Pargyline_555-57-7_DataSheet_MedChemExpress
碧云天生物技术 Beyotime Biotechnology 生物素标记EMSA探针说明书

碧云天生物技术/Beyotime Biotechnology订货热线:400-168-3301或800-8283301订货e-mail:******************技术咨询:*****************网址:碧云天网站微信公众号生物素标记EMSA探针-β-Catenin/TCF (0.2μM)产品编号产品名称包装GS018B 生物素标记EMSA探针-β-Catenin/TCF (0.2µM) 200µl产品简介:生物素标记EMSA探针-β-Catenin/TCF是用于EMSA(也称gel shift)研究的生物素(Biotin)标记的β-Catenin/TCF consensus oligonucleotide。
这个生物素标记的双链寡核苷酸含有公认的β-Catenin/TCF结合位点,可以用作EMSA研究时的探针。
β-Catenin/TCF consensus oligo的序列如下:5'-CCC TTT GAT CTT ACC-3'3'-GGG AAA CTA GAA TGG-5'本生物素标记EMSA探针已经过纯化,可以直接用于EMSA结合反应。
本生物素标记EMSA探针可以和碧云天的化学发光法EMSA试剂盒(GS009)配套使用。
一个包装的生物素标记探针可以进行约200-400个样品的EMSA检测。
包装清单:产品编号产品名称包装GS018B 生物素标记EMSA探针-β-Catenin/TCF (0.2µM) 200µl—说明书1份保存条件:-20ºC保存,一年有效。
注意事项:避免加热到40ºC以上,温度过高会导致双链DNA探针解聚成单链。
而单链无法用于EMSA研究。
对于基于生物素标记的EMSA检测的详细操作可以参考碧云天的化学发光法EMSA试剂盒(GS009)的使用说明。
本产品仅限于专业人员的科学研究用,不得用于临床诊断或治疗,不得用于食品或药品,不得存放于普通住宅内。
hss-p-5.75.09 - hyaluronic acid derivatives说明书

5.75.09Section:Prescription DrugsEffective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject:Hyaluronic Acid DerivativesPage:1 of 7Last Review Date:March 13, 2020Hyaluronic Acid DerivativesDescriptionDurolane, Euflexxa, GelSyn-3, GenVisc 850, Hyalgan , SodiumHyaluronate, Supartz , Synojoynt*, Triluron, TriVisc, Visco-3 (sodium hyaluronate)Gel-ONE , Hymovis, Monovisc, Orthovisc (hyaluronan)Synvisc, Synvisc-One (hylan G-F 20)Bolded medications are the preferred products*These medications are included in this policy but are not available in the market as of yetBackgroundOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint . The goal of therapy is torestore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1).The American College of Rheumatology (ACR) updated its guidelines for the treatment of osteoarthritis (OA) of the knee in 2012. In mild symptomatic OA, treatment may be limited toFederal Employee Program® 1310 G Street, N.W.Washington, D.C. 20005 202.942.1000Fax 202.942.1125Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 2 of 7patient education, physical and occupational therapy and other non-pharmacologic modalities. Nonpharmacologic modalities strongly recommended for the management of knee OA were aerobic, aquatic, and/or resistance exercises as well as weight loss for overweight patients. Nonpharmacologic modalities conditionally recommended for knee OA included medial wedge insoles for valgus knee OA, subtalar strapped lateral insoles for varus knee OA, medially directed patellar taping, manual therapy, walking aids, thermal agents, tai chi, self-management programs, and psychosocial interventions. Pharmacologic modalities conditionally recommended for the initial management of patients with knee OA included acetaminophen, oral and topical NSAIDs, tramadol, and intraarticular corticosteroid injections (1).Regulatory StatusFDA-approved indication: Hyaluronic acid derivatives are indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy, simple analgesics (e.g., acetaminophen), NSAIDs, tramadol, or intra-articular steroid injections (2-18).The hyaluronic acid derivatives are contraindicated for use in patients with known hypersensitivity to hyaluronan (sodium hyaluronate) preparations. Orthovisc lists hypersensitivity to gram positive bacterial proteins as an additional contraindication (4). Caution should be exercised when Gel-One, Hyalgan, Visco-3, Synvisc, Synvisc-One, Supartz, and Triluron are administered to patients with allergies to avian proteins, feathers, and egg products (3-8, 18).Hyaluronic acid derivatives are contraindicated to treat patients with knee joint infections, infections or skin diseases in the area of the injection site (2-17).A treatment cycle for most of the hyaluronan derivatives typically involves multiple weekly injections. Euflexxa, GelSyn-3, Sodium Hyaluronate, Synvisc, Triluron, TriVisc, and Visco-3 are given for a total of three injections. Orthovisc is given for three or four injections. GenVisc 850, Supartz and Hyalgan are given for a total of three or five injections. Durolane, Gel-One, Synojoynt, and Synvisc-One differ from the other hyaluronan derivatives in that it only requires one injection. Repeat courses of hyaluronan derivatives may be administered if symptoms return (2-18).Upon the basis of high quality supporting evidence, the American Academy of Orthopedic Surgeons cannot recommend using hyaluronic acid for patients with symptomatic osteoarthritis of the knee (19).Related policiesSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 3 of 7Hyaluronate PowderPolicyThis policy statement applies to clinical review performed for pre-service (Prior Approval, Precertification, Advanced Benefit Determination, etc.) and/or post-service claims.Hyaluronic acid derivatives may be considered medically necessary for the treatment of osteoarthritis of the knee and if the conditions indicated below are met.Hyaluronic acid derivatives may be considered investigational for all other indications.Prior-Approval RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Inadequate response to TWO or more of the following conservative non-pharmacologic therapy:a. Cardiovascular (aerobic) activity, such as: walking, biking, stationarybike, aquatic exerciseb. Resistance exercisec. Weight reduction (for persons who are overweight)d. Participation in self-management programse. Wear of medially directed patellar tapingf. Wear of wedged insolesg. Thermal agentsh. Walking aidsi. Physical therapyj. Occupational therapy2. Inadequate response, intolerance, or contraindication to TWO or more of thefollowing:Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 4 of 7a. Acetaminophenb. Oral NSAIDsc. Topical NSAIDs3. Inadequate response, intolerance, or contraindication to intra-articularsteroid injections in which efficacy lasted less than 8 weeks4. Radiologic confirmation of Kellgren-Lawrence Scale score of grade 2 orgreater5. NO dual therapy with another hyaluronic acid injectable6. Non-preferred medications only: Patient MUST have tried at least TWO ofthe preferred products unless the patient has a valid medical exception (e.g.inadequate treatment response, intolerance, contraindication)Prior – Approval Renewal RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Documentation of improvement in pain with previous course of treatment2. At least 12 months has elapsed since last injection of the prior treatmentcycle3. Documentation of reduction of dosing of NSAIDs or other analgesicsduring the 12 month period following the last injection of the prior treatmentcycle4. NO dual therapy with another hyaluronic acid injectable5. Non-preferred medications only: Patient MUST have tried at least TWOof the preferred products unless the patient has a valid medical exception(e.g. inadequate treatment response, intolerance, contraindication) Policy GuidelinesPre - PA AllowanceNoneSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 5 of 7Prior - Approval LimitsDuration12 monthsQuantity One course of therapy for each kneePrior – Approval Renewal LimitsSame as aboveRationaleSummaryOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint. The goal of therapy is to restore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1-18).Prior approval is required to ensure the safe, clinically appropriate and cost effective use of the hyaluronic acid derivatives while maintaining optimal therapeutic outcomes.References1. American College of Rheumatology, Subcommittee on Osteoarthritis Guidelines.Recommendations for the medical management of osteoarthritis of the hip and knee:2012 update. Arthritis Care & Research 2012; 64(4):465-474.2. Euflexxa [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc.; July 2016.3. Hyalgan [package insert]. Parsippany, NJ: Fidia Pharma USA Inc.; May 2014.4. Orthovisc [package insert]. Woburn, MA: Anika Therapeutics; June 2005.5. Supartz [package insert]. Durham, NC: Bioventus LLC; April 2015.6. Synvisc [package insert]. Ridgefield, NJ: Genzyme Corp.; December 2014.7. Synvisc-One [package insert]. Ridgefield, NJ: Genzyme Corp.; September 2014;8. Gel-One [package insert]. Warsaw, IN: Zimmer Inc.; May 2011.9. Monovisc [package insert]. Bedford, MA: Anika Therapeutics; December 2013.10. Hymovis [package insert]. Parsippany, NJ: O Fidia Pharma USA Inc.; October 2015.Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 6 of 711. GenVisc 850 [package insert]. Doylestown, PA: OrthogenRx Inc.; January 2015.12. GelSyn-3 [package insert]. Durham, NC: Bioventus LLC; January 2016.13. Durolane [package insert]. Durham, NC: Bioventus LLC; November 2017.14. Visco-3 [package insert]. Warsaw, IN: Zimmer, Inc.; May 2017.15. Sodium Hyaluronate [package insert]. North Wales, PA: Teva Pharmaceuticals USA,Inc.; March 2019.16. Synojoynt [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.;September 2019.17. TriVisc [package insert]. Doylestown, PA: OrthogenRx, Inc.; September 2018.18. Triluron [package insert]. Florham Park, NJ: Fidia Pharma USA Inc.; March 2019.19. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee.Evidence-based guideline 2nd edition. May 2013.Policy HistoryDate Action ReasonJanuary 2012 Added minimum age - only approved for adultsDecember 2012 Annual editorial review and reference updateDecember 2013 Annual editorial review and reference updateMarch 2014 Annual editorial reviewAddition of examples of non-pharmacological agents and agents of priorfailure medications.April 2014 Line-Addition of Monovisc to PAMarch 2015 Annual criteria review and reference updateMarch 2016 Change from one tried and failed to two tried and failed non-pharmacologic and pharmacologic therapies and addition of the tried and failed of intra-articular steroid and radiologic confirmation of Kellgren-Lawrence Scalescore of grade 2 or greaterAddition of HymovisPolicy # change from 5.11.04 to 5.75.09May 2016 Addition of GelSyn-3 and GenVisc 850December 2016 Annual editorial review and reference updateAdded: no dual therapy with another hyaluronic acid injectableMarch 2017 Bolded preferred products in the title pageJuly 2017 GelSyn-3 has been changed to preferredSeptember 2017 Annual reviewDecember 2017 Addition of Durolane and Visco-3March 2018 Annual editorial reviewRemoval of Tramadol from the T/F listSeptember 2019 Annual review and reference update. Addition of Sodium Hyaluronate,Synojoynt, and TriViscSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 7 of 7December 2019 Annual review. Addition of requirement to trial preferred products January 2020 Addition of TriluronMarch 2020 Annual reviewKeywordsThis policy was approved by the FEP® Pharmacy and Medical Policy Committee on March 13, 2020 and is effective on April 1, 2020.。
L755507_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:L755507 is a potent, selective agonist of β3–AR with an IC 50 of 35 nM.IC50 & Target: IC50: 35 nM (β3–AR)[1]In Vitro: L755507 causes a robust concentration–dependent increase in cAMP accumulation (pEC 50 values of 8.5 and 12.3,respectively). Maximal cAMP accumulation with zinterol and L755507 is increased after pretreatment with pertussis toxin. In contrast to cAMP, zinterol, L755507 and L748337 increase phosphorylation of extracellular signal–regulated kinase 1/2 (Erk1/2) with very high potency (pEC 50 values of 10.9, 11.7 and 11.6)[1]. L755507 and Scr7 do not reduce cell viability significantly. Scr7 does not affect cell cycle distribution in a range of 10 to 200 μM. L755507 significantly decreases the proportion of cells in the G2/M phase at 10 μM or 40μM and increases the S–phase cells at 10 μM compare with the DMSO–treated cells [2]. PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[1]The cytosensor microphysiometer is used to measure β3–AR–mediated increases in ECAR . In brief, CHO β3 cells areseeded into 12–mm Transwell inserts at 5×105 cells/cup and left to adhere overnight. On the day of experiment, cells are equilibrated for 2 h, and cumulative concentration–response curves to L755507, zinterol, or L748337 are constructed in paired sister cells with each concentration of drug exposed to cells for 14 min. Results are expressed as a percentage of the maximal response to L755507. In experiments examining the effect of inhibitors, cells are treated for 30 min before stimulation with appropriate drugs. All drugs are diluted in modified RPMI 1640 medium. These results are expressed as a percentage of the maximal response to L755507, zinterol, or L748337 over basal [1].References:[1]. Sato M, et al. The beta3–adrenoceptor agonist4–[[(Hexylamino)carbonyl]amino]–N–[4–[2–[[(2S)–2–hydroxy–3–(4–hydroxyphenoxy)propyl]amino]ethyl]–phenyl]–benzenesulfonamide (L755507) andantagonist (S)–N–[4–[2–[[3–[3–(acetamidomethyl)phenoxy]–2–hydroxypropyl]amino]–ethyl]phenyl]benzenesulfonamide (L748337) activate different signaling pathways in Chinese hamster ovary–K1 cells stably expressing the human beta3–adrenoceptor. Mol Pharmacol. 2008 Nov;74(5):1417–28.[2]. Guoling Li, et al. Small molecules enhance CRISPR/Cas9–mediated homology–directed genome editing in primary cells. Sci Rep. 2017; 7: 8943.Product Name:L755507Cat. No.:HY-19334CAS No.:159182-43-1Molecular Formula:C30H40N4O6S Molecular Weight:584.73Target:Adrenergic Receptor Pathway:GPCR/G Protein Solubility:DMSO: 125 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
稳定性英文版

HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFLUOXETINE HClC17H18F3NO•HClM.W. = 345.79CAS — 59333-67-4STABILITY INDICATINGA S S A Y V A L I D A T I O NMethod is suitable for:ýIn-process controlþProduct ReleaseþStability indicating analysis (Suitability - US/EU Product) CAUTIONFLUOXETINE HYDROCHLORIDE IS A HAZARDOUS CHEMICAL AND SHOULD BE HANDLED ONLY UNDER CONDITIONS SUITABLE FOR HAZARDOUS WORK.IT IS HIGHLY PRESSURE SENSITIVE AND ADEQUATE PRECAUTIONS SHOULD BE TAKEN TO AVOID ANY MECHANICAL FORCE (SUCH AS GRINDING, CRUSHING, ETC.) ON THE POWDER.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationTABLE OF CONTENTS INTRODUCTION........................................................................................................................ PRECISION............................................................................................................................... System Repeatability ................................................................................................................ Method Repeatability................................................................................................................. Intermediate Precision .............................................................................................................. LINEARITY................................................................................................................................ RANGE...................................................................................................................................... ACCURACY............................................................................................................................... Accuracy of Standard Injections................................................................................................ Accuracy of the Drug Product.................................................................................................... VALIDATION OF FLUOXETINE HCl AT LOW CONCENTRATION........................................... Linearity at Low Concentrations................................................................................................. Accuracy of Fluoxetine HCl at Low Concentration..................................................................... System Repeatability................................................................................................................. Quantitation Limit....................................................................................................................... Detection Limit........................................................................................................................... VALIDATION FOR META-FLUOXETINE HCl (POSSIBLE IMPURITIES).................................. Meta-Fluoxetine HCl linearity at 0.05% - 1.0%........................................................................... Detection Limit for Fluoxetine HCl.............................................................................................. Quantitation Limit for Meta Fluoxetine HCl................................................................................ Accuracy for Meta-Fluoxetine HCl ............................................................................................ Method Repeatability for Meta-Fluoxetine HCl........................................................................... Intermediate Precision for Meta-Fluoxetine HCl......................................................................... SPECIFICITY - STABILITY INDICATING EVALUATION OF THE METHOD............................. FORCED DEGRADATION OF FINISHED PRODUCT AND STANDARD..................................1. Unstressed analysis...............................................................................................................2. Acid Hydrolysis stressed analysis..........................................................................................3. Base hydrolysis stressed analysis.........................................................................................4. Oxidation stressed analysis...................................................................................................5. Sunlight stressed analysis.....................................................................................................6. Heat of solution stressed analysis.........................................................................................7. Heat of powder stressed analysis.......................................................................................... System Suitability stressed analysis.......................................................................................... Placebo...................................................................................................................................... STABILITY OF STANDARD AND SAMPLE SOLUTIONS......................................................... Standard Solution...................................................................................................................... Sample Solutions....................................................................................................................... ROBUSTNESS.......................................................................................................................... Extraction................................................................................................................................... Factorial Design......................................................................................................................... CONCLUSION...........................................................................................................................ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationBACKGROUNDTherapeutically, Fluoxetine hydrochloride is a classified as a selective serotonin-reuptake inhibitor. Effectively used for the treatment of various depressions. Fluoxetine hydrochloride has been shown to have comparable efficacy to tricyclic antidepressants but with fewer anticholinergic side effects. The patent expiry becomes effective in 2001 (US). INTRODUCTIONFluoxetine capsules were prepared in two dosage strengths: 10mg and 20mg dosage strengths with the same capsule weight. The formulas are essentially similar and geometrically equivalent with the same ingredients and proportions. Minor changes in non-active proportions account for the change in active ingredient amounts from the 10 and 20 mg strength.The following validation, for the method SI-IAG-206-02 , includes assay and determination of Meta-Fluoxetine by HPLC, is based on the analytical method validation SI-IAG-209-06. Currently the method is the in-house method performed for Stability Studies. The Validation was performed on the 20mg dosage samples, IAG-21-001 and IAG-21-002.In the forced degradation studies, the two placebo samples were also used. PRECISIONSYSTEM REPEATABILITYFive replicate injections of the standard solution at the concentration of 0.4242mg/mL as described in method SI-IAG-206-02 were made and the relative standard deviation (RSD) of the peak areas was calculated.SAMPLE PEAK AREA#15390#25406#35405#45405#55406Average5402.7SD 6.1% RSD0.1ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::PRECISION - Method RepeatabilityThe full HPLC method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method repeated six times and the relative standard deviation (RSD) was calculated.SAMPLENumber%ASSAYof labeled amountI 96.9II 97.8III 98.2IV 97.4V 97.7VI 98.5(%) Average97.7SD 0.6(%) RSD0.6PRECISION - Intermediate PrecisionThe full method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method was repeated six times by a second analyst on a different day using a different HPLC instrument. The average assay and the relative standard deviation (RSD) were calculated.SAMPLENumber% ASSAYof labeled amountI 98.3II 96.3III 94.6IV 96.3V 97.8VI 93.3Average (%)96.1SD 2.0RSD (%)2.1The difference between the average results of method repeatability and the intermediate precision is 1.7%.