LY2228820_COA_04790_MedChemExpress
Study_on_the_pharmacological_activities_and_chemic

ReviewStudy on the pharmacological activities and chemicalstructures of Viburnum dilatatumZhiheng Gao, Yufei Xi, Man Wang, Xiaoxiao Huang*, Shaojiang Song*Key Laboratory of Computational Chemistry-Based Natural Antitumor Drug Research &Development, Liaoning Province, School of Traditional Chinese Materia Medica, ShenyangPharmaceutical University, Shenyang 110016, ChinaAbstractViburnum dilatatum (jiami in Chinese), belonging to the Caprifollaceae family, is widely distributed in Japan and China. Phytochemical investigations of Viburnum dilatatum (V. dilatatum) have resulted in the isolation of triterpenoids, phenolic glycosides essential oil, norisoprenoids, etc. Research results have shown that the chemical constituents of V. dilatatum possess various pharmacological activities, including antihyperglycemic, antioxidant activity and antiulcer effects. This study reviewed the chemical constituents and pharmacological activities of V. dilatatum to provide practical and useful information for further research and development of this plant.Keywords: Viburnum dilatatum; pharmacological activity; chemical structures1 IntroductionViburnum dilatatum (called jiami in Chinese, gamazumi in Japanese and snowball tree in English), beloinging to family Caprifoliaceae, is a deciduous low tree distributed widely in the hills of northern China and Japan [1]. There are many types of chemical constituents in Viburnum dilatatum (V. dilatatum), including triterpenoids, * Author to whom correspondence should be addressed. Address:School of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, 103 Wenhua Rd., Shenyang 110016, China; Tel.: +86-24-43520793 (Xiaoxiao Huang); +86-24-43520707 (ShaojiangSong);E-mail:*******************(XiaoxiaoHuang); ****************(ShaojiangSong).Received: 2021-04-16 Accepted: 2022-08-28phenolic glycosides and norisoprenoids [2-4]. The leaves have been utilized as a traditional Chinese medicine, and phenolic compounds have been reported as the main active chemical component of the leaves. Many researchers have analyzed the functions of these medicinal components and found that these components have good antioxidant antihyperglycemic and antiulcer effects. For example, the gamazumi crude extract obtained from the squeezed juice of the fruit prevented oxidative injury in rats [5]. This review described the chemical structures and pharmacological activities of V. dilatatum, so as to help readers understand comprehensively the research progress of V. dilatatum and provide help for the development of V. dilatatum.2 Chemical constituents and structuresPrevious reports have indicated that the main chemical constituents of V. dilatatum are phenolic glycosides and triterpenoids.2.1 Phenolic