Effects of Particle Size and Content of Silicon Powder on Strength and Microstructure of Coked A
激光粒度仪湿法测定粒径时粉体的分散方法

激光粒度仪湿法测定粒径时粉体的分散方法1.搅拌粉体样品并加入适量的溶剂,使其充分分散。
Mix the powder sample and add an appropriate amount of solvent to ensure full dispersion.2.使用超声波或搅拌器对粉体样品进行处理,以增加其分散性。
Use ultrasonication or a stirrer to treat the powder sample to enhance its dispersibility.3.确保搅拌过程中不产生气泡或振动,以防止分散效果的降低。
Ensure that no bubbles or vibrations are created during the stirring process to prevent the reduction of dispersion effects.4.使用适当的分散剂来增强粉体样品的分散性能。
Use appropriate dispersants to enhance the dispersibility of the powder sample.5.将分散后的样品放置一段时间,使其达到稳定状态。
Allow the dispersed sample to stand for a period of time to reach a stable state.6.避免在分散过程中引入过多的能量,以免影响后续的粒径检测结果。
Avoid introducing too much energy during the dispersion process to avoid affecting the subsequent particle size measurement results.7.在分散后及时进行粒度检测,以确保分散状态的准确性。
Conduct particle size measurements promptly after dispersion to ensure the accuracy of the dispersion state.8.对于难分散的样品,可以考虑采用特殊的分散技术来提高其分散效果。
碳酸钠、氢氧化钠与水玻璃复合激发对地聚物胶凝材料性能的影响

第43卷第3期2024年3月硅㊀酸㊀盐㊀通㊀报BULLETIN OF THE CHINESE CERAMIC SOCIETY Vol.43㊀No.3March,2024碳酸钠㊁氢氧化钠与水玻璃复合激发对地聚物胶凝材料性能的影响蒋明屾,李㊀飞,周理安,宁佳蕊,张㊀政(北京建筑大学,北京节能减排与城乡可持续发展省部共建协同创新中心,北京㊀100044)摘要:采用碳酸钠替代氢氧化钠调节水玻璃模数制备复合碱激发剂,研究不同碱掺量下碳酸钠掺入比例对地聚物胶凝材料净浆流动度㊁凝结时间及抗压强度的影响,并通过FT-IR㊁XRD 和SEM 试验分析地聚物胶凝材料水化产物的物相组成及微观形貌㊂结果表明,氢氧化钠与碳酸钠共同复合水玻璃的激发剂激发效果优于二者单独与水玻璃复合的激发剂,当碱掺量为6%(质量分数)㊁碳酸钠替代比例为40%(质量分数)时,地聚物胶凝材料净浆流动度为185mm,28d 抗压强度为94.4MPa㊂碳酸钠替代比例增加可延长地聚物胶凝材料凝结时间,当替代比例为100%时,地聚物胶凝材料初凝时间㊁终凝时间可达372和420min㊂不同碱组分激发剂作用时,地聚物胶凝材料水化产物相似,均以无定形铝硅酸盐C-(A)-S-H 凝胶为主㊂关键词:复合碱激发剂;地聚物;流动度;凝结时间;抗压强度中图分类号:TU528㊀㊀文献标志码:A ㊀㊀文章编号:1001-1625(2024)03-0929-09Effects of Sodium Carbonate ,Sodium Hydroxide and Water Glass Composite Activation on Properties of Geopolymer Cementitious MaterialsJIANG Mingshen ,LI Fei ,ZHOU Li an ,NING Jiarui ,ZHANG Zheng(Beijing Collaborative Innovation Center for Energy Saving and Emission Reduction and Urban-Rural Sustainable Development,Beijing University of Civil Engineering and Architecture,Beijing 100044,China)Abstract :Composite alkali activator was prepared by using sodium carbonate instead of sodium hydroxide to adjust the modulus of water glass.The effects of different alkali content and sodium carbonate replacement ratio on fluidity,setting time,and compressive strength of geopolymer cementitious materials were studied.The phase composition and microstructure of hydration products of geopolymer cementitious materials were analyzed through FT-IR,XRD,and SEM experiments.The results show that the combined effects of sodium hydroxide and sodium carbonate combined with composite water glass activators are superior to the effects of their individual combined with water glass activators.When alkali content is 6%(mass fraction)and the replacement ratio of sodium carbonate is 40%(mass fraction),the fluidity of geopolymer cementitious materials reaches 185mm,and 28d compressive strength reaches 94.4MPa.The increase of replacement ratio of sodium carbonate can prolong the setting time of geopolymer cementitious materials.When the replacement ratio reaches 100%,the initial setting time and final setting time of geopolymer cementitious materials reach372and 420min.When different alkali components are used as activators,similar hydration products are observed in geopolymer cementitious materials,mainly consist of amorphous aluminosilicate C-(A)-S-H gel.Key words :composite alkali activator;geopolymer;fluidity;setting time;compressive strength 收稿日期:2023-09-12;修订日期:2023-11-22基金项目:国家重点研发计划(2022YFC3803404);北京市西城区财经科技专项资助项目(XCSTS-TI2022-12)作者简介:蒋明屾(1999 ),男,硕士研究生㊂主要从事建筑材料㊁地聚物材料方面的研究㊂E-mail:jimish1999@通信作者:李㊀飞,博士,教授㊂E-mail:lifei@0㊀引㊀言随着城镇化建设的推进和基础设施的迅速发展,我国混凝土用量占世界年产量的一半以上,传统硅酸盐930㊀资源综合利用硅酸盐通报㊀㊀㊀㊀㊀㊀第43卷水泥作为制备混凝土的常用材料,其制备工业属于高消耗和高排放行业,硅酸盐水泥生产相关的CO2排放量占全球人为CO2排放量的5%~10%[1-2],降低水泥混凝土行业的碳排放是我国实现 双碳 目标的关键环节之一㊂近年来,地聚物胶凝材料因具有利废㊁节能㊁减碳等特点,受到高度关注,其制备工艺简单,无需高温煅烧,兼具良好的力学性能和耐久性能[3-5],极有可能成为替代水泥的绿色新型胶凝材料㊂地聚物胶凝材料常用的激发剂主要是水玻璃㊁氢氧化钠和碳酸钠,采用上述单一激发剂激发时效果均不理想,因此一般采用氢氧化钠调节水玻璃模数制备复合碱激发剂,但氢氧化钠-水玻璃复合碱激发剂存在凝结时间过快㊁碱度大㊁成本高等缺点,限制了其在实际工程领域的应用㊂碳酸钠是一种强碱弱酸盐,与氢氧化钠相比具有较低的pH值,价格低廉且更加环保,一些研究[6-9]表明在激发剂中引入碳酸钠有利于地聚物胶凝材料力学性能的发展,但也存在凝结硬化时间过长㊁强度发展非常缓慢等问题[10-11]㊂目前对于碳酸钠与氢氧化钠共同调节水玻璃模数制备复合碱激发剂的系统研究并不常见㊂氢氧化钠复合水玻璃激发地聚物存在凝结时间过短的问题,而碳酸钠激发地聚物存在凝结时间过长的问题,若采用水玻璃㊁氢氧化钠与碳酸钠混合作为激发剂可获得理想的凝结时间㊂本试验采用氢氧化钠与碳酸钠复合水玻璃制备碱激发剂,研究复合碱激发剂对地聚物胶凝材料净浆流动度㊁凝结时间及抗压强度的影响,借助FTIR㊁XRD与SEM微观测试技术进一步分析水化产物的组成及形貌,并对宏观性能作出解释㊂1㊀实㊀验1.1㊀原材料矿渣:市售S95矿渣粉,白色粉末,密度为2.89g/cm3,流动度为102%,烧失量为0.13%,7d活性指数为80%,28d活性指数为95%;根据化学组成计算[12],质量系数K=2.21,碱性系数M0=1.32,活性系数M