Efficient conversion of ES cells into myogenic lineage using the gene-inducible system
产甘油假丝酵母过量合成甘油的机理

产甘油假丝酵母过量合成甘油的机理英文版Glycerol is a key component in the production of biofuels, pharmaceuticals, and cosmetics. One of the most efficient ways to produce glycerol is through the overproduction of glycerol by engineered strains of the yeast Saccharomyces cerevisiae, also known as glycerol-overproducing yeast. In this article, we will explore the mechanism by which glycerol-overproducing yeast synthesizes glycerol in excess.The overproduction of glycerol in yeast is achieved through the manipulation of key enzymes involved in glycerol biosynthesis. The key enzymes involved in glycerol biosynthesis are glycerol-3-phosphate dehydrogenase (GPD) and glycerol-3-phosphatase (GPP). GPD catalyzes the conversion of dihydroxyacetone phosphate (DHAP) to glycerol-3-phosphate (G3P), while GPP catalyzes the dephosphorylation of G3P to glycerol.In glycerol-overproducing yeast, the expression of GPD is upregulated, leading to an increased conversion of DHAP to G3P. This results in an accumulation of G3P, which is then converted to glycerol by GPP. The overexpression of GPP further enhances the conversion of G3P to glycerol, leading to the overproduction of glycerol in the yeast cells.Overall, the overproduction of glycerol in yeast is achieved through the coordinated upregulation of GPD and GPP, resulting in the efficient conversion of DHAP to glycerol. This mechanism allows for the production of glycerol in excess, making glycerol-overproducing yeast an attractive option for industrial applications.完整中文翻译产甘油是生产生物燃料、药品和化妆品的关键组成部分。
离子交换膜英文

离子交换膜英文Ionic Exchange MembranesIonic exchange membranes are a critical component in various electrochemical and separation processes, playing a vital role in diverse applications ranging from water treatment to energy storage. These specialized membranes possess the unique ability to selectively transport specific ions while rejecting others, making them invaluable in a wide array of industries.At the core of an ionic exchange membrane lies a polymer matrix, typically composed of a network of charged functional groups. These functional groups can be either positively charged (cationic) or negatively charged (anionic), and they serve as the foundation for the membrane's ion-exchange capabilities. The charged groups within the membrane attract and bind to oppositely charged ions, effectively creating a pathway for the selective transport of these ions across the membrane.One of the primary functions of ionic exchange membranes is in the field of water treatment. In processes such as desalination, reverse osmosis, and electrodialysis, these membranes are used to removedissolved salts and other ionic contaminants from water. The charged functional groups within the membrane attract and trap the unwanted ions, allowing for the production of high-quality, purified water. This technology is particularly crucial in regions with limited access to clean water, as it enables the conversion of brackish or seawater into a potable resource.Beyond water treatment, ionic exchange membranes find extensive applications in the energy sector. In fuel cells, these membranes act as the electrolyte, facilitating the transport of protons (H+ ions) between the anode and cathode. This proton exchange allows for the efficient conversion of chemical energy into electrical energy, making fuel cells a promising alternative to traditional combustion-based power generation. Similarly, in rechargeable batteries, ionic exchange membranes play a vital role in the movement of ions during the charging and discharging cycles, contributing to the overall performance and safety of the energy storage system.The versatility of ionic exchange membranes extends to the field of electrochemical synthesis and processing. In the production of various chemicals and materials, these membranes can be used to selectively separate and purify desired products, improving the efficiency and purity of the manufacturing process. Additionally, they are employed in the production of hydrogen gas through water electrolysis, where the membrane facilitates the separation ofhydrogen and oxygen.The development of ionic exchange membranes has undergone significant advancements in recent years, driven by the increasing demand for efficient and sustainable technologies. Researchers and engineers have been exploring new materials, designs, and manufacturing techniques to enhance the performance, durability, and cost-effectiveness of these membranes.One area of active research focuses on the development of novel polymer materials with improved ion-exchange properties. By tailoring the chemical structure and composition of the polymer matrix, scientists aim to create membranes with higher ion-exchange capacity, better selectivity, and enhanced resistance to fouling and degradation. This includes the exploration of hybrid materials, such as organic-inorganic composites, which can combine the advantages of different components to achieve enhanced performance.Another key area of innovation is the optimization of membrane fabrication processes. Techniques like phase inversion, electrospinning, and 3D printing are being investigated to produce membranes with precisely controlled pore structures, thickness, and surface properties. These advancements can lead to improved mass transfer, reduced resistance to ion transport, and enhanced mechanical stability, all of which contribute to the overall efficiencyand reliability of the membrane-based systems.In addition to material and manufacturing advancements, researchers are also exploring novel applications and integration strategies for ionic exchange membranes. For instance, the use of these membranes in redox flow batteries, water electrolyzers, and bioelectrochemical systems is an active area of investigation, as they offer the potential to enhance energy storage, hydrogen production, and wastewater treatment capabilities.As the global demand for sustainable and efficient technologies continues to grow, the importance of ionic exchange membranes is expected to increase. These versatile and essential components will play a crucial role in addressing the pressing challenges faced by various industries, from water scarcity and renewable energy to environmental remediation and chemical production.Through ongoing research, innovation, and collaborative efforts, the field of ionic exchange membranes is poised to witness further advancements, leading to the development of more efficient, cost-effective, and environmentally friendly solutions that will shape the future of various industries and communities around the world.。
太阳能发电英文
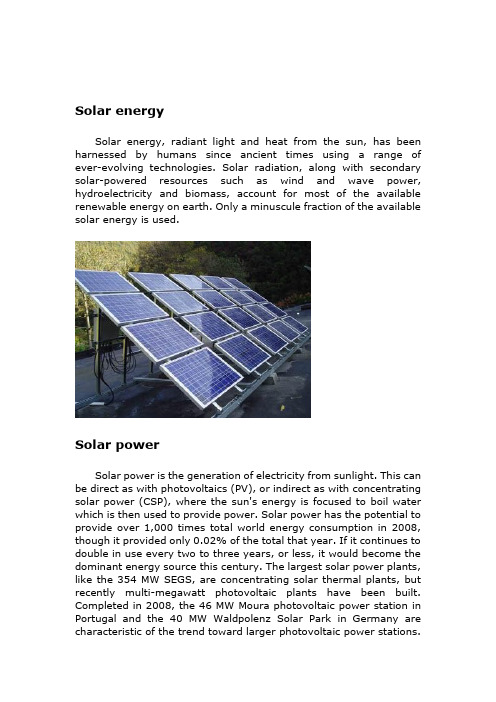