glycosidesThirteen phenolic glycosides were isolated and identified from V. dilatatum by extensive spectroscopic methods, namely p -hydroxyphenyl-6-O -trans-caffeoyl-β-D -glucoside (1) [6], p -hydroxyphenyl-6-O -trans-caffeoyl-β-D -alloside (2) [6], 4-allyl-2-methoxyphenyl-6-O -β-D -apiosyl(1→6)-β-D -glucoside (3) [6], 1-(4’-hydroxy-3’-methoxypheny1)-2-[2’’-hydroxy-4’’-(3’’’-hydroxypropyl)]-1,3-propanediol-l-O -β-D -glucopyranoside (erythro isomer) (4-7) [7], neochlorogenic acid methyl ester (8-9) [7], cryptochlorogenic acid methyl ester (10-11) [7], cyanidin-3-sambubioside (Cy-3-sam) (12) [8], cyanidin-3-glucoside (Cy-3-glc) (13) [8], 5-O -caffeoyl-4-methoxyl quinic acid (4-MeO-5-CQA) (14) [8], chlorogenic acid (5-CQA) (15) [8], quercetin (16) [8], 2-(glucopyranosyloxy)-benzyl-3-(glucopyranosyloxy)-benzoate (17) [9] and jiamizioside E (18) [10]. These structures are shown in Fig. 1.Fig. 1 Phenolic glycosides isolated from V . dilatatumContinued fig. 12.2 TriterpenoidsThere were about seventeen triterpenoids isolated and characterized from V. dilatatum , such as viburnols A (19) [11], viburnols B (20) [11], viburnols C (21) [11], viburnols D (22) [11], viburnols E (23) [11], viburnols F (24) [12], viburnols G (25) [12], viburnols H (26) [12], viburnols I (27) [12], viburnols J (28) [12],viburnols K (29) [12], viburnudienone B 2methyl ester (30) [13], viburnenone H 2 (31) [13],v i b u r n e n o n e B 2 m e t h y l e s t e r (32) [13], viburnudienone B 1 methyl ester (33) [13], viburnenone H 1 (34) [13], and viburnenone B 2 methyl ester (35) [13]. The structures are shown in Fig. 2.Continued fig. 23 Pharmacological activities3.1 Antioxidant activityOxidative stress caused by free radicals and their derivatives leads to disturbances in redox homeostasis. Reactive oxygen species (ROS) are not only endogenously produced during intracellular metabolic processes but also generated by exogenous stimuli such as UV radiation, pollutants, smoke and drugs. The cell triggers its defense systems or undergoes apoptosis when intracellular oxidative status increases. It influences numerous cellular processes including core signaling pathways, which are associated with development of systematic and chronic disorders, such as aging and cancer. Therefore, it is critical to remove cellular oxidants and restore redox balance.solution of V. dilatatum (GSS) had strong antioxidant activity in vivo and prevent stress-induced oxidative damage by the XYZ-dish method and the澳electron spin resonance (ESR) method [14]. The experimental result showed that the concentrations of lipid peroxide in plasma, liver and stomach in the GSS group were reduced. Furthermore, the activities of plasma lactic dehydrogenase, amylase and creatine phosphokinase are ordinarily increased by stress. However, these activities