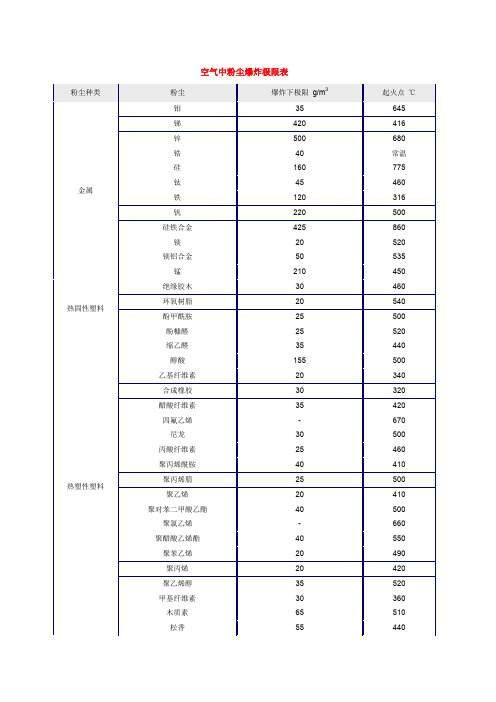
Titanium Dioxide Zeolite
粉尘临床表现和不良健康效应

PC-TWA:
总尘:5mg/m3
20
中文名:聚乙烯粉尘
英文名:Polyethylene dust
CAS号:9002-88-4
侵入途径:呼吸道
主要症状:急性中毒,接触加热后降解产物出现呼吸道和神经系统的症状和体征,
慢性影响,长期吸入PE粉尘,可引起刺激性炎症反应,如咳嗽、咳痰、胸闷等。沉积在肺内,会引起异物反应性改变。局限于粉尘沉着部位,可引起轻度纤维化,但一般不含特殊增生。
棉尘肺:主要为胸部X射线表现:肺纹理增多、增粗、紊乱,肺中上肺叶有不规则与淡类圆状小阴影,结节阴影很少出现,大多数出现局限性肺气肿。病变进展较慢,愈合较好。
尘肺
PC-TWA:
总尘:8mg/m3
呼尘:4mg/m3
2
中文名:玻璃钢粉尘
英文名:Fiberglass reinforced plastic dust
CAS号:/
侵入途径:呼吸道
主要症状:长期接触后,可引起鼻塞、流涕、干咳、咯少量痰等粘膜刺激症状,但轻微而不明显。胸部X射线表现为肺纹理增多、增粗、紊乱,可出现密度低的小阴影。
上呼吸道及皮肤刺激;尘肺
PC-TWA:
总尘:5mg/m3
5
中文名:大理石粉尘
英文名:Marble dust
CAS号:1317-65-3
侵入途径:呼吸道
主要症状:长期反复接触后,可出现咳嗽、干咳、咯少量痰,一般症状轻微、病程较长,中后期常有继发感染,表现为慢性支气管炎和气喘综合症。严重者并发阻碍性肺气肿、肺心病和肺功能不全。胸部X射线主要表现为肺纹理增多、增粗、紊乱。肺叶模糊,肺门可增宽。可见不规则的细小阴影、胸膜增厚等。肺功能,早期正常或轻度损害,晚期以混合性通气性功能障碍为主。
钛白粉介绍

钛白粉钛白粉学名为二氧化钛(Titanium Dioxide)成分结构它有金红石型(Rutile R型)和锐钛型(Anatase 型)二种结构,金红石晶体结构致密,比较稳定,光学活性小,因而耐候性好,同时有较高的遮盖力,消色力。
应用领域钛白粉在橡胶行业中既作为着色剂,又具有补强、防老化、填充作用。
在白色和彩色橡胶制品中加入钛白粉,在日光照射下,耐日晒,不开裂、不变色,伸展率大及耐酸碱。
橡胶用钛白粉,主要用于汽车轮胎以及胶鞋、橡胶地板、手套、运动器材等,一般以锐钛型为主。
但用于汽车轮胎生产量,常加入一定量的金红石型产品,以增强抗臭氧和抗紫外线能力。
钛白粉在化妆品中应用也日趋广泛。
由于钛白粉无毒,远比铅白优越,各种香粉几乎都用钛白粉来代替铅白和锌白。
香粉中只须加入5%-8%的钛白粉就可以得到永久白色,使香料更滑腻,有附着力、吸收力和遮盖力。
在水粉和冷霜中钛白粉可减弱油腻及透明的感觉。
其他各种香料、防晒霜、皂片、白色香皂和牙膏中也可用钛白粉。
用钛白粉制得的瓷釉透明度强,具有质量小、抗冲击力强、机械性能好、色彩鲜艳、不易污染等特点。
食品和医药用钛白粉为纯度很高、重金属含量低、遮盖力强的钛白粉。
GR复合钛白颜料---替代钛白粉使用----钛白粉---钛白---钛白颜料----复合钛白颜料----二氧化钛GR复合钛白颜料是精选结构外型和粒度分布等与钛白类似,且表面具有反应活性的无机粉体为基本原料(内核),TiO2为包膜物,应用新材料改性复合制备、无机包膜、粉体均化等高新技术,通过二者的分割细化,表面羟基化改造和机械力化学反应等方式制备而成的新型复合白色颜料,它具有与钛白粉相同或近似的物化性质,因而具有二氧化钛颜料性质。
广泛应用于涂料、塑料、橡胶、油墨行业,可取得与使用钛白粉相同的技术性能,且较大幅度地降低原材料的使用成本。
GR复合钛白颜料的生产和应用,是解决钛白粉生产中的钛资源短缺、降低环境污染、提高钛白资源利用率的有效途径。
tio分子量

tio分子量什么是tio分子量tio分子量是指二氧化钛(Titanium dioxide)的分子量,也被称为titanium dioxide molecular weight。
二氧化钛是一种常见的化合物,化学式为TiO2。
它是一种白色固体,常用作颜料、涂料和陶瓷这类应用中。
tio分子量的计算要计算出tio分子量,我们需要知道元素钛和氧的原子量。
根据元素周期表的数据,钛的原子量为47.867 g/mol,氧的原子量为15.999 g/mol。
二氧化钛(TiO2)的分子式中包含一个钛原子和两个氧原子。
因此,我们可以将二氧化钛的分子量计算公式表示为:分子量(TiO2)= 原子量(钛)+ 2 × 原子量(氧)将具体的数值代入计算公式,我们可以得到:分子量(TiO2)= 47.867 g/mol + 2 × 15.999 g/mol分子量(TiO2)= 47.867 g/mol + 31.998 g/mol分子量(TiO2)= 79.865 g/mol因此,二氧化钛的分子量为79.865 g/mol。
tio分子量的应用tio分子量广泛应用于材料科学、化学工程和化妆品等领域。
1. 材料科学二氧化钛是一种常见的材料,被广泛应用于制备陶瓷、涂料和塑料等材料。
掌握二氧化钛的分子量可以帮助科学家了解其化学性质和应用特性,并优化材料设计和制备过程。
2. 化学工程二氧化钛在化学工程中有着重要的应用。
例如,它可以作为催化剂,在化学反应中起到催化作用。
了解二氧化钛的分子量,有助于工程师确定合适的催化剂用量,并优化反应条件。
3. 化妆品二氧化钛是许多化妆品的主要成分之一。
它常常被用作防晒剂,能够对抗紫外线的辐射。
掌握二氧化钛的分子量可以帮助化妆品生产企业确定产品中二氧化钛的含量,从而保证产品的品质和功效。
tio分子量的重要性tio分子量的正确计算和理解对于相关领域的研究和应用至关重要。
首先,准确的分子量信息可以帮助科学家更好地理解和预测化合物的性质和行为。
空气中粉尘爆炸极限表

酚糠醛
25
缩乙醛
35
醇酸
155
乙基纤维 20
素
热
合成橡胶
30
塑性
塑料
醋酸纤维
35
素
四氟乙烯
-
尼龙
30
丙酸纤维
25
0
52 0
44 0
50 0
34 0
32 0
42 0
67 0
50 0
46
素
聚丙烯酰 40
胺
聚丙烯腈
25
聚乙烯
20
聚对苯二 40
甲酸乙酯
聚氯乙烯
-
聚醋酸乙 40
烯酯
聚苯乙烯
20
聚丙烯
20
聚乙烯醇
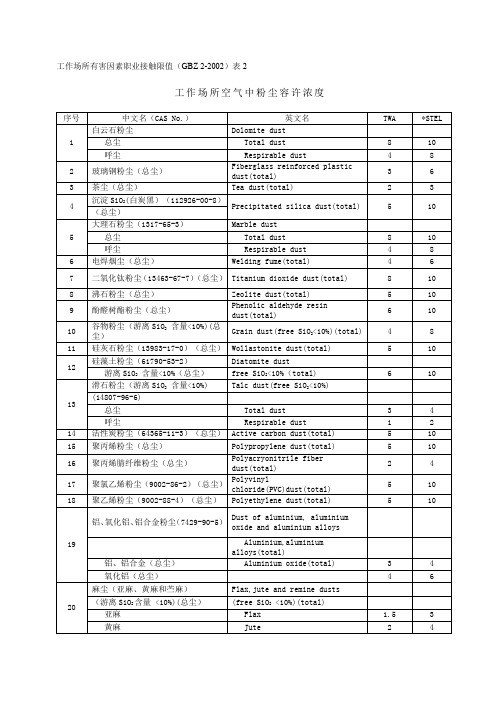
Asbestos (asbestos >10%)
1 硅灰石粉尘 1 (13983-17-0)
Wollastonite dust(total)
硅藻土粉尘 1 (61790-53-2) 2
游离 SiO2 含量
Diatomite dust free SiO2<10%(total)
PC-TW 超
A(mg/m3) 限
总 呼倍
尘 尘数
2
2
5
2
8
42
4
2
8
2
5
号
英文名
3 茶尘
Tea dust(total)
沉淀 SiO2(白炭黑) 4
(112926-00-8)
Precipitated silica dust(total)
大理石粉尘 5 (1317-65-3)
Marble dust
6 电焊烟尘
Welding fume(total)
硫酸法生产钛白粉工艺

由于它是白色和浅白色最好的遮盖颜料和消色颜料, 由于它是白色和浅白色最好的遮盖颜料和消色颜料,因此被广泛地应用于需 要着白色或浅白色的涂料、纸张、塑料、油墨、橡胶等领域。 要着白色或浅白色的涂料、纸张、塑料、油墨、橡胶等领域。随着世界经济 的发展和人类科技的进步,人们对钛白粉的认识将越来越深, 的发展和人类科技的进步,人们对钛白粉的认识将越来越深,钛白粉的应用 领域将越来越广,钛白粉的市场需求将越来越大, 领域将越来越广,钛白粉的市场需求将越来越大,钛白粉有着及其宽广的发 展和应用前景。 展和应用前景。
2.沉清 2.沉清/沉降 沉清/
冷却酸解液、 冷却酸解液、固体惰性物质和未反应的原料残余物溶液从酸解罐的底部全部排放 到宽底的地位沉淀池/沉降池中 沉降池中。 到宽底的地位沉淀池 沉降池中。 此处是将由钛矿杂质形成的可溶性残余物去掉。这些残余物可能包括硅石、锆石 硫 此处是将由钛矿杂质形成的可溶性残余物去掉。这些残余物可能包括硅石、锆石/硫 酸石、白钛石和/或金红石 加入酪蛋白、淀粉或其他有机絮凝剂, 或金红石。 酸石、白钛石和 或金红石。加入酪蛋白、淀粉或其他有机絮凝剂,液体便通过简单 的重力分解沉淀在沉降池中。可溶性残余物的沉降可以在此阶段辅以硫化锑(SbS3) 的重力分解沉淀在沉降池中。可溶性残余物的沉降可以在此阶段辅以硫化锑( 沉淀的形式进行。为此,需在酸解阶段将氧化锑加入到最初的原料中, 沉淀的形式进行。为此,需在酸解阶段将氧化锑加入到最初的原料中,沉降时加入硫 化钠以沉淀SbS3。 化钠以沉淀 用旋转耙从沉降池中将固体物质去除。通常,在沉降池底部有一集中排放点。 用旋转耙从沉降池中将固体物质去除。通常,在沉降池底部有一集中排放点。固 体物质排出后,先用废酸洗涤以回收未反应的原料,然后用水洗掉残留液。 体物质排出后,先用废酸洗涤以回收未反应的原料,然后用水洗掉残留液。沉降后的 钛液通过精滤除掉细小的残余粒子。 钛液通过精滤除掉细小的残余粒子。这些精滤滤渣与沉降池中收集的其他固体物合在 一起送往许可的堆放场。 一起送往许可的堆放场。 整个沉降过程大约8h。 整个沉降过程大约 。
钛白粉产品报告(丰奇琼)修改

钛白粉一、定义:钛白粉学名为二氧化钛( Titanium Dioxide),分子式为TiO2,相对分子质量79.90。
CAS 登录号:13463-67-7 ,EINECS 登录号:236-675-5.也称钛白。
是一种白色颜料,具有无毒、最佳的不透明性、最佳白度和光亮度,是目前世界尚性能最好的一种白色颜料。
二、分类:1.按结晶形态不同分为:金红石型(Rutile R型)和锐钛型(Anatase A型)二种结构。
2.按包膜程度不同分为:A1、A2、R1、R2、R33.按生产工艺的不同分为:硫酸法钛白粉和氯化法钛白粉。
4.按表面极性的不同分为:亲水性、亲油性和两水性三种。
三、成分结构它有金红石型(Rutile R型)和锐钛型(Anatase A型)二种结构。
四、性能指标1、含量锐钛型≧98% 金红石型≧92%2、白度:白度标注大都采用和标样进行比较或采用 L、a、b 表示。
几乎所有的应用行业都要求良好的白度。
钛白粉的杂质和粒子对白度有显著的影响。
CIE(commission internationals de eclairage)是国际照明协会的简称 CIE 制定了 L,a和 b值来测量色值,这种测量方法称为 CIELAB。
L代表着明度,从明亮(此时 L=100)到黑暗(此时 L=0)之间变化。
A值表示颜色从绿色(-a)到红色(+a)之间变化,而b值表示颜色从黄色(+b)到蓝色(-b)之间变化。
影响白度的主要因素:良好的粒径分布是产品白度色相稳定的基本条件。
它包括两个方面:1)适中的力度大小,一般情况下颜料粒径的直径为可见光的波长的一半时,可以达到最好的白度,即 0.15-0.35um 2)集中的力度范围,颜料颗粒直径的分布范围要小。
表示在图像上就是要窄。
3、消色力:钛白粉与其他颜料混合后显示他本身颜色的能力,一般是同标样比较或用雷诺数来表示:和标样比较的是相对消色力。
雷诺数是利用色差计(CR-300 色差仪)测得各个光学数据后计算而得。
空气中粉尘爆炸极限表
10
47
**其他粉尘
Particles not otherwise regulated
