Organic Syntheses, Coll. Vol. 4, p.780 (1963); Vol. 35, p.91 (1955).
Organic Syntheses, Coll. Vol. 5, p.157 (1973); Vol. 44, p.15 (1964).

Organic Syntheses, Coll. Vol. 5, p.157 (1973); Vol. 44, p.15 (1964).t-BUTYL AZIDOFORMATE[Formic acid, azido-, tert-butyl ester]Submitted by Louis A. Carpino, Barbara A. Carpino, Paul J. Crowley, Chester A. Giza, and PaulH. Terry1.Checked by Virgil Boekelheide and S. J. Cross.1. ProcedureIn a 1-l. round-bottomed flask fitted with a mechanical stirrer are placed 82 g. (0.62 mole) of t-butyl carbazate,2 72 g. of glacial acetic acid, and 100 ml. of water. The solution is cooled in an ice bath, and 47.0 g. (0.68 mole) of solid sodium nitrite is added over a period of 40–50 minutes, the temperature being kept at 10–15° (Note 1). The mixture is allowed to stand in the ice bath for 30 minutes, 100 ml. of water is added, and the floating oil is extracted into four 40-ml. portions of ether. The combined ether extracts are washed twice with 50-ml. portions of water and with 40-ml. portions of 1M sodium bicarbonate solution until no longer acidic (about three washings are required). The solution is dried over magnesium sulfate, and the ether is removed by distillation from a water bath maintained at 40–45°; water aspirator pressure of 140–150 mm. is used. The pressure is then lowered to 70 mm., and the water bath temperature is raised to 90–95°. The azide is distilled (Caution! (Note 2)) using a Claisen flask and is collected at 73–74° (70 mm.), n24D 1.4227, after a few drops of fore-run. The yield is 57–72.8 g. (64–82%) (Note 3) and (Note 4).2. Notes1. The sodium nitrite may be added as a concentrated aqueous solution.2. It is recommended that the distillation be carried out behind a safety shield. The submitters have distilled this compound several hundred times without incident under the conditions given on a scale up to 300–400 g. per run. On the other hand, Prof. P. G. Katsoyannis (University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania) has reported that an explosion took place in the receiving flask while the compound was being distilled under conditions previously used without incident. The reason for the explosion could not be traced. According to Prof. R. Schwyzer (Ciba, Ltd., Basel, Switzerland) tests at a Swiss Federal Institute showed that the compound could not be exploded by mere heating: it simply decomposes. For explosion, one must apply a primary explosive such as lead azide or silver azide. An attempt by the submitters to distil the azide at atmospheric pressure resulted in vigorous carbonization, but no explosion occurred. In view of the potential hazard some investigators prefer not to distil the azide; they use the crude material after removal of solvent. High yields of carbo-t-butoxy derivatives may be obtained in this way.3. When freshly distilled, the azide is water-white. When the azide is allowed to stand for several weeks, it slowly develops a light yellow color; however, this does not appear to affect its reactivity as an acylating agent.34. The azide should be handled with adequate ventilation. Careless inhalation of the substance was accompanied by development of a painful throbbing headache or a sensation of giddiness or both. These effects disappeared within several hours upon exposure to fresh air.3. Discussiont-Butyl azidoformate has been prepared by a variety of procedures,3,4,5,6,7,8,9,10,11,12 of which the present procedure and that described elsewhere in this series12appear most satisfactory.Because of the instability of t-butyl chloroformate a number of carbonic acid derivatives have been prepared and studied as reagents for the introduction of the carbo-t-butoxy group. A listing of these reagents and references to their preparation may be found in reference 13. In spite of some disadvantages the most widely used reagent is still t-butyl azidoformate, although t-butyl 2,4,5-trichlorophenyl-carbonate appears to be another potentially useful reagent. t-Butyl azidoformate is a convenient reagent for the acylation of amines, hydrazines, and similar compounds.3The acylation product of hydroxylamine, t-butyl N-hydroxycarbamate,5 is a valuable intermediate in the synthesis of O-substituted hydroxylamines such as O-acyl- and O-sulfonylhydroxylamines, many of which are valuable aminating agents and have not be obtained in any other way.14,15 This preparation is referenced from:z Org. Syn. Coll. Vol. 5, 160z Org. Syn. Coll. Vol. 6, 199z Org. Syn. Coll. Vol. 6, 203z Org. Syn. Coll. Vol. 6, 207z Org. Syn. Coll. Vol. 6, 418z Org. Syn. Coll. Vol. 7, 70References and Notes1.Department of Chemistry, University of Massachusetts, Amherst, Massachusetts.2.L. A. Carpino, D. Collins, and S. Göwecke, this volume, p. 166.3.L. A. Carpino, J. Am. Chem. Soc., 79, 4427 (1957).4.L. A. Carpino, J. Am. Chem. Soc., 79, 98 (1957).5.L. A. Carpino, C. A. Giza, and B. A. Carpino, J. Am. Chem. Soc., 81, 955 (1959).6.K. P. Polzhofer, Chimia (Aarau), 23, 298 (1969).7.H. Yajima and H. Kawatani, Chem. Pharm. Bull. (Tokyo), 16, 182 (1968).8.M. Itoh and D. Morino, Experientia, 24, 101 (1968).9.Y. A. Kiryushkin and A. I. Miroshnikov, Experientia, 21, 418 (1965).10.K. Inouye, M. Kanayama, and H. Otsuka, Nippon Kagaku Zasshi, 85, 599 (1964).11. D. S. Tarbell, Accounts Chem. Res., 2, 296 (1969).12.M. A. Insalaco and D. S. Tarbell, Org. Syntheses, 50, 9 (1970).13.L. A. Carpino, K. N. Parameswaran, R. K. Kirkley, J. W. Spiewak, and E. Schmitz, J. Org.Chem., 35, 3291 (1970).14.L. A. Carpino, J. Am. Chem. Soc., 82, 3133 (1960).15.L. A. Carpino, J. Am. Chem. Soc., 85, 2144 (1963).AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)acetic acid (64-19-7)ether (60-29-7)sodium bicarbonate (144-55-8)sodium nitrite(7632-00-0)hydroxylamine (7803-49-8)magnesium sulfate (7487-88-9)silver azidet-BUTYL AZIDOFORMATE,Formic acid, azido-, tert-butyl ester (1070-19-5)t-butyl carbazate (870-46-2)t-butyl chloroformatet-butyl 2,4,5-trichlorophenyl-carbonate (16965-08-5)t-butyl N-hydroxycarbamate (36016-38-3)lead azideCopyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
经典化学合成反应标准操作醛酮的合成

