Toll-like_receptor_modulator_COA_00094_MedChemExpress
LTE_3GPP_36.213-860(中文版)

3GPP
Release 8
3
3GPP TS 36.213 V8.6.0 (2009-03)
Contents
Foreword ...................................................................................................................................................... 5 1 2 3
Internet
Copyright Notification No part may be reproduced except as authorized by written permission. The copyright and the foregoing restriction extend to reproduction in all media.
© 2009, 3GPP Organizational Partners (ARIB, ATIS, CCSA, ETSI, TTA, TTC). All rights reserved. UMTS™ is a Trade Mark of ETSI registered for the benefit of its members 3GPP™ is a Trade Mark of ETSI registered for the benefit of its Members and of the 3GPP Organizational Partners LTE™ is a Trade Mark of ETSI currently being registered for the benefit of i ts Members and of the 3GPP Organizational Partners GSM® and the GSM logo are registered and owned by the GSM Association
cadence中calculator阻值函数 -回复

cadence中calculator阻值函数-回复Cadence中的calculator阻值函数是一项非常重要的功能,它可以帮助工程师和设计师计算电路中的阻抗值。
在本文中,我们将逐步回答有关这个函数的一些常见问题,包括如何使用它以及它的优势和应用。
首先,我们需要了解Cadence是什么。
Cadence是一种用于电子设计自动化的软件工具,提供了一系列功能强大的设计工具,用于创建和分析电路,模拟电路行为以及优化电路性能。
Cadence的calculator阻值函数是其中一个重要的功能,可以帮助用户计算电路中的阻抗。
那么,什么是阻抗?在电路中,阻抗是电路中的电阻或复阻抗的一种表示。
它是电压和电流之间相互作用的一种度量,通常以欧姆(Ω)为单位。
阻抗的大小和相位决定了电路对电压和电流的响应方式。
在Cadence中,calculator阻值函数可以通过一些简单的输入,帮助用户计算电路的阻抗。
下面是一些常见的问题和它们的回答:问题1:如何使用Cadence中的calculator阻值函数?回答:要使用calculator阻值函数,首先需要打开Cadence软件。
然后,在工具栏上选择“计算器”选项。
接下来,在弹出的计算器窗口中选择“阻值”选项。
在输入框中输入电路元件的参数,例如电阻值、电感值或电容值。
点击“计算”按钮,即可得到计算结果。
问题2:calculator阻值函数有哪些优势?回答:calculator阻值函数具有多个优势。
首先,它提供了一种快速、方便且准确的计算阻抗的方法。
用户只需输入电路元件的参数,即可立即得到计算结果。
其次,它支持复杂电路的计算,包括并联和串联电路,以及由多个元件组成的复杂电路。
此外,它还提供了多种单位选项,如欧姆、千欧姆和兆欧姆等,适应不同的计量需求。
问题3:calculator阻值函数的应用范围是什么?回答:calculator阻值函数可以广泛应用于各种电路设计和分析中。
例如,在功率电子学中,阻抗是控制电路响应和改变功率传输的关键参数。
Native Instruments MASCHINE MK3 用户手册说明书

The information in this document is subject to change without notice and does not represent a commitment on the part of Native Instruments GmbH. The software described by this docu-ment is subject to a License Agreement and may not be copied to other media. No part of this publication may be copied, reproduced or otherwise transmitted or recorded, for any purpose, without prior written permission by Native Instruments GmbH, hereinafter referred to as Native Instruments.“Native Instruments”, “NI” and associated logos are (registered) trademarks of Native Instru-ments GmbH.ASIO, VST, HALion and Cubase are registered trademarks of Steinberg Media Technologies GmbH.All other product and company names are trademarks™ or registered® trademarks of their re-spective holders. Use of them does not imply any affiliation with or endorsement by them.Document authored by: David Gover and Nico Sidi.Software version: 2.8 (02/2019)Hardware version: MASCHINE MK3Special thanks to the Beta Test Team, who were invaluable not just in tracking down bugs, but in making this a better product.NATIVE INSTRUMENTS GmbH Schlesische Str. 29-30D-10997 Berlin Germanywww.native-instruments.de NATIVE INSTRUMENTS North America, Inc. 6725 Sunset Boulevard5th FloorLos Angeles, CA 90028USANATIVE INSTRUMENTS K.K.YO Building 3FJingumae 6-7-15, Shibuya-ku, Tokyo 150-0001Japanwww.native-instruments.co.jp NATIVE INSTRUMENTS UK Limited 18 Phipp StreetLondon EC2A 4NUUKNATIVE INSTRUMENTS FRANCE SARL 113 Rue Saint-Maur75011 ParisFrance SHENZHEN NATIVE INSTRUMENTS COMPANY Limited 5F, Shenzhen Zimao Center111 Taizi Road, Nanshan District, Shenzhen, GuangdongChina© NATIVE INSTRUMENTS GmbH, 2019. All rights reserved.Table of Contents1Welcome to MASCHINE (25)1.1MASCHINE Documentation (26)1.2Document Conventions (27)1.3New Features in MASCHINE 2.8 (29)1.4New Features in MASCHINE 2.7.10 (31)1.5New Features in MASCHINE 2.7.8 (31)1.6New Features in MASCHINE 2.7.7 (32)1.7New Features in MASCHINE 2.7.4 (33)1.8New Features in MASCHINE 2.7.3 (36)2Quick Reference (38)2.1Using Your Controller (38)2.1.1Controller Modes and Mode Pinning (38)2.1.2Controlling the Software Views from Your Controller (40)2.2MASCHINE Project Overview (43)2.2.1Sound Content (44)2.2.2Arrangement (45)2.3MASCHINE Hardware Overview (48)2.3.1MASCHINE Hardware Overview (48)2.3.1.1Control Section (50)2.3.1.2Edit Section (53)2.3.1.3Performance Section (54)2.3.1.4Group Section (56)2.3.1.5Transport Section (56)2.3.1.6Pad Section (58)2.3.1.7Rear Panel (63)2.4MASCHINE Software Overview (65)2.4.1Header (66)2.4.2Browser (68)2.4.3Arranger (70)2.4.4Control Area (73)2.4.5Pattern Editor (74)3Basic Concepts (76)3.1Important Names and Concepts (76)3.2Adjusting the MASCHINE User Interface (79)3.2.1Adjusting the Size of the Interface (79)3.2.2Switching between Ideas View and Song View (80)3.2.3Showing/Hiding the Browser (81)3.2.4Showing/Hiding the Control Lane (81)3.3Common Operations (82)3.3.1Using the 4-Directional Push Encoder (82)3.3.2Pinning a Mode on the Controller (83)3.3.3Adjusting Volume, Swing, and Tempo (84)3.3.4Undo/Redo (87)3.3.5List Overlay for Selectors (89)3.3.6Zoom and Scroll Overlays (90)3.3.7Focusing on a Group or a Sound (91)3.3.8Switching Between the Master, Group, and Sound Level (96)3.3.9Navigating Channel Properties, Plug-ins, and Parameter Pages in the Control Area.973.3.9.1Extended Navigate Mode on Your Controller (102)3.3.10Navigating the Software Using the Controller (105)3.3.11Using Two or More Hardware Controllers (106)3.3.12Touch Auto-Write Option (108)3.4Native Kontrol Standard (110)3.5Stand-Alone and Plug-in Mode (111)3.5.1Differences between Stand-Alone and Plug-in Mode (112)3.5.2Switching Instances (113)3.5.3Controlling Various Instances with Different Controllers (114)3.6Host Integration (114)3.6.1Setting up Host Integration (115)3.6.1.1Setting up Ableton Live (macOS) (115)3.6.1.2Setting up Ableton Live (Windows) (116)3.6.1.3Setting up Apple Logic Pro X (116)3.6.2Integration with Ableton Live (117)3.6.3Integration with Apple Logic Pro X (119)3.7Preferences (120)3.7.1Preferences – General Page (121)3.7.2Preferences – Audio Page (126)3.7.3Preferences – MIDI Page (130)3.7.4Preferences – Default Page (133)3.7.5Preferences – Library Page (137)3.7.6Preferences – Plug-ins Page (145)3.7.7Preferences – Hardware Page (150)3.7.8Preferences – Colors Page (154)3.8Integrating MASCHINE into a MIDI Setup (156)3.8.1Connecting External MIDI Equipment (156)3.8.2Sync to External MIDI Clock (157)3.8.3Send MIDI Clock (158)3.9Syncing MASCHINE using Ableton Link (159)3.9.1Connecting to a Network (159)3.9.2Joining and Leaving a Link Session (159)3.10Using a Pedal with the MASCHINE Controller (160)3.11File Management on the MASCHINE Controller (161)4Browser (163)4.1Browser Basics (163)4.1.1The MASCHINE Library (163)4.1.2Browsing the Library vs. Browsing Your Hard Disks (164)4.2Searching and Loading Files from the Library (165)4.2.1Overview of the Library Pane (165)4.2.2Selecting or Loading a Product and Selecting a Bank from the Browser (170)4.2.2.1[MK3] Browsing by Product Category Using the Controller (174)4.2.2.2[MK3] Browsing by Product Vendor Using the Controller (174)4.2.3Selecting a Product Category, a Product, a Bank, and a Sub-Bank (175)4.2.3.1Selecting a Product Category, a Product, a Bank, and a Sub-Bank on theController (179)4.2.4Selecting a File Type (180)4.2.5Choosing Between Factory and User Content (181)4.2.6Selecting Type and Character Tags (182)4.2.7List and Tag Overlays in the Browser (186)4.2.8Performing a Text Search (188)4.2.9Loading a File from the Result List (188)4.3Additional Browsing Tools (193)4.3.1Loading the Selected Files Automatically (193)4.3.2Auditioning Instrument Presets (195)4.3.3Auditioning Samples (196)4.3.4Loading Groups with Patterns (197)4.3.5Loading Groups with Routing (198)4.3.6Displaying File Information (198)4.4Using Favorites in the Browser (199)4.5Editing the Files’ Tags and Properties (203)4.5.1Attribute Editor Basics (203)4.5.2The Bank Page (205)4.5.3The Types and Characters Pages (205)4.5.4The Properties Page (208)4.6Loading and Importing Files from Your File System (209)4.6.1Overview of the FILES Pane (209)4.6.2Using Favorites (211)4.6.3Using the Location Bar (212)4.6.4Navigating to Recent Locations (213)4.6.5Using the Result List (214)4.6.6Importing Files to the MASCHINE Library (217)4.7Locating Missing Samples (219)4.8Using Quick Browse (221)5Managing Sounds, Groups, and Your Project (225)5.1Overview of the Sounds, Groups, and Master (225)5.1.1The Sound, Group, and Master Channels (226)5.1.2Similarities and Differences in Handling Sounds and Groups (227)5.1.3Selecting Multiple Sounds or Groups (228)5.2Managing Sounds (233)5.2.1Loading Sounds (235)5.2.2Pre-listening to Sounds (236)5.2.3Renaming Sound Slots (237)5.2.4Changing the Sound’s Color (237)5.2.5Saving Sounds (239)5.2.6Copying and Pasting Sounds (241)5.2.7Moving Sounds (244)5.2.8Resetting Sound Slots (245)5.3Managing Groups (247)5.3.1Creating Groups (248)5.3.2Loading Groups (249)5.3.3Renaming Groups (251)5.3.4Changing the Group’s Color (251)5.3.5Saving Groups (253)5.3.6Copying and Pasting Groups (255)5.3.7Reordering Groups (258)5.3.8Deleting Groups (259)5.4Exporting MASCHINE Objects and Audio (260)5.4.1Saving a Group with its Samples (261)5.4.2Saving a Project with its Samples (262)5.4.3Exporting Audio (264)5.5Importing Third-Party File Formats (270)5.5.1Loading REX Files