CPSC-CH-E1002-08_2儿童非金属产品中总铅测试标准程序
铅含量测试

4.检测方法4.1铅含量测定4.1.1原理二价铅离子在PH=5~5.5的条件下与EDTA形成稳定的络合物4.1.2试剂和材料4.1.2.1 NaOH:10%4.1.2.2 HNO3:1:14.1.2.3乙酸-乙酸铵缓冲溶液:PH=5.0称取80克乙酸铵(CH3COONH4),溶于水,加16ml冰乙酸,稀释至1000ml4.1.2.4二甲酚橙:0.2%水溶液4.1.2.5铬黑T :0.5%乙醇溶液称取0.5g铬黑T,2g盐酸羟胺与乙醇研磨溶解,稀释至100m4.1.2.6 EDTA标准溶液0.05mol/LA:溶液配置称取20克乙二胺四乙酸二钠,用1000ml水溶解,储存于试剂瓶中。
B:溶液标定称取0.15克(精确至0.0001克)在800±50℃灼烧至恒重的基准氧化锌,用少量水湿润,加2ml盐酸溶液(20%)溶解,加100ml水,用氨水溶液(10%)调节PH至7~8,加10ml 氨-氯化氨缓冲溶液,及5滴0.5%铬黑T指示剂,用配好的乙二胺四乙酸二钠溶液滴定,溶液由紫色变为纯兰色,同时做空白C:浓度计算C(EDTA)=m×1000/[(V1-V2)]/81.398]m:氧化锌的质量gV1:EDTA的体积mlV2:空白消耗EDTA的体积ml81.398:氧化锌的分子量4.1.3 分析步骤A、对于含锌的稳定剂按照以下方法测定称取试样0.2g左右(准确至0.0002g)置于250mL锥形瓶中,加入硝酸(1+1)10mL,装上球形冷凝管,缓缓加热保持微沸,直至试样分解完全,油脂成透明状,停止加热,以热蒸馏水冲洗冷凝管管壁和瓶塞四周。
取下冷凝管,冷却至室温,加入浓氨水8ml,摇匀冷却至室温(1h)过滤(锥形瓶需要多次洗涤保证全部加入漏斗过滤),用蒸馏洗涤沉淀至无钙、锌离子。
将滤渣和滤纸使用加1:1硝酸溶解10ml,在250ml三角瓶中,使用10%NaOH调节PH值至刚果红试纸变色(PH=5~6),加10mL乙酸—乙酸铵缓冲溶液,3—4滴二甲酚橙指示液,用0.05mol/L EDTA标准溶液滴定至溶液由红色变成亮黄色(或者橘黄色)为终点。
CPSC-CH-E1004-11cadmiumjewelrytest

United State美國Consumer Product Safety Commission消費品安全委員會Directorate for Laboratory Sciences實驗室科學理事會Division of Chemistry化學部10901 Darnestown RoadGaithersburg, MD 20878Test Method測試方法: CPSC-CH-E1004-11Standard Operating Procedure for Determining Cadmium(Cd) Extractability from Children’s Metal Jewelry兒童金屬珠寶中总鎘测定的标准操作步驟February 03, 20111. Scope范圍This document provides detailed information on two test methodologies that will be used by the U.S. Consumer Product Safety Commission’s (CPSC) testing laboratory (LSC) in the analysis of metal items, such as children’s metal jewelry, for extractable cadmium. Utilizing different sample preparation techniques, both methods will determine whether a children’s jewelry item contains extractable cadmium content. This information then can be used in the development of a risk assessment to support findings under the Federal Hazardous Substances Act (FHSA).本文件提供兩個測試方法學的詳細信息, 將被美國消費品安全委員用于分析金屬物品中可提取的鎘,例如,兒童的金屬珠寶。
CPLIA儿童产品测试程序

3. Standards Referenced参考标准
3.1 CPSC对CPSIA第102条要求的申明草案
3.2 16 CFR 1107产品认证的测试及标签
2.培训内容
施加不正当影响的例子
1)要求第三方符合性评估机构修改不合格的测试报告(将破坏实验室测试数据的真实性)
非施加不正当影响的例子
1)有理由认为第三方符合性评估机构颁布的报告是错误的。(咨询第三方)
2)在儿童产品证书颁布前,调查不合格测试结果的原因并采取行动来处理不合格的测试结果。(基于1107.20(d)的要求商讨)
通知CPSC
若有任何人员违背该方针并在调查时证实有对测试结果进行隐瞒或施加不当影响,东莞市新创玩具有限公司将立即通知CPSC。可以秘密地将不正当影响汇报給CPSC。
违规处罚
1)民事处罚(CPSA第20条):任何人故意违反CPSA第19条,对于每次违反将最多处不超过5000美金的罚款,对于相关的一系列违法行为累计罚款不超过1250000美金。
3.3 BV深圳2011年3月31日研讨会的介绍材料
3.4 BV2011年10月2日发布的CPSC通过关于产品的认证测试及标签和保留合理化测试程序的最终条款周报。
3.5 CPSC 16 CFR 1107最终条款
文件更改简历
版本
更改类别
更改内容简要
生效日期
增加
删除
日期
部门:
□总经理□包装部□采购部
□行政部□注塑部□计划部
□SMT□仓库部□财务部
《有害物质质量保证协议书》
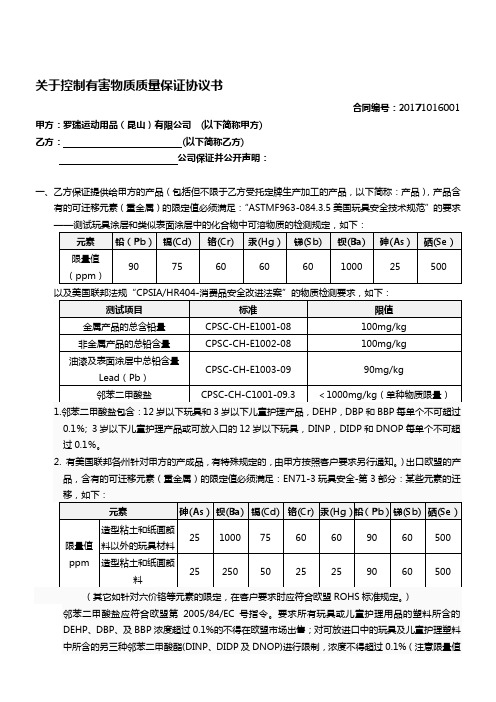
关于控制有害物质质量保证协议书合同编号:20171016001 甲方:罗瑞运动用品(昆山)有限公司(以下简称甲方)乙方:(以下简称乙方)公司保证并公开声明:一、乙方保证提供给甲方的产品(包括但不限于乙方受托定牌生产加工的产品,以下简称:产品),产品含有的可迁移元素(重金属)的限定值必须满足:“ASTMF963-084.3.5美国玩具安全技术规范”的要求为三种物质累加总量)。
化学品:直接用于甲方产成品的,应符合上述所有标准要求同时提供MSDS。
非直接使用于甲方产成品的,由乙方提供MSDS(化学品安全说明书)即可。
包装材料:应符合美国《包装用品之毒性要求》。
即TPCH。
其重金属控制等同欧盟包装物指令94/62/EC。
具体限量如下:Pb、Cd、Hg、Cr6+总和不超过100 ppm。
其它:乙方所提供的产品必须能满足甲方相关产成品(如滑板车、电动车等等)的国家标准、产品使用地区或国家的相关标准要求。
针对某些产品的特殊要求,乙方应按照甲方针对发行的标准要求提供合格产品。
二、乙方必须定期(至少每季度)向甲方提供由双方认可的第三方机构测试的产品合格测试报告。
同时,乙方须制定书面的有害物质控制计划,并建立书面的有害物质追溯机制,甲方可随时对执行的状况作确认。
三、甲方对乙方提供的产品进行抽样检测。
凡发现测试存在不合格的现象(按材料成分主要分为重金属、邻苯二甲酸盐、甲醛、阻燃性等,但不限于此),乙方应承担3万元/次(RMB)的违约金给甲方。
如果乙方对测试结果存在异议的,以甲方发现的该不合格样品作为送测样品,经双方共同确认样品,送双方认可的检测机构判定,以权威检测机构的测试报告为准,权威测试报告合格,由甲方承担测试费用;权威测试报告不合格,则乙方承担测试费用,并向甲方支付违约金3万元(RMB)。
此外因为影响甲方出货造成的所有的经济损失。
全部由乙方承担。
四、包括但不限于喷涂件\喷粉件\电泳件\喷油\喷漆\布套\塑料件等外协加工供应商,对涉及到有害物质的二级供应商,须经甲方许可。
cpc测试标准

CPC测试标准一、检测项目CPC测试主要包括以下项目:1. 产品质量检测:对产品的各项指标进行检测,包括外观、尺寸、功能、安全性等。
2. 性能检测:对产品的性能进行检测,包括产品的工作效率、稳定性、可靠性等。
3. 成分检测:对产品的成分进行检测,包括产品的原材料、配方、含量等。
4. 安全性检测:对产品的安全性进行检测,包括产品的使用安全、环境安全等。
二、样品要求CPC测试需要提交符合要求的样品,具体要求如下:1. 样品数量:根据产品种类和测试项目的要求,确定所需样品的数量。
2. 样品质量:样品应具有一定的代表性,能够反映该批次产品的整体质量。
3. 样品有效期:样品应处于有效期内,以保证测试结果的准确性。
4. 样品包装:样品的包装应符合运输和储存的要求,能够保证样品在运输过程中不受损坏。
三、检测方法CPC测试采用以下方法进行检测:1. 外观检测:采用目视、触摸等方法对产品的外观进行检测。
2. 尺寸检测:采用测量工具对产品的尺寸进行检测。
3. 功能检测:按照产品说明书和相关标准对产品的功能进行检测。
4. 安全性检测:采用专业设备和方法对产品的安全性进行检测。
