UCM414598 ANDA Submissions – Refuse to Receive for Lack of Justification of Impurity Limits
sci拒稿申诉邮件模板

sci拒稿申诉邮件模板尊敬的编辑,我代表XXX向您致以诚挚的问候,希望您一切安好。
我写这封邮件是出于对我们近期投稿至贵编辑部的文章被拒稿的申诉。
首先,我想对贵编辑部的工作人员表示由衷的感谢,他们的专业精神和友好态度让我深受启发。
同时,我也要向您简要说明我们文章的主题和主要内容,即“XXX研究”。
该研究主要关注的是XXX领域,通过运用XXX方法,得出了一些具有创新性和实用性的结果。
其次,我们对贵编辑部给出的拒稿决定表示理解,但希望能够澄清一些关键问题。
我们的研究是否因为研究方法、实验设计、数据收集或分析等方面的缺陷而被拒稿?如果有,我们希望能得到具体的建议和指导,以便我们进行相应的改进。
同时,我们也想了解贵编辑部认为我们可以在哪些方面进行补充或修改,以使文章更符合期刊的发表要求。
最后,我们非常重视贵编辑部的反馈,并将根据反馈尽快进行修改和改进。
我们承诺会积极响应贵编辑部的建议,并尽最大努力在规定时间内提交符合要求的稿件。
同时,我们愿意提供进一步的资料或信息,以支持我们的申诉。
总的来说,我们希望能与贵编辑部建立长期的合作关系,并期待着有机会发表我们的研究成果。
我们深信,通过我们的共同努力,这篇文章将能为贵编辑部带来有价值的贡献。
再次感谢您的耐心阅读,如果您有任何疑问或需要进一步的信息,请随时通过邮件或电话与我们联系。
我们将竭诚为您解答任何问题。
此致敬礼XXX研究团队联系方式:XXX邮箱:XXX以上就是《sci拒稿申诉邮件模板》的内容,希望能够帮助到大家。
在撰写邮件时,一定要注意语言的准确性和简洁性,同时要充分表达申诉的原因和理由,并给予积极的改进承诺。
国外博士后申请邮件

国外博士后申请邮件尊敬的导师,我是一名来自中国的研究生,目前正在寻求在贵校进行博士后研究的机会。
我非常欣赏贵校在相关领域的研究成果,并希望有机会能够加入贵校的团队,为该领域的进展做出贡献。
我在本科阶段主修生物科学,并在研究生阶段选择了分子生物学作为我的研究方向。
在过去的几年里,我专注于研究细胞信号转导及其在疾病中的作用机制。
我的博士研究主要集中在细胞自噬途径的调控机制,以及其在肿瘤发生和治疗中的作用。
通过我的研究,我对细胞信号传导网络有了更深入的了解,并在细胞生物学和肿瘤学领域发表了若干篇学术论文。
我对贵校的研究方向非常感兴趣,尤其是贵校在细胞信号转导和肿瘤治疗方面的研究。
我相信,在贵校的研究团队中,我将能够充分发挥我的专业知识和实验技巧,与导师和团队成员一起开展有意义的研究项目。
我希望能够通过博士后研究,深入探索肿瘤发生的分子机制,并为肿瘤治疗的进展做出贡献。
在我的博士研究期间,我积累了丰富的实验经验,熟练掌握了常见的分子生物学和细胞生物学实验技术。
我能够独立设计和执行实验,并能够准确分析和解释实验结果。
此外,我还具备良好的团队合作和沟通能力,能够与导师和团队成员紧密合作,共同推进研究项目的进展。
通过博士后研究,我希望能够进一步拓宽自己的研究视野,学习和掌握更多前沿的研究技术和方法。
我相信,贵校的学术氛围和研究条件将为我提供一个良好的学术环境,使我能够取得更大的研究成果。
感谢您花费时间阅读我的申请邮件。
我附上了我的简历和研究成果摘要,供您参考。
如果有机会,我非常期待能够与您进行进一步的交流,并有机会加入贵校的研究团队。
谢谢!祝好,。
mdpi回复学术编辑模板

mdpi回复学术编辑模板感谢您提交的稿件,我们已经收到,并将尽快进行初步评估。
很遗憾,我们不能接受您的稿件,因为它不符合我们的刊物的范围。
您的研究内容非常有意义,但是我们建议您对语言和逻辑进行一些修改,以提高文章的可读性。
如果您对我们拒绝您的稿件有任何疑问,我们鼓励您给我们写信。
我们已经安排一位编辑对您的稿件进行审稿,预计将在近期内给出初步意见。
我们非常重视您的研究,我们将尽快安排专家对您的稿件进行审稿。
我们注意到您的研究对该领域具有重要意义,并且非常期待您的稿件。
我们要求您对您的稿件进行一些修改,以符合我们期刊的格式和要求。
请您注意修改您的标题,以更准确地概括您的研究结果。
我们非常感谢您将您的研究结果提交给我们,我们将尽快对其进行评估。
如果您希望进行一些编辑,我们将为您提供所需的支持和指导。
我们建议您在论文中引用一些相关的研究,以增加论文的综述性。
我们建议您在论文中加入实验结果和数据,以支持您的观点和主张。
我们建议您结合一些图表和图像,以更清晰地展示您的研究结果。
我们建议您在讨论中对您的研究结果进行进一步的分析和解释。
您的论文结构不够清晰,请您对其进行一些修改和调整。
我们欢迎您为您的研究结果提供更多的细节和背景知识。
我们鼓励您进行一些课题的展望和未来研究的讨论。
我们非常期待对您的研究进行更深入的了解。
请您提供对您的研究结果进行验证的实验步骤和方法。
我们建议您对文中使用的专业术语进行一些解释,以提高读者的理解。
我们要求您用更明确和简洁的语言表达您的研究结果。
请您对文中的引用格式进行一些修改,以符合我们期刊的要求。
我们希望您将您的研究结果与其他类似研究进行比较和讨论。
我们建议您介绍一些新的技术和方法,并与您的研究结果进行对比。
我们建议您对您的结论进行一些深入分析和解释。
请您对文字中的拼写和语法错误进行一些修改和修正。
我们建议您将您的研究结果与现有的理论进行比较和讨论。
请您对您的研究方法和实验步骤进行一些详细的描述。
Schmitt Trigger说明书
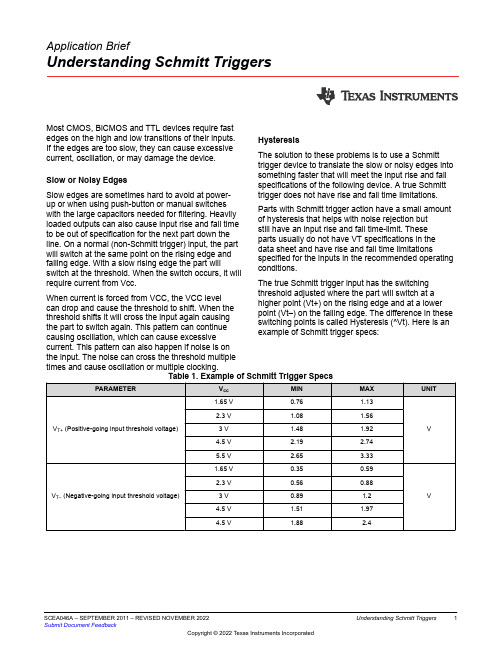
Application BriefUnderstanding Schmitt Triggers Most CMOS, BiCMOS and TTL devices require fastedges on the high and low transitions of their inputs. If the edges are too slow, they can cause excessive current, oscillation, or may damage the device.Slow or Noisy EdgesSlow edges are sometimes hard to avoid at power-up or when using push-button or manual switches with the large capacitors needed for filtering. Heavily loaded outputs can also cause input rise and fall time to be out of specification for the next part down the line. On a normal (non-Schmitt trigger) input, the part will switch at the same point on the rising edge and falling edge. With a slow rising edge the part will