Solar energySolar energy, radiant light and heat from the sun, has been harnessed by humans since ancient times using a range of ever-evolving technologies. Solar radiation, along with secondary solar-powered resources such as wind and wave power, hydroelectricity and biomass, account for most of the available renewable energy on earth. Only a minuscule fraction of the available solar energy is used.Solar powerSolar power is the generation of electricity from sunlight. This can be direct as with photovoltaics (PV), or indirect as with concentrating solar power (CSP), where the sun's energy is focused to boil water which is then used to provide power. Solar power has the potential to provide over 1,000 times total world energy consumption in 2008, though it provided only 0.02% of the total that year. If it continues to double in use every two to three years, or less, it would become the dominant energy source this century. The largest solar power plants, like the 354 MW SEGS, are concentrating solar thermal plants, but recently multi-megawatt photovoltaic plants have been built. Completed in 2008, the 46 MW Moura photovoltaic power station in Portugal and the 40 MW Waldpolenz Solar Park in Germany are characteristic of the trend toward larger photovoltaic power stations.Much larger ones are proposed, such as the 100 MW Fort Peck Solar Farm, the 550 MW Topaz Solar Farm, and the 600 MW Rancho Cielo Solar Farm.Solar power is amazing. On average, every square meter of Earth's surface receives 164 watts of solar energy. In other words, you could stand a really powerful (150 watt) table lamp on every square meter of Earth's surface and light up the whole planet with the Sun's energy! Or, to put it another way, if we covered just one percent of the Sahara desert with solar panels, we could generate enough electricity to power the whole world. That's the good thing about solar power: there's an awful lot of it—much more than we could ever use.But there's a downside too. The energy the Sun sends out arrives on Earth as a mixture of light and heat. Both of these are incredibly important—the light makes plants grow, providing us with food, while the heat keeps us warm enough to survive—but we can't use either the Sun's light or heat directly to run a television or a car. We have to find some way of converting solar energy into other forms of energy we can use more easily, such as electricity. And that's exactly what solar panels do.Solar cellA solar cell is a device that converts the energy of sunlight directly into electricity by the photovoltaic effect. Sometimes the term solar cell is reserved for devices intended specifically to capture energy from sunlight such as solar panels and solar cells, while the term photovoltaic cell is used when the light source is unspecified. Assemblies of cells are used to make solar panels, solar modules, or photovoltaic arrays. Photovoltaics is the field of technology andresearch related to the application of solar cells in producing electricity for practical use. The energy generated this way is an example of solar energy.History of solar cellsThe development of the solar cell stems from the work of the French physicist Antoine-César Becquerel in 1839. Becquerel discovered the photovoltaic effect while experimenting with a solid electrode in an electrolyte solution; he observed that voltage developed when light fell upon the electrode. About 50 years later, Charles Fritts constructed the first true solar cells using junctions formed by coating the semiconductor selenium with an ultrathin, nearly transparent layer of gold. Fritts's devices were very inefficient, transforming less than 1 percent of the absorbed light into electrical energy.By 1927 another metalÐsemiconductor-junction solar cell, in this case made of copper and the semiconductor copper oxide, had been demonstrated. By the 1930s both the selenium cell and the copper oxide cell were being employed in light-sensitive devices, such as photometers, for use in photography. These early solar cells, however, still had energy-conversion efficiencies of less than 1 percent. This impasse was finally overcome with the development of the silicon solar cell by Russell Ohl in 1941. In 1954, three other American researchers, G.L. Pearson, Daryl Chapin, and Calvin Fuller, demonstrated a silicon solar cell capable of a 6-percent energy-conversion efficiency when used in direct sunlight. By the late 1980s silicon cells, as well as those made of gallium arsenide, with efficiencies of more than 20 percent had been fabricated. In 1989 a concentrator solar cell, a type of device in which sunlight is concentrated onto the cell surface by means of lenses, achieved an efficiency of 37 percent due to the increased intensity of the collected energy. In general, solar cells of widely varying efficiencies and cost are now available.StructureModern solar cells are based on semiconductor physics -- they are basically just P-N junction photodiodes with a very large light-sensitive area. The photovoltaic effect, which causes the cell toconvert light directly into electrical energy, occurs in the three energy-conversion layers.The first of these three layers necessary for energy conversion in a solar cell is the top junction layer (made of N-type semiconductor ). The next layer in the structure is the core of the device; this is the absorber layer (the P-N junction). The last of the energy-conversion layers is the back junction layer (made of P-type semiconductor).As may be seen in the above diagram, there are two additional layers that must be present in a solar cell. These are the electrical contact layers. There must obviously be two such layers to allow electric current to flow out of and into the cell. The electrical contact layer on the face of the cell where light enters is generally present in some grid pattern and is composed of a good conductor such as a metal. The grid pattern does not cover the entire face of the cell since grid materials, though good electrical conductors, are generally not transparent to light. Hence, the grid pattern must be widely spaced toallow light to enter the solar cell but not to the extent that the electrical contact layer will have difficulty collecting the current produced by the cell. The back electrical contact layer has no such diametrically opposed restrictions. It need simply function as an electrical contact and thus covers the entire back surface of the cell structure. Because the back layer must be a very good electrical conductor, it is always made of metal.How do solar cells workA solar cell is a sandwich of n-type silicon (blue) and p-type silicon (red).1.When sunlight shines on the cell, photons (light particles)bombard the upper surface.2.The photons (yellow blobs) carry their energy down through thecell.3.The photons give up their energy to electrons (green blobs) inthe lower, p-type layer.4.The electrons use this energy to jump across the barrier into theupper, n-type layer and escape out into the circuit.5.Flowing around the circuit, the electrons make the lamp lightup.Solar Power - Advantages and Disadvantages Solar Power AdvantagesThere are many advantages of solar energy. Just consider the advantages of solar energy over that of oil:· Solar energy is a renewable resource. Although we cannot utilize the power of the sun at night or on stormy, cloudy days, etc., we can count on the sun being there the next day, ready to give us more energy and light. As long as we have the sun, we can have solar energy (and on the day that we no longer have the sun, you can believe that we will no longer have ourselves, either).