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationLINEARITYStandard solutions were prepared at 50% to 200% of the nominal concentration required by the assay procedure. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over the concentration range required. Y-Intercept was found to be insignificant.RANGEDifferent concentrations of the sample (IAG-21-001) for the 20mg dosage form were prepared, covering between 50% - 200% of the nominal weight of the sample.Conc. (%)Conc. (mg/mL)Peak Area% Assayof labeled amount500.20116235096.7700.27935334099.21000.39734463296.61500.64480757797.52000.79448939497.9(%) Average97.6SD 1.0(%) RSD 1.0ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::RANGE (cont.)The results demonstrate linearity as well over the specified range.Correlation coefficient (RSQ)0.99981 Slope11808.3Y -Interceptresponse at 100%* 100 (%) 0.3%ACCURACYACCURACY OF STANDARD INJECTIONSFive (5) replicate injections of the working standard solution at concentration of 0.4242mg/mL, as described in method SI-IAG-206-02 were made.INJECTIONNO.PEAK AREA%ACCURACYI 539299.7II 540599.9III 540499.9IV 5406100.0V 5407100.0Average 5402.899.9%SD 6.10.1RSD, (%)0.10.1The percent deviation from the true value wasdetermined from the linear regression lineHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::ACCURACY OF THE DRUG PRODUCTAdmixtures of non-actives (placebo, batch IAG-21-001 ) with Fluoxetine HCl were prepared at the same proportion as in a capsule (70%-180% of the nominal concentration).Three preparations were made for each concentration and the recovery was calculated.Conc.(%)Placebo Wt.(mg)Fluoxetine HCl Wt.(mg)Peak Area%Accuracy Average (%)70%7079.477.843465102.27079.687.873427100.77079.618.013465100.0101.0100%10079.6211.25476397.910080.8011.42491799.610079.6011.42485498.398.6130%13079.7214.90640599.413080.3114.75632899.213081.3314.766402100.399.618079.9920.10863699.318079.3820.45879499.418080.0820.32874899.599.4Placebo, Batch Lot IAG-21-001HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION OF FLUOXETINE HClAT LOW CONCENTRATIONLINEARITY AT LOW CONCENTRATIONSStandard solution of Fluoxetine were prepared at approximately 0.02%-1.0% of the working concentration required by the method SI-IAG-206-02. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over this range.ACCURACY OF FLUOXETINE HCl AT LOW CONCENTRATIONThe peak areas of the standard solution at the working concentration were measured and the percent deviation from the true value, as determined from the linear regression was calculated.SAMPLECONC.µg/100mLAREA FOUND%ACCURACYI 470.56258499.7II 470.56359098.1III 470.561585101.3IV 470.561940100.7V 470.56252599.8VI 470.56271599.5(%) AverageSlope = 132.7395299.9SD Y-Intercept = -65.872371.1(%) RSD1.1HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSystem RepeatabilitySix replicate injections of standard solution at 0.02% and 0.05% of working concentration as described in method SI-IAG-206-02 were made and the relative standard deviation was calculated.SAMPLE FLUOXETINE HCl AREA0.02%0.05%I10173623II11503731III10103475IV10623390V10393315VI10953235Average10623462RSD, (%) 5.0 5.4Quantitation Limit - QLThe quantitation limit ( QL) was established by determining the minimum level at which the analyte was quantified. The quantitation limit for Fluoxetine HCl is 0.02% of the working standard concentration with resulting RSD (for six injections) of 5.0%. Detection Limit - DLThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected. The detection limit of Fluoxetine HCl is about 0.01% of the working standard concentration.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION FOR META-FLUOXETINE HCl(EVALUATING POSSIBLE IMPURITIES)Meta-Fluoxetine HCl linearity at 0.05% - 1.0%Relative Response Factor (F)Relative response factor for Meta-Fluoxetine HCl was determined as slope of Fluoxetine HCl divided by the slope of Meta-Fluoxetine HCl from the linearity graphs (analysed at the same time).F =132.7395274.859534= 1.8Detection Limit (DL) for Fluoxetine HClThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected.Detection limit for Meta Fluoxetine HCl is about 0.02%.Quantitation Limit (QL) for Meta-Fluoxetine HClThe QL is determined by the analysis of samples with known concentration of Meta-Fluoxetine HCl and by establishing the minimum level at which the Meta-Fluoxetine HCl can be quantified with acceptable accuracy and precision.Six individual preparations of standard and placebo spiked with Meta-Fluoxetine HCl solution to give solution with 0.05% of Meta Fluoxetine HCl, were injected into the HPLC and the recovery was calculated.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES].Approx.Conc.(%)Known Conc.(µg/100ml)Area in SpikedSampleFound Conc.(µg/100mL)Recovery (%)0.0521.783326125.735118.10.0521.783326825.821118.50.0521.783292021.55799.00.0521.783324125.490117.00.0521.783287220.96996.30.0521.783328526.030119.5(%) AVERAGE111.4SD The recovery result of 6 samples is between 80%-120%.10.7(%) RSDQL for Meta Fluoxetine HCl is 0.05%.9.6Accuracy for Meta Fluoxetine HClDetermination of Accuracy for Meta-Fluoxetine HCl impurity was assessed using triplicate samples (of the drug product) spiked with known quantities of Meta Fluoxetine HCl impurity at three concentrations levels (namely 80%, 100% and 120% of the specified limit - 0.05%).The results are within specifications:For 0.4% and 0.5% recovery of 85% -115%For 0.6% recovery of 90%-110%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES]Approx.Conc.(%)Known Conc.(µg/100mL)Area in spikedSample Found Conc.(µg/100mL)Recovery (%)[0.4%]0.4174.2614283182.66104.820.4174.2614606187.11107.370.4174.2614351183.59105.36[0.5%]0.5217.8317344224.85103.220.5217.8316713216.1599.230.5217.8317341224.81103.20[0.6%]0.6261.3918367238.9591.420.6261.3920606269.81103.220.6261.3920237264.73101.28RECOVERY DATA DETERMINED IN SPIKED SAMPLESHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::REPEATABILITYMethod Repeatability - Meta Fluoxetine HClThe full method (as described in SI-IAG-206-02) was carried out on the finished drug product representing lot number IAG-21-001-(1). The HPLC method repeated serially, six times and the relative standard deviation (RSD) was calculated.IAG-21-001 20mg CAPSULES - FLUOXETINESample% Meta Fluoxetine % Meta-Fluoxetine 1 in Spiked Solution10.0260.09520.0270.08630.0320.07740.0300.07450.0240.09060.0280.063AVERAGE (%)0.0280.081SD 0.0030.012RSD, (%)10.314.51NOTE :All results are less than QL (0.05%) therefore spiked samples with 0.05% Meta Fluoxetine HCl were injected.