Dabigatran_DataSheet_MedChemExpress
ML324_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:ML324 is a potent JMJD2 demethylase inhibitor with demonstrated antiviral activity.IC50 value: 920 nM(JMJD2E) [1]Target: JMJD2 demethylase inhibitorML324 is a probe molecule that displays submicromolar inhibitory activity toward JMJD2E (in vitro) and possesses excellent in vitro ADME properties. In contrast to previously reported inhibitors of the JMJD proteins, ML324 displays excellent cell permeabilityproviding an opportunity for more extensive cell–based studies of JMJD2 enzymes to be undertaken. In addition, ML324 demonstrates potent anti–viral activity against both herpes simplex virus (HSV) and human cytomegalovirus (hCMV) infection via inhibition viral IE gene expression. ML324 suppresses the formation of HSV plaques, even at high MOI, and blocks HSV–1 reactivation in a mouse ganglia explant model of latently infected mice.PROTOCOL (Extracted from published papers and Only for reference)JMJD2E qHTS FDH Assay [1]:Enzyme and buffer solutions (3 μL) were dispensed into a 1,536–well Greiner black solid–bottom assay plate. The library compounds (23 nL) were transferred using a Kalypsys pintool equipped with 1,536–pin array. The plate was incubated at room temperature (15min), and then a 1 μL aliquot of substrate solution was added to initiate the reaction. The plate was transferred to ViewLux imager where an initial reading using standard UV optics (Ex 340 nm, Em 450 nm) was obtained. The plate was then removed from the reader,incubated for 30 minutes at room temperature, and returned to the reader for a second fluorescence reading. A fully automated robotic screening system (Kalypsys Inc, San Diego, CA) was used to perform the above steps as described previously. Compound plates containing DMSO as a vehicle–only control were included at regular interval throughout the screen to monitor any systematic trend in the assay signal associated with reagent dispenser variation or decreases in enzyme specific activity. For activity calculations,percent values were computed as the difference in fluorescence intensity between last and first time points. The percentage activity was calculated from the median values of the catalyzed, or neutral control, and the uncatalyzed, or 100% inhibited, control,respectively, using in–house software.Inhibition of viral infection in cell culture [1]:Cells were treated with DMSO, LSD1 inhibitor (TCP, tranylcypromine, Sigma P8511), or JMJD2 inhibitorsand infected with HSV–1 or hCMV as described below. cDNA was produced from total RNA and quantitated using an ABI 7900HT (ABI SDS 2.3 Software). Viral yields were determined by titration.References:Product Name:ML324Cat. No.:HY-12725CAS No.:1222800-79-4Molecular Formula:C 21H 23N 3O 2Molecular Weight:349.43Target:Histone Demethylase Pathway:Epigenetics Solubility:DMSO: ≥ 33 mg/mL[1]. Rai G, et al. Discovery of ML324, a JMJD2 demethylase inhibitor with demonstrated antiviral activity.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Dabrafenib_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Dabrafenib is an ATP–competitive inhibitor of BRAF with IC 50s of 5 nM and 0.6 nM for CRAF and BRAF V600E , respectively.IC50 & Target: IC50: 0.6 nM (BRAF V600E ), 5 nM (CRAF)[4]In Vitro: Dabrafenib (GSK2118436, 1 μM) with 0.01 μM GSK1120212 inhibits more than 90% of cell growth in the NRAS mutant clones. GSK2118436 is sufficient to reduce S6P phosphorylation in A375[1]. Dabrafenib suppresses the PolyP–mediated vascular barrier