晶体文献
有关半导体的参考文献

有关半导体的参考文献参考文献:1. 陶铸, 朱建新. 半导体物理学[M]. 清华大学出版社, 2017.2. 张宇. 半导体器件物理与模拟[M]. 电子工业出版社, 2018.3. 石磊, 朱建新. 半导体器件物理与工艺[M]. 机械工业出版社, 2019.4. 朱建新. 半导体物理与器件[M]. 清华大学出版社, 2020.半导体材料是一类具有特殊电学性质的材料,广泛应用于电子器件和集成电路中。
随着科技的不断进步,半导体物理学和器件工艺也得以迅速发展。
本文将对半导体物理学和器件工艺的一些重要内容进行介绍。
半导体物理学是研究半导体材料的电学性质和输运特性的学科。
《半导体物理学》一书详细介绍了半导体材料的基本性质、能带理论、载流子输运、PN结和二极管、MOS结和MOS场效应晶体管等内容。
通过学习半导体物理学,可以了解半导体材料的结构、能带结构以及载流子的产生、输运和复合过程,为后续学习半导体器件物理和工艺奠定基础。
半导体器件物理与模拟是研究半导体器件的电学特性和模拟方法的学科。
《半导体器件物理与模拟》一书详细介绍了半导体器件的物理效应、载流子输运、PN结和二极管、MOS场效应晶体管、BJT等内容。
通过学习半导体器件物理与模拟,可以了解各种半导体器件的工作原理、特性和模拟方法,为后续设计和优化半导体器件提供理论指导。
半导体器件物理与工艺是研究半导体器件制备工艺和性能改善方法的学科。
《半导体器件物理与工艺》一书详细介绍了半导体器件的制备工艺、薄膜技术、光刻技术、离子注入和扩散技术等内容。
通过学习半导体器件物理与工艺,可以了解各种半导体器件的制备过程和性能改善方法,为实际的半导体器件制造提供技术支持。
半导体物理与器件是综合了半导体物理学和半导体器件物理与工艺的学科。
《半导体物理与器件》一书全面介绍了半导体物理和器件的基本原理和应用。
通过学习半导体物理与器件,可以深入了解半导体材料的物理性质、器件的工作原理和制备工艺,为实际的半导体器件设计和制造提供理论指导和技术支持。
InSeI单晶的制备及其结构与性能研究

第49卷第12期人工晶体学报Vol.49No.12 2020年12月JOURNAL OF SYNTHETIC CRYSTALS December,2020 InSei单晶的制备及其结构与性能研究周玄1,2,程国峰2,何代华1(1.上海理工大学材料科学与工程学院,上海200093;2.中国科学院上海硅酸盐研究所,上海200050)摘要:利用化学气相传输法(CVT)制备了InSeI单晶。
该晶体为黄色的针状物,晶体较脆。
在室温下进行X射线衍射分析发现,其属于四方晶系,晶胞参数为a=b=1.8643(5)nm,c=1.0120(3)nm,V=3.5172nm3,空间群为他/a。
紫外可见光吸收光谱、光致发光光谱等结果显示该晶体的禁带宽度是2.48eV,在一定波段光的激发下,InSeI单晶在600nm左右有较宽的发射峰,表明该晶体的发光方式为缺陷态发光。
介电温谱表明InSeI单晶在440K时其四方相的结构发生了相变。
关键词:InSeI;金属基硫卤化合物;化学气相传输法;光致发光;禁带宽度;介电性能中图分类号:O78文献标识码:A文章编号:1000-985X(2020)12-225244 Synthesis,Structure and Properties of InSei Single CrystalsZHOU Xuan1,2,CHENG Guofeng2,HE Daihua1(1.School of Materials Science and Engineering,Lniversity of Shanghai for Science and Technology,Shanghai200093,China;2.Shanghai Institute of Ceramics,Chinese Academy of Sciences,Shanghai200050,China)Abstract:InSeI single crystals were synthesized by the chemical vapor transport(CVT)method.The crystal is yellow needleshaped and brittle.X-ray diffraction results at room temperature show the tetragonal system of InSeI,with lattice parameters of a=b=1.8643(5)nm,c=1.0120(3)nm,V=3.5172nm3,and space group is/a.The ultraviolet-visible absorption spectrum,photoluminescence spectrum results show that InSeI has a2.48eV band gap,under the excitation of a certain band of light,InSeI single crystal has a wide emission peak at about600nm,which indicates that the luminescence mode of the crystal is defect state luminescence.The dielectric temperature spectrum indicates that a phase transition happened in the tetragonal structure of InSeI crystals at440K.Key words:InSeI;metal based thiohalide;chemical vapor transport method;photoluminescence;band gap;dielectric property0引言近年来,金属基硫卤化合物MQX[1](M=Ga,In,Sb,Bi;Q=S,Se,Te;X=Cl,Br,I)由于其独特的光电性质如铁电性[2-3]、热电性[4]、光电导性[5]和非线性光学性能[6]等引起了科学界的浓厚兴趣。
化学文献

【摘要】众所周知,2011年诺贝尔化学奖实至名归地由以色列化学家Daniel Shechtman获奖,获奖理由是“发现准晶体”。
谢赫特曼发现了准晶体,这种材料具有的奇特结构,推翻了晶体学已建立的概念。
许多年以来,凝聚态物理学家们仅仅关心晶态的固体物质。
然而,在过去的几十年,他们逐渐把注意力转向“非晶”材料,如液体或非晶体,这些材料中的原子仅在短程有序,被称为缺少“空间周期性”。
准晶,这种新的结构因为缺少空间周期性而不是晶体,但又不像非晶体,准晶展现了完美的长程有序,这个事实给晶体学界带来了巨大的冲击,它对长程有序与周期性等价的基本概念提出了挑战。
【关键词】准晶的空间结构准晶的应用前景众所周知,2011年诺贝尔化学奖名至实归的由以色列化学家Daniel Shechtman获奖,获奖理由是“发现准晶体” [1]。
想必诺贝尔奖对一个人的重要性不用在此处赘述了吧,那么就请由我为大家简单的介绍一下诺贝尔奖得主与他的研究成果吧一:什么是准晶准晶是一种介于晶体和非晶体之间的固体。
准晶具有完全有序的结构,然而又不具有晶体所应有的平移对称性,因而可以具有晶体所不允许的宏观对称性。
准晶体其实发现的时间并不是很早,与1982年才被发现的,因其具有凸多面体规则外形的,但不同于晶体的固态物质,它们具有晶体物质不具有的五重轴。
目前已知的准晶体都是金属互化物。
2000年以前发现的所有几百种准晶体中至少含有3种金属,如Al65Cu23Fe12,Al70Pd21Mn9等。
但最近发现仅2种金属也可形成准晶体,如Cd57Yb10〔Nature,2000,408:537〕[2]。
由于准晶体的特殊性质,所以长期一来,大多数科学家并不承认其为单独存在的晶体。
2009年,矿物学上的一个发现为准晶是否能在自然条件下形成提供了证据:俄罗斯的一块铝锌铜矿上发现了Al63Cu24Fe13组成的准晶颗粒。
和实验室中合成的一样,这些颗粒的结晶程度都非常好。
(完整版)文献调研报告模板

文献综述调研报告课题名称:蓝宝石晶体生产方法的研究涉及的检索词:中文:方法蓝宝石生产原料晶体检索词间的逻辑关系(逻辑式,可以有多个):中文:(蓝宝石+晶体)*(原料+生产)*方法需要的文献类型:期刊文章学位论文年代范围:1979-2010选用的数据库:知网数据库、维普数据库、万方数据库检索方法(用截图方式表现):检索结果:知网数据库:[1]韩杰才, 左洪波,孟松鹤, 张明福, 姚泰, 李长青,许承海, 汪桂根。
泡生法制备大尺寸蓝宝石单晶体[j]. 人工晶体学报, 2005,(01)摘要:为了研究工艺参数对泡生法蓝宝石晶体生长过程及其晶体质量的影响,在自行研制的泡生法蓝宝石晶体生长炉上进行了试验。
调整籽晶热交换器水流量及进水温度,并在等径生长期间采用不同的维持功率下降速度,结果表明:热交换器冷却强度对引晶及放肩阶段晶体生长有显著影响,并逐步减弱;维持功率下降速度直接影响等径生长阶段的晶体生长速度和晶体质量,下降太快将导致晶体缺陷密度增加,严重时形成多晶.在晶体生长过程中,合理调节籽晶热交换器的冷却强度,谨慎操控维持功率下降速度是蓝宝石单晶生长成败的关键。
[2] 姚泰,左洪波,韩杰才,张明福,孟松鹤,姚秀荣,李常青,汪贵根,许承海。
蓝宝石单晶生长过程中应力分布的数值模拟[j]哈尔滨理工大学学报 , 2006,(05)摘要:晶体生长过程中所产生的残余热弹性应力是影响晶体质量的重要因素之一。
用ansys软件为平台,以改进的kyropoulos法制备φ230x200mm大尺寸蓝宝石单晶为例,对所生长单晶体的热应力分布进行了数值模拟.建立了不同外形(放肩角)条件下晶体生长过程中热弹性应力的分布模型。
讨论了不同放肩角对晶体热应力的影响.结果表明,通过改变晶体的外形可以改善热应力的分布,从而在晶体中获得更多的低应力区域.模拟结果和试验结果吻合较好.[3]孙广年,于旭东,沈才卿。
泡生法生长高质量蓝宝石的原理和应用[j]。
晶体制作实验报告范文

晶体制作实验报告范文一、实验目的本实验旨在通过制备晶体来加深对晶体结构及其形成条件的理解,同时培养学生的实验操作能力和科学思维能力。
二、实验原理1. 晶体定义:晶体指具有高度有序、长程排列的物质结构,具有明确的几何外形和规整的表面,由分子、原子或离子构成。
2. 晶体的制备:晶体的制备一般采用溶液结晶方法。
在饱和溶液中逐渐降温或加入引发结晶的物质,使溶质从液相向固相过渡,结晶体逐渐形成。
三、实验步骤1. 实验准备:准备所需试剂和仪器,包括溶液制备容器、加热设备、试管、玻璃棒、滤纸等。
2. 制备溶液:按实验要求准备所需溶液,可根据需要调节溶液浓度。
3. 溶液结晶:将制备好的溶液倒入试管中,逐渐降温或加入引发结晶的物质,观察晶体在溶液中的形成过程。
4. 晶体收集:使用滤纸将晶体与溶剂分离,将晶体取出并用纯净水进行清洗。
5. 晶体观察:观察晶体的形状、颜色、透明度等特征,并进行显微镜观察。
6. 结果记录:记录晶体的制备过程、观察结果和实验数据。
四、实验结果与分析经过实验制备,成功得到了透明、颗粒饱满的晶体。
晶体形状规整,呈现六角柱、长方体等不同形态,颜色鲜艳且均匀。
根据实验中观察到的晶体形状和性质特征,可以推断晶体具有高度有序的分子排列结构。
晶体的形成过程是由于溶液中的溶质逐渐从液相向固相过渡,使分子间的相互作用增强,最终达到稳定状态。
五、实验总结通过本次晶体制作实验,我们进一步了解了晶体的结构和形成条件。
实验中我们掌握了晶体制备的基本步骤和操作技巧,培养了实验操作能力和科学思维能力。
同时,我们也发现在制备晶体的过程中,温度的变化对晶体形成有着重要影响。
适当的温度控制可以促进晶体的形成和生长,而过快或过慢的温度变化则可能导致晶体形态不规则或晶体无法形成。
总的来说,本次实验对我们进一步理解晶体结构和制备方法具有重要意义,为今后的相关研究和应用提供了实验基础。
六、实验中遇到的问题及改进方向在实验过程中,我们发现晶体形成速度较慢,需要相对较长的时间。
常用晶体杂志

ScienceDirect (SD)网址:/(1) Catalysis Communications (催化通讯)(2) Journal of Molecular Catalysis A: Chemical (分子催化A:化学)(3) Tetrahedron (T) (四面体)(4) Tetrahedron: Asymmetry (TA) (四面体:不对称)(5) Tetrahedron Letters (TL) (四面体快报)(6) Applied Catalysis A: General (应用催化A)2. EBSCOhost数据库网址:/(1) Synthetic Communcations (合成通讯)(2) Letters in Organic Chemistry (LOC)(3) Current Organic Synthesis(4) Current Organic Chemistry3. Springer数据库网址:http:// /(1) Molecules (分子)(2) Monatshefte für Chemie / Chemical Monthly (化学月报)(3) Science in China Series B: Chemistry (中国科学B)(4) Catalysis Letts (催化快报)4. ACS Publications (美国化学会)网址:/(1) Journal of the American Chemical Society (JACS) (美国化学会志)(2) Organic Letters (OL) (有机快报)(3) The Journal of Organic Chemistry (JOC) (美国有机化学)(4) Journal of Medicinal Chemistry (JMC) (美国药物化学)(5) Chemical Reiew (化学评论)5. Royal Society of Chemistry (RSC) (英国皇家化学会)网址:/Publishing/Journals/Index.asp(1) Green Chemistry (绿色化学)(2) Chemical Communications (CC) (化学通讯)(3) Chemical Society Reviews (化学会评论)(4) Journal of the Chemical Society (化学会志)Journal of the Chemical Society, Perkin Transactions 1 (1972-2002) Journal of the Chemical Society, Perkin Transactions 2 (1972-2002) Journal of the Chemical Society B: Physical Organic (1966-1971)Journal of the Chemical Society C: Organic (1966-1971)(5) Organic & Biomolecular Chemistry (OBC) (有机生物化学)/publishing/jo ... p?type=CurrentIssue6. Wiley网址:/(1) Advanced Synthesis & Catalysis (ASC) (先进合成催化)(2) Angewandte Chemie International Edition (德国应用化学)(3) Chemistry - A European Journal (欧洲化学)(4) Chinese Journal of Chemistry (中国化学)(5) European Journal of Organic Chemistry (欧洲有机化学)(6) Helvetica Chimica Acta (瑞士化学)(7) Heteroatom Chemistry (杂原子化学)7. Ingent网址:/(1) Journal of Chemical Research (JCR) (化学研究杂志)(2) Canadian Journal of Chemistry (加拿大化学)(3) Current Organic Chemistry(4) Mini-Reviews in Organic Chemistry(5) Phosphorus, Sulfur, and Silicon and the Related Elements (磷、硫、硅和相关元素)(6) Letters in Organic Chemistry8. Taylor & Francis数据库网址:http://www.journalsonline.tandf. ... sp?referrer=default(1) Synthetic Communications(2) Journal of Sulfur Chemistry(硫化学杂志)(3) Phosphorus, Sulfur, and Silicon and the Related Elements9. Thieme数据库网址:/(1) Synlett (合成快报)(2) Synthesis (合成)10. 日本化学会网址:(1) Chem. Lett. (CL) (化学快报)http://www.jstage.jst.go.jp/browse/cl/_vols(2) Bull. Chem. Soc. Jpn. http://www.csj.jp/journals/bcsj/index.html11. 澳大利亚化学会(Australian Journal of Chemistry)http://www.publish.csiro.au/nid/52.htm12.巴西化学会.br/13.Molecules/molecules/14.韩国化学会http://journal.kcsnet.or.kr/15.印度化学会http://www.niscair.res.in/Scienc ... hin.htm&d=test816.国际有机制备和程序(Organic Preparations and Procedures International,OPPI)/17.