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::Intermediate Precision - Meta-Fluoxetine HClThe full method as described in SI-IAG-206-02 was applied on the finished product IAG-21-001-(1) .It was repeated six times, with a different analyst on a different day using a different HPLC instrument.The difference between the average results obtained by the method repeatability and the intermediate precision was less than 30.0%, (11.4% for Meta-Fluoxetine HCl as is and 28.5% for spiked solution).IAG-21-001 20mg - CAPSULES FLUOXETINESample N o:Percentage Meta-fluoxetine% Meta-fluoxetine 1 in spiked solution10.0260.06920.0270.05730.0120.06140.0210.05850.0360.05560.0270.079(%) AVERAGE0.0250.063SD 0.0080.009(%) RSD31.514.51NOTE:All results obtained were well below the QL (0.05%) thus spiked samples slightly greater than 0.05% Meta-Fluoxetine HCl were injected. The RSD at the QL of the spiked solution was 14.5%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSPECIFICITY - STABILITY INDICATING EVALUATIONDemonstration of the Stability Indicating parameters of the HPLC assay method [SI-IAG-206-02] for Fluoxetine 10 & 20mg capsules, a suitable photo-diode array detector was incorporated utilizing a commercial chromatography software managing system2, and applied to analyze a range of stressed samples of the finished drug product.GLOSSARY of PEAK PURITY RESULT NOTATION (as reported2):Purity Angle-is a measure of spectral non-homogeneity across a peak, i.e. the weighed average of all spectral contrast angles calculated by comparing all spectra in the integrated peak against the peak apex spectrum.Purity Threshold-is the sum of noise angle3 and solvent angle4. It is the limit of detection of shape differences between two spectra.Match Angle-is a comparison of the spectrum at the peak apex against a library spectrum.Match Threshold-is the sum of the match noise angle3 and match solvent angle4.3Noise Angle-is a measure of spectral non-homogeneity caused by system noise.4Solvent Angle-is a measure of spectral non-homogeneity caused by solvent composition.OVERVIEWT he assay of the main peak in each stressed solution is calculated according to the assay method SI-IAG-206-02, against the Standard Solution, injected on the same day.I f the Purity Angle is smaller than the Purity Threshold and the Match Angle is smaller than the Match Threshold, no significant differences between spectra can be detected. As a result no spectroscopic evidence for co-elution is evident and the peak is considered to be pure.T he stressed condition study indicated that the Fluoxetine peak is free from any appreciable degradation interference under the stressed conditions tested. Observed degradation products peaks were well separated from the main peak.1® PDA-996 Waters™ ; 2[Millennium 2010]ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFORCED DEGRADATION OF FINISHED PRODUCT & STANDARD 1.UNSTRESSED SAMPLE1.1.Sample IAG-21-001 (2) (20mg/capsule) was prepared as stated in SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 98.5%.SAMPLE - UNSTRESSEDFluoxetine:Purity Angle:0.075Match Angle:0.407Purity Threshold:0.142Match Threshold:0.4251.2.Standard solution was prepared as stated in method SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 100.0%.Fluoxetine:Purity Angle:0.078Match Angle:0.379Purity Threshold:0.146Match Threshold:0.4272.ACID HYDROLYSIS2.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of conc. HCl was added to this solution The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system after filtration.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 98.8%.SAMPLE- ACID HYDROLYSISFluoxetine peak:Purity Angle:0.055Match Angle:0.143Purity Threshold:0.096Match Threshold:0.3712.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask. 20mL Diluent were added. 2mL of conc. HCl were added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 97.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSTANDARD - ACID HYDROLYSISFluoxetine peak:Purity Angle:0.060Match Angle:0.060Purity Threshold:0.099Match Threshold:0.3713.BASE HYDROLYSIS3.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weight into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 99.3%.SAMPLE - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.063Match Angle:0.065Purity Threshold:0.099Match Threshold:0.3623.2.Standard stock solution was prepared as per method SI-IAG-206-02 : About 22mg Fluoxetine HCl was weighed into a 50mL volumetric flask. 20mL Diluent was added. 2mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH=5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease - 99.5%.STANDARD - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.081Match Angle:0.096Purity Threshold:0.103Match Threshold:0.3634.OXIDATION4.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02. An equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent added and the solution sonicated for 10 minutes.1.0mL of 30% H2O2 was added to the solution and allowed to stand for 5 hours, then made up to volume with Diluent, filtered and injected into HPLC system.Fluoxetine peak intensity decreased to 95.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSAMPLE - OXIDATIONFluoxetine peak:Purity Angle:0.090Match Angle:0.400Purity Threshold:0.154Match Threshold:0.4294.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask and 25mL Diluent were added. 2mL of 30% H2O2 were added to this solution which was standing for 5 hours, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity decreased to 95.8%.STANDARD - OXIDATIONFluoxetine peak:Purity Angle:0.083Match Angle:0.416Purity Threshold:0.153Match Threshold:0.4295.SUNLIGHT5.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1hour. The BST was set to 35°C and the ACT was 45°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak decreased to 91.2% and the dark control solution showed assay of 97.0%. The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak was observed at RRT of 1.5 (2.7%).The total percent of Fluoxetine peak with the degradation peak is about 93.9%.SAMPLE - SUNLIGHTFluoxetine peak:Purity Angle:0.093Match Angle:0.583Purity Threshold:0.148Match Threshold:0.825 ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSUNLIGHT (Cont.)5.2.Working standard solution was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1.5 hour. The BST was set to 35°C and the ACT was 42°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak was decreased to 95.2% and the dark control solution showed assay of 99.5%.The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak were observed at RRT of 1.5 (2.3).The total percent of Fluoxetine peak with the degradation peak is about 97.5%. STANDARD - SUNLIGHTFluoxetine peak:Purity Angle:0.067Match Angle:0.389Purity Threshold:0.134Match Threshold:0.8196.HEAT OF SOLUTION6.1.Sample solution of IAG-21-001-(2) (20 mg/capsule) was prepared as in method SI-IAG-206-02 . Equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution was sonicated for 10 minutes and made up to volume with Diluent. 4mL solution was transferred into a suitable crucible, heated at 105°C in an oven for 2 hours. The sample was cooled to ambient temperature, filtered and injected into the HPLC system.Fluoxetine peak was decreased to 93.3%.SAMPLE - HEAT OF SOLUTION [105o C]Fluoxetine peak:Purity Angle:0.062Match Angle:0.460Purity Threshold:0.131Match Threshold:0.8186.2.Standard Working Solution (WS) was prepared under method SI-IAG-206-02 . 4mL of the working solution was transferred into a suitable crucible, placed in an oven at 105°C for 2 hours, cooled to ambient temperature and injected into the HPLC system.Fluoxetine peak intensity did not decrease - 100.5%.ED. N0: 04Effective Date:APPROVED::。
