Antiferroelectric lead zirconate thin films by pulsed laser ablation
压电陶瓷的种类

压电陶瓷的种类1 铁电陶瓷ferroelecteic ceramics具有重铁电性的陶瓷称为铁电陶瓷。
从晶体结构来看,铁电陶瓷的晶体的主晶相具有钙钛矿结构,钨青铜结构,铋层状结构和焦绿石结构等。
2 反铁电陶瓷antiferroelectric ceramics具有反铁电性的陶瓷称为反铁电陶瓷。
3 压电陶瓷piezoelectric ceramics具有压电效应的陶瓷称为压电陶瓷,由于末经过极化处理的铁电陶瓷的自发极化随机取向,故没有压电性。
极化处理使其自发极化沿极化方向择优取向。
在撤去电场后,陶瓷体仍保留着一定的总体剩余极化,故使陶瓷体有了压电性,成为压电陶瓷。
在高温的高温度梯度场中定向析晶的非铁电极性玻璃陶瓷也具有压电性。
4 钛酸钡陶瓷barium titanate ceramics钛酸钡陶瓷是一种具有典型钙钛矿结构的铁电陶瓷。
它通常是以碳酸钡和二氧化钛为主要原料,预先合成后再在高温下烧结而成的。
5 钛酸铅陶瓷lead titanate ceramics钛酸铅陶瓷是具有钙钛矿性结构的铁电陶瓷。
它通常是由四氧化三铅{或氧化铅}和二氧化钛以及少量添加物预先合成后再在高温下烧结而成的。
6 二元系陶瓷binary system ceramies二元系压电陶瓷是俩种化学通式ABO3型结构的化学物所形成的固溶体,其中A 代表二价的正离子Pb2+,Ba2+,Mg2+,Ca2+,Sr2+,等或一价正离子K+,Na+等,B代表四价的正离子Zr4+,Ti4+或五价的Nb5+等。
最常见的二元系压电陶瓷是PbZrxTi{1-x}O3。
通过调节两种ABO3型结构的克分子比,以及用取代元素和添加物改性的方法,可以获得各种不同用途的材料。
7 锆钛酸铅陶瓷Lead zirconate ceramic锆钛酸铅陶瓷通常简称为PZT陶瓷,这种压电陶瓷目前受到广泛应用。
它是PbZrO3和PbTiO3的固溶体,具有钙钛矿型结构,当锆钛比为53/47左右{即共晶相界附近}时,具有最强的压电性能。
反铁电TEM 研究

In situ transmission electron microscopy study of the electric field-induced transformation of incommensurate modulations in a Sn-modified lead zirconate titanate ceramicH. He and X. TanCitation: Appl. Phys. Lett. 85, 3187 (2004); doi: 10.1063/1.1805179View online: /10.1063/1.1805179View Table of Contents: /resource/1/APPLAB/v85/i15Published by the American Institute of Physics.Related ArticlesComplete set of elastic, dielectric, and piezoelectric constants of [011]C poled rhombohedral Pb(In0.5Nb0.5)O3-Pb(Mg1/3Nb2/3)O3-PbTiO3:Mn single crystalsJ. Appl. Phys. 113, 074106 (2013)Compressible spherical dipolar glass model of relaxor ferroelectricsJ. Appl. Phys. 112, 114122 (2012)Tunable self-biased magnetoelectric response in homogenous laminatesAppl. Phys. Lett. 101, 232905 (2012)Self-assembled NaNbO3-Nb2O5 (ferroelectric-semiconductor) heterostructures grown on LaAlO3 substrates Appl. Phys. Lett. 101, 132902 (2012)Pressure and electric field effects on piezoelectric responses of KNbO3J. Appl. Phys. 112, 064106 (2012)Additional information on Appl. Phys. Lett.Journal Homepage: /Journal Information: /about/about_the_journalTop downloads: /features/most_downloadedInformation for Authors: /authorsIn situ transmission electron microscopy study of the electric field-induced transformation of incommensurate modulations in a Sn-modified lead zirconate titanate ceramicH.He and X.Tan a )Department of Materials Science and Engineering,Iowa State University,Ames,Iowa 50011(Received 7May 2004;accepted 11August 2004)Electric field-induced transformation of incommensurate modulations in a Sn-modified lead zirconate titanate ceramic was investigated with an electric field in situ transmission electron microscopy technique.It is found that the spacing between the ͑1/x ͕͒110͖satellite spots and the fundamental reflections do not change with external electric field,indicating that the modulation wavelength stays constant under applied field.The intensity of these satellites starts to decrease when the field level reaches a critical value.Further increase in the field strength eventually leadsto the complete disappearance of the satellite reflections.In addition,the 12͕111͖-type superlattice reflections showed no response to electrical stimuli.©2004American Institute of Physics .[DOI:10.1063/1.1805179]Incommensurate modulations have been observed experimentally with transmission election microscopy (TEM )in many perovskite ferroelectric ceramics,such as PbZrO 3,1Pb ͑Zr 1−x Ti x ͒O 3,2,3Sn-modified Pb ͑Zr 1−x Ti x ͒O 3(PZST ),4–7La-modified Pb ͑Zr 1−x Ti x ͒O 3(PLZT ),8–10Pb ