Norethindrone_DataSheet_MedChemExpress
炔诺酮 Norethindrone-贝尔卡 低价高纯甾体激素原料药

医药有限公司"。 公司主要生产基地位于通山,占地面积 3000 亩,主要生产雄性激素、雌性激素、孕激素、糖皮质激
素等甾体激素原料药。公司联合了武汉大学、华中科技大学、武汉工程大学等相关专家教授了组建了一批
技术专家团队,专门负责甾体合成工艺的优化和绿色生产工艺的开发,确保贝尔卡的生产技术处于国际领 先水平。贝尔卡生产的多个甾体产品已通过国家 GMP 认证、COS 认证,遍及亚洲、欧洲、北美、南美等 各个国家。
产品应用 复方炔诺酮片
少数妇女可有恶心、呕吐、头晕、乏力、嗜睡等类早孕反应及不规则出血、闭经、乳房胀、皮 备注
疹等,一般可自行消失。
品名 生产日期 生产批号 批量
炔诺酮 2014-12-25 20141225 50kg
Cas.No 报告日期 有效日期 检验标准
68-22-4 2014-12-26 2016-12-24 USP34
贝尔卡生物医药
产品采购请点击
药理作用
孕激素类药。本品有较强的孕激素样作用,能使子宫内膜转化为蜕膜样变,其抑制垂体分泌促 性腺激素作用呈明显剂量关系,并有一定的抗雌激素作用,具有较弱的雄激素活性和蛋白同化 作用。可抑制排卵,使宫颈粘液变稠,不利于精子穿透。
主要用途 孕激素类药,用于月经不调、子宫功能性出血、子宫内膜异位症等
核验员:梁辉
检验结果
符合规定 合格 202.5~207℃ 正性反应 符合 符合 合格 合格 -34.5° 0.23% 8.27% 99.06%
质检科长:高进
贝尔卡生物医药
产品采购请点击
武汉贝尔卡生物医药有限公司创建于 2001 年,原名通山县医药原料厂,是国家级高新技术企业。2014 年,公司董事会从战略发展角度出发,决定响应打造国家级产业基地的号召,将公司总部迁入武汉市生物 医药园。与此同时,公司借力园区政策优势,进行产研合作,引入外商投资,正式更名为"武汉贝尔卡生物
阿替洛尔 结构

阿替洛尔1. 介绍阿替洛尔(Atorvastatin)是一种用于降低胆固醇和脂蛋白水平的药物。
它属于一类称为他汀类药物的药物家族,可以有效地降低低密度脂蛋白胆固醇(LDL-C)水平,并增加高密度脂蛋白胆固醇(HDL-C)水平。
阿替洛尔是世界上最常用的他汀类药物之一,广泛应用于治疗高胆固醇和心血管疾病。
2. 药理作用阿替洛尔通过抑制3-羟基-3-甲基戊二酸还原酶(HMG-CoA还原酶)的活性来发挥其降胆固醇作用。
HMG-CoA还原酶是胆固醇合成过程中的一个关键酶,通过抑制其活性,阿替洛尔可以减少肝脏内胆固醇的合成,从而降低血液中的总胆固醇和LDL-C水平。
此外,阿替洛尔还可以通过增加肝脏上清除LDL-C的受体的表达,促进LDL-C的清除和代谢。
同时,阿替洛尔还可以抑制氧化低密度脂蛋白(ox-LDL)的形成,并减少血小板聚集和炎症反应。
3. 适应症阿替洛尔主要用于治疗高胆固醇和心血管疾病。
具体适应症包括: - 原发性高胆固醇血症:用于降低总胆固醇、LDL-C、载脂蛋白B(ApoB)、非高密度脂蛋白胆固醇(non-HDL-C)和三酰甘油(TG)水平,以及增加HDL-C水平。
- 混合型高血脂症:用于降低总胆固醇、LDL-C、ApoB、non-HDL-C和TG水平,以及增加HDL-C水平。
