoeia 293Ta lentiviral packaging cells or comparable cells in a 10-cm dish in 10 ml of DMEM supplemented with 10% heat-inactivated fetal bovine serum so that the cells are 70–80% confluent at the moment of transfection. Incubate the cells at 37°C with 5% CO2.Note: Plating the packaging cells 2 days prior to transfection significantly increases the titer of lentivirus.Use heat-inactivated fetal bovine serum for lentivirus production. Heat-inactivated serum can be purchased from other vendors or prepared by incubating thawed serum for 30 minutes at 56°C with gentle shaking.2. Prepare DNA/EndoFectin Lenti complexIn a sterile polypropylene tube, dilute 2.5 µg of lentiviral ORF/shRNA expression plasmid and 5.0 µl (0.5 µg/µl) of Lenti-Pac HIV mix into 200 µl of Opti-MEM® I (Invitrogen). In a separate tube, dilute 15 µl of EndoFectin Lenti into 200 µl of Opti-MEM I. Add diluted EndoFectin Lenti reagent drop-wise to the DNA solution while gently vortexing the DNA-containing tube. Do not reverse the addition sequence. Use round-bottom polypropylene tubes such as Falcon® 5-ml or 14-ml tubes (BD) for larger volumes. Incubate the mixture for 10–25 minutes atroom temperature to allow the DNA-EndoFectin complex to form.Note: The DNA-EndoFectin complex must be formed in the absence of proteins even though the complex is able to transfect cells in the presence of proteins such as 10% serum. Opti-MEM I is recommended for diluting both DNA and EndoFectin Lenti reagent. Serum-free DMEM can be used in place of Opti-MEM I but the transfection efficiency will be compromised. The ratio of 3.0 µl of EndoFectin Lenti per 1 µg of plasmid has been found to be optimal. Increasing the ratio does not further improve transfection efficiency.3. Transfect packaging cellsAdd the DNA-EndoFectin Lenti complex directly to each dish and gently swirl the dish to distribute the complex.Incubate the cells in a CO2 incubator at 37°C overnight (8–14 hours). Replace the overnight culture medium with fresh DMEM medium supplemented with 2–5% heat-inactivated fetal bovine serum and penicillin-streptomycin. Add 1/500 volume of the TiterBoost reagent to the culture medium and continue incubation in the CO2 incubator at 37°C.Note:a. Replace the culture medium that contains the DNA-EndoFectin Lenti complex within 16 hours post-transfection.b. TiterBoost reagent at working concentration (1×) typically boosts the titer of lentivirus products 5-10 fold.This reagent is readily removed during commonly used lentivirus concentration/purification procedures such as ultracentrifugation, ultrafiltration and other chromatographic methods. If crude lentiviral particles are used directly to transduce target cells, the volume of lentiviral particles should not exceed 1/10 of that of culture medium; otherwise some adverse effects may occur. TiterBoost at 1/20× or lower concentrations has negligible effects on commonly used mammalian cell lines. It's advised to test the effects of TiterBoost on your target cells beforehand if large volumes of crude lentiviral particles are used.4. Harvest lentivirusCollect the pseudovirus-containing culture medium in sterile capped tubes 48 hours post transfection and centrifuge the tubes at 500 x g for 10 minutes to get rid of cell debris. Following centrifugation, filter the supernatant through 0.45 µm polyethersulfone (PES) low protein-binding filters.Note:a. Peak virus production is normally achieved 24–48 hours post transfection. Alternatively, lentivirus-containing medium may be collected multiple times at 36, 48 and 60 hours post-transfection. Lentiviral containing supernatants should be replaced with fresh DMEM supplemented with 2–5% heat-inactivated fetal bovine serum, penicillin-streptomycin and TiterBoost reagent.b. Do not use nitrocellulose filters as nitrocellulose is known to bind lentivirus and reduce titers.The supernatant containing lentiviral particles can be used directly to determine the titer and to transduce target cells in vitro as long as the target cells can survive in conditioned medium. Lentiviral