in the GSS group decreased to that in the control group. It was concluded that gastric ulcer formation, increase of lipid peroxidation in plasma and tissues and elevation of plasma enzymatic activities were confirmed in rats with water immersion restraint stress. It was also found that intake of GSS could protect the stomach and other tissues from oxidative damage.Kim et al. identified and isolated two major anthocyanins by NMR and LC-ESI-MS/MS, namely, cyanidin 3-sambubioside (I) and kuromanin (II) [15]. By the electron spin resonance method, the superoxide anion radical scavenging activities of I and II were evaluated with the IC 50 values of 17.3 and 69.6 µM, and their activities on hydroxyl radicals were evaluated with the IC 50 values of 4.3 and 53.2 mM. As the positive control, the IC 50 values of ascorbic acid were 74.2 µM on superoxide anion radicals and 3.0 mM on hydroxyl radicals, respectively. The above results suggested that these anthocyanins with radical scavenging properties might be the key compounds contributing to the antioxidant activity and physiological effects of V . dilatatum fruits.Woo et al. determined the free radical scavenging capacity of VD (the leaves of V. dilatatum ) [16]. Anti-oxidant activity of the extracts was assessed by the ability to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) or 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radicals. Butylated hydroxytoluene (BHT), a synthetic antioxidant, or α-tocopherol, was used as the positive control in these assays. The experimental result showed that VD inducedincrease in radical scavenging activity. In addition, lipid peroxidation inhibitory activity was determined via measurement of MDA (Malondialdehyde) levels using mouse liver tissue homogenate treated with various concentrations of the extracts. The concentration-dependent decrease in MDA levels observed was consistent with radical scavenging activities of the extracts. To examine whether VD extracts could protect mam-malian cells from oxidative stress, cultures of a human mammary gland-derived epithelial cell line MCF-7 were treated with each extract prior to challenging them with tBHP. The intracellular ROS (Reactive oxygen species) production was determined with the relative intensity of dichlorofluorescein fluorescence. While intracellular ROS formation was significantly promoted by tBHP treatment, the augmented ROS level was significantly reduced after the treatment with VD extracts.3.2 Antihyperglycemic effectIwai et al. used an oral glucose tolerance test on the diabetic rats [17]. They found that the elevation of plasma glucose level after oral administration of 2 g/kg glucose was suppressed by the repeated administration of the freeze-dried powder of V. dilatatum fruit juice (CEV). The α-glucosidase inhibitory