a=0.45㊂粉煤灰:河南远恒环保工程有限公司生产的Ⅱ级粉煤灰,灰黑色粉末,密度为2.28g/cm3,需水量为97%,烧失量为2.86%,活性指数为77%㊂矿渣与粉煤灰的主要化学组成见表1,XRD谱见图1,粒径分布见图2㊂表1㊀矿渣与粉煤灰的主要化学组成Table1㊀Main chemical composition of slag and fly ashMaterial Mass fraction/%CaO SiO2Al2O3MgO Fe2O3TiO2SO3K2O Na2O MnO Other Slag46.4227.8812.43 6.790.42 1.45 2.710.700.550.370.28 Fly ash 5.5252.9928.750.78 5.85 1.280.71 2.760.680.100.58图1㊀矿渣与粉煤灰的XRD谱Fig.1㊀XRD patterns of slag and fly ash激发剂采用水玻璃㊁氢氧化钠㊁碳酸钠,试验用水玻璃为浙江省嘉兴市嘉善县优瑞耐火材料有限公司生第3期蒋明屾等:碳酸钠㊁氢氧化钠与水玻璃复合激发对地聚物胶凝材料性能的影响931㊀产的钠水玻璃,无色透明黏稠液体,技术指标见表2㊂氢氧化钠为片状NaOH,纯度不小于98%,碳酸钠为颗粒状Na 2CO 3,纯度不小于99.8%㊂图2㊀矿渣与粉煤灰的粒径分布Fig.2㊀Particle size distribution of slag and fly ash表2㊀水玻璃的技术指标Table 2㊀Technical indicators of water glassModulus Na 2O content /%SiO 2content /%Solid content /%Concentration /ʎBéDensity /(g㊃cm -3)2.2513.7529.9943.5050 1.5㊀㊀Note:%represents mass fraction.1.2㊀配合比本试验固定水玻璃模数M s =1.2(即激发剂中SiO 2与Na 2O 物质的量之比为1.2),采用Na 2CO 3替代NaOH 调节水玻璃模数至1.2,替代比例为0%~100%(质量分数),碱掺量为4%㊁6%㊁8%(按激发剂中总Na 2O 质量占地聚物胶凝材料质量百分比计)㊂胶凝材料中矿渣与粉煤灰的质量比为4ʒ1,激发剂溶液配合比见表3,水胶比为0.36,附加水质量为水胶比计算所得用水量减去各激发剂中的水含量㊂表3㊀激发剂溶液的配合比Table 3㊀Mix ratio of activator solutionSample No.Alkali content /%Na 2CO 3replacement ratio /%Na 2CO 3content /g NaOH content /g Water glass content /g Additional water content /g J4-0J4-20J4-40J4-60J4-80J4-10040012.0420 3.199.6340 6.387.23609.57 4.828012.77 2.4110015.96077.56133.66J6-0J6-20J6-40J6-60J6-80J6-10060018.0620 4.7914.45409.5710.846014.367.238019.15 3.6110023.940116.34110.48J8-0J8-20J8-40J8-60J8-80J8-10080024.0820 6.3819.274012.7714.456019.159.638025.53 4.8210031.910155.1387.31932㊀资源综合利用硅酸盐通报㊀㊀㊀㊀㊀㊀第43卷1.3㊀试验方法试验前1d 配制激发剂溶液,按表3称取每组试验所需氢氧化钠和碳酸钠与附加水充分搅拌溶解,冷却至室温,再将碱溶液与原水玻璃溶液混合,搅拌至溶液不再分层,用保鲜膜密封静置24h㊂试验时按原材料比例称取粉煤灰㊁矿渣,在搅拌锅中均匀混合后加入激发剂溶液,搅拌4min,其中慢搅2min,中间停歇15s,再快搅2min㊂搅拌结束后参照‘混凝土外加剂匀质性试验方法“(GB /T 8077 2012)㊁‘水泥标准稠度用水量㊁凝结时间㊁安定性检测方法“(GB /T 1346 2011)进行流动度㊁初凝时间和终凝时间测试㊂成型试件尺寸为40mm ˑ40mm ˑ40mm,在标准养护条件下养护至测试龄期,采用YAW-300液压机进行抗压强度测试,加载速率为2400N /s㊂使用德国ZEISS Gemini SEM 300扫描电子显微镜㊁岛津XRD-610衍射仪㊁Thermo Nicolet iS5红外光谱分析仪分析水化产物形貌及物相组成,试件养护至测试龄期后放入无水乙醇终止水化,微观测试前对样品进行烘干㊁研磨处理㊂2㊀结果与讨论2.1㊀流动度图3㊀复合碱激发剂对地聚物胶凝材料流动度的影响Fig.3㊀Effect of composite alkali activator on fluidity of geopolymer cementitious materials 图3为复合碱激发剂对地聚物胶凝材料流动度的影响㊂由图3可见,不同碱掺量下,地聚物净浆流动度均随碳酸钠比例增加呈先增大后减小的趋势,当碳酸钠替代比例为40%时,净浆流动度最大,为175~185mm,当碳酸钠替代比例由80%提升至100%时,净浆流动度显著下降㊂这是由于Na 2CO 3水解产生的CO 2-3与体系中的Ca 2+反应生成CaCO 3沉淀,覆盖在未水化颗粒表面起到润滑作用从而提高流动性㊂然而,当碳酸钠掺量较高时,在反应过程中由于同离子效应,液相中会生成Na 2CO 3㊃10H 2O,导致浆体黏度增大,流动性降低[6]㊂碳酸钠掺量相同时,净浆流动度随碱掺量的增加均呈先增大后减小的趋势㊂当碱掺量为6%时,流动度最大㊂这是因为OH -的极性作用能够加速地聚物中玻璃体Si O 和Al O 的解聚,促进粉煤灰㊁矿渣溶解从而减小颗粒间内摩擦力,提高净浆流动性[13]㊂当碱掺量过高时,较高的溶液浓度以及早期反应中产生的较多凝胶是导致浆体黏度增大㊁流动度降低的主要因素[14]㊂2.2㊀凝结时间保持碱掺量6%不变,碳酸钠掺量对地聚物胶凝材料凝结时间的影响如图4所示㊂地聚物胶凝材料初凝㊁终凝时间均随碳酸钠掺量的增加而延长㊂当碳酸钠替代比例从0%增至80%时,地聚物初凝时间从19min 延长至28min,仅延长了9min,终凝时间从25min 延长至38min,仅延长了13min,碳酸钠的缓凝效果并不明显㊂此时激发剂中氢氧化钠占据主导作用,OH -浓度较高时能加速矿渣溶解,同时抑制CO 2-3参与反应[4]㊂当替代比例提升至100%时,浆体初凝时间㊁终凝时间激增,高达372和420min㊂补充碳酸钠替代比例75%㊁85%㊁90%㊁95%四组配比,以便更清晰地反映碳酸钠掺量对地聚物凝结时间的影响,如图4所示,碳酸钠对地聚物凝结时间的影响依然为连续变化㊂碳酸钠具有缓凝作用主要是因为CO 2-3会优先与体系中的Ca 2+结合生成CaCO 3,阻碍C-(A)-S-H 凝胶的形成,延缓地聚物凝结硬化进程[8,15]㊂同时,大量CO 2-3的存在会降低SiO 4-4参与化学反应的程度,起到缓凝效果[16]㊂保持碳酸钠替代比例40%不变,碱掺量对地聚物胶凝材料凝结时间的影响如图5所示㊂地聚物胶凝材料初凝时间随碱掺量增加线性递增㊂当碱掺量从4%增加至8%时,地聚物胶凝材料初凝时间从10min 增加至35min,当碱掺量为4%和6%时,地聚物胶凝材料终凝时间没有明显差别,均为28min,当碱掺量为8%时,终凝时间延长至43min㊂这是由于随着碱掺量的增加,地聚物中低聚合度前驱体受抑制程度加深,延缓了体系中硅铝酸盐解聚㊁聚合过程[17],这与Hadi 等[18]㊁王玲玲等[19]研究结果一致㊂第3期蒋明屾等:碳酸钠㊁氢氧化钠与水玻璃复合激发对地聚物胶凝材料性能的影响933㊀图4㊀碳酸钠掺量对地聚物胶凝材料凝结时间的影响Fig.4㊀Effect of sodium carbonate content on setting time of geopolymer cementitiousmaterials 图5㊀碱掺量对地聚物胶凝材料凝结时间的影响Fig.5㊀Effect of alkali content on setting time of geopolymer cementitious materials2.3㊀抗压强度地聚物胶凝材料抗压强度随碳酸钠替代比例增加的变化趋势如图6所示㊂由图6可知,随着碳酸钠掺量增加,不同碱掺量地聚物胶凝材料的3㊁7d 抗压强度均呈下降趋势,当碱掺量为8%㊁碳酸钠替代比例0%时,3㊁7d 抗压强度达到最大值,分别为66.2和76.3MPa㊂随着碳酸钠掺量增加,地聚物胶凝材料均呈先升高后降低的趋势,当碳酸钠掺量为4%时,28d 抗压强度在碳酸钠替代比例为20%时达到最大值,为85.2MPa;当碳酸钠掺量为6%与8%时,28d 抗压强度在碳酸钠替代比例为40%时达到最大值,分别为94.4和93.9MPa㊂图6㊀不同碱掺量下碳酸钠替代比例对地聚物胶凝材料抗压强度的影响Fig.6㊀Effect of sodium carbonate replacement ratio on compressive strength of geopolymer cementitious materials with different alkalicontent 图7㊀地聚物胶凝材料3㊁28d 的抗压强度比Fig.7㊀Compressive strength ratio 3and 28d ofgeopolymer cementitious materials 地聚物胶凝材料3㊁28d 强度比如图7所示㊂当碳酸钠替代比例为0%时,即使用氢氧化钠与水玻璃复合激发时,地聚物胶凝材料早期强度增长迅速,后期强度增长缓慢,3d 抗压强度可以达到28d 抗压强度的63.6%~80.1%㊂当碱掺量为8%时,28d 抗压强度82.6MPa 相比于3d 抗压强度66.2MPa,仅提升了24.8%㊂当碳酸钠替代比例100%时,即用碳酸钠与水玻璃复合激发时,3d 抗压强度为28d 抗压强度的44.9%~60.1%㊂对比可知当体系中引入碳酸钠时,地聚物早期强度发展缓慢,后期强度发展相对迅速㊂当碱掺量为4%时,3d 抗压强度为28.1MPa,28d 