· Oil, on the other hand, is not renewable. Once it is gone, it is gone. Yes, we may find another source to tap, but that source may run out, as well.· Solar cells are totally silent. They can extract energy from the sun without making a peep. Now imagine the noise that the giant machines used to drill for and pump oil make!· Solar energy is non-polluting. Of all advantages of solar energy over that of oil, this is, perhaps, the most important. The burning of oil releases carbon dioxide and other greenhouse gases and carcinogens into the air.·Solar cells require very little maintenance (they have no moving parts that will need to be fixed), and they last a long time.· Although solar panels or solar lights, etc., may be expensive to buy at the onset, you can save money in the long run. After all, you do not have to pay for energy from the sun. On the other hand, all of us are aware of the rising cost of oil.· Solar powered lights and other solar powered products are also very easy to install. You do not even need to worry about wires.Here are the disadvantages of solar energy:•The initial cost is the main disadvantage of installing a solar energy system, largely because of the high cost of thesemi-conducting materials used in building one.•The cost of solar energy is also high compared tonon-renewable utility-supplied electricity. As energy shortages are becoming more common, solar energy is becoming moreprice-competitive.•Solar panels require quite a large area for installation to achievea good level of efficiency.•The efficiency of the system also relies on the location of the sun, although this problem can be overcome with the installation of certain components.•The production of solar energy is influenced by the presence of clouds or pollution in the air.•Similarly, no solar energy will be produced during nighttime although a battery backup system and/or net metering willsolve this problem.Development, deployment and economicsBeginning with the surge in coal use which accompanied the Industrial Revolution, energy consumption has steadily transitioned from wood and biomass to fossil fuels. The early development of solar technologies starting in the 1860s was driven by an expectation that coal would soon become scarce. However development of solar technologies stagnated in the early 20th century in the face of the increasing availability, economy, and utility of coal and petroleum.The 1973 oil embargo and 1979 energy crisis caused a reorganization of energy policies around the world and brought renewed attention to developing solar technologies.Deployment strategies focused on incentive programs such as the Federal Photovoltaic Utilization Program in the US and the Sunshine Program in Japan. Other efforts included the formation of research facilities in the US (SERI, now NREL), Japan (NEDO), and Germany (Fraunhofer Institute for Solar Energy Systems ISE).Between 1970 and 1983 photovoltaic installations grew rapidly, but falling oil prices in the early 1980s moderated the growth of PV from 1984 to 1996.Photovoltaic production growth has averaged 40% per year since 2000 and installed capacity reached 10.6 GW at the end of 2007,and 14.73 GW in 2008.Since 2006 it has beeneconomical for investors to install photovoltaics for free in return for a long term power purchase agreement. 50% of commercial systems were installed in this manner in 2007 and it is expected that 90% will by 2009. Nellis Air Force Base is receiving photoelectric power for about 2.2 ¢/kWh and grid power for 9 ¢/kWh.Commercial concentrating solar thermal power (CSP) plants were first developed in the 1980s. CSP plants such as SEGS project in the United States have a levelized energy cost (LEC) of 12–14 ¢/kWh.The 11 MW PS10 power tower in Spain, completed in late 2005, is Europe's first commercial CSP system, and a total capacity of 300 MW is expected to be installed in the same area by 2013.In August 2009, First Solar announced plans to build a 2 GW photovoltaic system in Ordos City, Inner Mongolia, China in four phases consisting of 30 MW in 2010, 970 MW in 2014, and another 1000 MW by 2019. As of June 9, 2009, there is a new solar thermal power station being built in the Banaskantha district in North Gujarat. Once completed, it will be the world's largest.。
氨燃料电池电极反应式

氨燃料电池电极反应式英文回答:Ammonia fuel cells are a type of fuel cell that use ammonia as the fuel source. The electrode reactions in an ammonia fuel cell involve the oxidation of ammonia at the anode and the reduction of oxygen at the cathode.At the anode, ammonia is oxidized to nitrogen gas and protons according to the following reaction:2NH3 + 6OH-> N2 + 6H2O + 6e-。
This reaction releases electrons, which flow through an external circuit to the cathode. At the cathode, oxygen from the air is reduced to water by accepting electrons and protons:O2 + 4e+ 2H2O -> 4OH-。
The overall reaction of an ammonia fuel cell can be represented as:2NH3 + 5O2 -> N2 + 6H2O.In this reaction, ammonia and oxygen combine to produce nitrogen gas and water. The protons generated at the anode travel through an electrolyte membrane to the cathode, where they combine with oxygen and electrons to form water.Ammonia fuel cells have several advantages. Firstly, ammonia is a readily available and relatively inexpensive fuel source. It can be produced from renewable sources such as biomass or from the Haber-Bosch process, which converts nitrogen and hydrogen into ammonia. Secondly, ammonia has a high energy density, meaning that a small amount of ammonia can produce a large amount of energy. This makes ammonia fuel cells suitable for applications that require high power density, such as electric vehicles or portable electronic devices.Furthermore, ammonia fuel cells have a high conversionefficiency. The oxidation of ammonia at the anode and the reduction of oxygen at the cathode are both highlyefficient processes, resulting in a high overall efficiency of the fuel cell. This means that a larger percentage of the energy in the ammonia fuel is converted into electrical energy, making ammonia fuel cells more energy-efficient than other types of fuel cells.In addition, ammonia fuel cells have a long lifespan and are resistant to poisoning. Unlike other fuel cell technologies, ammonia fuel cells are not easily affected by impurities or contaminants in the fuel. This makes them more durable and reliable, as they can operate for longer periods of time without the need for maintenance or replacement.中文回答:氨燃料电池是一种使用氨作为燃料的燃料电池。
可再生能源化工 英语