into Sound Slots (270)5.5.2Importing MPC Programs to Groups (271)6Playing on the Controller (275)6.1Adjusting the Pads (275)6.1.1The Pad View in the Software (275)6.1.2Choosing a Pad Input Mode (277)6.1.3Adjusting the Base Key (280)6.1.4Using Choke Groups (282)6.1.5Using Link Groups (284)6.2Adjusting the Key, Choke, and Link Parameters for Multiple Sounds (286)6.3Playing Tools (287)6.3.1Mute and Solo (288)6.3.2Choke All Notes (292)6.3.3Groove (293)6.3.4Level, Tempo, Tune, and Groove Shortcuts on Your Controller (295)6.3.5Tap Tempo (299)6.4Performance Features (300)6.4.1Overview of the Perform Features (300)6.4.2Selecting a Scale and Creating Chords (303)6.4.3Scale and Chord Parameters (303)6.4.4Creating Arpeggios and Repeated Notes (316)6.4.5Swing on Note Repeat / Arp Output (321)6.5Using Lock Snapshots (322)6.5.1Creating a Lock Snapshot (322)6.5.2Using Extended Lock (323)6.5.3Updating a Lock Snapshot (323)6.5.4Recalling a Lock Snapshot (324)6.5.5Morphing Between Lock Snapshots (324)6.5.6Deleting a Lock Snapshot (325)6.5.7Triggering Lock Snapshots via MIDI (326)6.6Using the Smart Strip (327)6.6.1Pitch Mode (328)6.6.2Modulation Mode (328)6.6.3Perform Mode (328)6.6.4Notes Mode (329)7Working with Plug-ins (330)7.1Plug-in Overview (330)7.1.1Plug-in Basics (330)7.1.2First Plug-in Slot of Sounds: Choosing the Sound’s Role (334)7.1.3Loading, Removing, and Replacing a Plug-in (335)7.1.3.1Browser Plug-in Slot Selection (341)7.1.4Adjusting the Plug-in Parameters (344)7.1.5Bypassing Plug-in Slots (344)7.1.6Using Side-Chain (346)7.1.7Moving Plug-ins (346)7.1.8Alternative: the Plug-in Strip (348)7.1.9Saving and Recalling Plug-in Presets (348)7.1.9.1Saving Plug-in Presets (349)7.1.9.2Recalling Plug-in Presets (350)7.1.9.3Removing a Default Plug-in Preset (351)7.2The Sampler Plug-in (352)7.2.1Page 1: Voice Settings / Engine (354)7.2.2Page 2: Pitch / Envelope (356)7.2.3Page 3: FX / Filter (359)7.2.4Page 4: Modulation (361)7.2.5Page 5: LFO (363)7.2.6Page 6: Velocity / Modwheel (365)7.3Using Native Instruments and External Plug-ins (367)7.3.1Opening/Closing Plug-in Windows (367)7.3.2Using the VST/AU Plug-in Parameters (370)7.3.3Setting Up Your Own Parameter Pages (371)7.3.4Using VST/AU Plug-in Presets (376)7.3.5Multiple-Output Plug-ins and Multitimbral Plug-ins (378)8Using the Audio Plug-in (380)8.1Loading a Loop into the Audio Plug-in (384)8.2Editing Audio in the Audio Plug-in (385)8.3Using Loop Mode (386)8.4Using Gate Mode (388)9Using the Drumsynths (390)9.1Drumsynths – General Handling (391)9.1.1Engines: Many Different Drums per Drumsynth (391)9.1.2Common Parameter Organization (391)9.1.3Shared Parameters (394)9.1.4Various Velocity Responses (394)9.1.5Pitch Range, Tuning, and MIDI Notes (394)9.2The Kicks (395)9.2.1Kick – Sub (397)9.2.2Kick – Tronic (399)9.2.3Kick – Dusty (402)9.2.4Kick – Grit (403)9.2.5Kick – Rasper (406)9.2.6Kick – Snappy (407)9.2.7Kick – Bold (409)9.2.8Kick – Maple (411)9.2.9Kick – Push (412)9.3The Snares (414)9.3.1Snare – Volt (416)9.3.2Snare – Bit (418)9.3.3Snare – Pow (420)9.3.4Snare – Sharp (421)9.3.5Snare – Airy (423)9.3.6Snare – Vintage (425)9.3.7Snare – Chrome (427)9.3.8Snare – Iron (429)9.3.9Snare – Clap (431)9.3.10Snare – Breaker (433)9.4The Hi-hats (435)9.4.1Hi-hat – Silver (436)9.4.2Hi-hat – Circuit (438)9.4.3Hi-hat – Memory (440)9.4.4Hi-hat – Hybrid (442)9.4.5Creating a Pattern with Closed and Open Hi-hats (444)9.5The Toms (445)9.5.1Tom – Tronic (447)9.5.2Tom – Fractal (449)9.5.3Tom – Floor (453)9.5.4Tom – High (455)9.6The Percussions (456)9.6.1Percussion – Fractal (458)9.6.2Percussion – Kettle (461)9.6.3Percussion – Shaker (463)9.7The Cymbals (467)9.7.1Cymbal – Crash (469)9.7.2Cymbal – Ride (471)10Using the Bass Synth (474)10.1Bass Synth – General Handling (475)10.1.1Parameter Organization (475)10.1.2Bass Synth Parameters (477)11Working with Patterns (479)11.1Pattern Basics (479)11.1.1Pattern Editor Overview (480)11.1.2Navigating the Event Area (486)11.1.3Following the Playback Position in the Pattern (488)11.1.4Jumping to Another Playback Position in the Pattern (489)11.1.5Group View and Keyboard View (491)11.1.6Adjusting the Arrange Grid and the Pattern Length (493)11.1.7Adjusting the Step Grid and the Nudge Grid (497)11.2Recording Patterns in Real Time (501)11.2.1Recording Your Patterns Live (501)11.2.2The Record Prepare Mode (504)11.2.3Using the Metronome (505)11.2.4Recording with Count-in (506)11.2.5Quantizing while Recording (508)11.3Recording Patterns with the Step Sequencer (508)11.3.1Step Mode Basics (508)11.3.2Editing Events in Step Mode (511)11.3.3Recording Modulation in Step Mode (513)11.4Editing Events (514)11.4.1Editing Events with the Mouse: an Overview (514)11.4.2Creating Events/Notes (517)11.4.3Selecting Events/Notes (518)11.4.4Editing Selected Events/Notes (526)11.4.5Deleting Events/Notes (532)11.4.6Cut, Copy, and Paste Events/Notes (535)11.4.7Quantizing Events/Notes (538)11.4.8Quantization While Playing (540)11.4.9Doubling a Pattern (541)11.4.10Adding Variation to Patterns (541)11.5Recording and Editing Modulation (546)11.5.1Which Parameters Are Modulatable? (547)11.5.2Recording Modulation (548)11.5.3Creating and Editing Modulation in the Control Lane (550)11.6Creating MIDI Tracks from Scratch in MASCHINE (555)11.7Managing Patterns (557)11.7.1The Pattern Manager and Pattern Mode (558)11.7.2Selecting Patterns and Pattern Banks (560)11.7.3Creating Patterns (563)11.7.4Deleting Patterns (565)11.7.5Creating and Deleting Pattern Banks (566)11.7.6Naming Patterns (568)11.7.7Changing the Pattern’s Color (570)11.7.8Duplicating, Copying, and Pasting Patterns (571)11.7.9Moving Patterns (574)11.7.10Adjusting Pattern Length in Fine Increments (575)11.8Importing/Exporting Audio and MIDI to/from Patterns (576)11.8.1Exporting Audio from Patterns (576)11.8.2Exporting MIDI from Patterns (577)11.8.3Importing MIDI to Patterns (580)12Audio Routing, Remote Control, and Macro Controls (589)12.1Audio Routing in MASCHINE (590)12.1.1Sending External Audio to Sounds (591)12.1.2Configuring the Main Output of Sounds and Groups (596)12.1.3Setting Up Auxiliary Outputs for Sounds and Groups (601)12.1.4Configuring the Master and Cue Outputs of MASCHINE (605)12.1.5Mono Audio Inputs (610)12.1.5.1Configuring External Inputs for Sounds in Mix View (611)12.2Using MIDI Control and Host Automation (614)12.2.1Triggering Sounds via MIDI Notes (615)12.2.2Triggering Scenes via MIDI (622)12.2.3Controlling Parameters via MIDI and Host Automation (623)12.2.4Selecting VST/AU Plug-in Presets via MIDI Program Change (631)12.2.5Sending MIDI from Sounds (632)12.3Creating Custom Sets of Parameters with the Macro Controls (636)12.3.1Macro Control Overview (637)12.3.2Assigning Macro Controls Using the Software (638)12.3.3Assigning Macro Controls Using the Controller (644)13Controlling Your Mix (646)13.1Mix View Basics (646)13.1.1Switching between Arrange View and Mix View (646)13.1.2Mix View Elements (647)13.2The Mixer (649)13.2.1Displaying Groups vs. Displaying Sounds (650)13.2.2Adjusting the Mixer Layout (652)13.2.3Selecting Channel Strips (653)13.2.4Managing Your Channels in the Mixer (654)13.2.5Adjusting Settings in the Channel Strips (656)13.2.6Using the Cue Bus (660)13.3The Plug-in Chain (662)13.4The Plug-in Strip (663)13.4.1The Plug-in Header (665)13.4.2Panels for Drumsynths and Internal Effects (667)13.4.3Panel for the Sampler (668)13.4.4Custom Panels for Native Instruments Plug-ins (671)13.4.5Undocking a Plug-in Panel (Native Instruments and External Plug-ins Only) (675)13.5Controlling Your Mix from the Controller (677)13.5.1Navigating Your Channels in Mix Mode (678)13.5.2Adjusting the Level and Pan in Mix Mode (679)13.5.3Mute and Solo in Mix Mode (680)13.5.4Plug-in Icons in Mix Mode (680)14Using Effects (681)14.1Applying Effects to a Sound, a Group or the Master (681)14.1.1Adding an Effect (681)14.1.2Other Operations on Effects (690)14.1.3Using the Side-Chain Input (692)14.2Applying Effects to External Audio (695)14.2.1Step 1: Configure MASCHINE Audio Inputs (695)14.2.2Step 2: Set up a Sound to Receive the External Input (698)14.2.3Step 3: Load an Effect to Process an Input (700)14.3Creating a Send Effect (701)14.3.1Step 1: Set Up a Sound or Group as Send Effect (702)14.3.2Step 2: Route Audio to the Send Effect (706)14.3.3 A Few Notes on Send Effects (708)14.4Creating Multi-Effects (709)15Effect Reference (712)15.1Dynamics (713)15.1.1Compressor (713)15.1.2Gate (717)15.1.3Transient Master (721)15.1.4Limiter (723)15.1.5Maximizer (727)15.2Filtering Effects (730)15.2.1EQ (730)15.2.2Filter (733)15.2.3Cabinet (737)15.3Modulation Effects (738)15.3.1Chorus (738)15.3.2Flanger (740)15.3.3FM (742)15.3.4Freq Shifter (743)15.3.5Phaser (745)15.4Spatial and Reverb Effects (747)15.4.1Ice (747)15.4.2Metaverb (749)15.4.3Reflex (750)15.4.4Reverb (Legacy) (752)15.4.5Reverb (754)15.4.5.1Reverb Room (754)15.4.5.2Reverb Hall (757)15.4.5.3Plate Reverb (760)15.5Delays (762)15.5.1Beat Delay (762)15.5.2Grain Delay (765)15.5.3Grain Stretch (767)15.5.4Resochord (769)15.6Distortion Effects (771)15.6.1Distortion (771)15.6.2Lofi (774)15.6.3Saturator (775)15.7Perform FX (779)15.7.1Filter (780)15.7.2Flanger (782)15.7.3Burst Echo (785)15.7.4Reso Echo (787)15.7.5Ring (790)15.7.6Stutter (792)15.7.7Tremolo (795)15.7.8Scratcher (798)16Working with the Arranger (801)16.1Arranger Basics (801)16.1.1Navigating Song View (804)16.1.2Following the Playback Position in Your Project (806)16.1.3Performing with Scenes and Sections using the Pads (807)16.2Using Ideas View (811)16.2.1Scene Overview (811)16.2.2Creating Scenes (813)16.2.3Assigning and Removing Patterns (813)16.2.4Selecting Scenes (817)16.2.5Deleting Scenes (818)16.2.6Creating and Deleting Scene Banks (820)16.2.7Clearing Scenes (820)16.2.8Duplicating Scenes (821)16.2.9Reordering Scenes (822)16.2.10Making Scenes Unique (824)16.2.11Appending Scenes to Arrangement (825)16.2.12Naming Scenes (826)16.2.13Changing the Color of a Scene (827)16.3Using Song View (828)16.3.1Section Management Overview (828)16.3.2Creating Sections (833)16.3.3Assigning a Scene to a Section (834)16.3.4Selecting Sections and Section Banks (835)16.3.5Reorganizing Sections (839)16.3.6Adjusting the Length of a Section (840)16.3.6.1Adjusting the Length of a Section Using the Software (841)16.3.6.2Adjusting the Length of a Section Using the Controller (843)16.3.7Clearing a Pattern in Song View (843)16.3.8Duplicating Sections (844)16.3.8.1Making Sections Unique (845)16.3.9Removing Sections (846)16.3.10Renaming Scenes (848)16.3.11Clearing Sections (849)16.3.12Creating and Deleting Section Banks (850)16.3.13Working with Patterns in Song view (850)16.3.13.1Creating a Pattern in Song View (850)16.3.13.2Selecting a Pattern in Song View (850)16.3.13.3Clearing a Pattern in Song View (851)16.3.13.4Renaming a Pattern in Song View (851)16.3.13.5Coloring a Pattern in Song View (851)16.3.13.6Removing a Pattern in Song View (852)16.3.13.7Duplicating a Pattern in Song View (852)16.3.14Enabling Auto Length (852)16.3.15Looping (853)16.3.15.1Setting the Loop Range in the Software (854)16.4Playing with Sections (855)16.4.1Jumping to another Playback Position in Your Project (855)16.5Triggering Sections or Scenes via MIDI (856)16.6The Arrange Grid (858)16.7Quick Grid (860)17Sampling and Sample Mapping (862)17.1Opening the Sample Editor (862)17.2Recording Audio (863)17.2.1Opening the Record Page (863)17.2.2Selecting the Source and the Recording Mode (865)17.2.3Arming, Starting, and Stopping the Recording (868)17.2.5Using the Footswitch for Recording Audio (871)17.2.6Checking Your Recordings (872)17.2.7Location and Name of Your Recorded Samples (876)17.3Editing a Sample (876)17.3.1Using the Edit Page (877)17.3.2Audio Editing Functions (882)17.4Slicing a Sample (890)17.4.1Opening the Slice Page (891)17.4.2Adjusting the Slicing Settings (893)17.4.3Live Slicing (898)17.4.3.1Live Slicing Using the Controller (898)17.4.3.2Delete All Slices (899)17.4.4Manually Adjusting Your Slices (899)17.4.5Applying the Slicing (906)17.5Mapping Samples to Zones (912)17.5.1Opening the Zone Page (912)17.5.2Zone Page Overview (913)17.5.3Selecting and Managing Zones in the Zone List (915)17.5.4Selecting and Editing Zones in the Map View (920)17.5.5Editing Zones in the Sample View (924)17.5.6Adjusting the Zone Settings (927)17.5.7Adding Samples to the Sample Map (934)18Appendix: Tips for Playing Live (937)18.1Preparations (937)18.1.1Focus on the Hardware (937)18.1.2Customize the Pads of the Hardware (937)18.1.3Check Your CPU Power Before Playing (937)18.1.4Name and Color Your Groups, Patterns, Sounds and Scenes (938)18.1.5Consider Using a Limiter on Your Master (938)18.1.6Hook Up Your Other Gear and Sync It with MIDI Clock (938)18.1.7Improvise (938)18.2Basic Techniques (938)18.2.1Use Mute and Solo (938)18.2.2Use Scene Mode and Tweak the Loop Range (939)18.2.3Create Variations of Your Drum Patterns in the Step Sequencer (939)18.2.4Use Note Repeat (939)18.2.5Set Up Your Own Multi-effect Groups and Automate Them (939)18.3Special Tricks (940)18.3.1Changing Pattern Length for Variation (940)18.3.2Using Loops to Cycle Through Samples (940)18.3.3Using Loops to Cycle Through Samples (940)18.3.4Load Long Audio Files and Play with the Start Point (940)19Troubleshooting (941)19.1Knowledge Base (941)19.2Technical Support (941)19.3Registration Support (942)19.4User Forum (942)20Glossary (943)Index (951)1Welcome to MASCHINEThank you for buying MASCHINE!MASCHINE is a groove production studio that implements the familiar working style of classi-cal groove boxes along with the advantages of a computer based system. MASCHINE is ideal for making music live, as well as in the studio. It’s the hands-on aspect of a dedicated instru-ment, the MASCHINE hardware controller, united with the advanced editing features of the MASCHINE software.Creating beats is often not very intuitive with a computer, but using the MASCHINE hardware controller to do it makes it easy and fun. You can tap in freely with the pads or use Note Re-peat to jam along. Alternatively, build your beats using the step sequencer just as in classic drum machines.Patterns can be intuitively combined and rearranged on the fly to form larger ideas. You can try out several different versions of a song without ever having to stop the music.Since you can integrate it into any sequencer that supports VST, AU, or AAX plug-ins, you can reap the benefits in almost any software setup, or use it as a stand-alone application. You can sample your own material, slice loops and rearrange them easily.However, MASCHINE is a lot more than an ordinary groovebox or sampler: it comes with an inspiring 7-gigabyte library, and a sophisticated, yet easy to use tag-based Browser to give you instant access to the sounds you are looking for.What’s more, MASCHINE provides lots of options for manipulating your sounds via internal ef-fects and other sound-shaping possibilities. You can also control external MIDI hardware and 3rd-party software with the MASCHINE hardware controller, while customizing the functions of the pads, knobs and buttons according to your needs utilizing the included Controller Editor application. We hope you enjoy this fantastic instrument as much as we do. Now let’s get go-ing!—The MASCHINE team at Native Instruments.MASCHINE Documentation1.1MASCHINE DocumentationNative Instruments provide many information sources regarding MASCHINE. The main docu-ments should be read in the following sequence:1.MASCHINE Getting Started: This document provides a practical approach to MASCHINE viaa set of tutorials covering easy and more advanced tasks in order to help you familiarizeyourself with MASCHINE.2.MASCHINE Manual (this document): The MASCHINE Manual provides you with a compre-hensive description of all MASCHINE software and hardware features.Additional documentation sources provide you with details on more specific topics:▪Controller Editor Manual: Besides using your MASCHINE hardware controller together withits dedicated MASCHINE software, you can also use it as a powerful and highly versatileMIDI controller to pilot any other MIDI-capable application or device. This is made possibleby the Controller Editor software, an application that allows you to precisely define all MIDIassignments for your MASCHINE controller. The Controller Editor was installed during theMASCHINE installation procedure. For more information on this, please refer to the Con-troller Editor Manual available as a PDF file via the Help menu of Controller Editor.▪Online Support Videos: You can find a number of support videos on The Official Native In-struments Support Channel under the following URL: https:///NIsupport-EN. We recommend that you follow along with these instructions while the respective ap-plication is running on your computer.Other Online Resources:If you are experiencing problems related to your Native Instruments product that the supplied documentation does not cover, there are several ways of getting help:▪Knowledge Base▪User Forum▪Technical Support▪Registration SupportYou will find more information on these subjects in the chapter Troubleshooting.1.2Document ConventionsThis section introduces you to the signage and text highlighting used in this manual. This man-ual uses particular formatting to point out special facts and to warn you of potential issues. The icons introducing these notes let you see what kind of information is to be expected:This document uses particular formatting to point out special facts and to warn you of poten-tial issues. The icons introducing the following notes let you see what kind of information can be expected:Furthermore, the following formatting is used:▪Text appearing in (drop-down) menus (such as Open…, Save as… etc.) in the software and paths to locations on your hard disk or other storage devices is printed in italics.▪Text appearing elsewhere (labels of buttons, controls, text next to checkboxes etc.) in the software is printed in blue. Whenever you see this formatting applied, you will find the same text appearing somewhere on the screen.▪Text appearing on the displays of the controller is printed in light grey. Whenever you see this formatting applied, you will find the same text on a controller display.▪Text appearing on labels of the hardware controller is printed in orange. Whenever you see this formatting applied, you will find the same text on the controller.▪Important names and concepts are printed in bold.▪References to keys on your computer’s keyboard you’ll find put in square brackets (e.g.,“Press [Shift] + [Enter]”).►Single instructions are introduced by this play button type arrow.→Results of actions are introduced by this smaller arrow.Naming ConventionThroughout the documentation we will refer to MASCHINE controller (or just controller) as the hardware controller and MASCHINE software as the software installed on your computer.The term “effect” will sometimes be abbreviated as “FX” when referring to elements in the MA-SCHINE software and hardware. These terms have the same meaning.Button Combinations and Shortcuts on Your ControllerMost instructions will use the “+” sign to indicate buttons (or buttons and pads) that must be pressed simultaneously, starting with the button indicated first. E.g., an instruction such as:“Press SHIFT + PLAY”means:1.Press and hold SHIFT.2.While holding SHIFT, press PLAY and release it.3.Release SHIFT.Unlabeled Buttons on the ControllerThe buttons and knobs above and below the displays on your MASCHINE controller do not have labels.。
