chapter 5 Cycloalkanes
Cycloalkane-ye

I. Nomenclature
1. Monocycloalkanes 2. Bicyclic bridged hydrocarbons
4
1. Monocycloalkanes Prefix: cyclo;
Cyclopropane Cyclobutane Cyclopentane Cyclohexane Cyclooctane
12
1. Ring Strain
unstable stable [109.5°- 60°] =49.5° [109.5°- 90°] =19.5°
Heats of combustion:
(CH2)n +
3 2
n
O2
n CO 2 + n H2O + heat
Table Heats of Combustion of Cycloalkanes(kJ·mol-1)
Chapter 2Alkanes & Cycloalkanes
Section 2 Cycloalkanes
Cycloalkanes contain rings of carbon atoms.
Ⅰ. Nomenclature II. Structure III. Properties
1
Classification of cycloalkanes
18
2. Conformational isomerism in cyclohexane
Chair form Boat form
5 6
4
3
1 2
5 4
3
6 1
2
chair form
H
5
HH H
4
3
烃类热裂解

petrochemical industry
Boiling point and its use of various petroleum products
Petroleum gas Crude gasoline Petroleum ether Gasoline Solvent oil Kerosene Aviation kerosene Kerosene Diesel Machine oil Vaseline Paraffin wax Fuel oil Asphalt Petroleum coke
异构烷烃裂解规律 Cracking rules of isoalkanes
(1)比正构烷烃容易裂解或脱氢 The pyrolysis or dehydrogenation of the isoalkane is easier than that of the normal alkane. (2)脱氢能力与分子结构有关,难易顺序为叔氢>仲氢>伯氢
We studied the catalyst performance and utilization, and the mass balance calculation of the reaction process.
石油加工的两大体系 Two system of petroleum processing
3.1 热裂解过程的化学反应
(chemical reaction of thermal cracking process)
一、烃类裂解的反应规律 (Reaction rules of thermal cracking of hydrocarbon)
烃类热裂解过程是十分复杂的,即使是纯组分裂解,得到的产物也是很 复杂的。包括:脱氢、断裂、异构化、环化、岐化、聚合、焦化、二烯 合成等。 The process of hydrocarbon pyrolysis is very complex, and products are also very complex even if pure component is cracked. Thermal cracking of hydrocarbon contains the reactions of dehydrogenation, chain scission, isomerization, cyclization, disproportionation, polymerization, diene synthesis, etc.
chapter 5 nomenclature

As an introduction to the IUPAC nomenclature system, we shall first consider compounds that have no specific functional groups. Such compounds are composed only of carbon and hydrogen atoms bonded together by sigma bonds (all carbons are sp3 hybridized). 5-3 Alkanes Hydrocarbons having no double or triple bond functional groups are classified as alkanes or cycloalkanes, depending on whether the carbon atoms of the molecule are arranged only in chains or also in rings. Although these hydrocarbons have no functional groups, they constitute the framework of other classes of compounds, and provide an ideal starting point for studying and naming organic compounds. The alkanes and cycloalkanes are also members of a larger class of compounds referred to as aliphatic. Simply put, aliphatic compounds are compounds that do not incorporate any aromatic rings in their molecular structure.
化学第一章org2alkanes.cycloalkanes
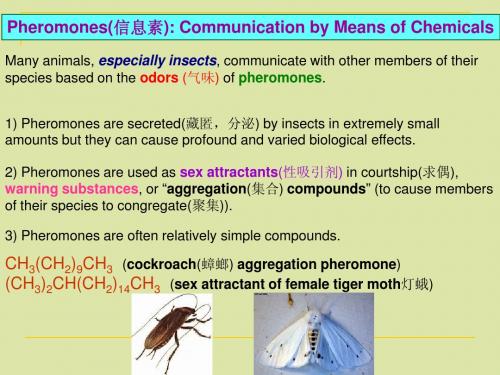
Sequence rule(次序规则)
a system of rules based on atomic number. CH3 4-甲基-8-异丙基十一烷 CH3CH2CH2CHCH2CH2CH2CHCH2CH2CH3 CH(CH3)2
about single bonds
H
H
CH4
H
C H
H
H H H
constitutional formula configurational formula
H
H C H
H C H
H
C 2 H6
C 3 H8
H
C H
H
H
CH3-CH3
H
C H
C
H
H
CH3-CH2-CH3
CH3-CH2-CH2-CH3
7 6 5 H3C CH 2 CH
4 CH 3 2 1 CH CH CH CH 3 3 CH 3 CH 35' 2 CH 2' CH 2CH 3 6' 7'
1
2,3,5-三甲基-4-丙基庚烷
CH3
2,7,8 3,4,9
H3C C C CH2 C H2C CH CH CH2 CH3 2,7,8-三甲基癸烷 10 9 H 8 3 7 6H2 5 4 2 1 CH3 CH3
Nanotubes, a new class of carbon-based materials with strength roughly one hundred times that of steel, also have an exceptional toughness.