有机化学/index.htm有机合成:Organic Syntheses(有机合成手册), John Wiley & Sons (免费)/Named Organic Reactions Collection from the University ofOxford (有机合成中的命名反应库) (免费)/thirdyearcomputing/NamedOrganicReac...有机化学资源导航Organic Chemistry Resources Worldwide/有机合成文献综述数据库Synthesis Reviews (免费)/srev/srev.htmCAMEO (预测有机化学反应产物的软件)/products/cameo/index.shtmlCarbohydrate Letters (免费,摘要)/Carbohydrate_Letters/Carbohydrate Research (免费,摘要)/locate/carresCurrent Organic Chemistry (免费,摘要)/coc/index.htmlElectronic Encyclopedia of Reagents for Organic Synthesis (有机合成试剂百科全书e-EROS) /eros/European Journal of Organic Chemistry (免费,摘要)/jpages/1434-193X/Methods in Organic Synthesis (MOS,有机合成方法)/is/database/mosabou.htmOrganic Letters (免费,目录)/journals/orlef7/index.htmlOrganometallics (免费,目录)/journals/orgnd7/index.htmlRussian Journal of Bioorganic Chemistry (Bioorganicheskaya Khimiya) (免费,摘要)http://www.wkap.nl/journalhome.htm/1068-1620Russian Journal of Organic Chemistry (Zhurnal Organicheskoi Khimii) (免费,摘要)http://www.maik.rssi.ru/journals/orgchem.htmScience of Synthesis: Houben-Weyl Methods of Molecular Transformation/Solid-Phase Synthesis database (固相有机合成)/chem_db/sps.htmlSynthetic Communications (免费,摘要)/servlet/product/productid/SCCSyntheticPages (合成化学数据库) (免费)/The Complex Carbohydrate Research Center (复杂碳水化合物研究中心)/合成材料老化与应用 (免费,目录)/default.html金属卡宾络合物催化的烯烃复分解反应 (免费)/html/books/O61BG/b1/2002/2.6%20.htm上海化学试剂研究所/英国化学数据服务中心CDS (Chemical Database Service)/cds/cds.html英国皇家化学会碳水化合物研究组织 (Carbohydrate Group of the Royal Society of Chemistry) /lap/rsccom/dab/perk002.htm有机反应催化学会 (ORCS, Organic Reaction Catalysis Society)/有机合成练习 (免费)/中国科学院成都有机化学研究所:催化与环境工程研究发展中心/MainIndex.htm金属有机及元素有机化学:CASREACT - Chemical Reactions Database(CAS的化学反应数据库)/CASFILES/casreact.html日本丰桥大学 Jinno实验室的研究数据库(液相色谱、多环芳烃/药物/杀虫剂的紫外谱、物性) (免费) http://chrom.tutms.tut.ac.jp/JINNO/ENGLISH/RESEARCH/research...A New Framework for Porous Chemistry (金属有机骨架) (免费)/alchem/articles/1056983432324.htmlActa Crystallographica Section B (免费,摘要)/b/journalhomepage.htmlActa Crystallographica Section E (免费,摘要)/e/journalhomepage.htmlBibliographic Notebooks for Organometallic Chemistryhttp://www.ensc-lille.fr/recherche/cbco/bnoc.htmlBiological Trace Element Research (生物痕量元素研究杂志) (免费,摘要)/JournalDetail.pasp?issn=0163-4984...Journal of Organometallic Chemistry (免费,摘要)/locate/jnlabr/jomOrganic Letters (免费,目录)/journals/orlef7/index.htmlOrganometallics (免费,目录)/journals/orgnd7/index.htmlSyntheticPages (合成化学数据库) (免费)/金属卡宾络合物催化的烯烃复分解反应 (免费)/html/books/O61BG/b1/2002/2.6%20.htm金属有机参考读物:The Organometallic HyperTextBook by Rob Toreki/organomet/index.html金属有机化学国家重点实验室,中国科学院上海有机所/元素有机化学国家重点实验室(南开大学)/在线网络课程:有机金属反应和均相催化机理 (Dermot O'Hare 主讲)/icl/dermot/organomet/药物化学:Fisher Scientific/PubMed: MEDLINE和PREMEDLINE (免费)/PubMed/生物医药:BioMedNet: The World Wide Club for the Biological and Medical Community/AIDSDRUGS (艾滋病药物) (免费)/pubs/factsheets/aidsinfs.htmlautodock (分子对接软件) (免费)/pub/olson-web/doc/autodock/DIRLINE (卫生与生物医药信息源库) (免费)/HISTLINE (医药史库) (免费)/TOXNET (化合物毒性相关数据库系列) (免费)/日本药典,第14版 (免费)http://jpdb.nihs.go.jp/jp14e/index.html小分子生物活性数据库ChemBank (免费)/Ashley Abstracts Database (药物研发、市场文献摘要) (免费)/databases/ashley/search.aspBIOSIS/BIOSIS/ONLINE/DBSS/biosisss.html从检索药物交易信息库PharmaDeals (部分免费)/从ChemWeb检索有机药物用途及别名库Negwer: organic-chemical drugs and their synonyms (部分免费) /negwer/negwersearch.html美国常用药品索引库RxList (免费)/美国国家医学图书馆NLM的免费在线数据库 (免费)/hotartcl/chemtech/99/tour/internet.html制药公司目录(Pharmaceutical Companies on Virtual Library: Pharmacy Page)/company.html37℃医学网/AAPS PharmSci (免费,全文)/Abcam Ltd.有关抗体、试剂的销售,抗体的搜索)/Acta Pharmaceutica (免费,摘要)http://public.srce.hr/acphee/Advanced Drug Delivery Reviews (免费,摘要)http://www.elsevier.nl/locate/drugdelivAmerican Journal of Drug and Alcohol Abuse (免费,摘要)/servlet/product/productid/ADAAmerican Journal of Pharmaceutical Education (AJPE) (免费,全文)/Amgen Inc. (医药)/Anita's web picks (药学与药物化学信息导航)http://wwwcmc.pharm.uu.nl/oyen/webpicks.htmlAnnals of Clinical Microbiology and Antimicrobials (免费,全文)/Annual Review of Pharmacology and Toxicology (免费,摘要)/Anti-Cancer Drug Design (免费,摘要)/antcan/生物有机化学:ScienceDirect: 在线访问Elsevier的1100种期刊全文 (免费目录) (免费)/生命、环境科学综合性资源TheScientificWorld (sciBASE)/生物医药:BioMedNet: The World Wide Club for the Biological and Medical Community /BIOETHICSLINE (BIOETHICS onLINE) (免费)/BIOME (生命科学资源导航)/browse/Directory of P450-containing Systems(P450酶系目录)http://p450.abc.hu/DIRLINE (卫生与生物医药信息源库) (免费)/百名最佳生物技术网站列表 (Top 100 Biotechnology WWW Sites)/top100.asp从ChemWeb检索《化学工程与生物技术文摘》库CEABA (部分免费)/课程材料:MIT生物学超文本教材http://esg-[url]:8001/esgbio/7001main.html[/url]生物材料网 (Biomaterials Network)/生物信息学资源导航,上海生物化学所/bio/index.htm小分子生物活性数据库ChemBank (免费)/英国剑桥医学研究委员会:分子生物学实验室LMB/biology site of the network./生物有机化学:ScienceDirect: 在线访问Elsevier的1100种期刊全文 (免费目录) (免费)/生命、环境科学综合性资源TheScientificWorld (sciBASE)/生物医药:BioMedNet: The World Wide Club for the Biological and Medical Community /BIOETHICSLINE (BIOETHICS onLINE) (免费)/BIOME (生命科学资源导航)/browse/Directory of P450-containing Systems(P450酶系目录)http://p450.abc.hu/DIRLINE (卫生与生物医药信息源库) (免费)/百名最佳生物技术网站列表 (Top 100 Biotechnology WWW Sites)/top100.asp从ChemWeb检索《化学工程与生物技术文摘》库CEABA (部分免费)/课程材料:MIT生物学超文本教材http://esg-[url]:8001/esgbio/7001main.html[/url]生物材料网 (Biomaterials Network)/生物信息学资源导航,上海生物化学所/bio/index.htm小分子生物活性数据库ChemBank (免费)/英国剑桥医学研究委员会:分子生物学实验室LMB/biology site of the network./。
晶体英文文献
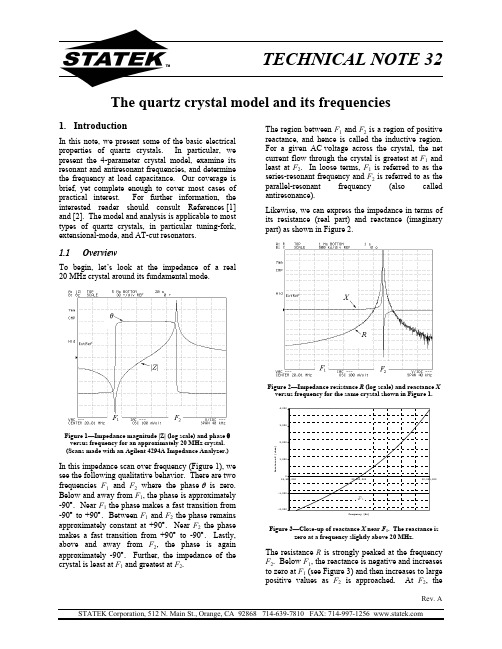
The quartz crystal model and its frequencies1. IntroductionThe region between F 1 and F 2 is a region of positive reactance, and hence is called the inductive region. For a given AC voltage across the crystal, the net current flow through the crystal is greatest at F 1 and least at F 2. In loose terms, F 1 is referred to as the series-resonant frequency and F 2 is referred to as the parallel-resonant frequency (also called antiresonance).In this note, we present some of the basic electrical properties of quartz crystals. In particular, we present the 4-parameter crystal model, examine its resonant and antiresonant frequencies, and determine the frequency at load capacitance. Our coverage is brief, yet complete enough to cover most cases of practical interest. For further information, the interested reader should consult References [1] and [2]. The model and analysis is applicable to most types of quartz crystals, in particular tuning-fork, extensional-mode, and AT-cut resonators.Likewise, we can express the impedance in terms of its resistance (real part) and reactance (imaginary part) as shown in Figure 2.1.1 OverviewTo begin, let’s look at the impedance of a real 20 MHz crystal around its fundamental mode.Figure 2—Impedance resistance R (log scale) and reactance X versus frequency for the same crystal shown in Figure 1.Frequency [Hz]R e a c t a n c e X [o h m s ]Figure 1—Impedance magnitude |Z| (log scale) and phase θ versus frequency for an approximately 20 MHz crystal. (Scans made with an Agilent 4294A Impedance Analyzer.)In this impedance scan over frequency (Figure 1), we see the following qualitative behavior. There are twofrequencies F 1 and F 2 where the phaseθ is zero. Below and away from F 1, the phase is approximately -90°. Near F 1 the phase makes a fast transition from -90° to +90°. Between F 1 and F 2 the phase remains approximately constant at +90°. Near F 2 the phase makes a fast transition from +90° to -90°. Lastly, above and away from F 2, the phase is again approximately -90°. Further, the impedance of the crystal is least at F 1 and greatest at F 2.Figure 3—Close-up of reactance X near F 1. The reactance iszero at a frequency slightly above 20 MHz.The resistance R is strongly peaked at the frequency F 2. Below F 1, the reactance is negative and increases to zero at F 1 (see Figure 3) and then increases to large positive values as F 2 is approached. At F 2, the1.2.3Frequency at load capacitancereactance quickly decreases to large negative values and then again steadily