实验一微藻培养基配制
藻类培养基
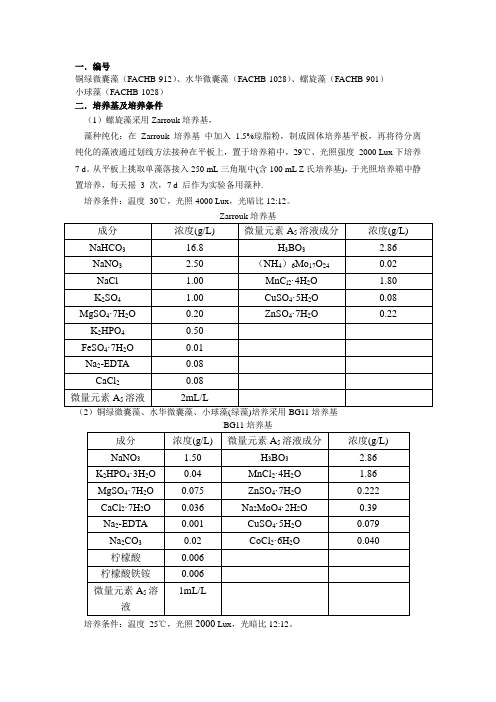
一.编号
铜绿微囊藻(FACHB-912)、水华微囊藻(FACHB-1028)、螺旋藻(FACHB-901)
小球藻(FACHB-1028)
二.培养基及培养条件
(1)螺旋藻采用Zarrouk培养基,
藻种纯化:在Zarrouk 培养基中加入 1.5%琼脂粉,制成固体培养基平板,再将待分离纯化的藻液通过划线方法接种在平板上,置于培养箱中,29℃、光照强度2000 Lux下培养7 d。
从平板上挑取单藻落接入250 mL三角瓶中(含100 mL Z氏培养基),于光照培养箱中静置培养,每天摇3 次,7 d 后作为实验备用藻种.
培养条件:温度30℃,光照4000 Lux,光暗比12:12。
(2)铜绿微囊藻、水华微囊藻、小球藻(绿藻)培养采用BG11培养基
培养条件:温度25℃,光照2000 Lux,光暗比12:12。
实验室藻种培养基

按实验室保存藻种及适用方法统计(共计18种)1、ASP2培养基培养基成分:微量元素储备液*成分:Tris-HCl缓冲液(pH8.0, 1M)50mL配制:称取6.0546g Tris,配成25mL,即为2mol/L的Tris溶液,用NaOH或HCl调节pH值到8.0,加水定容至50mL。
注意培养液中MgSO4和CaCl2分别溶解再加入其余成分。
固体培养基,添加1.5%-2%的琼脂粉。
121℃,0.1Mpa,灭菌20min。
适用藻种:杜氏盐藻2、SM(+N/-N)培养基生长培养基成分(+N):微量元素储备液*成分:固体培养基,添加1.5%-2%的琼脂粉。
121℃,0.1Mpa,灭菌20min。
诱导培养基成分(-N):生长培养基中不加氮源(NH4NO3),同时可向培养物中加入45mmol/L 醋酸钠,450μmol/L硫酸亚铁。
适用藻种:雨生红球藻(生长+N)(诱导-N)3、Z氏培养基基础培养基成分:储备液灭菌后备用缓冲液可选用HEPES-NaOH < 5mmol/L。
适用藻种:螺旋藻4、人工海水培养基基础培养基(f/2)成分:基础培养基(f/2)可加缓冲液或用酸碱调pH7.6-7.8,再将灭菌后的储备液,微量元素及维生素按要求加入。
维生素不能高温灭菌,可用0.22μm膜过滤。
适用藻种:三角褐指藻、中肋骨条藻、金藻塔玛亚历山大藻、微小原甲藻(dead)5、葡萄藻培养基基础培养基:微量元素储备液成分:适用藻种:葡萄藻6、BG11(+N/-N)培养基参考文献:Stanier, R.Y., Kunisawa, R., Mandel, M.& Cohen-Bazire, G.1971. Purification and properties of unicellular blue-green algae (Order Chroococcales). Bacteriol. Rev. 35: 171-205.准备储备液Stocks见下表:培养基配置:用NaOH或HCl调节pH值到7.1,固体培养基加1.5%的琼脂粉。
藻类的实验室培养
微藻的实验室培养学生:林晓生学号:2120180414导师:杨缜教授一、藻类的概述藻类是原生生物界一类真核生物(有些也为原核生物,如蓝藻门的藻类)。
主要水生,无维管束,能进行光合作用。
藻类植物共约为2100属,27000种。
根据所含色素、细胞构造、生殖方法和生殖器官构造的不同,分为绿藻门、裸藻门、轮藻门、金藻门、黄藻门、硅藻门、甲藻门、蓝藻门、褐藻门和红藻门。
色素的颜色划分,藻可分为3类:绿藻、褐藻和红藻。
由于单胞藻具有利用太阳光能效率高、营养丰富、生长繁殖迅速、对环境的适应性强和容易培养等重要特性,因而受到重视。
微藻是一类在陆地、海洋分布广泛,营养丰富、光合利用度高的自养植物,细胞代谢产生的多糖、蛋白质、色素等,使其在食品、医药、基因工程、液体燃料等领域具有很好的开发前景。
二、微藻的营养模式和生长模式(二)、微藻的生长模式藻类在培养过程中,生长繁殖的速度,出现一定的起伏,这种生长模式可划分为五个时期(延缓期、指数生长期、相对生长下降期、静止期、死亡期)。
三、培养按培养的场所分室内培养和室外培养1)按培养基的形态分固体培养和液体培养2)按培养的纯度分纯种培养和单种培养3)按藻液的流动情况分静止培养和循环流动水培养4)按气体交换情况分充气培养和不充气培养5)按藻液与外界接触程度分封闭式培养和开放式培养6)按培养规模和目的分小型培养、中继培养和大量培养方式简单介绍其中的四种方式:(1)纯培养和单种培养纯培养(axenic culture):是无菌培养,指排除了包括细菌在内的一切生物的条件下进行的培养。
纯培养操作要求十分严格,要求有无菌室、超净台等设备,容器、工具、培养液等均须彻底灭菌。
培养成功率很高,是进行科学研究不可缺少的技术。
单种培养(single-species culture):指区别于纯培养的不排除细菌存在的培养(可以是生产性的,也可以是非生产性的)(2)封闭式培养和开放式培养封闭式培养(closed culture):指把培养液密封在透明的容器中,与外界空气隔离,暴露在阳光中,CO2完全采用人工供给的方法。
微藻养殖培养基介绍
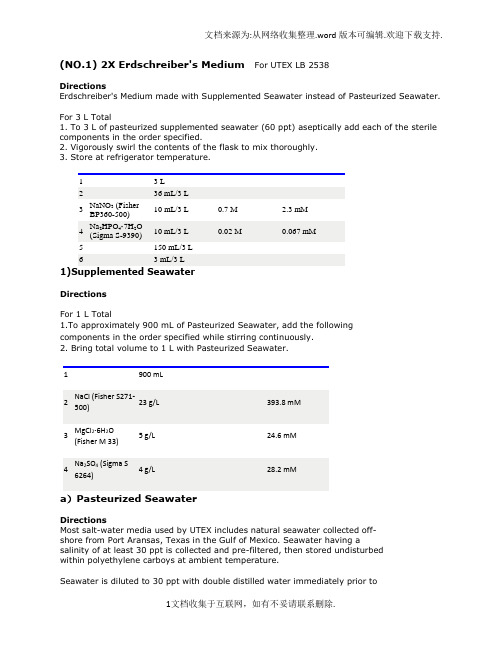
(NO.1) 2X Erdschreiber's Medium For UTEX LB 2538DirectionsErdschreiber's Medium made with Supplemented Seawater instead of Pasteurized Seawater. For 3 L Total1. To 3 L of pasteurized supplemented seawater (60 ppt) aseptically add each of the sterile components in the order specified.2. Vigorously swirl the contents of the flask to mix thoroughly.3. Store at refrigerator temperature.1 3 L2 36 mL/3 L3 NaNO3 (FisherBP360-500)10 mL/3 L 0.7 