activities of isolated compounds from CEV were also measured. Cyanidin 3-sambubioside and 5-caffeoyl quinic acid A showed inhibitory activity. These results suggested that V. dilatatum fruit had the antihyperglycemic effects.4 ConclusionV. dilatatum is distributed widely in the hills of northern China and Japan. Currently, the studies on V. dilatatum have been conducted at home and abroad, but few studies focus on its chemical components and pharmacological activities. Previousphytochemical investigations showed that the constituents of V. dilatatum included triterpenoids, phenolic glycosides, norisoprenoids and other compounds. This study describes thirteen phenolic glycosides and seventeen triterpenoids and their different degrees of antihyperglycemic, antioxidant activity and antiulcer effects, aiming to provide a reference for further studies on V. dilatatum and pharmaceutical development.References[1] Jeffrey B, Harborne A. Colour atlas of medicinal plantsof Japan. Phytochemistry, 1981, 20: 1467.[2] Miyazawa M, Hashidume S, Takahashi T, et al. Aromaevaluation of gamazumi (Viburnum dilatatum) by aroma extract dilution analysis and odour activity value.Phytochem Anal, 2012, 23: 208-213.[3] Kurihara T, Kikuchi M. Studies on the constituentsof flowers. IV. On the components of the flower of Viburnum dilatatum Thunb. J Health Sci, 1975, 95: 1098-1102.[4] Machida K, Kikuchi M. Norisoprenoids from Viburnumdilatatum. Phytochemistry, 1996, 41: 1333-1336. [5] Iwai K, Onodera A, Matsue H. Mechanism of preventiveaction of Viburnum dilatatum Thunb (gamazumi) crude extract on oxidative damage in rats subjected to stress. J Sci Food Agric, 2010, 83: 1593-1599.[6] Machida K, Nakano Y, Kikuchi M. Phenolic glycosidesfrom Viburnum dilatatum. Phytochemistry, 1991, 30: 2013-2014.[7] Machida K, Kikuchi M. Phenolic compounds fromViburnum dilatatum. Phytochemistry, 1992, 31: 3654-3656.[8] Kim MY, Iwai K, Matsue H. Phenolic compositions ofViburnum dilatatum Thunb. fruits and their antiradical properties. J Food Compos Anal, 2005, 18: 789-802. [9] Lu D, Yao S. Phenolic glycoside from the roots ofViburnum dilatatum. Nat Prod Commun, 2009, 4: 945-946.[10] Wu B, Zeng X, Zhang Y. New metabolite fromViburnum dilatatum. Nat Prod Commun, 2010, 5: 1097-1098.[11] Machida K, Kikuchi M. Viburnols: Novel triterpenoidswith a rearranged dammarane skeleton from Viburnum dilatatum. Tetrahedron Lett, 1996, 37: 4157-4160. [12] Machida K, Kikuchi M. Viburnols: Six noveltriterpenoids from Viburnum dilatatum. Tetrahedron Lett, 1997, 38: 571-574.[13] Machida K, Kikuchi M. Studies on the Constituents ofViburnum Species. XIX. Six New Triterpenoids from Viburnum dilatatum Thunb. Chem Pharm Bull, 1999, 47: 692-694.