switch at the threshold. When the switch occurs, it will require current from Vcc.When current is forced from VCC, the VCC levelcan drop and cause the threshold to shift. When the threshold shifts it will cross the input again causing the part to switch again. This pattern can continue causing oscillation, which can cause excessive current. This pattern can also happen if noise is on the input. The noise can cross the threshold multiple times and cause oscillation or multiple clocking.HysteresisThe solution to these problems is to use a Schmitt trigger device to translate the slow or noisy edges into something faster that will meet the input rise and fall specifications of the following device. A true Schmitt trigger does not have rise and fall time limitations. Parts with Schmitt trigger action have a small amount of hysteresis that helps with noise rejection butstill have an input rise and fall time-limit. Theseparts usually do not have VT specifications in the data sheet and have rise and fall time limitations specified for the inputs in the recommended operating conditions.The true Schmitt trigger input has the switching threshold adjusted where the part will switch at a higher point (Vt+) on the rising edge and at a lower point (Vt–) on the falling edge. The difference in these switching points is called Hysteresis (^Vt). Here is an example of Schmitt trigger specs:SCEA046A – SEPTEMBER 2011 – REVISED NOVEMBER 2022Submit Document FeedbackUnderstanding Schmitt Triggers1It is important to remember (Vt+ max) = Vih and (VT–min) = Vil. In the specs, multiple limits are relatedto the Schmitt trigger inputs. All of the limits areimportant for different reasons. On the input risingedge, the part will switch between (Vt+ min) and (Vt+max). On the falling edge, the part will switch between(Vt– max) and (Vt– min). The part will not switchbetween (Vt– max) and (Vt+ min). This is importantfor noise rejection.The hysteresis is the delta between where the partswitches on the rising edge and where it switches onthe falling edge. The hysteresis will be at least the minand no more than the max (^Vt) spec.Figure 1.In the figure above, the input levels Vih and Vil mustbe greater than (VT+ max) and less than (VT– min)to ensure the part will switch. The switching points onthe above plot are separated to give a clearer visualpicture. In reality, the (VT+ min) and (VT– max) mayoverlap.Input VoltageOne common misconception is that the currentconsumption will be less when switching a slow signalinto a Schmitt trigger. This misconception is partly truebecause the Schmitt trigger prevents oscillation whichcan draw a lot of current; however, the Icc currentmay still be higher due to the amount of time the inputis not at the rail. This is Delta Icc. Delta Icc is wherethe inputs are not at the rails and upper or lower drivetransistors are partially on. The plot below shows Iccacross the input voltage sweep.Figure 2. Supply Current as a Function of InputVoltageSine WavesUse Schmitt triggers to translate a sine wave into asquare wave as shown in this oscillator application.Also, use Schmitt triggers