5. 成分检测:采用化学分析、光谱分析等方法对产品的成分进行检测。
四、检测结果处理CPC测试结束后,将对测试结果进行处理,主要包括以下方面:1. 数据分析:对测试数据进行统计和分析,得出产品的各项指标。
2. 结果判定:根据测试数据和相关标准对产品进行判定,确定其是否符合要求。
3. 报告编写:根据测试结果编写CPC测试报告,内容包括测试项目、样品信息、检测方法、检测结果等。
4. 结果审核:对CPC测试报告进行审核,确保报告的准确性和完整性。
如需进一步处理,可根据审核结果对样品进行重新检测或调整检测方案。
五、检测报告CPC测试完成后,将出具相应的检测报告,报告内容应包括以下信息:1. 样品信息:包括样品名称、规格型号、生产厂家等。
2. 检测项目:列明所进行的各项检测项目及其对应的标准要求。
保健用品理化检测方法 铅的测定

保健用品理化检测方法 第1部分:铅的测定警示——使用本标准的人员应有正规实验室工作的实践经验。
本标准并未指出所有可能的安全问题。
使用者有责任采取适当的安全和健康措施,并保证符合国家有关法规规定的条件。
1 范围本标准规定了保健用品中铅的石墨炉原子吸收光谱法和电感耦合等离子体质谱法。
本标准适用于保健用品理化指标中铅含量的测定。
2 规范性引用文件下列文件对于本文件的应用是必不可少的。
凡是注日期的引用文件,仅所注日期的版本适用于本文件。
凡是不注日期的引用文件,其最新版本(包括所有的修改单)适用于本文件。
GB/T 6682 分析实验室用水规格和试验方法第一法 石墨炉原子吸收光谱法3 原理试样消解处理后,经石墨炉原子化,在283.3 nm处测定吸光度。
在一定浓度范围内铅的吸光度值与铅含量成正比,与标准系列比较定量。
4 试剂和材料除非另有说明,本方法所用试剂均为优级纯,水为GB/T 6682规定的一级水。
4.1 试剂4.1.1 硝酸(HNO3)。
4.1.2 高氯酸(HClO4)4.1.3 磷酸二氢铵(NH4H2PO4)。
4.1.4 硝酸钯[Pd(NO3)2]4.2 试剂配制4.2.1 硝酸溶液(1+99):量取10 mL硝酸,缓慢加入到990 mL水中,混匀。
4.2.2 硝酸溶液(1+9):量取50mL硝酸,缓慢加入到450 mL水中,混匀。
4.2.3 磷酸二氢铵-硝酸钯溶液:称取0.02 g硝酸钯,加少量硝酸溶液(1+9)溶解后,再加入2 g磷酸二氢铵,溶解后用硝酸溶液(1+99)定容至100 mL,混匀。
4.3 标准品经国家认证并授予标准物质证书的1000 mg/L的铅标准溶液。
4.4 标准溶液配制4.4.1 铅标准使用液准确吸取铅标准液(1000 mg/L)1.00 mL于1000 mL容量瓶中,加硝酸溶液(1+99)至刻度,混匀,得到1.00 mg/L的铅标准使用液。
4.4.2 铅标准系列溶液分别吸取铅标准使用液(1.00 mg/L)0 mL、1.00 mL、2.00 mL、3.00 mL、4.00 mL和6.00 mL于l00 mL容量瓶中,加硝酸溶液(1+99)至刻度,混匀。
铅测定标准检验操作规程
1.目的:建立铅的标准检验操作程序,保证产品生产安全、质量稳定。
2.适用范围:本标准适用于原料、辅料、溶剂及半成品、成品中铅的测定3.职责:质量管理部负责本规程的执行。
4.控制要求与内容4.1原理试样经消化后,在pH 8.5~9.0 时,铅离子与二硫腙生成红色络合物,溶于三氯甲烷。
加入柠檬酸铵、氰化钾和盐酸羟胺等,防止铁、铜、锌等离子干扰,与标准系列比较定量。
4.2试剂和材料4.2.1 氨水(1+1);4.2.2 盐酸(1+1):量取100 mL 盐酸,加入100 mL 水中;4.2.3 酚红指示液(1 g/L):称取0.10 g 酚红,用少量多次乙醇溶解后移入100 mL 容量瓶中并定容至刻度;4.2.4 盐酸羟胺溶液(200 g/L):称取20.0 g 盐酸羟胺,加水溶解至50 mL,加2 滴酚红指示液,加氨水(1+1),调pH 至8.5~9.0(由黄变红,再多加2 滴),用二硫腙-三氯甲烷溶液(22.10)提取至三氯甲烷层绿色不变为止,再用三氯甲烷洗二次,弃去三氯甲烷层,水层加盐酸(1+1)至呈酸性,加水至100 mL;4.2.5 柠檬酸铵溶液(200 g/L):称取50 g 柠檬酸铵,溶于100 mL 水中,加2 滴酚红指示液(22.3),加氨水(22.1),调pH 至8.5~9.0,用二硫腙-三氯甲烷溶液(22.10)提取数次,每次10 mL~20 mL,至三氯甲烷层绿色不变为止,弃去三氯甲烷层,再用三氯甲烷洗二次,每次5 mL,弃去三氯甲烷层,加水稀释至250 m;4.2.6 氰化钾溶液(100 g/L):称取10.0 g 氰化钾,用水溶解后稀释至100 mL;4.2.7 三氯甲烷:不应含氧化物;4.2.7.1 检查方法:量取10 mL 三氯甲烷,加25 mL 新煮沸过的水,振摇3 min,静置分层后,取10 mL水溶液,加数滴碘化钾溶液(150 g/L)及淀粉指示液,振摇后应不显蓝色;4.2.7.2 处理方法:于三氯甲烷中加入 1/10~1/20 体积的硫代硫酸钠溶液(200 g/L)洗涤,再用水洗后加入少量无水氯化钙脱水后进行蒸馏,弃去最初及最后的十分之一馏出液,收集中间馏出液备用;4.2.8 淀粉指示液:称取0.5 g 可溶性淀粉,加5 mL 水搅匀后,慢慢倒入100 mL 沸水中,边倒边搅拌,煮沸,放冷备用,临用时配制;4.2.9 硝酸(1+99):量取1 mL 硝酸,加入99 mL 水中;4.2.10 二硫腙-三氯甲烷溶液(0.5 g/L):保存冰箱中,必要时用下述方法纯化。
有害物质质量保证协议书(201112)
关于控制有害物质质量保证协议书合同编号:甲方:罗瑞运动用品(昆山)有限公司(以下简称甲方)乙方:(以下简称乙方)公司保证并公开声明:一、乙方保证提供给甲方的产品(包括但不限于乙方受托定牌生产加工的产品,以下简称:产品),产品含有的可迁移元素(重金属)的限定值必须满足:“ASTMF963-084.3.5美国玩具安全技术规范”的要求——测试玩具涂层和类似表面涂层中的化合物中可溶物质的检测规定,如下:以及美国联邦法规“CPSIA/HR404-消费品安全改进法案”的物质检测要求,如下:化学品:直接用于甲方产成品的,应符合上述所有标准要求同时提供MSDS。
非直接使用于甲方产成品的,由乙方提供MSDS(化学品安全说明书)即可。
包装材料:应符合美国《包装用品之毒性要求》。
即TPCH。
其重金属控制等同欧盟包装物指令94/62/EC。
具体限量如下:Pb、Cd、Hg、Cr6+总和不超过100 ppm。
其它:乙方所提供的产品必须能满足甲方相关产成品(如滑板车、电动车等等)的国家标准、产品使用地区或国家的相关标准要求。
针对某些产品的特殊要求,乙方应按照甲方针对发行的标准要求提供合格产品。
二、乙方必须定期(至少每季度)向甲方提供由双方认可的第三方机构测试的产品合格测试报告。
同时,乙方须制定书面的有害物质控制计划,并建立书面的有害物质追溯机制,甲方可随时对执行的状况作确认。
三、甲方对乙方提供的产品进行抽样检测。
凡发现测试存在不合格的现象(按材料成分主要分为重金属、邻苯二甲酸盐、甲醛、阻燃性等,但不限于此),乙方应承担3万元/次(RMB)的违约金给甲方。
如果乙方对测试结果存在异议的,以甲方发现的该不合格样品作为送测样品,经双方共同确认样品,送双方认可的检测机构判定,以权威检测机构的测试报告为准,权威测试报告合格,由甲方承担测试费用;权威测试报告不合格,则乙方承担测试费用,并向甲方支付违约金3万元(RMB)。
此外因为影响甲方出货造成的所有的经济损失。
中国合格评定国家认可委员会
中国合格评定国家认可委员会实验室认可证书附件(AS L3430)名称:联合厂商会检定中心(上海)有限公司地址:上海市宝庆路8号科技大厦6楼签发日期:2009年06月11日有效期至:2011年04月13日附件1-1 认可的检测能力范围CHINA NATIONAL ACCREDITATION SERVICE FOR CONFORMITY ASSESSMENT APPENDIX OF LABORATORY ACCREDITATION CERTIFICATE(No. CNAS L3430)NAME:CMA Testing and Certification Laboratories(Shanghai) Co., Ltd.ADDRESS:6/F, Keji Building, No.8, Baoqing Road, Shanghai,ChinaDate of issue: 2009-06-11 Date of expiry: 2011-04-13 APPENDIX1-1 LIST OF ACCREDITED TESTING SCOPE中国合格评定国家认可委员会实验室认可证书附件(AS L3430)名称:联合厂商会检定中心(上海)有限公司地址:上海市宝庆路8号科技大厦6楼签发日期:2009年06月11日有效期至:2011年04月13日附件2 认可的授权签字人及其授权签字领域CHINA NATIONAL ACCREDITATION SERVICE FOR CONFORMITY ASSESSMENT APPENDIX OF LABORATORY ACCREDITATION CERTIFICATE(No. CNAS L3430)NAME:CMA Testing and Certification Laboratories(Shanghai) Co., Ltd.ADDRESS:6/F, Keji Building, No.8, Baoqing Road, Shanghai,ChinaDate of issue: 2009-06-11 Date of expiry: 2011-04-13 APPENDIX2 LIST OF ACCREDITED SIGNATORY AND SCOPE。