M 2.3 mM4 Na2HPO4·7H2O(Sigma S-9390)10 mL/3 L 0.02 M 0.067 mM5 150 mL/3 L6 3 mL/3 L1)Supplemented SeawaterDirectionsFor 1 L Total1.To approximately 900 mL of Pasteurized Seawater, add the following components in the order specified while stirring continuously.2. Bring total volume to 1 L with Pasteurized Seawater.1 900 mL2 NaCl (Fisher S271-500)23 g/L 393.8 mM3 MgCl2·6H2O(Fisher M 33)5 g/L 24.6 mM4 Na2SO4 (Sigma S6264)4 g/L 28.2 mMa)Pasteurized SeawaterDirectionsMost salt-water media used by UTEX includes natural seawater collected off-shore from Port Aransas, Texas in the Gulf of Mexico. Seawater having a salinity of at least 30 ppt is collected and pre-filtered, then stored undisturbed within polyethylene carboys at ambient temperature.Seawater is diluted to 30 ppt with double distilled water immediately prior topasteurization. A three-liter batch of seawater at 30 ppt in a 4-liter Erlenmeyer flask is covered with a small inverted glass petri plate and an inverted 250-ml beaker, then "pasteurized" in a steamer for 45 minutes. The pasteurized content of the flask is allowed to cool and left undisturbed at ambient temperature for approximately 24 hr. It is then again steam-pasteurized for 45 min., as on the previous day. After the flask cools the second time, the inverted-petri-plate lid is sealed in place with Parafilm and the flask is stored at refrigerator temperature until it is used to prepare culture medium. This pasteurized seawater may used immediately or may be stored for several months prior to use.Procedure:In various laboratories marine algae are cultured in media prepared from "pasteurized" seawater that is assumed to be heated to exactly 73 degrees C. The procedure described here heats 3-L batches of seawater to over 95 degrees C for two consecutive days, although it does not reach boiling temperature.This procedure generally does not cause precipitation of seawater, although excessive agitation of flasks, the use of scratched or etched flasks, or pasteurization of seawater at higher salinity may result in salt precipitation during heating.Pasteurized seawater prepared as described above appears to be nearly sterile, although it is not used to culture axenic UTEX cultures without further heating in agar. Liquid unialgal cultures grown in media prepared from seawater that has been pasteurized by this method can be sub-cultured for many years without the introduction of invasive contamination.2) P-IV Metal SolutionDirectionsFor 1 L TotalNote final concentration listed is for the stock solution.1.To approximately 950 mL of dH2O, add the nutrients in the order listed while stirring continuously.Note: The Na2EDTA should be fully dissolved