Organic Syntheses, Coll. Vol. 6, p.762 (1988); Vol. 52, p.109 (1972).
经典化学合成反应标准操作醛酮的合成

经典化学合成反应标准操作醛酮的经典合成目录1.前言 (4)2.由醇合成醛酮 (4)2.1铬(VI)试剂 (4)2.1.1 Jones氧化(Cr2O3/H2SO4/acetone) (4)2.1.2 Collins氧化(Cr2O3.2Py) (5)2.1.3 PCC(Pyrindium Chlorochromate)氧化 (8)2.1.4 PDC(Pyrindium Dichromate)氧化 (9)2.2 用活性MnO2氧化 (10)2.2.1 用活性MnO2氧化示例一: (10)2.3用DMSO氧化 (11)2.3.1 DMSO-(COCl)2氧化(Swern Oxidation) (11)2.3.2 DMSO-SO3-Pyridine (12)2.4 用氧铵盐氧化 (13)2.4.1 用氧铵盐氧化示例: (13)2.5 用高价碘试剂氧化 (14)2.5 .1 Dess-Martin氧化反应示例: (14)2.5.2 IBX氧化反应示例: (15)2.6 亚硝酸钠和醋酐氧化 (15)2.6.1 亚硝酸钠和醋酐氧化示例 (15)2.6 TPAP-NMO 氧化 (16)2.6.1 TPAP-NMO 氧化示例 (16)2.7 1,2-二醇的氧化 (16)2.7.1 1,2-二醇的氧化示例一: (17)2.7.1 其他1,2-二醇的氧化相关文献: (18)3.由卤化物合成醛酮 (18)3.1 由伯卤甲基和仲卤甲基的氧化合成醛酮 (18)3.1.1 用DMSO氧化(Kornblum反应) (18)3.1.2用硝基化合物氧化(Hass反应) (20)3.1.3用乌洛托品氧化(Sommelet反应) (21)3.1.4用对亚硝基二甲苯胺氧化吡啶翁盐氧化(Kröhnke反应) (22)3.1.5用胺氧化物氧化 (22)3.2 由二卤甲基或二卤亚甲基合成醛酮 (23)3.2.1 由二卤甲基合成醛反应示例: (23)3.3 由有机金属化合物的酰化合成醛酮 (24)3.3.1 由有机金属化合物的酰化合成醛酮示例 (25)3.4 由Pd催化反应合成醛 (25)4.由活泼甲基或活泼亚甲基烷烃合成醛酮 (25)4.1 用SeO2氧化合成醛酮 (26)4.1.1 用SeO2氧化合成醛酮示例 (26)4.2用空气氧化合成酮 (26)4.2.1用空气氧化合成酮反应示例: (27)4.3 用铬酸氧化合成酮 (27)4.3.1 用铬酸氧化合成酮示例 (27)4.4用高锰酸盐氧化合成酮 (29)4.5 用醌氧化合成酮 (29)5.由羧酸及其衍生物合成醛酮 (30)5.1由羧酸合成醛 (30)5.1.1用金属氢化物还原 (30)5.1.2由脱CO2合成醛 (31)5.1.3由羧酸合成酮 (31)5.2由酰氯及酸酐合成醛酮 (33)5.2.1用Rosenmund法合成 (33)5.2.2用金属氢化物还原 (34)5.3由酯及内酯合成醛 (35)5.3.1 酯通过DIBAL还原为醛示例: (36)5.4由酰胺合成醛酮 (36)5.4.1 由酰胺合成醛酮 (37)5.4.2 McFadyen-Stevens Reaction (38)5.5由酯或酰氯经Weinreb酰胺合成醛酮 (39)5.5.1 由Weinreb酰胺还原合成醛反应示例一 (40)5.5.2由Weinreb酰胺还原合成酮反应示例: (41)5.6由氰合成醛酮 (41)5.6.1DIBAL 还原腈到醛示例(最重要的方法) (42)5.6.2Li(EtO)3AlH 还原腈到醛示例(较重要的方法) (43)5.6.3Ranney Ni 加氢还原氰到合成醛示例 (43)5.6.4有机金属试剂对腈加成合成酮示例 (44)6. 由烯烃、芳环合成醛酮 (46)6.1 由烯烃臭氧氧化合成醛 (46)6.2 烯烃用OsO4/NaIO4氧化合成醛 (47)6.3 烯烃经由有机硼化合物中间体的烯烃甲酰化合成醛 (47)6.5 由烯烃的甲酰化合成醛 (48)6.5.1 Vilsmeyer反应 (48)6.5.2 Duff’s 甲酰化 (51)6.5.3 Reimer-Tiemann 甲酰化 (52)6.5.4 Gattermann甲酰化 (53)6.5.5 多聚甲醛/甲醇镁苯酚甲酰化 (53)6.5.6氯化锡/多聚甲醛苯酚甲酰化 (54)6.5.7重氮化后甲酰化 (54)6.6烯烃经加成-氧化反应合成酮 (56)6.6.1 烯烃经加成-氧化反应合成酮示例 (56)7. 由炔烃合成醛酮 (57)7.1 由加成-氧化反应合成醛酮 (57)7.2 由氧化反应合成酮 (57)7.3 由加成-水解反应合成酮 (58)7.4 由加成-还原反应合成酮 (59)7.5 由加成-烷基化,酰化等反应合成酮 (59)8. 由醚及环氧化合物合成醛酮 (59)8.1 Claisen重排 (59)8.2酸催化下环氧化物重排 (61)8.2.1 酸催化下环氧化物重排合成醛酮示例一 (61)8.3氧化法 (61)8.4 水解法缩醛或酮合成醛酮 (61)9. 由胺合成醛 (62)9.1胺的氧化 (62)9.1.1 胺的氧化合成醛反应示例: (63)9.2 由胺经由西佛碱的方法 (64)9.2.1 由胺经由西佛碱合成醛示例 (64)9.3 自苯胺衍生物合成 (64)10. 由硝基化合物合成醛酮 (64)11. 由Friedel-Crafts反应合成芳基酮 (65)11.1 由Friedel-Crafts反应合成芳基酮示例 (68)12. Dieckmann 缩合脱酸 (69)13. 由合成子合成醛酮 (71)14. 由砜合成醛酮 (71)15. Michael 反应和类似反应(Addition, Condensation) (71)1.前言醛和酮是一类重要的有机化合物,其合成在有机合成中占有非常重要的地位。
有机化学常用网址整理

有机化学常用网址整理有机合成:Organic Syntheses(有机合成手册), John Wiley & Sons (免费)/Named Organic Reactions Collection from the University ofOxford (有机合成中的命名反应库) (免费)/thirdyearcomputing/NamedOrganicReac...有机化学资源导航Organic Chemistry Resources Worldwide/有机合成文献综述数据库Synthesis Reviews (免费)/srev/srev.htmCAMEO (预测有机化学反应产物的软件)/products/cameo/index.shtmlCarbohydrate Letters (免费,摘要)/Carbohydrate_Letters/Carbohydrate Research (免费,摘要)/locate/carresCurrent Organic Chemistry (免费,摘要)/coc/index.htmlElectronic Encyclopedia of Reagents for Organic Synthesis (有机合成试剂百科全书e-EROS) /eros/European Journal of Organic Chemistry (免费,摘要)/jpages/1434-193X/Methods in Organic Synthesis (MOS,有机合成方法)/is/database/mosabou.htmOrganic Letters (免费,目录)/journals/orlef7/index.htmlOrganometallics (免费,目录)/journals/orgnd7/index.htmlRussian Journal of Bioorganic Chemistry (Bioorganicheskaya Khimiya) (免费,摘要)http://www.wkap.nl/journalhome.htm/1068-1620Russian Journal of Organic Chemistry (Zhurnal Organicheskoi Khimii) (免费,摘要)http://www.maik.rssi.ru/journals/orgchem.htmScience of Synthesis: Houben-Weyl Methods of Molecular Transformation/Solid-Phase Synthesis database (固相有机合成)/chem_db/sps.htmlSynthetic Communications (免费,摘要)/servlet/product/productid/SCCSyntheticPages (合成化学数据库) (免费)/The Complex Carbohydrate Research Center (复杂碳水化合物研究中心)/合成材料老化与应用(免费,目录)/default.html金属卡宾络合物催化的烯烃复分解反应(免费)/html/books/O61BG/b1/2002/2.6%20.htm上海化学试剂研究所/英国化学数据服务中心CDS (Chemical Database Service)/cds/cds.html英国皇家化学会碳水化合物研究组织(Carbohydrate Group of the Royal Society of Chemistry) /lap/rsccom/dab/perk002.htm有机反应催化学会(ORCS, Organic Reaction Catalysis Society)/有机合成练习(免费)/中国科学院成都有机化学研究所:催化与环境工程研究发展中心/MainIndex.htm金属有机及元素有机化学:CASREACT - Chemical Reactions Database(CAS的化学反应数据库)/CASFILES/casreact.html日本丰桥大学Jinno实验室的研究数据库(液相色谱、多环芳烃/药物/杀虫剂的紫外谱、物性) (免费)http://chrom.tutms.tut.ac.jp/JINNO/ENGLISH/RESEARCH/research...A New Framework for Porous Chemistry (金属有机骨架) (免费)/alchem/articles/1056983432324.htmlActa Crystallographica Section B (免费,摘要)/b/journalhomepage.htmlActa Crystallographica Section E (免费,摘要)/e/journalhomepage.htmlBibliographic Notebooks for Organometallic Chemistryhttp://www.ensc-lille.fr/recherche/cbco/bnoc.htmlBiological Trace Element Research (生物痕量元素研究杂志) (免费,摘要)/JournalDetail.pasp?issn=0163-4984...Journal of Organometallic Chemistry (免费,摘要)/locate/jnlabr/jomOrganic Letters (免费,目录)/journals/orlef7/index.htmlOrganometallics (免费,目录)/journals/orgnd7/index.htmlSyntheticPages (合成化学数据库) (免费)/金属卡宾络合物催化的烯烃复分解反应(免费)/html/books/O61BG/b1/2002/2.6%20.htm金属有机参考读物:The Organometallic HyperTextBook by Rob Toreki/organomet/index.html金属有机化学国家重点实验室,中国科学院上海有机所/元素有机化学国家重点实验室(南开大学)/在线网络课程:有机金属反应和均相催化机理(Dermot O'Hare 主讲)/icl/dermot/organomet/药物化学:Fisher Scientific/PubMed: MEDLINE和PREMEDLINE (免费)/PubMed/生物医药:BioMedNet: The World Wide Club for the Biological and Medical Community/AIDSDRUGS (艾滋病药物) (免费)/pubs/factsheets/aidsinfs.htmlautodock (分子对接软件) (免费)/pub/olson-web/doc/autodock/DIRLINE (卫生与生物医药信息源库) (免费)/HISTLINE (医药史库) (免费)/TOXNET (化合物毒性相关数据库系列) (免费)/日本药典,第14版(免费)http://jpdb.nihs.go.jp/jp14e/index.html小分子生物活性数据库ChemBank (免费)/Ashley Abstracts Database (药物研发、市场文献摘要) (免费)/databases/ashley/search.aspBIOSIS/BIOSIS/ONLINE/DBSS/biosisss.html从检索药物交易信息库PharmaDeals (部分免费)/从ChemWeb检索有机药物用途及别名库Negwer: organic-chemical drugs and their synonyms (部分免费)/negwer/negwersearch.html美国常用药品索引库RxList (免费)/美国国家医学图书馆NLM的免费在线数据库(免费)/hotartcl/chemtech/99/tour/internet.html制药公司目录(Pharmaceutical Companies on Virtual Library: Pharmacy Page)/company.html37℃医学网/AAPS PharmSci (免费,全文)/Abcam Ltd.