经典化学合成反应标准操作醛酮的经典合成目录1.前言 (4)2.由醇合成醛酮 (4)2.1铬(VI)试剂 (4)2.1.1 Jones氧化(Cr2O3/H2SO4/acetone) (4)2.1.2 Collins氧化(Cr2O3.2Py) (5)2.1.3 PCC(Pyrindium Chlorochromate)氧化 (8)2.1.4 PDC(Pyrindium Dichromate)氧化 (9)2.2 用活性MnO2氧化 (10)2.2.1 用活性MnO2氧化示例一: (10)2.3用DMSO氧化 (11)2.3.1 DMSO-(COCl)2氧化(Swern Oxidation) (11)2.3.2 DMSO-SO3-Pyridine (12)2.4 用氧铵盐氧化 (13)2.4.1 用氧铵盐氧化示例: (13)2.5 用高价碘试剂氧化 (14)2.5 .1 Dess-Martin氧化反应示例: (14)2.5.2 IBX氧化反应示例: (15)2.6 亚硝酸钠和醋酐氧化 (15)2.6.1 亚硝酸钠和醋酐氧化示例 (15)2.6 TPAP-NMO 氧化 (16)2.6.1 TPAP-NMO 氧化示例 (16)2.7 1,2-二醇的氧化 (16)2.7.1 1,2-二醇的氧化示例一: (17)2.7.1 其他1,2-二醇的氧化相关文献: (18)3.由卤化物合成醛酮 (18)3.1 由伯卤甲基和仲卤甲基的氧化合成醛酮 (18)3.1.1 用DMSO氧化(Kornblum反应) (18)3.1.2用硝基化合物氧化(Hass反应) (20)3.1.3用乌洛托品氧化(Sommelet反应) (21)3.1.4用对亚硝基二甲苯胺氧化吡啶翁盐氧化(Kröhnke反应) (22)3.1.5用胺氧化物氧化 (22)3.2 由二卤甲基或二卤亚甲基合成醛酮 (23)3.2.1 由二卤甲基合成醛反应示例: (23)3.3 由有机金属化合物的酰化合成醛酮 (24)3.3.1 由有机金属化合物的酰化合成醛酮示例 (25)3.4 由Pd催化反应合成醛 (25)4.由活泼甲基或活泼亚甲基烷烃合成醛酮 (25)4.1 用SeO2氧化合成醛酮 (26)4.1.1 用SeO2氧化合成醛酮示例 (26)4.2用空气氧化合成酮 (26)4.2.1用空气氧化合成酮反应示例: (27)4.3 用铬酸氧化合成酮 (27)4.3.1 用铬酸氧化合成酮示例 (27)4.4用高锰酸盐氧化合成酮 (29)4.5 用醌氧化合成酮 (29)5.由羧酸及其衍生物合成醛酮 (30)5.1由羧酸合成醛 (30)5.1.1用金属氢化物还原 (30)5.1.2由脱CO2合成醛 (31)5.1.3由羧酸合成酮 (31)5.2由酰氯及酸酐合成醛酮 (33)5.2.1用Rosenmund法合成 (33)5.2.2用金属氢化物还原 (34)5.3由酯及内酯合成醛 (35)5.3.1 酯通过DIBAL还原为醛示例: (36)5.4由酰胺合成醛酮 (36)5.4.1 由酰胺合成醛酮 (37)5.4.2 McFadyen-Stevens Reaction (38)5.5由酯或酰氯经Weinreb酰胺合成醛酮 (39)5.5.1 由Weinreb酰胺还原合成醛反应示例一 (40)5.5.2由Weinreb酰胺还原合成酮反应示例: (41)5.6由氰合成醛酮 (41)5.6.1DIBAL 还原腈到醛示例(最重要的方法) (42)5.6.2Li(EtO)3AlH 还原腈到醛示例(较重要的方法) (43)5.6.3Ranney Ni 加氢还原氰到合成醛示例 (43)5.6.4有机金属试剂对腈加成合成酮示例 (44)6. 由烯烃、芳环合成醛酮 (46)6.1 由烯烃臭氧氧化合成醛 (46)6.2 烯烃用OsO4/NaIO4氧化合成醛 (47)6.3 烯烃经由有机硼化合物中间体的烯烃甲酰化合成醛 (47)6.5 由烯烃的甲酰化合成醛 (48)6.5.1 Vilsmeyer反应 (48)6.5.2 Duff’s 甲酰化 (51)6.5.3 Reimer-Tiemann 甲酰化 (52)6.5.4 Gattermann甲酰化 (53)6.5.5 多聚甲醛/甲醇镁苯酚甲酰化 (53)6.5.6氯化锡/多聚甲醛苯酚甲酰化 (54)6.5.7重氮化后甲酰化 (54)6.6烯烃经加成-氧化反应合成酮 (56)6.6.1 烯烃经加成-氧化反应合成酮示例 (56)7. 由炔烃合成醛酮 (57)7.1 由加成-氧化反应合成醛酮 (57)7.2 由氧化反应合成酮 (57)7.3 由加成-水解反应合成酮 (58)7.4 由加成-还原反应合成酮 (59)7.5 由加成-烷基化,酰化等反应合成酮 (59)8. 由醚及环氧化合物合成醛酮 (59)8.1 Claisen重排 (59)8.2酸催化下环氧化物重排 (61)8.2.1 酸催化下环氧化物重排合成醛酮示例一 (61)8.3氧化法 (61)8.4 水解法缩醛或酮合成醛酮 (61)9. 由胺合成醛 (62)9.1胺的氧化 (62)9.1.1 胺的氧化合成醛反应示例: (63)9.2 由胺经由西佛碱的方法 (64)9.2.1 由胺经由西佛碱合成醛示例 (64)9.3 自苯胺衍生物合成 (64)10. 由硝基化合物合成醛酮 (64)11. 由Friedel-Crafts反应合成芳基酮 (65)11.1 由Friedel-Crafts反应合成芳基酮示例 (68)12. Dieckmann 缩合脱酸 (69)13. 由合成子合成醛酮 (71)14. 由砜合成醛酮 (71)15. Michael 反应和类似反应(Addition, Condensation) (71)1.前言醛和酮是一类重要的有机化合物,其合成在有机合成中占有非常重要的地位。
重氮化反应 氨基变卤素
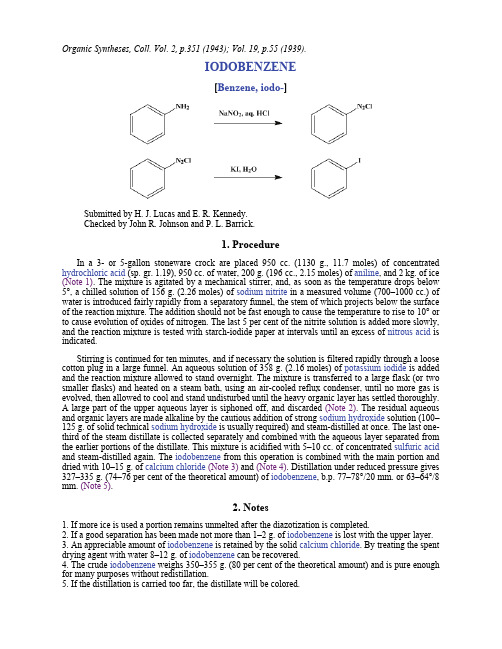
Organic Syntheses, Coll. Vol. 2, p.351 (1943); Vol. 19, p.55 (1939).IODOBENZENE[Benzene, iodo-]Submitted by H. J. Lucas and E. R. Kennedy.Checked by John R. Johnson and P. L. Barrick.1. ProcedureIn a 3- or 5-gallon stoneware crock are placed 950 cc. (1130 g., 11.7 moles) of concentrated hydrochloric acid (sp. gr. 1.19), 950 cc. of water, 200 g. (196 cc., 2.15 moles) of aniline, and 2 kg. of ice (Note 1). The mixture is agitated by a mechanical stirrer, and, as soon as the temperature drops below 5°, a chilled solution of 156 g. (2.26 moles) of sodium nitrite in a measured volume (700–1000 cc.) of water is introduced fairly rapidly from a separatory funnel, the stem of which projects below the surface of the reaction mixture. The addition should not be fast enough to cause the temperature to rise to 10° or to cause evolution of oxides of nitrogen. The last 5 per cent of the nitrite solution is added more slowly, and the reaction mixture is tested with starch-iodide paper at intervals until an excess of nitrous acid is indicated.Stirring is continued for ten minutes, and if necessary the solution is filtered rapidly through a loose cotton plug in a large funnel. An aqueous solution of 358 g. (2.16 moles) of potassium iodide is added and the reaction mixture allowed to stand overnight. The mixture is transferred to a large flask (or two smaller flasks) and heated on a steam bath, using an air-cooled reflux condenser, until no more gas is evolved, then allowed to cool and stand undisturbed until the heavy organic layer has settled thoroughly.A large part of the upper aqueous layer is siphoned off, and discarded (Note 2). The residual aqueous and organic layers are made alkaline by the cautious addition of strong sodium hydroxide solution (100–125 g. of solid technical sodium hydroxide is usually required) and steam-distilled at once. The last one-third of the steam distillate is collected separately and combined with the aqueous layer separated from the earlier portions of the distillate. This mixture is acidified with 5–10 cc. of concentrated sulfuric acid and steam-distilled again. The iodobenzene from this operation is combined with the main portion and dried with 10–15 g. of calcium chloride(Note 3) and (Note 4). Distillation under reduced pressure gives 327–335 g. (74–76 per cent of the theoretical amount) of iodobenzene, b.p. 77–78°/20 mm. or 63–64°/8 mm. (Note 5).2. Notes1. If more ice is used a portion remains unmelted after the diazotization is completed.2. If a good separation has been made not more than 1–2 g. of iodobenzene is lost with the upper layer.3. An appreciable amount of iodobenzene is retained by the solid calcium chloride. By treating the spent drying agent with water 8–12 g. of iodobenzene can be recovered.4. The crude iodobenzene weighs 350–355 g. (80 per cent of the theoretical amount) and is pure enough for many purposes without redistillation.5. If the distillation is carried too far, the distillate will be colored.3. DiscussionThe preparation of iodobenzene by iodination of benzene, with iodine and nitric acid, and a survey of preparative methods have been given in an earlier volume.1 The present procedure, based upon the method of Gattermann,2 gives a purer product.This preparation is referenced from:z Org. Syn. Coll. Vol. 5, 660z Org. Syn. Coll. Vol. 5, 665References and Notes. Syn. Coll. Vol. I, 1941, 323.2.Gattermann-Wieland, "Laboratory Methods of Organic Chemistry," p. 283. Translated from thetwenty-fourth German edition by W. McCartney, The Macmillan Company, New York, 1937.AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)oxides of nitrogencalcium chloride (10043-52-4)sulfuric acid (7664-93-9)hydrochloric acid (7647-01-0)Benzene (71-43-2)aniline (62-53-3)sodium hydroxide (1310-73-2)nitric acid (7697-37-2)potassium iodide (7681-11-0)sodium nitrite (7632-00-0)nitrous acid (7782-77-6)iodine (7553-56-2)Iodobenzene,Benzene, iodo-(591-50-4)Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