[14] Iwai K, Onodera A, Matsue H, et al. Antioxidant activityand inhibitory effect of Gamazumi (Viburnum dilatatum THUNB.) on oxidative damage induced by water immersion restraint stress in rats. Int J. Food Sci Nutr, 2001, 52: 443-451.[15] Kim MY, Iwai K, Onodera A, et al. Identification andAntiradical Properties of Anthocyanins in Fruits of Viburnum dilatatum Thunb. J Agric Food Chem, 2003, 51: 6173-6177.[16] Woo YJ, Lee HJ, Jeong YS, et al. Antioxidant Potentialof Selected Korean Edible Plant Extracts. Bio Med Res Int, 2017, 2017: 1-9.[17] Iwai K, Kim MY, Akio O, et al. Alpha-glucosidaseinhibitory and antihyperglycemic effects of polyphenols in the fruit of Viburnum dilatatum Thunb. J Agric Food Chem, 2006, 54: 4588-4592.。
Bedaquiline fumarate_845533-86-0_DataSheet_MedChemExpress
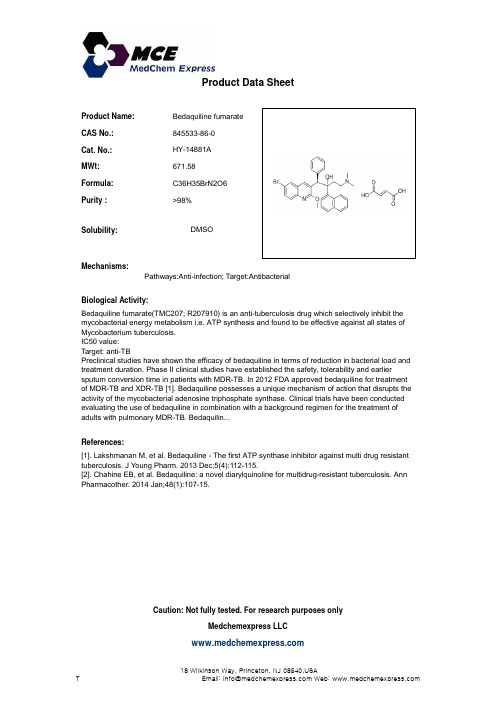
Product Name:Bedaquiline fumarate CAS No.:845533-86-0Cat. No.:HY-14881A Product Data SheetMWt:671.58Formula:C36H35BrN2O6Purity :>98%Solubility:DMSOMechanisms:Biological Activity:Bedaquiline fumarate(TMC207; R207910) is an anti-tuberculosis drug which selectively inhibit the Pathways:Anti-infection; Target:Antibacterial q (;)g y mycobacterial energy metabolism i.e. ATP synthesis and found to be effective against all states ofMycobacterium tuberculosis.IC50 value:Target: anti-TB Preclinical studies have shown the efficacy of bedaquiline in terms of reduction in bacterial load and treatment duration. Phase II clinical studies have established the safety, tolerability and earliersputum conversion time in patients with MDR-TB. In 2012 FDA approved bedaquiline for treatment of MDR-TB and XDR-TB [1]. Bedaquiline possesses a unique mechanism of action that disrupts the ti it f th b t i l d i t i h h t th Cli i l t i l h b d t d References:[1]. Lakshmanan M, et al. Bedaquiline - The first ATP synthase inhibitor against multi drug resistanttuberculosis. J Young Pharm. 2013 Dec;5(4):112-115.[2]Chahine EB et al Bedaquiline:a novel diarylquinoline for multidrug-resistant tuberculosis Ann activity of the mycobacterial adenosine triphosphate synthase. Clinical trials have been conducted evaluating the use of bedaquiline in combination with a background regimen for the treatment of adults with pulmonary MDR-TB. Bedaquilin...[2]. Chahine EB, et al. Bedaquiline: a novel diarylquinoline for multidrug-resistant tuberculosis. AnnPharmacother. 2014 Jan;48(1):107-15.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AT E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
HPLC法测定注射用艾迪(冻干)中紫丁香苷的含量

HPLC法测定注射用艾迪(冻干)中紫丁香苷的含量
王晓辉;李清;程维明;王志伟;陈晓辉;毕开顺
【期刊名称】《沈阳药科大学学报》
【年(卷),期】2006(23)12
【摘要】目的建立注射用艾迪(冻干)中紫丁香苷的含量测定方法。
方法采用HPLC法,色谱柱为Zorbax C18柱(250mm×4,6mm。
5μm),流动相为乙
腈-水(体积比为9:91),检测波长为265nm,柱温为40℃。
结果紫丁香苷在5,35~26.75mg·L^-1内峰面积与质量浓度呈良好的线性关系,相关系数为0,9998,平均回收率为99.7%,RSD为2.0%(n=9)。
结论采用HPLC法测
定注射用艾迪(冻干)中紫丁香苷的含量可作为注射用艾迪(冻干)质量控制的指标。
【总页数】3页(P782-784)
【关键词】注射用艾迪(冻干);紫丁香苷;高效液相色谱法;含量测定