有关抗体、试剂的销售,抗体的搜索)/Acta Pharmaceutica (免费,摘要)http://public.srce.hr/acphee/Advanced Drug Delivery Reviews (免费,摘要)http://www.elsevier.nl/locate/drugdelivAmerican Journal of Drug and Alcohol Abuse (免费,摘要)/servlet/product/productid/ADAAmerican Journal of Pharmaceutical Education (AJPE) (免费,全文)/Amgen Inc. (医药)/Anita's web picks (药学与药物化学信息导航)http://wwwcmc.pharm.uu.nl/oyen/webpicks.htmlAnnals of Clinical Microbiology and Antimicrobials (免费,全文)/Annual Review of Pharmacology and Toxicology (免费,摘要)/Anti-Cancer Drug Design (免费,摘要)/antcan/生物有机化学:ScienceDirect: 在线访问Elsevier的1100种期刊全文(免费目录) (免费)/生命、环境科学综合性资源TheScientificWorld (sciBASE)/生物医药:BioMedNet: The World Wide Club for the Biological and Medical Community/BIOETHICSLINE (BIOETHICS onLINE) (免费)/BIOME (生命科学资源导航)/browse/Directory of P450-containing Systems(P450酶系目录)http://p450.abc.hu/DIRLINE (卫生与生物医药信息源库) (免费)/百名最佳生物技术网站列表(Top 100 Biotechnology WWW Sites)/top100.asp从ChemWeb检索《化学工程与生物技术文摘》库CEABA (部分免费)/课程材料:MIT生物学超文本教材[url=:8001/esgbio/7001main.html]:8001/esgbio/7001main.html[/url]生物材料网(Biomaterials Network)/生物信息学资源导航,上海生物化学所/bio/index.htm小分子生物活性数据库ChemBank (免费)/英国剑桥医学研究委员会:分子生物学实验室LMB/biology site of the network./BIOSIS/BIOSIS/ONLINE/DBSS/biosisss.htmlCATH Protein Structure Classification (蛋白质结构分类) (免费)/bsm/cath/Databases and Tools for 3-D Protein Structure Comparison and Alignment (三维蛋白质结构对比) (免费)/ce.htmlLos Alamos National Laboratory Bioscience Division/Protein Data Bank (PDB, 蛋白质数据库) (免费)/pdb/计算分子生物学:Computational Molecular Biology at NIH/molbio/酶命名数据库(ENZYME-Enzyme nomenclature database) (免费)/enzyme/Access Excellence (有关生物、生命等的科学教育网站)/Acta Biochimica Polonica (免费,全文)http://www.actabp.pl/Acta Biotechnologica (生物技术学报) (免费,摘要)http://www.wiley-vch.de/publish/en/journals/alphabeticIndex/...American Institute of Biological Sciences (AIBS)/American Journal of Medical Genetics Part A (免费,摘要)/cgi-bin/jtoc?ID=33129Amos' WWW links page (生物大分子网络资源导航)/alinks.htmlAmersham International (英国,生物技术供应商)/....................杂:经典有机反应/ads/middle.htm有机合成手册/NIST 化学手册/chemistry/危险化学品数据库查询/erd/糖类(碳水化合物)拉曼谱图库SPECARBhttp://newton.foodsci.kvl.dk/users/engelsen/specarb/specarb.html 小型光谱数据库下载Optical Databases Download Page(免费) /download/db/uv_ir.html有机合成反应/Chemical Kinetics Database on the Web/index.php命名反应/display4.html图片演示实验(多个)/delights/texts/gif演示实验(多个)/delights/animations/bubble.html有机合成方法数据库/化学反应库/合成化学数据库/化学反应、合成方法库网络版/indexunten.htm固相有机合成/chem_db/sps.htmlScience of Synthesis/index.shtml英国曼彻斯特理工大学天体化学数据库/rate99.html保护基团数据库/chem_db/protectgrps.html金属合金物性数据库/prime/step1.asp几十个有机化合物的蒸气压数据/vp_data.html亨利常数http://www.mpch-mainz.mpg.de/~sander/res/henry.html高温材料热力学数据库/HiTempThermo/电离能数据表/keyan2/data/200412/822.html电解质溶液数据库/cds/datasets/physchem/elys.html侧重于放射化学的化学性质数据库/browse_compil.html#compilation_searchThermo Explorer/thermexpl.html光谱分析数据库软件/常用核磁共振NMR溶剂的性质http://www.chem.uni-potsdam.de/englisch/nmrsolv.htmlpKa Data/pKa/pKa.htmlPerry化学工程师手册(7th Edition)/knovel2/Toc.jsp?SpaceID=10093&BookID=48农药活性成分信息数据库/profiles/index.htmlMolecular Knowledge Systems, Inc./元素的电离能、电子亲和性、电负性数据表/keyan2/data/200412/812.html脂类数据库/home.stmBiochemical Compounds Declarative Database/klotho/水溶液反应库及平衡计算.au/jess/jess_home.htm瑞士芬美意香精香料公司Firmenich/portal/page?_pageid=655,143613&_dad=portal&_schema=PORTAL& cid=HO&sid=null杀虫剂毒性库EXTOXNET/ghindex.htmlChemical Abstracts/SciFinder/publications/scifinder/Combined Chemical Dictionary/scripts/ccdweb.exe?welcome-mainCRC handbook of chemistry and physics/search/t?SEARCH=crc+handbook+of+chemistryData Search for Species by Chemical Formula/chemistry/form-ser.html溶剂选择数据库/Index.htm?url=/Home.htm [组图]气体的临界性质Properties of Various Gases/ads/middle.htm美国NIST的标准参考数据产品/srd/有机化合物数据库/chemistry/cmp/cmp.html稀土物理化学性质数据库/wuxindex.htm稀土萃取数据库/cuiqindex.htm碳-13核磁共振波谱数据库free/c13index.htmUnits conversion / metric conversion online/en/[推荐]PHYSICS RESOURCES DATABASE.au/teach_res/db/d0001.htmEnergy provided by foods/keyan2/data/200412/790.html一些物质的熔点、冰点等/keyan2/data/200412/789.html。
关于羧酸酯还原为醇的原理

TBSO OH NMe2
Organic Syntheses,Coll.Vol.10,p.442;Vol.78,p.160
Work up: The reaction mixture is stirred for 15 min, diluted with ether(100 mL), and quenched by dropwise addition of water (9 mL). The resulting gray suspension is allowed to reach RT, and the mixture is stirred vigorously for an additional 60 min.The mixture is transferred to a 1.0-L Erlenmeyer flask and diluted with ether(350 mL).
NaBH4还原
NaBH4 H3CO2C N CO2CH3 HO N OH
Liebigs Ann.lRecueil 1997,707-720. 此法操作较为简单,安全.由于NaBH4的还原性不够强,因此此类反应一 般需要回流过夜.而且,反应初始阶段不要去加热,而是在室温下搅拌数小 时后再缓缓加热至回流,否则极易冲料.
关于羧酸酯还原为醇 的原理及操作
• 1.金属钠和醇为还原剂(BouveaultBlanc反应) • 2.金属氢化物为还原剂
金属钠和醇为还原剂
• 本反应是将羧酸酯用 金属钠和醇直接还原 生成相应的伯醇,主 要用于高级脂肪羧酸 酯的还原.