stocks should be aliquoted and stored at -80°C. Expect significant loss of viral titer with each freeze/thaw cycle.VI. Lentivirus Titer Estimation by TransductionAt this point it is recommended that the pseudoviral stock is titered to ensure it is viable and to test what fraction of target cells can be transduced. This enables the number of copies of viral construct per target cell to be controlled. There are several methods to determine the titer of pseudovirus stock. The procedure below is based on the transduction of H1299 or HT-1080 cells. Different cells can also be used but titers may vary up to several orders of magnitude.Day 1: Plate H1299 or HT-1080 cells1. Plate ~5 x 104 of the H1299 or HT-1080 cells per well in a 24-well plate 24 hours prior to viral infection. Use 0.5 ml of DMEM supplemented with 10% heat-inactivated fetal bovine serum and penicillin-streptomycin for each well. Incubate the cells at 37°C with 5% CO2 overnight.Day 2: Transduce H1299 or HT-1080 cells2. Dilute Polybrene to 10 µg/ml with DMEM containing 5% heat-inactivated fetal bovine serum and penicillin-streptomycin.3. Remove old culture medium from each well. Add 0.25 ml of Polybrene (from Step 2). The final concentration of Polybrene will be 5 µg/ml after adding diluted lentivirus. For each pseudoviral stock, use five wells.3a. For lentivirus containing a fluorescent marker and to be analyzed with flow cytometry (step 5a): Infect H1299 or HT-1080 cells by adding 0.1 μl of lentivirus (10 μl of 100-fold diluted viral stock) into the first well, 0.5 μl of lentivirus (50 μl of 100-fold diluted viral stock) into the second well, 2.0 μl into the third well, 10 μl into the fourth well, and 50 μl into the fifth well. Add appropriate amount of DMEM containing 5% heat-inactivated serum and penicillin-streptomycin so that the final volume reaches 0.5 ml per well.3b. For lentivirus to be analyzed by drug selection and colony counting (step 5b-10): Infect H1299 or HT-1080 cells by adding 0.001 μl of lentivirus (10 μl of 10,000-fold diluted viral stock) into the first well, 0.01 μl of lentivirus (10 μl of 1,000-fold diluted viral stock) into the second well, 0.1 μl of lentivirus (10 μl of 100-fold diluted viral stock) into the third well, 0.5 μl of lentivirus (50 μl of 100-fold diluted viral stock) into the fourth well, and 2.0 μl into the fifth well. Add appropriate amount of DMEM containing 5% heat-inactivated serum and penicillin-streptomycin so that the final volume reaches 0.5 ml per well.Place the plates for 2 hours at 4-8°C; then transfer the plates to a 37°C incubator with 5% CO2 and incubate cells overnight.Note:a. Dilute lentivirus with only culture medium. Do not use water or other buffers.b. The optimal concentration of Polybrene depends on cell type and may need to be empirically determined,but is usually in the range of 2–10 µg/ml. Prepare enough for an extra well as a negative control.c. Excessive exposure to Polybrene (>12 hr) can be toxic to some cells.Day 3: Replace medium/split cell culture4. Trysinize and transfer the cells to 6-well plates (each 6-well plate will be used for one lentivirus with one control well of non-transduced cells). Incubate in DMEM supplemented with 10% fetal bovine serum and penicillin-streptomycin for additional 48 hours.Day 5: Determine titer by fluorescence analysis (step 5a) or drug selection (5b)5a. The fraction of eGFP fluorescent cells can be counted by FACS (fluorescent activated cell sorting). Alternatively the eGFP fluorescence may be visualized under a fluorescent microscope. Normally 10 random fields of view are used to estimate the overall fraction of fluorescing cells in each well. The cells are then trypsinized, suspended with complete DMEM, and the total number of cells in each well is determined by using a hemocytometer. The averaged fraction of fluorescent cells is multiplied by the corresponding total cell numbers, then divided by the actual volume of added lentivirus supernatant (in ml, e.g. 0.1 μl equals 10–4 ml) to determine the titer of the