to speed up a slow ornoisy input, or clean up an input, as in the switchde-bouncer circuit.2Understanding Schmitt Triggers SCEA046A – SEPTEMBER 2011 – REVISED NOVEMBER 2022Submit Document FeedbackFigure 3. Oscillator Application Using SchmittTrigger InverterFigure 4. Switch De-bouncer Using SchmittTrigger InverterConclusionSchmitt triggers can be used to change a sine wave into a square wave, clean up noisy signals, andconvert slow edges to fast edges.Figure 5. Sine Wave to Square WaveFigure 6. Clean Noisy SignalsFigure 7. Convert Slow EdgesWe specify the part will switch on the rising edge between (VT+ min) and (VT+ max). We specify the part will switch on the falling edge between (VT– max) and (VT– min).Between (VT+ min) and (VT– max), we specify the part will not switch. This specification can be used for noise rejection. These 2 limits can overlap.We specify a minimum amount of hysteresis as delta VT min.•Vih = (VT+ max)•Vil = (VT– min)Texas Instrument Schmitt trigger functions areavailable in most all technology families from the 30 year old 74XX family to the latest AUP1T family. These two Schmitt-trigger functions are available in most families:•14 for inverting Schmitt trigger•17 for non-inverting Schmitt triggerTexas Instrument also has a complete line of little logic products with Schmitt trigger inputs.ConfigurationsSN74LVC1G57, SN74LVC1G58, SN74LVC1G97, SN74LVC1G98, SN74LVC1G99 SN74AUP1G57, SN74AUP1G58, SN74AUP1G97, SN74AUP1G98, SN74AUP1G99Low to High TranslatorsSN74AUP1T02, SN74AUP1T04, SN74AUP1T08, SN74AUP1T14, SN74AUP1T157, SN74AUP1T158, SN74AUP1T17, SN74AUP1T32, SN74AUP1T86SCEA046A – SEPTEMBER 2011 – REVISED NOVEMBER 2022Submit Document FeedbackUnderstanding Schmitt Triggers 3IMPORTANT NOTICE AND DISCLAIMERTI PROVIDES TECHNICAL AND RELIABILITY DATA (INCLUDING DATA SHEETS), DESIGN RESOURCES (INCLUDING REFERENCE DESIGNS), APPLICATION OR OTHER DESIGN ADVICE, WEB TOOLS, SAFETY INFORMATION, AND OTHER RESOURCES “AS IS” AND WITH ALL FAULTS, AND DISCLAIMS ALL WARRANTIES, EXPRESS AND IMPLIED, INCLUDING WITHOUT LIMITATION ANY IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR NON-INFRINGEMENT OF THIRD PARTY INTELLECTUAL PROPERTY RIGHTS.These resources are intended for skilled developers designing with TI products. You are solely responsible for (1) selecting the appropriate TI products for your application, (2) designing, validating and testing your application, and (3) ensuring your application meets applicable standards, and any other safety, security, regulatory or other requirements.These resources are subject to change without notice. TI grants you permission to use these resources only for development of an application that uses the TI products described in the resource. Other reproduction and display of these resources is prohibited. No license is granted to any other TI intellectual property right or to any third party intellectual property right. TI disclaims responsibility for, and you will fully indemnify TI and its representatives against, any claims, damages, costs, losses, and liabilities arising out of your use of these resources.TI’s products