permeability, upregulation of inflammatory biomarkers, adhesion/migration of leukocytes, and activation and/or production of nuclear factor–κB, tumor necrosis factor–α, and interleukin–6[2]. Dabrafenib inhibits the release of HMGB1 and downregulates HMGB1–dependent inflammatory responses by enhancing the expressions of cell adhesion molecules (CAMs) in human endothelialcells [3].In Vivo: Dabrafenib–treated females have mostly immature reproductive tracts with no evidence of ovulation, similar toage–matched controls; however, DAB–treated females have keratinized and histologically open vaginas [5].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[1]For longer term proliferation assays, cells are plated and treated with compound or combination of compounds inRMPI–1640 containing 10% FBS for 12 days. Compound treatments are replaced at least once during the assay. After 12 days, cells are stained with 0.5% methylene blue in 50% ethanol. Images are captured using flatbed scanner.Animal Administration: Dabrafenib is formulated as a suspension in vehicle, 0.5% hydroxypropylmethylcellulose K15M, and 0.1%(v/v) Tween80 in purified water.[5]The rat pups selected as the test system are derived from 26 10–week–old, time–mated,virus–antibody–free SD (Crl:CD[SD]) female rats. Mated females are observed for natural deliveries from Day 20 to 23 pc (dayparturition completed is designated PND 0). Litter examinations are conducted when parturition is complete, on PNDs 3 and 6, and included gender identification, individual pup weights, and external morphologic examinations. Parturient dams and their litters are selected for study based on clinical signs and body weights, and selected dams and their litters are randomized into study groups based on clinical observations and PND 3 litter mean body weights. On PND 3 or 4, litters are culled to four males and five females,with minimal fostering only when necessary to obtain the desired sex ratio, such that natural litters are maintained as much aspossible. Records are kept of fostered pups of original and foster dams. All pups are identified by paw tattoo. To the extent possible,nonlittermates are assigned to subsets. DAB is formulated as a suspension in vehicle, 0.5% hydroxypropylmethylcellulose K15M, and 0.1% (v/v) Tween80 in purified water, and is given to juvenile male and female rats orally by gavage at a dose volume of 5 ml/kg,based on daily body weight.References:[1]. Greger JG, et al. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib,Product Name:Dabrafenib Cat. No.:HY-14660CAS No.:1195765-45-7Molecular Formula:C 23H 20F 3N 5O 2S 2Molecular Weight:519.56Target:Raf Pathway:MAPK/ERK Pathway Solubility:DMSO: ≥ 33 mg/mLmediated by NRAS or MEK mutations. Mol Cancer Ther, 2012, 11(4), 909–920.[2]. Lee S, et al. Anti–inflammatory effects of dabrafenib on polyphosphate–mediated vascular disruption. Chem Biol Interact. 2016 Jul 22.[3]. Jung B, et al. Anti–septic effects of dabrafenib on HMGB1–mediated inflammatory responses. BMB Rep. 2016 Apr;49(4):214–9.[4]. Alexander M Menzies, et al. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des Devel Ther. 2012; 6: 391–405.[5]. Posobiec LM, et al. Early Vaginal Opening in Juvenile Female Rats Given BRAF–Inhibitor Dabrafenib Is Not Associated with Early Physiologic Sexual Maturation. Birth Defects Res B Dev Reprod Toxicol. 2015 Dec;104(6):244–52.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Emricasan-DataSheet-MedChemExpress
Hale Waihona Puke DescriptionEmricasan is an irreversible pan-caspase inhibitor.