pseudovirus in the supernatant.5b. For HT-1080 or H1299 cells transduced with lentiviral stocks lacking a fluorescent marker, replace the old medium with fresh complete DMEM containing an appropriate selection drug. (Note: The concentration of the selection drug should be determined empirically beforehand.) Then follow steps 6–10 below:Days 6-14: Select stably transduced cells and count colonies (continued from step 5b)6. Replace medium with fresh complete medium containing the appropriate selection drug every 3–4 days until drug-resistant colonies become visible (generally 7–14 days after selection). There should not be any colonies in the mock well control.7. Remove medium and wash dishes twice with cold PBS.8. Add enough 10% formalin to cover each dish. Incubate for 5 minutes at room temperature to fix the cells.9. Decant 10% formalin, add 0.5% crystal violet and incubate for 10 minutes at room temperature.10. Remove the crystal violet solution and wash the dishes with tap water until there is no background staining. Count the blue colonies and calculate the titer of lentiviral stock.Note:Viral titers determined by drug selection/colony counting are significantly lower than those determined by FACS.VII. Transduction of Target Cells with LentivirusesThe transduction efficiency depends upon the target cells and experimental procedure. It is recommended that the titrated pseudoviral stock containing the positive control eGFP is used to determine the concentration of pseudoviral particles required for the desired MOI of the target cells. After these test transductions are performed, it should be possible to determine the optimum concentration of pseudoviral particles for transduction based on eGFP fluorescence.Day 1: Plate cells1. Plate 2–10 x 104 of the target cells per well in a 24-well plate 24 hours prior to viral infection. Use 0.5 ml of DMEM supplemented with 5% heat-inactivated serum and penicillin-streptomycin for each well. Incubate the cells at 37°C with 5% CO2 overnight.Day 2: Transduce target cells2. For each well, prepare 0.5 ml of virus suspension diluted in complete medium with Polybrene at a final concentration of 5–8 µg/ml.Note:Use several dilutions of pseudoviral stock (0.1 μl to 100 μl). In addition, we recommend including a transduction with the eGFP control and other appropriate positive and negative controls. Mix the virus with the medium gently by rotation or inversion. Do not vortex.3. Infect the target cells by removing the old culture medium and replacing it with 0.5 ml of diluted viral supernatant. For one well (mock well control), add 0.5 ml of complete DMEM with Polybrene. Place the plates in a 37°C incubator with 5% CO2 and incubate cells overnight. (Optional: Place the plates for 2 hours at 4-8°C; then transfer the plates to a 37°C incubator with 5% CO2 and incubate cells overnight.)Note:Incubating cells with lentivirus for 2 hours at low temperatures can significantly increase the transduction efficiency. But it may be omitted if the cells cannot tolerate low temperatures.Day 3: Replace medium/Split cell culture4. Remove the culture medium and replace with 0.5 ml of complete medium (without Polybrene). Or split the cells 1:3 to 1:5 depending on the type of cells, and continue incubating for 48 hours in DMEM.Day 5: Analyze transduced cells or start drug selection of stably transduced cells5a. The infected target cells can be analyzed for transient expression of transgenes using an appropriate biological assay. If you have used an internal eGFP control, determine the percentage of infected cells by counting fluorescing cells by flow cytometry or with a fluorescent microscope.5b. To select stably transduced cells, replace old medium with fresh complete medium containing the appropriate selection drug every 3–4 days until drug-resistant colonies become visible (generally 7–14 days after selection).VIII. Limited Use License and WarrantyLimited Use LicenseFollow ing terms and conditions apply to use of all OmicsLink™ ORF Expression Clones in all lentiviral