Total Lead (Pb)铅含量测试
Total Lead (Pb)铅含量测试您的产品中“铅”是否超标?您有没有因为“铅”超标而影响生产周期?Lead / Pd(铅)是什么?铅是一种金属化学元素,其化学符号是Pb.,是原子量最大的非放射性元素。
铅被广泛应用于各种各样的日常消费品,如玩具、儿童珠宝、包装材料、食品容器、陶瓷产品、家具、文具、金属配件等等,与人们的生活密切相关。
Lead / Pd(铅)超标的危害目前根据国际惯用标准,结合我国实际,一般认为血铅的相对安全标准不应超过10—14微克/升。
长期接触铅化合物或吸入金属铅尘埃,都会引起不同程度的“铅中毒”病症(血清铅浓度大于每百毫升40μg) 。
人体吸入过多会危害人的神经系统、心脏和呼吸系统,从而导致不同程度的铅中毒。
人体中铅能与多种酶结合从而干扰有机体多方面的生理活动,导致对全身器官产生危害。
儿童发生铅中毒的机会远远超过成人,其临床特点为剧烈腹绞痛、贫血、中毒性肝病、中毒性肾病、多发性周围神经病。
表现头晕全身无力、肌肉关节酸痛、不能进食、便秘或腹泻、肝脏肿大、肝区压痛、黄疽、血压升高实验室检查:除铅中毒指标明显升高外,胆红质升高、ALT升高;尿中可见红细胞、白细胞、尿卟胆原阳性;血色素和红细胞均下降。
神经系统检查,可发现四肢末端呈手套袜子型感觉减退,肌肉萎缩及肌无力。
总铅含量测试限值1、根据美国CPSIA《2008美国消费品改进法案》,2009年2月10日之后,ASTM F963将成为强制性标准,含铅涂料中的铅含量限值降至90ppm(虽目前该项要求已被CPSC暂缓执行,但是生产者还是有义务满足限量要求);2、根据2009年6月,加拿大政府拟议的一项法案,限制与一切可能与嘴接触的消费品(包括为3岁以下儿童设计的可入口的部位)中的铅含量限值由现在的600ppm改为90ppm;3、根据美国CPSIA《2008美国消费品改进法案》,2009年2月10日之后,总铅含量限制为600ppm;2009年8月14日之后,总铅含量限限值为300ppm;2011年8月14日之后,总铅含量限制为100ppm。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
U NITED S TATESC ONSUMER P RODUCT S AFETY C OMMISSIOND IRECTORATE FOR L ABORATORY S CIENCESD IVISION OF C HEMISTRY5R ESEARCH P LACER OCKVILLE,MD20850Test Method: CPSC-CH-E1002-08.2Standard Operating Procedure for Determining Total Lead (Pb) in NonmetalChildren’s Products, RevisionApril 10, 2012*This document provides detailed information on the test method that will be used by the U.S. Consumer Product Safety Commission’s testing laboratory (LSC) in the analysis of nonmetal children’s products for lead (Pb) content. This method is divided into three sections. The first section describes how to digest samples to determine the total lead content in crystal, ceramic, and other siliceous materials and contains a subsection on the use of X-ray fluorescence spectrometry (XRF) for determination of lead in such siliceous materials. The second section describes how to digest samples to determine the total lead content in polymeric (including natural and synthetic polymers), or plastic materials, and it contains a subsection on the use of X-ray fluorescence spectrometry (XRF) for determination of lead in such polymeric materials. The third section describes how to analyze the digested samples from the first two sections. This revision recognizes use of X-ray fluorescent spectroscopy measurement techniques in additional materials with certain limitations and acceptable ranges and replaces the previously issued Test Method CPSC-CH-E1002-08.1.The method applies to most nonmetal components other than paint, but it is not recommended by CPSC staff for materials, that when combined with the specified acid(s), results in an inappropriate combination of materials that would be inconsistent with safe laboratory practices. In such cases, the chemist should make a knowledge-based decision on the proper modifications of the method to maintain laboratory safety, while following the general approach described here.The general approach is to grind or cut any accessible component part of a sample into small pieces or a powder; digest an aliquot completely in nitric acid or for siliceous products in a combination of hot, concentrated nitric and hydrofluoric acids; and analyze by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). Other analytical methods, such as Inductively Coupled Plasma – Mass Spectrometry (ICP-MS), Flame Atomic Absorption Spectroscopy (FLAA), and Graphite Furnace Atomic Absorption Spectroscopy (GFAA), may be used under appropriate conditions as an alternative to ICP-OES, using applicable,* This is a revision of Test Method CPSC-CH-E1002-08.1. This document was prepared by CPSC staff, has notbeen reviewed or approved by, and may not necessarily reflect the views of, the Commission.recognized analytical techniques for