O n-C11H23 OEt Na,EtOH toluene n-C11H23 OH
NaBH4-ZnCl2还原
EtOOC OTIPS COOEt Zn(BH4)2 THF EtOOC OTIPS OH
Organic Syntheses有机合成

Organic Syntheses(有机合成手册), John Wiley & Sons (免费)/Named Organic Reactions Collection from the University ofOxford (有机合成中的命名反应库) (免费)http://www.chem.ox.ac. ... rganicReac...有机化学资源导航Organic Chemistry Resources Worldwide/有机合成文献综述数据库Synthesis Reviews (免费)/srev/srev.htmCAMEO (预测有机化学反应产物的软件)http://zarbi.chem.yale ... o/index.shtmlCarbohydrate Letters (免费,摘要)/Carbohydrate_Letters/Carbohydrate Research (免费,摘要)/locate/carresCurrent Organic Chemistry (免费,摘要)/coc/index.htmlElectronic Encyclopedia of Reagents for Organic Synthesis (有机合成试剂百科全书e-EROS)/eros/European Journal of Organic Chemistry (免费,摘要)http://www.interscienc ... es/1434-193X/Methods in Organic Synthesis (MOS,有机合成方法)/is/database/mosabou.htmOrganic Letters (免费,目录)/jo ... f7/index.htmlOrganometallics (免费,目录)/jo ... d7/index.htmlRussian Journal of Bioorganic Chemistry (Bioorganicheskaya Khimiya) (免费,摘要) http://www.wkap.nl/journalhome.htm/1068-1620Russian Journal of Organic Chemistry (Zhurnal Organicheskoi Khimii) (免费,摘要) http://www.maik.rssi.ru/journals/orgchem.htmScience of Synthesis: Houben-Weyl Methods of Molecular Transformation/Solid-Phase Synthesis database (固相有机合成)/chem_db/sps.htmlSynthetic Communications (免费,摘要)/ ... productid/SCCSyntheticPages (合成化学数据库) (免费)/The Complex Carbohydrate Research Center (复杂碳水化合物研究中心)/合成材料老化与应用(免费,目录)http://hccllhyyy.perio ... /default.html金属卡宾络合物催化的烯烃复分解反应(免费) ... 02/2.6%20.htm上海化学试剂研究所/英国化学数据服务中心CDS (Chemical Database Service)/cds/cds.html英国皇家化学会碳水化合物研究组织(Carbohydrate Group of the Royal Society of Chemistry)/lap/rsccom/dab/perk002.htm有机反应催化学会(ORCS, Organic Reaction Catalysis Society)/有机合成练习(免费)/中国科学院成都有机化学研究所:催化与环境工程研究发展中心/MainIndex.htm金属有机及元素有机化学:CASREACT - Chemical Reactions Database(CAS的化学反应数据库)/CASFILES/casreact.html日本丰桥大学Jinno实验室的研究数据库(液相色谱、多环芳烃/药物/杀虫剂的紫外谱、物性) (免费)http://chrom.tutms.tut ... H/research...A New Framework for Porous Chemistry (金属有机骨架) (免费) ... 83432324.htmlActa Crystallographica Section B (免费,摘要)http://journals.iucr.o ... homepage.htmlActa Crystallographica Section E (免费,摘要)http://journals.iucr.o ... homepage.htmlBibliographic Notebooks for Organometallic Chemistryhttp://www.ensc-lille. ... bco/bnoc.htmlBiological Trace Element Research (生物痕量元素研究杂志) (免费,摘要)http://www.humanapress ... =0163-4984...Journal of Organometallic Chemistry (免费,摘要)/locate/jnlabr/jomOrganic Letters (免费,目录)/jo ... f7/index.htmlOrganometallics (免费,目录)/jo ... d7/index.htmlSyntheticPages (合成化学数据库) (免费)/金属卡宾络合物催化的烯烃复分解反应(免费) ... 02/2.6%20.htm金属有机参考读物:The Organometallic HyperTextBook by Rob Toreki/organomet/index.html金属有机化学国家重点实验室,中国科学院上海有机所/元素有机化学国家重点实验室(南开大学)/在线网络课程:有机金属反应和均相催化机理(Dermot O'Hare 主讲)http://www.chem.ox.ac. ... ot/organomet/药物化学:Fisher Scientific/PubMed: MEDLINE和PREMEDLINE (免费)/PubMed/生物医药:BioMedNet: The World Wide Club for the Biological and Medical Community /AIDSDRUGS (艾滋病药物) (免费) ... aidsinfs.htmlautodock (分子对接软件) (免费) ... doc/autodock/DIRLINE (卫生与生物医药信息源库) (免费)/HISTLINE (医药史库) (免费)/TOXNET (化合物毒性相关数据库系列) (免费)/日本药典,第14版(免费)http://jpdb.nihs.go.jp/jp14e/index.html小分子生物活性数据库ChemBank (免费)/Ashley Abstracts Database (药物研发、市场文献摘要) (免费) ... ey/search.aspBIOSIS/BIOSIS/ONLINE/DBSS/biosisss.html从检索药物交易信息库PharmaDeals (部分免费)/从ChemWeb检索有机药物用途及别名库Negwer: organic-chemical drugs and their synonyms (部分免费)http://cwgen.chemweb.c ... ersearch.html美国常用药品索引库RxList (免费)/美国国家医学图书馆NLM的免费在线数据库(免费)/ho ... internet.html制药公司目录(Pharmaceutical Companies on Virtual Library: Pharmacy Page)/company.html37℃医学网/AAPS PharmSci (免费,全文)/Abcam Ltd.有关抗体、试剂的销售,抗体的搜索)/Acta Pharmaceutica (免费,摘要)http://public.srce.hr/acphee/Advanced Drug Delivery Reviews (免费,摘要)http://www.elsevier.nl/locate/drugdelivAmerican Journal of Drug and Alcohol Abuse (免费,摘要)/ ... productid/ADAAmerican Journal of Pharmaceutical Education (AJPE) (免费,全文)/Amgen Inc. (医药)/Anita's web picks (药学与药物化学信息导航)http://wwwcmc.pharm.uu.nl/oyen/webpicks.htmlAnnals of Clinical Microbiology and Antimicrobials (免费,全文)/Annual Review of Pharmacology and Toxicology (免费,摘要)/Anti-Cancer Drug Design (免费,摘要)/antcan/生物有机化学:ScienceDirect: 在线访问Elsevier的1100种期刊全文(免费目录) (免费)/生命、环境科学综合性资源TheScientificWorld (sciBASE)/生物医药:BioMedNet: The World Wide Club for the Biological and Medical Community /BIOETHICSLINE (BIOETHICS onLINE) (免费)/BIOME (生命科学资源导航)/browse/Directory of P450-containing Systems(P450酶系目录)http://p450.abc.hu/DIRLINE (卫生与生物医药信息源库) (免费)/百名最佳生物技术网站列表(Top 100 Biotechnology WWW Sites)/top100.asp从ChemWeb检索《化学工程与生物技术文摘》库CEABA (部分免费)/课程材料:MIT生物学超文本教材 ... 7001main.html生物材料网(Biomaterials Network)/生物信息学资源导航,上海生物化学所/bio/index.htm小分子生物活性数据库ChemBank (免费)/英国剑桥医学研究委员会:分子生物学实验室LMB/biology site of the network./BIOSIS/BIOSIS/ONLINE/DBSS/biosisss.htmlCATH Protein Structure Classification (蛋白质结构分类) (免费)/bsm/cath/Databases and Tools for 3-D Protein Structure Comparison and Alignment (三维蛋白质结构对比) (免费)/ce.htmlLos Alamos National Laboratory Bioscience Division/Protein Data Bank (PDB, 蛋白质数据库) (免费)/pdb/计算分子生物学:Computational Molecular Biology at NIH/molbio/酶命名数据库(ENZYME-Enzyme nomenclature database) (免费) /enzyme/Access Excellence (有关生物、生命等的科学教育网站)/Acta Biochimica Polonica (免费,全文)http://www.actabp.pl/Acta Biotechnologica (生物技术学报) (免费,摘要)http://www.wiley-vch.d ... eticIndex/...American Institute of Biological Sciences (AIBS)/American Journal of Medical Genetics Part A (免费,摘要) http://www3.interscien ... jtoc?ID=33129Amos' WWW links page (生物大分子网络资源导航)/alinks.htmlAmersham International (英国,生物技术供应商)/。
库尔提斯重排反应-推荐下载
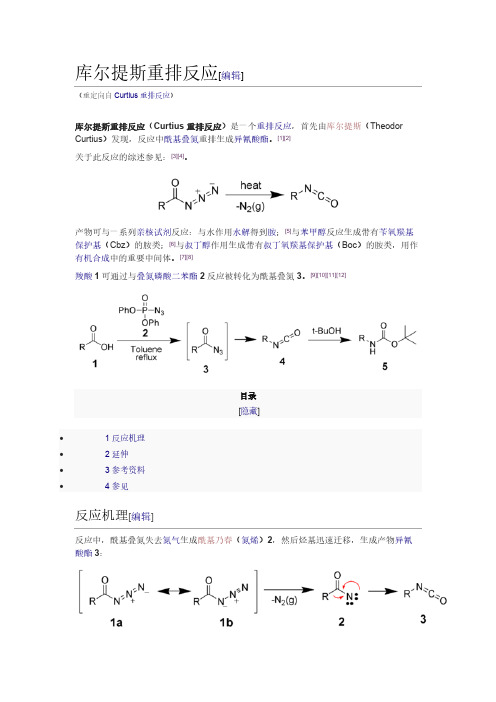
在一个研究中[5],研究者使用电脑模拟丙酮肟在贝克曼溶剂中的重排反应,并考虑到了溶 剂分子和取代物的影响。模拟表明,有三个乙酸分子和一个质子(以氧鎓的形式存在)参 与了反应。形成亚胺中间体后(σ 配合物),甲基通过协同反应迁徙到氮上,并推走羟基。 羟基中氧原子受到三个乙酸分子的稳定。接下来,一分子水进攻亲电的碳原子,其中一个 氢原子被一个乙酸接收,生成的中间体为 N-甲基乙酰氨酸,其中氧原子为四配位。最后异 构化形成稳定的产物酰胺。
p.48 (1971).Article 6. ^ Ende, D. J. a.; DeVries, K. M.; Clifford, P. J.; Brenek, S. J. Org. Proc. Res.