Organic Syntheses有机合成

Organic Syntheses(有机合成手册), John Wiley & Sons (免费)/Named Organic Reactions Collection from the University ofOxford (有机合成中的命名反应库) (免费)http://www.chem.ox.ac. ... rganicReac...有机化学资源导航Organic Chemistry Resources Worldwide/有机合成文献综述数据库Synthesis Reviews (免费)/srev/srev.htmCAMEO (预测有机化学反应产物的软件)http://zarbi.chem.yale ... o/index.shtmlCarbohydrate Letters (免费,摘要)/Carbohydrate_Letters/Carbohydrate Research (免费,摘要)/locate/carresCurrent Organic Chemistry (免费,摘要)/coc/index.htmlElectronic Encyclopedia of Reagents for Organic Synthesis (有机合成试剂百科全书e-EROS)/eros/European Journal of Organic Chemistry (免费,摘要)http://www.interscienc ... es/1434-193X/Methods in Organic Synthesis (MOS,有机合成方法)/is/database/mosabou.htmOrganic Letters (免费,目录)/jo ... f7/index.htmlOrganometallics (免费,目录)/jo ... d7/index.htmlRussian Journal of Bioorganic Chemistry (Bioorganicheskaya Khimiya) (免费,摘要) http://www.wkap.nl/journalhome.htm/1068-1620Russian Journal of Organic Chemistry (Zhurnal Organicheskoi Khimii) (免费,摘要) http://www.maik.rssi.ru/journals/orgchem.htmScience of Synthesis: Houben-Weyl Methods of Molecular Transformation/Solid-Phase Synthesis database (固相有机合成)/chem_db/sps.htmlSynthetic Communications (免费,摘要)/ ... productid/SCCSyntheticPages (合成化学数据库) (免费)/The Complex Carbohydrate Research Center (复杂碳水化合物研究中心)/合成材料老化与应用(免费,目录)http://hccllhyyy.perio ... /default.html金属卡宾络合物催化的烯烃复分解反应(免费) ... 02/2.6%20.htm上海化学试剂研究所/英国化学数据服务中心CDS (Chemical Database Service)/cds/cds.html英国皇家化学会碳水化合物研究组织(Carbohydrate Group of the Royal Society of Chemistry)/lap/rsccom/dab/perk002.htm有机反应催化学会(ORCS, Organic Reaction Catalysis Society)/有机合成练习(免费)/中国科学院成都有机化学研究所:催化与环境工程研究发展中心/MainIndex.htm金属有机及元素有机化学:CASREACT - Chemical Reactions Database(CAS的化学反应数据库)/CASFILES/casreact.html日本丰桥大学Jinno实验室的研究数据库(液相色谱、多环芳烃/药物/杀虫剂的紫外谱、物性) (免费)http://chrom.tutms.tut ... H/research...A New Framework for Porous Chemistry (金属有机骨架) (免费) ... 83432324.htmlActa Crystallographica Section B (免费,摘要)http://journals.iucr.o ... homepage.htmlActa Crystallographica Section E (免费,摘要)http://journals.iucr.o ... homepage.htmlBibliographic Notebooks for Organometallic Chemistryhttp://www.ensc-lille. ... bco/bnoc.htmlBiological Trace Element Research (生物痕量元素研究杂志) (免费,摘要)http://www.humanapress ... =0163-4984...Journal of Organometallic Chemistry (免费,摘要)/locate/jnlabr/jomOrganic Letters (免费,目录)/jo ... f7/index.htmlOrganometallics (免费,目录)/jo ... d7/index.htmlSyntheticPages (合成化学数据库) (免费)/金属卡宾络合物催化的烯烃复分解反应(免费) ... 02/2.6%20.htm金属有机参考读物:The Organometallic HyperTextBook by Rob Toreki/organomet/index.html金属有机化学国家重点实验室,中国科学院上海有机所/元素有机化学国家重点实验室(南开大学)/在线网络课程:有机金属反应和均相催化机理(Dermot O'Hare 主讲)http://www.chem.ox.ac. ... ot/organomet/药物化学:Fisher Scientific/PubMed: MEDLINE和PREMEDLINE (免费)/PubMed/生物医药:BioMedNet: The World Wide Club for the Biological and Medical Community /AIDSDRUGS (艾滋病药物) (免费) ... aidsinfs.htmlautodock (分子对接软件) (免费) ... doc/autodock/DIRLINE (卫生与生物医药信息源库) (免费)/HISTLINE (医药史库) (免费)/TOXNET (化合物毒性相关数据库系列) (免费)/日本药典,第14版(免费)http://jpdb.nihs.go.jp/jp14e/index.html小分子生物活性数据库ChemBank (免费)/Ashley Abstracts Database (药物研发、市场文献摘要) (免费) ... ey/search.aspBIOSIS/BIOSIS/ONLINE/DBSS/biosisss.html从检索药物交易信息库PharmaDeals (部分免费)/从ChemWeb检索有机药物用途及别名库Negwer: organic-chemical drugs and their synonyms (部分免费)http://cwgen.chemweb.c ... ersearch.html美国常用药品索引库RxList (免费)/美国国家医学图书馆NLM的免费在线数据库(免费)/ho ... internet.html制药公司目录(Pharmaceutical Companies on Virtual Library: Pharmacy Page)/company.html37℃医学网/AAPS PharmSci (免费,全文)/Abcam Ltd.有关抗体、试剂的销售,抗体的搜索)/Acta Pharmaceutica (免费,摘要)http://public.srce.hr/acphee/Advanced Drug Delivery Reviews (免费,摘要)http://www.elsevier.nl/locate/drugdelivAmerican Journal of Drug and Alcohol Abuse (免费,摘要)/ ... productid/ADAAmerican Journal of Pharmaceutical Education (AJPE) (免费,全文)/Amgen Inc. (医药)/Anita's web picks (药学与药物化学信息导航)http://wwwcmc.pharm.uu.nl/oyen/webpicks.htmlAnnals of Clinical Microbiology and Antimicrobials (免费,全文)/Annual Review of Pharmacology and Toxicology (免费,摘要)/Anti-Cancer Drug Design (免费,摘要)/antcan/生物有机化学:ScienceDirect: 在线访问Elsevier的1100种期刊全文(免费目录) (免费)/生命、环境科学综合性资源TheScientificWorld (sciBASE)/生物医药:BioMedNet: The World Wide Club for the Biological and Medical Community /BIOETHICSLINE (BIOETHICS onLINE) (免费)/BIOME (生命科学资源导航)/browse/Directory of P450-containing Systems(P450酶系目录)http://p450.abc.hu/DIRLINE (卫生与生物医药信息源库) (免费)/百名最佳生物技术网站列表(Top 100 Biotechnology WWW Sites)/top100.asp从ChemWeb检索《化学工程与生物技术文摘》库CEABA (部分免费)/课程材料:MIT生物学超文本教材 ... 7001main.html生物材料网(Biomaterials Network)/生物信息学资源导航,上海生物化学所/bio/index.htm小分子生物活性数据库ChemBank (免费)/英国剑桥医学研究委员会:分子生物学实验室LMB/biology site of the network./BIOSIS/BIOSIS/ONLINE/DBSS/biosisss.htmlCATH Protein Structure Classification (蛋白质结构分类) (免费)/bsm/cath/Databases and Tools for 3-D Protein Structure Comparison and Alignment (三维蛋白质结构对比) (免费)/ce.htmlLos Alamos National Laboratory Bioscience Division/Protein Data Bank (PDB, 蛋白质数据库) (免费)/pdb/计算分子生物学:Computational Molecular Biology at NIH/molbio/酶命名数据库(ENZYME-Enzyme nomenclature database) (免费) /enzyme/Access Excellence (有关生物、生命等的科学教育网站)/Acta Biochimica Polonica (免费,全文)http://www.actabp.pl/Acta Biotechnologica (生物技术学报) (免费,摘要)http://www.wiley-vch.d ... eticIndex/...American Institute of Biological Sciences (AIBS)/American Journal of Medical Genetics Part A (免费,摘要) http://www3.interscien ... jtoc?ID=33129Amos' WWW links page (生物大分子网络资源导航)/alinks.htmlAmersham International (英国,生物技术供应商)/。
一种二氯乙腈的合成方法[发明专利]
![一种二氯乙腈的合成方法[发明专利]](https://img.taocdn.com/s3/m/fb49bb013868011ca300a6c30c2259010202f3fd.png)