vectors and Packaging Kit (the Product). If the terms and conditions are not acceptable, the Product in its entirety must be returned to GeneCopoeia within 5 calendar days. A limited End-User license is granted to the purchaser of the Product. The Product shall be used by the purchaser for internal research purposes only. The Product is expressly not designed, intended, or warranted for use in humans or for therapeutic or diagnostic use. The Product must not be resold, repackaged or modified for resale, or used to manufacture commercial products without prior written consent from GeneCopoeia. This Product should be used in accordance with the NIH guidelines developed for recombinant DNA and genetic research. Use of any part of the Product constitutes acceptance of the above terms.Limited WarrantyGeneCopoeia warrants that the Product meets the specifications described in the accompanying Product Datasheet. If it is proven to the satisfaction of GeneCopoeia that the Product fails to meet these specifications, GeneCopoeia will replace the Product. In the event a replacement cannot be provided, GeneCopoeia will provide the purchaser with a refund. This limited warranty shall not extend to anyone other than the original purchaser of the Product. Notice of nonconforming products must be made to GeneCopoeia within 30 days of receipt of the Product. GeneCopoe ia’s liability is expressly limited to replacement of Product or a refund limited to the actual purchase price. GeneCopoeia’s liability does not extend to any damages arising from use or improper use of the Product, or losses associated with the use of additional materials or reagents. This limited warranty is the sole and exclusive warranty. GeneCopoeia does not provide any other warranties of any kind, expressed or implied, including the merchantability or fitness of the Product for a particular purpose.GeneCopoeia is committed to providing our customers with high-quality products. If you should have any questions or concerns about any GeneCopoeia products, please contact us at (301)-762-0888.© 2009, GeneCopoeia, Inc.GeneCopoeia, Inc.9620 Medical Center Drive, #101Rockville, Maryland 20850Tel: 301-762-0888 Fax: 301-762-8333Email:***********************Web: GeneCopoeia Products are for Research Use Only Copyright © 2009 GeneCopoeia, Inc.。
慢病毒包装体系使用说明

慢病毒包装体系使用说明本说明书适用于以下产品:名称货号慢病毒包装体系KLV3501慢病毒包装体系(含293V细胞)KLV3502慢病毒包装体系(含转染试剂)KLV3503慢病毒包装体系(含293V细胞、转染试剂)KLV3504北京英茂盛业生物科技有限公司Web site:1北京英茂盛业生物科技有限公司/产品内容KLV3501KLV3502KLV3503KLV3504慢病毒载体(过表达或RNA 干扰载体任选一种) 3333辅助载体pH1 3 3 3 3 辅助载体pH2 3 3 3 3 HEK293V 细胞 3 3 Polyfect-V 转染试剂33载体采用质粒形式发货,请在收到质粒后放-20℃冻存,也可以直接转化大肠杆菌感受态进行质粒扩增。
HEK293V 细胞采用干冰或培养瓶发货。
请在收到细胞后根据附带说明书进行复苏或传代。
慢病毒载体慢病毒载体中含有病毒整合和表达所需原件及表达外源目的基因的元件。
外源基因通过载体中的多克隆位点插入慢病毒载体中进行表达。
pLV-EGFP-C 的载体图谱见下,本公司的其它慢病毒载体结构与之基本相似。
其它载体信息见本公司网站 或本说明书后面的附表。
慢病毒包装载体慢病毒包装载体包括pH1和pH2,表达生产病毒颗粒所需的病毒蛋白。
载体图谱见下:HEK293V细胞包装细胞的状态对病毒包装效果有直接影响。
我公司保存的293V细胞为低次代293V细胞,细胞性状稳定。
在高密度下生长3天仍可保持贴壁状态,持续产生病毒颗粒,因此可多次收获病毒,降低病毒包装实验成本。
Polyfect‐V转染试剂Polyfect-V转染试剂专为293V细胞转染及慢病毒包装研制,可以在细胞铺板同时进行转染,缩短病毒包装时间;无需要求细胞处于生长对数期,细胞转染时密度可以很高;细胞毒性极低;质粒和转染试剂用量是普通转染试剂的1/3到1/2等显著优点;包装病毒时转染效率接近100%,能提高病毒产量3-5倍。
3北京英茂盛业生物科技有限公司/慢病毒包装概述:病毒包装前需制备转染级慢病毒载体及两种包装载体。
[高等教育]慢病毒包装操作说明
![[高等教育]慢病毒包装操作说明](https://uimg.taocdn.com/a4ea667dfc4ffe473368abe1.webp)
Clontech-Lenti-X™ Lentiviral Expression Systems User ManualProtocol No. PT5135-1慢病毒包装操作说明A.用Lenti-X HTX Packaging System生产慢病毒悬浮物为了获得最高效价的病毒悬液,用Lenti-X 293T细胞系,严格尊守以下说明,尤其尊守(1)培养体系和培养量(2)DNA的量和转染质量(3)无四环素血清(4)孵育时间。
所有的Xfect™转染成份,量和条件最好用Lenti-X Vectors,Lenti-X HTX 包装混合物,Lenti-X 293T细胞。
用10cm组织培养板并确保血清无四环素,四环素污染的血清对表达包装成份是有害的。
所有的实验步骤均在无菌组织培养器血中完成。
包装病毒需要有微生物安全等级2的生物安全柜中进行,注意重组的假性慢病毒包装颗粒能够感染人。
1.转染24小时前,在10cm培养板接种4-5×106个293T细胞,添加10ml的生长培养基。
在37℃,5%CO2℃条件下过夜。
在进行第7步前确保培养血有80-90%的覆盖率。
2.充分混均Xfect Polymer。
3.每个转染样品需准备两个离心管,按顺序添加如下试剂Tube 1(Plasmid DNA) Tube 2(Polymer)557µl Xfect Reaction Buffer 592.5µl Xfect Reaction Buffer 36µl Lenti-X HTX Packaging Mix 7.5µl Xfect Polymer7µl Lenti-X Vector DNA(1µg/µl)600µl 总量600µl 总量注意:Xfect Polymer 不要在室温下搁置长于30min4.充分混均每个管5.把Tube2添加到Tube1中,中速涡旋10秒。