有机化学课件Chapter环烷烃和芳香烃

9
2
1
3
8 6
4
7
5
8-甲基二环[4. 3. 0]壬烷
8-methylbicyclo[4. 3. 0]nonane
7
6 54
1 2
3
2, 7, 7-三甲基二环[2. 2. 1]庚烷
2, 7, 7-trimethylbicyclo [2. 2. 1]heptane
7
4
5
3
1
6
2 用","隔开
三环[2. 2. 1. 02, 6]庚烷
23
第四章 环烷烃和芳香烃
有机化学课件
24
第四章 环烷烃和芳香烃
4
Boat Conformer
Conformational Energy
有机化学课件
25
第四章 环烷烃和芳香烃
有机化学课件
26
第四章 环烷烃和芳香烃
直立键和平伏键(Axial and Equatorial Bonds)
四、单取代环己烷
Long-chain
有机化学课件
14
第四章 环烷烃和芳香烃
二、环大小与化学性质
五元以上 环烷烃
性质相似
链状烷烃
Cl2 / hv
Cl 自由基取代反应
H2 / Pt 催化加氢
HI
不反应 不反应
有机化学课件
15
第四章 环烷烃和芳香烃
三、小环化合物的特殊性质 —— 开环加成
1. 催化加氢
H2 / Pt, 50oC or Ni, 80oC
椅式构象的画法
有机化学课件
35
第四章 环烷烃和芳香烃
有机化学课件
36
烷烃

3-Ethyl-5-methylh eptane
(n ot 3-methyl-5-ethylheptane)
2-14
Nomenclature - IUPAC
7. The prefixes di-, tri-, tetra-, etc. are not included in alphabetization • alphabetize the names of substituents first and then insert these prefixes
2-6
Constitutional Isomerism
Constitutional
isomers: compounds with the same molecular formula but a different connectivity of their atoms
• example: C4H10
Alk enes (Chap ters 5-6) One or more carb on -carbon double b on ds H C C H H Eth ene H
A lkynes (Ch apter 7) One or more carbon-carb on trip le bonds H-C C-H Acetylen e
Primary
(1°) C: a carbon bonded to one other
carbon
• 1° H: a hydrogen bonded to a 1° carbon
Secondary
(2°) C: a carbon bonded to two other
第四章 烷烃和环烷烃
构造异构
(一)、碳链异构 (isomerism): 具有相同分子式,仅由于碳链结构不同而产
生的同分异构现象称为碳链异构。
由于碳链的连接次序不同而产生的 异构现象称为碳链异构
CH3 CH2 CH2 CH2 CH3
CH3 CH CH2 CH3 CH3 CH3
2, 4, 5 2, 3, 5
1. 确定主链: 最长链为主链。 号: 第一行 取代基编号为2, 4, 5; 第二行 取代基编号为2, 3, 5; 根据最低系列原则, 用第二行编号。 3. 命 名: 中文名称:2,3,5-三甲基己烷 英文名称:2,3,5-trimethylhexane
8 例1 CH3-CH2
4 3
43
2 5
61
2,4-二甲基已烷
(最长碳链;编号方向,从先遇到取代基 的一端开始编号;相同的取代基合并) 命名书写中常见错误: 2-二甲基己烷、 2,4-甲基己烷、 2,4-2甲基己烷、 2,4二甲基己烷
实 例 一
2. 编
1 2 3 4 5 6 6 5 4 3 2 1 CH3CHCH2CHCHCH3 CH3 H3C CH3
在英文命名中,取代基按词首的字母排列顺序先后列出
3-ethyl-4-isopropyl-3-methyl-5-propyloctane
课堂练习:写出化合物的名称,并指出各碳原子的类型。
C2H5 CH3 CH3 CH3 (2) CH3 CH3 CH3CCH2CCH3 CH3 CH3
(1) H3C
四、烷烃的同分异构现象 (The phenomenon of isomerism)
支链
CH3CH2CH2CH3
烷烃和环烷烃
烷烃分子中只有σ键,化学性质比较稳定, 不易发生化学反应。可以燃烧,也可以发生 卤代反应。
1、 燃烧
CnH2n+2
+
3n 1
(
2
) O2
n CO2 + (n+1)H2O
陈明
2、 卤代反应