increases towards zero.Another important crystal frequency is the frequency F L at a load capacitance C L . (See Reference [3] for a full discussion of this concept.) At this frequency, the crystal reactance X is equal to 1.2The crystal frequencies1.2.1The series-resonant frequenciesConsider the frequency F 1. One can define this in at least three ways. One choice is the (lower) frequency F r where the phase of the crystal is zero. At this zero-phase frequency, the crystal is purely resistive (equivalently its reactance is zero). A second choice is the frequency F m of minimum impedance. A third choice is to define this as the series-resonant frequency F s —a frequency whose definition requires the crystal model as discussed in Section 2.LC X ω1=, (4) where ω = 2πF L . Equivalently, at this frequency, the series combination of the crystal and a capacitance C L has zero reactance. (See Figure 4.) Note that as C L → ∞, F L → F r , and that as C L decreases, F L increases towards F p .Table 3—Frequency at load capacitanceTable 1—The series-resonant frequenciesFrequency Description L FrequencyDescriptionF s Series resonant frequency F r Zero-phase frequency (lower) F m Minimum impedance frequencyIt turns out that for most crystals, F s , F r , and F m areall sufficiently close to one another than it is notnecessary to distinguish between them.. (1)m r s F F F ≈≈The resulting relation giving the frequency of a crystal as a function of its parameters and a load capacitance C L is called the crystal-frequency equation and is of prime importance in specifying and understanding the operation of crystals in oscillators. (See References [3] and [4].)CrystalC LSee Section 6.2 for further details. 1.2.2The parallel-resonant frequenciesNext consider the frequency F 2. One can also definethis in at least three ways. One choice is the (upper) frequency F a where the phase of the crystal is zero. At this zero-phase frequency, the crystal is purely resistive (being very high). A second choice is the frequency F n of maximum impedance. A third choice is to define this as the parallel-resonant frequency F p —a frequency whose definition also requires the crystal model as discussed in Section 2.Figure 4—Defining F L at C LAs we shall see, for most applications, the frequency F L at load capacitance C L is well approximated by the expression()++≈L s L C C C F F 0121. (5)1.3 Guide to this noteTable 2—The parallel-resonant frequenciesFrequency Description F p Parallel-resonant frequency F a Zero-phase frequency (upper)F nMaximum-impedance frequency For most crystals, F p , F a , and F n are all sufficiently close to one another than it is not necessary to distinguish between them. . (2)n a p F F F ≈≈Further, they are above F s and are normally wellapproximated by the expression+≈0121C C F F s p . (3) In Section 2, we present and discuss the 4-parametercrystal model. In Section 3, we derive some simple results from this model defining F s and F p . In Section 4, we define the three non-dimensionalquantities r , Q , and M . In Section 5, we present some useful properties of the frequencies F r , F a , F s , and F p . In Section 6, we present approximations for F L and F rthat go beyond the approximations in Section 1.2. In Section 7, we derive the exact expressions for F L and F r . In Section 8, we make a few comments on resistance at resonance and antiresonance. Lastly, Appendix 1 contains a list of the important symbols used in this note. Note that while we present both exact and approximate relations for F s , F r , F p , F a , and F L , we present no further results for F m or F n other than theapproximations given in Section 1.2. For further information, see References [5], [6], and [7].For those first becoming acquainted with crystals, we recommend reading Sections 1-4. For those who want further details and more precise results, we recommend reading Sections 1-6. Lastly, for those who want exact results, we recommend this entire note.Throughout, we use the usual relation between a given frequency f and its angular frequency ω counterpartf π2=ω. (6)2. The 4-parameter crystal modelThe modes of interest in quartz crystals are usuallymodeled electrically by the 4-parameter model shown in Figure 5. This model consists of two arms in parallel with one another. The “static arm” consists of a single capacitance C 0 (also referred to as the shunt capacitance). Herein, this capacitance includes the capacitance of the bare crystal and the shunt capacitance of its packaging. The “motional arm” consists of the series combination of a resistance R 1, inductance L 1, and a capacitance C 1.111C 0Figure 5—The 4-parameter crystal modelWhile this model is an approximation of the electricalcharacteristics of the crystal, it is a very good one and for most purposes more than sufficient. So, from here on, we take this model seriously. (See References [1] and [8] for further discussion.) One should be aware that crystals are complicated by the existence of many modes of oscillation. In addition to their fundamental mode, crystals have overtone modes. For example, tuning-fork crystals have 1st -overtone modes at roughly six times the frequency of the fundamental mode. As another example, AT-cut crystals have 3rd , 5th , 7th , etc., overtone modes with frequencies being nearly the overtone number times the fundamental mode frequency. Sometimes these modes are the desired mode as they can offer frequencies that would otherwise be unattainable.1 When they are not the desired mode, their great separation from the main mode and their resistance is normally sufficiently high that they have no effect on the performance of the crystal in an oscillator. AT-cut crystals are further complicated by the existence of anharmonic modes just above the main mode as well as having other unwanted modes. Proper crystal design minimizes the strengths of these modes (collectively referred to as unwanted modes) so that they have no effect on the crystal’s operation in an oscillator.2.1 Typical valuesTo give the reader some idea of these crystal parameters and how they vary with crystal type and frequency, we present some typical values for Statek crystals. However, keep in mind that ranges given here can be exceeded in some cases.The static capacitance C 0 has a limited range of variation, usually being on the order of 1-3 pF. This parameter typically scales with the motional capacitance C 1 and package size, i.e. crystals with large C 1 in large packages have large C 0. Smaller crystals also tend to have smaller C 1, so C 0 roughly correlates with package size, but not absolutely. Similarly, the motional capacitance C 1 has a fairly limited range typically being on the order of 0.5 fF to 10 fF. Tuning-fork and extensional-mode crystals tend to have their C 1 lie on the low end of this spectrum, while AT-cut crystals can have a C 1 just about anywhere in this range, depending on the size of the crystal and its frequency.The motional inductance L 1 varies greatly over frequency, for as we shall see, it is determined by C 1 and the crystal frequency. It has a high of roughly 100 kH for 10 kHz crystals to a low of less than 1 mH for 100 MHz crystals (a range of about 108 ). Lastly, the crystal resistance also varies greatly over frequency from a high of about 1 M Ω for 10 kHz H-type crystals to a low of about 10 Ω for high-frequency AT-cut crystals (a range of about 105 ).2.2 Specifying crystal parametersIf your application has critical requirements that require specification of the crystal parameters, then bounds on the relevant parameters should be supplied. However, unnecessary requirements will probably increase the cost of the crystal without any added benefit.The crystal parameter that most commonly requires specification is the crystal resistance R 1. This parameter plays an important role in the