慢病毒载体的构建

慢
慢病毒载体是一种常用的基因转移工具,其安全性评估主要 包括对病毒的毒力、致病性和传播性的评估。在构建慢病毒 载体时,应确保所选用的慢病毒毒株无致病性,且不具有传 播性。
慢病毒载体的生物安全性
在构建慢病毒载体时,应确保载体无外源污染,如细菌、真 菌、支原体等污染。同时,应进行逆转录酶活性检测,以确 保载体无逆转录酶活性,从而避免潜在的基因重组和插入突 变风险。
慢病毒载体的构建流程
01
02
03
04
目的基因的克隆
将目的基因克隆到慢病毒载体 中,常用的克隆方法包括限制
性酶切、连接和转化等。
慢病毒载体的包装
将目的基因与包装信号共同转 染包装细胞,包装细胞能够产 生具有感染力的慢病毒颗粒。
慢病毒的纯化
通过离心、过滤等方法将慢病 毒颗粒从包装细胞中分离出来
,并进行纯化。
生物学功能分析
对目的基因进行生物学功能分析, 如报告基因实验、细胞活性实验、 动物模型实验等,以评估慢病毒 载体对目的基因功能的改善效果。
安全性评估
对慢病毒载体进行安全性评估, 包括对宿主细胞的毒性、免疫反 应、致瘤性等方面进行检测,以 确保慢病毒载体的应用安全可靠。
05
慢病毒载体的安全性与伦理问题
慢病毒的滴度测定
测定纯化后慢病毒的滴度,即 每毫升或每毫克病毒颗粒的数
量。
02
慢病毒载体的设计
包装元件的选择
01
02
03
包装元件
选择合适的包装元件是构 建慢病毒载体的关键步骤, 包括病毒的复制酶、转录 酶和整合酶等。
安全性
确保所选的包装元件无致 病性,不会对宿主细胞造 成不良影响。
兼容性
确保所选的包装元件与载 体的其他元件兼容,能够 实现有效转导和表达。
汉恒生物-慢病毒生产及使用操作手册第二版
2. 感染细胞最佳 MOI 的测定 MOI(Multiplicity of Infection,感染复数)是指每个细胞感染的病毒
数,通常 MOI 越高,病毒整合到染色体的数量以及目的蛋白的表达量越高。 对于分裂活跃的细胞,比如 Hela、293 细胞,MOI=1~3 时,80%以上的细胞均表 达目的基因。而对于非分裂细胞,比如原代细胞,感染效率较低。需要进行 MOI 梯度摸索实验,选择适合的 MOI 进行实验。
四、慢病毒包装和浓缩 (一)质粒扩增
构建好的慢病毒载体和辅助质粒需经过大量抽提,浓度大于 1ug/ul,A260/280 在 1.7-1.8 间方可用以包毒。推荐使用 Qiagen 大抽试剂盒进 行质粒的大量去内毒素抽提。 (二)传 293T 细胞
将培养 293T 细胞 T75 瓶中的培养基吸净,加入 2mL 4 度冰箱取出的 0.25% 胰酶,使其均匀覆盖瓶底,置于 37 度培养箱中 3-5min,取出,摇晃可发现细胞 于底部脱离,将其全部晃下,加入 3mL 37 度水浴中预热的 10% DMEM,移液枪 用 10mL 移液管进行吹打,较大力吹打 6-8 次即可,不留死角,瓶口处较难吹打 可将移液管对准培口,小力将培养基打出即可覆盖到接近瓶口的细胞。之后,将 所有细胞吸出,置于 15mL 离心管中,取 50ul 混匀后的细胞于 1.5mL eppendorf
当细胞传代次数过多,细胞状态变差时,或者细胞出现污染事故时,需要丢 弃并对最初冻存的细胞进行复苏。
1、设置温度为 37~42℃的水浴。 2、查看细胞库记录,根据记录从液氮罐中取出冻存的细胞(需戴上棉手套, 防止被冻伤),迅速丢入水浴锅中并快速晃动,尽量在 1~2min 内使细胞溶液完全 溶解。 3、将细胞溶液转移到 15ml 离心管中,并在其中加上 1ml 新鲜的完全培养 基,混匀后离心,1000rpm,5min。 4、去掉上清,加入 5ml 新鲜的完全培养基,混匀沉淀后,转入 6cm 培养皿。 5、将培养皿平稳放入 37℃、5%CO2 和 95%相对湿度的培养箱中培养。 6、第二天观察细胞存活率。给细胞换一下培养基。以后每天观察细胞生长 情况。
慢病毒载体构建和包装操作手册

慢病毒载体构建及包装操作手册目录慢病毒收到后的注意事项一、整体实验流程二、实验材料三、慢病毒包装和浓缩四、感染目的细胞附1. 汉恒生物慢病毒质粒列表附2. 慢病毒滴度测定方法简介附3. 慢病毒MOI感染参数附4. 汉恒生物各病毒载体感染目的细胞比较慢病毒安全使用和注意事项➢慢病毒安全使用注意事项(*非常重要!!!*)1)慢病毒相关实验请在生物安全柜(BL-2级别)内操作。
2)操作病毒时请穿实验服,佩戴口罩和手套,尽量不要裸露双手及手臂的皮肤。
3)操作病毒时特别小心病毒溅出。
如果操作时超净工作台有病毒污染,请立即用70%乙醇加1%的SDS溶液擦拭干净。
接触过病毒的枪头,离心管,培养板,培养液请于84消毒液浸泡后统一处理。
4)如需要离心,应使用密封性好的离心管,如有必要请用封口膜封口后离心。
5)病毒相关的废弃物需要特殊收集,统一经高温灭菌处理。
6)实验完毕用香皂清洗双手。
➢慢病毒收到后的注意事项1)慢病毒的储存用户收到病毒液后在短期内即使用慢病毒进行实验,可以将病毒暂时放置于4 ℃保存(尽量一周内用完);如需长期保存请分装后放置于-80℃。
注:a.反复冻融会降低病毒滴度(每次冻融会降低病毒滴度10%-50%);在病毒使用过程中应尽量避免反复冻融,所以我们前期对病毒进行了分装(200 l/tube),收到后直接放置-80℃保存即可。
b.如果病毒储存时间超过6个月,我们建议在使用前重新测定病毒滴度。
2)慢病毒的稀释用户需要稀释病毒时,请将病毒取出置于冰浴融解后,使用培养目的细胞用PBS或无血清培养基(含血清或含双抗不影响病毒感染)混匀分装后置于4℃保存(请尽量一周内用完)。
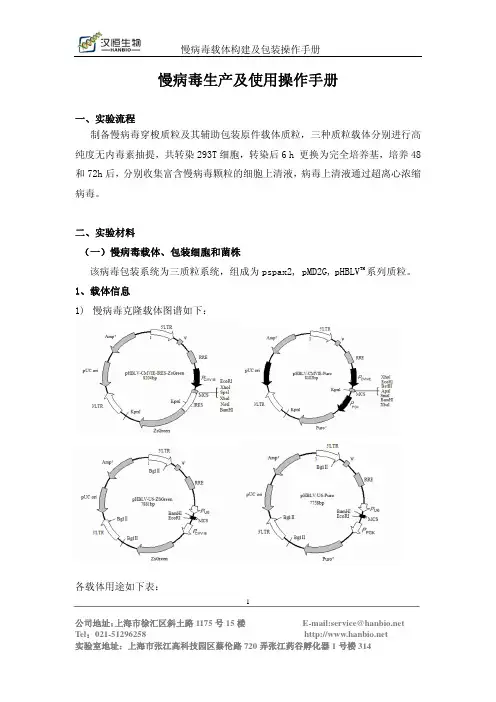
一、整体实验流程二、实验材料(一)慢病毒载体、包装细胞和菌株该病毒包装系统为三质粒系统,组成为psPAX2, pMD2.G, pHBLV TM系列质粒。
1、载体信息(见附表1)2、细胞株:我们采用293T作为慢病毒的包装细胞。
该细胞系为贴壁依赖型成上皮样细胞,生长培养基为DMEM+10% FBS+双抗。
慢病毒包装SOP
慢病毒包装标准操作细则1. 目的:规范慢病毒包装标准操作程序2. 范围:适用于慢病毒包装操作工序3. 操作程序3.1器材准备无菌10ml玻璃吸管、电动移液枪、,移液枪(量程1000μL)、10cm培养皿、各种枪头,1.5mL EP管、试管架,酒精灯、振荡混匀仪、荧光显微镜、CO2培养箱、生物安全柜3.2溶液准备1640培养基,Opti-MEM,Lipo转染试剂,293T细胞,重组慢病毒穿梭质粒,重组慢病毒骨架质粒(H1,H2)。
3.3操作步骤3.3.1转染前一天,用胰蛋白酶消化对数生长期的293T细胞,以含10%血清的培养基调整细胞密度为6x 106细胞,重新接种于10 cm细胞培养皿,37 ℃、5% CO2培养箱内培养。
24 h待细胞密度达~80%时即可用于转染。
细胞状态对于病毒包装至关重要,因此需要保证良好的细胞状态和较少的传代次数。
3.3.2第二天转染前用电动移液器将细胞培养基更换为无血清的1640培养基或是opti-MEM培养基。
3.3.3准备两个灭菌EP管,依次向一灭菌的EP管中加入10ug目的载体质粒,12ug pHelper 1.0质粒, 10ug pHelper 2.0质粒、相应体积的Opti-MEM培养基,总体积调整为500ul,轻轻混匀。
3.3.4在另一灭菌EP管中,加入460ul的Opti-MEM培养基和40ul Lipo转染试剂轻轻混匀,室温孵育5分钟。
3.3.5把两管含有载体的溶液与转染试剂的溶液进行混合,轻轻颠倒混匀,总体积为1ml,室温孵育20分钟。
将混合液滴加入细胞培养皿中,37 ℃, 5% CO2细胞培养箱中孵育6h~8 h。
3.3.6培养6-8 h后换液,弃去含有转染混和物的培养基,每盘细胞加入10 ml的10% FBS的1640培养基,于37 ℃, 5% CO2细胞培养箱中培养。
3.3.7转染24h后观察转染效率并拍照。
3.4清场3.4.1物品集中在废液桶中灭菌后清洗。
慢病毒生产及使用操作手册
慢病毒生产及使用操作手册一、实验流程制备慢病毒穿梭质粒及其辅助包装原件载体质粒,三种质粒载体分别进行高纯度无内毒素抽提,共转染293T细胞,转染后6 h 更换为完全培养基,培养48和72h后,分别收集富含慢病毒颗粒的细胞上清液,病毒上清液通过超离心浓缩病毒。
以下内容由汉恒生物科技(上海)有限公司精心整理总结。
二、实验材料(一)慢病毒载体、包装细胞和菌株该病毒包装系统为三质粒系统,组成为pspax2, pMD2G,pHBLV TM系列质粒。
1、载体信息(见附录)2、细胞株 293T,慢病毒的包装细胞,为贴壁依赖型成上皮样细胞,生长培养基为DMEM(含10% FBS)。
贴壁细胞经培养生长增殖形成单层细胞。
3、菌株大肠杆菌菌株DH5α。
用于扩增慢病毒载体和辅助包装载体质粒。
三、包装细胞293T细胞的培养(一) 293T细胞的冻存随着传代的次数增加,293T细胞会出现生长状态下降、突变等。
为了防止此类现象的出现,我们需要在开始就对细胞进行大量冻存,以保证实验的稳定性和持续性。
在细胞对数生长期进行冻存,增加细胞复苏成活率。
1、去掉上清液,加入PBS洗去残留的培养基;2、加入0.25%的胰酶,消化1~2min后,镜下观察细胞变圆,细胞间间隙加大时,去除胰酶,加入新鲜培养基吹打混匀,移入离心管中。