before adding other components.2. Bring total volume to 1 L with dH2O.3. Store at refrigerator temperature.1 Na2EDTA·2H2O(Sigma ED255)0.75 g/L 2 mM2 FeCl3·6H2O(Sigma F-1513)0.097 g/L 0.36 mM3 MnCl2·4H2O(Baker 2540)0.041 g/L 0.21 mM4 ZnCl2 (Sigma Z-0152)0.005 g/L 0.037 mM5 CoCl2·6H2O(Sigma C-3169)0.002 g/L 0.0084 mM6 Na2MoO4·2H2O 0.004 g/L 0.018 mMconsiderations are probably important, including the following:1. The soil should be a loam, with a mixture of particle sizes(sand, silt, clay).2. It should contain a moderate amount (15 - 20%) of very-well-decomposed organic matter.3. It must not contain pesticides, especially herbicides.4. It should be soil that has been aged (preferably for 6 monthsor more) under moist conditions and not, for example, freshpotting soil, soil that contains fresh manure, or soil to which acommercial fertilizer was recently applied.5. A slightly acidic soil derived from granite or other igneousrock is preferable to soil obtained from calcareous soils. Calcium carbonate can be added to the soilwater medium when it isprepared if a slightly alkaline medium is required.6. Particulate matter in the soil such as gravel, Perlite, orvermiculite are not necessarily damaging but can be ofconsiderable nuisance when wishing to quantitate the amount ofsoil used in the medium or when handling algae that arephysically associated with the soil. Particulate organic matter,such as compost that is only partially degraded, should beavoided altogether.4) Vitamin B12DirectionsFor 200 mL Total1. Prepare 200 mL of HEPES buffer (50 mM).2. Adjust the pH to 7.8.3. Add Vitamin B12 (0.1 mM) wait until fully dissolved.4. Sterilize by 0.45 µm Millipore filter. Store in dark at freezer temperature. ** The amount of vitamins added can vary from medium to medium so the final concentration is not listed.1 HEPES buffer pH7.8 (Sigma H-3375)2.4 g/200 mLdH2O2 Vitamin B12 (cyanocobalamin,(Sigma V-6629)0.027 g/200 mLdH2O(NO.2)Proteose Medium UTEX 32DirectionsGeneral purpose freshwater medium suitable for axenic cultures. Modified bristol's medium.For 1 L Total pH ~6.81. Add proteose peptone to Bristol Medium.*For 1.5% agar medium add 15 g of agar into the flask; do not mix.2. Cover and autoclave medium.1 1 L2 Proteose Peptone(BD 211684)1 g/L1)Bristol MediumDirectionsH.C. Bold's modification of Bristol's recipe (Bold 1949). General purpose freshwater medium and as bristol's solution, an essential component of other media--see Bold 1NV, Bold 3N, Bristol-NaCl, LDM, Proteose, Soil extract, and Trebouxia.For 1 L Total1. To approximately 900 mL of dH2O add each of the components in the order specified while stirring continuously.2. Bring total volume to 1 L with dH2O.