8
10
XXX记录
公司名称:
记录人:
记录时间:年月日~年月日
Respirable dust
1.5
3
44
珍珠岩粉尘(93763-70-3)
Perlite dust
总尘
Total dust
8
10
呼尘
Respirable dust
4
8
45
蛭石粉尘(总尘)
Vermiculite dust(total)
3
5
46
重晶石粉尘(7727-43-7)(总尘)
Barite dust (total)
4
8
36
碳化硅粉尘(409-21-2)
Silicon carbide dust
总尘
Total dust
8
10
呼尘
Respirable dust
4
8
37
碳纤维粉尘(总尘)
Carbon fiber dust(total)
3
6
38
矽尘(14808-60-7)
Silica dust
总尘
Total dust
含10%~50%游离SiO2的粉尘
Containing 10%~50% free SiO2
0.7
1
含50%~80%游离SiO2
Containing 50%~80% free SiO2
0.3
0.5
含80%以上游离SiO2
Containing >80% free SiO2
0.2
工作场所粉尘限值
10
47
**其他粉尘
Particles not otherwise regulated
8
10
Fibrous glass dust(total)
3
5
矿渣棉粉尘(总尘)
Slag wool dust(total)
3
5
岩棉粉法(总尘)
Rock wool dust(total)
3
5
28
桑蚕丝尘(总尘)
Mulberry silk dust(total)
8
10
29
砂轮磨尘(总尘)
Grinding wheel dust(total)
Asbestos fibre and dusts Containing>10%
(1332-21-4)
asbestos
总尘
Total dust
0.8
1.5
纤维
Asbestos fibre
0.8f/ml
1.5f/ml
33
石墨粉尘(7782-42-5)
Graphite dust
总尘
Total dust
4
6
铝、铝合金(总尘)
Aluminium oxide(total)
3
4
氧化铝(总尘)
4
6
20
麻尘(亚麻、黄麻和苎麻)
Flax,jute and remine dusts
(游离SiO2含量<10%)(总尘)
(free SiO2<10%)(total)
亚麻
Flax
1.5
3
黄麻
Jute
2
4
苎麻
Ramie
3
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Titanium Dioxide/Zeolite Catalytic Adsorbent for the Removal of NO and Acetone VaporsYan-Huei Jan,Liang-Yi Lin,Mani Karthik,and Hsunling BaiInstitute of Environmental Engineering,National Chiao Tung University,Hsinchu,Taiwan,Republicof ChinaABSTRACTThis study delineates a simple and versatile approach forthe removal of nitrogen monoxide (NO)and volatile or-ganic vapors over composites of titanium dioxide (TiO2)catalyst/zeoliteadsorbent under ultraviolet (UV)irradia-tion at ambient temperature.The catalytic adsorbentswith different TiO 2/H-ZSM-5zeolite ratios were preparedby a simple insipient wetness impregnation method.Itwas found that a 60%/40%weight ratio of TiO2/H-ZSM-5composite is most effective and can achieve over 90%efficiency for the removal of NO and acetone vapors.Thiscomposite also showed a better long-term activity thanthat of bulk TiO 2photocatalyst or zeolite adsorbent.Theexperimental results revealed that photocatalytic decom-position of NO was dramatically enhanced in the pres-ence of acetone.NO also promoted the acetone oxidationunder humid conditions.Furthermore,the co-existenceof acetone and NO in the gaseous stream could reduceacid accumulation on the surface of the catalyst as con-firmed by Fourier-transform infrared spectroscopy.Thus,the TiO 2/zeolite catalytic adsorbent could have a highpotential for the removal of multiple air pollutants in theindoor air environment.INTRODUCTIONIndoor air pollution has been recognized as one of themost important environmental problems.1Indoor air pol-lutants are emitted from various sources such as combus-tion processes,cooking stoves,office equipment,con-struction materials,consumer products,etc.1,2Variouskinds of air pollutants may be presented in the indoor airbecause of different sources of indoor air pollution,butnitrogen oxides (NO x )and volatile organic compounds(VOCs)have always been two of the most common air pollutants.For the protection of indoor air quality,adsorption is one of the traditional pollutant control techniques.Ad-sorbent such as activated carbon is most commonly used for the removal of air pollutants because of its high ad-sorption capacity and low operation cost.However,the adsorbent merely transfers pollutants from the gaseous phase to the solid phase.3–5For the past 2decades,it has been recognized that photocatalytic oxidation (PCO)using titanium dioxide (TiO 2)semiconductors is one of the attractive and effi-cient techniques for the complete destruction of harmful pollutants into nontoxic final products.6,7Furthermore,the semiconductor catalysts are inexpensive and capable of effectively destroying the gaseous pollutants.In this regard,numerous investigations have been conducted on TiO 2semiconductors for the photocatalytic destruction of various air pollutants such as VOCs 8–10and NO x .11–13Several studies have been investigated on the photo-chemical reaction and photodegradation mechanism of VOCs and NO x .For example,Atkinson 14–16reviewed the gas-phase reaction and photodegradation mechanism of VOCs and NO x in the troposphere.Ao et al.3,17–19have also demonstrated that the photoreaction and mecha-nism of nitrogen monoxide (NO);benzene,toluene,ethyl benzene,and xylenes (BTEX);NO;nitrogen dioxide (NO 2);and carbon monoxide (CO)at typical indoor air levels using TiO 2as a photocatalyst.Although it is not possible to evaluate the interactions of all indoor air pol-lutants by photocatalysis,a study of the interactions be-tween the major and common air pollutants using pho-tocatalysis is feasible and valuable.Most of the studies in the literature have mainly described removal of air contaminants by adsorption or photocatalytic destruction methods.The research