STM32固件库使用手册的中文翻译版

因为该固件库是通用的,并且包括了所有外设的功能,所以应用程序代码的大小和执行速度可能不是最优 的。对大多数应用程序来说,用户可以直接使用之,对于那些在代码大小和执行速度方面有严格要求的应 用程序,该固件库驱动程序可以作为如何设置外设的一份参考资料,根据实际需求对其进行调整。
1.3.1 变量 ................................................................................................................................................ 28 1.3.2 布尔型 ............................................................................................................................................ 28 1.3.3 标志位状态类型 ........................................................................................................................... 29 1.3.4 功能状态类型 .............................................................................................................
惠普彩色激光打印机 Pro M454 和惠普彩色激光多功能一体机 Pro M479 维修手册说明书
Table -1 Revision history Revision number 1
Revision date 6/2019
Revision notes HP LaserJet Pro M454 HP LaserJet Pro MFP M479 Repair manual initial release
Additional service and support for HP internal personnel HP internal personnel, go to one of the following Web-based Interactive Search Engine (WISE) sites: Americas (AMS) – https:///wise/home/ams-enWISE - English – https:///wise/home/ams-esWISE - Spanish – https:///wise/home/ams-ptWISE - Portuguese – https:///wise/home/ams-frWISE - French Asia Pacific / Japan (APJ) ○ https:///wise/home/apj-enWISE - English ○ https:///wise/home/apj-jaWISE - Japanese ○ https:///wise/home/apj-koWISE - Korean ○ https:///wise/home/apj-zh-HansWISE - Chinese (simplified)
Find information about the following topics ● Service manuals ● Service advisories ● Up-to-date control panel message (CPMD) troubleshooting ● Install and configure ● Printer specifications ● Solutions for printer issues and emerging issues ● Remove and replace part instructions and videos ● Warranty and regulatory information
Ovation I O Reference Manual

This publication adds the Eight Channel RTD module to the Ovation I/O Reference Manual. It should be placed between Sections 19 and 20.Date: 04/03IPU No.243Ovation ® Interim Publication UpdatePUBLICATION TITLEOvation I/O Reference ManualPublication No. R3-1150Revision 3, March 2003Section 19A. Eight Channel RTDModule19A-1. DescriptionThe Eight (8) channel RTD module is used to convert inputs from Resistance Temperature Detectors (RTDs) to digital data. The digitized data is transmitted to the Controller.19A-2. Module Groups19A-2.1. Electronics ModulesThere is one Electronics module group for the 8 channel RTD Module:n5X00119G01 converts inputs for all ranges and is compatible only with Personality module 5X00121G01 (not applicable for CE Mark certified systems).19A-2.2. Personality ModulesThere is one Personality module groups for the 8 channel RTD Module:n5X00121G01 converts inputs for all ranges and is compatible only with Electronics module 5x00119G01 (not applicable for CE Mark certified systems).19A-2.3. Module Block Diagram and Field Connection WiringDiagramThe Ovation 8 Channel RTD module consists of two modules an electronics module contains a logic printed circuit board (LIA) and a printed circuit board (FTD). The electronics module is used in conjunction with a personalty module, which contains a single printed circuit board (PTD). The block diagram for the 8 channel RTD moduleis shown in Figure 19A-1.Table 19A-1. 8 Channel RTD Module Subsystem ChannelsElectronic Module Personality Module85X00119G015X00121G01Figure 19A-1. 8 Channel RTD Module Block Diagram and Field Connection Wiring Diagram19A-3. SpecificationsElectronics Module (5X00119)Personality Module (5X00121)Table 19A-2. 8 Channel RTD Module SpecificationsDescription ValueNumber of channels8Sampling rate50 HZ mode: 16.67/sec. normally. In 3 wire mode, leadresistance measurement occurs once every 6.45 sec.during which the rate drops to 3/sec.60 HZ mode: 20/sec. normally. In 3 wire mode, leadresistance measurement occurs once every 6.45 sec.during which the rate drops to 2/sec.Self Calibration Mode: Occurs on demand only. The ratedrops to 1/sec. once during each self calibration cycle.RTD ranges Refer to Table 19A-3.Resolution12 bitsGuaranteed accuracy (@25°C)0.10% ±[0.045 (Rcold/Rspan)]% ± [((Rcold + Rspan)/4096 OHM)]% ± [0.5 OHM/Rspan]% ±10 m V ± 1/2LSBwhere:Rcold and Rspan are in Ohms.Temperature coefficient 10ppm/°CDielectric isolation:Channel to channel Channel to logic 200V AC/DC 1000 V AC/DCInput impedance100 M OHM50 K OHM in power downModule power 3.6 W typical; 4.2 W maximumOperating temperature range0 to 60°C (32°F to 140°F)Storage temperature range-40°C to 85°C (-40°F to 185°F)Humidity (non-condensing)0 to 95%Self Calibration On Demand by Ovation ControllerCommon Mode Rejection120 dB @ DC and nominal power line frequency+/- 1/2%Normal Mode Rejection100 dB @ DC and nominal power line frequency+/- 1/2%Table 19A-3. 8 Channel RTD RangesScale #(HEX)Wires Type Tempo FTempo CRcold(ohm)Rhot(ohm)Excitationcurrent(ma)Accuracy± ±countsAccuracy± ±% ofSPAN1310OhmPL0 to1200–18 t o6496106.3 1.090.222310OhmCU 0 to302–18 t o1508.516.5 1.0 130.32D350OhmCU 32 to2840 to1405080 1.0110.2711350OhmCU 32 to2300 to1105378 1.0120.30193100Ohm PL –4 to334–16 t o16892163.671.0110.27223100Ohm PL 32 to5200 to269100200 1.0100.25233100Ohm PL 32 to10400 to561100301 1.0100.25253120Ohm NI –12 t o464–11 t o240109360 1.0100.25263120Ohm NI 32 to1500 to70120170 1.0130.32283120Ohm NI 32 to2780 to122120225 1.0110.27804100Ohm PL 32 to5440 to290100 208 1.0100.25814100Ohm PL 356 t o446180 t o230168 186 1.0300.74824200Ohm PL 32 to6980 to370200 473 1.0120.30834200Ohm PL 514 t o648268 t o342402452 1.0290.71844100Ohm PL 32 to1240 to51100120 1.0190.47854100Ohm PL 32 to2170 to103100 140 1.0130.3286 4100Ohm PL 32 to4120 to211100 180 1.0110.27874100Ohm PL 32 to7140 to379100 240 1.0100.25884120Ohm PL 511 t o662266 t o350200230 1.0240.5919A-4. 8 Channel RTD Terminal Block Wiring Information19A-4.1. Systems Using Personality Module 5X00121G01 Each Personality module has a simplified wiring diagram label on its side, which appears above the terminal block. This diagram indicates how the wiring from the field is to beconnected to the terminal block in the base unit. The following table lists and defines the abbreviations used in this diagram.Table 19A-4. Abbreviations Used in the DiagramAbbreviation Definition+IN, -IN Positive and negative sense input connectionEarth ground terminal. Used for landing shields when the shield is to begrounded at the module.PS+, PS-Auxiliary power supply terminals.RTN Return for current source connection.SH Shield connector. used for landing shields when the shield is to begrounded at the RTD.SRC Current source connection.Note:PS+ and PS- are not used by this module.19A-5. 8 Channel RTD Module Address Locations19A-5.1. Configuration and Status RegisterWord address 13 (D in Hex) is used for both module configuration and module status. The Module Status Register has both status and diagnostic information. The bit information contained within these words is shown in Table 19A-5.Definitions for the Configuration/Module Status Register bits:Bit 0:This bit configures the module (write) or indicates the configuration state of the module (read). A “1” indicates that the module is configured. Note that until the module is configured, reading from addresses #0 through #11 (B in Hex) will produce an attention status.Bit 1:This bit (write “1”) forces the module into the error state, resulting in the error LED being lit. The read of bit “1” indicates that there is an internal module error,or the controller has forced the module into the error state. The state of this bit is always reflected by the module’s Internal Error LED. Whenever this bit is set,an attention status is returned to the controller when address #0 through #11(B in Hex) are read.Table 19A-5. 