*For 1.5% agar medium add 15 g of agar into the flask; do not mix.3. Cover and autoclave medium.4. Store at refrigerator temperature.1 NaNO3 (FisherBP360-500)10 mL/L10 g/400mLdH2O2.94 mM2 CaCl2·2H2O(Sigma C-3881)10 mL/L 1 g/400mL dH2O 0.17 mM3 MgSO4·7H2O(Sigma 230391)10 mL/L 3 g/400mL dH2O 0.3 mM4 K2HPO4 (Sigma P3786)10 mL/L 3 g/400mL dH2O 0.43 mM5 KH2PO4 (Sigma P0662)10 mL/L 7 g/400mL dH2O 1.29 mM6 NaCl (FisherS271-500)10 mL/L 1 g/400mL dH2O 0.43 mM(NO.3) MES-volvox Medium UTEX 2505DirectionsGeneral purpose medium for freshwater strains, especially those requiring ammonium. Suitable for xenic and axenic cultures. Modified volvox medium. For 1 L Total1. To approximately 950 mL of dH2O, add each of the components in the order specified (except vitamins) while stirring continuously.2. Adjust the pH to 6.7.3. Bring the total volume to 1 L with dH2O.*For 1.5% agar medium add 15 g of agar into the flask; do not mix.4. Cover and autoclave medium.5. When cooled add vitamins.*For agar medium add vitamins, mix, and dispense before agar solidifies.6. Store at refrigerator temperature.1 Ca(NO3)2·4H2O (Sigma C5676)1 mL/L11.8 g/100 mLdH200.5 mM2 MgSO4·7H2O (Sigma 1 mL/L 4 g/100 mL 0.16 mM230391) dH203 Na2glycerophosphate.5H2O(Sigma G 6501 )1 mL/L5 g/100 mLdH200.16 mM4 KCl (Fisher P 217) 1 mL/L5 g/100 mLdH200.67 mM5 MES (Sigma M-8250) 1.95 g/L 10 mM6 6 mL/L7 NH4Cl (Fisher A 649-500) 1 mL/L 2.67 g/100 mLdH200.5 mM8 1 mL/L9 1 mL/La)Biotin Vitamin SolutionDirectionsFor 200 mL Total1. Prepare 200 mL of HEPES buffer (50 mM).2. Adjust the pH to 7.8.3. Add biotin (0.1 mM) wait until fully dissolved.4. Sterilize by 0.45 µm Millipore filter. Store in dark at freezer temperature. ** The amount of vitamins added can vary from medium to medium so the final concentration is not listed.1 HEPES buffer pH7.8 (Sigma H-3375)2.4 g/200 mLdH2O2 Biotin(Sigma B-4639)0.005 g/200 mLdH2O(NO.4)Enriched Seawater Medium UTEX LB 1926DirectionsModification of L. Provasoli's ES-enrichment for seawater PES (Bold & Wynne 1978). General purpose marine medium for xenic cultures.1. Aseptically add 20 mL of sterile ES Enrichment Solution per liter of Pasteurized Seawater (30 ppt).2. Store at refrigerator temperature.1 1 L2 20 mL/L1)Enrichment Solution for Seawater MediumDirectionsFor 2 L Total1. To approximately 1 L of dH2O, add each of the components in the order specified (except vitamins) while stirring continuously.2. Adjust the pH to 7.8.3. Bring the total volume to 2 L with dH2O.4. Cover and autoclave medium.5. When cooled add vitamins.6. Store at refrigerator temperature.1 NaNO3 (Fisher BP360-500) 4.7 g/2 L 27.65 mM2 Na2glycerophosphate.5H2O(Sigma G 6501 )0.7 g/2 L 1.6 mM3 325 mL/2 