3、细胞计数,将细胞全部晃下,加入 3mL 37 ℃预热的 10% DMEM,用 10mL 移液管进行吹打,较大力吹打 6~8 次即可,不留死角,之后,将所有细胞吸出,置于15mL 离心管中,取 50ul 混匀后的细胞于 1.5mL eppendorf 管中,加入 450ul 10% DMEM,即为 10 倍稀释,混匀,取 10ul 细胞于计数板中计数。
计数板上共 4 大格,每大格 16 小格。
计数时,4 大格均计数,总数除以 4(得每大格细胞数),再乘以 10(10 倍稀释),即为实际 n万/mL 细胞浓度。
4、细胞离心,1000rpm,5min。
慢病毒载体的构建
慢病毒载体的构建一.构建原理:慢病毒属于逆转录病毒科, 但其基因组结构复杂, 除gag 、po l 和env 这3 个和单纯逆转录病毒相似的结构基因外, 还包括4 个辅助基因, vif 、vp r 、 nef 、vpu 和2 个调节基因tat 和rev 。
H IV 21 是慢病毒中最具特征性的病毒, 第一个慢病毒载体系统即以此病毒为基础进行构建的。
慢病毒载体的构建原理就是将H IV 21 基因组中的顺式作用元件(如包装信号、长末端重复序列) 和编码反式作用蛋白的序列进行分离。
载体系统包括包装成分和载体成分: 包装成分由H IV 21 基因组去除了包装、逆转录和整合所需的顺式作用序列而构建, 能反式提供产生病毒颗粒所需的蛋白; 载体成分与包装成分互补, 含有包装、逆转录和整合所需的H IV 21 顺式作用序列。
同时具有异源启动子控制下的多克隆位点及在此位点插入的目的基因。
为降低两种成分同源重组产生有复制能力的病毒(RCV ) 的可能性, 将包装成分的5′ L TR 换成巨细胞病毒(CMV ) 立即早期启动子, 3′ L TR 换成SV 40 po lyA 位点等。
将包装成分分别构建在两个质粒上, 即一个表达gag 和po l 、另一个表达env 。
二.实验操作举例:一)磷酸钙法转染HEK293T 细胞1. 试剂配制:无菌水ddw: 高温灭菌; 分装;2XHBS: 280mM NaCl10mM KCl1.5 mM Na2HPO412 mM glucose50 mM HEPESAdjust the pH , every 0.05pH from 7.00 to 7.45 using 10N NaOH, then add ddw. to the final volume. 过滤灭菌,分装;2M CaCl2: 过滤灭菌,分装.2. 实验过程:1. 铺细胞: 选择状态良好的293T 细胞传代, 2-3x105个细胞/35mm dish.2. 20-24h 后,待细胞长至铺满瓶底约50-70%的时候,进行转染.下面就35mm dish 为例,采用以下转染体系:ddw: 105ulplasmid: 2 ug (如0.5ug/ul, 即用4ul)2M CaCl2: 16.5ul2XHBS: 125ul按上述顺序,往eppendorf管中依次加入上述四种试剂.先将前三者混匀,最后加2XHBS.一种方法是,加2XHBS时要逐滴加入,吹打至微现乳白色,立即均匀滴入培养皿,轻轻摇匀后置于培养箱中,6-8h后换液.另一种方法是,加入2XHBS后立即吹打约40下(不过这个要根据每个人的力道和吹打的频率而定,建议做一个梯度实验,吹打不同的次数看哪次的转染效率高以后就按这个次数来吹打),之后步骤同上,立即均匀滴入培养皿,轻轻摇匀后置于培养箱中,6-8h后换液.二)转染方法2-Fugene转染病毒包装1.传293T细胞,将T75用明胶包被,每瓶T75加1.5-2mL明胶,置于37度培养箱10min。
慢病毒包装试剂盒说明书
YRGene 慢病毒包装试剂盒说明书产品编号:LPK010 产品规格:10个10cm 皿 产品简介:赢润生物的慢病毒包装试剂盒包括如下成分:(1) 优化配比的慢病毒包装辅助质粒混合物,可兼容大多数慢病毒表达载体。
(2) 高效转染试剂(Invitrogen Lip2000原装产品分装,293T 细胞转染效率接近 100%)。
(3 )高效率的慢病毒浓缩液,无需超速离心,也不需要价格昂贵的过滤柱,快速富集病毒粒子,其优 势在于操作安全简单,对设备要求低,产毒效率高,能够快速、高效地收获高滴度病毒。
产品组成:1. 转染前,传代 293FT 细胞于10cm 培养皿中(例如,接种 1 x 107细胞于10cm 培养皿中,使用完全 培养基DMEM+10%FBS培养),当细胞密度能够达到 90-95%即可进行转染。
Tips :培养基里面不要添加抗生素。
2. 转染前1-3小时,更换培养基,加入7ml 新鲜的完全培养基(DMEM+10%FBS ),注意不要添加抗生素。
3. 准备转染。
在5ml 离心管中,分别配制 A 管与B 管试剂(Tube A and Tube B )配好后,放置5min ,然后将A 管缓慢加入B 管,混合均匀。
室温放置20min ,使得脂质体-DNA 混合物形成。
Tips :混合后可能会出现淡淡的乳白状,不会影响转染。
然后将混合液逐滴均匀加入 10cm 培养皿,轻微混匀。
置于37C, 5% CO ?培养箱中培养过夜。
4. 第三天,更换培养基,加入 10ml 的完全培养基,同样注意不要加抗生素。
5. 转染后48-72h 后收取上清,转移至 15ml 离心管。
Tips :上清里面含有病毒,请小心操作。
6. 3000rpm 在4C 离心15min ,去除沉淀。
7. 上清液用0.45卩m 滤器过滤后转移到新的离心管中。
慢病毒浓缩:1. 每10ml 过滤后的病毒初始液,加入 Concen Solution 3ml ,每20-30min 混合一次,共进行 3-5次。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
过表达慢病毒载体构建和包装手册Version1.0吉凯基因二零一一年五月目录简介 (3)第一部分过表达慢病毒载体的制备实验流程 (4)实验材料 (5)过表达克隆制备 (6)第二部分慢病毒包装与滴度检测实验流程 (17)实验材料 (18)L e n t i v i r u s病毒包装 (21)病毒的收获及浓缩 (22)L e n t i v i r u s滴度测定 (24)参考文献 (33)简介慢病毒(Lentivirus)载体是以人类免疫缺陷型病毒(HIV)为基础发展起来的基因治疗载体,它对分裂细胞和非分裂细胞均具有感染能力,并可以在体内较长期的表达且安全性高。
吉凯基因提供的慢病毒为“自杀”性病毒,即病毒感染目的细胞后不会再感染其他细胞,也不会利用宿主细胞产生新的病毒颗粒。
慢病毒中的毒性基因已经被剔除并被外源性目的基因所取代,属于假型病毒。
但该病毒仍然具有可能的潜在的生物学危险,吉凯基因建议不要使用编码已知或可能会致癌的基因的假型病毒,除非已经完全公认某个基因肯定没有致癌性,否则均不建议采用假型病毒进行生物学实验。
吉凯基因慢病毒载体系统由GV慢病毒载体系列、pHelper 1.0载体和pHelper 2.0载体三质粒组成。
GV慢载体中含有HIV的基本元件5’LTR和3’LTR以及其他辅助元件,例如WRE (woodchuck hepatitis virus posttranscriptional regulatory element)。
通常根据不同的实验目的针对GV载体改造以进行基因功能研究。
pHelper 1.0载体中含有HIV病毒的gag基因,编码病毒主要的结构蛋白;pol基因,编码病毒特异性的酶;rev基因,编码调节gag和pol基因表达的调节因子。
pHelper 2.0载体中含有单纯疱疹病毒来源的VSV-G基因,提供病毒包装所需要的包膜蛋白。
吉凯基因过表达慢病毒产品可通过对GV慢病毒载体的改造和病毒包装,获得带有特定基因序列的慢病毒颗粒,以满足不同的实验需求。
本手册为吉凯基因RNAi慢病毒载体的构建和病毒包装的通用操作流程,目的是为了方便大家交流使用,部分细节内容未能做到一一详述,敬请谅解。
同时希望大家能够针对手册中的错误和问题,提出宝贵的意见。
第一部分过表达慢病毒载体的制备一、流程图从含有目的基因的质粒中,利用PCR方法钓取目的基因,将目的载体进行酶切,酶切产物电泳回收后进行交换,其产物转化细菌感受态细胞。
对长出的克隆先进行菌落PCR鉴定,再对PCR 鉴定阳性的克隆进行测序和比对分析,比对正确的即为构建成功的目的质粒。