works of Ao and co-workers 3,17–20have been the few references that have conducted the enhancement or inhibition ef-fects due to the simultaneous presence of multiple air pollutants.The composite materials of photocatalyst/ad-sorbent they used were TiO 2supported on activated car-bon (AC).Tao and co-workers 21,22have investigated the photocatalytic removal of methanol over a dual func-tional TiO 2/AC composite material.They found that a well dispersion of TiO 2particles on AC composite mate-rial prepared by dry impregnation method has a high photocatalytic activity for methanol removal.However,IMPLICATIONSIndoor air pollution has always been a problem that involvesmore than one air pollutant.However,there is usually onlyone air cleaner for removing several air pollutants,so thecombination of effective adsorbents/catalysts in one aircleaning device is a viable method to achieve this goal.Inthis study,the composite of TiO 2/H-ZSM-5as photocata-lytic sorbent under UV irradiation has been used to study itseffectiveness in removing common air pollutants of NO andvolatile organic compounds.The promotion effect on theremoval of these two air pollutants has been observed,andthis composite material has shown its high potential appli-cation to indoor air cleaning.TECHNICAL PAPER ISSN:1047-3289J.Air &Waste Manage.Assoc.59:1186–1193DOI:10.3155/1047-3289.59.10.1186Copyright 2009Air &Waste Management Association1186Journal of the Air &Waste Management Association Volume 59October 2009Thevenet et al.23conducted the photocatalytic enhance-ment of acetylene on TiO 2/AC and TiO 2/zeolite and com-pared with the bulk TiO 2,they found that zeolite signif-icantly improved the photoactivity,whereas AC was only identified as a pollutant pool.TiO 2-supported zeolites have attracted research inter-est as an active composite photocatalyst for the removal of toxic contaminants.24,25However,there is still limited information available on the photochemical interactions between VOCs and NO x during the simultaneous adsorp-tion and PCO process.In this regard,the purpose of this study was to evaluate the combined effects of TiO 2cata-lyst and zeolite adsorbent for the removal of NO and acetone vapors under ultraviolet (UV)irradiation at am-bient temperature.The removal efficiency of the catalyst/adsorbent composites was compared with those of bulk TiO 2(ST-01)catalyst and zeolite (H-ZSM-5)adsorbent,and optimal catalyst/adsorbent composition was ob-tained.Furthermore,the promotion effects of NO and acetone removal efficiencies because of the co-presence of the two pollutants were addressed.EXPERIMENTAL PROCEDURESPreparation of TiO 2/Zeolite Composite Materials The Na-form of ZSM-5zeolite (silicon/aluminum ratio 50,particle size 1.5–2m;Zeotyst,Ltd.)was converted into the H-form by repeated ion exchange.The zeolite and 0.05M ammonium nitrate solution (15mL/g of zeolite)were stirred at 80°C for 12hr.Then the material was filtered and washed with distilled water.The resulting filtered material was dried at 120°C for 6hr and subse-quently calcined at 550°C for 6hr.This H-form of ZSM-5zeolite was used as an adsorbent and support in this study.The TiO 2/zeolite-based composite photocatalysts were prepared by a simple incipient wetness impregnation method.An appropriate amount of TiO 2(ST-01,particle size 7nm,Ishihara Sangyo Kaisha,Ltd.)was added into 20mL of deionized (DI)water and stirred for 1hr.Then H-ZSM-5zeolite was added into the aqueous colloidal suspension of TiO 2.The above colloidal mixture was thor-oughly stirred for additional 1hr and agitated with son-ication bath (Tohama DC-400)for 10min.Then the above mixture was sprayed on a heating glass plate of 110°C.The total weight of all samples for comparison tests was fixed at 0.1g regardless of the weight percentageof TiO 2or zeolite.Repeated spraying,cooling,and weighting of the composite samples confirmed the total weight of sample.Characterization of TiO 2/Zeolite Composite Materials The crystalline nature of the calcined samples was ob-tained with Rigaku X-ray diffractometer equipped with nickel-filtered CuK ␣(ϭ1.5405Å)radiation.The diffrac-tograms of the calcined samples were recorded in the 2range between 2°and 60°in steps of 1°with a count time of 60sec at each point.The BET (Brunauer-Emmett-Teller)specific surface areas of the samples were measured by nitrogen gas (N 2)adsorption-desorption isotherms at 77K using a surface area analyzer (Micromeritics,ASAP 2000).All of the samples were degassed for 6hr at 350°C under vacuum (10Ϫ6mbar)before the adsorption experiments.The elemental titanium in the samples was analyzed by a SCIEX ELAN 5000inductively coupled plasma mass spec-trometer (ICP-MS).The infrared (IR)spectra of the sam-ples were obtained from a Fourier-transform IR (FTIR;FT-730)spectrophotometer using a potassium bromide pellet technique.Adsorption and Photocatalytic Experiments A