the alternative analytical method. Nonmetal materials may also be analyzed, using XRF, following the standard test method of ASTM F2617-081 or ASTM F2853-10e1,2 with limitations described below. The general approach in that case is to consider any XRF result to be indeterminate and in need of digestion and ICP analysis if that result falls within 30 percent of the Consumer Product Safety Improvement Act (CPSIA) limit.CPSC staff has concluded that the test methodologies provided in detail below are sufficient to determine lead content in most products. Knowledge-based adjustments on a case-by-case basis may be necessary for products made from certain materials.Definitions1.Sample–An individual consumer product or a group of identical consumer productsfrom a batch to be tested.ponent Part–An individual subunit within the total sample. An item, such as abracelet, may be broken into component parts, such as a bead, crystal, a hook, and apendant, with those component parts individually analyzed.3.Instrument Detection Limit (IDL)–3 times the standard deviation of 10 replicatemeasurements of reagent blank.4.Method Detection Limit (MDL)–Reagent blank fortified with 2–3 times the IDL.Seven replicate measurements are made. Calculate the MDL as follows: MDL = t X S, t= 3.14 (99 percent confidence level for 7 replicates), S= standard deviation.boratory Reagent Blank (LRB)–An aliquot of the digestion reagents that is treatedexactly as a sample, including exposure to glassware, digestion media, apparatus, and conditions used for a particular Pb test but with no added sample. LRB data are used to assess contamination from the laboratory environment.6.Calibration Blank–Deionized water acidified with nitric acid (3 ml concentrated nitricacid diluted to 100 ml with deionized water).7.Stock Standard Solution–1,000 ppm solution of Pb purchased from a reputablecommercial source, used to prepare calibration standards. Replace before expirationdate.8.Calibration Standards–Solutions containing 0 to 25 ppm of Pb in 3 percent nitric acidmatrix are used. A minimum of 4 calibration standards are used. Calibrationstandards should be prepared on a biweekly basis at minimum.9.Quality Control Sample (QCS)–A solution containing Pb that is used to evaluate theperformance of the instrument system. QCS is obtained from a source external to the laboratory and Stock Standard Solution.10.Certified Reference Material (CRM)–CRMs are materials with a similar matrix as testsamples with known lead levels. CRMs are used to verify digestion and analysismethods. For example, standard reference materials (SRMs) are CRMs that are1 Standard Test Method for Identification of Chromium, Bromine, Cadmium, Mercury, and Lead in Polymeric Material Using Energy Dispersive X-ray Spectrometry.2Standard Test Method for Determination of Lead in Paint Layers and Similar Coatings or in Substrates and Homogenous Materials by Energy Dispersive X-Ray Fluorescence Spectrometry Using Multiple Monochromatic Excitation Beams.available from the National Institute of Standards and Technology (NIST), such asthose listed in the Equipment and Supplies section below. Appropriate CRMs fromother sources are also acceptable.Equipment and Supplies: The materials used for sampling and analyses are as follows:1.Nitric Acid, Trace Metal Grade2.Hydrofluoric Acid, Trace Metal Grade3.Distilled Water4.Microwave Digestion Apparatus5.Cryogenic Mill6.Liquid Nitrogen7.CRMs such as ERM®-EC680k3 and EC681k, low-density polyethylene materials thatcontain lead and NIST SRM 89 and 610, leaded glass.8.Internal Standard (such as yttrium, from a stock standard solution of that elementappropriate to the instrument parameters of the ICP used for the analysis)I.Total Lead in Ceramics, Glass and Crystal, and other Siliceous MaterialsA.Acid DigestionWhen preparing a sample, the laboratory should make every effort to ensure that thealiquot removed from a component part of a sample is