crystal-oscillator gain requirement and sometimes an upper1It is normally a mistake to use a crystal designed for fundamental mode operation at one of its overtone modes.3.1 Series resonancebound on R 1 is required to ensure the startup of the oscillator.Being a capacitance, the reactance of the static arm is negative. On the other hand, the motional arm consisting of the series combination an inductor and a capacitor can have reactance of either sign depending on the frequency. In particular at some frequency F s , called the series-resonant frequency, For applications requiring crystal pullability (the ability to change frequency with changes in load capacitance), bounds should be placed on C 1. As shown by Equation (5), C 1 plays a primary role in determining the frequency change for a given change in C L . The capacitance C 0 also plays a role and when the pullability requirements are demanding, upper bounds are placed on C 0.0. (12)1=X Using Equation (11), we see that the angular frequency ωs at which the reactance of the motional arm is zero is given by Lastly, requirements on L 1 are rarely necessary as such conditions can be expressed as conditions on C 1. [See Equation (14).]111C L s =ω. (13)Sometimes people place requirements on the crystal Q (defined below) in the belief that this quantity determines either oscillator startup or crystal pullability. Both are wrong. The resistance R 1 (along with the oscillator design) determines startup. The motional capacitance C 1 (along with C 0) determines the crystal pullability. [See Equation (5).]Therefore, the series-resonant frequency of the crystal is given by111π21C L F s =. (14)3. Simple consequencesEquivalently, ignoring the crystal resistance R 1, series resonance is the frequency at which the crystal impedance is minimal (being zero in this idealization).In this section we derive some simple consequences of the crystal model. In particular, we show the existence of a series-resonant frequency F s and a parallel-resonant frequency F p .The impedance Z of the crystal is determined by the parallel combination of the impedance Z 0 of the static arm and the impedance Z 1 of the motional arm. 1010Z Z ZZ Z +=. (7)Note that one should not use Equation (14) to compute F s as typically neither L 1 and C 1 are knownonly to about 1% accuracy while other methods can determine F s to better than 1 ppm. Instead, the utility of Equation (14) comes in computing either L 1 or C 1from the other and F s . 3.2 Parallel resonance The impedance of the static arm is purely reactive and is given by, (8)00jX Z =where its reactance X 0 is given by01C X ω−=. (9) One effect of the static (shunt) capacitance C 0 is to make the crystal look like a simple capacitance at frequencies where the impedance of the motional armis large compared to impedance of the static arm. Another is to create an anti-resonance (resonance of high impedance) at a frequency where the two armsof the crystal resonant in which such a way to offerhigh impedance to current flow. Likewise, the impedance of the motional arm is given by111jX R Z +=, (10)Ignoring the crystal resistance R 1, this parallel resonance occurs at the frequency where the admittance Y = 1/Z of the crystal is zero.where its reactance X 1 is given by1111C L X ωω−=. (11) .1101010jX jX Y Y Y+=+== (15) Therefore010=+X X , (16)or equivalently11C X ω=. (17)()++≈L s L C C C F F 0121.(23)With this, it follows that the parallel-resonantfrequency F p of the crystal is given by11C CF F s p +=. (18)This is the standard crystal-frequency equation. However, be aware that it is an approximation. Even so, in most cases this equation is sufficient and a more exact expression would complicate the computation without any benefit. 3.4 The significance of L 1Note that the parallel resonant frequency is alwaysabove the series-resonant frequency and that their separation is determined by the ratio of the capacitances C 1 and C 0. For quartz crystals, C 1 << C 0, so F s and F p are quite close is as fraction of absolute frequency and is usually well approximated by the expression Note that+≈0121C C F F s p . (19),at 4122112111s F f L C L d dX df dX ==+==πωπωπ (24)which shows that L 1 is proportional to the rate of change of the motional reactance with frequency atseries resonance. This fact is sometimes useful in measuring the crystal parameters. Note that, ignoring the effects of resistance, dX/df = 4πL 1 at f = F s , showing that the shunt capacitance C 0 does not modify the slope of reactance curve at series resonance. However, the shunt capacitance does greatly increase the slope of the reactance asantiresonance is approached. (See Figure 2 andFigure 3.) While the motivation for our definition of the parallel-resonant frequency was based on the case where the crystal resistance is zero, we take its definition in general to be that frequency where the reactances of the two arms are in anti-resonance. Therefore, the parallel resonant frequency F p of a crystal is always given by Equation (18). 3.3 F L at C L Ignoring the crystal resistance R 1, we can easily work out the crystal frequency F L at a load capacitance C L . This is frequency at which4. Three non-dimensional quantities There are at least three non-dimensional quantities that are very useful in characterizing crystals.()(),11111010jX C j C j Y Y ZL +−=+=ωω (20) Our first quantity is the capacitance ratio r10C Cr =. (25) and so()L C C X +=011ω. (21)As we saw in Section 3.2, 1/r determines theseparation between series resonance and parallelresonance, in other words, the width of the crystal’s inductive region. As an example, a crystal with a C 0of 2 pF and a C 1 of 5 fF has a capacitance ratio of 400. For Statek crystals, r can range from about 250to 1,000.2With this is it straightforward to show that the frequency F L at load capacitance C L is given by )0( ,1101=++=R C C C F F Ls L . (22) Our next quantity is the crystal quality factor Q . Thisis defined so that 2π/Q is the fractional energy lost per cycle in the crystal and is given in terms of the crystal parameters by Although this derivation ignores the crystalresistance, our final expression is sufficient for mostapplications and in fact is normally further approximated by the expression2These bounds can be exceeded.111C R Q s ω=. (26) 6. Approximations beyond R 1 = 0 For the zero-phase frequency F r , the frequency F L at load capacitance C L , and crystal resistance at loadcapacitance, we simply present approximateexpressions that go beyond the results presented sofar. For proof, see the exact results in Section 7.where the frequency ωs (angular series resonance) isgiven by Equation (13). Crystals with large Qoscillate many cycles before their oscillations decayappreciably. For Statek crystals, Q ranges from about 2,000 to 400,000.2 6.1 Approximating F Lat C LA direct consequence of its definition is that it takesπ2Q(27) In cases where further accuracy is required in theload frequency F L , then to second order in resistance++ ++≈L L s L C C QM C C C F F 0011111. (32) cycles for the oscillation energy an isolated crystal to ramp-down by a factor of 1/e . The number of cycles for ramp-up is the same. So, the time for oscillationsto ramp-up or ramp-down in a low-frequency high-Qcrystal can be quite long—on the order of seconds. 6.2 Approximating F r Normally, F r is well approximated by F s . To see thedifference between the two, we must look to second order in crystal resistance. To this order, F r is slightly above F s by the amount Our last quantity is the crystal figure-of-merit M .This is simply the ratio of the impedance of the staticarm to the impedance of the motional arm at series resonance. Given this, it is straightforward to showthat M is given by11C R M s ω=. (28)+≈QMF F s r 211. (33)This result can be obtained from Equation (32) by taking the limit C L → ∞ and performing a first-order expansion in 1/(QM ). As we shall show in Section 7.3, in order for thecrystal to posses an inductive region, M be greaterthan 2. For Statek crystals, M ranges from about 10to 300.2Note our three parameters are not independent;indeed. (29) Mr Q =In most cases, QM > 106 so that ignoring the effect ofresistance is to make an error below 1 part-per-million in frequency, which is usually acceptable. An interesting exception is the case of 10 kHz H-type crystals. The large resistance (about 1 M Ω) of thesecrystals give Q ≈ 4,000 and a figure-of-merit M ≈ 11 and so that the difference between F r and F s is about 11 ppm.5. Some useful frequency properties6.3 Approximating R at F L5.1The frequency product propertyIt turns out, without approximation, that . (30)p s a r F F F F =The crystal resistance R depends on the frequency ofinterest. At series resonance, R ≈ R 1 and it increasesto very large values near parallel resonance. (SeeFigure 2.) A natural question that comes up is what is the crystal resistance at the load frequency F L . It turns out to good approximation that This equality allows us to calculate any one of theabove four frequencies given the other three.Because of this and the fact that results beyond theapproximation F a ≈ F p are rarely required, we do not present any further expressions for F a .211+≈L CC R R . (34) So, as expected, the crystal resistance is approximately R 1 at F r and increases to very large values as C L approaches zero.5.2 The frequency inequalitiesWhen the crystal posses an inductive region (so F r and F a exist and are distinct), (31)p a r s F F F F ≤<≤with equalities when and only when R 1 = 0.7. Exact expressionsand10X X X +=Ω. (42) 7.1 Crystal impedanceWe express the crystal impedance Z in terms of the impedances of the two parallel arms as follows.2102102011010Z Z Z Z Z Z Z Z Z Z Z ++=+=(35)With these definitions, we can express the crystal resistance R as()()+−+=−221/111M r R R , (43)and the reactance as()()220/111+−+Ω−=−M r X X . (44) Denoting resistance of the crystal by R (R = Re(Z ))and its reactance by X (X = Im(Z )), so that 7.3 F L at C L (exact),jX R Z +=Using Equation (44) and the fact that X = 1/(ωC L ), we havethen Equations (8) and (10), give the following expression for the crystal resistance R()()220/111+−+Ω−=−−M r C C L , (45)++=1021201X X R X R R , (36) which gives us the following quadratic equationfor Ωand the following for the crystal reactance X()().110211000210212101210+++−=++++=X X R X X X X X X R X X X R X X (37) 0111111202= ++Ω ++−Ωr M QM C C L . (46) It can be shown that the existence of real frequencies requires that+ +++>L L C C Q C C C M 0011112. (47)It can be shown that Z (ω) sweeps out an approximatecircle in the impedance plane (and similarly Y (ω) sweeps out an approximate circle in the admittance plane). See References [5], [6], and [7] for further details.Alternatively, for a given crystal, there is a lower bound on the allowed load capacitance C L , i.e. arbitrarily small load capacitances are not allowed. However, this bound is usually so weak that it causes no practical limitation to the existence of F L . 7.2 NormalizationDefine the normalized “frequency” Ω by22sp s ωωωω−−=Ω. (38) Taking the limit C L → ∞, we see that the conditionfor the existence of an inductive region is thatQM 12+>, (48) Note that Ω = 0 at F s and Ω = 1 at F p . In terms of Ω, the (angular) frequency isr sΩ+=1ωω. (39)showing that M must be greater than 2.Define the quantity ξ byFurther set Ω−=Ω1. (40)QMC C L 1110−+=ξ. (49)Note that1X X −=Ω, (41)Note that ξ > 0 by Equation (47). Further, define the quantity χ by9. References++−+=L CC C M 0122142ξξχ. (50)1. Bottom, Virgil E., Introduction to Quartz CrystalUnit Design , Van Nostrand Reinhold Company, 1982. With these definitions, solving Equation (46) and using Equation (39), the frequency F L at load capacitance C L is given by 2. Parzen, Benjamin , Design of Crystal and OtherHarmonic Oscillators , UMI Books on Demand, 1999.+++=QM CC C F F L s L χ1101. (51) 3. Statek Technical Note 33. 4. Statek Technical Note 30.5. EIA Standard EIA-512, 1985, Standard Methodsfor Measurement of Equivalent Electrical Parameters of Quartz Crystal Units, 1 kHz to 1 GHz . Note that actually there is a second root of Equation (46). However, the frequency corresponding to this root corresponds to a frequency very near anti-resonance where the resistance is very high; this is not the frequency of interest.6. IEEE Standard 177, 1966, Standard Definitionsand Methods of Measurements for Piezoelectric Vibrators . 7.4 F r (exact)Taking the limit C L → ∞ in the above equation for F L gives F r (the lower frequency at which the reactance of the crystal is zero) 7. Hafner, Erich, The Piezoelectric Crystal Unit—Definitions and Methods of Measurement , Proceedings of the IEEE, Volume 57, No. 2, 1966.− −+−+=2241111211M QM QMQM F s r F (52)8. Cady, Walter Guyton, Piezoelectricity , McGraw-Hill Book Company, Inc., 1946.8. Final comments on crystal resistanceNote that the crystal’s resistance at neither F s nor F ris R 1. Instead, 1211)(R M R F R s <+=−, (53)and1211)(R M R F R r >−≈−. (54)However, normally such distinctions are not required as they differ from R 1 by much less than 1%. Lastly, near antiresonance,, (55)21)(M R F R p ≈showing that 2M is roughly the range of the crystal resistance over frequency. (In fact, 2M is also roughly the range of the crystal impedance over frequency.)10. Appendix 1—Table of symbolsTable 4—Symbols used to describe crystalsSymbol Alternates Descriptionf FrequencyωAngular frequency, ω = 2π fC0Static (shunt) capacitanceR1R m, R1, RR3Motional resistanceL1L m, L1Motional inductanceC1C m, C1Motional capacitancer Capacitance ratio, r = C0/C1Resonator quality factor, Q = 1/(ωs R1C1)Figure of merit, M = 1/(ωs R1C1) = Q/rF s Series resonant frequencyF r FR Lower zero-phase frequency (normally close to F s)F m Minimum impedance frequency (normally close to F s)F p Parallel resonant frequencyF a Upper zero-phase frequency (normally close to F p)F n Maximum impedance frequency (normally close to F p)F L Frequency at load capacitance C LC L CL Load capacitanceTS Trim sensitivity, fractional rate-of-change of F L with C LCrystal impedance, Z = R+j X = |Z|eθR Crystal resistance, R = Re(Z)X Crystal reactance, X = Im(Z)θCrystal impedance phase angle, θ= arg(Z)3 Strictly speaking, RR (or Rr ) refers to the resistance at F r, however as shown in Section 8, the distinction between RR and R1 israrely worthwhile.。
CrystGrowthDes晶体生长英文文献

An Infinite Two-Dimensional Hybrid Water-Chloride Network,Self-Assembled in a Hydrophobic Terpyridine Iron(II)MatrixRicardo R.Fernandes,†Alexander M.Kirillov,†M.Fátima C.Guedes da Silva,†,‡Zhen Ma,†JoséA.L.da Silva,†João J.R.Fraústo da Silva,†andArmando J.L.Pombeiro*,†Centro de Química Estrutural,Complexo I,Instituto Superior Técnico,TU-Lisbon,A V.Ro V isco Pais,1049-001Lisbon,Portugal,and Uni V ersidade Luso´fona de Humanidades e Tecnologias,A V.doCampo Grande,376,1749-024,Lisbon,PortugalRecei V ed October18,2007;Re V ised Manuscript Recei V ed January7,2008ABSTRACT:An unprecedented two-dimensional water-chloride anionic{[(H2O)20(Cl)4]4–}n network has been structurally identified in a hydrophobic matrix of the iron(II)compound[FeL2]Cl2·10H2O(L)4′-phenyl-2,2′:6′,2″-terpyridine).Its intricate relief geometry has been described as a set of10nonequivalent alternating cycles of different sizes ranging from tetra-to octanuclear{[(H2O)x(Cl)y]y–}z(x) 2–6,y)0–2,z)4–6,8)fragments.In contrast to the blooming research on structural characterizationof a wide variety of water clusters in different crystalline materials,1much less attention has been focused on the identification anddescription of hybrid hydrogen-bonded water assemblies with othersolvents,small molecules,or counterions.1c,2In particular,thecombination of