抗压强度为62.7MPa,抗压强度增长率高达123.1%㊂934㊀资源综合利用硅酸盐通报㊀㊀㊀㊀㊀㊀第43卷碱浓度对地聚物胶凝材料早期强度发展起到决定作用,加入碳酸钠会使体系中pH值降低,削弱激发剂对地聚物中玻璃体结构的解聚效果,延长水化进程,导致强度发展缓慢㊂同时,较长的凝结硬化时间有利于C-A-S-H的形成,促使强度随龄期进一步发展[9]㊂当碳酸钠替代比例较高时,反应会存在一段时间的休眠期[20],早期水化产物大多为不同形态的碳酸钙,如方解石和文石以及少量的单斜钠钙石,CO2-3浓度成为控制反应进程的主要因素㊂随着水化进程发展,单斜钠钙石积累和水滑石形成会不断消耗CO2-3,导致体系中CO2-3浓度下降,逐渐释放Ca2+与[SiO4]4-结合形成C-(A)-S-H凝胶[21],促进硬化体强度发展㊂不同碱掺量时,28d强度最大值均出现在三种激发剂复合作用的情况下,这可能是因为在氢氧化钠-水玻璃激发体系中加入适量的碳酸钠参与孔溶液化学反应有助于优化水泥石结构,减小孔隙从而提升强度㊂2.4㊀物相组成图8(a)㊁(b)为地聚物胶凝材料养护水化3㊁28d后的FTIR谱㊂使用不同激发剂时,地聚物胶凝材料水化产物具有较高的相似度㊂1414~1488cm-1处的双峰和870cm-1附近的吸收带是由不同水化产物中O C O(CO2-3)非对称拉伸和平面外弯曲振动引起的[22],3d时随碳酸钠含量增大,特征峰强度显著提高,养护㊁研磨过程中样品的碳化与风化导致J6-0中也存在该特征峰㊂980~1020cm-1附近的吸收峰对应C-(A)-S-H中SiO4四面体的Si O伸缩振动㊂水玻璃或NaOH激发的地聚物C-(A)-S-H凝胶Si O Si伸缩带一般在950~1000cm-1,随着碳酸钠含量提高,该特征峰向高波数移动,表明C-(A)-S-H中Si含量相对较高[23],水化产物聚合度增大㊂450cm-1处的吸收峰归属于Si O Si的平面弯曲振动,这与体系中无定形铝硅酸盐沸石结构有关[24-25]㊂1650cm-1附近的微弱 峰包 与硬化体中化学结合水H O H弯曲振动有关㊂图8㊀地聚物胶凝材料水化3㊁28d的FTIR谱Fig.8㊀FTIR spectra of geopolymer cementitious materials hydration for3and28d 图9(a)㊁(b)为地聚物胶凝材料水化3㊁28d后的XRD谱㊂XRD谱整体呈弥散状驼峰,表明地聚物胶凝材料的水化产物多为无定形凝胶,结晶性较差㊂反应早期矿渣溶解释放的Ca2+优先与孔隙溶液中的CO2-3结合,少量Ca2+与[SiO4]4-结合,主要水化产物为CaCO3㊁C-(A)-S-H凝胶㊂随着碳酸钠含量增加,CaCO3衍射峰强度明显提高,单斜钠长石(Na2Ca(CO3)2㊃5H2O)与钠沸石(Na12Al12Si12O48-n H2O)衍射峰开始出现㊂CO2-3对Ca2+的消耗促使钠沸石中Si和Al达到饱和[8]㊂水化28d时,可以明显观察到单斜钠长石与钠沸石衍射峰强度降低,水滑石(Mg6Al2(OH)16CO3㊃4H2O)衍射峰开始出现㊂一些学者[8,26]认为,单斜钠长石与钠沸石在水化产物中的存在形式并不稳定,随龄期发展会进一步向C-(A)-S-H㊁水滑石㊁钙沸石(CaAl2Si7O18㊃n H2O)等稳定产物进行转化,对比图9(a)㊁(b)可证明反应前㊁后期产物发生转变㊂此外,石英与莫来石的衍射峰来自体系中未水化的粉煤灰㊂2.5㊀微观形貌使用J6-0㊁J6-40㊁J6-100激发时,地聚物胶凝材料3㊁28d的SEM照片如图10所示㊂使用不同激发剂激发水化3d后,体系中均存在未反应的粉煤灰颗粒,随着激发剂中碳酸钠含量增多,早期水化产物㊁硬化体结构逐渐由致密变得松散,28d水化产物整体呈块状㊂仅使用氢氧化钠与水玻璃复合激发时,3d出现较为致㊀第3期蒋明屾等:碳酸钠㊁氢氧化钠与水玻璃复合激发对地聚物胶凝材料性能的影响935密的块状凝胶结构,28d水化结构与3d相差不大,证明水化反应主要发生在早期㊂当使用碳酸钠与水玻璃复合激发时,早期水化产物极为疏松,甚至可以观察到未反应的矿渣,所以此时地聚物胶凝材料强度较低㊂水化28d后,在硬化体表面可以观察到不规则晶体,这与詹疆淮等[27]观察到的三维无定形类沸石结构铝硅酸盐凝胶类似,产物结构相对致密㊂三种激发剂复合作用时,28d硬化体结构非常致密,微裂缝数量减少,裂缝宽度明显减小,从微观层面进一步解释了该组地聚物胶凝材料抗压强度更高的原因㊂图9㊀地聚物胶凝材料3㊁28d的XRD谱Fig.9㊀XRD patterns of geopolymer cementitious materials for3and28d图10㊀地聚物胶凝材料3㊁28d的SEM照片Fig.10㊀SEM images of geopolymer cementitious materials for3and28d936㊀资源综合利用硅酸盐通报㊀㊀㊀㊀㊀㊀第43卷3㊀结㊀论1)地聚物胶凝材料净浆流动度随碱掺量㊁碳酸钠含量增加均呈先增大后减小的趋势㊂当碱掺量为6%㊁碳酸钠替代比例为40%时,净浆流动度达到最大值,为185mm㊂2)地聚物胶凝材料凝结时间随碱掺量增加而增大㊂当碳酸钠替代比例为0%~80%时,碳酸钠含量对地聚物胶凝材料凝结时间的影响并不显著㊂当替代比例为80%~100%时,地聚物胶凝材料凝结时间随碳酸钠含量增加显著提高㊂当替代比例为100%时,地聚物胶凝材料初凝时间㊁终凝时间可达372和420min㊂3)地聚物胶凝材料3㊁7d抗压强度均随碱掺量增加而提高,随碳酸钠含量增加而降低㊂28d抗压强度相差不大,碱掺量为6%与8%时略大于碱掺量为4%时,且随着碳酸钠含量增加先提高后降低㊂当碱掺量为6%㊁碳酸钠替代比例为40%时,28d抗压强度达到最大值,为94.4MPa㊂4)不同碱组分激发地聚物胶凝材料水化产物相似㊂氢氧化钠复合水玻璃作为激发剂时,不同龄期水化产物均以无定形铝硅酸盐C-(A)-S-H凝胶为主㊂碳酸钠复合水玻璃作为激发剂时,早期水化产物中含有大量碳酸钙并出现单斜钠长石与钠沸石,后期水化产物中单斜钠长石与钠沸石含量降低,出现水滑石㊁钙沸石以及C-(A)-S-H凝胶㊂参考文献[1]㊀SCRIVENER K L,KIRKPATRICK R J.Innovation in use and research on cementitious material[J].Cement and Concrete Research,2008,38(2):128-136.[2]㊀黄㊀华,郭梦雪,张㊀伟,等.粉煤灰-矿渣基地聚物混凝土力学性能与微观结构[J].哈尔滨工业大学学报,2022,54(3):74-84.HUANG H,GUO M X,ZHANG W,et al.Mechanical property and microstructure of geopolymer concrete based on fly ash and slag[J].Journal of Harbin Institute of Technology,2022,54(3):74-84(in Chinese).[3]㊀吴小缓,张㊀杨,袁㊀鹏,等.地质聚合物的研究进展与应用[J].硅酸盐通报,2016,35(12):4032-4037.WU X H,ZHANG Y,YUAN P,et al.Research progress and applications of geopolymer[J].Bulletin of the Chinese Ceramic Society,2016, 35(12):4032-4037(in Chinese).[4]㊀LI N,FARZADNIA N,SHI C J.Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation[J].Cement andConcrete Research,2017,100:214-226.[5]㊀ZHANG P,ZHENG Y X,WANG K J,et al.A review on properties of fresh and hardened geopolymer mortar[J].Composites Part B:Engineering,2018,152:79-95.[6]㊀王㊀新.碳酸钠对碱矿渣水泥性能的影响研究[D].重庆:重庆大学,2016.WANG X.Study on the influence of sodium carbonate on the properties of alkali slag cement[D].Chongqing:Chongqing University,2016(in Chinese).[7]㊀LI N,SHI C J,ZHANG Z H.Understanding the roles of activators towards setting and hardening control of alkali-activated slag cement[J].Composites Part B:Engineering,2019,171:34-45.[8]㊀BERNAL S A,PROVIS J L,MYERS R J,et al.Role of carbonates in the chemical evolution of sodium carbonate-activated slag binders[J].Materials and Structures,2015,48(3):517-529.[9]㊀BERNAL S A,NICOLAS R S,VAN DEVENTER J S J,et al.Alkali-activated slag cements produced with a blended sodium carbonate/sodiumsilicate activator[J].Advances in Cement Research,2016,28(4):262-273.[10]㊀CHEAH C B,TAN L E,RAMLI M.The engineering properties and microstructure of sodium carbonate activated fly ash/slag blended mortarswith silica fume[J].Composites Part B:Engineering,2019,160:558-572.[11]㊀DURAN ATIŞC,BILIM C,ÇELIKÖ,et al.Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar[J].Construction and Building Materials,2009,23(1):548-555.[12]㊀董㊀刚.粉煤灰和矿渣在水泥浆体中的反应程度研究[D].