L4 325 mL/2 L5 HEPES buffer (Sigma H-3375)6.5 g/2 L 14 mM6 3 mL/2 L7 3 mL/2 L8 3 mL/2 La)ES Fe SolutionDirectionsFor 2 L Total1. To approximately 1.5 L of dH2O, add the following components in the order listed while stirring continuously.2. Bring total volume to 2 L with dH2O.3. Store at refrigerator temperature.1 Fe(NH4)2(SO4)2·6H2O(Sigma F-1513)1.4 g/2 L 1.8 mM2 Na2EDTA·2H2O(Sigma ED255)1.2 g/2 L 1.6 mMb)P-II Metal SolutionDirectionsFor 100 mL Total1. To approximately 70 mL of dH2O, add each of the components in the order specified while stirring continuously.2. Bring total volume to 100 mL with dH2O.3. Store at refrigerator temperature.Note: CoCL2 can be interchancable with CoSO4.1 Na2EDTA·2H2O(Sigma ED255)0.1 g/100 mL 0.27 mM2 H3BO3 (Baker 0.114 g/100 mL 1.8 mM0084)3 FeCl3·6H2O(Sigma F-1513)4.9 mg/100 mL 0.018 mM4 MnSO4·H2O(Sigma M8179)16.4 mg/100 mL 0.097 mM5 ZnSO4·7H2O(Sigma Z 0251)2.2 mg/100 mL 0.007 mM6 CoCl2·6H2O(Sigma C-3169)0.48 mg/100 mL 0.002 mMc)Thiamine Vitamin SolutionDirectionsFor 50 mL Total1. Prepare 50 mL of HEPES buffer (50 mM).2. Adjust the pH to 7.8.3. Add Thiamine (6.5 mM) wait until fully dissolved.4. Sterilize by 0.45 µm Millipore filter. Store in dark at freezer temperature. ** The amount of vitamins added can vary from medium to medium so the final concentration is not listed.1 HEPES buffer pH7.8 (Sigma H-3375)1.2 g/100 mLdH202 Thiamine (SigmaT-1270)0.11 g/100 mLdH20(NO.5)Soil Extract + Sodium Metasilicate UTEX 640DirectionsModification of Soil Extract Medium for diatoms.For 1 L Total1. Prepare 1 L of Bristol Medium and thoroughly mix.2. Discard 40 mL of the Bristol Medium.3. Add 40 mL of previously prepared GR+ Medium [Note: The GR+ should be filtered to remove soil particles].* or For 1.5% agar medium add 15 g of agar into the flask; do not mix.4. Cover and autoclave medium.5. When cooled, add filter sterilized sodium metasilicate.*For agar medium, add sodium metasilicate, mix, and dispense before agar solidifies.6. Store at refrigerator temperature.1 960 mL2 40 mL of supernatant3 Sodium Metasilicate 1 mL 200 mM 200 µM (NO.6)Erdschreiber'sMedium UTEX LB 1002and 2307DirectionsModified from the original Plymouth seawater recipe. General purpose marine medium for xenic cultures [for bacteria-free cultures see reprints in Rosowski & Parker (1971)].For 3 L Total1. To 3 L of pasteurized filtered seawater (30 ppt) aseptically add each of the sterile components in the order specified.2. Vigorously swirl the contents of the flask to mix thoroughly.3. Store at refrigerator temperature.1 3 L2 36 mL/3 L3 NaNO3(autoclave beforeadding) (FisherBP360-500)10 mL/3 L 0.7 M 2.3 mM4 Na2HPO4·7H2O(autoclave beforeadding) (Sigma S-9390)10 mL/3 L 0.02 M 0.067 mM5 150 mL/3 L6 3 mL/3 L(NO.7)TAP medium (original )from Gorman, D.S., and R.P. Levine (1965) Proc. Natl. Acad. Sci. USA54, 1665-1669. This is probably the most widely-used medium at present for experimental work.Make the following stock solutions:1. TAP saltsNH4Cl 15.0 gMgSO4 . 