(1) 甲烷的氯代
H
H
D or hv
H C H + Cl2
HC
H
H
H
D or hv
D or hv
H C Cl
H
根据碳原子数称某烷,前面不加“正”。
CH3CH2CH2CH3 丁烷 CH3(CH2)13CH3 十五烷
2) 支链烷烃 (1)把其看作直链烷烃的衍生物,把支链 作为取代基。在整个名称中包括母体和取代 基两部分,取代基部分在前,母体部分在后。
陈明
CH3CHCH2CH2CH3
CH3
2-甲基戊烷
(2)主链的选择: a、最长碳链为主链
陈明
Step 1 链的引发
D or hv
Cl Cl
Cl + Cl
Step 2 链的增长
H
H
H C H + Cl
H C + HCl
H
H
H
H
H C + Cl Cl
H C Cl+ Cl
H
H
陈明
Step 1 链的中止
H
HC
H H
+ Cl H
HC H
+ CH H
Cl + Cl
H H C Cl
H HH H CC H HH
陈明
二、命名( Nomenclature)
1、烷基 (alkyl) 的名称
烷基是烷烃去掉一个或几个 H 后剩下的 原子基团,用 R-表示,通式:CnH2n+1; 甲基常表示为 Me、乙基表示为 Et 。
有机化学习题集
第一章 绪 论Chaper1 intrduction1.将下列凯库勒式改写成路易斯式。
(1)(2)(3)(4)HH H H HH H HHHH HC C CC C C N OOO O ClO OO C HHHHH(5)(6)C 2H 5OH浓 H 2SO 4CH 2=CH 2 + H 2O 的反应热试计算23. 某化合物仅含碳、氢、氧元素,燃烧纯的该化合物 2.642mg ,得到CO 2 7.638mg,H 2O2.518mg ,试求其经验式。
4. 某化合物含碳45.44%、氢9.11%、氮21.20%、分子量132,求其分子式。
5. 试举例说明有机化合物在日常生活中的应用。
第二章 烷烃 Chapter 2 alkanes1、 写出下列化合物的构造式:1、 正戊烷 异戊烷 新戊烷2、 2,2,3-三甲基戊烷3、四甲基丁烷4、3-甲基-4-乙基己烷5、3-甲基-3-乙基-6-异丙基壬烷6、 2,5-二甲基-3,4-二氯己烷 2、 写出下列烷基的名称:CH 3CHCH 231.CH 3CH 2CH32.CH 3CHCH 2CH 2CH 233.(CH 3)3C CH 24.(CH 3)3C5.CH 33CH 36.3、 将下列化合物用系统命名法命名:(CH 3CH 2)22CH 22CH 3CH 3CH(CH 3)21. 2.CH 3CH 22CH 2CH 3CH(CH 3)23.(CH 3)2CCHCH 2CH 3CH 2CH 2CH 3CH 2CH 2CH 3(CH 3)2CHCHCHCCH 2CH 3CH 3CH 32CH 2CH 34.5.CH 3CH 2CHC 2CH 3CH 3CH 32CH 2CH 3CH 36.(CH 3CH 2CCH 2CH 2CH 3)3CHCH 3CH 37.CH 3CH 2CH 2CHCH 2CH 2CH 33)3(CH 3)3CCH 2CH(CH 2CH 3)28.4、 将下列烷烃的沸点按从高到低的顺序排列,然后与手册核对。
5 pigments and colorants print
Yellow
Red
Violet or black (crystaline forms) Blue or green (associated with a protei来自)Chapter 5-18
• Two main groups of carotenoids:
1) The carotenes(烃类胡萝卜素), which contain only (烃类胡萝卜素) hydrogen and carbon, and may be cyclic or linear. Of the carotenes, beta-carotene is well known as a vitamin A precursor. Lycopene(番茄红素), one of (番茄红素) the red pigment in tomatoes, is a carotene. 2) Oxycarotenoids(氧合叶黄素), which contain oxygen (氧合叶黄素) in the form of hydroxy, epoxy(环氧), or oxy groups, (环氧) as well as hydrogen and carbon. These compounds are also referred to as xanthophylls(叶黄素). Lutein (叶黄素) 叶黄素) (叶黄素), found in egg yolk, is a modified betacarotene that is di-hydroxylated.