二、实验材料描述a)主要试剂试剂名称试剂来源cat.No. 1kp DNA ladder Marker Fermentas公司#SM0311 250bp DNA ladder Marker 捷瑞公司DL250+,100T 琼脂糖赛百盛公司GA4-100 限制性内切酶NEB公司R0136VIn-Fusion™ PCR Cloning Kit clontech 639626 Taq polymerase SinoBio E001-02B dNTP Takara D4030APrimer 捷瑞生物Plasmid 抽提Kit Promega A1460b)主要仪器仪器名称仪器来源cat. No.PCR仪Applied Biosystems公司2720 thermalcyclerpositive clone 测序美季生物技术ABI3730型稳压DNA电泳仪BioRad公司凝胶成像仪天能公司细菌摇床华利达实验设备公司HI-9211K 细菌培养箱上海一恒科学仪器有限公司Gilson移液器吉尔森公司高速离心机日立公司TGL-16G-A三、过表达克隆制备——以STK39基因为例描述详细实验过程1.基因信息:Symbol:STK39Accession:NM_013233Organism:HumanDescription:Homo sapiens serine threonine kinase 39 (STK39), mRNA. 2.工具载体:3.载体酶切:酶切反应温度:37℃酶切反应时间:2 h酶切反应体系:1.1.1 载体酶切结果:1.2 目的基因片段的获取 1.2.1 引物引物说明:含交换配对碱基、酶切位点(下划线标记的),表达增强序列(双下划线记的)以及目的基因5’端部分序列用于PCR 钓取目的基因1.2.2 PCR 扩增目的基因片段PCR 反应体系:PCR 反应条件:94℃ 5 min 94℃ 30 sec 55℃ 30 sec 72℃ 2 min 72℃ 10 min 4℃ ∞1.2.3 PCR 结果30 cycle1.3重组质粒构建模式图1.3.1PCR产物交换入线性化表达载体反应条件:25℃30分钟→42℃15分钟反应体系:反应体系(µL): 阳性对照自连对照实验组dd H2O 17.5-X-Y 17.5-X 17.5-X-Y10 ×In-Fusion交换酶缓冲液 2 2 2In-Fusion交换酶0.5 0.5 0.5线性化载体DNA X X X纯化后PCR产物片段Y 0 YTotal 20 20 20说明:a)加入的线性化载体DNA和纯化的PCR产物的摩尔数比例为:1:3~1:9之间b)阳性对照加入的纯化的PCR产物为GAPDH基因(同样带有交换臂)1.3.2制备感受态细胞:配置下述溶液:1)0.1M CaCl2溶液,以0.22µm过滤除菌。
2)250mM KCl2溶液。
3)2M MgCl2溶液,高压灭菌。
4)SOB: 1ml 250mM KCl2溶液加入100ml LB,以5M NaOH 调PH值至7.0,高压灭菌,临用前加入0.5ml 2M MgCl2溶液。
用氯化钙制备新鲜的大肠杆菌感受态细胞1)从于37o C培养16小时的新鲜平板中挑取一个单菌落,转到一个含有100 ml LB培养基的1 L烧瓶中。
于37o C剧烈振摇培养3 小时(旋转摇床,300转/分)。
2)在无菌条件下将细菌转移到一个无菌、一次性使用的、用冰预冷的50 ml聚丙烯管中,在冰上放置10分钟,使培养物冷却至0o C。
3)于4 o C, 以4000转/分离心10分钟,回收细胞。
4)倒出培养液,将管倒置1分钟,使最后残留的痕量培养液流尽。
5)以10 ml用冰预冷的0.1 mol/L CaCl2重悬每份沉淀,放置于冰浴上。
6)于4o C,以4000转/分离心10分钟,回收细胞。
7)倒出培养液,将管倒置1分钟,使最后残留的痕量培养液流尽。
8)每50 ml初始培养物用2 ml用冰预冷的0.1 mol/L CaCl2重悬每份细胞沉淀。
9)将细胞分装成小份,放于-70o C冻存。
(感受态细胞制备参考:分子克隆实验指南第二版55页)1.3.3转化:1)用冷却的无菌吸头从每种感受态细胞悬液中各取200 µL转移到无菌的微量离心管中,每管加10 µL 连接液,轻轻旋转以混匀内容物,在冰中放置30分钟。
2)将管放到预加温到42℃的循环水浴中放好的EP管架上,恰恰放置90秒,不要摇动EP管架。
3)快速将管转移到冰浴中,使细胞冷却l—2分钟。
4)每管加800 µL LB培养基。
用水浴将培养基加温至37℃,然后将管转移到37℃摇床上,温育45分钟使细菌复苏。
5)将150 µL已转化的感受态细胞转移到AMP抗性(100ug/ml)的LB琼脂培养基上。
6)将平板置于室温直至液体被吸收。
7)倒置平皿,于37℃培养,16小时。
8)长出的克隆进行后续PCR鉴定。
(转化操作参考:分子克隆实验指南第二版55-56页)1.4阳性克隆的PCR鉴定1.4.1引物引物说明:本对引物一个位于目的基因中,一个位于载体上,用于菌落PCR 鉴定转化子1.4.2 PCR 鉴定PCR 反应体系:PCR 反应条件: 94℃ 3 min94℃ 30 sec60℃ 30 sec72℃ 30 sec72℃ 5 min4℃ ∞1.4.3 PCR 结果30 cycle样品说明:接种阳性转化子,37℃培养16小时后保存为甘油菌,分装200µL送测序1.5阳性克隆测序结果及结果分析:>ref|NM_016866.2| Mus musculus serine/threonine kinase 39, STE20/SPS1 homolog (yeast)(Stk39), mRNAgb|BC064443.1| Mus musculus serine/threonine kinase 39, STE20/SPS1 homolog (yeast),mRNA (cDNA clone MGC:69607 IMAGE:6843981), completecdsLength=3268GENE ID: 53416 Stk39 | serine/threonine kinase 39, STE20/SPS1 homolog (yeast) [Mus musculus] (Over 10 PubMed links)Score = 3083 bits (1669), Expect = 0.0Identities = 1669/1669 (100%), Gaps = 0/1669 (0%)Strand=Plus/PlusQuery 84 CATGGCGGAGCCGAGCGGCTCGCCTGTGCACGTCCAGCTTTCCCAGCA ggcggccccggt 143 |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| Sbjct 104 CATGGCGGAGCCGAGCGGCTCGCCTGTGCACGTCCAGCTTTCCCAGCAGGCGGCCCCGGT 163Query 144 gacagcggcggcggcgacggccccggcggccgcgacatcagcaccagccccggccccggc 203 |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| Sbjct 164 GACAGCGGCGGCGGCGACGGCCCCGGCGGCCGCGACATCAGCACCAGCCCCGGCCCCGGC 223Query 204 cccggctccggcggcgtccgcagccccggccccggctccagctgctgctccagccccggc 263 |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| Sbjct 224 CCCGGCTCCGGCGGCGTCCGCAGCCCCGGCCCCGGCTCCAGCTGCTGCTCCAGCCCCGGC 283Query 264 ccc GGCAGCTCAGGCGGTCGGCTGGCCCATCTGCAGGGACGCGTACGAGCTCCAGGAGGT 323 |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| Sbjct 284 CCCGGCAGCTCAGGCGGTCGGCTGGCCCATCTGCAGGGACGCGTACGAGCTCCAGGAGGT 343Query 324 TATCGGCAGCGGAGCAACTGCGGTGGTTCAGGCAGCCCTATGCAAACCCAGGCAAGAACG 383 |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| Sbjct 344 TATCGGCAGCGGAGCAACTGCGGTGGTTCAGGCAGCCCTATGCAAACCCAGGCAAGAACG 403Query 384 CGTAGCCATAAAGCGGATCAACTTGGAAAAATGCCAGACGAGTATGGATGAACTCCTGAA 443 |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| Sbjct 404 CGTAGCCATAAAGCGGATCAACTTGGAAAAATGCCAGACGAGTATGGATGAACTCCTGAA 463Query 444 AGAAATTCAAGCCATGAGCCAATGCAGCCATCCCAACGTAGTGACTTATTACACCTCCTT 503 |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| Sbjct 464 AGAAATTCAAGCCATGAGCCAATGCAGCCATCCCAACGTAGTGACTTATTACACCTCCTT 523Query 504 TGTGGTCAAAGATGAGCTGTGGCTGGTCATGAAATTACTAAGTGGAGGTTCCATGTTGGA 563 |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| Sbjct 524 TGTGGTCAAAGATGAGCTGTGGCTGGTCATGAAATTACTAAGTGGAGGTTCCATGTTGGA 583Query 564 TATCATCAAATACATCGTTAATCGAGGAGAACATAAAAATGGTGTCCTAGAAGAGGCGAT 623 |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| Sbjct 584 