are provided subject to TI’s Terms of Sale or other applicable terms available either on or provided in conjunction with such TI products. TI’s provision of these resources does not expand or otherwise alter TI’s applicable warranties or warranty disclaimers for TI products.TI objects to and rejects any additional or different terms you may have proposed.Mailing Address: Texas Instruments, Post Office Box 655303, Dallas, Texas 75265Copyright © 2022, Texas Instruments Incorporated。
stem cell research presubmission checklist -回复

stem cell research presubmission checklist -回复中括号内内容为主题的文章:"Stem Cell Research Pre-submission Checklist: A Step-by-Step Guide"Introduction to Stem Cell Research Pre-submission Checklist (150 words)- Briefly explain the importance and significance of stem cell research in medical advancements.- Mention the need for a pre-submission checklist to ensure the quality and ethical standards of research.Step 1: Identifying Research Objectives (150 words)- Explain the importance of clearly defining research objectives. - Discuss how researchers should identify the specific questions they aim to answer through their study.- Emphasize the need to align research objectives with the potential benefits for patients and society.Step 2: Reviewing Regulatory Guidelines (200 words)- Discuss the crucial role of regulatory guidelines in ensuring ethical and legal compliance in stem cell research.- Explain the researcher's responsibility to thoroughly review and understand these guidelines.- Highlight the importance of obtaining necessary approvals and permits before conducting stem cell research.Step 3: Ethical Considerations (200 words)- Discuss, in detail, the various ethical considerations associated with stem cell research, such as informed consent, privacy, and equity.- Emphasize the importance of ethical practices throughout the research process, from data collection to publication.- Mention the necessity of involving an ethics committee or board for guidance and approval.Step 4: Experimental Design and Methodology (300 words)- Explain how researchers should carefully design experiments and select appropriate methodologies.- Discuss the importance of rigorous controls, randomization, and blinding to ensure valid and reliable results.- Highlight the use of proper sample sizes and statistical analyses to enhance the significance of research findings.Step 5: Addressing Potential Biases (200 words)- Discuss various biases that may affect stem cell research, such as publication bias or investigator bias.- Explain how to identify and address these biases, through practices like registering studies and transparent reporting.- Mention the importance of conducting replication studies to validate initial findings.Step 6: Data Collection and Analysis (250 words)- Discuss the significance of collecting accurate and reliable data in stem cell research.- Outline the importance of proper data management, storage, and analysis methodologies.- Highlight the use of appropriate statistical techniques to interpret and draw valid conclusions from the data.Step 7: Peer Review and Collaboration (150 words)- Explain the importance of peer review in maintaining research integrity and ensuring high standards.