- 高三酰甘油血症:用于降低TG水平。
- 家族性高胆固醇血症:用于降低总胆固醇、LDL-C、ApoB和non-HDL-C水平。
- 心血管疾病的二级预防:用于降低心血管事件的风险,包括非致命性心肌梗死、心脏血管事件、冠状动脉重建术、卒中或缺血性心脏病死亡。
4. 用法和剂量阿替洛尔通常以口服片剂的形式给予。
剂量根据患者的具体情况而定,应由医生根据患者的胆固醇水平、年龄、性别和其他相关因素进行调整。
一般情况下,阿替洛尔的初始剂量为10-20毫克(mg)/天,可在4周后逐渐增加。
维持剂量通常为10-80mg/天。
对于需要更强降胆固醇作用的患者,最大剂量可以增加到80mg/天。
诺适得说明书

核准日期:2011年12月31日修改日期:2014年02月18日2014年10月31日2016年01月12日2017年01月16日2018年04月28日2018年11月08日2018年11月21日2020年04月07日2020年06月24日2021年XX月XX日雷珠单抗注射液说明书请仔细阅读说明书并在医师指导下使用。
【药品名称】通用名称:雷珠单抗注射液商品名称:诺适得®/Lucentis®英文名称:Ranibizumab Injections汉语拼音:Leizhu Dankang Zhusheye【成份】活性成份:雷珠单抗化学名称:G1,抗-(人血管内皮生长因子)Fab片断(人-鼠单克隆rhuFabV2γ1-链),二硫键结合人-鼠单克隆rhuFabV2κ-链分子量:48KD处方组成:1mL含10mg雷珠单抗。
本品所含辅料为:α,α-海藻糖二水合物;组氨酸;盐酸组氨酸一水合物;聚山梨醇酯20。
【性状】透明至微乳白色液体。
【适应症】雷珠单抗注射液适用于成人:∙用于治疗湿性(新生血管性)年龄相关性黄斑变性(AMD)。
∙用于治疗糖尿病性黄斑水肿(DME)引起的视力损害。
∙用于治疗糖尿病视网膜病变(DR)[增殖性糖尿病视网膜病变(PDR)和中重度至重度非增殖性糖尿病视网膜病变(NPDR)]。
∙用于治疗继发于视网膜静脉阻塞(RVO)(视网膜分支静脉阻塞(BRVO)或视网膜中央静脉阻塞(CRVO))的黄斑水肿引起的视力损害。
∙用于治疗脉络膜新生血管(CNV,即继发于病理性近视(PM)和其它原因的CNV)导致的视力损害。
雷珠单抗注射液适用于早产儿:∙用于治疗I区(1+、2+、3或3+期)、II区(3+期)早产儿视网膜病变(ROP)和AP-ROP(急进性后极部ROP)。
【规格】10mg/mL,每瓶装量0.20mL。
【用法用量】本品应在有资质的医院和眼科医生中使用。
医院应具备疾病诊断和治疗所需的相关仪器设备和条件,眼科医生应具备确诊湿性年龄相关性黄斑变性,糖尿病性黄斑水肿,糖尿病视网膜病变(DR)[增殖性糖尿病视网膜病变(PDR)和中重度至重度非增殖性糖尿病视网膜病变(NPDR)],继发于视网膜静脉阻塞(RVO)的黄斑水肿,脉络膜新生血管疾病以及早产儿视网膜病变的能力和丰富的玻璃体内注射经验。
常用蛋白酶切割位点
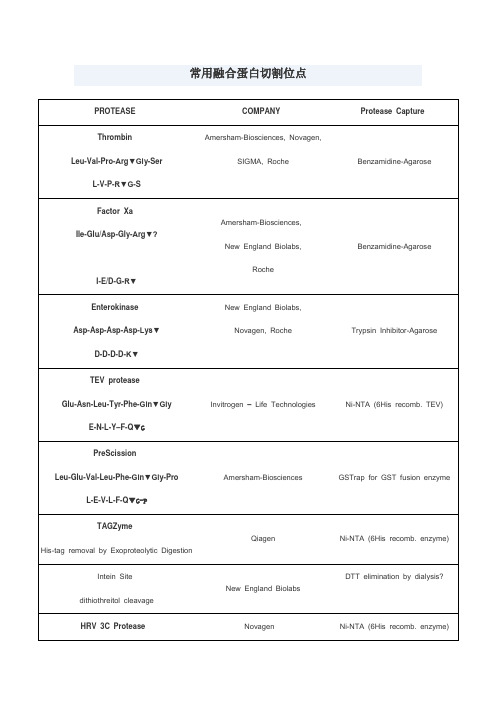
4.溴化氰处理,专一性的切割甲硫氨酸羧基端的肽键。
SIGMA, Roche
Benzamidine-Agarose
Factor Xa
Ile-Glu/Asp-Gly-Arg▼?