photoreactor (dimensions of 5.5-cm height ϫ63-cm length ϫ10-cm width)was made up of acrylic resin-type material and a glass plate with 300mm of length,50mm of width,and 1mm of thickness was used as a coating substrate that was placed inside of the photoreactor.The photocatalysts were dispersed in DI water by using soni-cation method.The photocatalytic degradation of NO and acetone vapors was carried out using a continuous flow photoreactor under UV irradiation at ambient tem-perature.Figure 1shows a schematic diagram of the ex-perimental setup used in this study.A feed gas of reactant mixture containing NO (10parts per million by volume [ppmv]),acetone (110ppmv),and air (balance)were passed through two different mass flow controllers to the photoreactor.It is noted that typ-ical concentrations of indoor air pollutants such as VOCs and NO x are usually in a few parts per million (ppm)to sub-ppm levels.26,27However,for the sake of analytical quantification of the deterioration of photocatalyst dueto Figure 1.Schematic diagram of the continuous gas flow photoreactor system.Jan,Lin,Karthik,and BaiVolume 59October 2009Journal of the Air &Waste Management Association 1187the formation of nitric acid and to speed up the adsorp-tion breakthrough time,NO and acetone vapors were tested at relatively higher concentration levels in this study.The concentration of acetone was selected to be much higher than that of NO because the total concen-tration of volatile organic vapors in ambient air is usually much higher than that of the NO x .The source of NO gas was supplied from cylinder and diluted by a separate airstream.The concentration of acetone (VOC)vapors was controlled by passing carrier gas through an impinger containing acetone and the impinger was kept in a con-stant-temperature controller maintained at Ϫ10°C.The diluted air was controlled by a mass flow controller and passed into an impinger containing DI water to adjust the relative humidity (RH)to be approximately 55Ϯ5%in the outlet gas flow,which corresponds to a water vapor concentration of around 17,500ppmv under 25°C and 1atm.The level of RH used in this study was in the range of recommended levels by the American Society of Heating,Refrigerating and Air-Conditioning Engineers 28(30–60%)and by the Taiwan Indoor Air Association 29(40–70%).The desired flow of gas stream of NO,acetone,and water vapors was controlled by a mass flow controller and the gas stream was premixed by using a stainless steel mixer.The concentrations of the gas mixture were mea-sured before each experimental test to ensure the desired stable concentrations.The total flow rate of gas mixture was set at two different flow rates of 180and 500mL/min,which correspond to residence times of 75and 27sec,respectively.The photocatalytic decomposition reaction was carried out under UV irradiation with a 365-nm UV lamp (Sparkie UVA-S;10W)being used as a light source.The UV lamp was horizontally placed at the upper part of the reactor,and the photocatalyst-coated glass plate was horizontally fixed at the lower part of the reactor at a vertical distance of approximately 20cm from the UV lamp.UV intensity measured in all experiments was 2.5mW/cm 2as measured on the surface of the photocata-lysts.The inlet and outlet concentrations of NO and NO 2were continuously monitored by an online NO x (SIR Model S5012)analyzer,which monitors NO,NO 2,and NO x ,and then the selectivity of NO 2was calculated.The inlet and outlet concentrations of acetone samples were taken at designated time intervals during the experiment using a microsyringe,and the samples were analyzed by a gas chromatograph (GC;Model-SRI 8610)using a capil-lary column (30m)equipped with a flame-ionization detector (FID).RESULTS AND DISCUSSION X-Ray Diffraction and BET Analysis The crystalline nature of H-ZSM-5,TiO 2(ST-01),and TiO 2/H-ZSM-5composite materials was confirmed by X-ray diffraction (XRD)analysis and the results are depicted in Figure 2.It can be seen that the XRD pattern of TiO 2clearly indicates characteristic peaks of the anatase form of TiO 2crystals.30The major peaks that appeared in the 2range between 22°and 25°and 7°and 9°correspond to the specific peaks of H-ZSM-5zeolite.31As the content of H-ZSM-5zeolite is increased in the TiO 2/zeolite compos-ites,the gradual increase in the intensity of the zeolite peaks and the decrease in the intensity of the TiO 2peaks are clearly observed.To confirm the accuracy of the weight percentage mixing of the TiO 2/zeolite composites,the titanium con-tents of the TiO 2/H-ZSM-5composite materials were an-alyzed by ICP-MS,and the specific surface areas of H-ZSM-5,TiO 2,and TiO 2/H-ZSM-5composites were analyzed by nitrogen adsorption-desorption measure-ments.ICP-MS and BET results of the samples are pre-sented in Table 1.Because the surface area of H-ZSM-5zeolite (410m 2/g)is much higher than that of