可再生能源化工英语English:Renewable energy chemical engineering is a field of study and work that focuses on the development and utilization of chemical processes and technologies for the production, storage, and utilization of energy from renewable sources such as solar, wind, hydro, and bioenergy. This interdisciplinary field combines principles of chemical engineering, materials science, and environmental science to develop innovative solutions for the transition towards a more sustainable and low-carbon energy system. In renewable energy chemical engineering, professionals work on designing and optimizing processes for the conversion of renewable resources into clean energy products, such as biofuels, hydrogen, and electricity. They also focus on developing advanced energy storage technologies, such as batteries and fuel cells, to enhance the integration of renewable energy into the existing energy infrastructure. Furthermore, renewable energy chemical engineers are also involved in the development of sustainable materials and technologies that can reduce the environmental impact of energy production and utilization. Overall, renewable energy chemicalengineering plays a crucial role in addressing the challenges of climate change and promoting a more sustainable and resilient energy future.中文翻译:可再生能源化工是一门致力于开发和利用化学工艺和技术,以生产、储存和利用太阳能、风能、水能和生物能等可再生能源的领域。
纳米技术的利用英语作文

纳米技术的利用英语作文英文回答:In the realm of scientific advancement, nanotechnology has emerged as a game-changer, offering unprecedented opportunities to manipulate matter at the atomic and molecular scale. This extraordinary field holds immense promise for revolutionizing diverse industries and sectors, spanning from healthcare and energy to manufacturing and environmental protection.Healthcare:Nanotechnology presents groundbreaking possibilities in healthcare by enabling targeted drug delivery, early disease detection, and regenerative medicine. Nanoparticles can be engineered to encapsulate and deliver therapeutic agents directly to diseased cells, enhancing efficacy and reducing side effects. Nanosensors can detect minute concentrations of disease markers, facilitating earlydiagnosis and personalized treatment plans. Moreover, nanomaterials can serve as scaffolds for tissue engineering, offering hope for organ repair and regeneration.Energy:Nanotechnology holds the key to unlocking clean and sustainable energy sources. Nano-structured materials can enhance the efficiency of solar cells and fuel cells, enabling more efficient conversion of renewable energy. Nanomaterials also find applications in energy storage systems, improving the capacity and lifespan of batteries. By paving the way for cleaner energy generation and storage, nanotechnology contributes to a more sustainable future.Manufacturing:In the manufacturing sector, nanotechnology introduces novel materials with tailored properties. Nanoscalecoatings can enhance the durability and performance of surfaces, while nano-engineered composites offer improved strength and lightness. Nanotechnology also enables thefabrication of miniaturized and multifunctional devices, leading to advancements in electronics, robotics, and sensing technologies.Environmental Protection:Nanotechnology has the potential to revolutionize environmental protection. Nanoparticles can be employed as catalysts for the degradation of pollutants, reducing air and water contamination. Nanofilters can effectively remove contaminants from water sources, ensuring clean and safe drinking water. Moreover, nanomaterials can enhance the efficiency of solar energy conversion, promoting sustainability and mitigating greenhouse gas emissions.Challenges and Future Directions:Despite its immense potential, the deployment of nanotechnology faces ethical and safety considerations. The potential risks associated with the environmental and human health implications of nanomaterials require careful evaluation. Ongoing research focuses on developingsustainable and biocompatible nanomaterials, as well as establishing robust regulatory frameworks for their responsible use.As we move forward, nanotechnology is poised to shape the future across multiple disciplines. By unlocking new frontiers of scientific exploration and technological innovation, it has the potential to address global challenges, improve human health, and forge a sustainable path for generations to come.中文回答:纳米技术在各个领域中的应用。
猪诱导多能干细胞可定向分化为前脑GABA能神经元前体

doi 10.12122/j.issn.1673-4254.2021.06.03
J South Med Univ, 2021, 41(6): 820-827
猪诱导多能干细胞可定向分化为前脑 GABA 能神经元前体
朱 缓 1,孙婷婷 2,王圆圆 2,王 铁 2,马彩云 2,王春景 2,刘长青 2,郭 俣 1 蚌埠医学院 1检验医学院,2生命科学学院,安徽 蚌埠 233000
摘A 能神经元前体的方法体系。方法 猪 iPSCs 诱导分化为 GABA 能神经元前体遵循两个阶段,第 1 阶段,猪 iPSCs 悬浮培养,第 3 天时形成类胚体,采用神经诱导培养基 NIM(SB431542、 DMH1、FGF2)继续诱导,第 12 天分化为原始神经上皮细胞。第 2 阶段,使用含 Pur、B27 的 NIM 培养基悬浮培养形成神经球,至 第 21 天时形成 GABA 能神经元前体。CM-DiI 标记后,定向移植帕金森(PD)模型大鼠黑质纹状体,检测其在宿主脑内存活、迁 移及分化状况。结果 猪 iPSCs 在饲养层细胞上稳定传代,表达多能性标记 OCT4、Nanog、SSEA1 和 TRA-160,并且核型分析显 示没有其他物种来源细胞污染。第 12 天经诱导分化获得原始神经上皮细胞能够形成玫瑰花环结构,并表达其表面标记物 (PAX6、SOX2 和 Nestin)与神经微管蛋白标志物 Tuj1。第 21 天诱导细胞高表达 GABA 能神经元前体的表面特异性抗原 NKX2.1 和前脑标志物 FOXG1。移植 8 周后,体内可分化为 GABA 能神经元与多巴胺能神经元,明显改善 PD 大鼠运动行为。 结论 结合无血清培养基筛选法逐步定向诱导猪 iPSCs 高效分化为前脑 GABA 能神经元前体,移植后能够显著改善 PD 大鼠的 运动功能障碍,为诱导 GABA 能神经元前体移植治疗神经损伤疾病奠定基础。 关键词:诱导多能干细胞,猪;细胞分化;原始神经上皮细胞;GABA 能神经元前体;帕金森模型大鼠
想象生活中的纳米技术作文

想象生活中的纳米技术作文English Response:Nanotechnology in Everyday Life.Nanotechnology, the manipulation of matter on an atomic and molecular scale, has become increasingly prevalent in our daily lives. From electronics to medicine, its applications are vast and diverse.In the realm of electronics, nanotechnology has revolutionized the way we interact with technology. Take, for example, the ubiquitous smartphone. The touchscreen technology relies on nanoscale materials such as indium tin oxide, allowing for sensitive touch recognition and vibrant displays. Furthermore, the miniaturization of transistors using nanotechnology has led to faster processors and increased memory capacity, enhancing the performance of electronic devices.In the medical field, nanotechnology holds tremendous promise for diagnosing and treating diseases. Nanoparticles can be engineered to target specific cells or tissues, enabling precise drug delivery while minimizing side effects. For instance, researchers are exploring the use of nanobots to deliver drugs directly to cancer cells, sparing healthy tissue from damage.Moreover, nanotechnology has found its way into everyday consumer products, such as clothing and sunscreen. Nanomaterials embedded in fabrics can impart propertieslike stain resistance and UV protection. Similarly, nanoparticles in sunscreen formulations provide enhanced protection against harmful UV rays without leaving a white residue on the skin.In transportation, nanotechnology plays a crucial role in improving fuel efficiency and reducing emissions. Lightweight nanocomposites are utilized in vehicle manufacturing to enhance structural integrity while minimizing weight. Additionally, catalytic converters containing nanomaterials facilitate more efficientconversion of harmful exhaust gases into less harmful substances, thereby reducing air pollution.In summary, nanotechnology permeates various aspects of our lives, enhancing convenience, improving health outcomes, and promoting sustainability. As the field continues to advance, we can expect even more innovative applicationsthat redefine the boundaries of what is possible.