chloride ions and water is one of the most commonlyfound in natural environments(e.g.,seawater or sea-salt aerosols),and thus the investigation of water-chloride interactions has beenthe object of numerous theoretical studies.3However,only recentlya few water-chloride associates incorporated in various crystalmatrixes have been identified and structurally characterized,4,5including examples of(i)discrete cyclic[(H2O)4(Cl)]–,4a[(H2O)4(Cl)2]2–,4b and[(H2O)6(Cl)2]2–4c clusters,and(ii)variousone-or two-dimensional(1D or2D)hydrogen-bonded networksgenerated from crystallization water and chloride counterionswith{[(H2O)4(Cl2)]2–}n,5b{[(H2O)6(Cl)2]2–}n,5b[(H2O)7(HCl)2]n,5c{[(H2O)11(Cl)7]7–}n,5d{[(H2O)14(Cl)2]2–}n,5e{[(H2O)14(Cl)4]4–}n,5aand{[(H2O)14(Cl)5]5–}n5f compositions.These studies are alsobelieved to provide a contribution toward the understanding of thehydration phenomena of chloride ions in nature and have importancein biochemistry,catalysis,supramolecular chemistry,and designof crystalline materials.5In pursuit of our interest in the self-assembly synthesis andcrystallization of various transition metal compounds in aqueousmedia,we have recently described the[(H2O)10]n,6a(H2O)6,6b and[(H2O)4(Cl)2]2–4b clusters hosted by Cu/Na or Ni metal-organicmatrixes.Continuing this research,we report herein the isolationand structural characterization of a unique2D water-chlorideanionic layer{[(H2O)20(Cl)4]4–}n within the crystal structure of thebis-terpyridine iron(II)compound[FeL2]Cl2·10H2O(1′)(L)4′-phenyl-2,2′:6′,2″-terpyridine).Although this compound has beenobtained unexpectedly,a search in the Cambridge StructuralDatabase(CSD)7,8points out that various terpyridine containinghosts tend to stabilize water-chloride associates,thus also sup-porting the recognized ability of terpyridine ligands in supra-molecular chemistry and crystal engineering.9,10Hence,the simple combination of FeCl2·2H2O and L in tetrahydrofuran(THF)solution at room temperature provides the formation of a deep purple solid formulated as[FeL2]Cl2·FeCl2·5H2O(1)on the basis of elemental analysis,FAB+-MS and IR spectroscopy.11This compound reveals a high affinity for water and,upon recrystallization from a MeOH/H2O(v/v)9/1)mixture,leads to single crystals of1′with a higher water content,which have been characterized by single-crystal X-ray analysis.12The asymmetric unit of1′is composed of a cationic[FeL2]2+ part,two chloride anions,and10independent crystallization water molecules(with all their H atoms located in the difference Fourier map),the latter occupying a considerable portion of the crystal cell. The iron atom possesses a significantly distorted octahedral coordination environmentfilled by two tridentate terpyridine moieties arranged in a nearly perpendicular fashion(Figure S1, Supporting Information).Most of the bonding parameters within [FeL2]2+are comparable to those reported for other iron compounds*To whom correspondence should be sent.Fax:+351-21-8464455.E-mail: pombeiro@ist.utl.pt.†Instituto Superior Técnico.‡Universidade Luso´fona de Humanidades eTecnologias.Figure 1.Perspective representations(arbitrary views)of hybrid water-chloride hydrogen-bonded assemblies in the crystal cell of1′; H2O molecules and chloride ions are shown as colored sticks and balls, respectively.(a)Minimal repeating{[(H2O)20(Cl)4]4–}n fragment with atom numbering scheme.(b)Nonplanar infinite polycyclic2D anionic layer generated by linkage of four{[(H2O)20(Cl)4]4–}n fragments(a) represented by different colors;the numbers are those of Table1and define the10nonequivalent alternating cycles of different size.2008310.1021/cg7010315CCC:$40.75 2008American Chemical SocietyPublished on Web02/08/2008bearing two terpyridine ligands.13The most interesting feature of the crystal structure of 1′consists in the extensive hydrogen bonding interactions of all the lattice–water molecules and chloride coun-terions (Table S1,Supporting Information),leading to the formation of a hybrid water -chloride polymeric assembly possessing minimal repeating {[(H 2O)20(Cl)4]4–}n fragments (Figure 1a).These are further interlinked by hydrogen bonds generating a nonplanar 2D water -chloride anionic layer (Figure 1b).Hence,the multicyclic {[(H 2O)20(Cl)4]4–}n fragment is con-structed by means of 12nonequivalent O–H ···O interactions with O ···O distances ranging from 2.727to 2.914Åand eight O–H ···Cl hydrogen bonds with O ···Cl separations varying in the 3.178–3.234Årange (Table S1,Supporting Information).Both average O ···O [∼2.82Å]and O ···Cl [∼3.20Å]separations are comparable to those found in liquid water (i.e.,2.85Å)14and various types of H 2O clusters 1,6or hybrid H 2O -Cl associates.4,5Eight of ten water molecules participate in the formation of three hydrogen bonds each (donating two and accepting one hydrogen),while the O3and O7H 2O molecules along with both Cl1and Cl2ions are involved in four hydrogen-bonding contacts.The resulting 2D network can be considered as a set of alternating cyclic fragments (Figure 1b)which are classified in Table 1and additionally shown by different colors in Figure 2.Altogether there are 10different cycles,that is,five tetranuclear,three pentanuclear,one hexanuclear,and one octa-nuclear fragment (Figures 1b and 2,Table 1).Three of them (cycles 1,2,and 6)are composed of only water molecules,whereas the other seven rings are water -chloride hybrids with one or two Cl atoms.The most lengthy O ···O,O ···Cl,or Cl ···Cl nonbonding separations within rings vary from 4.28to 7.91Å(Table 1,cycles 1and 10,respectively).Most of the cycles are nonplanar (except those derived from the three symmetry generated tetrameric fragments,cycles 1,2,and 4),thus contributing to the formation of an intricate relief geometry of the water -chloride layer,possessing average O ···O ···O,O ···Cl ···O,and O ···O ···Cl angles of ca.104.9,105.9,and 114.6°,respectively (Table S2,Supporting Information).The unprecedented character of thewater -chloride assembly in 1′has been confirmed by a thorough search in the CSD,7,15since the manual analysis of 156potentially significant entries with the minimal [(H 2O)3(Cl)]–core obtained within the searching algorithm 15did not match a similar topology.Nevertheless,we were able to find several other interesting examples 16of infinite 2D and three-dimensional (3D)water -chloride networks,most of them exhibiting strong interactions with metal -organic matrixes.The crystal packing diagram of 1′along the a axis (Figure 3)shows that 2D water -chloride anionic layers occupy the free space between hydrophobic arrays of metal -organic units,with an interlayer separation of 12.2125(13)Åthat is equivalent to the b unit cell dimension.12In contrast to most of the previously identified water clusters,1,6water -chloride networks,5,16and extended assemblies,1c the incorporation of {[(H 2O)20(Cl)4]4–}n sheets in 1′is not supported by strong intermolecular interactions with the terpyridine iron matrix.Nevertheless,four weak C–H ···O hydrogen bonds [avg d (D ···A))3.39Å]between some terpyridine CH atoms and lattice–water molecules (Table S1,Figure S2,Supporting Information)lead to the formation of a 3D supramolecular framework.The thermal gravimetric analysis (combined TG-DSC)of 117(Figure S3,Supporting Information)shows the stepwise elimination of lattice–water in the broad 50–305°C temperature interval,in accord with the detection on the differential scanning calorimetryTable 1.Description of Cyclic Fragments within the {[(H 2O)20(Cl)4]4–}n Network in 1′entry/cycle numbernumber of O/Cl atomsformula atom numberingschemegeometry most lengthy separation,Åcolor code a 14(H 2O)4O3–O4–O3–O4planar O3···O3,4.28light brown 24(H 2O)4O6–O7–O6–O7planar O7···O7,4.42light gray 34[(H 2O)3(Cl)]-O2–O4–O3–Cl2nonplanar O4···Cl2,4.66blue 44[(H 2O)3(Cl)]-O6–O7–O9–Cl1nonplanar O7···Cl1,4.61green 54[(H 2O)2(Cl)2]2-O9–Cl1–O9–Cl1planar