(19)中华人民共和国国家知识产权局(12)发明专利(10)授权公告号 (45)授权公告日 (21)申请号 201910004035.5(22)申请日 2019.01.03(65)同一申请的已公布的文献号申请公布号 CN 109608361 A (43)申请公布日 2019.04.12(73)专利权人 山东国邦药业有限公司地址 261108 山东省潍坊市滨海经济开发区先进制造产业园香江西一街02131号院内专利权人 国邦医药集团股份有限公司(72)发明人 王友杰 李琦斌 王伟宏 (74)专利代理机构 潍坊正信致远知识产权代理有限公司 37255代理人 刘德林(51)Int.Cl.C07C 253/18(2006.01)C07C 255/03(2006.01)(56)对比文件CN 106278945 A ,2017.01.04CN 108424375 A ,2018.08.21US 5770757 A ,1998.06.23Yuecheng Zhang等.Amination of allyl alcohol to …… hydrogenation reactions.《Applied Catalysis A: General》.2013,第467卷154-162.Yuecheng Zhang等.Amination of allyl alcohol to propionitrile over a Zn30Cr4.5/γ-Al2O3 bimetallic catalyst via coupled dehydrogenation –hydrogenation reactions.《Applied Catalysis A: General》.2013,第467卷154-162.审查员 姜雪(54)发明名称一种二氯乙腈的合成方法(57)摘要本发明公开了一种二氯乙腈的合成方法,包括以下步骤:向四氯乙烯加入二氯甲烷,然后向二氯甲烷‑四氯乙烯体系中缓慢通入氨气升压,并使用水浴进行升温,并保温保压进行反应,反应结束后降温,并排清剩余的氨气,通过蒸馏收集馏分得到二氯乙腈。
LDA的制备(标准)

Organic Syntheses, Coll. Vol. 10, p.107; Vol. 78, p.82EFFICIENT SYNTHESIS OF HALOMETHYL-2,2'-BIPYRIDINES:4,4'-BIS(CHLOROMETHYL)-2,2'-BIPYRIDINE[ 2,2'-Bipyridine, 4,4'-bis(chloromethyl)- ]Submitted by Adam P. Smith, Jaydeep J. S. Lamba, and Cassandra L. Fraser 1 .Checked by Motoki Yamane and Koichi Narasaka. 1. ProcedureA. 4,4'-Bis[(trimethylsilyl)methyl]-2,2'-bipyridine . A 500-mL, two-necked, round-bottomed flask (Note 1), equipped with a nitrogen inlet, magnetic stirrer, and rubber septum is charged with tetrahydrofuran (THF) (90 mL) (Note 2) and diisopropylamine (9.8 mL, 69.7 mmol) (Note 3). The reaction mixture is cooled to −78°C and a solution of butyllithium (n-BuLi) (1.7 M in hexanes, 36.0 mL, 61.4 mmol) (Note 4) is added. The solution is stirred at −78°C for 10 min, warmed to 0°C and stirred for 10 min, then cooled back to −78°C. A solution of 4,4'-dimethyl-2,2'-bipyridine (5.14 g, 27.9 mmol) (Note 5) in THF (130 mL) (Note 2), prepared in a 250-mL, two-necked, round-bottomed flask under a nitrogen atmosphere, is added via cannula to the cold lithium diisopropylamide (LDA) solution. The resulting maroon-black reaction mixture is stirred at −78°C for 1 hr, then chlorotrimethylsilane (TMSCl) (8.85 mL, 69.7 mmol) (Note 6) is rapidly added via syringe. After the solution becomes pale blue-green (≈10 sec after the TMSCl addition), the reaction is quenched by rapid addition of absolute ethanol (10 mL). (Note: the reaction should be quenched regardless of color change after a maximum of 15 seconds to avoid over silylation). The cold reaction mixture is poured into a separatory funnel (1 L) containing aqueous saturated sodium bicarbonate (NaHCO 3, ≈200 mL) and allowed to warm to ≈25°C. The product is extracted with dichloromethane (CH 2Cl 2, 3 × 300 mL); the combined organic fractions are shaken with brine (≈200 mL) and dried over sodium sulfate (Na 2SO 4). Filtration and concentration on a rotary evaporator affords 8.85 g (97%) of 4,4'-bis[(trimethylsilyl)methyl]-2,2'-bipyridine as a slightly off-white crystalline solid (Note 7).B. 4,4'-Bis(chloromethyl)-2,2'-bipyridine . Into a 500-mL, two-necked, round-bottomed flask (Note 1) equipped with a magnetic stirring bar are placed 5.22 g (15.9 mmol) of 4,4'-bis[(trimethylsilyl)methyl]-2,2'-bipyridine , 15.1 g (63.6 mmol) of hexachloroethane (Cl 3CCCl 3, Note 8) and 9.65 g (63.6 mmol) of cesium fluoride (CsF, Note 9) at 25°C under a nitrogen atmosphere. Acetonitrile (260 mL) (Note 10) is added and the heterogeneous reaction mixture is stirred at 60°C for ≈3.5 hr (or until TLC indicates that all TMS starting material is consumed). After the mixture is cooled to 25°C, it is poured into a separatory funnel containing ethyl acetate (EtOAc) and water (H 2O, ≈100 mL each). The product is extracted with EtOAc (3 × 100 mL); the combined organic fractions are shaken with brine (≈200 mL) and dried over Na 2SO 4. Filtration and concentration on a rotary evaporator, followed by flashchromatography using deactivated silica gel (60% EtOAc: 40% hexanes)(Note 11), gives 3.67 g (91%) of the chloride as a white solid (Note 12).2. Notes1. Before use, all glassware, needles, and syringes were dried overnight in a 120°C oven.2. THF was dried and purified by passage through alumina solvent purification columns 2 or by distillation over sodium /benzophenone .3. Diisopropylamine was purchased from Aldrich Chemical Company, Inc. , and distilled over calcium hydride (CaH 2) prior to use.4. A 1.7 M solution of n-BuLi in hexanes was obtained from Aldrich Chemical Company, Inc. The n-BuLi is titrated prior to its use in each reaction using the following procedure.3 To a 50-mL, round-bottomed flask (Note 1), equipped with nitrogen inlet and a magnetic stirrer is added N-benzylbenzamide (854 mg, 4.0 mmol) (as received from Aldrich Chemical Company, Inc.) and THF (40 mL) (Note 2). The solution is cooled to −42°C (acetonitrile /dry ice) and n-BuLi is added dropwise to the blue endpoint (color persists for >30 sec). The molarity is calculated using a 1:1 stoichiometric ratio of N-benzylbenzamide to n-BuLi. (Just greater than 1 equivalent of alkyllithium is needed to reach the endpoint).5. 4,4'-Dimethyl-2,2'-bipyridine was obtained from GFS Chemicals, Inc. or Tokyo Chemical Industry Co. and used as received.6. Chlorotrimethylsilane (TMSCl) was purchased from Aldrich Chemical Company, Inc. , and used as obtained.7. The following characterization data was obtained: mp 90-92°C; 1H NMR (CDCl 3, 300 MHz) δ: 0.04 (s, 18 H), 2.21 (s, 4 H), 6.94 (d, 2 H, J = 5.01),8.05 (br s, 2 H), 8.46 (d, 2 H, J = 5.00) ; 13C NMR (CDCl 3, 75 MHz) δ: −2.2, 27.1, 120.4, 123.0, 148.3, 150.8, 155.5 . Anal. Calcd for C 18H 28N 2Si 2: C, 65.79; H, 8.59; N, 8.53. Found: C, 65.78; H, 8.43; N, 8.76. It has been noted that desilylation occurs after standing in deuterochloroform (CDCl 3) overnight. The resulting methyl derivatives have also been observed in certain purified TMS bipyridine samples when stored over time. Therefore, it is best to convert these intermediates to the corresponding halides in a timely fashion. 8. Hexachloroethane (Cl 3CCCl 3), obtained from Aldrich Chemical Company, Inc. , was used as received.9. Cesium fluoride was purchased from Acros Organics, Inc. or Soekawa Chemicals Co. and stored in a dry box prior to use. 10. Acetonitrile was distilled over CaH 2 and stored in a 500-mL Kontes flask prior to use. 11. Silica gel used for flash chromatography (particle size 0.035-0.075 mm) was obtained from VWR Scientific Products . Silica chromatography columns were deactivated by flushing with 10% triethylamine in hexanes and then were washed with hexanes prior to use. 12. Spectral properties are as follows: mp 98-100°C; 1H NMR (CDCl 3, 300 MHz) δ: 4.63 (s, 4 H), 7.38 (dd, 2 H, J = 1.9, 5.0), 8.43 (s, 2 H), 8.70 (d, 2 H, J = 4.6) ; 13C NMR (CDCl 3, 75 MHz) δ: 43.9, 120.1, 122.8, 146.7, 149.4, 155.8 . Anal. Calcd for C 12H 10Cl 2N 2: C, 56.94; H, 3.98; N, 11.07. Found: C, 56.82; H, 4.04; N, 11.01. 