TATCATCAAATACATCGTTAATCGAGGAGAACATAAAAATGGTGTCCTAGAAGAGGCGAT 643Query 624 AATTGCAACAATTCTTAAGGAGGTTTTGGAAGGTTTAGACTATCTGCACAGAAACGGTCA 683 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 644 AATTGCAACAATTCTTAAGGAGGTTTTGGAAGGTTTAGACTATCTGCACAGAAACGGTCA 703Query 684 GATCCATAGGGATTTGAAAGCTGGAAATATTCTTCTGGGTGAGGATGGCTCAGTACAAAT 743 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 704 GATCCATAGGGATTTGAAAGCTGGAAATATTCTTCTGGGTGAGGATGGCTCAGTACAAAT 763Query 744 AGCAGATTTTGGAGTAAGCGCATTCTTAGCCACAGGGGGTGATGTTACACGGAATAAAGT 803 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 764 AGCAGATTTTGGAGTAAGCGCATTCTTAGCCACAGGGGGTGATGTTACACGGAATAAAGT 823Query 804 CAGAAAAACATTTGTTGGTACCCCATGTTGGATGGCCCCTGAAGTCATGGAACAGGTGAG 863 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 824 CAGAAAAACATTTGTTGGTACCCCATGTTGGATGGCCCCTGAAGTCATGGAACAGGTGAG 883Query 864 AGGCTATGACTTCAAGGCTGATATGTGGAGTTTTGGAATAACTGCCATTGAATTAGCAAC 923 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 884 AGGCTATGACTTCAAGGCTGATATGTGGAGTTTTGGAATAACTGCCATTGAATTAGCAAC 943Query 924 CGGAGCAGCGCCTTACCACAAATACCCTCCAATGAAAGTGCTAATGCTGACTTTGCAAAA 983 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 944 CGGAGCAGCGCCTTACCACAAATACCCTCCAATGAAAGTGCTAATGCTGACTTTGCAAAA 1003Query 984 TGACCCGCCCACTTTAGAAACTGGTGTAGAGGATAAAGAAATGATGAAAAAATACGGCAA 1043 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1004 TGACCCGCCCACTTTAGAAACTGGTGTAGAGGATAAAGAAATGATGAAAAAATACGGCAA 1063Query 1044 GTCCTTCAGAAAGTTACTGTCACTGTGTCTTCAGAAAGATCCTTCCAAAAGGCCCACAGC 1103 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1064 GTCCTTCAGAAAGTTACTGTCACTGTGTCTTCAGAAAGATCCTTCCAAAAGGCCCACAGC 1123Query 1104 AGCAGAACTTTTAAAATGCAAGTTCTTCCAGAAAGCCAAGAACAGAGAGTACCTGATCGA 1163 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1124 AGCAGAACTTTTAAAATGCAAGTTCTTCCAGAAAGCCAAGAACAGAGAGTACCTGATCGA 1183Query 1164 GAAGCTGCTGACAAGAACCCCAGACATAGCCCAAAGAGCCAAGAAGGTAAGGCGAGTTCC 1223 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1184 GAAGCTGCTGACAAGAACCCCAGACATAGCCCAAAGAGCCAAGAAGGTAAGGCGAGTTCC 1243Query 1224 TGGGTCGAGCGGTCACCTTCATAAGACTGAAGACGGCGACTGGGAGTGGAGCGATGACGA 1283 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1244 TGGGTCGAGCGGTCACCTTCATAAGACTGAAGACGGCGACTGGGAGTGGAGCGATGACGA 1303Query 1284 GATGGATGAGAAGAGCGAGGAGGGGAAGGCAGCCGCCTCCCAAGAGAAGTCACGAAGAGT 1343 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1304 GATGGATGAGAAGAGCGAGGAGGGGAAGGCAGCCGCCTCCCAAGAGAAGTCACGAAGAGT 1363Query 1344 AAAAGAAGAGAACTCAGAGATCTCGGTGAATGCTGGTGGCATCCCTGAGCAAATACAGTC 1403 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1364 AAAAGAAGAGAACTCAGAGATCTCGGTGAATGCTGGTGGCATCCCTGAGCAAATACAGTC 1423Query 1404 TCTCTCTGTGCACGACTCTCAGGCCCAACCAAACGCTAATGAAGACTACAGAGAAGGTCC 1463 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1424 TCTCTCTGTGCACGACTCTCAGGCCCAACCAAACGCTAATGAAGACTACAGAGAAGGTCC 1483Query 1464 TTGTGCAGTCAACCTTGTTTTAAGATTAAGAAACTCCAGAAAGGAACTTAATGACATACG 1523 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1484 TTGTGCAGTCAACCTTGTTTTAAGATTAAGAAACTCCAGAAAGGAACTTAATGACATACG 1543Query 1524 ATTTGAGTTTACTCCAGGAAGAGATACAGCAGACGGTGTGTCTCAGGAGCTCTTCTCTGC 1583 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1544 ATTTGAGTTTACTCCAGGAAGAGATACAGCAGACGGTGTGTCTCAGGAGCTCTTCTCTGC 1603Query 1584 TGGCTTGGTTGACGGCCATGATGTAGTTATAGTGGCTGCTAATTTACAGAAGATTGTAGA 1643 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1604 TGGCTTGGTTGACGGCCATGATGTAGTTATAGTGGCTGCTAATTTACAGAAGATTGTAGA 1663Query 1644 TGACCCCAAAGCTTTAAAAACGTTGACATTTAAGTTGGCTTCTGGCTGCGATGGGTCGGA 1703 ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1664 TGACCCCAAAGCTTTAAAAACGTTGACATTTAAGTTGGCTTCTGGCTGCGATGGGTCGGA 1723Query 1704 GATTCCTGATGAAGTGAAGCTGATCGGGTTCGCCCAGTTGAGTGTGAGC 1752|||||||||||||||||||||||||||||||||||||||||||||||||Sbjct 1724 GATTCCTGATGAAGTGAAGCTGATCGGGTTCGCCCAGTTGAGTGTGAGC 1772比对说明:Query是我们得到的测序结果;Sbjct是目标基因序列;Identities =100%,说明测序结果与目标序列完全一致第二部分慢病毒包装与滴度检测一、实验流程制备编码慢病毒颗粒的重组病毒质粒及其两种辅助包装原件载体质粒,三种质粒载体分别进行高纯度无内毒素抽提,按Invitrogen公司Lipofectamine 2000使用说明进行共转染293T 细胞,转染后8 h 更换为完全培养基,培养48h后,收集富含慢病毒颗粒的细胞上清液,对其浓缩后得到高滴度的慢病毒浓缩液,在293T细胞中测定并标定病毒滴度。