Dev. 1998, 2, 382-392. 7. ^ Lebel, H.; Leogane, O.; Org. Lett. 2005, 7(19), 4107-
延伸[编辑]
在 Curtius 重排反应的基础上,Darapasky 递降反应(A. Darapsky, 1936)以 α-氰基酯 为原料,通过重排反应生成氨基酸。[13]
参考资料[编辑]
1. ^ Curtius, T. Ber. 1890, 23, 3023. 2. ^ Curtius, T. J. Prakt. Chem. 1894, 50, 275. 3. ^ Smith, P. A. S. Org. React. 1946, 3, 337-449. (Review) 4. ^ Scriven, E. F.; Turnbull, K.; Chem. Rev. 1988, 88, 297-368. Review 5. ^ Kaiser, C.; Weinstock, J. Organic Syntheses, Coll. Vol. 6, p.910 (1988); Vol. 51,
有机化学常用期刊网址

1. ScienceDirect (SD)网址:/(1) Catalysis Communications (催化通讯)(2) Journal of Molecular Catalysis A: Chemical (分子催化A:化学)(3) Tetrahedron (T) (四面体)(4) Tetrahedron: Asymmetry (TA) (四面体:不对称)(5) Tetrahedron Letters (TL) (四面体快报)(6) Applied Catalysis A: General (应用催化A)2. EBSCOhost数据库网址:/(1) Synthetic Communcations (合成通讯)(2) Letters in Organic Chemistry (LOC)(3) Current Organic Synthesis(4) Current Organic Chemistry3. Springer数据库网址:http:// /(1) Molecules (分子)(2) Monatshefte für Ch emie / Chemical Monthly (化学月报)(3) Science in China Series B: Chemistry (中国科学B)(4) Catalysis Letts (催化快报)4. ACS Publications (美国化学会)网址:/(1) Journal of the American Chemical Society (JACS) (美国化学会志)(2) Organic Letters (OL) (有机快报)(3) The Journal of Organic Chemistry (JOC) (美国有机化学)(4) Journal of Medicinal Chemistry (JMC) (美国药物化学)(5) Chemical Reiew (化学评论)5. Royal Society of Chemistry (RSC) (英国皇家化学会)网址:/Publishing/Journals/Index.asp(1) Green Chemistry (绿色化学)(2) Chemical Communications (CC) (化学通讯)(3) Chemical Society Reviews (化学会评论)(4) Journal of the Chemical Society (化学会志)Journal of the Chemical Society, Perkin Transactions 1 (1972-2002) Journal of the Chemical Society, Perkin Transactions 2 (1972-2002) Journal of the Chemical Society B: Physical Organic (1966-1971) Journal of the Chemical Society C: Organic (1966-1971)(5) Organic & Biomolecular Chemistry (OBC) (有机生物化学)/publishing/jo ... p?type=CurrentIssue6. Wiley网址:/(1) Advanced Synthesis & Catalysis (ASC) (先进合成催化)(2) Angewandte Chemie International Edition (德国应用化学)(3) Chemistry - A European Journal (欧洲化学)(4) Chinese Journal of Chemistry (中国化学)(5) European Journal of Organic Chemistry (欧洲有机化学)(6) Helvetica Chimica Acta (瑞士化学)(7) Heteroatom Chemistry (杂原子化学)7. Ingent网址:/(1) Journal of Chemical Research (JCR) (化学研究杂志)(2) Canadian Journal of Chemistry (加拿大化学)(3) Current Organic Chemistry(4) Mini-Reviews in Organic Chemistry(5) Phosphorus, Sulfur, and Silicon and the Related Elements (磷、硫、硅和相关元素)(6) Letters in Organic Chemistry8. Taylor & Francis数据库网址:http://www.journalsonline.tandf. ... sp?referrer=default(1) Synthetic Communications(2) Journal of Sulfur Chemistry(硫化学杂志)(3) Phosphorus, Sulfur, and Silicon and the Related Elements 9. Thieme数据库网址:/(1) Synlett (合成快报)(2) Synthesis (合成)10. 日本化学会网址:(1) Chem. Lett. (CL) (化学快报)http://www.jstage.jst.go.jp/browse/cl/_vols(2) Bull. Chem. Soc. Jpn. http://www.csj.jp/journals/bcsj/index.html11. 澳大利亚化学会(Australian Journal of Chemistry)http://www.publish.csiro.au/nid/52.htm12.巴西化学会.br/13.Molecules/molecules/14.韩国化学会http://journal.kcsnet.or.kr/15.印度化学会http://www.niscair.res.in/Scienc ... hin.htm&d=test816.国际有机制备和程序(Organic Preparations and Procedures International,OPPI)/17.有机化学/index.htm有机合成:Organic Syntheses(有机合成手册), John Wiley & Sons (免费)/Named Organic Reactions Collection from the University ofOxford (有机合成中的命名反应库) (免费)/thirdyearcomputing/NamedOrganicReac...有机化学资源导航Organic Chemistry Resources Worldwide/有机合成文献综述数据库Synthesis Reviews (免费)/srev/srev.htmCAMEO (预测有机化学反应产物的软件)/products/cameo/index.shtmlCarbohydrate Letters (免费,摘要)/Carbohydrate_Letters/Carbohydrate Research (免费,摘要)/locate/carresCurrent Organic Chemistry (免费,摘要)/coc/index.htmlElectronic Encyclopedia of Reagents for Organic Synthesis (有机合成试剂百科全书e-EROS)/eros/European Journal of Organic Chemistry (免费,摘要)/jpages/1434-193X/Methods in Organic Synthesis (MOS,有机合成方法)/is/database/mosabou.htmOrganic Letters (免费,目录)/journals/orlef7/index.htmlOrganometallics (免费,目录)/journals/orgnd7/index.htmlRussian Journal of Bioorganic Chemistry (Bioorganicheskaya Khimiya) (免费,摘要)http://www.wkap.nl/journalhome.htm/1068-1620Russian Journal of Organic Chemistry (Zhurnal Organicheskoi Khimii) (免费,摘要)http://www.maik.rssi.ru/journals/orgchem.htmScience of Synthesis: Houben-Weyl Methods of Molecular Transformation /Solid-Phase Synthesis database (固相有机合成)/chem_db/sps.htmlSynthetic Communications (免费,摘要)/servlet/product/productid/SCCSyntheticPages (合成化学数据库) (免费)/The Complex Carbohydrate Research Center (复杂碳水化合物研究中心) /合成材料老化与应用 (免费,目录)/default.html金属卡宾络合物催化的烯烃复分解反应 (免费)/html/books/O61BG/b1/2002/2.6%20.htm上海化学试剂研究所/英国化学数据服务中心CDS (Chemical Database Service)/cds/cds.html英国皇家化学会碳水化合物研究组织 (Carbohydrate Group of the Royal Society of Chemistry)/lap/rsccom/dab/perk002.htm有机反应催化学会 (ORCS, Organic Reaction Catalysis Society)/有机合成练习 (免费)/中国科学院成都有机化学研究所:催化与环境工程研究发展中心/MainIndex.htm金属有机及元素有机化学:CASREACT - Chemical Reactions Database(CAS的化学反应数据库)/CASFILES/casreact.html日本丰桥大学 Jinno实验室的研究数据库(液相色谱、多环芳烃/药物/杀虫剂的紫外谱、物性) (免费)http://chrom.tutms.tut.ac.jp/JINNO/ENGLISH/RESEARCH/research...A New Framework for Porous Chemistry (金属有机骨架) (免费)/alchem/articles/1056983432324.htmlActa Crystallographica Section B (免费,摘要)/b/journalhomepage.htmlActa Crystallographica Section E (免费,摘要)/e/journalhomepage.htmlBibliographic Notebooks for Organometallic Chemistryhttp://www.ensc-lille.fr/recherche/cbco/bnoc.htmlBiological Trace Element Research (生物痕量元素研究杂志) (免费,摘要) /JournalDetail.pasp?issn=0163-4984... Journal of Organometallic Chemistry (免费,摘要)/locate/jnlabr/jomOrganic Letters (免费,目录)/journals/orlef7/index.htmlOrganometallics (免费,目录)/journals/orgnd7/index.htmlSyntheticPages (合成化学数据库) (免费)/金属卡宾络合物催化的烯烃复分解反应 (免费)/html/books/O61BG/b1/2002/2.6%20.htm金属有机参考读物:The Organometallic HyperTextBook by Rob Toreki /organomet/index.html金属有机化学国家重点实验室,中国科学院上海有机所/元素有机化学国家重点实验室(南开大学)/在线网络课程:有机金属反应和均相催化机理 (Dermot O'Hare 主讲)/icl/dermot/organomet/药物化学:Fisher Scientific/PubMed: MEDLINE和PREMEDLINE (免费)/PubMed/生物医药:BioMedNet: The World Wide Club for the Biological and Medical Community/AIDSDRUGS (艾滋病药物) (免费)/pubs/factsheets/aidsinfs.htmlautodock (分子对接软件) (免费)/pub/olson-web/doc/autodock/DIRLINE (卫生与生物医药信息源库) (免费)/HISTLINE (医药史库) (免费)/TOXNET (化合物毒性相关数据库系列) (免费)/日本药典,第14版 (免费)http://jpdb.nihs.go.jp/jp14e/index.html小分子生物活性数据库ChemBank (免费)/Ashley Abstracts Database (药物研发、市场文献摘要) (免费)/databases/ashley/search.aspBIOSIS/BIOSIS/ONLINE/DBSS/biosisss.html从检索药物交易信息库PharmaDeals (部分免费)/从ChemWeb检索有机药物用途及别名库Negwer: organic-chemical drugs and their synonyms (部分免费)/negwer/negwersearch.html美国常用药品索引库RxList (免费)/美国国家医学图书馆NLM的免费在线数据库 (免费)/hotartcl/chemtech/99/tour/internet.html制药公司目录(Pharmaceutical Companies on Virtual Library: Pharmacy Page) /company.html37℃医学网/AAPS PharmSci (免费,全文)/Abcam Ltd.有关抗体、试剂的销售,抗体的搜索)/Acta Pharmaceutica (免费,摘要)http://public.srce.hr/acphee/Advanced Drug Delivery Reviews (免费,摘要)http://www.elsevier.nl/locate/drugdelivAmerican Journal of Drug and Alcohol Abuse (免费,摘要)/servlet/product/productid/ADAAmerican Journal of Pharmaceutical Education (AJPE) (免费,全文)/Amgen Inc. (医药)/Anita's web picks (药学与药物化学信息导航)http://wwwcmc.pharm.uu.nl/oyen/webpicks.htmlAnnals of Clinical Microbiology and Antimicrobials (免费,全文)/Annual Review of Pharmacology and Toxicology (免费,摘要)/Anti-Cancer Drug Design (免费,摘要)/antcan/生物有机化学:ScienceDirect: 在线访问Elsevier的1100种期刊全文 (免费目录) (免费) /生命、环境科学综合性资源TheScientificWorld (sciBASE)/生物医药:BioMedNet: The World Wide Club for the Biological and MedicalCommunity/BIOETHICSLINE (BIOETHICS onLINE) (免费)/BIOME (生命科学资源导航)/browse/Directory of P450-containing Systems(P450酶系目录)http://p450.abc.hu/DIRLINE (卫生与生物医药信息源库) (免费)/百名最佳生物技术网站列表 (Top 100 Biotechnology WWW Sites)/top100.asp从ChemWeb检索《化学工程与生物技术文摘》库CEABA (部分免费)/课程材料:MIT生物学超文本教材:8001/esgbio/7001main.html生物材料网 (Biomaterials Network)/生物信息学资源导航,上海生物化学所/bio/index.htm小分子生物活性数据库ChemBank (免费)/英国剑桥医学研究委员会:分子生物学实验室LMB/biology site of the network./生物有机化学:ScienceDirect: 在线访问Elsevier的1100种期刊全文 (免费目录) (免费) /生命、环境科学综合性资源TheScientificWorld (sciBASE)/生物医药:BioMedNet: The World Wide Club for the Biological and Medical Community/BIOETHICSLINE (BIOETHICS onLINE) (免费)/BIOME (生命科学资源导航)/browse/Directory of P450-containing Systems(P450酶系目录)http://p450.abc.hu/DIRLINE (卫生与生物医药信息源库) (免费)/百名最佳生物技术网站列表 (Top 100 Biotechnology WWW Sites) /top100.asp从ChemWeb检索《化学工程与生物技术文摘》库CEABA (部分免费) /课程材料:MIT生物学超文本教材:8001/esgbio/7001main.html生物材料网(Biomaterials Network)/生物信息学资源导航,上海生物化学所/bio/index.htm小分子生物活性数据库ChemBank (免费)/英国剑桥医学研究委员会:分子生物学实验室LMB/biology site of the network./。
Organic Syntheses, Coll. Vol. 6, p.1019 (1988); Vol. 51, p.142 (1971).

