中学化学物质的英文命名
完整版化合物英文命名规则

Nomenclature of Inorganic Compounds 无机化合物的命名(Prefix 词头,前缀Suffix 词尾,后缀Stem 词根) 1.Trivial Names 俗名H2O water 不说dihydrogen oxideNH3 ammonia 不说nitrogen trihydrideCaO quicklimeCaCO3 limestone 2.Systematic Nomenclature 系统命名1)Oxide 氧化物——先命名非氧元素ZnOzinc oxideCaO calcium oxideCO carbon oxideNa2O2 sodium peroxideH2O2hydrogen peroxide 注:peroxide 过氧化物2)Hydroxide 氢氧化物(base 碱)Ba(OH)2 barium hydroxideKOH potassium hydroxide3)Acid 酸Hydro acid 氢酸General formula 通式:HnX 命名:hydro- + stem of X + -ic acid H2S hydrosulfuric acid(英) hydrosulphuric(美)氢硫酸S:sulfur(英)、sulphur(美)HBr 氢溴酸hydrobromic acidBr: bromine HCl 氢氯酸(盐酸)hydrochloric acidCl: chlorine HF 氢氟酸hydrofluoric acidF: fluorineOxoacid or Oxyacid 含氧酸General formula 通式:HnXOm 命名:Stem of X + -ic acid 注:oxo- (oxy-)含氧, 氧代H2SO4 sulfuric acid(英) sulphuric acid(美)H2CO3 carbonic acidH3PO4 phosphoric acid P: phosphorus H3BO3 boric acid B: boron HNO3 nitric acid N: nitrogen If X has two oxidation states :-ic:the higher oxidation state-ous:the lower oxidation stateH2SO4 sulfuric acidH2SO3 sulfurous acid 1/5HNO3 nitric acidHNO2 nitrous acidIf X (such as halogens) has more than two oxidation states :halogen 卤素per- (过,高) + -ic :the still higher oxidation state hypo- (次,在,下) + -ous:the still lower oxidation state HClO3 chloric acidHClO2 chlorous acidHClO4 perchloric acidHClO hypochlorous acidHIO hypoiodous acid4)Salt 盐General formula 通式:MnXm 命名:Name of M stem of X + -ide (- ide, 化物)Oxide 、chloride 、nitride 、hydrideKI potassium iodideAl2S3 aluminum sulfideLiH lithium hydrideOxysalt 含氧酸盐Name the metal ion first and then the anion Naming anions:-ate anions derived from the -ic acid(the higher oxidation state of X) -ite anions derived from the -ous acid (the lower oxidation state of X) HNO3 nitric acidNaNO3 sodium nitrateHNO2 nitrous acidNaNO2 sodium nitriteSO42- sulfateSO32- sulfite AgClO4 silver perchlorateNaIO3 sodium iodateKClO2 potassium chlorite KBrO potassium hypobromite MnO42- manganate MnO4- permanganate Acid salt 酸式盐Using “ hydrogen ” to specify NaHSO4 sodium hydrogen sulfate NaH2PO4 sodium dihydrogen phosphate Na2HPO4 disodium hydrogen phosphate P: phosphorus phosphate 磷酸盐(根)Using prefix bi- + name of anion if only one acid salt existsNaHSO4 sodium bisulfateH”NaHSO3 sodium bisulfiteKHCO3 potassium bicarbonate5)Metals (M )with more than one oxidation state 2/5Two methods: ①后缀法: 早期使用stem of M + -ic the higher oxidation state of M stem of M + -ous the lower oxidation state of M HgI2 mercuric iodideHg2I2 mercurous iodide Hg:mercury Cr2+ chromousCr3+ chromic Cr: chromium注:In most cases, Latin stem is used if the metal has symbol derived from its Latin name.