北京:中国建筑材料科学研究总院,2008.DONG G.Study on reaction degree of fly ash and slag in cement paste[D].Beijing:China Building Materials Academy,2008(in Chinese).[13]㊀殷素红,管海宇,胡㊀捷,等.碱激发粉煤灰-矿渣灌浆材料的流变性与流动性[J].华南理工大学学报(自然科学版),2019,47(8):120-128+135.YIN S H,GUAN H Y,HU J,et al.Rheological properties and fluidity of alkali-activated fly ash-slag grouting material[J].Journal of South China University of Technology(Natural Science Edition),2019,47(8):120-128+135(in Chinese).[14]㊀王丽娟,刘玉娟.碱激发矿渣/粉煤灰体系流动性及力学性能试验研究[J].矿业研究与开发,2022,42(6):141-147.WANG L J,LIU Y J.Experimental study on the fluidity and mechanical properties of alkali-activated slag/fly ash system[J].Mining Research㊀第3期蒋明屾等:碳酸钠㊁氢氧化钠与水玻璃复合激发对地聚物胶凝材料性能的影响937 and Development,2022,42(6):141-147(in Chinese).[15]㊀SHI C J,DAY R L.A calorimetric study of early hydration of alkali-slag cements[J].Cement and Concrete Research,1995,25(6):1333-1346.[16]㊀FERNÁNDEZ-JIMÉNEZ A,PUERTAS F.Effect of activator mix on the hydration and strength behaviour of alkali-activated slag cements[J].Advances in Cement Research,2003,15(3):129-136.[17]㊀SHI Z G,SHI C J,WAN S,et al.Effect of alkali dosage and silicate modulus on carbonation of alkali-activated slag mortars[J].Cement andConcrete Research,2018,113:55-64.[18]㊀HADI M N S,ZHANG H Q,PARKINSON S.Optimum mix design of geopolymer pastes and concretes cured in ambient condition based oncompressive strength,setting time and workability[J].Journal of Building Engineering,2019,23:301-313.[19]㊀王玲玲,司晨玉,李㊀畅,等.氢氧化钾-钠水玻璃激发剂对碱激发矿渣胶凝材料性能的影响[J].硅酸盐通报,2022,41(8):2654-2662+2695.WANG L L,SI C Y,LI C,et al.Effect of potassium hydroxide-sodium water glass activator on properties of alkali-activated slag cementitious materials[J].Bulletin of the Chinese Ceramic Society,2022,41(8):2654-2662+2695(in Chinese).[20]㊀YUAN B,YU Q L,BROUWERS H J H.Reaction kinetics,reaction products and compressive strength of ternary activators activated slagdesigned by Taguchi method[J].Materials&Design,2015,86:878-886.[21]㊀YUAN B,YU Q L,BROUWERS H J H.Time-dependent characterization of Na2CO3activated slag[J].Cement and Concrete Composites,2017,84:188-197.[22]㊀ISHWARYA G,SINGH B,DESHWAL S,et al.Effect of sodium carbonate/sodium silicate activator on the rheology,geopolymerization andstrength of fly ash/slag geopolymer pastes[J].Cement and Concrete Composites,2019,97:226-238.[23]㊀PALACIOS M,PUERTAS F.Effect of carbonation on alkali-activated slag paste[J].Journal of the American Ceramic Society,2006,89(10):3211-3221.[24]㊀HUANG G D,YANG K,SUN Y H,et al.Influence of NaOH content on the alkali conversion mechanism in MSWI bottom ash alkali-activatedmortars[J].Construction and Building Materials,2020,248:118582.[25]㊀FERNÁNDEZ-JIMÉNEZ A,PALOMO A.Mid-infrared spectroscopic studies of alkali-activated fly ash structure[J].Microporous andMesoporous Materials,2005,86(1/2/3):207-214.[26]㊀ABDALQADER A F,JIN F,AL-TABBAA A.Development of greener alkali-activated cement:utilization of sodium carbonate for activating slagand fly ash mixtures[J].Journal of Cleaner Production,2016,113:66-75.[27]㊀詹疆淮,李宏波,傅㊀博,等.不同碱当量㊁粉煤灰和矿渣掺量对碱激发粉煤灰-矿渣地聚物力学性能及微观结构的影响[J].科学技术与工程,2021,21(28):12218-12224.ZHAN J H,LI H B,FU B,et al.Effect of different alkali equivalent,fly ash and slag content on the mechanical properties and microstructure of alkali-activated fly ash-slag geopolymer[J].Science Technology and Engineering,2021,21(28):12218-12224(in Chinese).。
欧盟禁止使用二氧化钛文件

II(Non-legislative acts)REGULATIONSCOMMISSION REGULATION (EU) 2022/63of 14 January 2022amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of theCouncil as regards the food additive titanium dioxide (E 171)(Text with EEA relevance)THE EUROPEAN COMMISSION,Having regard to the Treaty on the Functioning of the European Union,Having regard to Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008on food additives(1), and in particular Article 10(3) thereof,Having regard to Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings(2), and in particular Article 7(5) thereof,Whereas:(1)Annex II to Regulation (EC) No 1333/2008 lays down a Union list of food additives approved for use in foods andtheir conditions of use.(2)Annex III to Regulation (EC) No 1333/2008 lays down a Union list of food additives approved for use in foodadditives, food enzymes, food flavourings, nutrients and their conditions of use.(3)Titanium dioxide (E 171) is a substance authorised as a colour in certain foods, in accordance with Annex II toRegulation (EC) No 1333/2008.(4)Pursuant to Article 3(1) of Regulation (EC) No 1331/2008, the Union list of food additives may be updated either onthe initiative of the Commission or following an application.