I-E/D-G-R▼
Amersham-Biosciences,
New England Biolabs,
Roche
Benzamidine-Agarose
Enterokinase
Asp-Asp-Asp-Asp-Lys▼
羧肽酶
羧肽酶B可以切割C端的Lys或Arg;羧肽酶A可以切割C端除了Lys、Arg、Pro的氨基酸,但如果倒数第二个氨基酸为Pro两种羧肽酶均不能作用
1.胰蛋白酶属肽链内切酶,能把多肽链中Lys和Arg残基中的羧基侧切断。
2.胰凝乳蛋白酶(亦称糜蛋白酶)属肽链内切酶,主要切断多肽链中的芳香族氨基酸(Phe、Trp、Tyr)残基的羧基一侧。
LifeSensors
Ni-NTA (6His recomb. enzyme)
Kex-2
-Arg-X-Lys/Arg-Arg▼
Invitrogen – Life Technologies,
Ni-NTA (6His recomb. enzyme)
KEX2对arg的专一性高,要求最重要。
Arg前为lys效率最高,不切-Arg-lys,Pro影响KEX2切割
Ni-NTA (6His recomb. TEV)
PreScission
Leu-Glu-Val-Leu-Phe-Gln▼Gly-Pro
VX-765-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-12-2018Print Date:Oct.-12-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :VX-765Catalog No. :HY-13205CAS No. :273404-37-81.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, oral (Category3), H301Skin irritation (Category 3), H316Eye irritation (Category 2B), H320Reproductive toxicity (Category 2), H3612.2 GHS Label elements, including precautionary statementsPictogram No data availableSignal word No data availableHazard statement(s)No data availablePrecautionary statement(s)No data available2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Belnacasan;VX765;VX 765Formula:C24H33ClN4O6Molecular Weight:509.00CAS No. :273404-37-84. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Drospirenone_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Drospirenone(Dihydrospirorenone) is a synthetic progestin that is an analog to spironolactone.Target: Progesterone ReceptorDrospirenone is a novel progestin under clinical development that is similar to the natural hormone progesterone, combining potent progestogenic with antimineralocorticoid and antiandrogenic activities. drospirenone was devoid of glucocorticoid activity. Both progestins did not show any antiglucocorticoid action. Furthermore, drospirenone and progesterone both showed considerable antimineralocorticoid activity and weak mineralocorticoid activity [1]. the pharmacological profile of drospirenone is more closely related to that of the natural hormone progesterone than is that of any other synthetic progestogen in use today. Therefore,drospirenone is anticipated to give rise to a number of additional health benefits both for users of oral contraceptives and hormone replacement therapy recipients [2]. The combination of 17beta–estradiol and drospirenone has a positive effect on BMD and apotentially beneficial effect on lipids. Although endometrial thickness increased slightly, the safety of the endometrium was assured,as no cases of hyperplasia or cancer occurred [3].Clinical indications: Acne; Dysmenorrhea; Endometriosis; Female contraception; Folic acid deficiency; Premenstrual syndrome References:[1]. Fuhrmann, U., et al., The novel progestin drospirenone and its natural counterpart progesterone: biochemical profile and antiandrogenic potential.Contraception, 1996. 54(4): p. 243–51.[2]. Muhn, P., et al., Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Pharmacological characterization in animal models. Contraception, 1995. 51(2): p. 99–110.[3]. Warming, L., et al., Safety and efficacy of drospirenone used in a continuous combination with 17beta–estradiol for prevention of postmenopausal osteoporosis. Climacteric, 2004. 7(1): p. 103–11.Product Name:Drospirenone Cat. No.:HY-B0111CAS No.:67392-87-4Molecular Formula:C 24H 30O 3Molecular Weight:366.49Target:Progesterone Receptor Pathway:Others Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
加州65有害物质清单2013中文版

Listing Mechanism SQE SQE SQE SQE SQE AB FR
FR FR
CAS No. --15972-60-8 --309-00-2 302-79-4 107-05-1 28981-97-7 645-05-6 665-66-7 39831-55-5 117-79-3 60-09-3 97-56-3 92-67-1 81-49-2 6109-97-3 153-78-6 125-84-8 --82-28-0 712-68-5 119-34-6 54-62-6 19774-82-4 33089-61-1 61-82-5 14028-44-5 51264-14-3 994-05-8 ----63-05-8 --62-53-3 142-04-1