(mercury is an exception)Cu:cuprum (拉丁),copper (英)Cu+ cuprousCu2+ cupricCuI cuprous iodideCuS cupric sulfide Sn:stannum (拉丁), tin ( 英) SnCl2 stannous chlorideSnO2 stannic oxideFe:ferrum ( 拉丁), iron ( 英)Fe(OH)2 ferrous hydroxideFeBr3 ferric bromide② IUPAC Rule 1957 年开始使用English name of metal(Roman numeral)CuBr copper(I) bromideCuF2 copper(II) fluorideSnO tin(II) oxideSnS2 tin(IV) sulfideFe(NO3)2 iron(II) nitrateFe2(SO4)3 iron(III) sulfateUse Greek prefixes 希腊文前缀Mon (0)—di 二tri 三tetr (a)四pent (a) 五hex (a) 六hepta 七octa 八nona 九1.to specify the number of each atom in the chemical formula.NO2 nitrogen dioxidePCl5 phosphorus pentachlorideCO2 carbon dioxide 2.to specify the number of identical central atoms in condensed acids and their corresponding anions.condensed acid 缩酸H3PO4 (mono)phosphoric acidH4P2O7 diphosphoric acid3/5H2SO4 sulfuric acidH2S3O10 trisulfuric acidCrO42- 铬酸盐(根) chromateCr2O72- 重铬酸盐(根)dichromate3. to indicate extent of substitutionPO43- phosphatePS2O23- dithiophosphate thio-硫代”硫的,含硫的注:The prefixes ortho- and meta- have been used to distinguish acids differing in the “content of water. ”ortho- [ 希腊词头] 正、原(无机酸用)邻(位) (有机化合物命名)meta- [希腊词头] 偏(无机酸用)间(位) (有机化合物命名)ortho-acid 原酸;meta-acid 偏酸H3BO3 orthoboric acid(or boric acid) (原)硼酸(HBO3)n metaboric acid 偏硼酸H4SiO4 orthosilicic acid(or silicic acid) 原硅酸H2SiO3 metasilicic acid 硅酸(习惯上不叫偏硅酸)H3PO4 orthophosphoric acid (or phosphoric acid) (正)磷酸(HPO3)n metaphosphoric acid 偏磷酸。
简单的化学物质的英文命名法

简单的化学物质的英文命名法在命名化学物质时,最基本的是词根和词缀,掌握这些之后,命名就变得简单。
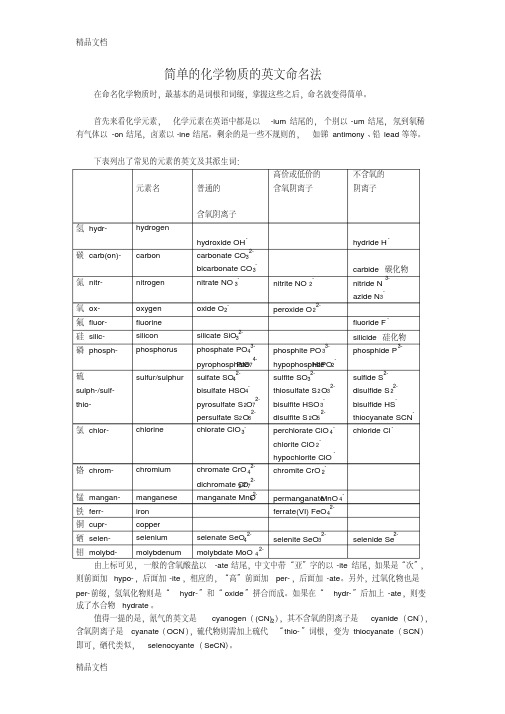
首先来看化学元素,化学元素在英语中都是以-ium结尾的,个别以-um结尾,氖到氡稀有气体以-on结尾,卤素以-ine结尾。
剩余的是一些不规则的,如锑antimony、铅lead等等。
下表列出了常见的元素的英文及其派生词:由上标可见,一般的含氧酸盐以-ate结尾,中文中带“亚”字的以-ite结尾,如果是“次”,则前面加hypo-,后面加-ite,相应的,“高”前面加per-,后面加-ate。
另外,过氧化物也是per-前缀,氢氧化物则是“hydr-”和“oxide”拼合而成。
如果在“hydr-”后加上-ate,则变成了水合物hydrate。
值得一提的是,氰气的英文是cyanogen((CN)2),其不含氧的阴离子是cyanide(CN-),含氧阴离子是cyanate(OCN-),硫代物则需加上硫代“thio-”词根,变为thiocyanate(SCN-)即可,硒代类似,selenocyante(SeCN-)。
如果阴离子遇到氢离子,成为酸,那么叫法就改变了,如硫酸sulfuric acid,“亚硫酸”sulfurous acid。
由此可见,一般的酸加上-ic即可,如果低于正常价态,一般加上-ous。
金属阳离子的变化和酸一样,如Fe3+为ferric,Fe2+为ferrous,Cu2+为cupric,Cu+为cuprous等等。
此外,还有另一种方法命名,就是在元素后加上罗马数字,如Fe3+为iron(III),FeO42-为ferrate(VI),MnO4-为manganate(VII)等。
现在我们知道了阴阳离子的命名,现在就剩组合起来了。
比如NaCl的中文是氯化钠,英文则要反过来——“钠·氯化”,即sodium chloride,乙酸锌为“锌·乙酸盐”zinc acetate。