representative of the component to be tested and is free of contamination. Each unique component type from a subsample is analyzed for total Pb content. CPSC staff uses a method based on EPA 30524(/epawaste/hazard/testmethods/sw846/pdfs/3052.pdf) for determining lead content in ceramic or crystal materials. Certified reference materials, such as NIST SRM 89 and 610, which closely match the material of the tested product, should be used to verify accuracy of digestion and analysis methods. After digesting the sampleaccording to this procedure, it should be tested by ICP, as described below in Section III.1.Weigh out a 30–100 mg piece of crystal, glass, or ceramic item into an appropriatemicrowave vessel equipped with a controlled pressure-relief mechanism. Ceramicitems generally weigh several grams or more, and consist of the base ceramic with aglaze and decoration fired on. The lead in ceramics is generally in the glaze ordecoration. When analyzing ceramics or glass, the entire item, including the glaze,decoration, and ceramic base material should be ground in a cryogenic mill and 30–100 mg of the ground ceramic/glass powder weighed in an appropriate microwavevessel. If used, the grinding apparatus must be cleaned thoroughly to prevent cross-contamination. Record actual weight to the nearest 0.1 mg.2.At room temperature, add 3 ml of concentrated nitric acid and 1 ml of concentratedhydrofluoric acid to each vessel. Wait for completion of the initial reaction of theacid and the sample before sealing vessels. Seal vessels in accordance with themanufacturer’s directions.3 European Reference Material, produced and certified under Institute for Reference Material and Measurements (IRMM).4 Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices.3.The microwave method should involve increasing the temperature of each sample toat least 180°C in approximately 5.5 minutes, and holding at 180°C for 9.5 minutes.4.Allow the samples to cool for a minimum of 5 minutes before removal frommicrowave. Vent the microwave vessels in fume hood before uncapping.5.Add 30 ml of 4 percent (w/w) boric acid to each vessel to permit the complexation offluoride to protect the ICP quartz plasma torch. Quantitatively transfer the sample toa 50 ml plastic volumetric flask or disposable volumetric digestion cup. Dilute to 50ml with deionized water.Caution: The analyst should wear protective gloves and face protection and must n ot at any time permit solution containing hydrofluoric acid to come in contact with skin orlungs. This document does not address all safety concerns; additional safety precautions are necessary for all steps, particularly when using hydrofluoric acid. This method is not to be used except by qualified, properly trained workers.B.Identification and Quantification of Pb in Siliceous Materials Using EnergyDispersive XRF Spectrometry Using Multiple Monochromatic Excitation Beams Alternately, Energy Dispersive XRF Spectrometry Using Multiple MonochromaticExcitation Beams (HDXRF) can be used with limitations to determine quantitatively the amount of Pb in siliceous materials by following ASTM F 2853-10e1. This standard isapplicable only for homogeneous siliceous materials and for XRF instruments meetingthe requirements given in the ASTM method. The following limitations apply:1.Applicable only for analysis of homogeneous materials. It is not suitable for testingglazed ceramics.2.Multiple measurements on different locations of the sample component part should beperformed to ensure some degree of spatial homogeneity. If the relative standarddeviation on 3 or more XRF measurements of a sample componen t part exceeds 30percent, analysis using wet chemical procedures (after preparing a homogenized aliquot by grinding sufficient sample) should be done before determining that the items meetCPSIA requirements for lead.3.Any XRF measurement of lead concentration, where the interval comprised of thereported