(5)Article 32(1) of Regulation (EC) No 1333/2008 provides that all food additives that were already permitted in theUnion before 20 January 2009are subject to a new risk assessment by the European Food Safety Authority (‘the Authority’).(6)On 14 September 2016, the Authority published a scientific opinion on the re-evaluation of the safety of titaniumdioxide (E 171) as a food additive(3)concluding that the margins of safety calculated in the opinion would not be of concern. Nevertheless, the Authority recommended additional toxicological testing, an extended 90-day study or a multigeneration or extended-one generation reproduction toxicity study according to the current OECD guidelines, in order to be able to establish a health-based guidance value (acceptable daily intake – ADI) for titanium dioxide (E 171). The Authority also recommended amendments to the Union specifications for titanium dioxide (E 171) by introducing a characterisation of the particle size distribution and the percentage of particles in the nanoscale present in titanium dioxide (E 171) used as a food additive, and revising the maximum limits for impurities of toxic elements.(1)OJ L 354, 31.12.2008, p. 16.(2)OJ L 354, 31.12.2008, p. 1.(7)On 30 January 2017, the Commission launched a public call for scientific and technological data on titaniumdioxide (E 171), targeting the data needs identified in the scientific opinion on the re-evaluation of this substance asa food additive.(8)On 2 October 2017and 29 June 2018, in view of the recommendations made by the Authority, business operatorsmade a proposal for the amendment of the specifications for titanium dioxide (E 171) and submitted the necessary data. On 7 August 2018, the Commission requested the Authority to provide a scientific opinion on whether the data provided adequately support the proposed amendment of the specifications for titanium dioxide (E 171). (9)On 12 July 2019, the Authority published a scientific opinion on the proposed amendments of the specifications fortitanium dioxide (E 171) used as a food additive. The Authority concluded on additional parameters related to particle size distribution to be included in the specifications and recommended a revision of the definition of the food additive titanium dioxide (E 171) in the Union specifications. The Authority also concluded that, based on the proposed change in the specifications, revisiting the toxicological database on titanium dioxide (E 171) as a food additive should consequently be conducted in line with the data requirements specified in the 2018 ‘Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain’(4).(10)On 6 March 2020, the Commission requested the Authority to assess the safety of the food additive titanium dioxide(E 171), taking into account the proposed amendments of the specifications, the data from an extended onegeneration reproductive study submitted by a consortium of interested business operators in reply to the public call for data launched in 2017 as well as all new relevant data available since the completion of the re-evaluation of titanium dioxide (E 171) in 2016, including the data considered to be in line with the data requirements specified in the 2018 Guidance on nanotechnology.(11)On 6 May 2021, the Authority published a scientific opinion on the safety assessment of titanium dioxide (E 171) asa food additive(5). In light of the opinion on the proposed amendments of the specifications and following the 2018Guidance on nanotechnology, the opinion also takes into account, in addition to all new relevant data, the data on the potential genotoxicity of titanium dioxide nanoparticles published before 2016, that had not previously been identified as relevant for the 2016 re-evaluation. In its opinion the Authority indicated that, based on all the evidence available, a concern for genotoxicity could not be ruled out, and given the many uncertainties, it concluded that titanium dioxide (E 171) can no longer be considered safe when used as a food additive. The Authority neither identified nor recommended any new studies that could alleviate the genotoxicity concern and other remaining uncertainties.(12)In light of the conclusion of the 2021 Authority’s opinion about the safety of titanium dioxide (E 171) when used asa food additive, it is appropriate to remove the authorisation to use titanium dioxide (E 171) in foods. Accordingly,titanium dioxide (E 171) may no longer be used in foods. As titanium dioxide (E 171) would no longer be authorised for use in foods, it is also appropriate to remove the reference to it from the entry on the use of potassium aluminium silicate (E 555) as a carrier laid down in Part 1 of Annex III to Regulation (EC) No 1333/2008.