IC₅₀ & Target
Caspase
In Vivo
Emricasan (IDN-6556) is orally active that is retained in the liver for prolonged period of time. TUNEL-positive cells are considerably increased by five-fold in mice fed a HFD and are reduced under Emricasan treatment. In accordance with this observation caspase-3 and -8 are increased in HFD-fed mice by 1.5- and 1.3-fold respectively and are significantly decreased by Emricasan treatment[1].When comparing efficacy by multiple routes of administration, Emricasan is administered i.p., p.o., i.m., or i.v. (0.03-3 mg/kg). Caspase 3-like activities, measured as DEVD-AMC cleavage, dose dependently decreased with a 92.5% reduction after the highest dose of Emricasan (3 mg/kg). Emricasan is initially tested in the α-Fas model of liver injury, marked hepatocellular apoptosis, and peak ALT activities
L-DABA-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-08-2018Print Date:Oct.-08-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :L-DABACatalog No. :HY-101414CAS No. :1758-80-11.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:L-2,4-Diaminobutyric acidFormula:C4H10N2O2Molecular Weight:118.13CAS No. :1758-80-14. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Tozasertib_639089-54-6_DataSheet_MedChemExpress

Product Name:Tozasertib CAS No.:639089-54-6Cat. No.:HY-10161Product Data SheetMWt:464.59Formula:C23H28N8OS Purity :>98%Solubility:DMSO ≥90mg/mL Watery Mechanisms:Biological Activity:Tozasertib (MK 0457;VX 680)is the inhibitor of Aurora A B C kinases with Ki values of 0618Pathways:Cell Cycle/DNA Damage; Target:Aurora Kinase g <1.2mg/mL Ethanol <1.2mg/mLTozasertib (MK-0457; VX-680) is the inhibitor of Aurora-A, -B, -C kinases with Ki values of 0.6, 18,4.6 nM respectively.IC50 Value: 0.6 nM(Ki for Aurora A); 18 nM(Ki for Aurora B); 4.6 nM(Ki for Aurora C)Target: Aurora Kinase in vitro: VX-680 induces similar cytotoxicity with IC50 of approximately 300 nM and exhibits an AUR B-like inhibitory phenotype of G2/M arrest, endoreduplication and apoptosis in BaF3 cells transfected with ABL or FLT-3 (mutant and wild type) kinases. VX-680 prevents the CAL-62proliferation in a time-dependent manner. VX-680 treatment for 14 days significantly decreases the number and size of colonies by approximately 70% in the 8305C and 90% in the CAL-62, 8505C References:[1]. Harrington EA, Bebbington D, Moore J, et al. VX-680, a potent and selective smallmoleculeinhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004; 10:262-7.[2]. Salah E, Ugochukwu E, Barr AJ, von Delft F, Knapp S, Elkins JM.Crystal structures of ABL-y pp yand BHT-101. Treatment of the different ATC cells with VX-680 inhibits proliferation with the IC50between 25 and 150 ?nM. The VX-680 significantly impairs the ability of the differe...[]g pp yrelated gene (ABL2) in complex with imatinib, tozasertib (VX-680), and a type I inhibitor of thetriazole carbothioamide class.J Med Chem. 2011 Apr 14;54(7):2359-67. Epub 2011 Mar 18.[3]. Oliveira TM, Ahmad R, Engh RA.VX680 binding in Aurora A: π-π interactions involving the conserved aromatic amino acid of the flexible glycine-rich loop.J Phys Chem A. 2011 Apr28;115(16):3895-904. Epub 2011 Feb 9.[4]. Li Y, Zhang ZF, Chen J, Huang D, Ding Y, Tan MH, Qian CN, Resau JH, Kim H, Teh BT.VX680/MK-0457, a potent and selective Aurora kinase inhibitor, targets both tumor and endothelia...Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