bulk TiO 2(82m 2/g),the specific surface area of the TiO 2/H-ZSM-5composites gradually decreased with increasing TiO 2loading.One can see that the surface area of the TiO 2/H-ZSM-5composites and the titanium content corre-sponded well to the weight percentage of thecomposites.Figure 2.XRD patterns of the bulk TiO 2,H-ZSM-5,and TiO 2/H-ZSM-5composite catalysts.Table 1.Physicochemical characterization of the photocatalytic sorbents.Photocatalytic SorbentCatalyst/Sorbent Content (wt%)ICP-MS Titanium Contents (wt%)Surface Area (m 2/g)TiO 2(ST-01)Zeolite (H-ZSM-5)H-ZSM-5–100–410TiO 2/H-ZSM-520809.42343TiO 2/H-ZSM-5406019.47292TiO 2/H-ZSM-5604030.24224TiO 2/H-ZSM-5802040.53158TiO 2100––82Jan,Lin,Karthik,and Bai1188Journal of the Air &Waste Management Association Volume 59October 2009Removal of NO and Acetone VaporsBlank tests on the removal of NO and acetone vapors were conducted first.The photochemical reaction was tested in the absence of catalyst and zeolite.The blank test results indicated that there was no photochemical reaction un-der UV irradiation in the absence of catalyst for NO and acetone vapors;hence,the results are not shown.Further-more,blank tests were also conducted on TiO2/zeolitecomposites in the absence of UV irradiation to evaluatethe acetone and NO removal via pure adsorption.The adsorption results indicated that there was no adsorption of NO on the surface of the TiO 2/zeolite composites,but the acetone vapors were predominately adsorbed on the surface of the catalysts.Figure 3a shows the acetone removal efficiency over H-ZSM-5,TiO 2,and 40and 60wt %TiO 2/H-ZSM-5com-posites as a function of operation time.It can be seen that the acetone removal efficiencies are in the order of H-ZSM-5zeolite Ͼ40wt %TiO 2/H-ZSM-5Ͼ60wt %TiO 2/H-ZSM-5ϾTiO 2.The higher acetone removal efficiency of H-ZSM-5zeolite as compared with that of TiO 2could be due to its higher BET surface area.This was confirmed bythe correlation between specific surface area of the mate-rial and the saturated adsorption capacity of acetone as shown in Figure 3b.It can be clearly seen that the adsorp-tion capacity of acetone is linearly correlated to the spe-cific surface area of the material.Adsorption and Photocatalytic Effects The adsorption and photocatalytic degradation for mix-ture of NO and acetone vapors were carried out in the continuous flow photoreactor system under UV irradia-tion at ambient temperature.Figure 4shows the NO re-moval over bulk TiO 2,H-ZSM-5,and TiO 2-supported ze-olite composites as a function of irradiation time.As can be expected,when no TiO 2was present,there was no photocatalytic reaction at all.As the weight percentage of TiO 2on the H-ZSM-5zeolite was increased to 20%(w/w),the initial NO removal rate was gradually increased with increasing irradiation time and it reached approximately 100%removal after 100min of irradiation time.On the other hand,at high TiO 2loading of 80wt %TiO 2/H-ZSM-5composite and bulk TiO 2catalyst,they showed quite high initial NO removal of approximately 100%up to 80min,then the NO removal decreased with increas-ing irradiation time.This could be ascribed to the deacti-vation of the catalyst by accumulation of unreactive by-products.The deactivation rate is much faster as the TiO 2content increases.As a result,the best TiO 2/zeolite com-posites appeared to be 40and 60wt %TiO 2/zeolite,the NO removal of which was near 100%for all irradiation time.The adsorption and PCO of acetone vapors from streams of NO and acetone mixture by H-ZSM-5,bulk TiO 2,and TiO 2/H-ZSM-5composites are depicted in Fig-ure 5.The acetone vapor removal by H-ZSM-5zeolite,which is expected to be due to its adsorption effect,was around 90%up to 60min and then decreased to 0%because of saturated adsorption.On the other hand,the removal efficiency of acetone over bulk TiO 2catalyst,Figure 3.(a)The acetone removal from streams of NO ϩacetone mixture as a function of operation time without UV irradiation under a flow residence time of 75sec.(b)The correlation between specific surface area of the material and the saturated adsorption capacity ofacetone.Figure 4.Effect of TiO 2content in the TiO 2/zeolite composites on the NO removal from streams of NO ϩacetone mixture under a flow residence time of 75sec.Jan,Lin,Karthik,and Bai Volume 59October 2009Journal of the Air &Waste Management Association 1189which is expected to be due to photocatalytic effect,was above 80%initially then gradually decreased to around 70%.For the TiO 2/zeolite composites,it showed that 20and 40wt %TiO 2-supported zeolites have less acetone removal efficiency as compared with those of 60and 80wt %TiO 2-supported zeolite and even bulk TiO 2catalyst.This could be because the TiO 2loading is not enough so that the ad-sorption process dominates and the photocatalytic reac-tion can proceed only to some extent.The results reveal that the optimal composite