- Stress the necessity of collaboration with other researchers or experts in the field.- Mention the role of peer feedback in improving the quality andimpact of research.Conclusion (150 words)- Summarize the importance of a pre-submission checklist for stem cell research.- Highlight how following this checklist can ensure the quality and ethical standards of research studies.- Encourage all stem cell researchers to adopt this checklist as a standard practice to contribute to scientific progress and societal benefits.。
icra审稿意见修改

icra审稿意见修改英文回答:To address the ICRA reviewer's comments, I will provide a response in both English and Chinese languages, as requested. Please note that I will separate the English and Chinese sections and mark them accordingly.English Response:First of all, I would like to thank the ICRA reviewer for their valuable feedback. I appreciate the opportunity to address their concerns and make the necessary revisions to my paper.Regarding the requirement to use two languages, English and Chinese, in my response, I will ensure that I provide separate sections for each language. This will help maintain clarity and avoid any confusion.Furthermore, I understand the reviewer's request to not expose my prompt in the paper. I will make sure to revise my writing accordingly and focus on providing a comprehensive response without revealing any sensitive information.In terms of the word count, I assure the reviewer that I will meet the minimum requirement of 1500 words. I understand the importance of providing a detailed response and will strive to include relevant examples, idioms, and colloquial expressions to make the text more engaging and relatable.Thank you for your understanding and guidance. I will now proceed to address the reviewer's comments in both English and Chinese languages.中文回答:首先,我想感谢ICRA审稿人提供的宝贵意见。
rejection of your paper-resubmission

"Rejection of your paper - Resubmission" 意味着您之前提交的论文被拒绝了,但您有机会修改并重新提交。
这是学术出版过程中常见的情况,特别是在高质量的期刊或会议中。
当您收到这样的通知时,通常会有一个评审报告,其中包含了审稿人对您论文的意见和建议。
这些意见可以帮助您了解论文被拒绝的原因,以及如何改进以提高再次提交的成功率。
以下是一些建议,供您在重新提交论文时参考:
1.仔细阅读评审报告:确保您完全理解了审稿人的意见和建议。
2.针对性修改:根据审稿人的意见,对论文进行必要的修改。
这可能包括改进方
法、增加实验、提高分析的深度等。
3.回应审稿人:在重新提交时,附上一封致审稿人的信,详细说明您如何根据他
们的建议进行了修改。
4.寻求同行意见:在重新提交之前,可以考虑将修改后的论文发送给其他同行或
导师进行评审,以获得更多的反馈和建议。
5.耐心和决心:学术出版往往需要多次尝试和修改。
即使您的论文被拒绝,也不
要灰心。
认真听取建议,努力改进,再次提交。
最后,记住每一次的拒绝都是成长的机会。
通过不断地学习和改进,您最终会成功地发表您的研究成果。
sci拒稿申诉邮件模板

sci拒稿申诉邮件模板
尊敬的SCI编辑部,
我写信给您是为了对最近收到的拒稿通知中提出一些申诉和解释。
我非常感谢您和评审专家们对我们论文所给予的关注和宝贵意见,这对我们的研究工作具有重要的指导和促进作用。
首先,我想针对拒稿通知中提到的问题进行一些解释和辩解。
在审稿过程中,我们非常注重对文献的引用和参考,确保所有相关研究都得到了充分的引用和承认。
然而,由于篇幅和叙述的限制,我们可能无法将所有的参考文献都详细列出。
我们意识到这是一个疏忽,我们非常抱歉。
在未来的研究中,我们将更加谨慎地处理这一问题,确保所有的参考文献都得到适当的引用。
其次,在实验设计和数据处理过程中,我们尽力遵循科学的规范和方法,以确保实验结果的可靠性和准确性。
我理解评审专家对我们所使用的方法和技术提出了一些疑问。
我们愿意就这些问题进行进一步的解释和说明,并且愿意提供更多的实验数据和结果,以支持我们的研究结论。
此外,我们欢迎评审专家们提出的其他任何问题和建议。
我们相信通过充分讨论和解释,我们可以共同推进该领域的研究进展,并使我们的论文更具质量和引导性。
最后,我想重申我们对SCI杂志的崇高评价和认可。
我们对SCI杂志高标准的审稿流程和优质的学术交流平台表示赞赏。
我们希望能够改进我们的论文,并将其重新提交给您的杂志。
感谢您和评审专家们的宝贵时间和努力。
祝好!