中文回答:纳米技术在日常生活中的应用。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Efficient conversion of ES cells into myogenic lineage usingthe gene-inducible systemShiro Ozasa a ,Shigemi Kimura a,*,Kaori Ito a,b ,Hiroe Ueno a ,Makoto Ikezawa a ,Makoto Matsukura a,b ,Kowashi Yoshioka a ,Kimi Araki c ,Ken-ich Yamamura c ,Kuniya Abe d ,Hitoshi Niwa e ,Teruhisa Miike aaDepartment of Child Development,Kumamoto University Graduate School,1-1-1Honjo,Kumamoto City,Kumamoto 862-8556,JapanbLaboratory of Clinical Pharmacology and Therapeutics,Sojo University,Kumamoto,Japan cDivision of Developmental Genetics,Kumamoto University Graduate School,Kumamoto,JapandResearch &Development Team for Mammalian Cellular Dynamics,RIKEN Tsukuba Institute,Tsukuba,JapaneLaboratory of Pluripotent Cell Studies,RIKEN Center for Developmental Biology,Kobe,JapanReceived 2April 2007Available online 17April 2007AbstractWe established genetically engineered ES (ZHTc6-MyoD)cells that harbor a tetracycline-regulated expression vector encoding myogenic transcriptional factor MyoD,for the therapy of muscle diseases,especially Duchenne muscular dystrophy (DMD).Almost all the ZHTc6-MyoD cells were induced into muscle lineage after removal of tetracycline.The undifferentiated ZHTc6-MyoD cells are Sca-1+and c-kit+,but CD34À,all well-known markers for mouse hematopoietic stem cells.In addition,they are able to maintain themselves in the undifferentiated state,even after one month of culture.Therefore,it is possible to obtain a large quantity of ZHTc6-MyoD cells in the undifferentiated state that maintain the potential to differentiate only into muscle lineage.Additionally,at two weeks post-injection of these cells into muscle of mdx ,a model mouse of DMD,clusters of dystrophin-positive myofibers were observed at the injection site.Therefore,ES cells have considerable therapeutic potential for treating muscle diseases.Ó2007Elsevier Inc.All rights reserved.Keywords:Embryonic stem cells;Tetracycline-regulatable system;Myogenic differentiation;Duchenne muscular dystrophyDuchenne muscular dystrophy (DMD)is a severe mus-cle degenerative disorder caused by mutations in the dys-trophin gene [1].Stem cell transplantation is considered to be one of the most promising therapies for the disease.Specifically,side population cells (SP cells)[2]derived from bone marrow have garnered considerable interest because of their ability to migrate into,and undergo myogenic spec-ification,in affected muscles [3].Similarly,SP cells derived from muscles have been shown to have similar characteris-tics as those from bone marrow.The migration of these cells into muscle after intravenous injection is insufficient for ameliorating the cumulative effect of muscle weaknesscaused by the disease [4,5]and a large dosage of cells may therefore need to be injected.However,given that less than 1%of these cells can be isolated from bone marrow,a limiting factor of this approach has been that these cells cannot be obtained in sufficiently large quantities.In addi-tion,these cells are difficult to culture in an undifferentiated condition for extended periods of time.By contrast,embryonic stem (ES)cells have consider-able advantages over SP cells:they can be cultured for extended periods in an undifferentiated and pluripotent condition.The possibility of invoking specific myogenic differentiation has been investigated in several studies.Thus far,however,these approaches have not succeeded in achieving the differentiation of ES cells only into muscle lineage.0006-291X/$-see front matter Ó2007Elsevier Inc.All rights reserved.doi:10.1016/j.bbrc.2007.04.032*Corresponding author.Fax:+81963735200.E-mail address:kimusige@kaiju.medic.kumamoto-u.ac.jp (S.Kimura)./locate/ybbrcBiochemical and Biophysical Research Communications 357(2007)957–963The tetracycline-regulatable(Tet-Off)gene expression system[6]has been shown to confer inducibility of trans-gene expression in several cell types in culture and in vivo.However,establishing the system in ES cells has proven problematic.This is probably due to the combina-tion of the relatively toxic effects of the tet repressor-VP16 fusion(tTA)and the tendency of ES cells to undergo trans-gene silencing.However,an ES cell line,ZHTc6,has been isolated,which is regulated by the Tet-Offsystem[7].Here, we propose a novel approach to controlling specific muscle lineage differentiation of ES cells by introducing MyoD cDNA into a tetracycline regulatory expression site in ZHTc6cells.Materials and methodsCell culture conditions and staining.ZHTc6are feeder-free ES cells derived from CGR8,which are129/Ola mouse-derived wild-type ES cells (Fig.1A)[7].ZHTc6cells carry a targeted integration of IRESzeo in one Oct3/4allele.They also carry an integrated gene trap construct, IRES hph:CAGtTA,that confers stable expression of the tetracycline-responsive tTA transactivator and a randomly integrated hCMV*-1-Oct3/ 4-IRES b geopA transgene.The hCMV*-1-Oct3/4-IRES b geopA transgene is comprised of the tetracycline-inducible promoter hCMV*-1(derived from pUHD10-3),the rabbit b-globin second intron,full-length Oct3/4 cDNA,and IRES b geopA unit[8].The ZHTc6ES cells were grown in maintenance medium,according to the protocols of Dr Niwa et al.[8,7].To induce myogenesis,maintenance medium was changed to differentiation medium[leukemia inhibitory fac-tor(LIF)(Invitrogen,CA,USA)was removed,and15%Knockout SR (Invitrogen,CA,USA)was changed to4%fetal bovine serum(FBS)].4%, 8%,and20%FBS were tested to see which concentration produced the best differentiation medium.The4%FBS was best,though the differen-tiation of ZHTc6-MyoD cells to myotubes also was observed with8%and 20%FBS(data not shown).X-gal staining was performed by standard protocol,and alkaline phosphatase activity was analyzed using an alkaline phosphatase staining kit(Sigma,MO,USA),Supertargeting.STV-MyoD was constructed for Supertargeting,as shown in Fig.1A[8].After the introduction of STV-MyoD to ZHTc6cells by electroporation,the cells were selected in the presence of200or300l g/ mL G418and100ng/mL doxycycline.Targeted clones were maintained in the presence of100ng/mL doxycycline and80l g/mL hygromycin (Invitrogen,CA,USA).Southern blotting.Genomic DNA from cells was digested with restriction enzyme Sac I(Takara,Mie,Japan),and Southern blot analysis was performed according to standard protocol.