Estrone_53-16-7_DataSheet_MedChemExpress

Product Name:Estrone CAS No.:53-16-7Cat. No.:HY-B0234Product Data SheetMWt:270.37Formula:C18H22O2Purity :>98%Solubility:DMSO 55 mg/mL; Water <1 mg/mL y Mechanisms:Biological Activity:Estrone is an estrogenic hormonePathways:Others; Target:Estrogen Receptor/ERR g gEstrone is an estrogenic hormone.Target: Estrogen Receptor/ERR Estrone (E1) is an estrogenic hormone secreted by the ovary as well as adipose tissue with the chemical name of 3-hydroxyestra-1,3,5(10)-triene-17-one and the chemical formula C18H22O2.Estrone is one of several natural estrogens, which also include estriol and estradiol. Estrone is the least abundant of the three hormones; estradiol is present almost always in the reproductive female body, and estriol is abundant primarily during pregnancy. Estrone is relevant to health and disease states because of its conversion to estrone sulfate, a long-lived derivative. Estrone sulfate acts as areservoir that can be converted as needed to the more active estradiol. It is the predominantReferences:[1]. Hoffmann, B., et al., [Profiles of estrone, estrone sulfate and progesterone in donkey (Equusasinus) mares during pregnancy]. Tierarztl Prax Ausg G Grosstiere Nutztiere, 2014. 42(1): p. 32-9.[2]. Oneson, I.B. and S.L. Cohen, The nature of the conjugated estrone in human pregnancy urine.pestrogen in postmenopausal women [1, 2]. ...Endocrinology, 1952. 51(3): p. 173-82.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
Emricasan_254750-02-2_DataSheet_MedChemExpress

Product Name:Emricasan CAS No.:254750-02-2Cat. No.:HY-10396Product Data SheetMWt:569.50Formula:C26H27F4N3O7Purity :>98%Solubility:DMSOMechanisms:Biological Activity:Emricasan (IDN 6556PF 03491390)is a potent irreversible pan caspase inhibitor Pathways:Apoptosis; Target:CaspaseEmricasan (IDN-6556, PF-03491390) is a potent irreversible pan-caspase inhibitor.IC50 Value: 10 mg/kg was required to prevent liver injury [1]Target: pan-caspase in vitro: Three caspase inhibitors (IDN-8066, IDN-1965, and IDN-6556) effectively attenuated SEC apoptosis and caspase 3 activation. The most potent inhibitor, IDN-6556, reduced SEC apoptosis and caspase 3 activity by 55% and 94%, respectively. Prevention of SEC apoptosis byIDN-6556 was not reduced when this agent was administered only during the cold preservation period [1]. in vivo: In the mouse alpha-Fas model of liver injury, i.p. administration of IDN-6556 resulted inmarked reduction of alanine aminotransferase (ALT), apoptosis, and caspase activities at a dose ofReferences:[1]. Natori, S., et al., The caspase inhibitor IDN-6556 prevents caspase activation and apoptosis insinusoidal endothelial cells during liver preservation injury. Liver Transpl, 2003. 9(3): p. 278-84.[2]. Hoglen, N.C., et al., Characterization of IDN-6556 (3-[2-(2-tert-butyl-phenylaminooxalyl)-amino]-()p p p 3 mg/kg. At this dose, IDN-6556 was also effective when given up to 2 h before alpha-Fas and as late as 4 h after alpha-Fas administratio...[]g ,,,([(y p y y )]propionylamino]-4-oxo-5-(2,3,5,6-te trafluoro-phenoxy)-pentanoic acid): a liver-targeted caspaseinhibitor. J Pharmacol Exp Ther, 2004. 309(2): p. 634-40.[3]. McCall, M., et al., The caspase inhibitor IDN-6556 (PF3491390) improves marginal massengraftment after islet transplantation in mice. Surgery, 2011. 150(1): p. 48-55.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