result, plus or minus the instrument’s reported 95 percent uncertainty, includes the range with 30 percent above or below the CPSIA limit, shall be considered“inconclusive.” An average of at least 3 measurements, none of which is“inconclusive,” as defined in this paragraph, should be obtained in order to have a“conclusive” result.55 For example, if the XRF instrument reports a result of 65 ppm lead with an uncertainty of 10 ppm lead for a material subject to the CPSIA limit of 100 ppm lead content, this measurement would be consideredinconclusive because 65 ppm +10 ppm = 75 ppm, which is less than 30 percent below the applicable limit of 100 ppm. A reported result of 60 ppm with a reported uncertainty of 8 ppm would be a conclusive measurement of a material subject to the CPSIA limit of 100 ppm as 68 ppm is more than 30 percent below the applicable limit of 100 ppm.4.For “inconclusive” results, additional testing is necessary in order to make adetermination, such as by digestion and ICP analysis, per sections IA and III.C. Identification and Quantification of Pb in Siliceous Materials Using Other Formsof XRF SpectrometryOther types of XRF spectrometers that do not meet the requirements of ASTM F2853-10 can be used to determine quantitatively the amount of Pb in siliceous materials, withlimitations. The following limitations, in addition to those outlined in Section I-B forHDXRF, apply:1.Follow sampling, testing, calibration, quality control guidelines described in section 6of International Electrotechnical Commission (IEC) Method 62321 ED 1.0 B.2. A set of at least 4 glass calibration standards should be used to validate that theinstrument is suitable for testing for Pb in siliceous materials. The calibrationstandards should cover the applicable range to certify that the sample meets CPSIAlead content requirements (0-2000 mg/kg). At least 1 standard in each calibration setshould have lead concentration less than 100 mg/kg.3.Verify the instrument performance daily, by analyzing one or more reference materialsof the same matrix or metal type as the materials on which analyses will be performed.The lead concentration of the reference material should be in the range of 50–300mg/kg, and the determined concentration from the measurement must be in agreementwith the known or certified value. The measured result with the given uncertainty (at95 percent confidence) should overlap with the reported certified values and givenuncertainty of the reference materials.4.The limit of detection (LOD) for lead in glass should be determined followingguidelines in section 6 of IEC 62321. The lead LOD shall be equal to or less than 30mg/kg for the specific material or metal type tested. Some types of XRFspectrometers may not have sensitivity to obtain sufficient LOD for certifying to leadrequirements.II. Total Lead in Plastics, Polymers, and Other Non-Siliceous MaterialsA.Acid DigestionWhen preparing a sample, the laboratory should make every effort to ensure that thealiquot removed from a component part of a sample is representative of the component to be tested and is free of contamination. Each unique component type from a subsample is analyzed for total Pb content. CPSC staff uses a method based on methodology found in Canada Product Safety Bureau Method C-02.36 (http://www.hc-sc.gc.ca/cps-spc/prod-test-essai/_method-chem-chim/c-02_3-eng.php) for determining lead content in plasticmaterials, such as polyethylene and polyvinyl chloride (PVC). EPA Method 3051A7(/epawaste/hazard/testmethods/sw846/pdfs/3051a.pdf), with6 Determination of Total Lead in Polyvinyl Chloride Products by Closed Vessel Microwave Digestion.7 Microwave Assisted Acid Digestion of Sediments, Sludges, Soil, and Oils.modifications in sample weight, temperature, time, and acid volumes to match those given below, is also acceptable. Certified reference materials that closely match the material of the tested product, such as ERM®-EC680k and