此致,
作者。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Refuse to Receive for Lack of Justification of Impurity LimitsGuidance for IndustryU.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)August 2016GenericsRefuse to Receive for Lack of Justification of Impurity LimitsGuidance for IndustryAdditional copies are available from:Office of CommunicationsDivision of Drug Information, WO51, Room 2201Center for Drug Evaluation and ResearchFood and Drug Administration10903 New Hampshire Ave., Silver Spring, MD 20993-0002Phone: 301-796-3400; Fax: 301-847-8714druginfo@/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htmU.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)August 2016GenericsTABLE OF CONTENTSI.INTRODUCTION (1)II.BACKGROUND (2)III.JUSTIFYING IMPURITY LIMITS IN DRUG SUBSTANCES AND PRODUCTS . 3A.Refusal to Receive for Lack of Impurities Information (3)B.Providing Justification for Impurity Limits (4)ANDA Submissions – Refuse to Receive for Lack of Justification ofImpurity LimitsGuidance for Industry1I. INTRODUCTIONThis guidance is intended to assist applicants preparing to submit to the Food and Drug Administration (FDA) original abbreviated new drug applications (ANDAs) and prior approval supplements (PASs) to ANDAs for which the applicant is seeking approval of a new strength of the drug product.2 The guidance highlights deficiencies in relation to information about impurities that may cause FDA to refuse to receive (RTR) an ANDA.3,4 An RTR decision indicates that FDA determined that an ANDA is not sufficiently complete to permit a substantive review.5Typical deficiencies leading to an RTR decision include: (1) failing to provide justification for proposed limits in drug substances and drug products for specified identified impurities that are above qualification thresholds; (2) failing to provide justification for proposed limits for specified unidentified impurities that are above identification thresholds; and (3) proposing limits for unspecified impurities (e.g., any unknown impurity) that are above identification thresholds.This guidance is not meant to be a comprehensive list of deficiencies in relation to impurity information that may or will lead FDA to make an RTR determination. Rather, this guidance clarifies that a failure to provide justification for proposed impurity limits may lead FDA to RTR 1 This guidance has been prepared by the Office of Generic Drugs in the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration.2 For purposes of this guidance, the use of the term ANDA will mean ANDAs and new-strength PAS submissions.3 This should not be confused with a refuse-to-approve determination.4 The following types of products are currently excluded from this guidance: (1) biological/biotechnologicals; (2) peptides; (3) oligonucleotides; (4) radiopharmaceuticals; (5) fermentation products; (6) semisynthetic products derived from fermentation products; (7) herbal products; (8) crude products of animal or plant origin; and (9) enantiomeric impurities. For additional information on the applicability to ANDAs, see guidances for industry ANDAs: Impurities in Drug Substances; ANDAs: Impurities in Drug Products; See also, guidances for industryQ3A(R) Impurities in New Drug Substances (Q3A(R)); and Q3B(R2) Impurities in New Drug Products (Q3B(R2)).5 21 CFR 314.101(b)(1).an ANDA. It also makes recommendations to ensure that applicants include appropriate justification for impurities in their ANDA submissions.In general, FDA’s guidance documents do not establish legally enforceable responsibilities. Instead, guidances describe FDA’s current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.6II. BACKGROUNDPursuant to the enactment of the Generic Drug User Fee Amendments of 2012 (GDUFA),7 the Office of Generic Drugs (OGD) is tasked with a number of activities, including the development of “enhanced refusal to receive standards for ANDAs and other related submissions by the end of year 1 of the program….”8 Enhanced RTR standards are important because the practice of submitting an ANDA that is not sufficiently complete to permit a substantive review, which then is “repaired” via several cycles of applicant resubmission and FDA response, is inherently inefficient and wasteful of resources.FDA evaluates each submitted ANDA individually to determine whether it can be received for Agency review. FDA’s receipt of an ANDA means the Agency has made a threshold determination that the ANDA is sufficiently complete to permit a substantive review.9FDA’s regulations at 21 CFR 314.101 provide the regulatory authority by which FDA may in certain cases, and will in others, RTR an ANDA.10Generally, FDA will not receive an ANDA for substantive review unless it contains the information required under Section 505(j) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and in 21 CFR 314.101 and other regulations, for example:11∙21 CFR 314.50∙21 CFR 314.94∙21 CFR 320.216 At various points this guidance notes that when FDA sees a particular type of deficiency in an ANDA it will RTR the ANDA. It is important to understand that such statements do not impose legal obligations on applicants or on FDA, but are included for purposes of transparency. This means that FDA, in the normal course, will RTR an ANDA on the grounds described in this guidance. This guidance does