7H2O 4.0 gCaCl2 . 2H2O 2.0 gwater to 1 liter2. phosphate solutionK2HPO428.8 gKH2PO414.4 gwater to 100 ml3. Hutner's trace elements (follow this )To make the final medium, mix the following:2.42 g Tris25 ml solution #1 (salts)0.375 ml solution #2 (phosphate)1.0 ml solution #3 (trace elements)1.0 ml glacial acetic acidwater to 1 literFor solid medium, add 15 g agar per literAutoclave.For Tris-minimal medium omit the acetic acid and titrate the final solution to pH 7.0 with HCl(1)Hutner's trace elementsHutner et al. (1950) Proc. Am. Philos. Soc.94, 152-170This mixture is used both in and in the medium.For a detailed analysis of how well this trace elements solution meets the nutritional requirements of C. reinhardtii, see Merchant et al. (2006) Biochim. Biophys. Acta1763, 578-594.For 1 liter final mix, dissolve each compound in the volume of water indicated.文档来源为:从网络收集整理.word版本可编辑.欢迎下载支持.The EDTA should be dissolved in boiling water, and the FeSO4 should be prepared last to avoid oxidation.compound amount waterEDTA disodium salt 50 g 250 mlZnSO4 . 7 H2O 22 g 100 mlH3BO311.4 g 200 mlMnCl2 . 4 H2O 5.06 g 50 mlCoCl2. 6 H2O 1.61 g 50 mlCuSO4 . 5 H2O 1.57 g 50 ml(NH4)6Mo7O24. 4 H2O 1.10 g 50 mlFeSO4. 7 H2O 4.99 g 50 mlMix all solutions except EDTA. Bring to boil, thenadd EDTA solution. The mixture should turn green.When everything is dissolved, cool to 70 degrees C.Keeping temperature at 70, add 85 ml hot 20% KOHsolution (20 grams / 100 ml final volume). Do NOTuse NaOH to adjust the pH.Bring the final solution to 1 liter total volume. Itshould be clear green initially. Stopper the flask witha cotton plug and let it stand for 1-2 weeks, shaking itonce a day. The solution should eventually turnpurple and leave a rust-brown precipitate, which canbe removed by filtering through two layers ofWhatman#1 filter paper, repeating the filtration ifnecessary until the solution is clear. Storerefrigerated or frozen convenient aliquots. Somepeople shorten the time for formation of theprecipiate by bubbling the solution with filtered air.If no precipitate forms, the solution is still usable.However, you might want to check the pH in thiscase and adjust it to around 7.0 using either KOH orHCl as needed.To prepare sulfur-free trace elements for hydrogengeneration, the sulfate salts can be replaced withequimolar chloride salts (ZnCl2 10.0 g; CuCl2 . 2H2O 1.00 g; FeCl2 . 4 H2O, 3.60 g). .11文档收集于互联网,如有不妥请联系删除.。