【作者】王晓辉;李清;程维明;王志伟;陈晓辉;毕开顺
【作者单位】沈阳药科大学药学院
【正文语种】中文
【中图分类】R917
【相关文献】
1.HPLC同时测定注射用艾迪(冻干)中异嗪皮啶和芒丙花素的含量 [J], 王晓辉;李清;程维明;陈晓辉;毕开顺
2.RP-HPLC法测定刺五加注射液中紫丁香苷、紫丁香树脂苷的含量 [J], 焦正花;顾秀琰;杨小源;金录胜
3.HPLC法测定艾迪注射液中紫丁香苷含量 [J], 陈靖;庞燕军;曹春英;张悫
4.SPE-HPLC测定注射用红景天(冻干)中红景天苷和酪醇的含量 [J], 孟健;王淑芬;韩飞;李三鸣
因版权原因,仅展示原文概要,查看原文内容请购买。
结直肠癌患者尿液中20种氨基酸靶向代谢组特征研究

结直肠癌患者尿液中20种氨基酸靶向代谢组特征研究祁峰;孙玉琳;刘佳琦;郭正光;刘晓燕;孙海丹;何成彦;赵晓航;孙伟【期刊名称】《中国实验诊断学》【年(卷),期】2024(28)4【摘要】目的探究20种常见氨基酸在结直肠癌(CRC)患者的尿液特征;寻找CRC 诊断的生物学标志物。
方法纳入100名健康对照者和113名CRC患者(Ⅰ期27例,Ⅱ期28例,Ⅲ期34例,Ⅳ期24例),基于超高效液相色谱串联质谱法对尿液中的20种常见氨基酸应用多反应监测技术进行靶向定量分析,应用统计学方法分析CRC 患者与健康对照氨基酸含量差异,并构建CRC诊断模型。
结果CRC患者尿液中16种氨基酸含量与健康对照差异有统计学意义,Ⅰ,Ⅱ,Ⅲ和Ⅳ期各自有9种,15种,14种,17种氨基酸含量与健康对照差异有统计学意义。
由异亮氨酸,天冬酰胺,脯氨酸,组氨酸,酪氨酸,苯丙氨酸6种氨基酸构建的生物标志物组合用于CRC诊断的AUC 值在发现组/验证组分别为0.857/0.851,在Ⅰ,Ⅱ,Ⅲ和Ⅳ期CRC的AUC值分别为0.847,0.843,0.897,0.896。
结论尿液中20种氨基酸含量变化可以用于CRC诊断,为CRC的早期诊断提供了新的研究思路。
【总页数】7页(P395-401)【作者】祁峰;孙玉琳;刘佳琦;郭正光;刘晓燕;孙海丹;何成彦;赵晓航;孙伟【作者单位】中国医学科学院基础医学研究所·北京协和医学院基础学院药理系;国家癌症中心·国家癌症临床研究中心·中国医学科学院癌症医院分子肿瘤国家重点实验室;吉林大学中日联谊医院检验科【正文语种】中文【中图分类】R446.12;R735.3【相关文献】1.miR-128-3p靶向xCT基因在结直肠癌中的分子机制及其与肠癌患者临床病理特征的相关性2.弥漫大B细胞淋巴瘤患者血清氨基酸谱的靶向代谢组学研究3.基于非靶向代谢组学的老年胃癌患者术前衰弱与代谢综合征代谢特征研究4.基于液相色谱-串联质谱技术的氨基酸代谢组学在结直肠癌研究中的应用5.基于16S rRNA和非靶向代谢组测序分析结直肠癌患者肠道菌群及代谢物的变化因版权原因,仅展示原文概要,查看原文内容请购买。
一种叶酸顺式乌头酸酐键阿霉素人血清白蛋白(FACADHSA)组合物及其制备方法和应用[发明专利]
![一种叶酸顺式乌头酸酐键阿霉素人血清白蛋白(FACADHSA)组合物及其制备方法和应用[发明专利]](https://img.taocdn.com/s3/m/dda194f8a48da0116c175f0e7cd184254b351be1.png)
专利名称:一种叶酸/顺式乌头酸酐键阿霉素/人血清白蛋白(FA/CAD/HSA)组合物及其制备方法和应用
专利类型:发明专利
发明人:李红玉,王加涛,邹玥,余兰,乔志伟,李洋
申请号:CN202111553281.X
申请日:20211217
公开号:CN114306356A
公开日:
20220412
专利内容由知识产权出版社提供
摘要:本发明涉及一种叶酸/顺式乌头酸酐键阿霉素/人血清白蛋白(FA/CAD/HSA)组合物及其制备方法和应用。
该组合物是由叶酸、顺式乌头酸酐键阿霉素和人血清白蛋白通过化学偶联而成的靶向给药系统,具有叶酸主动靶向和pH敏感释药功能,能够降低阿霉素毒性,对肿瘤细胞产生强大的杀伤作用。
申请人:兰州大学
地址:730000 甘肃省兰州市城关区天水南路222号
国籍:CN
更多信息请下载全文后查看。
丹酚酸A在制备抗肿瘤细胞上皮间质转化药物中的应用[发明专利]
![丹酚酸A在制备抗肿瘤细胞上皮间质转化药物中的应用[发明专利]](https://img.taocdn.com/s3/m/465ca43fce2f0066f4332240.png)
专利名称:丹酚酸A在制备抗肿瘤细胞上皮间质转化药物中的应用
专利类型:发明专利
发明人:毕蕾,陈卫平,颜晓静
申请号:CN201410467822.0
申请日:20140911
公开号:CN104666289A
公开日:
20150603
专利内容由知识产权出版社提供
摘要:本发明公开了丹酚酸A用于制备抗肿瘤细胞上皮间质转化药物的应用。
本发明的重要之处是发现了丹酚酸A对肿瘤细胞上皮间质转化具有显著的抑制作用,对上皮间质转化关键蛋白基因有明显的影响,具有制备抗肿瘤细胞上皮间质转化药物的应用前景。
申请人:南京中医药大学
地址:210023 江苏省南京市栖霞区仙林大学城仙林大道138号
国籍:CN
更多信息请下载全文后查看。
毛蕊花糖苷在制备预防和治疗神经退行性疾病药物中的应用[发明专利]
![毛蕊花糖苷在制备预防和治疗神经退行性疾病药物中的应用[发明专利]](https://img.taocdn.com/s3/m/dfedba3b5022aaea988f0f41.png)
专利名称:毛蕊花糖苷在制备预防和治疗神经退行性疾病药物中的应用
专利类型:发明专利
发明人:米娜,木塔力甫·艾买提,伊力亚斯·艾萨,古丽努尔·阿不力米提,李飞
申请号:CN202010590753.8
申请日:20200624
公开号:CN111632058A
公开日:
20200908
专利内容由知识产权出版社提供
摘要:本发明为毛蕊花糖苷在制备预防和治疗神经退行性疾病药物中的应用。
本发明首先发现毛蕊花糖苷对鱼藤酮诱导的神经细胞损伤具有修复作用;研究发现,毛蕊花糖苷可以显著降低神经细胞活性氧、保护神经细胞线粒体的完整性、诱导细胞自噬,以及降低帕金森病相关α‑突触核蛋白
(α‑Synuclein,α‑Syn)的堆积,抗细胞凋亡。
实验结果表明,毛蕊花糖苷通过诱导细胞自噬而发挥其对神经细胞的损伤修复功能,具有抗神经退行性疾病的活性。
本发明提出毛蕊花糖苷具有确切的预防和治疗神经退行性疾病新型药物的应用前景。
申请人:新疆医科大学
地址:830000 新疆维吾尔自治区乌鲁木齐市新医路393号
国籍:CN
代理机构:北京鼎佳达知识产权代理事务所(普通合伙)
更多信息请下载全文后查看。