(Amersham,NJ,USA). An Eco RI–Sac I fragment of the LacZ gene as probe(Fig.1A)was labeled with[a-32P]dCTP(Amersham,NJ,USA)by using a Megaprime DNA labeling system(Amersham,NJ,USA).Western blotting.The cells were homogenized in100l l of a proteolytic solution(62.5l M Tris–HCl(pH6.8),20%Glycerol,2%SDS,0.05%b-mercaptoethanol,0.00125%bromophenol blue),boiled for10min,and then centrifuged.Two hundred micrograms of total proteins was taken from the supernatants and loaded on an SDS–polyacrylamide gel,withthe Fig.1.Generation of an inducible MyoD transgene integration by supertargeting.(A)Schematic of the supertargeting strategy for introduction of MyoD into a tetracycline-regulatable expression site.ZHTc6ES cells contain a tetracycline-regulated transgene comprising the hCMV*-1promoter,b-globin second intron,Oct-3/4open reading frame,and IRESgeopA selection marker.(B)Analysis of the supertargeting event in Gp ES cells.X-gal-positive clones(Gp)and X-gal-negative clones(Gn)were analyzed by Southern hybridization.C,control(the original ZHTc6cells before supertargeting);IRES, encephalomyocarditis internal ribosome entry site;hph,hygromycin phosphotransferase;CAG,CAG expression unit(cytomegalovirus enhancer,chicken b-actin promoter,and rabbit b-globin poly(A)signal);tTA,tetracycline-controlled transactivator;hCMV*-1,tTA-dependent promoter(tetR binding motif+human cytomegalovirus minimal promoter);b-geo,Escherichia coli b-galactosidase(LacZ)+neomycin phosphotransferase(neo r)fusion gene; loxP,site-specific recombination target sequence recognized by Cre recombinase;MC1tk,herpes simplex virus thymidine kinase(HSV tk)gene without pA under the control of MC1expression unit(PyF101mouse polyoma virus mutant enhancer+HSV tk minimal promoter).958S.Ozasa et al./Biochemical and Biophysical Research Communications357(2007)957–963use of a3%stacking gel and a4–12%gradient resolving gel.The frac-tionated proteins were transferred to nitrocellulose membranes(Bio-Rad, CA,USA)and then incubated with anti-MyoD1(Dako,Copenhagen, USA,Cat.No.M3512)at1:10,monoclonal anti-desmin(Sigma,MO, USA,Cat.No.D1033)at1:200,monoclonal anti-myogenin(Sigma,MO, USA,Cat.No.M5815)at1:20,monoclonal anti-dystrophin(Novocastra, Newcastle,USA,Cat.No.NCL-DYS2)at1:10dilutions.The resulting immune complexes were detected with affinity-purified anti-mouse IgG (H+L)(Vector Lab.Inc.,CA,USA)using ABC method(Vector Lab. Inc.,CA,USA).Flow cytometry.Undifferentiated and3-day-differentiated Gp1cells were stained withfluorescein isothiocyanate(FITC)-conjugated anti-mouse-CD34antibody(BD Bioscience,CA,USA,Cat.No.553733), phycoerythrin(PE)-conjugated anti-mouse-c-kit antibody(BD Bioscience, CA,USA,Cat.No.553355),and allophycocyanin(APC)-conjugated anti-mouse-Sca1antibody(BD Bioscience,CA,USA,Cat.No.17-5981-81). Approximately1·106cells were stained,and5000–10,000live cells were analyzed using a FACScalibur(BD Bioscience,CA,USA).Positively stained cells were quantitated using CellQuest software(BD Bioscience, CA,USA).Immunofluorescent microscopy of cells.Cells to be immunostained were grown on collagen type I-coated4-chamber slide glasses(IWAKI,Tokyo, Japan).After the cells werefixed,the immunostainings of cells were per-formed using the following antibodies:mouse monoclonal anti-Pax7 (R&D Systems,MN,USA,Cat.No.MAB1675)at1:20,the above anti-MyoD1at1:20,the above anti-desmin at1:100,mouse monoclonal anti-fast myosin heavy chain(fMHC)(Sigma,MO,USA,Cat.No.M4276)at 1:100,and rabbit polyclonal anti-dystrophin(Santa Cruz,CA,USA,Cat. No.SC-15376)at1:20dilutions.Secondary antibodies used were as fol-lows:rhodamine-conjugated goat anti-mouse IgG(Chemicon,CA,USA) at1:100for mouse antibodies,FITC-conjugated goat anti-rabbit IgG (Chemicon,CA,USA)at1:100,dilutions for rabbit antibody.After rinsing,thefluorescein was visualized using appropriatefilter sets.The percentages of positive cells and standard deviations for each specific antibody were calculated by counting the number of positive and negative cells based onfive high-powerfield digital images,paired with differential interference contrast microscopic images,taken randomly at200·.Intramuscular injection of Gp cells into mdx mice.Undifferentiated Gp1 cells were cultured for3days in the absence of doxycycline before injec-tion.After anesthesia,1·106Gp1cells were injected intramuscularly into the gastrocnemius muscles of8-week-old immunodeficient mdx-nude mice,and immunocompetent mdx mice.Two mdx-nude mice were sacri-ficed at7weeks post-injection for histochemistry of tissue sections.Four and two mdx mice were sacrificed at2and10weeks,respectively.Immunofluorescent microscopy of specimens.For histochemistry of tissue sections,samples were frozen in liquid nitrogen-cooled isopentane and cryostat-sectioned.The rabbit polyclonal anti-dystrophin(Santa Cruz,CA,USA,Cat.No.SC-15376)was used asfirst antibody,and FITC-conjugated anti-rabbit IgG antibody(Chemicon,CA,USA)was used as second antibody.For the quantification of dystrophin-positive myofibers,the gastrocnemius muscle sections with the highest number of dystrophin-positive myofibers were selected and the number of total dystrophin-positivefibers counted.C57BL/10wild-type mice(B10)were used for positive controls,and mdx mice were used for negative controls.Detection of injected cells by RT-PCR analysis.Total RNA from40to 50pieces of serial frozen sections with20l m thickness each,i.e.,the same segments of the injected mdx muscle used for immunohistochemistry,were isolated using TRIZOL Reagent(Invitrogen,CA,USA),according to the manufacturer’s instructions.After treatment with DNase I,the isolated RNA was reverse transcribed using Superscript II reverse transcriptase (Invitrogen,CA,USA)and random hexamer primers,according to the manufacturer’s instructions.RT-PCR analysis was performed in order to distinguish ES cells(wild-type)-differentiated myofibers from myofibers in mdx mouse,using the specific primers50-CTCTGCAAAGTTCTTTGA AAGAGTAA(forward)and50-GAAGTTTATTCATATGTTCTTCT AGC(reverse)[9].The PCR product includes exon23of the dystrophin gene,in which mdx mouse has the relevant point mutation.Additionally, the PCR product was digested by Mae III.ResultsIsolation of mouse-derived ES cell lines by tetracycline regulation(Tet-Off)Supertargeting is an effective strategy for producing Tet-OffSystem-regulated ES cells for controlling the MyoD gene[7,8](Fig.1A).G418-resistant clones were duplicated and screened for b-galactosidase expression in the presence of doxycycline by X-gal staining.b-Galactosidase-positive (Gp)and b-galactosidase-negative(Gn)clones were ana-lyzed by Southern hybridization(Fig.1B).A3.2-kb Sac I fragment was detected with a probe(Fig.1A)from the 50-end of LacZ gene in the Gp samples,which is indicative of the correct replacement of the Oct-3/4cDNA sequence with MyoD sequence.The Gn clone retained the4.8-kb fragment diagnostic for the original Oct-3/4transgene inte-gration in ZHTc6cells.Three clones,Gp1,Gp2and Gp3, were then analyzed further.They were named ZHTc6-MyoD cells.Induction of differentiation of Gp clonesThe Gp1,Gp2,and Gp3clones were observed to main-tain alkaline phosphatase activity even after one month of culture.After change to differentiation medium,the mor-phology of the cells