ratio seems to be 60–80wt %TiO 2/H-ZSM-5composites.Therefore it seems that com-bining a small amount of H-ZSM-5zeolite with the TiO 2photocatalyst can help to enhance acetone removal and prevent the deactivation of the photocatalysts.Effect of Residence Time on the Optimal TiO 2/Zeolite Composite RatioFigure 6shows the influence of residence time on removal of NO and acetone over TiO 2,H-ZSM-5,and TiO 2/H-ZSM-5composites.In this study,two residence times of 75and 27sec were chosen to evaluate the removal effi-ciency of the catalysts.When decreasing the residence time from 75to 27sec,the acetone removal was drasti-cally decreased over all catalysts.On the other hand,the removal of NO does not seem to be significantly affected by the residence time.It implies that the acetone mole-cules might first be adsorbed on the surface of the catalyst and then the photocatalytic decomposition of acetone takes place.But the NO molecule might be immediately photodecomposed into N 2or oxidized to NO 2.32,33The best TiO 2/zeolite composites appeared to be 20–60wt %TiO 2supported on H-ZSM-5zeolite for the removal of NO,and 80wt %TiO 2supported on H-ZSM-5zeolite for the removal of acetone vapors.For the removal of NO and acetone vapors,60wt %TiO 2/H-ZSM-5composite was found to be the most effective and optimal catalyst/ad-sorbent composite.Effect of NO on Acetone Removal Figure 7illustrates the removal of acetone in the presence and absence of NO in the gaseous stream using 60wt %TiO 2/H-ZSM-5composite at a flow residence time of 75sec.It was found that the PCO of acetone was dramati-cally enhanced in the presence of NO and the photocat-alytic activity was almost the same up to 240min of irradiation time.On the other hand,the acetone removal under the absence of NO was initially very high at 100%,but it then decreased drastically to around 60–65%after 60min.On the basis of the results,it is possible to propose that the formation of some intermediate species from NO oxidation could react immediately with acetone mole-cules.Therefore,the oxidation of acetone is strongly pro-moted by some intermediate species formed from NO.During the photocatalytic reaction,NO is generally con-tinuously oxidized to form NO 2,and then NO 2isfurther Figure 5.Effect of TiO 2content in the TiO 2/zeolite composites on the acetone removal from streams of NO ϩacetone mixture under a flow residence time of 75sec.Figure 6.Effect of flow residence time on NO and acetone removal from streams of NO ϩacetone mixture at an irradiation time of 240min.Figure 7.PCO of acetone in the presence and absence of NO over 60wt %TiO 2/H-ZSM-5composite.Jan,Lin,Karthik,and Bai1190Journal of the Air &Waste Management Association Volume 59October 2009converted into nitric acid (HNO 3)under PCO in the at-mosphere.The general reactions of NO oxidation by pho-tocatalysis are given in the following equations:H 2O ϩO 23OH ⅐ϩHO 2⅐(1)NO ϩHO 2⅐3NO 2ϩOH ⅐(2)NO 2ϩOH ⅐3HNO 3(3)Atkinson and co-workers 14–16demonstrated the rate con-stant and mechanism for the combination reaction of the OH radical with NO 2to form HNO 3in the tropo-sphere.They also calculated the rate constant for the gas-phase reactions of OH radical with acetone mole-cules with various temperature ranges.15The calculated room temperature rate constants for the reactions of OH radicals with acetone molecule and NO2is around 0.22ϫ10Ϫ12cm 3⅐molecule Ϫ1⅐sec Ϫ1and 1.42ϫ10Ϫ11cm 3⅐molecule Ϫ1⅐sec Ϫ1,respectively.14,34Furthermore,methods also exist for estimating rate constants for the reactions of VOCs with OH and NO x radicals,which can be used when data are not available.14–16Under the simultaneous presence of NO and acetone,the acetone could take up the OH radical formed in eq 2;hence,the acetone removal is enhanced.On the other hand,HNO 3is usually formed as a final byproduct.35This HNO3species is strongly adsorbed on the surface of thecatalyst,which causes catalyst deactivation and hence decreased photocatalytic activity with respect to irradia-tion time.13,33But if the OH radical is taken up by acetone,then the HNO 3formation of eq 3should be hindered.Effect of Acetone on NO RemovalTo further understand the mutual effect of NO and ace-tone,the experiment was also conducted over 60wt %TiO 2/H-ZSM-5composite in the presence and absence of acetone in the gas mixtures under UV irradiation.The effects of acetone on the NO removal and on the selec-tivity of NO 2are shown in Figure 8.It can be seen that thephotocatalytic decomposition of NO was significantly en-hanced in the presence of acetone.Thus,from Figures 7and 8it was found that the photocatalytic decomposition of acetone and NO was enhanced under the simultaneous presence of NO and acetone vapors.Similar results have also been identified by Higashimoto et al.36for the pho-tocatalytic decomposition of NO in the presence of pro-pane (C 3H 8)over VS-1zeolite used as a