13. In some cases, particularly if the solvent or reaction conditions are not thoroughly dry, 4,4'-dimethyl-2,2'-bipyridine is formed as a byproduct during the halogenation reaction. This compound may be separated from 4,4'-bis(chloromethyl)-2,2'-bipyridine by flash chromatography on silica gel (not deactivated with Et3N) using EtOAc as the mobile phase. Alternatively, 4,4'-bis(chloromethyl)-2,2'-bipyridine may be purified by recrystallization in hot/cold absolute EtOH, with no evidence of ether formation (e.g., 4,4'-di-Ethoxymethyl-2,2'-bipyridine) by 1H NMR.Waste Disposal InformationAll toxic materials were disposed of in accordance with "Prudent Practices in the Laboratory"; National Academy Press; Washington, DC, 1995.3. DiscussionHalomethylbipyridines, are typically synthesized either by radical halogenation 4 or from hydroxymethylbipyridine precursors.5Radical methods often give rise to mixtures of halogenatedspecies that are difficult to separate with flash chromatography. A solution to this problem, involving the selective reduction of polyhalogenated by-products with diisobutylaluminum hydride (DIBAL-H), has resulted in slight improvements in overall yields.6 While the synthesis of halomethyl compounds from hydroxymethyl precursors is more efficient than radical halogenation, such procedures involve many steps, each of which give intermediates in moderate to high yields.5 Direct trapping of bpy (CH 2Li)n with electrophiles has proved unsuccessful for the generation of halide products.5a The quenching of LDA-generated carbanions with TMSCl prior to halogenation as described here constitutes an efficient, high yield synthesis of halomethyl bpys substituted at various positions around the ring system.7,8Currently, 2,2'-bipyridine derivatives figure prominently in supramolecular assembly,9 in bioinorganic contexts,10 in studies of redox electrocatalysis 4a and in polymeric materials.11 Halomethyl bpys and their various metal complexes have also been used as initiators for controlled polymerizations of several different monomers including styrene and 2-alkyl-2-oxazolines.12TABLE I SYNTHESIS OF (TRIMETHYLSILYL)METHYL-2,2'-BIPYRIDINESProductR 1 R 2 R 3 R 4 Yield (%)4-(Trimethylsilyl)-methyl-2,2'-bipyridineTMSCH 2H H H 935-(Trimethylsilyl)-methyl-2,2'-bipyridineH TMSCH 2H H 996-(Trimethylsilyl)-methyl-2,2'-bipyridineH H TMSCH 2H 974,4'-Bis[(trimethylsilyl)-methyl]-2,2'-bipyridineTMSCH 2H H TMSCH 297TABLE II SYNTHESIS OF HALOMETHYL-2,2'-BIPYRIDINES Product R 1 R 2 R 3 R 4 Yield (%)4-Chloromethyl-2,2'-bipyridineClCH 2H H H94 5-Chloromethyl-2,2'-bipyridineH ClCH 2H H98 6-Chloromethyl-2,2'-bipyridineH H ClCH 2H 95 4,4'-Bis(chloromethyl)-2,2'-bipyridineClCH 2H H ClCH 291 4-Bromomethyl-2,2'-bipyridineBrCH 2H H H925-Bromomethyl-2,2'-bipyridineH BrCH 2H H986-Bromomethyl-2,2'-bipyridine H H BrCH 2H 99References and Notes1.Department of Chemistry, University of Virginia, Charlottesville, VA 22904-4319.2.Pangborn, A. B.; Giardello, M. A.; Grubbs, R. H.; Rosen, R. K.; Timmers, F. J. Organometallics1996, 15, 1518.3.Burchat, A. F.; Chong, J. M.; Nielsen, N. J. Organomet. Chem. 1997, 542, 281.4.(a) Gould, S.; Strouse, G. F.; Meyer, T. J.; Sullivan, B. P. Inorg. Chem. 1991, 30, 2942 andreferences therein; (b) Wang, Z.; Reibenspies, J.; Motekaitis, R. J.; Martell, A. E. J. Chem. Soc., Dalton Trans. 1995, 1511; (c) Rodriguez-Ubis, J.-C.; Alpha, B.; Plancherel, D.; Lehn, J.-M. Helv. Chim. Acta 1984, 67, 2264; (d) Newkome, G. R.; Puckett, W. E.; Kiefer, G. E.; Gupta, V. K.; Xia, Y.; Coreil, M.; Hackney, M. A. J. Org. Chem. 1982, 47, 4116.5.(a) Della Ciana, L.; Hamachi, I.; Meyer, T. J. J. Org. Chem. 1989, 54, 1731; (b) Della Ciana, L.;Dressick, W. J.; Von Zelewsky, A. J. Heterocycl. Chem. 1990, 27, 163; (c) Newkome, G. R.; Kiefer, G. E.; Kohli, D. K.; Xia, Y.-J.; Fronczek, F. R.; Baker, G. R. J. Org. Chem. 1989, 54, 5105; (d) Imperiali, B.; Prins, T. J.; Fisher, S. L. J. Org. Chem. 1993, 58, 1613.6.Uenishi, J.; Tanaka, T.; Nishiwaki, K.; Wakabayashi, S.; Oae, S.; Tsukube, H. J. Org. Chem.1993, 58, 4382.7.Fraser, C. L.; Anastasi, N. R.; Lamba, J. J. S. J. Org. Chem. 1997, 62, 9314.8.Savage, S. A.; Smith, A. P.; Fraser, C. L. J. Org. Chem. 1998, 63, 10048.9.(a) Boulas, P. L.; Gómez-Kaifer, M.; Echegoyen, L. Angew. Chem., Int. Ed. Engl. 1998, 37, 216;(b) Mamula, O.; von Zelewsky, A.; Bernardinelli, G. Angew. Chem., Int. Ed. Engl. 1998, 37, 290. 10.(a) Gray, H. B.; Winkler, J. R. Annu. Rev. Biochem. 1996, 65, 537; (b) Dandliker, P. J.; Holmlin,R. E.; Barton, J. K. Science 1997, 275, 1465.11.For a recent review see: Matyjaszewski, K., Ed. "Controlled Radical Polymerizations"; AmericanChemical Society: Washington, DC, 1998.12.(a) Collins, J. E.; Fraser, C. L. Macromolecules 1998, 31, 6715; (b) McAlvin, J. E.; Fraser, C. L. Macromolecules 1999, 32, 1341; (c) Wu, X.; Fraser, C. L. Macromolecules 2000, 33, 4053.AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)4,4'-Bis(chloromethyl)-2,2'-bipyridines :2,2'-Bipyridine, 4,4'-bis(chloromethyl)- (13); (138219-98-4)4,4'-Bis[(trimethylsilyl)methyl]-2,2'-bipyridine :2,2'-Bipyridine, 4,4'-bis[(trimethylsilyl)methyl]-(14); (199282-52-5) 4,4'-Bis(bromomethyl)-2,2'-bipyridineBrCH 2H H BrCH 297Diisopropylamine (8);2-Propanamine, N-(1-methylethyl)- (9); (108-18-9)Butyllithium:Lithium, butyl- (8,9); (109-72-8)4,4'-Dimethyl-2,2'-bipyridine:2,2'-Bipyridine, 4,4'-dimethyl- (9); (1134-35-6)Chlorotrimethylsilane:Silane, chlorotrimethyl- (8,9); (75-77-4)Hexachloroethane:Ethane, hexachloro- (8,9); (67-72-1)Cesium fluoride (8,9); (13400-13-0)Acetonitrile: TOXIC (8,9); (75-05-8)N-Benzylbenzamide:Benzamide, N-benzyl- (8);Benzamide, N-(phenylmethyl)- (9); (1485-70-7) Copyright © 1921-2002, Organic Syntheses, Inc. All Rights Reserved。
Organic Syntheses, Coll. Vol. 2, p.464 (1943); Vol. 13, p.84 (1933).