(13)However, given that the Authority did not identify an immediate health concern linked to titanium dioxide (E 171)used as a food additive and in order to allow for a smooth transition, it is appropriate that foods that contain titanium dioxide (E 171) used in accordance with the rules applicable before the date of entry into force of this Regulation may be placed on the market until six months after that date. Those foods may then continue to be marketed until their date of minimum durability or ‘use by’ date.(4)EFSA Journal 2018;16(7):5327.(14)Directive 2009/35/EC of the European Parliament and of the Council(6)restricts the use of colours in human andveterinary medicinal products to those authorised in accordance with Regulation (EC) No 1333/2008 on food additives, for which specifications are laid down in Commission Regulation (EU) No 231/2012(7). Uses of excipients other than colours in medicinal products are subject to the Union rules on medicinal products and are evaluated as part of the overall benefit risk profile of a medicinal product.(15)In response to a request from the Commission, the European Medicines Agency (EMA) provided on 8 September2021a scientific analysis on the technical purpose of the use of titanium dioxide (E 171) in medicinal products, the feasibility of replacement and possible timeframes for alternatives. In its conclusions, EMA indicated that titanium dioxide is mainly used in medicinal products as a colour and opacifier, even if it has multiple functions. It also stressed that titanium dioxide is used frequently in a number of essential medicinal products in oral-solid and oral semi-solid dosage forms. EMA also stressed that, from a technical point of view, it should be possible to find alternatives to replace titanium dioxide (E 171)-containing coatings, both as colour and for other uses. However, it also underlined that its feasibility is not confirmed at this stage, as replacing titanium dioxide (E 171) would impact in a negative manner the quality, safety and efficacy of medicinal products. EMA highlighted the need to carefully assess alternatives, notably to ensure their compatibility with the various components of individual pharmaceutical products. The replacement of titanium dioxide (E 171) in authorised medicinal products would require an individual review and assessment, possibly requiring bioequivalence studies. Furthermore, EMA concluded that it is difficult at this stage to recommend a precise transition period timeframe for the replacement of titanium dioxide (E 171) used in medicinal products, as the time needed to reformulate each individual product could take several years, depending on the complexity of reformulation and studies required. Finally, considering the scale of the use of this excipient and the volume of products impacted, and taking into account global supply chains, EMA stressed that a requirement to replace titanium dioxide (E 171) would almost certainly cause significant medicines shortages on the Union market.(16)On the basis of the EMA scientific analysis, and in order to avoid shortages of medicinal products that could haveimpacts on public health, titanium dioxide (E 171) should remain provisionally on the list of authorised additives to allow its use in medicinal products as a colour, pending the development of adequate alternatives to replace it while ensuring the quality, safety and efficacy of the medicinal products concerned. During this time, titanium dioxide (E 171) should however be included in the list of colours that may not be sold directly to consumers.(17)It is of critical importance that the pharmaceutical industry makes any possible efforts to accelerate the research anddevelopment of alternatives that would be used as a replacement for titanium dioxide (E 171) in medicinal products, and to submit the necessary variation to the terms of the marketing authorisations concerned. In the absence of such efforts, competent authorities may request the concerned stakeholders to submit objective and verifiable reason explaining the non-feasibility of the replacement.