cancer cancer cancer developmental cancer developmental cancer cancer cancer developmental, male cancer cancer developmental cancer
AB SQE AB
FR
SQE FR SQE AB AB
AB
26148-68-5 75-07-0 60-35-5 59-66-5 34256-82-1 546-88-3 53-96-3 62476-59-9 79-06-1 79-06-1 107-13-1 50-76-0 50-76-0 3688-53-7
1-Jan-90 2 1-Apr-88 90 (inhalation) 1-Jan-90 10 20-Aug-99 1-Jan-89 1-Apr-90 1-Jul-87 0.2 1-Jan-90 1-Jan-90 0.2 25-Feb-11 1-Jul-87 1-Oct-89 1-Oct-92 1-Jul-87
复方新诺明片美国药典标准

复方新诺明片甲氧苄氨嘧啶和磺胺甲噁唑含量:甲氧苄氨嘧啶含量:92.5~107.5%磺胺甲噁唑含量:92.5~107.5%储存:密封、避光保存鉴别:供试品溶液:将相当于含有甲氧苄氨嘧啶(4mg)的片剂粉末加入到10ml容量瓶中,再加入甲醇8ml,加温摇匀。
冷却,甲醇稀释至10ml,混匀,稍稍离心。
对照品溶液:将甲氧苄氨嘧啶(USPRS)用甲醇制成0.4mg/ml的溶液;将磺胺甲噁唑(USPRS)用甲醇制成0.2mg/ml的溶液。
点样量:5μl薄层板:薄层色谱硅胶板展距:3cm流动相:氯仿/异丙醇/二乙胺(6:5:1)干燥:暖空气中干燥。
检验:短波长紫外光检视。
结果判定:供试品溶液色谱图中甲氧苄氨嘧啶和磺胺甲噁唑的R F值与标准品一致。
溶解度:溶解介质:0.1N盐酸溶液,900ml仪器2:75转/分钟时间:60分钟检测:根据甲氧苄氨嘧啶和磺胺甲噁唑的溶解量,运用含量测定的检测方法,进行必须的容量校正(见色谱分析法621)。
根据对照品溶液中的相应峰,计算出各个活性成分的溶解度。
限值:在60分钟内,不低于70%的C10H11N3O3S和C14H18N4O3溶解。
含量均匀度(905):符合要求。
含量测定:流动相、对照品溶液、色谱分析系统与甲氧苄氨嘧啶和磺胺甲噁唑口服混悬液一致。
供试品溶液:将不少于20只片剂粉碎、称重。
称取相当于160mg磺胺甲噁唑的粉末至100ml 容量瓶,加入甲醇50ml,用声波处理,摇匀5分钟。
冷却至室温,甲醇稀释至100ml,混匀,过滤。
取5.0ml滤液至50ml容量瓶,用流动相定容,混匀。
检测:分别检测对照品溶液和供试品溶液约(20μl),记录色谱图,测量主峰面积。
根据公式1000C(r U / r S) 计算出检测片剂粉末中C10H11N3O3S和C14H18N4O3的质量(mg);C 是对照品溶液的浓度(mg/ml),r U和r S分别为供试品溶液和对照品溶液的峰面积。
甲氧苄氨嘧啶和磺胺甲噁唑口服混悬液的含量检测:流动相:将1400ml水、400ml乙腈、2.0ml三乙胺加至2000ml容量瓶中,用0.2N氢氧化钠和1%乙酸调节pH为5.9±0.1。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Norethindrone is a synthetic progestin, which mimic the actions of the endogenous ovarian hormone progesterone.
Target: Progesterone Receptor
Norethisterone, or, is a 19–nortestosterone derivative, that lacks a C19 methyl group and possesses C17 ethinyl substitution, and primarily displays progestational activity rather than androgenic activity and, to a lesser extent, has oestrogenic and anti–oestrogenic activity [1]. Norethisterone (50 nM) induces signi cant effects on rat osteoblast proliferation, differentiation, and mineralization processes, mimicking the effects of estradiol, which is mediated by estrogen receptor [2].
Norethisterone displays hormonal properties in vivo. Mean active dose (MAD) of Norethisterone s.c. in progestagenic test (McPhail),androgenic test (Hershberger), estrogenic test (Allen–Doisy), and in a progestagenic and estrogenic test (ovulation inhibition test) is 0.63 mg/kg, 2.5 mg/kg, 4 mg/kg, and 0.235 mg/kg, respectively, and the MAD for orally administration is 0.25 mg/kg, 20 mg/kg, 8mg/kg, and 12 mg/kg, respectively. Norethisterone shows five– to eight–fold weaker progesterone receptor binding and
transactivation activities than the Org 2058 (100%) and two–fold stronger than progesterone. Binding and transactivation activities of Norethisterone for androgen receptor (5α–dihydrotestosterone 100%) are 3.2 and 1.1%, respectively, for estrogen receptor none (estradiol 100%) and for glucocorticoid receptor below 1% (dexamethasone 100%) [3].
References:
[1]. Schindler, A.E., et al., Classification and pharmacology of progestins. Maturitas, 2003. 46 Suppl 1: p. S7–S16.
[2]. Enriquez, J., et al., The effects of synthetic 19–norprogestins on osteoblastic cell function are mediated by their non–phenolic reduced metabolites. J Endocrinol, 2007. 193(3): p. 493–504.
[3]. Schoonen, W.G., et al., Hormonal properties of norethisterone, 7alpha–methyl–norethisterone and their derivatives. J Steroid Biochem Mol Biol, 2000.74(4): p. 213–22.
Product Name:
Norethindrone Cat. No.:
HY-B0554CAS No.:
68-22-4Molecular Formula:
C 20H 26O 2Molecular Weight:
298.42Target:
Progesterone Receptor Pathway:
Others Solubility:
10 mM in DMSO
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