水合物则只需在最后加上带有数字前缀的hydrate即可。
化合物的英文命名方法大全

CO32- Carbonate
(2). Acid radicals for meta-salts (亚酸根 -ite )
Anion’s name = Central element’s root -ite
for example:
ClO2- Chlorite
PO33- Phosphite
IO2- Iodite
Monocrystalline Dioxide Triagonal tetragonal pyramid Pentagonal hexagonal
6
Naming metal ions (cations) for metal oxides, bases and salts
1. Single valence ions
Cu2(OH)2CO3 Dicopper(II) dihydroxycarbonate
Ca(OH)Cl Mg(OH)PO4 Calcium hydroxychloride Magnesium hydroxyphosphate
22
Hale Waihona Puke (4).复盐(Mixed salts):同正盐的读法。
Mixed salt = Cation + cation’ + anion
Cation’s name = Element for example: Na+ Sodium K+ Potassium Al3+ Aluminum Ca2+ Calcium
7
2.Multivalence ions
Cation’s name = Element(N)
For example:
Fe2+ Fe3+ Cr2+ Cr3+ Mn4+ Mn2+ Iron(II) or Ferrous Iron(III) or Ferric Chromium(II) Chromium(III) Manganese(IV) Manganese(II)
化合物的英文命名方法大全

如
FeO: iron(II) oxide 或 ferrous oxide
Fe2O3: iron (III) oxide或ferric oxide
Cu2O: copper(I) oxide 或cuprous oxide
CuO: copper(II) oxide或cupric oxide
9
Naming nonmetal ions (anions)
for example:
NaHSO4 Sodium hydrogen sulfate
Na2HPO4 Disodium hydrogen phosphate
NaH2PO4 Sodium dihydrogen phosphate
Ca(HSO4)2 Calcium bisulfate
NaHCO3 Sodium hydrogencarbonate
2
S-block Element
IA H Hydrogen Li Lithium Na Sodium K Potassium Rb Rubidium Cs Cesium Fr Francium
IIA
Be Beryllium Mg Magnesium Ca Calcium Sr Strontium Ba Barium Ra Radium
NaNH4SO4 Sodium ammonium sulfate
KNaCO3
Potassium
sodium
carbonate
NaNH4HPO4: sodium ammonium hydrogenphosphate
23
5)水合盐:结晶水读做water或hydrate 如 AlCl3∙6H2O: aluminum chloride 6-water
化学物质的英文命名法

化学物质的英文命名法
甲烷methane乙烷ethane丙烷propane 丁烷butane戊烷pentane己烷hexane 庚烷heptane辛烷octane
烷烃-ane 烯烃-ene 环烃-cyclo- 甲基methyl 乙基ethyl 二甲基dimethyl 二乙基diethyl 三。
tri- 氯chrolo 溴bromo 碘iodo 顺cis- 反trans-
二氯二甲基丙烷2-Chrolo-2-methylpropane 一溴一甲基环己烷1-Bromo-1-methylcyclohexane summary:首个字母大写,基团按字母表顺序,首字母在前的先写。
若有两个或三个重复的,di-,tri-不参与排列。
次序:bromo,chrolo,ethyl,methyl
一氯环己烯1-Methylcyclohexene 顺1,2二溴环戊烷trans-1,2-Dibromocyclopentane summary:cis-,trans-不为官能团,不用遵循首个字母大写。
异丁烯2-Methylpropene (普通命名Iso butyl ene) 二甲基一,三丁二烯2-Methyl-1,3-butadiene (普通命名Iso pr ene) 2,2-二甲基-3-乙基-3-庚烯3-Ethyl-2,2-dimethyl-3-heptene。
(完整版)化合物英文命名规则