Organic Syntheses, Coll. Vol. 6, p.1019 (1988); Vol. 51, p.142 (1971).TRIMETHYLOXONIUM TETRAFLUOROBORATE[Oxonium, trimethyl- tetrafluoroborate(1-)]Submitted by T. J. Curphey1Checked by A. Eschenmoser, R. Keese, and A. Daniel.1. ProcedureA 500-ml., three-necked flask fitted with a mechanical stirrer, a Dewar condenser (Note 1) connected by a T-tube to a mineral oil bubbler and a source of dry nitrogen, and a gas-inlet tube connected to a source of dry dimethyl ether(Note 2) is charged with 80 ml. of dichloromethane and 38.4 g. (33.3 ml., 0.271 mole) of boron trifluoride diethyl etherate(Note 3). After establishing a nitrogen atmosphere in the flask, the condenser is filled with an acetone–dry ice mixture. With gentle stirring, dimethyl ether is passed into the solution until approximately 75 ml. has collected (Note 4). The gas-inlet tube is replaced with a pressure-equalizing dropping funnel containing 28.4 g. (24.1 ml., 0.307 mole) of epichlorohydrin, which is added dropwise with vigorous stirring over a 15-minute period. The mixture is stirred overnight under an atmosphere of nitrogen(Note 5). The stirrer is replaced by a filter stick, and the supernatant liquid is drawn off from the crystalline trimethyloxonium tetrafluoroborate, while keeping the mixture under nitrogen. The oxonium salt is washed with two 100-ml. portions of anhydrous dichloromethane and two 100-ml. portions of sodium-dried diethyl ether(Note 6), and dried by passing a stream of nitrogen over the salt until the odor of ether is no longer detected, yielding 28–29g. (92.5–96.5%) of a white crystalline solid, m.p. (sealed tube) 179.6–180.0° (dec.), (Note 7) and (Note8).2. Notes1. A Kontes K-45750 condenser was used.2. Dimethyl ether and nitrogen were dried by passage through columns of Drierite. Boron trifluoride etherate (Eastman Practical Grade) was redistilled. Epichlorohydrin (Eastman Organic Chemicals) and dichloromethane (Fisher Scientific Company) were used as received.3. According to 1H NMR analysis the use of boron trifluoride etherate does not cause any detectable introduction of ethyl groups into the product.4. This may conveniently be done by placing, prior to conducting the reaction, a mark on the reaction flask at a level of 190 ml., and collecting dimethyl ether up to the mark. The exact amount of dimethyl ether used is not critical.5. After 2–3 hours of stirring the reaction appears to be over, and the dry ice in the condenser need no longer be renewed. The reaction mixture may be worked up at this point without appreciable reduction in the product yield or purity.6. According to analysis by 1H NMR the use of diethyl ether at this point does not cause any detectable exchange of methyl by ethyl groups in the oxonium salt. A user2 has reported obtaining the best samples of oxonium salt by using boron fluoride dimethyl etherate instead of the diethyl etherate and omitting the diethyl ether washing of the product. Oxonium salt prepared in this way was used to prepare methyl esters (from the corresponding amides) with no detectable (by GC analysis) ethyl esters.7. The melting point of trimethyloxonium tetrafluoroborate apparently depends upon the procedure by which it is prepared and the method of melting-point determination. It has, for example, been reported to melt at 124.5°,3 141–143° [Org. Synth., Coll. Vol. 5, 1096 (1973)], and 175°.4] The 1H NMRspectrum, determined (liquid SO 2, purissimum Fluka AG) in a sealed tube at room temperature shows a single methyl resonance at δ 4.54; a trace of impurity is discernible as a singlet at δ 3.39. 8. When prepared as described, the oxonium salt is stable and nonhygroscopic, and may readily be handled in the air for short periods of time. A sample kept in a desiccator over Drierite for 1 month at −20° showed no change in melting point, and batches stored in this manner for over a year have been successfully used for alkylations.3. DiscussionTrialkyloxonium salts were first discovered by Meerwein,3 who also investigated much of their chemistry. A discussion of the literature prior to 1963 has been published.5 Simple trialkyloxonium cations which have been prepared, other than trimethyl, include triethyl,6 tri-n -propyl,7 and tri-n -butyl,8 with tetrafluoroborate or hexachloroantimonate anions, in most cases. Methods used to prepare trimethyloxonium tetrafluoroborate , which are typical of the class as a whole, include the reaction of boron trifluoride with epichlorohydrin in the presence of dimethyl ether ,3,4,9 the reaction of dimethyloxonium tetrafluoroborate with diazomethane or diazoacetic ester,10 and the alkylation of dimethyl ether by triethyloxonium tetrafluoroborate 11 or dimethoxycarbonium tetrafluoroborate.12 Several of these reactions involve the initial formation of a mixed oxonium ion [R 1R 2OCH 3]+, which then methylates dimethyl ether , providing R 1R 2O and the trimethyloxonium ion. Of the available procedures, the one described here is probably the most convenient, involving as it does a single-step preparation from inexpensive, commercially available, and nonhazardous reagents. Under the proper conditions (Note 8), the resulting product has storage properties comparable to those of the less-accessible trimethyloxonium 2,4,6-trinitrobenzenesulfonate .13The trialkyloxonium salts are powerful alkylating agents. Trimethyl- and triethyloxonium tetrafluoroborates, in particular, have been widely employed for methylation and ethylation of sensitive or weakly nucleophilic functional groups. Alkylations of over 50 such functional groups have been reported in the literature. Examples include amides,4,7,14,15,16 lactams,16,17,18,19 sulfides,3,20 nitro compounds,9 enols and enolates,3,21 ethers,7,11,22 phenols,3 sulfoxides,3,7 amine oxides,3,7,23 carboxylic acids,3 lactones,3,4 ketones,3,16 metal carbonyls,12,24 thiophenes,25 and phosphonitriles.26. Oxonium salts have also been advantageously employed as quarternizing agents for a variety of heterocyclic amines.27,28,29,30,31,32,33,34 In this way the first diquarternary salts of several heterocyclic diazines have been prepared,30,31 as have reagents for peptide synthesis,33,34 for the synthesis of polycyclic ketones,32 and for cyanine dyes.28One of the major advantages of oxonium salts is that alkylations can be effected under reaction conditions that are generally much milder than those necessary with the more conventional alkyl halides or sulfonates. Triethyloxonium tetrafluoroborate , for example, has usually been employed at room temperature in dichloromethane or dichloroethane solution. Occasionally chloroform 17,23 or no solvent at all 4,21 is used. Difficult alkylations can be effected in refluxing dichloroethane .30,31 The less soluble trimethyloxonium tetrafluoroborate has been used as a suspension in dichloromethane or dichloroethane , or as a solution in nitromethane or liquid sulfur dioxide . Reports of alkylations in water 24 and trifluoroacetic acid 22 have also appeared. Direct fusion with trimethyloxonium tetrafluoroborate has succeeded in cases where other conditions have failed.26,31Alkylations by oxonium salts have added several new weapons to the synthetic chemist's armamentarium. For example, the O -alkylated products from amides [R 1C(OR)=NR 2R 3]+ (R=CH 3 or C 2H 5) may be hydrolyzed under mild conditions to amines and esters,15,35 reduced to the amines R 1CH 2NR 2R 3 by sodium borohydride ,14 converted to amide acetals R 1C(OR)2NR 2R 3 by alkoxides,4,16 and (for R 3=H) deprotonated to the imino esters R 1C(OR)=NR 2.17,18,19 Amide acetals and imino esters are themselves in turn useful synthetic intermediates. Indeed, oxonium salts transform the rather intractable amide group into a highly reactive and versatile functionality, a fact elegantly exploited in recent work on the synthesis of corrins.35Other reagents which approach or exceed the oxonium salts in alkylating ability include dialkoxycarbonium ions,36 alkyl trifluoromethanesulfonates,37 alkyl fluorosulfonates,38 dialkylhalonium ions,39 and alkyl halides in the presence of silver salts.25,37,40 In terms of availability, stability, and freedom from hazards,25 oxonium salts often appear to be the reagents of choice. When eithermethylation or ethylation is acceptable, methylation may be preferable. Triethyloxonium tetrafluoroborate must be stored under ether and handled in a dry box,6 whereas the trimethyl salt can be stored solvent-free in the freezing compartment of a refrigerator and dispensed in the open atmosphere. Moreover, while information on the relative alkylating ability of the oxonium salts is not extensive, a few cases