8 Channel RTD Configuration/Status Register (Address 13 0xD in Hex)Bit Data Description -Configuration Register (Write)Data Description -Status Register (Read)0Configure Module Module Configured(1 = configured; 0 = unconfigured)1Force errorInternal or forced error(1 = forced error; 0 = no forced error)250/60 Hz select (0 = 60Hz, 1 = 50Hz)50/60 Hz System (1 = 50Hz) d(read back)3SELF_CAL (Initiates Self Calibration)Warming bit (set during power up or configuration)40050060Module Not Calibrated 708CH.1 _ 3/4 Wire.CH.1 _ 3/4 Wire - Configuration (read back)9CH.2 _ 3/4 Wire.CH.2 _ 3/4 Wire - Configuration (read back)10CH.3 _ 3/4 Wire.CH.3 _ 3/4 Wire - Configuration (read back)11CH.4 _ 3/4 Wire.CH.4 _ 3/4 Wire - Configuration (read back)12CH.5 _ 3/4 Wire.CH.5 _ 3/4 Wire - Configuration (read back)13CH.6 _ 3/4 Wire.CH.6 _ 3/4 Wire - Configuration (read back)14CH.7 _ 3/4 Wire.CH.7 _ 3/4 Wire - Configuration (read back)15CH.8 _ 3/4 Wire.CH.8 _ 3/4 Wire - Configuration (read back)Bit 2:The status of this bit (read) indicates the conversion rate of the module, write to this bit configures the conversion rate of A/D converters as shown below.see Table 19A-6.Bit3:Write: This bit is used to initiate self-calibration. Read: This bit indicates that the module is in the “Warming” state. this state exists after power up and ter-minates after 8.16 seconds. the module will be in the error condition during the warm up period.Bit4 & 5:These bits are not used and read as “0” under normal operation.Bit 6:This bit (read) is the result of a checksum test of the EEPROM. A failure of this test can indicate a bad EEPROM, but it typically indicates that the module has not been calibrated. A “0” indicates that there is no error condition. If an error is present, the internal error LED is lit and attention status will be returned for all address offsets 0-11 (0x0 - 0xB). The “1” state of this bit indicates an unre-coverable error condition in the field.Bit 7:This bits is not used and read as “0” under normal operation.Bit 8 - 15:These bits are used to configure channels 1 - 8 respectively for 3 or 4 wire op-eration. A “0” indicates 3 wire and a “1” indicates 4 wire operation, see Table 19A-7 and Table 19A-8).Word address 12 (0xC) is used to configure the appropriate scales for Channels 1 - 4 (refer to Table 19A-7 and Table 19A-8).Table 19A-6. Conversion Rate Conversion Rate (1/sec.)Bit 260 (for 60Hz systems)050 (for 50Hz systems)1Table 19A-7. Data Format for the Channel Scale Configuration Register(0xC)Bit Data Description Configuration (Write)Data Description Status (Read)0 Configure Channel #1scale - Bit 0Channel #1 scale configuration (read back) - Bit 01Configure Channel #1scale - Bit 1Channel #1 scale configuration (read back) - Bit 12Configure Channel #1scale - Bit 2Channel #1 scale configuration (read back) - Bit 23Configure Channel #1scale - Bit 3Channel #1 scale configuration (read back) - Bit 34Configure Channel #2 scale - Bit 0Channel #2 scale configuration (read back) - Bit 05Configure Channel #2 scale - Bit 1Channel #2 scale configuration (read back) - Bit 16Configure Channel #2 scale - Bit 2Channel #2 scale configuration (read back) - Bit 27Configure Channel #2 scale - Bit 3Channel #2 scale configuration (read back) - Bit 38Configure Channel #3 scale - Bit 0Channel #3 scale configuration (read back) - Bit 09Configure Channel #3 scale - Bit 1Channel #3 scale configuration (read back) - Bit 1Caution:Configuring any or all channel scales while the system is running will cause all channels to return attention status for up to two seconds following the reconfiguration.Caution:Configuring any or all channel scales while the system is running will cause all channels to return attention status for up to two seconds following the reconfiguration.10Configure Channel #3 scale - Bit 2Channel #3 scale configuration (read back) - Bit 211Configure Channel #3 scale - Bit 3Channel #3 scale configuration (read back) - Bit 312Configure Channel #4 scale - Bit 0Channel #4 scale configuration (read back) - Bit 013Configure Channel #4 scale - Bit 1Channel #4 scale configuration (read back) - Bit 114Configure Channel #4 scale - Bit 2Channel #4 scale configuration (read back) - Bit 215Configure Channel #4 scale - Bit 3Channel #4 scale configuration (read back) - Bit 3Table 19A-8. Data Format for the Channel Scale Configuration Register(0xE)Bit Data Description Configuration (Write)Data Description Status (Read)0 Configure Channel #5 scale - Bit 0Channel #5 scale configuration (read back) - Bit 01Configure Channel #5 scale - Bit 1Channel #5 scale configuration (read back) - Bit 12Configure Channel #5 scale - Bit 2Channel #5 scale configuration (read back) - Bit 23Configure Channel #5 scale - Bit 3Channel #5 scale configuration (read back) - Bit 34Configure Channel #6 scale - Bit 0Channel #6 scale configuration (read back) - Bit 05Configure Channel #6 scale - Bit 1Channel #6 scale configuration (read back) - Bit 16Configure Channel #6 scale - Bit 2Channel #6 scale configuration (read back) - Bit 27Configure Channel #6 scale - Bit 3Channel #6 scale configuration (read back) - Bit 38Configure Channel #7 scale - Bit 0Channel #7 scale configuration (read back) - Bit 09Configure Channel #7 scale - Bit 1Channel #7 scale configuration (read back) - Bit 110Configure Channel #7 scale - Bit 2Channel #7 scale configuration (read back) - Bit 211Configure Channel #7 scale - Bit 3Channel #7 scale configuration (read back) - Bit 312Configure Channel #8 scale - Bit 0Channel #8 scale configuration (read back) - Bit 013Configure Channel #8 scale - Bit 1Channel #8 scale configuration (read back) - Bit 114Configure Channel #8 scale - Bit 2Channel #8 scale configuration (read back) - Bit 215Configure Channel #8 scale - Bit 3Channel #8 scale configuration (read back) - Bit 3Table 19A-7. Data Format for the Channel Scale Configuration Register(0xC)19A-6. Diagnostic LEDsTable 19A-9. 8 Channel RTD Diagnostic LEDsLED DescriptionP (Green)Power OK LED. Lit when the +5V power is OK.C (Green)Communications OK LED. Lit when the Controller is communicatingwith the module.I (Red)Internal Fault LED. Lit whenever there is any type of error with themodule except to a loss of power. Possible causes are:n - Module initialization is in progress.n - I/O Bus time-out has occurred.n - Register, static RAM, or FLASH checksum error.n - Module resetn - Module is uncalibrated.n - Forced error has been received from the Controllern - Communication between the Field and Logic boards failedCH1 - CH 8 (Red)Channel error. Lit whenever there is an error associated with a channel or channels. Possible causes are:n - Positive overrangen - Negative overrangen Communication with the channel has failed。
Activity-of-ETR1-Modulates-the-Interaction-of-Receptors-and-EIN2_2010_Molecular-Plant
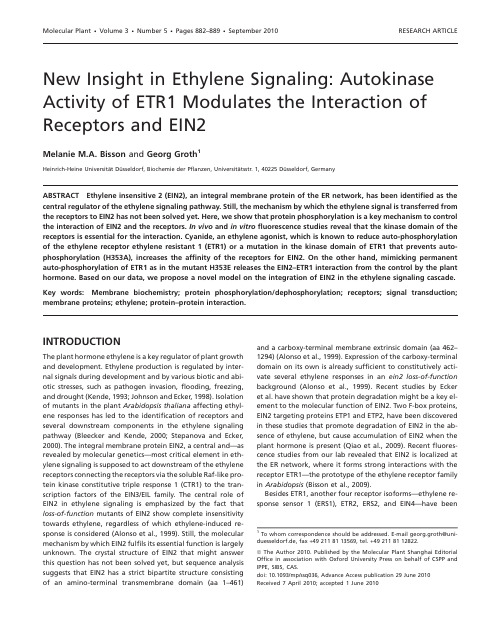
Molecular Plant•Volume3•Number5•Pages882–889•September2010RESEARCH ARTICLE New Insight in Ethylene Signaling:Autokinase Activity of ETR1Modulates the Interaction of Receptors and EIN2Melanie M.A.Bisson and Georg Groth1Heinrich-Heine Universita¨t Du¨sseldorf,Biochemie der Pflanzen,Universita¨tsstr.1,40225Du¨sseldorf,GermanyABSTRACT Ethylene insensitive2(EIN2),an integral membrane protein of the ER network,has been identified as the central regulator of the ethylene signaling pathway.Still,the mechanism by which the ethylene signal is transferred from the receptors to EIN2has not been solved yet.Here,we show that protein phosphorylation is a key mechanism to control the interaction of EIN2and the receptors.In vivo and in vitrofluorescence studies reveal that the kinase domain of the receptors is essential for the interaction.Cyanide,an ethylene agonist,which is known to reduce auto-phosphorylation of the ethylene receptor ethylene resistant1(ETR1)or a mutation in the kinase domain of ETR1that prevents auto-phosphorylation(H353A),increases the affinity of the receptors for EIN2.On the other hand,mimicking permanent auto-phosphorylation of ETR1as in the mutant H353E releases the EIN2–ETR1interaction from the control by the plant hormone.Based on our data,we propose a novel model on the integration of EIN2in the ethylene signaling cascade.Key words:Membrane biochemistry;protein phosphorylation/dephosphorylation;receptors;signal transduction; membrane proteins;ethylene;protein–protein interaction.INTRODUCTIONThe plant hormone ethylene is a key regulator of plant growth and development.Ethylene production is regulated by inter-nal signals during development and by various biotic and abi-otic stresses,such as pathogen invasion,flooding,freezing, and drought(Kende,1993;Johnson and Ecker,1998).Isolation of mutants in the plant Arabidopsis thaliana affecting ethyl-ene responses has led to the identification of receptors and several downstream components in the ethylene signaling pathway(Bleecker and Kende,2000;Stepanova and Ecker, 2000).The integral membrane protein EIN2,a central and—as revealed by molecular genetics—most critical element in eth-ylene signaling is supposed to act downstream of the ethylene receptors connecting the receptors via the soluble Raf-like pro-tein kinase constitutive triple response1(CTR1)to the tran-scription factors of the EIN3/EIL family.The central role of EIN2in ethylene signaling is emphasized by the fact that loss-of-function mutants of EIN2show complete insensitivity towards ethylene,regardless of which ethylene-induced re-sponse is considered(Alonso et al.,1999).Still,the molecular mechanism by which EIN2fulfils its essential function is largely unknown.The crystal structure of EIN2that might answer this question has not been solved yet,but sequence analysis suggests that EIN2has a strict bipartite structure consisting of an amino-terminal transmembrane domain(aa1–461)and a carboxy-terminal membrane extrinsic domain(aa462–1294)(Alonso et al.,1999).Expression of the carboxy-terminal domain on its own is already sufficient to constitutively acti-vate several ethylene responses in an ein2loss-of-function background(Alonso et al.,1999).Recent studies by Ecker et al.have shown that protein degradation might be a key el-ement to the molecular function of EIN2.Two F-box proteins, EIN2targeting proteins ETP1and ETP2,have been discovered in these studies that promote degradation of EIN2in the ab-sence of ethylene,but cause accumulation of EIN2when the plant hormone is present(Qiao et al.,2009).Recentfluores-cence studies from our lab revealed that EIN2is localized at the ER network,where it forms strong interactions with the receptor ETR1—the prototype of the ethylene receptor family in Arabidopsis(Bisson et al.,2009).Besides ETR1,another four receptor isoforms—ethylene re-sponse sensor1(ERS1),ETR2,ERS2,and EIN4—have been1To whom correspondence should be addressed.E-mail georg.groth@uni-duesseldorf.de,fax+492118113569,tel.