达比加群酯杂质
达比加群酯杂质
湖北扬信医药科技有限公司
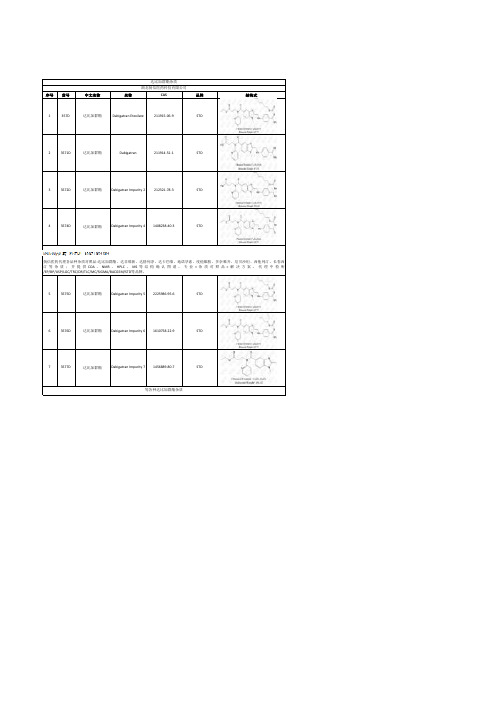
序号货号中文名称名称CAS品牌结构式
1357D达比加群酯Dabigatran Etexilate211915-06-9STD
23571D达比加群酯Dabigatran211914-51-1STD
33572D达比加群酯Dabigatran Impurity 2212321-78-3STD
43574D达比加群酯Dabigatran Impurity 41408238-40-3STD
扬信医药代理各品种杂质对照品:达比加群酯、达非那新、达格列净、达卡巴嗪、地诺孕素、度他雄胺、多奈哌齐、厄贝沙坦、西他列汀、长春西汀等杂质;并提供COA、NMR、HPLC、MS等结构确认图谱。
专业<杂质对照品>解决方案,代理中检所/EP/BP/USP/LGC/TRC/DR/TLC/MC/SIGMA/BACGEM/STD等品牌。
53575D达比加群酯Dabigatran Impurity 52225986-95-6STD
63576D达比加群酯Dabigatran Impurity 61610758-22-9STD
73577D达比加群酯Dabigatran Impurity 71456889-80-7STD
等各种达比加群酯杂质。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Dabigatran(BIB–953; BIBR 953ZW) is a reversible and selective, direct thrombin inhibitor (DTI) with Ki value of 4.5 nM.
IC50 Value: 4.5 nM (Ki); 10 nM(Thrombin–induced platelet aggregation) [1]
Target: thrombin
in vitro: Dabigatran selectively and reversibly inhibited human thrombin(Ki: 4.5 nM) as well as thrombin–induced platelet aggregation (IC(50): 10 nM), while showing no inhibitory effect on other platelet–stimulating agents.Thrombin generation in platelet–poor plasma (PPP), measured as the endogenous thrombin potential (ETP) was inhibited concentration–dependently (IC(50): 0.56 microM).
Dabigatran demonstrated concentration–dependent anticoagulant effects in various species in vitro, doubling the activated partial thromboplastin time (aPTT), prothrombin time (PT) and ecarin clotting time (ECT) in human PPP at concentrations of 0.23, 0.83 and 0.18 microM, respectively [1].
in vivo: Dabigatran prolonged the aPTT dose–dependently after intravenous administration in rats (0.3, 1 and 3 mg/kg) and rhesus monkeys (0.15, 0.3 and 0.6 mg/kg). Dose– and time–dependent anticoagulant effects were observed with dabigatran etexilate
administered orally to conscious rats (10, 20 and 50 mg/kg) or rhesus monkeys (1, 2.5 or 5 mg/kg), with maximum effects observed between 30 and 120 min after administration, respectively [1]. Patients treated with dabigatran etexilate experienced fewer ischaemic strokes (3.74 dabigatran etexilate vs 3.97 warfarin) and fewer combined intracranial haemorrhages and haemorrhagic strokes (0.43dabigatran etexilate vs 0.99 warfarin) per 100 patient–years [2].
Clinical trial: An Evaluation of the Pharmacokinetics and Pharmacodynamics of Oral Dabigatran Etexilate in Hemodialysis Patients .Phase1
References:
[1]. Wienen W, Stassen JM, Priepke H, In–vitro profile and ex–vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally activeprodrug, dabigatran etexilate. Thromb Haemost. 2007 Jul;98(1):155–62.
[2]. Kansal AR, Sorensen SV, Gani R, Cost–effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in UK patients withatrial fibrillation. Heart. 2012 Apr;98(7):573–8.
Product Name:
Dabigatran Cat. No.:
HY-10163CAS No.:
211914-51-1Molecular Formula:
C 25H 25N 7O 3Molecular Weight:
471.51Target:
Thrombin Pathway:
Metabolic Enzyme/Protease Solubility:DMSO: < 1 mg/mL; H 2
O: < 1 mg/mL
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