have been reported in which trimethyloxonium tetrafluoroborate effected alkylations which the triethyl analog did not.20,31 The trimethyloxonium salt, therefore, appears to be the more potent alkylating agent.This preparation is referenced from:z Org. Syn. Coll. Vol. 5, 1080z Org. Syn. Coll. Vol. 5, 1096z Org. Syn. Coll. Vol. 5, 1099z Org. Syn. Coll. Vol. 6, 576References and Notes1.Department of Chemistry, St. Louis University, St. Louis, Missouri 63156 [Present address:Department of Pathology, Dartmouth Medical School, Hanover, New Hampshire 03755].2.Robert F. Myers (with William S. Johnson), Department of Chemistry, Stanford University,Stanford, California 94305, private communication to the editor-in-chief, May 5, 1970.3.H. Meerwein, G. Hinz, P. Hofmann, E. Kroning, and E. Pfeil, J. Prakt. Chem., [2], 147, 257(1937).4.H. Meerwein, P. Borner, O. Fuchs, H. J. Sasse, H. Schrodt, and J. Spille, Chem. Ber., 89, 2060(1956).5.H. Meerwein, in "Methoden der Organischen Chemie" (Houben-Weyl), Vol. 6/3, Georg ThiemeVerlag, Stuttgart, 1965, p. 325.6.H. Meerwein, Org. Synth., Coll. Vol. 5, 1080 (1973).7.H. Meerwein, E. Battenberg, H. Gold, E. Pfeil, and G. Willfang, J. Prakt. Chem., [2], 154, 83(1939).8.G. Hilgetag and H. Teichmann, Chem. Ber., 96, 1446 (1963).9.N. Kornblum and R. A. Brown, J. Am. Chem. Soc., 86, 2681 (1964).10. F. Klages, H. Meuresch, and W. Steppich, Justus Liebigs Ann. Chem., 592, 81 (1955).11.H. Meerwein, Org. Synth., Coll. Vol. 5, 1096 (1973).12.R. B. Silverman and R. A. Olofson, Chem. Commun., 1313 (1968).13.G. K. Helmkamp and D. J. Pettitt, Org. Synth., Coll. Vol. 5, 1099 (1973).14.R. F. Borch, Tetrahedron Lett., 61 (1968).15.H. Muxfeldt, J. Behling, G. Grethe, and W. Rogalski, J. Am. Chem. Soc., 89, 4991 (1967).16.H. Meerwein, W. Florian, N. Schön, and G. Stopp, Justus Liebigs Ann. Chem., 641, 1 (1961).17.S. Petersen and E. Tietze, Justus Liebigs Ann. Chem., 623, 166 (1959).18. E. Vogel, R. Erb, G. Lenz, and A. A. Bothner-By, Justus Liebigs Ann. Chem., 682, 1 (1965).19.L. A. Paquette, T. Kakihana, J. F. Hansen, and J. C. Philips, J. Am. Chem. Soc., 93, 152 (1971).20.J. E. Baldwin, R. E. Hackler, and D. P. Kelly, J. Am. Chem. Soc., 90, 4768 (1968).21.G. Hesse, H. Broll, and W. Rupp, Justus Liebigs Ann. Chem., 697, 62 (1966).22.P. E. Peterson and F. J. Slama, J. Am. Chem. Soc., 90, 6516 (1968).23. C. Reichardt, Chem. Ber., 99, 1769 (1966).24.R. Aumann and E. O. Fischer, Chem. Ber., 101, 954 (1968).25.R. M. Acheson and D. R. Harrison, J. Chem. Soc. C, 1764 (1970).26.J. N. Rapko and G. Feistel, Inorg. Chem., 9, 1401 (1970).27.H. Balli and F. Kersting, Justus Liebigs Ann. Chem., 647, 1 (1961).28. C. Reichardt, Justus Liebigs Ann. Chem., 715, 74 (1968).29.H. Quast and S. Hünig, Chem. Ber., 99, 2017 (1966); Chem. Ber.101, 435 (1968); H. Quast andE. Schmitt, Chem. Ber., 101, 1137 (1968).30.T. J. Curphey, J. Am. Chem. Soc., 87, 2063 (1965).31.T. J. Curphey and K. S. Prasad, J. Org. Chem., 37, 2259 (1972).32.G. Stork, S. Danishefsky, and M. Ohashi, J. Am. Chem. Soc., 89, 5459 (1967).33.R. B. Woodward, R. A. Olofson, and H. Mayer, Tetrahedron, Suppl. 8, 321 (1966).34.R. A. Olofson and Y. L. Marino, Tetrahedron, 26, 1779 (1970).35. A. Eschenmoser, Q. Rev. Chem. Soc., 24, 366 (1970).36.S. Kabuss, Angew. Chem., 78, 714 (1966) [Angew. Chem. Int. Ed. Engl., 5, 675 (1966)] andreferences therein.37. A. J. Boulton, A. C. G. Gray, and A. R. Katritzky, J. Chem. Soc. B, 911 (1967).38.M. G. Ahmed, R. W. Alder, G. H. James, M. L. Sinnott, and M. C. Whiting, Chem. Commun.,1533 (1968).39.G. A. Olah and J. R. DeMember, J. Am. Chem. Soc., 92, 2562 (1970).40.H. Meerwein, V. Hederich, and K. Wunderlich, Arch. Pharm. Weinheim, Ger., 291, 541 (1958).AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)Drieriteboron fluoride dimethyl etherateliquid SO2dimethoxycarbonium tetrafluoroborateether,diethyl ether (60-29-7)chloroform (67-66-3)Epichlorohydrin (106-89-8)sulfur dioxide (7446-09-5)nitrogen (7727-37-9)dimethyl ether (115-10-6)Nitromethane (75-52-5)dichloromethane (75-09-2)Diazomethane (334-88-3)boron trifluoride (7637-07-2)boron trifluoride etherate,boron trifluoride diethyl etherate (109-63-7)dichloroethane(75-34-3)trifluoroacetic acid (76-05-1)sodium borohydride (16940-66-2)triethyloxonium tetrafluoroborate (368-39-8)Trimethyloxonium tetrafluoroborate,Oxonium, trimethyl- tetrafluoroborate(1-) (420-37-1)Trimethyloxonium 2,4,6-trinitrobenzenesulfonate (13700-00-0)dimethyloxonium tetrafluoroborateCopyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
Organic Syntheses, Coll. Vol. 9, p.4 (1998); Vol. 71, p.63 (1993).

eluant (Note 7) to give 9.0 g (59% overall) of (2R,3S)- and (2S,3S)-1,4-dioxa-2,3-dimethyl-2-(1-methylethenyl)-8-carboethoxy-8-azaspiro[4.5]decane, a 6:1 mixture of diastereomers, as a pale yellow oil (Note 8).B. (2S,3S)-3-Acetyl-8-carboethoxy-2,3-dimethyl-1-oxa-8-azaspiro[4.5]decane. Dry nitromethane (100 mL) (Note 9) is added through a rubber septum by syringe to a vacuum-dried, 500-mL, round-bottomed flask that contains the ketal mixture prepared in Step A (9.00 g, 31.8 mmol) and a magnetic stir bar. The solution is cooled to −23°C, tin(IV) chloride (SnCl4) (11 mL, 94 mmol) is added by syringe and the solution is stirred for 30 min at −23°C (Note 10). At this time the brown solution is warmed to 23°C and stirring is continued for an additional 30 min. Saturated aqueous NH4Cl (200 mL) is added and the mixture is concentrated under reduced pressure using a rotary evaporator to remove nitromethane. The resulting aqueous suspension is extracted with ethyl acetate (200 mL) and the organic extract is washed with brine (200 mL), dried over sodium sulfate (Na2SO4) and concentrated under reduced pressure using a rotary evaporator. The residue is subjected to flash chromatography on silica gel (250 g, 20 cm × 10 cm) using ethyl acetate:hexane (1:1) eluant (Note 7) to give 8.1 g (90%) of (2S,3S)-3-acetyl-8-carboethoxy-2,3-dimethyl-1-oxa-8-azaspiro[4.5]decane as a pale yellow oil (Note 11) and (Note 12).2. Notes1. Anhydrous tetrahydrofuran was prepared by distillation under argon from sodium benzophenone ketyl.2. 2-Bromopropene, obtained from Aldrich Chemical Company, Inc., was distilled and then passed through a plugof activity IV basic alumina immediately before use.3. The fine white emulsion formed at this stage was collected with the organic phase and was cleared in the subsequent brine washings.4. This crude material was acceptable for use in the second step, although more p-toluenesulfonic acid will be required if large amounts of tributylamine are present. The diol mixture, free from tributylamine, can be obtained by careful chromatography on silica gel using ethyl acetate-hexane (1:1). The purified sample has the following characteristics: 1H NMR (500 MHz, CDCl3, major isomer) δ: 1.10 (d, 3 H, J = 6.5, CH3), 1.37 (s, 3 H, CH3), 1.80 (s,3 H, CH3), 2.21 (br s, 2 H, 2 × OH), 3.77 (q, 1 H, J = 5.6, CH), 4.89 (d, 1 H, J = 1.1, CH=C), 5.06 (s, 1 H, CH=C); IR (film) cm−1: 3421, 3397, 3390, 3364, 2981, 2937, 1088; MS (Cl) m/z 113.0936 (113.0966 calcd for C7H14O2, MH –H2O).5. The major isomer is assigned the 3R, 4S stereochemistry on the expectation that the addition would occur preferentially with Cram (Felkin-Ahn) selectivity.3 This assignment was confirmed by 1H NMR DNOE experimentson the isobutyraldehyde acetal.6. 1-Carbethoxy-4-piperidone was obtained from Aldrich Chemical Company, Inc., and used as received.7. A series of 200-mL fractions was collected during flash chromatography. The product was eluted in fractions3–8 as indicated by TLC analysis using 4% ethanolic phosphomolybdic acid stain.8. This sample has the following characteristics: 1H NMR (500 MHz, CDCl3, major isomer) δ: 1.17 (d, 3 H, J =5.1, CH3), 1.26 (t, 3 H, J = 7.1, OCH2CH3), 1.45 (s, 3 H, CH3), 1.77 (s, 3 H, CH3C=), 1.60–1.81 (m, 5 H, 2 × CH2and CH), 3.43–3.75 (m, 4 H, 2 × CH2N), 4.13 (q, 2 H, J = 7.1, OCH2CH3), 4.96 (s, 2 H, CH2=C); IR (film) cm−1: 2977, 1702, 1433, 1238, 1122; MS (Cl) m/z 284.1850 (284.1861 calcd for C15H25NO4, MH). Anal. Calcd forC15H25NO4: C, 63.58; H, 8.89; N, 4.94. Found: C, 63.48; H, 8.90; N, 4.89.9. Nitromethane was dried by distillation of a 10:1 mixture of nitromethane and trifluoroacetic anhydride and collection of the center fraction that distilled at 100°C.10. Tin(IV) chloride (SnCl4) was obtained from Aldrich Chemical Company, Inc., and handled under an atmosphere of argon.11. Gas chromatographic analysis using a 25-m 10% SP 2100 silicone column showed that this sample was 94% pure and contained one major unidentified impurity. Bulb-to-bulb distillation (200°C, 0.6 mm) of a 7.4-g sample of