YM-155_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:YM155 (Sepantronium bromide) is a novel small molecule survivin suppressant with an IC50 of 0.54 nM for the inhibition of survivin promoter activity.IC50 Value: 0.54 nMTarget: SurvivinIn vitro: YM155 is not sensitive to survivn gene promoter–driven luciferase reporter activity even at 30 μM. YM155 significantly inhibits endogenous survivin expression in PC–3 and PPC–1 human HRPC cells with deficient p53 through transcriptional inhibition of the survivin gene promoter. On the contrary YM155 shows no sufficient effect on protein expression of c–IAP2, XIAP, Bcl–2, Bcl–xL, Bad,α–actin, and β–tubulin at 100 nM. YM155 indicates great apoptosis in human cancer cell lines including PC–3 and PPC–1 with a concomitant increase in caspase–3 activity. YM155 potently inhibits human cancer cell lines (mutated or truncated p53) including PC–3, PPC–1, DU145, TSU–Pr1, 22Rv1, SK–MEL–5 and A375 with IC50 from 2.3 to 11 nM, respectively [1]. YM155 increases thesensitivity of NSCLC cells to γ–radiation. The combination of YM155 and γ–radiation increases both the number of apoptotic cells and the activity of caspase–3. YM155 delays the repair of radiation–induced double–strand breaks in nuclear DNA [2].In vivo: YM155 completely inhibits the tumor growth of PC–3 s.c. xenografted prostate tumors at doses of 3 and 10 mg/kg, without body weight loss and blood cell count decrease. Pharmacokinetic analysis shows that YM155 is highly distributed to tumor tissue.Moreover, YM155 shows 80% TGI at a dose of 5 mg/kg in PC–3 orthotopic xenografts [1]. The combination therapy with YM155 and γ–radiation shows great antitumor activity against H460 or Calu6 xenografts in nude mice [2].PROTOCOL (Extracted from published papers and Only for reference)Enzyme assay [1]:The caspase–3 activity was measured with a CPP32/Caspase–3 Fluometric Protease Assay Kit (MBL) according to the manufacturer's instructions. After incubation with YM155 for 48 h, PC–3 and PPC–1 cells were lysed in 100 μL of a cell lysis buffer (provided with the kit) and equal volumes (50 μL) of cell lysate were obtained (100 μg of protein). After addition of 2× reaction buffer, the mixture was added to a black 96–well plate. The DEVD–AFC substrate (appended with the kit) was then added at 5 mL/well and the mixture incubated at 37°C for 30 min. Fluorescence emissions were quantified with a spectrofluorometer at an excitation wavelength of 390nm and an emission wavelength of 460 nm.Cell assay(growth inhibition) [1]:The antiproliferative activity of YM155 was measured by the method used at the National Cancer Institute. After treatment with YM155for 48 h, the cell count was determined by sulforhodamine B assay. The GI50 value was calculated by logistic analysis, which is thedrug concentration resulting in a 50% reduction in the net protein increase (as measured by sulforhodamine B staining) in control cells during the drug incubation. The assay was done in triplicate, and the mean GI50 value was obtained from the results of fourProduct Name:YM–155Cat. No.:HY-10194CAS No.:781661-94-7Molecular Formula:C 20H 19BrN 4O 3Molecular Weight:443.29Target:Survivin; Autophagy Pathway:Apoptosis; Autophagy Solubility:DMSO: 10.66 mg/mLindependent assays.Animal administration [1]:Five–week–old male nude mice (BALB/c nu/nu) were purchased from Charles River Japan, Inc. PC–3 cells (2 × 106–3 × 106) were injected into the flanks of the mice and allowed to reach a tumor volume of >100 mm3 in tumor volume (length × width2 × 0.5). YM155 was s.c. administered as a 3–day continuous infusion per week for 2 weeks using an implanted micro–osmotic pump (Alzet model 1003D, Durect) or i.v. administered five times a week for 2 weeks. The percentage of tumor growth inhibition 14 days after initial YM155 administration was calculated for each group using the following formula: MTV = 100 × {1–[(MTV of the treated group on day 14)–(MTV of the treated group on day 0)] / [(MTV of the control group on day 14)–(MTV of the control group on day 0)]}, where MTV is mean tumor volume. For both the frozen tumors and plasma samples, survivin expression levels were analyzed by Western blotting and YM155 drug concentration by high–performance liquid chromatography/triple quadrupole mass spectrometry (LC/MS/MS) using validated methods.References:[1]. Nakahara T, et al. YM155, a novel small–molecule survivin suppressant, induces regression of established human hormone–refractory prostate tumor xenografts. Cancer Res. 2007 Sep 1;67(17):8014–21.[2]. Iisa T, et al. Radiosensitizing effect of YM155, a novel small–molecule survivin suppressant, in non–small cell lung cancer cell lines. Clin Cancer Res. 2008 Oct 15;14(20):6496–504.[3]. Guo K, et al. A combination of YM–155, a small molecule survivin inhibitor, and IL–2 potently suppresses renal cell carcinoma in murine model. Oncotarget. 2015 Aug 28;6(25):21137–47.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Ampicillin_69-53-4_DataSheet_MedChemExpress

Product Name:Ampicillin CAS No.:69-53-4Cat No :HY-B0522Product Data SheetCat. No.:HY B0522MWt:349.40Formula:C16H19N3O4S Purity :>98%Solubility:Mechanisms:Biological Activity:Pathways:Anti-infection; Target:Antibacterial DMSOAmpicillin is an orally active broad-spectrum antibiotic.Target: Antibacterial Ampicillin is an antibiotic useful for the treatment of a number of bacterial infections. It is a beta-lactam antibiotic that is part of the aminopenicillin family and is roughly equivalent to its successor,amoxicillin in terms of spectrum and level of activity. Belonging to the penicillin group of beta-lactam antibiotics, ampicillin is able to penetrate Gram-positive and some Gram-negative bacteria. It differs from penicillin G, or benzylpenicillin, only by the presence of an amino group. That amino group helps the drug penetrate the outer membrane of Gram-negative bacteria. Ampicillin acts as an irreversible inhibitor of the enzyme transpeptidase which is needed by bacteria to make their cell References:[1]. /wiki/Ampicillin irreversible inhibitor of the enzyme transpeptidase, which is needed by bacteria to make their cell walls. It inhibits the third and final stage of bacterial cell wall synthesis in binary fission...Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
循能泰说明书(己酮可可碱外文说明书)
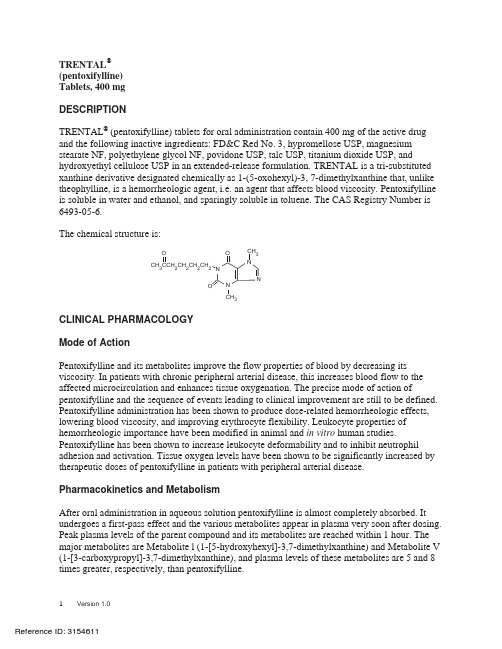