EC681k (described above), should be used to verify the accuracy of digestion and analysis methods. After digesting the sample according to this procedure, it should be tested by ICP, as described below in Section III.1.Cut the test specimen into small pieces. Hard to digest plasti cs may need to becryomilled to get finer powder. Weigh out 150 mg of the milled or cut plastic into an appropriate microwave vessel equipped with a controlled-pressure relief mechanism.Ensure that the milling apparatus is thoroughly clean between test specimens to avoid cross-contamination. Record actual weight to the nearest 0.1 mg.2.At room temperature, add 5 ml of concentrated nitric acid to each vessel. Wait forcompletion of the initial reaction of the acid and the sample before sealing vessels.Seal vessels in accordance with manufacturer’s directions.3.The microwave method should involve increasing temperature of each sample to atleast 200°C in approximately 20 minutes, and holding for 10 minutes.4.Allow the samples to cool for a minimum of 5 minutes before removal frommicrowave. Vent the microwave vessels in a fume hood before uncapping.5.Quantitatively transfer the sample to a 50 ml volumetric flask or disposable volumetricdigestion cup. Dilute to 50 ml with deionized water.B.Identification and Quantification of Pb in Polymeric and Other NonmetalMaterials Using XRFAlternately, Energy Dispersive XRF can be used with limitations to determine quantitatively the amount of Pb in polymeric materials by following ASTM F 2617-08 or ASTM F2853-10e1. These standards are applicable only for homogeneous polymeric materials and for XRF instruments meeting the requirements given in the ASTM methods. Components could be analyzed intact, without any modification, if they have suitable surface characteristics, geometry, and homogeneity. Destructive sample preparation techniques may be required for certain components to create a uniform sample for testing. Excessive curvature, rough surface texture, or specimen thickness less than 2 millimeters, may require sample preparation techniques, such as compression molding, as outlined in ASTM F 2617-08. Based on the interlaboratory study of reference materials reported in this standard and the fact that actual consumer products to be tested are likely to be less homogeneous than the reference materials, CSPC staff has concluded that analysis using wet chemical procedures outlined in Sections II-A and III should be done on any samples with Pb results determined using XRF to be greater than 70 percent of the Pb requirements of the Consumer Product Safety Improvement Act (CPSIA) before certifying that the item meets the CPSIA.Other homogeneous nonmetal materials such as wood and fabric can be analyzed by XRF following ASTM F2853-10e1 subject to the same limitations given in Section I-B aboveor using other types of XRF spectrometers that do not meet the requirements of ASTMF2853-10e1 subject to same limitions given in Section I-C above.III.Total Pb in Acid Digests of Polymeric or Siliceous Materials - Analysis of Sample Using ICP MethodAnalyze diluted samples for Pb concentration using an ICP spectrometer (or AtomicAbsorption spectrometer). Analysis procedures for ICP-OES and FLAA and GFAA are based on the methodology in ASTM E1613-04.8 ICP-MS may also be employed with appropriate procedures, such as EPA 6020A.9 Calculate total lead concentration in the component part from that of the diluted sample, accounting for all dilution. Report as percent by weight of the component part itself.ICP Operating Procedures and Quality Control MeasuresAnalysis1.Ignite plasma. Perform wavelength calibration or torch alignments per instrumentmanufacturer recommendations.2.Allow the instrument to become thermally stable before beginning.3.Ensure the following element and wavelength are selected in analytical method:a.Pb 220.353.One other Pb line, such as Pb 217.00, should be used to ensure spectral