Nomenclature of Inorganic Compounds无机化合物的命名(Prefix词头,前缀Suffix词尾,后缀Stem词根)1.Trivial Names俗名H2O water不说dihydrogen oxideNH3 ammonia不说nitrogen trihydrideCaO quicklimeCaCO3 limestone2.Systematic Nomenclature系统命名1)Oxide氧化物——先命名非氧元素ZnOzinc oxideCaO calcium oxideCO carbon oxideNa2O2 sodium peroxideH2O2hydrogen peroxide 注:peroxide过氧化物2)Hydroxide氢氧化物(base碱)Ba(OH)2 barium hydroxideKOH potassium hydroxide3)Acid酸Hydro acid氢酸General formula通式:HnX 命名:hydro- + stem of X + -ic acid H2S hydrosulfuric acid(英) hydrosulphuric(美) 氢硫酸S:sulfur(英)、sulphur(美) HBr 氢溴酸hydrobromic acidBr: bromine HCl 氢氯酸(盐酸)hydrochloric acidCl: chlorine HF 氢氟酸hydrofluoric acidF: fluorineOxoacid or Oxyacid含氧酸General formula通式:HnXOm 命名:Stem of X + -ic acid 注:oxo- (oxy-) 含氧, 氧代H2SO4 sulfuric acid(英) sulphuric acid(美)H2CO3 carbonic acidH3PO4 phosphoric acid P: phosphorus H3BO3 boric acid B: boron HNO3 nitric acid N: nitrogen If X has two oxidation states:-ic:the higher oxidation state-ous:the lower oxidation stateH2SO4 sulfuric acidH2SO3 sulfurous acid1/5HNO3 nitric acidHNO2 nitrous acidIf X (such as halogens) has more than two oxidation states:halogen卤素per- (过,高) + -ic:the still higher oxidation statehypo- (次,在…下) + -ous:the still lower oxidation stateHClO3 chloric acidHClO2 chlorous acidHClO4 perchloric acidHClO hypochlorous acidHIO hypoiodous acid4)Salt盐General formula通式:MnXm 命名:Name of M stem of X + -ide(-ide…化物)Oxide、chloride、nitride、hydrideKI potassium iodideAl2S3 aluminum sulfideLiH lithium hydrideOxysalt含氧酸盐Name the metal ion first and then the anionNaming anions:-ate anions derived from the -ic acid(the higher oxidation state of X)-ite anions derived from the -ous acid (the lower oxidation state of X)HNO3 nitric acidNaNO3 sodium nitrateHNO2 nitrous acidNaNO2 sodium nitriteSO42- sulfateSO32- sulfiteAgClO4 silver perchlorateNaIO3 sodium iodateKClO2 potassium chloriteKBrO potassium hypobromiteMnO42- manganateMnO4- permanganateAcid salt 酸式盐Using “hydrogen” to specify “H”NaHSO4 sodium hydrogen sulfateNaH2PO4 sodium dihydrogen phosphateNa2HPO4 disodium hydrogen phosphate P: phosphorus phosphate磷酸盐(根) Using prefix bi- + name of anion if only one acid salt existsNaHSO4 sodium bisulfateNaHSO3 sodium bisulfiteKHCO3 potassium bicarbonate5)Metals(M)with more than one oxidation state2/5Two methods:①后缀法: 早期使用stem of M + -ic the higher oxidation state of Mstem of M + -ous the lower oxidation state of MHgI2 mercuric iodideHg2I2 mercurous iodide Hg:mercury Cr2+ chromousCr3+ chromic Cr: chromium注:In most cases, Latin stem is used if the metal has symbol derived from itsLatin name.