(18)The Commission is committed to review the necessity to maintain titanium dioxide (E 171) or otherwise delete itfrom the Union list of food additives for exclusive use as a colour in medicinal products within three years after the date of entering into force of this Regulation. This review should be based on an updated assessment of the EMA to be performed before 1 April 2024. It should take into account the progress made during this period to develop alternatives to titanium dioxide (E 171) in medicinal products both for new products and for replacing it in authorised products, and possible impacts on quality, safety and efficacy, as well as on the availability of medicinal products. Where replacement of titanium dioxide (E 171) in medicinal products has not taken place or been initiated within this period, only objective verifiable reasons related to the lack of feasibility of its replacement should be taken into account.(19)Annexes II and III to Regulation (EC) No 1333/2008 should therefore be amended accordingly.(20)The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee onPlants, Animals, Food and Feed,(6)Directive 2009/35/EC of the European Parliament and of the Council of 23 April 2009 on the colouring matters which may be addedto medicinal products (OJ L 109, 30.4.2009, p. 10).(7)Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and IIIHAS ADOPTED THIS REGULATION:Article 1Annexes II and III to Regulation (EC) No 1333/2008 are amended in accordance with the Annex to this Regulation.Article 2Until 7 August 2022, foods produced in accordance with the rules applicable before 7 February 2022may continue to be placed on the market. After that date, they may remain on the market until their date of minimum durability or ‘use by’ date.Article 3The Commission shall, following consultation on the European Medicines Agency, review the necessity to maintain titanium dioxide (E 171) or to delete it from the Union list of food additives for the exclusive use as colour in medicinal products in Part B of Annex II to Regulation (EC) No 1333/2008 within three years after the date of entering into force of this Regulation.Article 4This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.This Regulation shall be binding in its entirety and directly applicable in all Member States.Done at Brussels, 14 January 2022.For the CommissionThe PresidentUrsula VON DER LEYENANNEX1.Annex II to Regulation (EC) No 1333/2008 is amended as follows:(a)in point 2 of Part A, point 5 is replaced by the following:‘5.The colours E 123, E 127, E 160b(i), E 160b(ii), E 161g, E 171, E 173 and E 180 and mixtures thereof may not be sold directly to the consumer.’(b)in Part B, point 1 ‘Colours’ is amended as follows:1.the entry for the food additive E 171 (Titanium dioxide) is replaced by the following:2.the following footnote (**) is added after footnote (*):‘(**)Titanium dioxide is not authorised in the food categories listed in Part D and E. The substance is in list B1 because it is used in medicinal products in accordance with Directive 2009/35/EC of the EuropeanParliament and of the Council (OJ L 109, 30.4.2009, p. 10).’;(c)in Part C, point (2) ‘Group II: Food colours authorised at quantum satis’, the entry for the food additive E 171(Titanium dioxide) is deleted;(d)Part E is amended as follows:1.In category 04.2.4.1 (Fruit and vegetable preparations excluding compote), the entry concerning the foodadditive E 171 (Titanium dioxide) is deleted;2.In category 09.2 (Processed fish and fishery products including molluscs and crustaceans), the three entriesconcerning the food additive E 171 (Titanium dioxide) are deleted;2.In Part 1 of Annex III to Regulation (EC) No 1333/2008, the entry concerning the food additive E 555 (Potassiumaluminium silicate) is replaced by the following:。
阻燃脱醇型RTV-1硅酮密封胶的制备及性能

Preparation and properties of flame retardant dealcohilized room temperature vulcanized one componentsilicone sealantA Dissertation Submitted for the Degree of MasterCandidate:Duan YuSupervisor:Prof. Ma WenshiMr. Zhong Hanrong(Senior Engineer)South China University of TechnologyGuangzhou, China摘要单组份室温硫化(RTV-1)硅酮密封胶因其优异的粘结密封性、对基材无腐蚀性、无臭味、在苛刻环境中能保持良好的物理性能及电性能,且使用方便,被广泛地应用于建筑、电子电气、航天航空、汽车工业、医药卫生等领域。
但RTV-1硅酮密封胶本身易燃,遇明火即会燃烧。
本论文选用两种价廉易得的无卤阻燃剂氢氧化铝(Al(OH)3)、铝硅酸盐,并通过阻燃剂之间的复配、协同阻燃作用,制备了综合性能良好的脱醇型RTV-1硅酮密封胶。
采用万能材料试验机对阻燃密封胶的力学性能进行了测试。
通过极限氧指数、垂直燃烧、锥形量热仪和热失重分析等方法分析了阻燃密封胶的阻燃性能。
采用加速老化的方法分析了阻燃密封胶的贮存稳定性,并使用扫描电子显微镜(SEM)分析了阻燃剂在密封胶基体中的分散状况。
具体的研究内容和成果包括:第一,选用Al(OH)3作阻燃剂,考察了其粒径、用量对阻燃密封胶的硫化速度、硬度、力学性能、阻燃性能、贮存稳定性及热稳定性的影响。
结果表明,Al(OH)3粒径越小,密封胶的力学性能及阻燃性能越好,但贮存稳定性能变差,综合考虑,本课题选用粒径为5μm的Al(OH)3。
随着Al(OH)3用量的增加,密封胶的极限氧指数不断提高,垂直燃烧等级先提高后不变,硫化速度变快,硬度增加,拉伸强度先增加后降低,断裂伸长率下降。
[整理]particle详解.