interferences are not occurring during analysis.4.An internal standard, such as 2 µg/ml yttrium, is used.5.Perform calibration using calibration blank and at least 3 standards. Calibrationshould be performed a minimum of once a day when used for analysis, or each timethe instrument is set up. Results for each standard should be within 5 percent of thetrue value, and the calibration blank should be < 5 times MDL. If the values do notfall within this range, recalibration is necessary.6.Analyze the QCS after the calibration and before any samples. The analyzed value ofPb should be within ±10 percent of the expected value. If Pb value is outside the ±10 percent limit, recalibration is required.a.At least one LRB must be analyzed with each sample set. If the Pb valueexceeds 10 times the MDL, laboratory or reagent contamination should beexpected. The source of the contamination should be identified and resolvedbefore continuing analyses. The LRBs should be the same acid concentrationsas added to the sample and should be taken through the same digestionprocedure.7.At least one certified reference material (CRM) should be analyzed with each batch ofsamples. The CRM should be a similar material as the test specimen with a knownamount of Pb. Analyte recoveries should be within ±20 percent of expected values. If recoveries are outside this limit, the source of the problem should be identified andresolved before continuing analyses.8.Dilute any samples that have Pb values exceeding 1.5 times the high calibrationstandard, and reanalyze.8 Standard Test Method for Determination of Lead by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES), Flame Atomic Absorption Spectrometry (FAAS), or Graphite Furnace Atomic Absorption Spectrometry (GFAAS) Techniques.9 Inductively Coupled Plasma-Mass Spectrometry.Calculations and Results ReportedResults for the Pb test methods are calculated and reported as follows:1.Total Pb - % Pb (wt/wt) = 0.10cd/wa.c= concentration of Pb detected (in units of ppm)b.d= dilution factor (in ml units)c.w= weight of aliquot digested (in mg units)Examples:Table 1: Total Pb Analysis(c) (d) (w)Item ppmPb DilutionfactorTotalPb(µg)Samplewt(mg)% PbCrystal 20 1,000 20,000 50 40Summary of changes in Revision CPSC-CH-E1002-08.11.Page 1, revised test method # and date.2.Page 1, last paragraph first sentence, allowed for polymeric materials to be cut intosmall pieces.3.Page 2, removed IDL and MDL CPSC lab values; not relevant to method and newinstruments will have different values.4.Page 2, definition 10, last sentence revised to include other sources for CRMs.5.Page 3, removed reference to NIST SRM 1412 and added 610. 1412 no longeravailable.6.Page 3, step 3, changed temperature requirements from “180±5°C” to “at least180°C”7.Page 4, step 1, removed requirement for cryomilling.8.Page 4, step 3, changed temperature requirements from “210±5°C” to “at least200°C.”9.Page 5, first paragraph, changed “>200mg/kg” to “greater than 70% of CPSIA Pblimit” to generalize for future changes to limit.Summary of Changes in Revision CPSC-CH-E-1002-8.21.Page 1, revised test method # and date, added statement that SOP contains asubsection on the use of X-ray fluorescence spectrometry (XRF) for determination oflead in such siliceous materials.2.Page 2, change to biweekly from weekly minimum time between calibration standardpreparation.3. Page 7, Analysis step 5, added statement that calibration blank should be < 5 timesMDL.4. Page 7, Analysis step 6, removed “immediately” after QCS and added” and beforeany sample” after calibration.5. Page 7, Analysis step 6a, changed “the LRB shall not exceed 3 times MDL” to“the LRB shall not exceed 10 times MDL.” This change reflects lower MDLsachieved on some instruments in particular ICP-MS and difficulty in LRB meeting3 times MDL.6. Page 4, added subsections B and C to section I that outline XRF testing proceduresfor determining Pb in glass or siliceous materials.。