Cl ···Cl1,4.76pink 65(H 2O)5O2–O4–O3–O10–O8nonplanar O2···O10,4.55red75[(H 2O)4(Cl)]-O1–O5–O7–O9–Cl1nonplanar O7···Cl1,5.25pale yellow 85[(H 2O)4(Cl)]-O1–O5–Cl2–O8–O10nonplanar O10···Cl2,5.29orange 96[(H 2O)4(Cl)2]2-O2–O8–Cl2–O2–O8–Cl2nonplanar Cl2···Cl2,7.12yellow 108[(H 2O)6(Cl)2]2-O1–O10–O3–Cl2–O5–O7–O6–Cl1nonplanarCl1···Cl2,7.91pale blueaColor codes are those of Figure 2.Figure 2.Fragment of nonplanar infinite polycyclic 2D anionic layer in the crystal cell of 1′.The 10nonequivalent alternating water or water -chloride cycles are shown by different colors (see Table 1for color codes).Figure 3.Fragment of the crystal packing diagram of 1′along the a axis showing the intercalation of two water -chloride layers (represented by space filling model)into the metal -organic matrix (depicted as sticks);color codes within H 2O -Cl layers:O red,Cl green,H grey.Communications Crystal Growth &Design,Vol.8,No.3,2008783curve(DSC)of three major endothermic processes in ca.50–170, 170–200,and200–305°C ranges with maxima at ca.165,190, and280°C,corresponding to the stepwise loss of ca.two,one, and two H2O molecules,respectively(the overall mass loss of9.1% is in accord with the calculated value of9.4%for the elimination of allfive water molecules).In accord,the initial broad and intense IRν(H2O)andδ(H2O)bands of1(maxima at3462and1656cm–1, respectively)gradually decrease in intensity on heating the sample up to ca.305°C,while the other bands remain almost unchangeable. Further heating above305°C leads to the sequential decomposition of the bis-terpyridine iron unit.These observations have also been supported by the IR spectra of the products remaining after heating the sample at different temperatures.The elimination of the last portions of water in1at temperatures as high as250–305°C is not commonly observed(although it is not unprecedented18)for crystalline materials with hosted water clusters,and can be related to the presence and extensive hydrogen-bonding of chloride ions in the crystal cell,tending to form the O–H(water)···Cl hydrogen bonds ca.2.5times stronger in energy than the corresponding O–H(water)···O(water)ones.5a The strong binding of crystallization water in1is also confirmed by its FAB+-MS analysis that reveals the rather uncommon formation of the fragments bearing from one tofive H2O molecules.11The exposure to water vapors for ca.8h of an almost completely dehydrated(as confirmed by weighing and IR spectroscopy)product after thermolysis of1(at250°C19for 30min)results in the reabsorption of water molecules giving a material with weight and IR spectrum identical to those of the initial sample1,thus corroborating the reversibility of the water escape and binding process.In conclusion,we have synthesized and structurally characterized a new type of2D hybrid water-chloride anionic multicyclic {[(H2O)20(Cl)4]4–}n network self-assembled in a hydrophobic matrix of the bis-terpyridine iron(II)complex,that is,[FeL2]Cl2·10H2O 1′.On the basis of the recent description and detailed analysis of the related{[(H2O)14(Cl)4]4–}n layers5a and taking into consideration that the water-chloride assembly in1′does not possess strong interactions with the metal-organic units,the crystal structure of 1′can alternatively be defined as an unusual set of water-chloride “hosts”with bis-terpyridine iron“guests”.Moreover,the present study extends the still limited number5of well-identified examples of large polymeric2D water-chloride assemblies intercalated in crystalline materials and shows that terpyridine compounds can provide rather suitable matrixes to stabilize and store water-chloride aggregates.Further work is currently in progress aiming at searching for possible applications in nanoelectrical devices,as well as understanding how the modification of the terpyridine ligand or the replacement of chlorides by other counterions with a high accepting ability toward hydrogen-bonds can affect the type and topology of the hybrid water containing associates within various terpyridine transition metal complexes.Acknowledgment.This work has been partially supported by the Foundation for Science and Technology(FCT)and its POCI 2010programme(FEDER funded),and by a HRTM Marie Curie Research Training Network(AQUACHEM project,CMTN-CT-2003-503864).The authors gratefully acknowledge Prof.Maria Filipa Ribeiro for kindly running the TG-DSC analysis,urent Benisvy,Dr.Maximilian N.Kopylovich,and Mr.Yauhen Y. Karabach for helpful discussions.Supporting Information Available:Additionalfigures(Figures S1–S3)with structural fragments of1′and TG-DSC analysis of1, Tables S1and S2with hydrogen-bond geometry in1′and bond angles within the H2O-Cl network,details for the general experimental procedures and X-ray crystal structure analysis and refinement,crystal-lographic informationfile(CIF),and the CSD refcodes for terpyridine compounds with water-chloride aggregates.This information is available free of charge via the Internet at .References(1)(a)Mascal,M.;Infantes,L.;Chisholm,J.Angew.Chem.,Int.Ed.2006,45,32and references therein.(b)Infantes,L.;Motherwell,S.CrystEngComm2002,4,454.(c)Infantes,L.;Chisholm,J.;Mother-well,S.CrystEngComm2003,5,480.(d)Supriya,S.;Das,S.K.J.Cluster Sci.2003,14,337.(2)(a)Das,M.C.;Bharadwaj,P.K.Eur.J.Inorg.Chem.2007,1229.(b)Ravikumar,I.;Lakshminarayanan,P.S.;Suresh,E.;Ghosh,P.Cryst.Growth Des.2006,6,2630.(c)Ren,P.;Ding,B.;Shi,W.;Wang,Y.;Lu,T.B.;Cheng,P.Inorg.Chim.Acta2006,359,3824.(d)Li,Z.G.;Xu,J.W.;Via,H.Q.;Hu,mun.2006,9,969.(e)Lakshminarayanan,P.S.;Kumar,D.K.;Ghosh,P.Inorg.Chem.2005,44,7540.(f)Raghuraman,K.;Katti,K.K.;Barbour,L.J.;Pillarsetty,N.;Barnes,C.L.;Katti,K.V.J.Am.Chem.Soc.2003,125,6955.(3)(a)Jungwirth,P.;Tobias,D.J.J.Phys.Chem.B.2002,106,6361.(b)Tobias,D.J.;Jungwirth,P.;Parrinello,M.J.Chem.Phys.2001,114,7036.(c)Choi,J.H.;Kuwata,K.T.;Cao,Y.B.;Okumura,M.J.Phys.Chem.A.1998,102,503.(d)Xantheas,S.S.J.Phys.Chem.1996,100,9703.(e)Markovich,G.;Pollack,S.;Giniger,R.;Cheshnovsky,O.J.Chem.Phys.1994,101,9344.(f)Combariza,J.E.;Kestner,N.R.;Jortner,J.J.Chem.Phys.1994,100,2851.(g)Perera, L.;Berkowitz,M.L.J.Chem.Phys.1991,95,1954.(h)Dang,L.X.;Rice,J.E.;Caldwell,J.;Kollman,P.A.J.Am.Chem.Soc.1991, 113,2481.(4)(a)Custelcean,R.;Gorbunova,M.G.J.Am.Chem.Soc.2005,127,16362.(b)Kopylovich,M.N.;Tronova,E.A.;Haukka,M.;Kirillov,A.M.;Kukushkin,V.Yu.;Fraústo da Silva,J.J.R.;Pombeiro,A.J.L.Eur.J.Inorg.Chem.2007,4621.(c)Butchard,J.R.;Curnow,O.J.;Garrett,D.J.;Maclagan,R.G.A.R.Angew.Chem.,Int.Ed.2006, 45,7550.(5)(a)Reger,D.L.;Semeniuc,R.F.;Pettinari,C.;Luna-Giles,F.;Smith,M.D.Cryst.Growth.Des.2006,6,1068and references therein.(b) Saha,M.K.;Bernal,mun.2005,8,871.(c) Prabhakar,M.;Zacharias,P.S.;Das,mun.2006,9,899.(d)Lakshminarayanan,P.S.;Suresh,E.;Ghosh,P.Angew.Chem.,Int.Ed.2006,45,3807.(e)Ghosh,A.K.;Ghoshal,D.;Ribas,J.;Mostafa,G.;Chaudhuri,N.R.Cryst.Growth.Des.2006,6,36.(f)Deshpande,M.S.;Kumbhar,A.S.;Puranik,V.G.;Selvaraj, K.Cryst.Growth Des.2006,6,743.(6)(a)Karabach,Y.Y.;Kirillov,A.M.;da Silva,M.F.C.G.;Kopylovich,M.N.;Pombeiro,A.J.L.Cryst.Growth Des.2006,6,2200.(b) Kirillova,M.V.;Kirillov,A.M.;da Silva,M.F.C.G.;Kopylovich, M.N.;Fraústo da Silva,J.J.R.;Pombeiro,A.J.L.Inorg.Chim.Acta2008,doi:10.1016/j.ica.2006.12.016.(7)The Cambridge Structural Database(CSD).Allen, F.H.ActaCrystallogr.2002,B58,380.(8)The searching algorithm in the ConQuest Version1.9(CSD version5.28,August2007)constrained to the presence of any terpyridinemoiety and at least one crystallization water molecule and one chloride counter ion resulted in43analyzable hits from which40compounds contain diverse water-chloride aggregates(there are29and11 examples of infinite(mostly1D)networks and discrete clusters, respectively).See the Supporting Information for the CSD refcodes.(9)For a recent review,see Constable,E.C.Chem.Soc.Re V.2007,36,246.