catalyst.The photocatalytic decomposition of NO in the ab-sence of acetone was found to be less efficient,as seen in Figure 8.This may be due to the rapid photooxidation of NO into NO 2and the active NO 2species reacting imme-diately with OH radical to form HNO 3,as shown in eq 3.Hence,the nitrate ions (NO 3Ϫ)from acid could be ad-sorbed on the surface active sites of the catalyst,which causes the catalyst deactivation.13,35On the other hand,the enhancement in NO removal in the presence of ace-tone could be expected from the rapid formation of OH radical that can be utilized for the simultaneous oxidation of NO and acetone.Therefore,the formation of HNO 3could be reduced with the co-existence of acetone and NO,and hence the photocatalytic decomposition of NO and acetone vapors could be maintained without any significant deactivation by HNO 3.As also observed in Figure 8,the selectivity of NO 2was increased with increasing irradiation time in the pres-ence and absence of acetone vapors.However,it is inter-esting to note that the selectivity of NO 2in the presence of acetone was lower than the selectivity of NO 2in the absence of acetone.Therefore eq 2may not be the only pathway for the further reaction of NO.It is possible that the NO reduction can also be favorable in addition to the NO oxidation mechanism.The formation of some inter-mediate organic radicals that reduced the NO x (NO and NO 2)into N 2and oxygen gas (O 2)because of the presence of hydrocarbons has been discussed by Higashimoto et al.36Therefore,oxidation and reduction of NO could oc-cur in the presence of acetone because acetone can act as a reducing agent in this system.However,because of instrument limitation,the NO reduction intermediates such as nitrous oxide (N 2O)and its final product,N 2,could not be detected at the present time.The confirma-tion of the NO oxidation/reduction mechanisms requires further study.On the other hand,the selectivity of NO 2was reached finally at approximately 100%in the absence of acetone.These results clearly indicate that the NO mole-cules were predominately oxidized into NO 2in the ab-sence of acetone.The generated NO 2may react immedi-ately with freely available OH radical to form HNO 3by eqs 2and 3;hence,the acid accumulation can cause the catalyst deactivation.To further confirm this,catalyst samples for the PCO of NO in the presence and absence of acetone were analyzed by FT-IR and the results are de-picted in Figure 9.It can be seen that the peaks appearing at 1385cm Ϫ1are due to the formation of nitrate species on the surface of the catalyst.11,35The FT-IR spectrum of the sample in the presence of NO and acetone clearly indicates that it has a less intense peak at 1385cm Ϫ1,which suggests less formation of HNO 3on the surface of the catalyst.On the other hand,when only NO was pre-sented in the gas stream,the increase in peak intensityat Figure 8.Removal of NO and the corresponding NO 2selectivity in the presence and absence of acetone over 60wt %TiO 2/H-ZSM-5composite.The flow residence time was 75sec.Jan,Lin,Karthik,and Bai Volume 59October 2009Journal of the Air &Waste Management Association 11911385cm Ϫ1indicates the formation of more HNO3on the surface of the catalyst.Thus the hypothesis that the co-existence of acetone and NO in the gaseous stream could diminish the acid formation is confirmed.CONCLUSIONSCombined effects of TiO 2catalyst/zeolite adsorbent com-posite was suggested as an effective photocatalytic sys-tem for the simultaneous removal of multiple pollutants in the ambient air under UV irradiation.The 60wt %TiO2/H-ZSM-5composite was found to be the optimalcomposite ratio for better removal efficiencies (Ն90%)of NO and acetone vapors than those of commercial TiO 2(ST-01)catalyst and zeolite (H-ZSM-5)adsorbent under humid conditions.It was found that the co-existence of acetone and NO in the gaseous stream could reduce the acid accumulation on the surface of the catalyst and enhance their removal as compared with their individual presence in the gas stream.The results on the selectivity of NO 2also indicated that the possibility of simultaneous NO oxidation/reduction mechanisms are present with the co-existence of NO and acetone in a humid environ-ment;however,further studies are required to confirm this hypothesis.ACKNOWLEDGMENTSThe authors gratefully acknowledge the financial support received from the National Science Council (NSC)of Tai-wan through research grant no.95-2221-E-009-112-MY3.M.Karthik is thankful for the postdoctoral research fel-lowship from NSC grant no.95-2221-E-009-112-MY3.REFERENCES1.Wang,S.;Ang,H.M.;Tade,M.O.Volatile Organic Compounds in Indoor Environment and Photocatalytic Oxidation:State of the Art;Environ.Int.2007,33,694-705.2.Namiesnik,J.;Gorecki,T.;Kozdronzabiegala,B.;Lukasiak,J.Indoor Air-Quality (IAQ),Pollutants,Their Sources and Concentration