was round initially,and then became long and spindly in appearance.By day7,almost all the cells began to fuse into myotubes,and occasional twitching of fused ES cells was observed,with some parts of the twitching myotubes becoming detached from the surface of the dish(Fig.2A and B)MyoD expression in the differ-entiated cells was confirmed by Western blot analysis (Fig.2C).Desmin expression in these differentiated cells was also detected from day4onwards after induction of differentiation,myogenin from day4onwards,and dystro-phin from day8onwards(Fig.2C).Pax7,MyoD,desmin,fMHC,and dystrophin expres-sion also were investigated by immunocytochemical analy-sis.Pax7expression was transiently observed only from day3to day5(Supplementary Figure1).The percentage of pax7-positive cells was highest on day3(68±11%) and declined to zero percent by day7.MyoD expression was observed from day3to day14and was localized in the nuclei(Supplementary Figure1);desmin expression was detected from day5to day14.Once MyoD-positive and desmin-positive cells appeared,the percentage of MyoD and desmin-positive cells observed reached nearly 100%,and these cells preserved their expression to the end of the experimental period.Fast myosin heavy chain and dystrophin expression were also detected,mainly on myotubes,from day10,and positive-percentages reached 66±18%and23±13%,respectively,by day14(Supple-mentary Figure1).By day14,more than half of the myo-tubes were detached from the dish,probably by contraction.However,the differentiated Gp1,Gp2,andS.Ozasa et al./Biochemical and Biophysical Research Communications357(2007)957–963959Gp3cells were unexpectedly negative for LacZ activity.The LacZ gene is driven by the MC1promoter,which is constitutive in itself.To further investigate the loss of LacZ expression upon differentiation,we performed RT-PCR analysis of Gp1cells.Transcripts of the LacZ and neo resistance genes were detected in the undifferentiatedGp1Fig.2.Phase contrast micrographs of Gp cells in the presence or absence of doxycycline and analysis of muscle-specific gene expressions after differentiation.(A)Gp1cells cultured in the presence of doxycycline formed colonies,indicative of undifferentiated state.(B)Gp1cells cultured for 10days in the absence of doxycycline morphed into long spindly cells.Bar,200l m.(C)Expression of dystrophin,desmin,MyoD,and myogenin detected by Western blot analysis during in vitro differentiation of Gp1cells investigated from day 0to 14.The MyoD expression in the differentiated cells was confirmed from day 2after the removal of doxycycline.Desmin was also detected from day 4,myogenin from day 4and dystrophin from day8.Fig.3.Flow cytometric analysis of undifferentiated and differentiated Gp cells.(A)Undifferentiated and (B)6-day-differentiated Gp1cells were stained for the expression of Sca1,c-kit,and CD34.In the undifferentiated cells,Sca1and c-kit were doubly expressed,whereas in the differentiated cells,only low expression of c-kit and Sca1was detected.CD34was rarely present in either undifferentiated or differentiated cells.960S.Ozasa et al./Biochemical and Biophysical Research Communications 357(2007)957–963cells but not in3-day-differentiated Gp1cells(data not shown).The cause of this problem may have been due to MC1promoter activity,which is suitable for gene expres-sion in immature cells,such as stem cells,but not in differ-entiated cells.Analysis of surface antigens of Gp clonesThe cell surface antigens,Sca1,c-kit,and CD34,are all well-established markers of mouse hematopoietic stem cells.The changes of these cell surface antigens in Gp1cells Fig.4.Detection of dystrophin expression of injected ZHTc6-MyoD cells in muscle of mdx-nude and mdx mouse.(A,B)A few dystrophin-positivefibers were detected in the tumor at7weeks post-injection into mdx-nude.(C)The clusters of dystrophin-positivefibers were observed at two weeks post-injection.(D)The serial section of(C).Arrows show dystrophin-negativefibers.(E)Positive control(B10mouse).(F)Negative control(mdx mouse).(A, C,E,and F)an immunohistochemical study with anti-dystrophin antibodies;(B,D)haematoxylin–eosin staining.Bar,50l m.(G)RT-PCR analysis for dystrophin expression of ES cell.Gp1-derived wild-type dystrophin expression is indicated by the25-bp ne1,DNA molecular weight marker XIII (50–750bp)(Roche,Germany);lane2,control B10muscle;lane3,control mdx muscle;lane4,injected mdx muscle.S.Ozasa et al./Biochemical and Biophysical Research Communications357(2007)957–963961pre-and post-differentiation were compared byflow cyto-metric analysis.The undifferentiated Gp1cells expressed Sca1and c-kit intensely(99.3%±0.6%and90.5%±0.8%,respectively), but expression levels decreased dramatically by day6 post-differentiation(13.3%±0.1%and 6.8%±0.3%, respectively;Fig.3).Conversely,little expression of CD34 was observed for both undifferentiated and differentiated Gp1cells(0.05%±0.02%and0.11%±0.08%,respec-tively;Fig.3).Transplantation of Gp cells into mdx miceTumor formation(20mm in diameter)was observed at the injected area of two mdx-nude mice at7weeks post-injec-tion.A few dystrophin-positivefibers at sarcolemma of myofibers,surrounded by non-myofiber cells that are thought to be non-differentiated injected ES cells,were detected at the injection areas(Fig.4A and B).However, the area did not include various organs like teratoma.In con-trast,tumors were not detected in the mdx mice,even at10 weeks post-injection,and clusters of many dystrophin-posi-tive myofibers in the injected area were observed(Fig.4C). Haematoxylin-eosin staining showed variation infiber size and centrally nucleated myofibers in these areas(Fig.4D). The average and standard deviation of the numbers of total dystrophin-positivefibers in the four injected mdx muscles was2727±330.The dystrophin-positive myofibers were negative for LacZ activity by X-gal assay(data not shown), which was consistent with the in vitro data shown above. Detection of dystrophin transcripts in mdx mice injected with Gp CellsThe normal dystrophin gene gave a150-bp band plus two25-bp fragments,whereas the mutated dystrophin gave a150-bp band plus a single50-bp fragment.Wild-type dys-trophin mRNAs were found in the muscles of injected mice (Fig.4G),suggesting that transplanted Gp1cells partici-pated in myogenesis.DiscussionWe established a mouse ES cell line(ZHTc6-MyoD), which not only proliferates indefinitely in an undifferenti-ated state in the presence of doxycycline,but also retains the potential to differentiate almost exclusively into myogenic lineage when exposed to a doxycycline-free environment.Morphologically,undifferentiated ZHTc6-MyoD cells are round,but fusing and twitching was observed at7days after differentiation.In addition,the myogenic conversion of the cells was confirmed by the expression of the myo-genic markers,desmin,myogenin(which is a skeletal mus-cle-specific transcriptional factor),and dystrophin[1]. These muscle-specific gene expressions were also confirmed by immunocytochemical