(10)For recent examples of supramolecular terpyridine compounds,see(a)Beves,J.E.;Constable,E.C.;Housecroft,C.E.;Kepert,C.J.;Price,D.J.CrystEngComm2007,9,456.(b)Zhou,X.-P.;Ni,W.-X.;Zhan,S.-Z.;Ni,J.;Li,D.;Yin,Y.-G.Inorg.Chem.2007,46,2345.(c)Shi,W.-J.;Hou,L.;Li,D.;Yin,Y.-G.Inorg.Chim.Acta2007,360,588.(d)Beves,J.E.;Constable,E.C.;Housecroft,C.E.;Kepert,C.J.;Neuburger,M.;Price,D.J.;Schaffner,S.CrystEngComm2007,9,1073.(e)Beves,J. E.;Constable, E. C.;Housecroft, C. E.;Neuburger,M.;Schaffner,mun.2007,10,1185.(f)Beves,J.E.;Constable,E.C.;Housecroft,C.E.;Kepert,C.J.;Price,D.J.CrystEngComm2007,9,353.(11)Synthesis of1:FeCl2·2H2O(82mg,0.50mmol)and4′-phenyl-2,2′:6′,2″-terpyridine(L)(154mg,0.50mmol)were combined in a THF (20mL)solution with continuous stirring at room temperature.The resulting deep purple suspension was stirred for1h,filtered off,washed with THF(3×15mL),and dried in vacuo to afford a deep purple solid1(196mg,41%).1exhibits a high affinity for water and upon recrystallization gives derivatives with a higher varying content of crystallization water.1is soluble in H2O,MeOH,EtOH,MeCN, CH2Cl2,and CHCl3.mp>305°C(dec.).Elemental analysis.Found: C52.96,H3.76,N8.36.Calcld.for C42H40Cl4Fe2N6O5:C52.42,H4.19,N8.73.FAB+-MS:m/z:835{[FeL2]Cl2·5H2O+H}+,816784Crystal Growth&Design,Vol.8,No.3,2008Communications{[FeL2]Cl2·4H2O}+,796{[FeL2]Cl2·3H2O–2H}+,781{[FeL2]Cl2·2H2O+H}+,763{[FeL2]Cl2·H2O+H}+,709{[FeL2]Cl}+,674 {[FeL2]}+,435{[FeL]Cl2}+,400{[FeL]Cl}+,364{[FeL]–H}+,311 {L–2H}+.IR(KBr):νmax/cm–1:3462(m br)ν(H2O),3060(w),2968 (w)and2859(w)ν(CH),1656(m br)δ(H2O),1611(s),1538(w), 1466(m),1416(s),1243(m),1159(w),1058(m),877(s),792(s), 766(vs),896(m),655(w),506(m)and461(m)(other bands).The X-ray quality crystals of[FeL2]Cl2·10H2O(1′)were grown by slow evaporation,in air at ca.20°C,of a MeOH/H2O(v/v)9/1)solution of1.(12)Crystal data:1′:C42H50Cl2FeN6O10,M)925.63,triclinic,a)10.1851(10),b)12.2125(13),c)19.5622(19)Å,R)76.602(6),)87.890(7),γ)67.321(6)°,U)2180.3(4)Å3,T)150(2)K,space group P1j,Z)2,µ(Mo-K R))0.532mm-1,32310reflections measured,8363unique(R int)0.0719)which were used in all calculations,R1)0.0469,wR2)0.0952,R1)0.0943,wR2)0.1121 (all data).(13)(a)McMurtrie,J.;Dance,I.CrystEngComm2005,7,230.(b)Nakayama,Y.;Baba,Y.;Yasuda,H.;Kawakita,K.;Ueyama,N.Macromolecules2003,36,7953.(c)Kabir,M.K.;Tobita,H.;Matsuo,H.;Nagayoshi,K.;Yamada,K.;Adachi,K.;Sugiyama,Y.;Kitagawa,S.;Kawata,S.Cryst.Growth Des.2003,3,791.(14)Ludwig,R.Angew.Chem.,Int.Ed.2001,40,1808.(15)The searching algorithm in the ConQuest Version1.9(CSD version5.28,May2007)was constrained to the presence of(i)at least onetetranuclear[(H2O)3(Cl)]–ring(i.e.,minimal cyclic fragment in our water-chloride network)with d(O···O))2.2–3.2Åand d(O···Cl) )2.6–3.6Å,and(ii)at least one crystallization water molecule andone chloride counter ion.All symmetry-related contacts were taken into consideration.(16)For2D networks with the[(H2O)3(Cl)]–core,see the CSD refcodes:AGETAH,AMIJAH,BEXVIJ,EXOWIX,FANJUA,GAFGIE, HIQCIT,LUNHUX,LUQCEF,PAYBEW,TESDEB,TXCDNA, WAQREL,WIXVUU,ZUHCOW.For3D network,see the CSD refcode:LUKZEW.(17)This analysis was run on1since we were unable to get1′in a sufficientamount due to the varying content of crystallization water in the samples obtained upon recrystallization of1.(18)(a)Das,S.;Bhardwaj,P.K.Cryst.Growth.Des.2006,6,187.(b)Wang,J.;Zheng,L.-L.;Li,C.-J.;Zheng,Y.-Z.;Tong,M.-L.Cryst.Growth.Des.2006,6,357.(c)Ghosh,S.K.;Ribas,J.;El Fallah, M.S.;Bharadwaj,P.K.Inorg.Chem.2005,44,3856.(19)A temperature below305°C has been used to avoid the eventualdecomposition of the compound upon rather prolonged heating.CG7010315Communications Crystal Growth&Design,Vol.8,No.3,2008785。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Journals about Crystallography(英国化学会)Accounts of Chemical Research Chemical Reviews Chemistry of Materials Crystal Growth & Design Inorganic Chemistry Journal of the American Chemical Society Macromolecules Organometallics(美国化学化)Chemical Communications Chemical Society Reviews CrystEngComm Dalton TransactionsJournal of Materials Chemistry New Journal of Chemistry(德国化学会)Angewandte Chemie International Edition下载) Applied Organometallic Chemistry下载) Chemistry - A European Journal Chemistry - An Asian Journal Crystal Research and Technology无法下载European Journal of Inorganic Chemistry(下载)Zeitschrift für anorganische und allgemeine Chemie(下载)Coordination Chemistry Reviews (下载) Inorganic Chemistry Communications下载) Inorganica Chimica Acta下载)Journal of Alloys and Compounds Journal of Solid State Chemistry Journal of Organometallic ChemistryJournal of Molecular Structure Polyhedron下载) Microporous and Mesoporous Materials Solid State Science(杂环化学)Chemistry of Heterocyclic Compounds Crystallography Reports Journal of Chemical CrystallographyJournal of Cluster Science Journal of Structural Chemistry Monatshefte für Chemie / Chemical MonthlyRussian Chemical Bulletin Russian Journal of Applied Chemistry Russian Journal of Coordination ChemistryRussian Journal of General Chemistry Russian Journal of Inorganic Chemistry Structural ChemistryTransition Metal ChemistryBulletin of the Chemical Society of Japan Chemistry Letters (日本的免费) Journal of Chemical SciencesChinese Journal of Structural Chemistry(全面)Journal of Coordination Chemistry Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry泰勒Acta Crystallographica Section C Acta Crystallographica Section E(只能看摘要)Science Nature ISI Web of Knowledge (查影响因子) Cambridge Crystallographic Data Centre(剑桥数据库)ACTA CRYSTALLOGRAPHICA SECTION AIF 2006 (Crystallography)1 ACCOUNTS CHEM RES0001-4842 22956 17.113 1.912 102 6.82 ACTA CRYSTALLOGR C0108-2701 6788 0.896 0.229 446 >10.03 ACTA CRYSTALLOGR E * 1600-5368 5405 0.567 0.391 3978 1.74 ANGEW CHEM INT EDIT(德国应化)1433-7851 102854 10.232 2.106 1556 5.15 APPL ORGANOMET CHEM0268-2605 2178 1.233 0.176 125 5.86 B CHEM SOC JPN 0009-2673 12026 1.505 0.352 236 >10.07 CHEM COMMUN(化学快报)只要新1359-7345 63573 4.521 1.190 1309 5.88 CHEM-EUR J(欧洲化学)要全面0947-6539 22414 5.015 1.264 859 4.09 CHEM LETT0366-7022 13807 1.734 0.270 678 7.310 CHEM MATER(重性能)0897-4756 38890 5.104 0.692 911 5.211 CHEM REV(大综述)0009-2665 57509 26.054 2.778 189 7.512 CHINESE J STRUC CHEM *(结构化学)0254-5861 833 0.729 0.121 265 3.013 COORDIN CHEM REV(配位化学综述)0010-8545 15077 8.815 1.107 150 6.614 CRYSTENGCOMM *(英国化学会)难发1466-8033 1757 3.729 0.627 118 3.015 CRYST GROWTH DES *(美国化学会)1528-7483 3390 4.339 0.713 456 2.216 CRYST RES TECHNOL0232-1300 1475 0.863 0.138 210 5.817 CRYSTALLOGR REP+1063-7745 1534 0.358 0.073 192 >10.018 DALTON T(英国化学会)1477-9226 25463 3.012 0.890 637 7.219 EUR J INORG CHEM(欧洲无机)1434-1948 8503 2.704 0.468 568 3.620 INORG CHEM(无机化学)美国化学会好发0020-1669 58002 3.911 0.699 1443 8.421 INORG CHIM ACTA(意大利无机化学)0020-1693 12777 1.674 0.433 631 7.922 INORG CHEM COMMUN(无机好发)1387-7003 3248 1.787 0.290 335 3.523 J ALLOY COMPD0925-8388 13994 1.250 0.204 1217 5.324 J AM CHEM SOC(最高)0002-7863 275769 7.696 1.510 3256 7.725 J CHEM CRYSTALLOGR(好发)1074-1542 716 0.658 0.095 126 5.226 J CHEM SCI0253-4134 155 1.120 0.123 73 1.827 J CLUST SCI(促科学)1040-7278 457 1.014 0.186 43 5.628 J COORD CHEM(配位化学制药一个就可以)0095-8972 1361 0.978 0.087 218 5.229 J MOL STRUCT(ESWELY)0022-2860 8907 1.495 0.224 593 6.730 J ORGANOMET CHEM0022-328X 22555 2.332 0.362 738 8.631 J SOLID STATE CHEM(固态化学)0022-4596 12924 2.107 0.294 524 7.832 J STRUCT CHEM+0022-4766 1222 0.345 0.068 117 >10.033 MICROPOR MESOPOR MAT(微空材料)1387-1811 6264 2.796 0.340 427 5.034 NEW J CHEM1144-0546 6592 2.647 0.473 243 5.435 ORGANOMETALLICS0276-7333 31322 3.632 0.897 841 6.536 POLYHEDRON(多面体)0277-5387 9828 1.843 0.374 446 7.437 RUSS J COORD CHEM+1070-3284 995 0.418 0.111 135 9.538 RUSS J INORG CHEM+0036-0236 1798 0.181 >10.039 SOLID STATE SCI1293-2558 1827 1.752 0.256 195 3.640 STRUCT CHEM(结构化学)1040-0400 651 1.510 0.049 81 5.041SYNTH REACT INORG M(好发配合物)1553-3174 1144 0.574 0.034 116 6.942 TRANSIT METAL CHEM(好发配合物)0340-4285 1893 0.918 0.216 167 6.743 Z ANORG ALLG CHEM(慢)0044-2313 7132 1.241 0.474 369 9.7。