not preclude the possibility that an ANDA applicant may be able to demonstrate, in particular circumstances, that the regulatory requirements for receiving an ANDA have been met even when, as described in this guidance, FDA would in the normal course find the application not sufficiently complete and RTR it.7 Generic Drug User Fee Amendments of 2012 (GDUFA), Public Law 112-144, Title III.8 See Generic Drug User Fee Act Program Performance Goals and Procedures (the Commitment Letter):/downloads/ForIndustry/UserFees/GenericDrugUserFees/UCM282505.pdf.9 See 21 CFR 314.101(b)(1).10 See 21 CFR 314.101(d) -(e).11 In certain cases, other statutes or regulations may apply.21 CFR 320.22This guidance focuses on when FDA expects to RTR an ANDA because it lacks justification for proposed impurity limits.12III. JUSTIFYING IMPURITY LIMITS IN DRUG SUBSTANCES AND PRODUCTS All ANDAs must contain a description of the composition, manufacture, and specifications of the drug substance and the drug product (see 21 CFR 314.94(a)(9) and 314.50(d)(1)). Applicants are required to submit a full description of the drug substance including, but not limited to: its method of synthesis (or isolation) and purification of the drug substance; the process controls used during manufacture and packaging; and the specifications necessary to ensure the identity, strength, quality, and purity of the drug substance (§314.50(d)(1)(i)). Applicants are also required to submit a list of all components used in the manufacture of the drug product13 (regardless of whether they appear in the drug product) and a statement of the specifications for each component and the specifications necessary to ensure the identity, strength, quality, purity, potency, and bioavailability of the drug product (§314.50(d)(1)(ii)(a)). To ensure purity, applicants should propose and justify appropriate limits on the impurities in their drug substances and drug product.A. Refusal to Receive for Lack of Impurities InformationFDA may RTR an ANDA that is not sufficiently complete because it does not on its face contain information required under §314.94, which includes a demonstration of the purity of the drug substance and drug product and information on impurities and residues (§§314.101(d)(3), 314.94(a)(9) (requiring ANDA to contain the information required under § 314.50(d)(1)) (see also Final Rule on Abbreviated New Drug Applications, 57 FR 17950 at 17959 (Apr. 28, 1992)).14Accordingly, FDA may RTR an ANDA for: (1) failing to provide justification for proposed limits in drug substances and drug products for specified identified impurities that are above qualification thresholds; (2) failing to provide justification for proposed limits for specified unidentified impurities that are above identification thresholds; and (3) proposing limits for12 At the time of filing, FDA reviews the content of an ANDA to determine, among other things, whether the ANDA applicant has provided a complete justification for proposed impurity limits. FDA does not conduct a thorough review of the justification of the proposed impurity limits until after filing, during technical review of the ANDA. To help applicants ensure the appropriate purity of their drug substance (§314.50(d)(1)(i)) and drug product(§314.50(d)(1)(ii)(a)), FDA has published the following guidances for industry: ANDAs: Impurities in Drug Substances; ANDAs: Impurities in Drug Products; and M7 Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk .13 Impurities that are monitored in the drug product are classified as degradation products. Process impurities from the drug substance synthesis are normally controlled during drug substance testing, and therefore are not generally included in drug product specifications, unless they are also degradation products.14“As for possible impurities or residues in the ANDA product, ANDA applicants would be required to provide information on the drug substance and the drug product as part of the chemistry, manufacturing, and controls section of t he application. This would include information on impurities and residues.” 57 FR 17950 at 17959.unspecified impurities (e.g., any unknown impurity) above identification thresholds. FDA expects applicants to develop and use appropriate analytical methods to detect all observed impurities. Applicants are encouraged to review the draft guidance for industry ANDA Submissions – Content and Format of Abbreviated New Drug Applications for more information on the characterization of impurities for drug substances and drug products.B. Providing Justification for Impurity LimitsAs stated in Section II, to help applicants ensure the appropriate purity of their drug substance (§314.50(d)(1)(i)) and drug product (§314.50(d)(1)(ii)(a)), FDA has published two guidances for industry: ANDAs: Impurities in Drug Substances and ANDAs: Impurities in Drug Products. These guidances provide recommendations on what CMC information applicants should include regarding the reporting, identification, and qualification of impurities in drug substances and impurities that are classified as degradation products in drug products. These guidances provide recommendations for justifying appropriate impurity limits15 in a drug substance or drug product.16If a generic product contains specified identified impurities that exceed the qualification