+492118112822.ªThe Author2010.Published by the Molecular Plant Shanghai Editorial Office in association with Oxford University Press on behalf of CSPP and IPPE,SIBS,CAS.doi:10.1093/mp/ssq036,Advance Access publication29June2010 Received7April2010;accepted1June2010identified in Arabidopsis(Bleecker et al.,1988;Chang et al., 1993;Hua et al.,1995,1998;Sakai et al.,1998;Hua and Meyerowitz,1998).All resemble typical bacterial histidine kinase signaling systems,but based on their sequence,they have been classified into two subfamilies.ETR1and ERS1,be-longing to subfamily-I,are characterized by a canonical histi-dine kinase domain and an amino-terminal sensor domain that binds ethylene via a copper co-factor.Receptors ETR2,ERS2, and EIN4form subfamily-II.They are characterized by an ad-ditional hydrophobic domain at their amino-terminus and a less conserved kinase domain that is lacking essential ele-ments such as the conserved histidine.Receptors ETR1, ETR2,and EIN4are termed‘hybrid-type histidine kinases’,as they contain a carboxy-terminal receiver domain that was shown to specifically interact with histidine phosphotransfer proteins(HPts),strengthening the idea that a multistep-phos-phorelay is involved in ethylene signaling(Urao et al.,2000; Hass et al.,2004;Scharein et al.,2008).Receptors ERS1and ERS2are lacking the carboxy-terminal two-component re-ceiver domain found in the hybrid-type receptors.All receptors form dimers,which—as in bacterial histidine kinases—reflect the functional unit of the receptors.Disulfide-linked homo-dimers have been described for subfamily-I receptors ETR1 and ERS1(Schaller et al.,1995;Hall et al.,2000).Recent inter-action studies in transiently transformed tobacco cells and in yeast indicate that the receptors also can form homomeric and heteromeric complexes of all combinations including subfam-ily-I–subfamily-II co-complexes(Grefen et al.,2008;Gao et al., 2008).The precise mechanism and the mode of action by which the receptors are linked to further downstream components—in particular to the central regulator EIN2—are unclear.Here, we use a combination of in vitro and in vivofluorescence studies to show that EIN2interacts with all members of the ethylene receptor family.Wefind furthermore that the kinase domain of the receptors is crucial for this interaction.The plant hormone itself modulates the EIN2–receptor interaction by shutting down the autokinase activity of the receptors.RESULTSEIN2Interacts with All Members of the Ethylene Receptor Family In PlantaWe previously showed that the central regulator of ethylene responses EIN2is localized at the ER membrane,where it shows specific interaction with the receptor protein ETR1(Bisson et al.,2009).To determine whether the interaction of ETR1 and EIN2reflects a general mechanism in ethylene signaling, we have analyzed the interaction of EIN2with the other mem-bers of the ethylene receptor family by further in planta Fluo-rescence Resonance Energy Transfer(FRET)studies using the fluorophors greenfluorescent protein(GFP)as donor and redfluorescent protein(RFP)as acceptor.The donor was fused to the carboxy-terminus of EIN2and RFP-fusions of the recep-tor proteins ETR1,ERS1,ETR2,ERS2,and EIN4(kindly provided by Klaus Harter)were used as acceptors.The fusion proteins were transiently expressed in tobacco epidermal leaf cells. Analysis by confocal laser scanning microscopy confirms that all members of the ethylene receptor family co-localize with EIN2at the ER membrane(Figure1).Energy transfer between donor and acceptor in planta,and thus interaction of EIN2and different ethylene receptor proteins(ERP),was demonstrated by the acceptor bleaching method(Karpova et al.,2003).FRET-efficiencies were calculated from thefluorescence intensity of the donor in the absence and in the presence of different ac-ceptor receptor proteins(ERP–RFP).Transfer efficiencies of 15–16%were obtained for subfamily-I receptors ETR1and ERS1.Efficiencies for receptors ETR2,ERS2,and EIN4,belonging to subfamily-II,were slightly lower,with11–12%(Figure2A). The lower efficiency may be attributed to the additional mem-brane-spanning helix in the amino-terminal ethylene binding domain of subfamily-II receptors resulting in an increased distance between the donor and acceptorfluorophors and a reduced FRET efficiency.In any case,bothfigures clearly dis-criminate from negative controls.Backgroundfluctuation of cells expressing EIN2–GFP on its own and cells co-expressing EIN2–GFP and CLAVATA2–mCherry(kindly provided by Ru¨diger Simon),a membrane protein involved in stem cell de-velopment that is located to the ER(Bleckmann et al.,2010), corresponds to transfer efficiencies of4–6%only.Positive con-trols using tandem labeled EIN2showed transfer efficiencies of16%,like those obtained with the subfamily-I receptors (Figure2B).Both negative and positive controls emphasize that transfer efficiencies obtained from cellsco-expressingFigure1.The Central Regulator of Ethylene Responses EIN2Co-Localizes with the Arabidopsis Ethylene Receptor Family at the ER Membrane in Transiently Transformed Tobacco Leaf Cells. Confocal images of epidermal leaf cells expressing EIN2–GFP and a RFP fusion protein of the ethylene receptor family.The upper row shows the emission channel for GFP,while the middle column represents RFPfluorescence measured with ETR1–RFP,ERS1–RFP, ETR2–RFP,ERS2–RFP,and EIN4–RFP.The yellow overlay color of the GFP and RFPfluorescence(lower row)demonstrates the co-localization of EIN2and the different members of the Arabidop-sis ethylene receptor family at the ER.Scale bars indicate5l m.Bisson&Groth d Phosphorylation Controls EIN2–Receptor Interaction|883EIN2–GFP and the different members of the ethylene receptor family (ERP–RFP)result from specific protein–protein interac-tions between EIN2and the receptor proteins rather than from non-specific binding of the overexpressed fluorescent probes.Due to the similar excitation and emission maxima,quantum yield,and fluorescence brightness (Shaner et al.,2004)of the acceptor fluorophors FRET-efficiencies obtained with RFP or mCherry,an enhanced RFP-derivate,which was applied as acceptor only in our control experiments,are highly compat-ible.Equivalence of transfer efficiencies using GFP–RFP or GFP–mCherry as donor–acceptor pair is further supported by FRET interaction studies on ETR1and EIN2.A FRET-efficiency of 18.362.3%was observed using ETR1–GFP:EIN2–mCherry (Bisson et al.,2009)that matches very well to the transfer efficiency of 16.462.1%obtained with EIN2–GFP:ETR1–RFP (this work).Tryptophan Quenching as an Analytical Tool to Quantify EIN2–ETR1Interactions In VitroThe data presented above show that the central regulator of ethylene responses EIN2associates with all members of the Arabidopsis ethylene receptor family.To quantify the inter-actions between EIN2and the ERP revealed by the in planta studies,we used the endogenous tryptophan residues in recombinant ETR1as reporter.Intrinsic tryptophan fluores-cence of purified proteins provides a sensitive probe to study protein–protein interactions in vitro .Quenching of fluores-cence upon addition of a tryptophan-less binding partner can be used to evaluate the apparent dissociation constant of the complex unraveling specificity and stability of the com-plex (Bisson et al.,2009).In order to obtain the tryptophan-less EIN2probe for the in vitro fluorescence studies,all nine endog-enous tryptophan residues in the soluble carboxy-terminal part of EIN2(aa 479–1294)were replaced by phenylalanine.To ensure that tryptophan substitution does not affect protein function,we transformed DNA encoding for a tryptophan-less carboxy-terminal domain of EIN2(aa 479–1294)in A.thaliana plants with an ein2loss-of-function background (ein2-1)and checked for phenotypic rescue based on triple-response anal-yses as described before (Alonso et al.,1999;Guzma´n and Ecker,1990).Triple response analysis revealed that comple-mented transgenic lines showed the same phenotype as the wild-type Columbia ecotype upon addition of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC)(Fig-ure 3C),confirming functionality of the tryptophan-less EIN2mutant.Quenching of tryptophan fluorescence of purified recombinant ETR1and truncated ETR1(aa 1–609)lacking the carboxy-terminal receiver domain and thereby mimicking the ERS1receptor showed no significant differences upon addition of tryptophan-less EIN2,respectively.Both receptor forms form specific complexes with the carboxy-terminal do-main of EIN2,which is underlined by the low dissociation con-stant (K d ;400nM)of the respective complexes (Figure 3A and 3B).Correct folding and structure of the purified recom-binant ETR1proteins were confirmed by circular dichroism (Supplemental Figure 1).Stability of the EIN2–ETR1Complex Is Modulated by the Ethylene Agonist CyanideTo study the effect of ethylene binding on the interaction of EIN2with the ethylene receptor family in fluorescence titration studies,we added cyanide—an ethylene agonist—together with the copper co-factor essential for ethylene binding (Figure 3A).Equivalence of cyanide and ethylene on ETR1autokinase activ-ity was demonstrated previously (Voet van Vormizeele and Groth,2008).Ethylene and cyanide reduce autophosphoryla-tion of purified recombinant ETR1.In vivo studies confirm the equivalence of cyanide and ethylene observed in the in vitro studies.They show that cyanide mimics ethylene action and response in planta (Sisler,1977)and efficiently competes with ethylene binding in living cyanide-resistant tissue(SolomosFigure 2.FRET Reveals Specific Interaction of EIN2with All Mem-bers of the Ethylene Receptor Family at the ER Membrane.