analysis,using anti-Pax7,MyoD,desmin,fMHC,and dystrophin antibodies.In particular, Pax7is an important transcription factor for progenitor cells destined to differentiate into skeletal muscles[10]. The results,therefore,demonstrated that the differentiation of ZHTc6-MyoD cells into the skeletal muscle lineage was controlled by MyoD under a Tet-Offsystem.We demonstrated that the undifferentiated ZHTc6-MyoD cells were Sca-1+and c-kit+,but CD34À,a profile that is partly reminiscent of the expression pattern of bone marrow SP cells[3,11].However,this is not unique to ZHTc6-MyoD cells,because undifferentiated ZHTc6cells are similar to ZHTc6-MyoD(data not shown)regarding surface antigens[12–15].These results and alkaline phos-phatase staining show that our ZHTc6-MyoD cells,before removal of doxycycline,still retain an undifferentiated state.When ZHTc6-MyoD cells were differentiated into muscle lineage,a few cells still remained round in spite of the induction to differentiate.In order to confirm that they were still in the undifferentiated state,these round cells were extracted,returned to the growth condition and allowed to grow,and then again induced to differen-tiate into myotubes by removal of doxycycline.Interest-ingly,these round cells differentiated to myotubes(data not shown).In addition,the ZHTc6-MyoD cells died in the presence of gancyclovir,because the cells have a thymidine kinase gene(tk)(Fig.1A)(data not shown). These data showed that undifferentiated round cells were not contaminated with b-galactosidase-negative(Gn) clones,and they might maintain their undifferentiated state like muscle quiescent satellite cells and reserve cells [16].We then investigated whether ZHTc6-MyoD cells have the potential to differentiate into myofibers in vivo.At two weeks post-injection of ZHTc6-MyoD cells to muscles of mdx,clusters of dystrophin-positive myofibers were detected at the injection area.The average number of dys-trophin-positivefibers of the four injected mdx muscles was 2727±330.About1%of dystrophin-positivefibers,i.e., revertantfiber,are usually seen in the muscle section of mdx,but such a large number of positivefibers has never been observed before[17].We also confirmed dystrophin expression at the injection site with RT-PCR.These results revealed that our ZHTc6-MyoD cells also had capacity to differentiate into muscle lineage in vivo.CGR8(ES cell)cells and their derivatives were demon-strated to produce tumors approximately80%of the time when injected into immunocompetent syngenic mice[18]. In fact,after injection of our ZHTc6-MyoD cells into the muscle of mdx-nude mice,tumor formation was observed, but it was not teratoma.In the case of injection into immu-nocompetent mdx mice,tumor was not observed.Further experimental work is needed in order to determine the best way to avoid tumor formation.In summary,we successfully isolated ES cell lines that differentiate into the myogenic lineage in vitro by the regu-lation of the Tet-Offsystem.This system is excellent for962S.Ozasa et al./Biochemical and Biophysical Research Communications357(2007)957–963inducing ES cells into muscle cells more efficiently than any other in vitro methods.Additionally,our results showed that ES cells might have potential uses as in vivo therapeu-tic agents for muscle diseases such as DMD.AcknowledgmentsThis work was supported by a research grant for Ner-vous and Mental Disorders from the Ministry of Health, Labour,and Welfare,a grant for research in Brain Science from the Ministry of Health,Labour and Welfare,and a grant from the Ministry of Education,Science,Culture and Sports of Japan.Appendix A.Supplementary dataSupplementary data associated with this article can be found,in the online version,at doi:10.1016/j.bbrc.2007.04.032.References[1]M.Koenig,E.P.Hoffman,C.J.Bertelson,A.P.Monaco,C.Feener,L.M.Kunkel,Complete cloning of the Duchenne muscular dystrophy (DMD)cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals,Cell50(1987)509–517. [2]M.A.Goodell,K.Brose,G.Paradis,A.S.Conner,R.C.Mulligan,Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo,J.Exp.Med.183(1996)1797–1806.[3]E.Gussoni,Y.Soneoka,C.D.Strickland,E.A.Buzney,M.K.Khan,A.F.Flint,L.M.Kunkel,R.C.Mulligan,Dystrophin expression inthe mdx mouse restored by stem cell transplantation,Nature401 (1999)390–394.[4]G.Ferrari,A.Stornaiuolo,F.Mavilio,Failure to correct murinemuscular dystrophy,Nature411(2001)1014–1015.[5]E.Gussoni,R.R.Bennett,K.R.Muskiewicz,T.Meyerrose,J.A.Nolta,I.Gilgoff,J.Stein,Y.M.Chan,H.G.Lidov,C.G.Bonnemann,A.Von Moers,G.E.Morris,J.T.Den Dunnen,J.S.Chamberlain,L.M.Kunkel,K.Weinberg,Long-term persistence of donor nuclei ina Duchenne muscular dystrophy patient receiving bone marrowtransplantation,J.Clin.Invest.110(2002)807–814.[6]M.Gossen,H.Bujard,Tight control of gene expression in mamma-lian cells by tetracycline-responsive promoters,Proc.Natl.Acad.Sci.USA89(1992)5547–5551.[7]H.Niwa,J.Miyazaki,A.G.Smith,Quantitative expression of Oct-3/4defines differentiation,dedifferentiation or self-renewal of ES cells, Nat.Genet.24(2000)372–376.[8]H.Niwa,T.Burdon,I.Chambers, A.Smith,Self-renewal ofpluripotent embryonic stem cells is mediated via activation of STAT3,Genes Dev.12(1998)2048–2060.[9]J.B.Shrager,A.Naji,A.M.Kelly,H.H.Stedman,A PCR-basedassay for the wild-type dystrophin gene transferred into the mdx mouse,Muscle Nerve15(1992)1133–1137.[10]P.Seale,L.A.Sabourin,A.Girgis-Gabardo,A.Mansouri,P.Gruss,M.A.Rudnicki,Pax7is required for the specification of myogenic satellite cells,Cell102(2000)777–786.[11]M.A.Goodell,M.Rosenzweig,H.Kim,D.F.Marks,M.DeMaria,G.Paradis,S.A.Grupp,C.A.Sieff,R.C.Mulligan,R.P.Johnson,Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34antigen exist in multiple species, Nat.Med.3(1997)1337–1345.[12]A.G.Elefanty,L.Robb,R.Birner,C.G.Begley,Hematopoietic-specific genes are not induced during in vitro differentiation of scl-null embryonic stem cells,Blood90(1997)1435–1447.[13]V.Ling,S.Neben,In vitro differentiation of embryonic stem cells:immunophenotypic analysis of cultured embryoid bodies,J.Cell Physiol.171(1997)104–115.[14]H.K.Mikkola,Y.Fujiwara,T.M.Schlaeger,D.Traver,S.H.Orkin,Expression of CD41marks the initiation of definitive hematopoiesis in the mouse embryo,Blood101(2003)508–516.[15]T.Miyagi,M.Takeno,H.Nagafuchi,M.Takahashi,N.Suzuki,Flk1+cells derived from mouse embryonic stem cells reconstitute hematopoiesis in vivo in SCID mice,Exp.Hematol.30(2002)1444–1453.[16]N.Yoshida,S.Yoshida,K.Koishi,K.Masuda,Y.Nabeshima,Cellheterogeneity upon myogenic differentiation:down-regulation of MyoD and Myf-5generates‘reserve cells’,J.Cell Sci.111(Pt6) (1998)769–779.[17]J.Zhao,K.Yoshioka,T.Miike,M.Miyatake,Developmental studiesof dystrophin-positivefibers in mdx,and DRP localization,J.Neurol.Sci.114(1993)104–108.[18]S.Gidekel,G.Pizov,Y.Bergman,E.Pikarsky,Oct-3/4is a dose-dependent oncogenic fate determinant,Cancer Cell4(2003)361–370.S.Ozasa et al./Biochemical and Biophysical Research Communications357(2007)957–963963。