藻类生物学实验

进行微藻培养时,可根据培养藻类对营养的要求,选用合适 的配方,在消毒水中按配方加入各种营养物质配成。所加肥料 应保持清洁,某些不清洁肥料必须消毒,预防敌害生物通过肥 料污染。
绿藻培养液配方:
①海洋三号扁藻培养液(海洋研究所,1960):
NaNO3
0.1g
2Na3C6H5O7﹒ 11H2O 0.02g
1、实验材料:海带、 裙带菜、紫菜、小球藻、球等鞭金藻等,选4种藻 2、实验仪器:紫外分光光度计(208实验室) 3、实验器材:研钵(每组一套)、15ml离心管(每组4支-依藻种类定)、
15ml刻度试管(每组4支)、0.45m的滤膜(每组3个),抽滤装置, 离心机(或用20ml针管和滤膜器) 4、试剂:丙酮(分析纯)、MgCO3
碘液,常用的配方是:将6克的碘化钾溶于20毫升水中,待完全溶解后加入4克碘,摇 荡,待碘完全溶解后,加入80毫升蒸馏水,贮存在棕色试剂瓶内。)
(3) 计数板与盖玻片洗净擦干――盖好盖玻片――摇荡藻液――吸取藻液 (干的微吸管)――迅速加样――1分钟后低倍镜下计数-计数任何对角两大 格(加盖玻片后每一大格即形成一个体积为0.1立方毫米的空间),然后取其 平均值。每个样品须重复计数两次。
三、实验步骤
3、分离:镜检待分离的藻液,调藻液浓度为5-6个藻细胞为宜,在已灭菌 的载玻片上滴加6滴消毒培养液,另取一载玻片滴一滴稀释的待分离藻 液,在显微镜下用微吸管吸取所需的藻细胞,放在第一滴消毒水中,清 洗,再用微吸管吸取所需的藻细胞放在备有培养液的试管中。
4、将装有藻细胞的试管置于适宜的条件下培养。
三、实验步骤
1、抽滤: 减压过滤5ml的藻类培养液到玻璃纤维滤片上,约加0.1g MgCO3 细粉,使均匀覆盖于薄膜上,倒入水样抽滤,注意避免高温、强光及压力
小球藻培养基

小球藻培养基Newly compiled on November 23, 2020
(一)淡水培养基
1. BG—11 培养基
另取(NO3),溶解在200ml纯水中,每次同A5一起,加入 1L的培养基中。
灭菌后PH 调至
2. Zarrouk 培养基
注意:NH4VO3很难溶解,注意充分搅拌,B6液使用前要充分的摇动。
A5和B6要单独配制,要低温或冷冻保存。
新培养基的PH值为—
3.紫球藻培养基
(二)海水培养基
4. f / 2 培养基
使用时,每1L海水中需加入1ml 上述母液;三种维生素不能高温灭菌处理。
5. f / 2 + Si 培养基
所有的试剂与配制剂使用的方法同上述的f / 2,只不过要额外添加Si源。
单独取1.5g Na2SiO3 9H2O溶于50ml 纯水中(所以母液浓度为30Mg/L)。
每1L海水中需加入1ml母液。
藻类生物学试验

二、实验材料及用具
1、实验材料:小球藻(500ml)、等鞭金藻、扁藻 2、实验仪器:可见分光光度计、显微镜(1部/组)、血球计数板(一个/
组)、电炉、灭菌锅、pH计、 500ml烧杯(1个/组)、胶头滴管(3支 /组)、 250ml锥形瓶(每组3个)、牛皮纸、细绵绳 3、试剂:蒸馏水、海水、碘化钾
碘液,常用的配方是:将6克的碘化钾溶于20毫升水中,待完全溶解后加入4克碘,摇 荡,待碘完全溶解后,加入80毫升蒸馏水,贮存在棕色试剂瓶内。)
5.E液 500 ml 1000倍
CuSO4.5H2O
0.0098 mg; ZnSO4.7H2O
0.022 mg
CaCl2.6H2O
0.01 mg; MgCl2.4H2O
0.180 mg
Na2MoO4.2H2O 0.0063 mg
A、B、D、E高压灭菌备用
(二)海水处理
沉淀 —— 砂池过滤 ——(抽滤)——灭菌(海水放于三角瓶 中,置于电炉上煮沸,可杀来一般细菌)
加入海泥抽出液20ml,效果更好。
硅藻培养液配方:
①艾伦-内尔森(Allen-Nelson)培养液{Allen,E.J.et al (1910)}:
A. KNO3
20.2g
纯水
100ml
B. Na2HPO4.12H2O 4.0g
CaCl.6H2O
4.0g
HCl(浓)
2.0ml
纯水
80ml
FeCl3(溶液) 2.0ml
K2HPO4
0.01g
Fe(SO4)3(1%溶液) 10滴
海水
1000ml
②亚心形扁藻培养液(湛江水产学院):
微藻工厂化培养经验分享(附单胞藻的培养配方)

微藻工厂化培养经验分享(附单胞藻的培养配方)在主要运用于大棚生物饵料方面 一定地点建立车间、浓缩之后近距离管道运输到养殖区域 、提取色素添加在饲料中,至于土塘泼洒,如何控制量、增氧和开口饵料这方面正在摸索,需要大家一起总结出经验。
今天我跟大家主要跟大家分享一下藻种的工厂化规模化培育。
群里面应该很多人都培育过藻,大家都知道藻种的培育分为一级、二级、三级培养,今天我是简单从一级、二级、三级培养过程可能中遇到的问题、日常管理、接种、藻种营养配方这些方面做一下简单的交流。
养殖品种的需求量也不同。
所以培养条件、营养盐配方等各有不同。
今天我主要是以金藻为例,引申出其他藻种的营养配方,让大家学习一下其中的相似点。
国内大部分水产育苗企业,在育苗生产中都是自备微藻养殖设施,自行生产各类饵料用微藻。
但是一般育苗场都普遍缺乏相应的专业技术力量,只能利用各自的藻池和天然水体粗放培养,在饵料微藻种质、生产技术和应用方法上都各自为正,导致微藻种质混乱、供应不稳定、营养成分不平衡、饵料效价低、缺乏多品种集约化生产应用技术;同时,受限于微藻高密度养殖、采收技术和浓缩液保藏技术的限制,国内几乎没有统一的、专业化的饵料微藻质量标准和集中供应点。
所以工厂化育苗需要及时的补充藻种,开口饵料非常重要。
首先从工艺流程上来说一级培养:主要用于保种,主要用的仪器是锥形瓶,其能够完全消毒,所以应用在保种上面特别多。
二级培养:主要是用塑料白桶(聚丙烯材料),生产上也用 的饮水桶,但是瓶口小,操作不方便,消毒也不彻底;而用氧气袋又易破裂。
在南方经常可以见到用玻璃制作的大型鱼缸和氧气袋。
回复举报 来自 客户端 楼•••••知名人士三级培养:主要有小型的 、 、 左右的室内水泥池,采光良好,白色透明玻璃钢瓦或塑料薄膜于车间顶部用于培育藻种。
接下来我主要简单的讲一下容器和工具的洗涤、消毒。