(A)FRET efficiencies detected with the acceptor bleaching method in cells coexpressing EIN2–GFP and the different ethylene receptor protein–RFP fusion constructs (ERP–RFP).FRET efficiencies calcu-lated from the fluorescence intensity of the donor before and after bleaching of the acceptor receptor proteins are about 15%for sub-family-I receptors ETR1and ERS1and about 11%for subfamily-II receptors ETR2,ERS2,and EIN4.(B)Controls expressing EIN2–GFP on its own (negative control)and a tandem construct of EIN2labeled with GFP and mCherry (positive control).In order to demonstrate that EIN2forms specific interac-tion with the ethylene receptors at the ER membrane,another con-trol is shown in which EIN2–GFP was co-expressed with the mCherry-labeled receptor-like protein CLAVATA2(CLV2),a mem-brane protein shown to localize to the ER membrane when expressed in the absence of the receptor kinase CORYNE.Data rep-resent the mean 6standard error (n =30cells).884|Bisson &GrothdPhosphorylation Controls EIN2–Receptor Interactionand Laties,1974).Titration studies with the purified recombi-nant proteins that are summarized in Figure 3B show that cy-anide substantially increases the stability of the ETR1–EIN2complex.The dissociation constant of the EIN2–ETR1complex in the presence of cyanide and copper is about 100nM.Hence,the ethylene agonist increases the affinity of the receptor for the binding of EIN2by a factor of four compared to the situ-ation in which the stimulus is lacking.Together with the in planta fluorescence studies that revealed interaction of EIN2with all members of the ethylene receptor family,no matter whether they contain a carboxy-terminal receiver domain or not,the in vitro titration studies suggest that the kinase domain of the receptors is essential for the interaction.Site-Directed Mutagenesis of the Predicted Phosphorylation Site H353in ETR1To further assess the role of the histidine kinase domain in the interaction of the ethylene receptors with EIN2,we employed two phosphorylation mutants of ETR1in the in vitro titration studies.In both mutants,the conserved histidine-353pre-dicted to be the site of phosphorylation (Gamble et al.,1998)was mutated.Phosphorylation was abolished in the H353A mutant,while in the other mutant (H353E),histidine-353was replaced by a negatively charged glutamate to mimic permanent phosphorylation of the receptor protein.Folding and structure of the mutant ETR1proteins were probed by CD-spectroscopy.Both proteins showed similar spectra to the ETR1wild-type,suggesting that both substitu-tion mutants adopt a well folded structure (Supplemental Figure 1).Interaction of both mutants with EIN2was studied in the absence and in the presence of pared to the wild-type receptor,the H353A mutant showed an in-creased affinity towards EIN2(K d ;100nM),regardless of the presence or absence of the ethylene antagonist cyanide.The wild-type receptor showed the same increase in affinity only in the presence of cyanide (Figure 3A and 3B).Hence,the mutant H353A simulates constitutive presence of the plant hormone.Addition of an ethylene agonist did notfurtherFigure 3.Analysis of Signaling Complexes Formed by EIN2and the Individual Members of the Arabidopsis Ethylene Receptor Family by Tryptophan Fluorescence.(A)Titration of purified recombinant ETR1,ETR1(aa 1–609)lacking the carboxy-terminal receiver domain,thereby mimicking a recep-tor like ERS1or the phosphorylation mutants H353A and H353E of receptor ETR1,respectively,with the purified recombinant tryptophan-less carboxy-terminal part of EIN2(aa 479–1294).Quenching data were analyzed according to Bisson et al.(2009)and were fitted to a model assuming a single binding site in the interacting partners.Interactions of the recombinant proteins were monitored in the absence (o)or in the presence (d )of the ethylene agonist cyanide and the copper co-factor that was shown essential for ethylene binding.(B)Summary of the dissociation constants K d for EIN2–ETR1inter-action calculated from the fluorescence data.Dissociation con-stants are given as mean 6maximal errors of three independent measurements.(C)Triple response analysis of ein2loss-of-function plants (ein2-1)complemented with the tryptophan-less carboxy-terminal part of EIN2(aa 479–1294).Wild-type A.thaliana Columbia seeds,ethylene-insensitive ein2-1seeds,as well as seeds of complemented transgenic line ein2-1:EIN2479–1294(D W)under control of 35S promo-tor were plated onto GM-Plates (see Methods section)and incubated for 8d in the dark at 21°C.In the absence of ethylene precursor ACC (left panel),no ethylene response could be observed.Wild-type seedlings on plates containing 50l M ACC (right panel)showed the typical triple response to ethylene,inhibition of root and hypo-cotyls elongation,radial swelling of hypocotyls,and exaggeration ofapical hook (Guzma´n and Ecker,1990).Seedlings of the ethylene-insensitive ein2-1mutant showed no such typical responses and had the same phenotype as in the absence of ACC,whereas seedlings of complemented line ein2-1:EIN2479–1294(D W)showed a triple response in the presence of ACC,verifying full functionality of the tryptophan-less carboxy-terminal part of EIN2.Bisson &GrothdPhosphorylation Controls EIN2–Receptor Interaction|885improve binding affinity of this mutant for EIN2.Affinity of the EIN2–ETR1complex was also not affected by cyanide in the H353E mutant.The mutant showed a reduced affinity of the complex(K d;400nM),in both the presence and absence of the ethylene agonist.For the wild-type protein,this reduced affinity is only observed the absence of cyanide(Figure3A and 3B).Hence,the H353E mutant mimics constitutive absence of the plant hormone.Taken together,these results show that the interaction between EIN2and the ethylene receptor family is controlled by the phosphorylation in the kinase domain of the receptors.The plant hormone itself triggers the interaction by controlling the phosphorylation status of the receptors.DISCUSSIONOur study provides compelling evidence that the central reg-ulator of ethylene responses EIN2forms contacts with all mem-bers of the Arabidopsis ethylene receptor family.Moreover, the results implicate a novel model for ethylene signaling. Phosphorylation seems to be a key mechanism to modulate in-teraction of EIN2and the subfamily-I receptors rather than serving(merely)for phosphotransfer on HPts and two compo-nent signaling.In the presence of the plant hormone,the auto-kinase activity of the receptors is inhibited and the non-phosphorylated kinase domain of the receptors binds tightly to the corresponding domain of EIN2(Figure4B).Iso-lated EIN2is subject to degradation by the proteasome, explaining the rapid protein turnover observed in the absence of the plant hormone(Qiao et al.,2009).In addition to the phosphorylation of the kinase domain of the receptor,the Raf-like protein kinase CTR1,a negative regulator of ethylene responses acting downstream of the receptors(Kieber et al., 1993;Huang et al.,2003),is assumed to play a critical role in the ETR1–EIN2interaction.In the absence of ethylene, CTR1is tightly associated with the receptor complex(Clark et al.,1998),preventing the receptor from interacting with EIN2(Figure4A).Upon binding of ethylene to the receptor, CTR1is released or a conformational shift in CTR1is triggered, allowing interaction of the kinase domain of the receptor with EIN2.The release of CTR1or the induced conformational shift, in turn,causes the inactivation of CTR1,resulting in phosphor-ylation and stabilization of the nuclear transcription factor EIN3(Yoo et al.,2008).Both mechanisms controlling the inter-action of EIN2with the receptors,modulation of the autoki-nase activity,and interaction with CTR1are complementary, like disulfide reduction in the c subunit and release of the in-hibitory e subunit in the activation of the chloroplast ATPase. Here,reduction in the disulfide decreases the affinity for sub-unit e(Soteropoulos et al.,1992),while removal of subunit e