H3C CH3 CH2 H N CH3 HN H3C N O
Chapter 5-10
Mg+2
NH
H3C
O
O
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Ring Strain and Stability
• Although the cycloalkanes show many of the same properties and chemistry as the acyclic alkanes, there are two additional features that result from the constraint of binding the chains into a ring: • Ring strain and conformational effect.
cyclobutane cyclopropane cyclopentane cyclohexane
Complex Cycloalkanes
• Naturally occurring materials contain cycloalkane structures • Examples: chrysanthemic acid (cyclopropane), prostaglandins (cyclopentane), steroids (cyclohexanes and cyclopentane)
Stereoisomers
• Compounds with atoms connected in the same order but which differ in three-dimensional orientation, are stereoisomers • The terms “cis” and “trans” should be used to specify stereoisomeric ring structures • Recall that constitutional isomers have atoms connected in different order
bicyclo[4.3.0]nonane
Spiro[4.5]decane
Bicyclic hydrocarbons
one-carbon bridge bridgehead carbons two-carbon bridge three-carbon bridge bicyclo[3.2.1]octane
Heats of formation per-CH2- for the cycloalkanes
n compound ΔHfo/n kcal/mol n 8 compound Cyclooctane ΔHfo/n kcal/mol -3.75
3 cyclopropane 4.25
4 cyclobutane
60o
90o
108o
120o
128.6o
135o
Conformation of Cycloalkane
Conformation of Cyclopropane
• 3-membered ring must have planar structure • Symmetrical with C–C–C bond angles of 60° • Requires that sp3 based bonds are bent (and weakened) – severe angle strain • All C-H bonds are eclipsed – torsional strain
Bicyclic and polycyclic compounds
• If two rings share two or more common atoms, the compound is called a bicyclic compound(桥环化合物). • If two rings have a single common atom, the result is called a spirocyclic compound (螺环化合物).
Conformations of Cyclobutane
• Cyclobutane has less angle strain than cyclopropane but more torsional strain because of its larger number of ring hydrogens • Cyclobutane is slightly bent out of plane - one carbon atom is about 25° above
5,7,7-Trimethyl-bicyclo[2.2.1]hept-2-ene
bicyclo[4.4.0]dec-1(6)-ene
Spirocyclic hydrocarbons
• The numbering of substituted bicyclic ring systems always assigns the 1 to one next to spiro-carbons in smaller ring. • Numbering proceeds around each bridge in turn, beginning with the smaller bridge. • Only after this rule is taken into account are the lowest numbers then assigned to double bonds and substituents
Bent bond
The Nature of Ring Strain
• Rings larger than 3 atoms are not flat • Cyclic molecules can assume nonplanar conformations to minimize angle strain and torsional strain by ring-puckering • Larger rings have many more possible conformations than smaller rings and are more difficult to analyze
bicyclo[4.3.0]nonane
carbon number of whole bicyclic ring carbon number of each bridge according bigger to small
• The numbering of substituted bicyclic ring systems always assigns the 1 to one of the bridgehead carbons. • Numbering proceeds around each bridge in turn, beginning with the largest bridge. • Only after this rule is taken into account are the lowest numbers then assigned to double bonds and substituents.
1.65
9
Cyclononane
-3.55
-4.95
5 cyclopentane -3.65
10 Cyclodecane
6 cyclohexane
-4.95
11 Cycloundecane
12 cyclododecane
-4.65
-4.55
7 cycloheptane -4.05
• Stability of cycloalkanes: 3<4<5<6>7>8>9
菊酸
前列腺素
可的松
Naming Cycloalkanes
• Rules are similar to those for open-chain alkanes • Count the number of carbons in the ring, and add the prefix cyclo- to the name of the corresponding alkane • If a substituent is present on the ring, count the number of carbon atoms in the ring and the number in the largest substituent chain. If the number of carbon atoms in the ring is equal to or greater than the number in the substituent, the compound is named as an alkyl-substituted cycloalkane • For substituted cycloalkane, start at a point of attachment as C1 and number the substituents on the ring. • If there are two or more substituents, begin numbering at the group with alphabetical priority, and give the other substituents the lowest possible number
Chapter 5 Cycloalkanes
Cycloalkanes
• Cycloalkanes are alkanes that have carbon atoms that form a ring (called alicyclic compounds) • Simple cycloalkanes rings of CH2 units, (CH2)n, or CnH2n • Structure is shown as a regular polygon with the number of vertices equal to the number of C’s (a projection of the actual structure)