培藻过程中所有的容器和工具,比如锥形瓶、矿泉水桶、搅拌棒等必须经过去污渍、肥皂等刷洗,之后再用消毒后的蒸馏水冲洗 遍。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
加入海泥抽出液20ml,效果更好。
硅藻培养液配方:
①艾伦-内尔森(Allen-Nelson)培养液{Allen,E.J.et al (1910)}:
A. KNO3
20.2g
纯水
100ml
B. Na2HPO4.12H2O 4.0g
CaCl.6H2O
4.0g
HCl(浓)
2.0ml
纯水
80ml
FeCl3(溶液) 2.0ml
K2HPO4
0.01g
Fe(SO4)3(1%溶液) 10滴
海水
1000ml
②亚心形扁藻培养液(湛江水产学院):
NaNO3
0.05g
维生素B12
200mμg
KH2PO4(1%溶液) 0.005g
人尿
2ml
FeC6H5O7 0.2ml
维生素B1
200μg
海水
1000ml
培养亚心形扁藻及其他绿藻使用,多用于小型培养和中继培养,如能
掌握单细胞微藻的实验室培养(藻种培养)方法,细胞生长曲线观察。
二、实验材料及用具
1、实验材料:小球藻(500ml)、等鞭金藻、扁藻 2、实验仪器:可见分光光度计、显微镜(1部/组)、血球计数板(一个/
组)、电炉、灭菌锅、pH计、 500ml烧杯(1个/组)、胶头滴管(3支 /组)、 250ml锥形瓶(每组3个)、牛皮纸、细绵绳 3、试剂:蒸馏水、海水、碘化钾
4.D液 500 ml 1000倍
盐酸硫铵素(VB1) 5 mg (试剂 50 mg/ml取100 μl)
Biotin VH
0.025 mg (试剂 0.1 mg/ml取250 μl)
VB12
0.025 mg (试剂 0.25 mg/ml取100 μl)
先用维生素分别用纯水溶解混合,稀HCl将pH调到4.5~5.0,最后用纯水 稀释至1升。膜过滤后冰冻保存。
实验一 微藻的培养基配制
一、实验目: 掌握微藻的培养基配制方法。 以海水单胞藻培养液通用配方“f/2”为例
二、实验原理 藻类的培养液必须具备藻类的生长繁殖所需要的各种营养元
素,并且营养盐的比例应该符合藻类生长繁殖的需要。不同种 类的微藻对营养盐的质和量的需求不同,各有其特殊性,因此, 制定某种微藻的一个培养液配方时,首先必须进行一系列的试 验,了解这该种藻类对营养的要求,并在使用中验证配方的效 果,加以ቤተ መጻሕፍቲ ባይዱ进,使配方达到更理想的水平。藻类对营养盐的需 求也有很多共同点,因而一些培养液配方(如F/2)可应用于多 种藻类的培养。
小球藻
扁藻
球等鞭金藻
1、血球计数板计数方法:
(1) 搅拌:由于藻类细胞在培养液中分布不均匀,所以在取产前必须进行 搅拌,搅拌后立即取样。 (2)固定、稀释:运动细胞需加碘液杀死才能计数。如细胞浓度过大,计数 困难,需把水样稀释到适宜的程度。(附:鲁哥氏液(Lugol‘s Solution)又称
进行微藻培养时,可根据培养藻类对营养的要求,选用合适 的配方,在消毒水中按配方加入各种营养物质配成。所加肥料 应保持清洁,某些不清洁肥料必须消毒,预防敌害生物通过肥 料污染。
绿藻培养液配方:
①海洋三号扁藻培养液(海洋研究所,1960):
NaNO3
0.1g
2Na3C6H5O7﹒ 11H2O 0.02g
培养液是根据培养液配方配制的。为了减少每次称量的麻 烦,固体的营养元素一般都配成母液(如浓缩1000倍的母 液),在使用时,只要吸取一定的量加入培养液中即成。主 要营养元素可以单项营养元素配成母液,也可以分成若干组, 每组2种或多种营养元素同配在一起。微量元素和辅助生长有 机物质也多种组合在一起,配成母液。在浓营养溶液中一般 生物因不能忍受而死亡。
三、实验步骤
(一) F/2 (+ Si)培养基母液的配制(用去离子水配制) 1.A液 500 ml 1000倍 NaNO3 37.5 g; NaH2PO4 2.5 g
2.B液 500 ml 1000倍 Fe-EDTA 2.5 g (FeCl3 1.6 g + EDTA 0.9 g)
3.C液 500 ml 1000倍 Na2SiO3.9H2O 10 g
三、实验材料及用具 1、器材 电炉;灭菌锅;pH计 500ml 试剂瓶(每组5个); 玻棒、500ml烧杯、50ml容量瓶(每组一个)/牛皮纸、细绵绳 2、试剂: 蒸馏水、海水 各种试剂 NaNO3; NaH2PO4;FeCl3 EDTA;Na2SiO3.9H2O;盐酸硫铵素 (VB1) ;Biotin VH
(三)F/2培养基配制
1000 ml (1升) F/2 (+ Si)培养基 = 1000 ml (1升)过滤灭菌海水 + 1ml A 液 + 1ml B液+ 1ml D液+ 1ml E液 (+ 1ml C液)
注意:煮沸的海水放置凉后(50度以下)再加母液,否则会出现沉淀。
实验二 单细胞藻类的培养
一、实验目的
5.E液 500 ml 1000倍
CuSO4.5H2O
0.0098 mg; ZnSO4.7H2O
0.022 mg
CaCl2.6H2O
0.01 mg; MgCl2.4H2O
0.180 mg
Na2MoO4.2H2O 0.0063 mg
A、B、D、E高压灭菌备用
(二)海水处理
沉淀 —— 砂池过滤 ——(抽滤)——灭菌(海水放于三角瓶 中,置于电炉上煮沸,可杀来一般细菌)
三、实验步骤 1、培养基配制:见实验一 2、接种:一般培养的接种量为1/3~1/5. 3、计数:分光光度计测定500nm(OD500)和750nm
(OD750)吸光值;细胞计数板计数。 4、每天测定一次,分别绘制以OD值和细胞绝对数目为纵坐
标、时间为横坐标的生长曲线,分析细胞数目与OD500和 OD750的相关性。 5、观察并描述微藻不同生长时期的特点
碘液,常用的配方是:将6克的碘化钾溶于20毫升水中,待完全溶解后加入4克碘,摇 荡,待碘完全溶解后,加入80毫升蒸馏水,贮存在棕色试剂瓶内。)
B液的配制法:先把氧化钙4g溶解于40ml纯水中。另外把磷酸氢二钙4g溶
解于40ml纯水中,再把三氯化铁溶融液2ml,缓慢滴入,摇动,产生白色
沉淀,加热,缓慢滴入浓盐酸2ml,沉淀的白色沉淀即重新溶解。把以上两
溶液混合。
使用时,取A液2ml和B液1ml,加入1000ml海水中,充分混合后加热消毒,
消毒后静置24h,然后将上层清夜倒出使用。