(mercury is an exception)Cu:cuprum (拉丁),copper (英)Cu+ cuprousCu2+ cupricCuI cuprous iodideCuS cupric sulfideSn:stannum (拉丁), tin (英)SnCl2 stannous chlorideSnO2 stannic oxideFe:ferrum (拉丁), iron (英)Fe(OH)2 ferrous hydroxideFeBr3 ferric bromide②IUPAC Rule 1957年开始使用English name of metal(Roman numeral)CuBr copper(I) bromideCuF2 copper(II) fluorideSnO tin(II) oxideSnS2 tin(IV) sulfideFe(NO3)2 iron(II) nitrateFe2(SO4)3 iron(III) sulfateUse Greek prefixes希腊文前缀Mon(o)一di二tri三tetr(a)四pent(a)五hex(a)六hepta七octa八nona九1.to specify the number of each atom in the chemical formula.NO2 nitrogen dioxidePCl5 phosphorus pentachlorideCO2 carbon dioxide2.to specify the number of identical central atoms in condensed acids and their corresponding anions.condensed acid缩酸H3PO4 (mono)phosphoric acidH4P2O7 diphosphoric acid3/5H2SO4 sulfuric acidH2S3O10 trisulfuric acidCrO42- 铬酸盐(根) chromateCr2O72- 重铬酸盐(根)dichromate3. to indicate extent of substitutionPO43- phosphatePS2O23- dithiophosphate thio-硫代…,硫的,含硫的注:The prefixes ortho- and meta- have been used to distinguish acids differingin the “content of water.”ortho- [希腊词头] 正、原(无机酸用)邻(位)(有机化合物命名)meta- [希腊词头] 偏(无机酸用)间(位)(有机化合物命名)ortho-acid 原酸;meta-acid 偏酸H3BO3 orthoboric acid(or boric acid)(原)硼酸(HBO3)n metaboric acid偏硼酸H4SiO4 orthosilicic acid(or silicic acid)原硅酸H2SiO3 metasilicic acid 硅酸(习惯上不叫偏硅酸)H3PO4 orthophosphoric acid (or phosphoric acid)(正)磷酸(HPO3)n metaphosphoric acid 偏磷酸。
最新简单的化学物质的英文命名法
水合物则只需在最后加上带有数字前缀的
hydrate 即可。如五水硫酸铜 copper(II) sulfate
pentahydrate 。 数字词缀如下表
1 mono-
6 hexa-
2 di-
7 hepta-
3 tri-
8 octa-
4 tetra-
9 nona-
5 penta-
10 deca-
以上为常用的数字, 此外,无水物是 anhydrate 。11 的词缀是 undeca- ,12 的词缀是 dodeca- ,
disulfide
S
22
-
bisulfide HS thiocyanate SCN-
chloride Cl -
permanganateMnO
4
ferrate(VI)
FeO
24
铜 cupr-
copper
硒 selen钼 molybd-
selenium molybdenum
selenate SeO42-
disulfite S 2O5 2-
perchlorate
ClO
4
chlorite
ClO
2
-
hypochlorite ClO
chromite
CrO
2
carbide 碳化物 nitride N 3azide N3-
fluoride F -
silicide 硅化物 phosphide P 3-
sulfide S2-
selenite SeO32-
molybdate
MoO
24
selenide Se2-
由上标可见, 一般的含氧酸盐以 -ate 结尾,中文中带“亚”字的以 -ite 结尾,如果是“次”,
化学专业英语---化合物的命名

24
5. Acids
(1). Per-, hydro-,normal acid (its salt-ate,-ide)
Acid = Central element’s root -ic + acid
举例: PH3: phosphine或phosphane SbH3: stibine或stibane CH4: methane B2H6: diborane
AsH3: arsine或arsane BiH3: bismuthane SiH4: silane
17
无氧酸
命名规则:hydro-词根-ic acid 举例: HCl: hydrochloric acid H2S : hydrosulfuric acid
VIA
VIIA
0
He Helium
O Oxygen
F Fluorine
Ne Neon
S Sulfur
Cl Chlorine
Ar Argon
Se Selenium Br Bromine
Kr Krypton
Te Tellurium
I Iodine
Xe Xenon
Po Polonium At Astatine
7
2.Multivalence ions