Organic Syntheses, Coll. Vol. 2, p.464 (1943); Vol. 13, p.84 (1933).NITROSOMETHYLURETHANE[Carbamic acid, methylnitroso-, ethyl ester]Submitted by W. W. Hartman and Ross Phillips.Checked by Louis F. Fieser and J. T. Walker.1. ProcedureTo 206 g. (2 moles) of ethyl N-methylcarbamate(p. 278) and 600 cc. of ordinary ethyl ether in a 5-l. flask is added, along with 200 g. of ice, 650 g. (9 moles) of 96 per cent sodium nitrite(Note 1) dissolved in 1 l. of cold water. The flask is provided with a stopper carrying a thermometer, a tube to lead off evolved nitric oxide, and a separatory funnel with an extension tube reaching to the bottom of the flask. A solution of 1.2 kg. (6.7 moles) of cold 35 per cent nitric acid, prepared by pouring 600 g. (426 cc.) of concentrated acid onto 600 g. of ice, is then cautiously added through the funnel in the course of one and one-half hours. The flask is given an occasional swirl to ensure some mixing, but most of the stirring is done by the evolved gases. Ice is added as required to keep the temperature below 15°. The ether layer first becomes pale red and gradually changes to a blue-green. As soon as the color has changed to green, the ether layer is separated (Note 2), washed twice with cold water, and then with cold potassium carbonate solution until carbon dioxide is no longer evolved. The solution is dried with solid potassium carbonate, and the ether is distilled from a water bath using a 1-l. flask with a 30-cm. column arranged for vacuum distillation. The vacuum is applied as soon as most of the ether has been removed, and the flask is heated gently so that the temperature of the liquid does not exceed 45–50° (Note 3) until the pressure has been reduced below 20 mm. The yield of nitrosomethylurethane boiling at 59–61/10 mm. is 200 g. (76 per cent of the theoretical amount). The density is 1.133 at 20°.2. Notes1. A large excess of sodium nitrite is required to give a satisfactory yield. This may be due to reaction according to the following equations: Nitric oxide (NO) is lost during the reaction. It is not thought advisable to use this by passing in oxygen because of the danger of an explosion, or by passing in air because of the loss of material by evaporation.2. Nitrosomethylurethane irritates the skin.3. According to the literature, nitrosomethylurethane explodes when attempts are made to distil it at normal pressure.3. DiscussionNitrosomethylurethane has been prepared by treating ethyl methylcarbamate with sodium nitrite and sulfuric acid,1 and by passing the gases generated from arsenious oxide and nitric acid into an ethereal solution of ethyl methylcarbamate.2This preparation is referenced from:z Org. Syn. Coll. Vol. 3, 119z Org. Syn. Coll. Vol. 4, 780z Org. Syn. Coll. Vol. 5, 842References and Notes1.Klobbie, Rec. trav. chim. 9, 139 (1890).2.v. Pechmann, Ber. 28, 856 (1895); Schmidt, ibid. 36, 2477 (1903); Brühl, ibid. 36, 3635 (1903).AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)arsenious oxideNitric oxide (NO)potassium carbonate (584-08-7)sulfuric acid (7664-93-9)ether,ethyl ether (60-29-7)nitric acid (7697-37-2)oxygen (7782-44-7)sodium nitrite (7632-00-0)carbon dioxide (124-38-9)nitric oxideNitrosomethylurethaneEthyl N-methylcarbamate,ethyl methylcarbamate (105-40-8)Carbamic acid, methylnitroso-, ethyl ester (615-53-2)Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
Organic Syntheses, Coll. Vol. 9, p.4 (1998); Vol. 71, p.63 (1993).

eluant (Note 7) to give 9.0 g (59% overall) of (2R,3S)- and (2S,3S)-1,4-dioxa-2,3-dimethyl-2-(1-methylethenyl)-8-carboethoxy-8-azaspiro[4.5]decane, a 6:1 mixture of diastereomers, as a pale yellow oil (Note 8).B. (2S,3S)-3-Acetyl-8-carboethoxy-2,3-dimethyl-1-oxa-8-azaspiro[4.5]decane. Dry nitromethane (100 mL) (Note 9) is added through a rubber septum by syringe to a vacuum-dried, 500-mL, round-bottomed flask that contains the ketal mixture prepared in Step A (9.00 g, 31.8 mmol) and a magnetic stir bar. The solution is cooled to −23°C, tin(IV) chloride (SnCl4) (11 mL, 94 mmol) is added by syringe and the solution is stirred for 30 min at −23°C (Note 10). At this time the brown solution is warmed to 23°C and stirring is continued for an additional 30 min. Saturated aqueous NH4Cl (200 mL) is added and the mixture is concentrated under reduced pressure using a rotary evaporator to remove nitromethane. The resulting aqueous suspension is extracted with ethyl acetate (200 mL) and the organic extract is washed with brine (200 mL), dried over sodium sulfate (Na2SO4) and concentrated under reduced pressure using a rotary evaporator. The residue is subjected to flash chromatography on silica gel (250 g, 20 cm × 10 cm) using ethyl acetate:hexane (1:1) eluant (Note 7) to give 8.1 g (90%) of (2S,3S)-3-acetyl-8-carboethoxy-2,3-dimethyl-1-oxa-8-azaspiro[4.5]decane as a pale yellow oil (Note 11) and (Note 12).2. Notes1. Anhydrous tetrahydrofuran was prepared by distillation under argon from sodium benzophenone ketyl.2. 2-Bromopropene, obtained from Aldrich Chemical Company, Inc., was distilled and then passed through a plugof activity IV basic alumina immediately before use.3. The fine white emulsion formed at this stage was collected with the organic phase and was cleared in the subsequent brine washings.4. This crude material was acceptable for use in the second step, although more p-toluenesulfonic acid will be required if large amounts of tributylamine are present. The diol mixture, free from tributylamine, can be obtained by careful chromatography on silica gel using ethyl acetate-hexane (1:1). The purified sample has the following characteristics: 1H NMR (500 MHz, CDCl3, major isomer) δ: 1.10 (d, 3 H, J = 6.5, CH3), 1.37 (s, 3 H, CH3), 1.80 (s,3 H, CH3), 2.21 (br s, 2 H, 2 × OH), 3.77 (q, 1 H, J = 5.6, CH), 4.89 (d, 1 H, J = 1.1, CH=C), 5.06 (s, 1 H, CH=C); IR (film) cm−1: 3421, 3397, 3390, 3364, 2981, 2937, 1088; MS (Cl) m/z 113.0936 (113.0966 calcd for C7H14O2, MH –H2O).5. The major isomer is assigned the 3R, 4S stereochemistry on the expectation that the addition would occur preferentially with Cram (Felkin-Ahn) selectivity.3 This assignment was confirmed by 1H NMR DNOE experimentson the isobutyraldehyde acetal.6. 