the crude product afforded 7.0 g (85%) of the product as a pale yellow oil, which was shown by GLC analysis to be of 100% purity. This sample has the following spectral characteristics: [α]D−79.1° (MeOH, c 1.0); 1H NMR (500 MHz, CDCl3) δ: 1.17 (d, 3 H, J = 6.6, CH3), 1.25 (m, 6 H, OCH2CH3 and CH3), 1.70–1.90 (m, 4 H, 2 × CH2), 2.19 (s, 3 H,CH3CO), 1.57 (d, 1 H, J = 13.5) 2.36 (d, 1 H, J = 13.5), 3.38–3.70 (m, 4 H, 2 × CH2N), 3.89 (q, 1 H, J = 6.6, CH)4.12 (q, 2 H, J = 7.1, OCH2CH3); 13C NMR (125 MHz, CDCl3) δ: 14.5, 15.6, 22.5, 28.3, 36.0, 37.0, 40.7, 41.1, 47.3, 58.4, 61.0, 79.1, 81.0, 155.5, 210.3; IR (film) cm−1: 2977, 2937, 1705, 1701, 1698, 1472, 1455, 1434, 1365, 1356, 1274, 1237; MS (Cl) m/z 284.1845 (284.1860 calcd for C15H25NO4, MH). Anal. Calcd for C15H25NO4: C, 63.58; H,8.89; N, 4.94. Found: C, 63.38; H, 8.87; N, 4.88.12. The enantiomeric excess of the product is >96%. This was determined by treating a sample of the ketone with sodium borohydride/methanol (NaBH4/MeOH) (23°C) and separating the resulting 3:2 mixture of alcohol diastereomers by flash chromatography (silica gel, 2:3 ethyl acetate-hexane). The major alcohol diastereomer wasconverted to its Mosher ester4 [2.5 eq of (+)-α-methoxytrifluoromethylphenylacetic acid, 3 eq of dicyclohexylcarbodiimide, and 0.2 eq of 4-(dimethylamino)pyridine, CH2Cl2] and the crude esterification reaction mixture was analyzed using 500 MHz 1H NMR. None of the minor diastereomer was observed while doping experiments established that 2% would have been detected [diagnostic signals: δ 1.80 (δ, J = 13.4, major ester diastereomer); δ 1.82 (δ, J = 14.1, minor ester diastereomer)].Waste Disposal InformationAll toxic materials were disposed of in accordance with "Prudent Practices in the Laboratory"; National Academy Press; Washington, DC, 1995.3. DiscussionThis procedure illustrates a fundamentally new method for constructing substituted tetrahydrofurans.5,6,7,8,9,10 This practical method assembles the tetrahydrofuran ring from allylic diol and carbonyl components and in the process forms three ring bonds: C(2)-C(3), C(4)-C(5) and O-C(5). Both aldehydes (eq 1) and ketones (illustrated in the present procedure) can be employed as the carbonyl component. Although it is often convenient to isolate the acetal intermediate, conversion to the 3-acyltetrahydrofuran can also be accomplished in many cases by the direct reaction of the diol and carbonyl components.8 High cis stereoselectivity (at least 20:1) is observed in the preparation of tetrahydrofurans that contain single side chains at carbons 2 and 5 (eq. 1). The kinetically controlled product also has the cis relationship of these side chains and the 3-acyl substituent.A definitive feature of this highly stereoselective new route to substituted tetrahydrofurans is that both syn and anti allylic diol stereoisomers typically afford identical tetrahydrofuran products. Thus, there is no need for stereoselective construction of the allylic diol reaction partner. The construction of substituted tetrahydrofurans in high enantiomeric purity from non-racemic allylic diol precursors has also been established.5,7 The rearrangement illustrated in eq. 2 is the key step in a recent synthesis of (+)-muscarine.The scope and mechanism of the SnCl4-promoted rearrangement of allylic acetals have been investigated in detail and these studies provide considerable guidance for using this new tetrahydrofuran synthesis.5,6,7,8,9 Three major limitations emerge from studies conducted to date: (1) When the tetrahydrofuran construction involves a ketone, and thus forms a quaternary center at C(5), allylic diols with alkene substituents more nucleophilic than terminal vinyl rearrange in highest yield. (2) Allylic acetals that are reluctant to ring open in the presence of acid catalysts to generate oxocarbenium ions often undergo decomposition, rather than conversion to acyltetrahydrofuran products. (3) Allylic acetals that form highly stabilized oxocarbeniums (e.g., cinnamaldehyde-derived acetals) do not undergo conversion to 3-acyltetrahydrofurans.This procedure illustrates the asymmetric synthesis of a spirobicyclic tetrahydrofuran from the reaction of readily available (S)-3-[[(1,1-dimethylethyl)diphenylsilyl]oxy]-2-butanone2 with cyclic ketones. The specific example describedargon (7440-37-1)sodium borohydride (16940-66-2)dicyclohexylcarbodiimide (538-75-0)tributylamine (102-82-9)trifluoroacetic anhydride (407-25-0)p-toluenesulfonic acid (104-15-4)ethyl acetate-hexane (2639-63-6)acetal carbon (463-57-0)Tetrabutylammonium fluoride (429-41-4)2-Bromopropene (557-93-7)4-(dimethylamino)pyridine (1122-58-3)tert-Butyllithium (594-19-4)(+)-α-methoxytrifluoromethylphenylacetic acid (56135-03-6)(2S,3S)-3-Acetyl-8-carboethoxy-2,3-dimethyl-1-oxa-8-azaspiro[4.5]decane (155534-75-1) 3-(S)-[(tert-Butyldiphenylsilyl)oxy]-2-butanone,(S)-3-[[(1,1-dimethylethyl)diphenylsilyl]oxy]-2-butanone (135367-18-9)tert-butyldiphenylsilyl1-Carbethoxy-4-piperidone (29976-53-2)isobutyraldehyde acetal3-methyl-4-pentene-2,3-diolCopyright © 1921-2007, Organic Syntheses, Inc. All Rights Reserved。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Organic Syntheses, Coll. Vol. 6, p.762 (1988); Vol. 52, p.109 (1972).PREPARATION AND REDUCTIVE CLEAVAGE OF ENOL PHOSPHATES: 5-METHYLCOPROST-3-ENE[Cholest-3-ene, 5-methyl-, (5β)-]Submitted by D. C. Muchmore1Checked by David G. Melillo and Herbert O. House.1. ProcedureA. Diethyl 5-methylcoprost-3-en-3-yl phosphate. A dry, 100-ml., three-necked flask equipped with a magnetic stirring bar, a pressure-equalizing dropping funnel, a nitrogen inlet tube, and a rubber septum is charged with 384 mg. (0.00201 mole) of copper(I) iodide(Note 1) and 20 ml. of anhydrous diethyl ether(Note 2). After the reaction vessel has been flushed with nitrogen, a static oxygen-free nitrogen atmosphere is maintained in the reaction vessel throughout the remainder of the reaction. The reaction mixture is cooled in an ice bath and an ether solution, containing 0.0040 mole of methyllithium(Note 3), is added with a hypodermic syringe, dropwise and with stirring. As the methyllithium is added, the initial yellow precipitate of polymeric methylcopper(I) redissolves, forming a colorless to pale-yellow solution of lithium dimethylcuprate(Note 4). To the resulting cold solution is added, dropwise and with stirring over 20 minutes, a solution of 576 mg. (0.00150 mole) of cholest-4-en-3-one(Note 5) in 20 ml. of ether(Note 2). During the addition of the enone, a yellow precipitate of polymeric methylcopper(I) separates from the reaction solution. After the addition is complete, the cooling bath is removed, and the reaction mixture is stirred for 2 hours at room temperature. The dropping funnel is replaced with a second dry dropping funnel which contains a loose plug of glass wool above the stopcock. The reaction mixture is again cooled in an ice bath and a mixture of 4.0 ml. of triethylamine(Note 6) and 2.00 g.(0.0115 mole) of diethyl phosphorochloridate (Note 7)is added from the dropping funnel to the reaction mixture, rapidly and with stirring. After this addition, the cooling bath is removed, and stirring is continued for one hour. Saturated aqueous sodium hydrogen carbonate is added to hydrolyze any remaining organometallic reagents before the reaction mixture is transferred to a separatory funnel and washed successively with two 50-ml. portions of cold (0°) 1 M ammonium hydroxide and a 50-ml. portion of water. The aqueous washes are extracted in turn with a 30-ml. portion of ether . The combined ether solutions are dried over anhydrous sodium sulfate and concentrated with a rotary evaporator. A solution of the residual liquid in 3 ml. of ether is applied to a 2.5 cm. by 15 cm. chromatographic column packed with a slurry of 50 g. of silica gel (Note 8) in ether . The column is eluted with ether . After the first 70 ml. of eluent has been collected and discarded, the next 120 ml. of ether eluent is collected and concentrated with a rotary evaporator, yielding 420–480 mg. of crude phosphate ester (Note 9), a colorless liquid, sufficiently pure for use in the following procedure.B. 5-Methylcoprost -3-ene. A dry, 100-ml., three-necked flask equipped with a polyethylene-coated magnetic stirring bar, two gas-inlet tubes, and a pressure-equalizing dropping funnel is immersed in a 2-propanol–dry ice cooling bath maintained at −15° to −20°. The reaction vessel is flushed with either helium or argon , and a static atmosphere of one of these gases is maintained in the reaction vessel throughout the reaction. Ethylamine (Note 10) is distilled through a tower of sodium hydroxide pellets into the cold reaction flask until 50 ml. of the liquid amine has been collected. A 70-mg. (0.010 g.