Cation’s name = Element(N)
For example:
Fe2+ Iron(II)
or Ferrous
Fe3+ Iron(III)
or Ferric
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
化合物的英文命名(Nomenclature of compounds)I 无机物的命名(Inorganic compounds)1 元素与单质的命名“元素”和“单质”的英文意思都是“element”,有时为了区别,在强调“单质”时可用“free element”。
因此,单质的英文名称与元素的英文名称是一样的。
下面给出的既是元素的名称,同时又是单质的名称。
S-block ElementIA IIAH Hydrogen Be BerylliumLi Lithium Mg Magnesiu mNa Sodium Ca Calcium K Potassium Sr Stronti umRb Rubidium Ba BariumCs CesiumFr Francium Ra Radi umP-block ElementIIIA IVA V AB BoronC CarbonN NitrogenAl Aluminium Si SiliconP PhosphorusGa Gallium Ge Germanium As ArsenicIn Indium Sn Tin Sb AntimonyTl Thallium Pb Lead Bi BismuthVIA VIIAHe HeliumO Oxygen F FluorineNe NeonS Sulfur Cl ChlorineAr ArgonSe Selenium Br BromineKr KryptonTe Tellurium I IodineXe XenonPo Polonium At AstatineRn RadonCommon Transition ElememtFe : iron Mn : manganeseCu: copper Zn: zincHg: mercury Ag: silverAu: gold2 化合物的命名化合物的命名顺序都是根据化学式从左往右读,这与中文读法顺序是相反的。
表示原子个数时使用前缀(1)mono-,(2)di -,(3)tri- ,(4)tetra – ,(5)penta- (6)hexa-,(7)hepta-, (8)octa-,(9)nona-,(10)deca-,但是在不会引起歧义时,这些前缀都尽可能被省去。
①Naming metal ions (cations) for metal oxides, bases and saltsi. Single valence ionsCation’s name = Elementfor example:Na+ Sodium Al3+ AluminumK+ Potassium Ca2+ Calciumii.Multivalence ionsCation’s name = Element(N)For example:Fe2+ Iron(II) or FerrousFe3+ Iron(III) or FerricCr2+ Chromium(II)Cr3+ Chromium(III)Mn4+ Manganese(IV)Mn2+ Manganese(II)对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous表示低价,-ic表示高价。
如 FeO: iron(II)oxide 或 ferrous oxideFe2O3: iron (III) oxide或ferric oxideCu2O: copper(I) oxide 或cuprous oxideCuO: copper(II)oxide或cupric oxide②Naming nonmetal ions (anions)i. Monatomic anionsAnion’s name = Element’s root -ideFor example:Cl- Chloride O2- OxideBr- Bromide OH- Hydroxide I- Iodide CN- CyanideS2- Sulfide H- Hydrideii. Polyatomic oxyanionsa. Acid radicals for normal salt(正酸根 -ate )Anion’s name = Central Element’s root -atefor example:ClO3- Chlor ate IO3- Iod atePO43- Phosph ate NO3- Nitr ateSO42- Sulf ate CO32- Carbon ateb. Acid radicals for meta-salts (亚酸根 -ite )Anion’s name = Central element’s root -itefor example:ClO2- Chlor ite IO2- Iod itePO33- Phosph ite NO2- Nitr iteSO32- Sulf itec. Acid radicals for hypo-salts (次酸根 -ite )Anion’s name = Hypo- Central element’s root -ite for example:ClO- Hypo chlor iteIO- Hypo iod itePO23- Hypo phosph ited. Acid radicals for persalts (高酸根Per -ate )Anion’s name =Per-central