OCH CCH CH CH CH 32223 TRENTAL ®(pentoxifylline)Tablets, 400 mg DESCRIPTIONTRENTAL ® (pentoxifylline) tablets for oral administration contain 400 mg of the active drugand the following inactive ingredients: FD&C Red No. 3, hypromellose USP, magnesium stearate NF, polyethylene glycol NF, povidone USP, talc USP, titanium dioxide USP, andhydroxyethyl cellulose USP in an extended-release formulation. TRENTAL is a tri-substitutedxanthine derivative designated chemically as 1-(5-oxohexyl)-3, 7-dimethylxanthine that, unliketheophylline, is a hemorrheologic agent, i.e. an agent that affects blood viscosity. Pentoxifyllineis soluble in water and ethanol, and sparingly soluble in toluene. The CAS Registry Number is6493-05-6.The chemical structure is:CLINICAL PHARMACOLOGYMode of ActionPentoxifylline and its metabolites improve the flow properties of blood by decreasing itsviscosity. In patients with chronic peripheral arterial disease, this increases blood flow to theaffected microcirculation and enhances tissue oxygenation. The precise mode of action ofpentoxifylline and the sequence of events leading to clinical improvement are still to be defined.Pentoxifylline administration has been shown to produce dose-related hemorrheologic effects,lowering blood viscosity, and improving erythrocyte flexibility. Leukocyte properties of hemorrheologic importance have been modified in animal and in vitro human studies.Pentoxifylline has been shown to increase leukocyte deformability and to inhibit neutrophiladhesion and activation. Tissue oxygen levels have been shown to be significantly increased bytherapeutic doses of pentoxifylline in patients with peripheral arterial disease.Pharmacokinetics and MetabolismAfter oral administration in aqueous solution pentoxifylline is almost completely absorbed. Itundergoes a first-pass effect and the various metabolites appear in plasma very soon after dosing.Peak plasma levels of the parent compound and its metabolites are reached within 1 hour. Themajor metabolites are Metabolite l (1-[5-hydroxyhexyl]-3,7-dimethylxanthine) and Metabolite V(1-[3-carboxypropyl]-3,7-dimethylxanthine), and plasma levels of these metabolites are 5 and 8times greater, respectively, than pentoxifylline.Following oral administration of aqueous solutions containing 100 to 400 mg of pentoxifylline, the pharmacokinetics of the parent compound and Metabolite l are dose-related and not proportional (non-linear), with half-life and area under the blood-level time curve (AUC) increasing with dose. The elimination kinetics of Metabolite V are not dose-dependent. The apparent plasma half-life of pentoxifylline varies from 0.4 to 0.8 hours and the apparent plasma half-lives of its metabolites vary from 1 to 1.6 hours. There is no evidence of accumulation or enzyme induction (Cytochrome P450) following multiple oral doses.Excretion is almost totally urinary; the main biotransformation product is Metabolite V. Essentially no parent drug is found in the urine. Despite large variations in plasma levels of parent compound and its metabolites, the urinary recovery of Metabolite V is consistent and shows dose proportionality. Less than 4% of the administered dose is recovered in feces. Food intake shortly before dosing delays absorption of an immediate-release dosage form but does not affect total absorption. The pharmacokinetics and metabolism of TRENTAL have not been studied in patients with renal and/or hepatic dysfunction. The pentoxifylline AUC was increased and elimination rate decreased in an older population (60-68 years, n=6) compared to younger individuals (22-30 years, n=6) (see PRECAUTIONS, Geriatric Use).After administration of the 400 mg extended-release TRENTAL tablet, plasma levels of the parent compound and its metabolites reach their maximum within 2 to 4 hours and remain constant over an extended period of time. Coadministration of TRENTAL tablets with meals resulted in an increase in mean C max and AUC by about 28% and 13% for pentoxifylline, respectively. C max for Metabolite 1 also increased by about 20%. The extended release of pentoxifylline from the tablet eliminates peaks and troughs in plasma levels for improved gastrointestinal tolerance.INDICATIONS AND USAGETRENTAL is indicated for the treatment of patients with intermittent claudication on the basis of chronic occlusive arterial disease of the limbs. TRENTAL can improve function and symptoms but is not intended to replace more definitive therapy, such as surgical bypass, or removal of arterial obstructions when treating peripheral vascular disease.CONTRAINDICATIONSTRENTAL should not be used in patients with recent cerebral and/or retinal hemorrhage or in patients who have previously exhibited intolerance to this product or methylxanthines such as caffeine, theophylline, and theobromine.PRECAUTIONSGeneralAt the first sign of anaphylactic/anaphylactoid reaction, TRENTAL must be discontinued.Patients with chronic occlusive arterial disease of the limbs frequently show other manifestations of arteriosclerotic disease. TRENTAL has been used safely for treatment of peripheral arterial disease in patients with concurrent coronary artery and cerebrovascular diseases, but there have been occasional reports of angina, hypotension, and arrhythmia. Controlled trials do not show that TRENTAL causes such adverse effects more often than placebo, but, as it is a methylxanthine derivative, it is possible some individuals will experience such responses. Patients on Warfarin should have more frequent monitoring of prothrombin times, while patients with other risk factors complicated by hemorrhage (e.g., recent surgery, peptic ulceration, cerebral and/or retinal bleeding) should have periodic examinations for bleeding including, hematocrit and/or hemoglobin.Drug InteractionsAlthough a causal relationship has not been established, there have been reports of bleedingand/or prolonged prothrombin time in patients treated with TRENTAL with and without anticoagulants or platelet aggregation inhibitors. Patients on Warfarin should have more frequent monitoring of prothrombin times, while patients with other risk factors complicated by hemorrhage (e.g., recent surgery, peptic ulceration) should have periodic examinations for bleeding including hematocrit and/or hemoglobin.Concomitant administration of TRENTAL and theophylline-containing drugs leads to increased theophylline levels and theophylline toxicity in some individuals. Such patients should be closely monitored for signs of toxicity and have their theophylline dosage adjusted as necessary.TRENTAL has been used concurrently with beta blockers, digitalis, diuretics, antidiabetic agents, and antiarrhythmics, without observed problems. Small decreases in blood pressure have been observed in some patients treated with TRENTAL plus nifedipine or captopril; periodic systemic blood pressure monitoring is recommended for patients receiving concomitant antihypertensive therapy. If indicated, dosage of the antihypertensive agents should be reduced.Postmarketing cases of increased anticoagulant activity have been reported in patients concomitantly treated with pentoxifylline and vitamin K antagonists. Monitoring of