Levels;Build.Environ.1992,27,339-356.3.Ao,C.H.;Lee,bination Effect of Activated Carbon with TiO 2for the Photodegradation of Binary Pollutants at Typical Indoor Air Level;J.Photochem.Photobiol.A Chem.2004,161,131-140.4.Guillemot,M.;Mijoin,J.;Mignard,S.;Magnoux,P.Volatile Organic Compounds (VOCs)Removal over Dual Functional Adsorbent/Cata-lyst System;Appl.Catal.B Environ.2007,75,249-255.5.Blocki,S.W.Hydrophobic Zeolite Adsorption:a Proven Advancement in Solvent Separation Technology;Environ.Prog.1993,12,226-230.6.Fujishima,A.;Rao,T.N.;Tryk,D.A.Titanium Dioxide Photocatalysis;J.Photochem.Photobiol.C Photochem.Rev.2000,1,1-21.7.Kitano,M.;Matsuoka,M.;Ueshima,M.;Anpo,M.Recent Develop-ments in Titanium Oxide-Based Photocatalysts;Appl.Catal.A Gen.2007,325,1-14.8.Coronado,J.M.;Kataoka,S.;Tejedor,I.T.;Anderson,M.A.Dynamic Phenomena during the Photocatalytic Oxidation of Ethanol and Ac-etone over Nanocrystalline TiO 2:Simultaneous FT-IR Analysis of Gas and Surface Species;J.Catal.2003,219,219-230.9.Deng,X.;Yue,Y.;Gao,Z.Gas-Phase Photo-Oxidation of Organic Compounds over Nanosized TiO 2Photocatalysts by Various Prepara-tion;Appl.Catal.B Environ.2002,39,135-147.10.Augugliaro,V.;Coluccia,S.;Loddo,V.;Marchese,L.;Martra,G.;Pal-misano,L.;Schiavello,M.Photocatalytic Oxidation of Gaseous Tolu-ene on Anatase TiO 2Catalyst:Mechanistic Aspects and FT-IR Investi-gation;Appl.Catal.B Environ.1999,20,15-27.11.Lim,T.H.;Jeong,S.M.;Kim,S.D.;Gyenis,J.Photocatalytic Decompo-sition of NO by TiO 2Particles;J.Photochem.Photobiol.A Chem.2000,134,209-217.12.Zhang,J.;Ayusawa,T.;Minagawa,M.;Kinugawa,K.;Yamashita,H.;Matsuoka,M.;Anpo,M.Investigations of TiO 2Photocatalysts for the Decomposition of NO in the Flow System:the Role of Pretreatment and Reaction Conditions in the Photocatalytic Efficiency;J.Catal.2001,198,1-8.13.Tseng,Y.H.;Kuo,C.-S.;Huang;C.-H.;Li,Y.-Y.;Chou,P.-W.;Cheng,C.-L.;Wong,M.-S.Visible-Light-Responsive Nano-TiO 2with Mixed Crystal Lattice and Its Photocatalytic Activity;Nanotechnology 2006,17,2490-2497.14.Atkinson,R.Atmospheric Chemistry of VOCs and NO x ;Atmos.Envi-ron.2000,34,2063-2101.15.Kwok,E.S.C.;Atkinson,R.Estimation of Hydroxyl Radical Reaction Rate Constants for Gas-Phase Organic Compounds Using a Structure-Reactivity Relationship:an Update;Atmos.Environ.1995,29,1685-1695.16.Atkinson,R.Gas Phase Tropospheric Chemistry of Volatile Organic Compounds:1.Alkanes and Alkenes,J.Phys.Chem.Ref.Data 1997,26,215-290.17.Ao,C.H.;Lee,S.C.Enhancement Effect of TiO 2Immobilized on Acti-vated Carbon Filter for the Photodegradation of Pollutants at Typical Indoor Air Level;Appl.Catal.B Environ.2003,44,191-205.18.Ao,C.H.;Lee,S.C.;Yu,J.Z.;Xu,J.H.Photodegradation of Formalde-hyde by Photocatalyst TiO 2:Effects on the Presences of NO,SO 2and VOCs;Appl.Catal.B Environ.2004,54,41-50.19.Ao,C.H.;Lee,S.C.;Mak,C.L.;Chan,L.Y.Photodegradation of Volatile Organic Compounds (VOCs)and NO for Indoor Air Purification Using TiO 2:Promotion Versus Inhibition Effect of NO;Appl.Catal.B Environ.2003,42,119-129.20.Yu,H.;Lee,S.C.;Yu,J.;Ao,C.H.Photocatalytic Activity of Dispersed TiO 2Particles Deposited on Glass Fibers;J.Mol.Catal.A Chem.2006,246,206-211.21.Tao,Y.;Wu,C.-Y.;Mazyck,D.W.Removal of Methanol from Pulp and Paper Mills Using Combined Activated Carbon Adsorption and Pho-tocatalytic Regeneration;Chemosphere 2006,65,35-42.22.Tao,Y.;Schwartz,S.;Wu,C.-Y.;Mazyck,D.W.Development of a TiO 2/AC Composite Photocatalyst by Dry Impregnation for the Treat-ment of Methanol in Humid Airstreams;Ind.Eng.Chem.Res.2005,44,7366-7372.23.Thevenet,F.;Guaitella,O.;Herrmann,J.M.;Rousseau,A.;Guillard,C.Photocatalytic Degradation of Acetylene over Various Titanium Diox-ide-Based Photocatalysts;Appl.Catal.B Environ.2005,61,58-68.24.Yamashita,H.;Ichihashi,Y.;Anpo,M.;Hashimoto,M.Louis,C.M.Photocatalytic Decomposition of NO at 275K on Titanium Oxides Included within Y-Zeolite Cavities:the Structure and Role of the Active Sites;J.Phys.Chem.1996,100,16041-16044.25.Hu,Y.;Rakhmawaty,D.;Matsuoka,M.;Takeuchi,M.;Anpo,M.Syn-thesis,Characterization and Photocatalytic Reactivity of Ti-Contain-ing Micro-and Mesoporous Materials;J.Porous Mat.2006,13,335-340.26.Ao,C.H.;Lee,S.C.;Zou,S.C.;Mak,C.L.Inhibition Effect of SO 2on NO x and VOCs during the Photodegradation of Synchronous Indoor Air Pollutants at Parts per Billion (ppb)Level by TiO 2;Appl.Catal.B Environ.2004,49,187-193.27.Chan,C.C.;Ozkaynak,H.;Spengler,J.D.;Sheldon,L.Driver Exposure to Volatile Organic Compounds,Carbon Monoxide,Ozone and Ni-trogen Dioxide under Different Driving Conditions;Environ.Sci.Tech-nol.1991,25,964-972.28.American Society of Heating,Refrigerating,and Air-Conditioning En-gineers;available at /education/page/1481(ac-cessed March 13,2009).Figure 9.FT-IR spectra of 60wt %TiO 2/H-ZSM-5composite catalyst before and after photocatalytic reaction.Jan,Lin,Karthik,and Bai1192Journal of the Air &Waste Management Association Volume 59October 2009。