Element’s root -atefor example:ClO4- Per chlor ateIO4- Per iod ateMnO4- Per mangan ate③Naming compoundsi. Metal oxideMetal oxide = Cation + oxidefor example:FeO Iron(II) oxide (Ferrous oxide)Fe2O3 Iron(III) oxide (Ferric oxide)Fe3O4 Ferroferric oxidePb3O4 Trilead tetroxideNa2O2 Sodium peroxideii. Nonmetal oxideNonmetal oxide = n-Nonmetal element + n-oxide for example:CO Carbon monoxideCO2 Carbon dioxideSO3 Sulfur trioxideN2O3 Dinitrogen trioxideP2O5 Diphosphorus pentoxideN2O4 Dinitrogen tetroxide(tetra-,mono-后缀中的a,o在后一o之前省去)有些物质常用俗称,如NO: nitric oxide N2O: nitrous oxideiii非金属氢化物除了水和氨气使用俗称water,ammonia以外,其它的非金属氢化物都用系统名称,命名规则根据化学式的写法不同而有所不同。
a.对于卤族和氧族氢化物,H在化学式中写在前面,因此将其看成与另一元素的二元化合物。
举例: HF hydrogen fluoride HCl hydrogen chlorideHBr hydrogen bromide HI hydrogen iodideH2S hydrogen sulfide H2Se hydrogen selenideH2Te hydrogen tellurideb对于其它族的非金属氢化物,H在化学式中写在后面,可加后缀-ane,氮族还可加-ine举例: PH3: phosphine或phosphane AsH3: arsine或arsaneSbH3: stibine或stibane BiH3: bismuthaneCH4: methaneSiH4: silaneB2H6: diboraneiv. Acids a. Per-, hydro-,normal acid (its salt-ate,-ide)Acid = Central element’s root -ic + acidfor example:H2CO3 Carbon ic acidH2SO4 Sulfur ic acidH3PO4 Phosphor ic acidHNO3 Nitr ic acidHClO4 Perchlor ic acidHCl Hydrochlor ic acidb Meta- and hypo-acid ( its salt-ite)Acid = Central element’s root -ous + acidfor example:H2SO3 Sulfur ous acidH3PO3 Phosphor ous acidHNO2 Nitr ous acidHClO Hypochlor ous acidHClO2 Chlor ous acid含氧酸与含氧酸根阴离子采用前后缀的不同组合显示不同价态的含氧酸和含氧酸根阴离子,价态相同的含氧酸及含氧酸根阴离子具有相同的前缀,不同的后缀。
高某酸 per-ic 正酸–ic 亚酸 -ous 次酸hypo-ous高某酸根 per-ate 正酸根–ate 亚酸根 -ite 次酸根hypo-ite其它的前缀还有 ortho-正 meta- 偏 thio-硫代举例:HClO4 perchloric acid ClO4- perchlorate ionHClO3 chloric acid ClO3- chlorate ionHClO2 chlorous acid ClO2- chlorite ionHClO hypochlorous acid ClO- hypochlorite ionH2SO4 sulfuricacid H2SO3 sulfurous acidHNO3 nitricacid HNO2 nitrous acidHPO3 metaphosphoric acid S2O32- thiosulfate ionH2SO4 sulfuric acidHCl hydrogen chloride or hydrochloric acidHNO3 nitric acidHNO2 nitrous acidHCN hydrogen cyanide or hydrocyanic acidNa2S sodium sulfideCuSO4 copper (II) sulfate or cupric sulfateFe(NO3)3 iron (III) nitrate or ferric nitrateHClO4 perchloric acidb无氧酸命名规则:hydro-词根-ic acid举例: HCl: hydrochloric acidH2S : hydrosulfuric acidvi. BasesBase = Metal cation + hydroxidefor example:Al(OH)3 Aluminum hydroxideNaOH Sodium hydroxideCa(OH)2 Calcium hydroxideBa(OH)2 Barium hydroxideCo(OH)2 Cobalt(II) hydroxidevii.盐(Salts)a. 正盐(Normal salt):根据化学式从左往右分别读出阳离子和阴离子的名称。