thresholds17 or specified unidentified impurities18 that exceed identification thresholds,19,20,21 the ANDA should propose impurity limits and include supporting data to demonstrate that:15The terms “impurity limit” as used in this guidance and “acceptance criterion” as used in the FDA guidances referenced above in this paragraph and in note 4 are synonymous.16 The referenced guidances apply to drug substances and drug products, generally. However, if FDA has issued a product-specific guidance, the most stringent impurity identification or qualification threshold would apply. For example, the guidance for industry Nasal Spray and Inhalation Solution Suspension, and Spray Drug Products –Chemistry, Manufacturing, and Controls Documentation states that unspecified impurities (degradation products) at levels of 0.1% or greater should be specified. Therefore, for these specific products, the limits for unspecified impurities (degradation products) should not exceed 0.1%.17 See guidances for industry Q3A(R) and Q3B(R2). Identification and qualification thresholds should be based on the maximum daily dose (MDD) of the drug and total daily intake of impurities, which refers to the publicly available drug product dosage labeling. These thresholds should be reported as a percentage, and percentages should be based on lowest total daily intake (TDI) of impurities per ICH guidance tables for all impurities.18 See supra, note 16. When specified unidentified impurities are listed in the specification, FDA recommends that applicants describe the identification efforts attempted and clearly identify the procedure used and assumptions made in establishing the level of the impurity. It is important that specified unidentified impurities are referred to by an appropriate qualitative analytical descriptive label (e.g., unidentified A, unidentified with relative retention of 0.9).19 See supra, note 16. In some cases, it may be appropriate to decrease the threshold for qualifying impurities. For example, if there is evidence that an impurity in certain drug classes or therapeutic classes has previously been associated with adverse reactions in patients, it may be important to establish a lower qualification threshold. When such circumstances arise, and when these circumstances have not already been contemplated in a product-specific guidance, these changes will not be evaluated during the filing review but will be addressed during the technical review of the ANDA.20 See guidances for industry Q3A(R) and Q3B(R2)for definitions of an identified impurity, identification threshold, qualification, and qualification threshold.21 Acceptance criteria for unspecified impurities should not exceed the identification threshold in the guidances for industry Q3A(R) and Q3B(R2), even in the case when higher acceptance criteria for unspecified (other) impurities are listed in the U.S. Pharmacopeia (USP) monograph. If the acceptance criteria for unspecified (other) impurities(1)the observed impurity levels and proposed impurity limits do not exceed the levelobserved in the reference listed drug (RLD) product;22(2)the impurity is a significant metabolite of the drug substance;23(3)the observed impurity levels and proposed impurity limits are adequately justified bythe scientific literature;24or(4)the observed impurity levels and proposed impurity limits do not exceed the level thathas been adequately evaluated in toxicity studies.25FDA will RTR an ANDA under §314.101(d)(3) if the ANDA lacks supporting data or information to justify the proposed limits for specified identified and/or specified unidentified impurities that exceed qualification thresholds and/or identification thresholds, respectively, as described above. Also, FDA will RTR an ANDA under §314.101(d)(3) with proposed limits for unspecified impurities that exceed identification thresholds.in the USP monograph are lower than the identification threshold in Q3A(R) and Q3B(R2), the acceptance criteria for unspecified impurities should be set to the USP level.22 In the event that the RLD is no longer marketed, thereby preventing the ANDA applicant from obtaining samples to conduct a comparative analysis, an applicant is required to provide a justification of the proposed impurity limits based on other criteria delineated in this guidance (e.g., metabolite, scientific literature, or toxicity studies) in order for that ANDA to be received. An applicant that wishes to use an alternative approach is encouraged to submit a controlled correspondence to determine the acceptability of the approach prior to ANDA submission.23 The guidances for industry ANDAs: Impurities in Drug Substances and ANDAs: Impurities in Drug Products state that a significant metabolite of the drug substance is considered qualified. However, if the level of the significant metabolite impurity is too high, other quality attributes, such as potency, could be significantly affected. In this case, FDA recommends that the acceptance criterion be set lower than the qualified level.24 If the applicant relies on published literature, complete and legible copies of each publication should be included in the ANDA submission.25The toxicity assessment should also include an evaluation of potentially genotoxic impurities (PGI) that may include in silico, in vitro and/or in vivo analyses.。