-atom) piece of lithium wire is cleaned by dipping it successively into methanol and pentane and added to the reaction flask. The resulting cold (−15°) mixture is stirred for 10 minutes to dissolve the lithium before a solution of the diethyl 5-methylcoprost-3-en-3-yl phosphate and 0.50 ml. (0.39 g., 0.0053 mole) of tert -butyl alcohol (Note 11) in 15 ml. of tetrahydrofuran (Note 2) is added, dropwise and with stirring over 15 minutes, to the cold, blue lithium–amine solution. The blue solution is stirred for an additional 15 minutes before 1 ml. of saturated ammonium chloride is added to consume excess lithium . The resulting colorless mixture is warmed, evaporating ethylamine , and the residue is diluted with 90 ml. of 10% aqueous sodium hydroxide and extracted with two 30-ml. portions of pentane . The combined organic solutions are washed with 50 ml. of aqueous sodium chloride , dried over anhydrous sodium sulfate , and concentrated with a rotary evaporator. The residual, viscous liquid is subjected to evaporative distillation, from a 25-ml. flask into a male 14–20 standard-taper glass joint as shown in Figure 1. The air bath is heated to 150–180° while the pressure in the system is maintained at 0.05 mm.to 0.4 mm. Distillation of 5-methylcoprost-3-ene yields 260–295 mg. (45–51%) of colorless liquid, n 25 1.5115–1.5123 (Note 12).Figure 1. Apparatus for evaporative distillation.2. Notes1. A purified grade of copper(I) iodide , purchased from Fisher Scientific Company, was used without purification.2. Reagent grade ether and tetrahydrofuran were distilled from lithium aluminum hydride immediately prior to use.3. Ethereal solutions ofmethyllithium are available from either Foote Mineral Company or Alpha DInorganics, Inc. These solutions should be titrated immediately before use with 2-butanol and 2,2-bipyridyl as an indicator [Org. Synth., Coll. Vol. 5, 211 (1973); Org. Synth., Coll. Vol. 6, 121 (1988)].2 In a typical run, 2.44 ml. of ethereal 1.64 M methyllithium was employed. 4. The appearance of a brown to black precipitate indicates either oxidative or thermal decomposition of the cuprate. If such decomposition has occurred, it is best to prepare the reagent again with greater care, avoiding molecular oxygen and/or excessive reaction temperatures. 5. A commercial sample of cholest-4-en-3-one from Eastman Organic Chemicals was used without further purification. The preparation of this ketone has also been described in Org. Synth., Coll. Vol. 4, 192 (1963). 6. The glass wool filter collects any of the insoluble triethylammonium chloride which might be formed. A reagent grade of triethylamine (b.p. 88°) was distilled from calcium hydride prior to use. N,N,N',N'-Tetramethylethylenediamine has also been found to be a satisfactory Lewis base for less reactive enolates 3,4. 7. Commercial diethyl phosphorochloridate , b.p. 60–62° (1.5 mm.), purchased from Eastman Organic Chemicals, was distilled prior to use in this reaction. 8. A good grade of silica gel, such as that available from E. Merck and Company, Darmstadt, is appropriate for this chromatography. 9. The 1H NMR spectrum (CDCl 3) of the crude product has absorption at δ 0.6–2.3 (m, ca. 52 H, aliphatic C H ), 3.9–4.4 (m, 4H, 2C H 2O), and 5.1 (m, 1H, vinyl H ). 10. Ethylamine (b.p. 17°) is available from Eastman Organic Chemicals. 11. A commercial grade of tert -butyl alcohol (b.p. 83°) should be distilled from calcium hydride before use. 12. The product exhibits end absorption in the UV (95% C 2H 5OH) with ε 330 at 210 nm and a series of 1H NMR peaks (CDCl 3) at δ 0.67, 0.82, 0.85, 0.88, and 0.92 (18H, 6C H 3) with a multiplet at δ 1.0–2.2 and a partially resolved multiplet attributable to two vinyl protons. This latter absorption corresponds approximately to signals at δ 5.28 (d of t, J = 8 and 1 Hz., 1H) and 5.60 (d of t, J = 8 and 2.8 Hz., 1H). The mass spectrum of the product has the following abundant peaks: m/e (rel. int.), 384 (100, M +), 369 (69), 355 (70), 229 (27), 122 (28), 109 (28), 107 (60), 95 (33), 93 (34), 81 (72), 55 (30), and 43 (31). The submitters report that ozonolysis of the product at −10° in a mixture of ethyl acetate and acetic acid , followed by reaction with hydrogen peroxide , formed 3,4-seco -5-methylcoprostan-3,4-dioic acid as crystals from ethyl acetate , m.p. 168–172° with prior softening at 130°.3. DiscussionThe conjugate addition of lithium dimethylcuprate and other organocopper reagents to α,β-unsaturated ketones is a reaction which has had wide application and has been fairly well studied.5 6 7 In order that the positional specificity which has been conferred upon the enolate anions generated by such additions might be maintained, these intermediates have been intercepted with acetic anhydride ,5 chlorotrimethylsilane ,8 9 diethyl phosphorochloridate ,4,10 tetramethyldiamidophosphorochloridate ,3,4 alkyl halides,11 12 aldehydes,13 ketones,14 acid chlorides,15 and Michael acceptors.16The reductive fission of enol phosphates to olefins is a modification of the procedure used by Kenner and Williams 17 to deoxygenate phenols. The enol phosphates, which have been reduced by the action of lithium in ammonia or alkylamines and by the action of titanium metal,18 have been prepared by treatment of α-bromoketones with triethyl phosphite ,4,19 by interception of enolates generated by the addition of lithium dimethylcuprate to α,β-unsaturated ketones,4,10 by interception of enolates resulting from treatment of unsaturated ketones with lithium in ammonia ,10 and by phosphorylation of enolates of ketones.20This preparation is referenced from:z Org. Syn. Coll. Vol. 7, 346 References and Notes1.Division of Chemistry and Chemical Engineering, Gates & Chellin Laboratories, CaliforniaInstitute of Technology, Pasadena, California 91109. [Present address: Institute of Molecular Biology, University of Oregon, Eugene, Oregon 97403.]2.S. C. Watson and J. F. Eastham, J. Organomet. Chem., 9, 165 (1967).3.R. E. Ireland, D. Muchmore, and U. Hengartner, J. Am. Chem. Soc., 94, 5098 (1972).4. D. Muchmore, Ph.D. dissertation, California Institute of Technology, Pasadena, 1971.5.H. O. House, W. L. Respess, and G. M. Whitesides, J. Org. Chem., 31, 3128 (1966);6.H. O. House and W. F. Fischer, Jr., J. Org. Chem., 33, 949 (1968);7.G. Posner, Org. React., 19, 1 (1972).8.G. Stork and P. F. Hudrlik, J. Am. Chem. Soc., 90, 4462 (1968);9.H. O. House, L. J. Czuba, M. Gall, and H. D. Olmstead, J. Org. Chem., 34, 2324 (1969).10.R. E. Ireland and G. Pfister, Tetrahedron Lett., 2145 (1969).11.P. A. Grieco and R. Finkelhor, J. Org. Chem., 38, 2100 (1973);12.R. K. Boeckman, Jr., J. Org. Chem., 38, 4450 (1973).13.K. Heng and R. Smith, Tetrahedron Lett., 589 (1975).14. F. Näf, R. Decorzant, and W. Thommen, Helv. Chim. Acta, 58, 1808 (1975).15.T. Tanaka, S. Kurozumi, T. Toru, M. Kobayashi, S. Miura, and S. Ishimoto, Tetrahedron Lett.,1535 (1975).16.R. K. Boeckman, Jr., J. Am. Chem. Soc., 95, 6867 (1973).17.G. W. Kenner and N. R. Williams, J. Chem. Soc., 522 (1955).18.S. Welch, J. Org. Chem., 43, 2715 (1978).19.M. Fetizon, M. Jurion, and N. T. Anh, J. Chem. Soc. D, 112 (1969).20.I. Borowitz, S. Firstenberg, F. Caspar, and R. Crouch, J. Org. Chem., 36, 3282 (1971).AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)acetic acid (64-19-7)ammonia (7664-41-7)ethyl acetate (141-78-6)methanol (67-56-1)ether,diethyl ether (60-29-7)acetic anhydride (108-24-7)ammonium chloride (12125-02-9)sodium hydroxide (1310-73-2)sodium hydrogen carbonate (144-55-8)sodium chloride (7647-14-5)sodium sulfate(7757-82-6)oxygen (7782-44-7)nitrogen (7727-37-9)hydrogen peroxide (7722-84-1)ammonium hydroxide (1336-21-6)Pentane (109-66-0)copper(I) iodide (7681-65-4)lithium (7439-93-2)Tetrahydrofuran (109-99-9) lithium aluminum hydride (16853-85-3) Methyllithium (917-54-4)Cholest-4-en-3-one (601-57-0)triethylamine (121-44-8)argon (7440-37-1)tert-butyl alcohol (75-65-0)Triethyl phosphite (122-52-1)ethylamine (75-04-7)calcium hydride (7789-78-8)helium (7440-59-7)2-Butanol (78-92-2)2,2-bipyridyl (366-18-7)diethyl phosphorochloridate (814-49-3)lithium dimethylcuprate CHLOROTRIMETHYLSILANE (75-77-4)methylcopper(I)5-Methylcoprost-3-ene (23931-38-6) Cholest-3-ene, 5-methyl-, (5β)-Diethyl 5-methylcoprost-3-en-3-yl phosphate (23931-37-5)triethylammonium chloridetetramethyldiamidophosphorochloridate (1605-65-8)titanium (7440-32-6)N,N,N',N'-tetramethylethylenediamine (110-18-9)3,4-seco-5-methylcoprostan-3,4-dioic acid Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