1-Carbethoxy-4-piperidone was obtained from Aldrich Chemical Company, Inc., and used as received.7. A series of 200-mL fractions was collected during flash chromatography. The product was eluted in fractions3–8 as indicated by TLC analysis using 4% ethanolic phosphomolybdic acid stain.8. This sample has the following characteristics: 1H NMR (500 MHz, CDCl3, major isomer) δ: 1.17 (d, 3 H, J =5.1, CH3), 1.26 (t, 3 H, J = 7.1, OCH2CH3), 1.45 (s, 3 H, CH3), 1.77 (s, 3 H, CH3C=), 1.60–1.81 (m, 5 H, 2 × CH2and CH), 3.43–3.75 (m, 4 H, 2 × CH2N), 4.13 (q, 2 H, J = 7.1, OCH2CH3), 4.96 (s, 2 H, CH2=C); IR (film) cm−1: 2977, 1702, 1433, 1238, 1122; MS (Cl) m/z 284.1850 (284.1861 calcd for C15H25NO4, MH). Anal. Calcd forC15H25NO4: C, 63.58; H, 8.89; N, 4.94. Found: C, 63.48; H, 8.90; N, 4.89.9. Nitromethane was dried by distillation of a 10:1 mixture of nitromethane and trifluoroacetic anhydride and collection of the center fraction that distilled at 100°C.10. Tin(IV) chloride (SnCl4) was obtained from Aldrich Chemical Company, Inc., and handled under an atmosphere of argon.11. Gas chromatographic analysis using a 25-m 10% SP 2100 silicone column showed that this sample was 94% pure and contained one major unidentified impurity. Bulb-to-bulb distillation (200°C, 0.6 mm) of a 7.4-g sample of the crude product afforded 7.0 g (85%) of the product as a pale yellow oil, which was shown by GLC analysis to be of 100% purity. This sample has the following spectral characteristics: [α]D−79.1° (MeOH, c 1.0); 1H NMR (500 MHz, CDCl3) δ: 1.17 (d, 3 H, J = 6.6, CH3), 1.25 (m, 6 H, OCH2CH3 and CH3), 1.70–1.90 (m, 4 H, 2 × CH2), 2.19 (s, 3 H,CH3CO), 1.57 (d, 1 H, J = 13.5) 2.36 (d, 1 H, J = 13.5), 3.38–3.70 (m, 4 H, 2 × CH2N), 3.89 (q, 1 H, J = 6.6, CH)4.12 (q, 2 H, J = 7.1, OCH2CH3); 13C NMR (125 MHz, CDCl3) δ: 14.5, 15.6, 22.5, 28.3, 36.0, 37.0, 40.7, 41.1, 47.3, 58.4, 61.0, 79.1, 81.0, 155.5, 210.3; IR (film) cm−1: 2977, 2937, 1705, 1701, 1698, 1472, 1455, 1434, 1365, 1356, 1274, 1237; MS (Cl) m/z 284.1845 (284.1860 calcd for C15H25NO4, MH). Anal. Calcd for C15H25NO4: C, 63.58; H,8.89; N, 4.94. Found: C, 63.38; H, 8.87; N, 4.88.12. The enantiomeric excess of the product is >96%. This was determined by treating a sample of the ketone with sodium borohydride/methanol (NaBH4/MeOH) (23°C) and separating the resulting 3:2 mixture of alcohol diastereomers by flash chromatography (silica gel, 2:3 ethyl acetate-hexane). The major alcohol diastereomer wasconverted to its Mosher ester4 [2.5 eq of (+)-α-methoxytrifluoromethylphenylacetic acid, 3 eq of dicyclohexylcarbodiimide, and 0.2 eq of 4-(dimethylamino)pyridine, CH2Cl2] and the crude esterification reaction mixture was analyzed using 500 MHz 1H NMR. None of the minor diastereomer was observed while doping experiments established that 2% would have been detected [diagnostic signals: δ 1.80 (δ, J = 13.4, major ester diastereomer); δ 1.82 (δ, J = 14.1, minor ester diastereomer)].Waste Disposal InformationAll toxic materials were disposed of in accordance with "Prudent Practices in the Laboratory"; National Academy Press; Washington, DC, 1995.3. DiscussionThis procedure illustrates a fundamentally new method for constructing substituted tetrahydrofurans.5,6,7,8,9,10 This practical method assembles the tetrahydrofuran ring from allylic diol and carbonyl components and in the process forms three ring bonds: C(2)-C(3), C(4)-C(5) and O-C(5). Both aldehydes (eq 1) and ketones (illustrated in the present procedure) can be employed as the carbonyl component. Although it is often convenient to isolate the acetal intermediate, conversion to the 3-acyltetrahydrofuran can also be accomplished in many cases by the direct reaction of the diol and carbonyl components.8 High cis stereoselectivity (at least 20:1) is observed in the preparation of tetrahydrofurans that contain single side chains at carbons 2 and 5 (eq. 1). The kinetically controlled product also has the cis relationship of these side chains and the 3-acyl substituent.A definitive feature of this highly stereoselective new route to substituted tetrahydrofurans is that both syn and anti allylic diol stereoisomers typically afford identical tetrahydrofuran products. Thus, there is no need for stereoselective construction of the allylic diol reaction partner. The construction of substituted tetrahydrofurans in high enantiomeric purity from non-racemic allylic diol precursors has also been established.5,7 The rearrangement illustrated in eq. 2 is the key step in a recent synthesis of (+)-muscarine.The scope and mechanism of the SnCl4-promoted rearrangement of allylic acetals have been investigated in detail and these studies provide considerable guidance for using this new tetrahydrofuran synthesis.5,6,7,8,9 Three major limitations emerge from studies conducted to date: (1) When the tetrahydrofuran construction involves a ketone, and thus forms a quaternary center at C(5), allylic diols with alkene substituents more nucleophilic than terminal vinyl rearrange in highest yield. (2) Allylic acetals that are reluctant to ring open in the presence of acid catalysts to generate oxocarbenium ions often undergo decomposition, rather than conversion to acyltetrahydrofuran products. (3) Allylic acetals that form highly stabilized oxocarbeniums (e.g., cinnamaldehyde-derived acetals) do not undergo conversion to 3-acyltetrahydrofurans.This procedure illustrates the asymmetric synthesis of a spirobicyclic tetrahydrofuran from the reaction of readily available (S)-3-[[(1,1-dimethylethyl)diphenylsilyl]oxy]-2-butanone2 with cyclic ketones. The specific example describedargon (7440-37-1)sodium borohydride (16940-66-2)dicyclohexylcarbodiimide (538-75-0)tributylamine (102-82-9)trifluoroacetic anhydride (407-25-0)p-toluenesulfonic acid (104-15-4)ethyl acetate-hexane (2639-63-6)acetal carbon (463-57-0)Tetrabutylammonium fluoride (429-41-4)2-Bromopropene (557-93-7)4-(dimethylamino)pyridine (1122-58-3)tert-Butyllithium (594-19-4)(+)-α-methoxytrifluoromethylphenylacetic acid (56135-03-6)(2S,3S)-3-Acetyl-8-carboethoxy-2,3-dimethyl-1-oxa-8-azaspiro[4.5]decane (155534-75-1) 3-(S)-[(tert-Butyldiphenylsilyl)oxy]-2-butanone,(S)-3-[[(1,1-dimethylethyl)diphenylsilyl]oxy]-2-butanone (135367-18-9)tert-butyldiphenylsilyl1-Carbethoxy-4-piperidone (29976-53-2)isobutyraldehyde acetal3-methyl-4-pentene-2,3-diolCopyright © 1921-2007, Organic Syntheses, Inc. All Rights Reserved。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Organic Syntheses, Coll. Vol. 4, p.780 (1963); Vol. 35, p.91 (1955).2-PHENYLCYCLOHEPTANONE[Cycloheptanone, 2-phenyl-]Submitted by C. David Gutsche and Herbert E. Johnson1.Checked by N. J. Leonard and F. P. Hauck, Jr..1. ProcedureA. Ethyl N-benzylcarbamate. A 12-l. three-necked flask fitted with a sturdy Hershberg-type stirrer and two 1-l. addition funnels is immersed in an ice bath and charged with 1 kg. (9.33 moles) of benzylamine, 500 ml. of ice water, and 1.5 kg. of chopped ice. To the stirred mixture 525 g. (4.83 moles) of ethyl chlorocarbonate is added dropwise while the temperature is maintained at 10–15° (1.0–1.5 hours) (Note 1). An additional 500 ml. of water and 1 kg. of chopped ice are then added to the flask, and a second 525-g. portion (4.83 moles) of ethyl chlorocarbonate is introduced. Simultaneously with this, an ice-cold solution of 400 g. (10 moles) of sodium hydroxide in 1.3 l. of water is added dropwise at such a rate that equal fractions of the ethyl chlorocarbonate and sodium hydroxide solutions are introduced over equal