![[整理]particle详解.](https://img.taocdn.com/s3/m/5b8f2724e45c3b3566ec8b4a.png)
Effects神奇的粒子特效系统,Particle Playground(粒子特效)和水波特效(Wave World)、散焦特效(Caustics)、泡沫特效(Foam)、爆炸特效(Shatter)同属于Simulation特效系统。
其中的Particle Playground(粒子特效)可以产生大量相似物体独立运动的模拟效果。
在今天我们来了解After Effects神奇的粒子特效系统,Particle Playground(粒子特效)和水波特效(Wave World)、散焦特效(Caustics)、泡沫特效(Foam)、爆炸特效(Shatter)同属于Simulation特效系统。
其中的Particle Playground (粒子特效)可以产生大量相似物体独立运动的模拟效果。
在自然界中存在很多个体独立而整体相似的物体运动,我们可以通过该粒子特效系统模拟这种符合自然规律的运动,比如雪花、雨点等都可以是我们模拟的对象。
象这种相互之间既有又有制约,整体相似个体不同的物质我们称之为粒子。
Particle Playground (粒子特效)特效可以从物理学和数学上对它们进行描述来模拟生产真实的粒子运动效果,比如纷飞的大雪,飘落的大雨等。
After Effects的粒子特效系统十分强大,我们在这里只对它进行一些入门级的介绍。
启动After Effects 6.5,新建一个项目文件,然后打开“Composition/New Composition”,新建一个Composition合成文件。
大小为720×576,其他选项默认即可。
然后打开“Layer/New/Solid”新建一个固态层,准备将来在这里显示粒子特效。
好了,现在可以增加粒子特效了。
选中Solid层,打开“Effect/Simulation/Particle Playground”菜单选项,就可以把粒子特效加入到图层中了。
默认情况下,你会在预览窗口中看到红色的粒子,按下小键盘上的0键预览,粒子就会以每秒100粒的速度朝窗口的顶部发射红色的粒子。
均质压力及喷雾干燥温度对鱼油微胶囊化的影响

2012年 第 37卷 第 10期 FOOD SCIENCE AND TECHNOLOGY
食品开发
均质压力及喷雾干燥温度对 鱼油微胶囊化的影响
刘盛楠,刘书来,丁玉庭* (浙江工业大学生物与环境工程学院,杭州 310014)
摘要:采用辛烯基琥珀酸酯淀粉Hi-Cap100和葡萄糖浆作为鱼油微胶囊的壁材。研究了不同均质 压力下乳化液黏度、粒径和粒径分布规律,考察了不同均质压力下乳化液特性与鱼油微胶囊包 埋率、表面油含量之间的相关性,探讨了喷雾干燥温度对包埋率和鱼油过氧化值(POV)的影响。 研究结果表明,随着均质压力的增加,乳化液黏度和平均粒径逐渐减小,而粒径分布离散度总 体呈下降趋势,在40 MPa时最小,说明此时粒径分布均一性最佳;喷雾干燥温度增加时,鱼油 包埋率先增后减,POV值先减后增,在进/出口温度为140 ℃/70 ℃时有最高的包埋率和最低的 POV值。通过分析确定最佳工艺参数如下,均质压力为40 MPa,喷雾干燥进口温度为140 ℃, 出口温度为 70 ℃。 在上述最适工艺条件下 , 鱼油微胶囊平均粒径为 5.97 μ m , 表面油含量为 2.03%,微胶囊化包埋率为95.6%,在扫描电镜下观察微胶囊表面和内部结构良好,具有良好的 包埋效果。 关键词:鱼油;微胶囊化;均质压力;喷雾干燥法;包埋率 中图分类号:TS 225.2+4 文献标志码:A 文章编号:1005-9989(2012)10-010-06
Influence of homogenization pressure and spray drying temperature on microencapsulated fish oil
LIU Sheng-nan1, LIU Shu-lai2 , DING Yu-ting1*
国外期刊英文论文
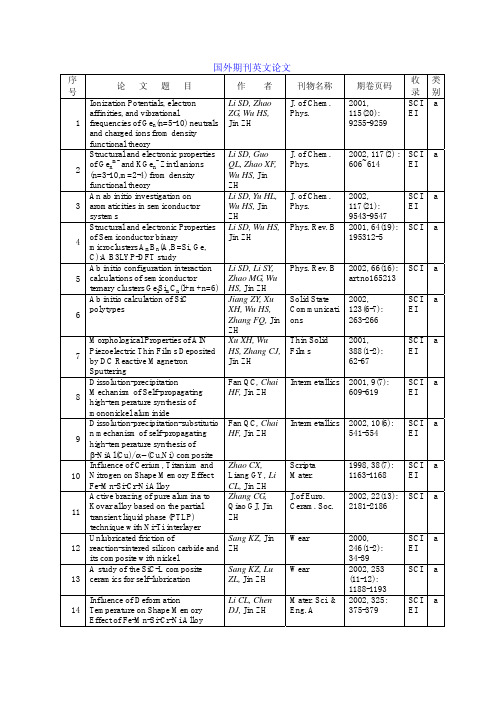
Influence of Deformation
Li CL, Chen Mater. Sci. & 2002, 325: SCI a
14 Temperature on Shape Memory
DJ, Jin ZH Eng. A
375-379
EI
Effect of Fe-Mn-Si-Cr-Ni Alloy
Wang TJ
Comparison between fatigue
Qiao GJ,
Int. J. Fatigue 2002, 24(5): SCI a
17
behavior of some ceramics: a new concept of intrinsic stress-corrosion
Wang HJ, Jin ZH
Ding HF, Jin
258-264
EI
ZH
The interfacial stability of the
Tang WM,
Mater. Chem. 2002, 77:
SCI a
21 coated-SiC/Fe couple
Zheng ZX,
Phys.
Ding HF, Jin
236-241
EI
ZH
31
Unlubricated wear of Si/SiC and its composite with nickel Si/SiC-Ni
Sang KZ, Jin ZH
Tribology Int.
2001, 34(5): SCI a
315 -319
EI
Effects of crystalline morphology Xu T, Yu J, Jin Mater.
AE常用particular粒子中英文对照表

Particular界面
(默认设置)
Emitter(发射器)
Particular/sec (粒子数量/秒)—100—每秒钟发射粒子的数量。
Velocity Distribution(速度分布)—0.5—
Velocity from Motion[%](继承运动速度【%】)20
——粒子在向外运动的同时也会跟随发射器的运动方向运动。
Emitter Size X(发射器尺寸X )50
Emitter Size Y (发射器尺寸Y )50
Emitter Size Z (发射器尺寸Z)50
Glow(辉光)
Size尺寸300
Opacity不透明度25
Feather羽化100
Transfer Mode(应用模式)——Glow与粒子的叠加模式
Normal普通
Add相加
Screen屏幕
Streaklet(条纹)——条状痕
Random Seed(随机种子)
No Streaks(无条纹)—7—条痕数,条状痕由几个粒子组成
粒子会向整个区域的百分之几运动。(即粒子发射方向有多宽)
X Rotation(X旋转)0x0
X Rotation (Y旋转)0x0
X Rotation (Z旋转)0x0
Velocity(速率)—100—粒子每秒钟运动的像素数。
Velocity Random[%](随机运动【%】)20
——每个粒子Velocity的随机性会随机增加或者减小每个粒子的Velocity。