anticoagulant activity in these patients is recommended when pentoxifylline is introduced or the dose is changed.Carcinogenesis, Mutagenesis and Impairment of FertilityLong-term studies of the carcinogenic potential of pentoxifylline were conducted in mice and rats by dietary administration of the drug at doses up to 450 mg/kg (approximately 19 times the maximum recommended human daily dose (MRHD) in both species when based on body weight; 1.5 times the MRHD in the mouse and 3.3 times the MRHD in the rat when based on body surface area). In mice, the drug was administered for 18 months, whereas in rats, the drug was administered for 18 months followed by an additional 6 months without drug exposure. In the rat study, there was a statistically significant increase in benign mammary fibroadenomas in females of the 450 mg/kg group. The relevance of this finding to human use is uncertain. Pentoxifylline was devoid of mutagenic activity in various strains of Salmonella (Ames test) andin cultured mammalian cells (unscheduled DNA synthesis test) when tested in the presence and absence of metabolic activation. It was also negative in the in vivo mouse micronucleus test. PregnancyCategory C. Teratogenicity studies have been performed in rats and rabbits using oral doses up to 576 and 264 mg/kg, respectively. On a weight basis, these doses are 24 and 11 times the maximum recommended human daily dose (MRHD); on a body-surface-area basis, they are 4.2 and 3.5 times the MRHD. No evidence of fetal malformation was observed. Increased resorption was seen in rats of the 576 mg/kg group. There are no adequate and well controlled studies in pregnant women. TRENTAL (pentoxifylline) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.Nursing MothersPentoxifylline and its metabolites are excreted in human milk. Because of the potential for tumorigenicity shown for pentoxifylline in rats, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.Pediatric UseSafety and effectiveness in pediatric patients have not been established.Geriatric UseClinical studies of TRENTAL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.The active metabolite is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.ADVERSE REACTIONSClinical trials were conducted using either extended-release TRENTAL tablets for up to 60 weeks or immediate-release TRENTAL capsules for up to 24 weeks. Dosage ranges in the tablet studies were 400 mg bid to tid and in the capsule studies, 200-400 mg tid. The table summarizes the incidence (in percent) of adverse reactions considered drug related, as well as the numbers of patients who received extended-release TRENTAL tablets, immediate-release TRENTAL capsules, or the corresponding placebos. The incidence of adverse reactions was higher in thecapsule studies (where dose related increases were seen in digestive and nervous system side effects) than in the tablet studies. Studies with the capsule include domestic experience, whereas studies with the extended-release tablets were conducted outside the U.S.The table indicates that in the tablet studies few patients discontinued because of adverse effects.INCIDENCE (%) OF SIDE EFFECTSExtended-Release Tablets Immediate-Release CapsulesCommercially Available Used only for Controlled ClinicalTrialsTRENTAL Placebo TRENTAL Placebo(Numbers of Patients at Risk) (321) (128) (177) (138)Discontinued for Side Effect 3.1 0 9.6 7.2CARDIOVASCULAR SYSTEMAngina/Chest Pain 0.3 - 1.1 2.2Arrhythmia/Palpitation -- 1.7 0.7Flushing -- 2.30.7 DIGESTIVE SYSTEMAbdominal Discomfort -- 4.0 1.4Belching/Flatus/Bloating 0.6 -9.0 3.62.9 Diarrhea --3.49.62.94.7Dyspepsia 2.88.728.80.8Nausea 2.20.7 Vomiting 1.2- 4.5NERVOUS SYSTEMAgitation/Nervousness -- 1.7 0.74.311.93.1Dizziness 1.95.8 Drowsiness -- 1.1Headache 1.2 1.6 6.2 5.82.2 Insomnia -- 2.3-0.8Tremor 0.3Blurred Vision -- 2.3 1.4TRENTAL has been marketed in Europe and elsewhere since 1972. In addition to the abovesymptoms, the following have been reported spontaneously since marketing or occurred in otherclinical trials with an incidence of less than 1%; the causal relationship was uncertain:Cardiovascular - dyspnea, edema, hypotension.Digestive - anorexia, cholecystitis, constipation, dry mouth/thirst.Nervous - anxiety, confusion, depression, seizures, aseptic meningitis.Respiratory - epistaxis, flu-like symptoms, laryngitis, nasal congestion.Skin and Appendages - brittle fingernails, pruritus, rash, urticaria, angioedema.Special Senses - blurred vision, conjunctivitis, earache, scotoma.Miscellaneous - bad taste, excessive salivation, leukopenia, malaise, sore throat/swollenneck glands, weight change.A few rare events have been reported spontaneously worldwide since marketing in 1972.Although they occurred under circumstances in which a causal relationship with pentoxifyllinecould not be established, they are listed to serve as information for physicians. Cardiovascular —angina, arrhythmia, tachycardia. Digestive — hepatitis, jaundice, cholestasis, increased liverenzymes; and Hemic and Lymphatic — decreased serum fibrinogen, pancytopenia, aplastic anemia, leukemia, purpura, thrombocytopenia. Immune system disorders — anaphylactic reaction, anaphylactoid reaction, anaphylactic shock.OVERDOSAGEOverdosage with TRENTAL has been reported in pediatric patients and adults. Symptoms appear to be dose related. A report from a poison control center on 44 patients taking overdoses of enteric-coated pentoxifylline tablets noted that symptoms usually occurred 4-5 hours after ingestion and lasted about 12 hours. The highest amount ingested was 80 mg/kg; flushing, hypotension, convulsions, somnolence, loss of consciousness, fever, and agitation occurred. All patients recovered. In addition to symptomatic treatment and gastric lavage, special attention must be given to supporting respiration, maintaining systemic blood pressure, and controlling convulsions. Activated charcoal has been used to absorb pentoxifylline in patients who have overdosed.DOSAGE AND ADMINISTRATIONThe usual dosage of TRENTAL in extended-release tablet form is one tablet (400 mg) three times a day with meals.While the effect of TRENTAL may be seen within 2 to 4 weeks, it is recommended that treatment be continued for at least 8 weeks. Efficacy has been demonstrated in double-blind clinical studies of 6 months duration.Digestive and central nervous system side effects are dose related. If patients develop these effects it is recommended that the dosage be lowered to one tablet twice a day (800 mg/day). If side effects persist at this lower dosage, the administration of TRENTAL should be discontinued.HOW SUPPLIEDTRENTAL (pentoxifylline) is available for oral administration as 400-mg pink film-coated oblong tablets imprinted Trental; supplied in bottles of 100 (NDC 0039-0078-10).Store between 59 and 86° F (15 and 30° C).Dispense in well-closed, light-resistant containers.Rx onlyRev. July 2010sanofi-aventis U.S. LLCBridgewater, NJ 08807©2010 sanofi-aventis U.S. LLC。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