significantly enhances the accessibility of the c-disulfide for reduction by thiol reagents(Richter et al.,1985).The pro-posed role of CTR1in our model should become testable once purified recombinant tryptophan-less CTR1becomes available or by in planta FRET studies usingfluorescent fusion proteins of CTR1,ETR1,and EIN2.Our proposed model(Figure4)is consistent with the central role of EIN2in ethylene signaling(Alonso et al.,1999),with the observed accumulation of EIN2upon ethylene exposure as well as with the lack of EIN2accumulation found in the etr1-1mutant(Qiao et al.,2009).Furthermore,it is consistent with the elevated level of EIN2in ctr1-1mutants(Qiao et al., 2009).Remaining association of EIN2with the phosphorylated receptors as found in our experiments(see Figures2and3) might be owed to the strong overexpression of thereceptorsFigure4.Signaling Complexes Formed at the ER Membrane in Re-sponse to Ethylene.Schematic model of the processes induced at the ER membrane by the binding of the plant hormone to the ethylene receptor com-plexes.Autokinase activity of ETR1and interaction with the soluble protein kinase CTR1control formation of signaling complexes of receptors and EIN2.Both processes are regulated by ethylene. (A)Without ethylene,binding receptor complexes are kept in their phosphorylated state due to their intrinsic autokinase activity(Voet van Vormizeele and Groth,2008)and combine with CTR1,a nega-tive regulator of ethylene signaling(Kieber et al,1993;Huang et al., 2003).Both processes distract the receptor complexes from interact-ing with the central regulator of ethylene signaling EIN2.Detached EIN2is promptly degraded by a proteasome-dependent pathway (Qiao et al.,2009).Hence,almost no EIN2accumulates in the ab-sence of the plant hormone.(B)Upon ethylene binding,autokinase activity of the receptors is inhibited,converting them to their non-phosphorylated status (Voet van Vormizeele and Groth,2008)and CTR1is inactivated by a conformational change or the release from the ER membrane (Gao et al.,2003).Both mechanisms result in a tight interaction of the ethylene receptor complexes with EIN2,causing the observed ethylene-dependent accumulation of EIN2at the ER membrane (Qiao et al.,2009).886|Bisson&Groth d Phosphorylation Controls EIN2–Receptor Interactionand of EIN2in planta and to the fact that no CTR1is present in the in vitro studies.Whether phosphorylation of subfamily-II on serine or thre-onine residues that has been shown in in vitro experiments with the isolated kinase domains of the receptors(Moussatche and Klee,2004)directly controls interaction with EIN2as well or whether this interaction is modulated by heterodimers of subfamily I and subfamily II receptors has to be addressed in further studies.METHODSCloning,Expression,and Purification of the Arabidopsis Ethylene Signaling Components ETR1and EIN2in Escherichia coliThe tryptophan-less carboxy-terminal part of EIN2(aa479–1294),full-length ETR1(aa1–738),and a truncated form of ETR1(aa1–609)lacking the receiver domain were cloned from Arabidopisis,expressed in Escherichia coli,and purified as de-scribed before(Bisson et al.,2009;Scharein et al.,2008).Phos-phorylation mutants H353A and H353E of the Arabidopsis ethylene receptor ETR1were obtained by site-directed muta-genesis of plasmid pET16b-ETR1expressing wild-type ETR1by sequential PCR steps as described by Cormack(1992).Amplifi-cation reactions were carried out in two sequential PCR-reactions in a BioMetra Gene Cycler using Pfu-polymerase, plasmid pET16b-ETR1as template and mutagenic oligonucleo-tides5’-AGCGGTTATGAACGCAGAAATGCGAACACC-3’for mu-tant H353A or5’-AGCGGTTATGAACGAAGAAATGCGAACACC-3’for mutant H353E,respectively.T7-terminator primer5’-GCTA-GTTATTGCTCAGCGG-3’was used as complementary primer in these reactions.Finally,T7-promotor sense primer5’-AGCGGT-TATGAACGCAGAAATGCGAACACC-3’was used to amplify the fragment encoding for full-length ETR1(aa1–738).Amplified fragments were agarose gel-purified,digested with Nde I and Bam HI,and ligated into the equivalent sites of vector pET16b. Inserted fragments were sequenced to confirm substitutions and to exclude additional mutations.Resulting plasmids pET16b-ETR1-H353A and pET16b-ETR1-H353E were expressed in E.coli C43(DE3)and proteins were purified following the protocol described for wild-type ETR1(Scharein et al.,2008). Protein concentrations of recombinant proteins were deter-mined by the bicinchoninic acid assay(Pierce Chemicals).Puri-fication of the proteins was analysed by SDS–PAGE according to Laemmli(1970).After electrophoresis,proteins were detected by silver staining(Heukeshoven and Dernick,1988).CD SpectroscopyFor CD measurements,purified recombinant ETR1constructs were dissolved in50mM potassium phosphate and0.03% (w/v)b-dodecyl-maltoside at afinal concentration of0.1–0.2 mg mlÀ1.Measurements were carried out in a Jasco720 spectropolarimeter at room temperature as described pre-viously(Voet van Vormizeele and Groth,2008).Cloning and Expression In PlantaFor transient expression in tobacco,the pDONR201-EIN2entry clone(Bisson et al.,2009)was combined with pMDC83(Curtis and Grossniklaus,2003)via the Gateway cloning system(Invi-trogen)to create a carboxy-terminal GFP fusion protein under control of a35S-promotor.Carboxy-terminal RFP-labeled ex-pression vectors containing cDNA fragments encoding for the different proteins of ethylene receptor family(ETR1, ERS1,ETR2,ERS2,EIN4)were kindly provided by Klaus Harter(Grefen et al.,2008).The transient expression vector encoding for mCherry-labeled CLAVATA2was kindly provided by Ru¨diger Simon(Bleckmann et al.,2010).Transient expres-sion in tobacco was performed as described in detail in Bisson et al.(2009).For stable expression of the tryptophan-less carboxy-terminal part of EIN2in Arabidopsis,the DNA fragment encoding this region of the protein was cloned via the Gateway system into the binary plant transformation plasmid pMDC32(Curtis and Grossniklaus,2003).Resulting ex-pression vector pMDC32-EIN2479–1294(D W)was transformed in-to ein2-1mutants of A.thaliana(obtained from the Nottingham European stock centre,ecotype Columbia(Guzma´n and Ecker, 1990))using thefloral dip method(Clough and Bent,1998). Transgenic lines were isolated by selection of the T1generation seeds on plates with GM-Medium(0.22%(w/v)Murashige and Skoog Medium(Duchefa),0.05%(w/v)MES,1%(w/v) sucrose, 1.2%(w/v)plant agar,pH7.8)containing50l M hygromycin.Fluorescence SpectroscopySteady-statefluorescence measurements on purified recombi-nant proteins were carried out in spectroluminometer LS55 (Perkin Elmer)at a controlled temperature of20°C using an excitation wavelength of295nm.Maximalfluorescence sig-nals were monitored at345nm emission wavelength.Titra-tions of EIN2479–1294(D W)to the different ETR1receptor constructs(ETR1,ETR11–609,ETR1-H353A,and ETR1-H353E) were performed according to the protocol described in Bisson et al.(2009).For measurements in the presence of the ethylene agonist cyanide,the different ETR1constructs were dissolved at a concentration of0.1l M in50mM Tris/HCl,pH7.6, 100mM potassium chloride,50mM NaCl,0.1%(w/v) b-dodecylmaltoside and pre-incubated with10l M copper chloride in order to provide the essential co-factor for binding of the ethylene agonist and10l M potassium cyanide.Titra-tions with EIN2479–1294(D W)were performed as in the absence of the ethylene agonist.The dissociation constant of the ETR1–EIN2complex was determined from thefluorescence data using the program GraFit(Erithacus Software)by afit of the experimental data to a model assuming a single binding site in the interacting partners.Confocal MicroscopyConfocal microscopy was performed using a LSM510Meta La-ser Scanning Microscope(Zeiss).GFPfluorescence was excitedBisson&Groth d Phosphorylation Controls EIN2–Receptor Interaction|887。
西门子技术问题总汇

文档标题
如何设置模拟量输入模板 SM 431-7KF00的温度补偿? 如何解决 SIMATIC BATCH 的 IL43基本设备上 hotfix 安装的问题? 如果通过 PCS7 V6.1 SP1 DVD 单独安装 SIMATIC BATCH Report 需要注意哪些设置? 为什么冗余模拟量输出模块的每个通道只有一半电流输出? 使用WinCC/Web Navigator V6.1 SP1需要什么样的操作系统和软件? 是否 COM PROFIBUS 可以使用所有版本的 GSD 文件? 如何在 WinCC flexible 中组态与S7 控制器的 Profinet 连接? 如何在操作面板上设定定时器时间, 同时如何输出定时器的剩余时间? 数据块初始值与实际值的含义 如何通过窗口对象滚动条步进调节过程值参数? 使用 SINAUT ST7 向电子邮箱接受方发送文本信息 SMS 需要做何设置? 可以使用CPU317-2PN/DP替代在iMap中组态的CPU315-2PN/DP吗? 什么情况下插入C-PLUG卡或者C-PLUG有什么作用? 通过一台PC,可以使用哪种方式访问与IWLAN/PB link PNIO或IE/PB link PNIO连接的PROFIBUS设备? 当在SINAUT网络中使用4线变压器应该注意哪些设置? 在 SINAUT 网络中,使用MD3拨号调制解调器作为专线调制解调器时,要进行哪些设置? 如何安装 DCF77 天线, 当选择 DCF77 天线时需要注意什么? 使用SINAUT ST7向传真机发送文本信息时,需要进行哪些设置? 在 SINAUT 项目中发送短消息必须进行哪些特殊服务的设置? 如何在S7-300 PN CPU和CP343-1之间建立一个open TCP 通讯连接,以及如何进行数据交换? 如何在两个S7-300 PN CPU之间建立一个open TCP 通讯连接,以及如何进行数据交换? 哪些控制系统可以成功与SINAUT ST7一起使用? 使用“零-Modem”电缆连接 TIM 模块应该注意什么? 当用 SINAUT 诊断工具的ST1协议进行诊断时,为什么TIM的状态不能显示? TIM 3V-IE 和 TIM 3V-IE Advanced 模块在以太网上通信时使用哪个端口号? 如何对没有接入网络的S7-200CPU编程? 掉电后,LOGO!的程序会丢失吗? 从 PCS7 V6.1 起,为什么没有分配任何 hierarchy (PH) 的 测量点(变量)通过编译不能在OS中自动创建相应的变量? 在SFC中,如何实现从一个 Sequencer 跳出后回到另一个 Sequencer 的某个固定位置并继续执行? 如何实现过程变量的平均值归档? 存储文件的目标路径和备份可选路径有何作用? WinCC变量归档中如何实现采集周期小于500ms的变量归档? 为什么在 OS 上会显示如下信息“时间跳变通知-永久切换为从站模式”? 在西门子A&D产品支持网站是否可以下载关于ET200M的手册? 在S7-400上怎样安装冗余电源? UDT改变后怎样更新使用UDT产生的数据块。 为什么在FB块中使用OUT变量赋值被调用FB块的IN变量时出现错误信息34:4469? 如何查看4-mation导入-导出错误 不能正确引导8212-1QU IBM/Lenovo M52 ThinkCentre 实时趋势更新缓慢的原因 如何保存变量名字典CSV文件的格式