periods of time, the temperature being maintained throughout at 10–15° (2.5–3.0 hours). The reaction mixture is stirred for an additional 30 minutes and is then filtered through a Büchner funnel. The solid product is washed with copious amounts of cold water and is air-dried to yield 1.6 kg. (96%) of glistening white crystals, m.p. 45–47°.B. Ethyl N-nitroso-N-benzylcarbamate(Note 2). In a 12-l. three-necked flask fitted with a thermometer, a 2-l. addition funnel (Note 3), and a gas outlet tube are placed a solution of 360 g. (2.0 moles) of ethyl N-benzylcarbamate in 2 l. of ether and a solution of 1.2 kg. (17.4 moles) of sodium nitrite in 2 l. of water. A stirrer is not used. The reaction mixture is cooled by means of a water bath to 20° and treated with a solution of 1 l. each of concentrated nitric acid and water, contained in the addition funnel. Enough of this solution is added to impart a permanent green color to the aqueous layer, and the remainder is then added over a period of 5 hours at such a rate as to keep the aqueous phase green (Note 4) and the temperature at 25–30°. The reaction mixture is allowed to stand an additional 30 minutes, and the layers are separated. The ether layer is washed with 200-ml. portions of 10% potassium carbonate solution (Note 5) until the evolution of gas ceases and is then dried over anhydrous potassium carbonate. The ether is removed under vacuum on a water bath kept below 50° (Note 6), a residue of 400–415 g. (95–99%) of a bright orange oil (Note 7) being left.C. 2-Phenylcycloheptanone. In a 2-l. three-necked flask fitted with a 500-ml. addition funnel, a sealed Hershberg stirrer, and a reflux condenser (Note 8) are placed 392 g. (4.0 moles) of freshly distilled cyclohexanone, 30 g. of finely powdered potassium carbonate, and 400 ml. of absolutemethanol . To the stirred mixture is added 415 g. (2.0 moles) of ethyl N-nitroso-N-benzylcarbamate over a period of 1.5 hours during which time the reaction temperature is maintained at 25° by means of an ice-water bath. The dark red reaction mixture is then allowed to stand at room temperature until the evolution of nitrogen has ceased (24–28 hours) (Note 9). The solid material is removed by filtration, the lower-boiling materials are removed by evaporation under reduced pressure on the steam bath (Note 10), and the residue is distilled through an efficient column. A fore-run consisting of 30–60 g. of material is discarded or refractionated (Note 10), and the fraction with b.p. 94–96°/0.4 mm. (124–126°/2 mm., 136–138°/4 mm.) is collected. It amounts to 155–177 g. (41–47%) of 2-phenylcycloheptanone , n 20 1.5395–1.5398, which is pure enough for most purposes, but which may be purified further by recrystallization from petroleum ether (b.p. 30–60°) and obtained as colorless, very long needles; m.p. 21–23° (Note 11).2. Notes1. During this time ethyl N-benzylcarbamate begins to separate from solution as a white solid.2. Although the benzyl nitroso compound appears to be a much less active vesicant than the methyl nitroso compound, it is, nevertheless, a wise precaution to wear heavy rubber gloves during the isolation of this product.3. The stem of the addition funnel should reach to the bottom of the flask.4. The color may appear yellow green, emerald green, or blue-green, depending upon the size of the run, the amount of nitric acid that has been added, and the room lighting.5. Seven to nine portions of carbonate solution are sufficient if each portion is shaken very thoroughly with the ether solution. Caution should be observed because of pressure build-up in the separatory funnel!6. Ethyl N-nitroso-N-benzylcarbamate is heat sensitive and, if the temperature is too high, may detonate violently . The submitters state that attempts to distil the nitroso compound under high vacuum have resulted in explosions.7. The submitters state that the nitroso compound is stable at low temperature and can be stored in a refrigerator for several months or longer with no signs of deterioration.8. The reflux condenser is an optional but convenient appendage for the third neck of the flask. To follow the evolution of nitrogen during the reaction, the exit from the condenser can be led either to a eudiometer tube (theoretical nitrogen evolution about 50 l. for the experiment described) or to a bubbler.9. It is necessary to allow the reaction mixture to stand for a rather prolonged period, since about 40% of the nitrogen is evolved during this time. 10. The lower-boiling material includes methyl benzyl ether , which may be isolated, by careful fractionation through an efficient column, in about 25% yield, b.p. 74–77°/30 mm. 11. In a similar fashion the following 2-arylcycloheptanones have been prepared by the submitters:3. DiscussionEthyl N-benzylcarbamate and its nitroso compound have been prepared by methods similar to those described for ethyl N-methylcarbamate and its nitroso compound.2,3 2-Phenylcycloheptanone has been prepared by the reaction of ethyl N-nitroso-N-benzylcarbamate 4 with cyclohexanone ,5 by the reaction of phenyldiazomethane with cyclohexanone ,6 by the reaction of ethyl N-nitroso-N-methylcarbamate with 2-phenylcyclohexanone ,5 and by the rearrangement of 1-phenyl-2-cyclohexylethylene oxide .7 References and Notes Yield, %Melting Point, or Refractive Index at 25°2-(o -Methylphenyl)cycloheptanone29 1.53482-(p -Methylphenyl)cycloheptanone2657–58°2-(o -Methoxyphenyl)cycloheptanone7 1.54072-(m -Methoxyphenyl)cycloheptanone42 1.54182-(p -Methoxyphenyl)cycloheptanone 2058–59°D1.Washington University, St. Louis, Missouri.. Syntheses Coll. Vol.2, 278 (1943).. Syntheses Coll. Vol.2, 464 (1943).4.v. Pechmann, Ber., 31, 2640 (1898).5.Gutsche, J. Am. Chem. Soc., 71, 3513 (1949); Gutsche and Johnson, J. Am. Chem. Soc., 77, 109(1955).6.Burger, Walter, Bennet, and Turnbull, Science, 112, 306 (1950); Gutsche and Jason, J. Am.Chem. Soc., 78, 1184 (1956).7.Tiffeneau, Weill, Gutmann, and Tchoubar, Compt. rend., 201, 277 (1935).AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)petroleum etherpotassium carbonate (584-08-7)methanol (67-56-1)ether (60-29-7)sodium hydroxide (1310-73-2)nitric acid (7697-37-2)Cyclohexanone (108-94-1)nitrogen (7727-37-9)sodium nitrite (7632-00-0)ethyl chlorocarbonate (541-41-3)benzylamine (100-46-9)Ethyl N-methylcarbamate (105-40-8)ethyl N-nitroso-N-methylcarbamate (615-53-2)2-Phenylcycloheptanone,Cycloheptanone, 2-phenyl- (14996-78-2)Ethyl N-benzylcarbamate (2621-78-5)Ethyl N-nitroso-N-benzylcarbamate (6558-76-5)methyl benzyl ether (538-86-3)phenyldiazomethane(766-91-6)2-phenylcyclohexanone (1444-65-1)1-phenyl-2-cyclohexylethylene oxide2-(o-Methylphenyl)cycloheptanone2-(p-Methylphenyl)cycloheptanone2-(o-Methoxyphenyl)cycloheptanone2-(m-Methoxyphenyl)cycloheptanone2-(p-Methoxyphenyl)cycloheptanone Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
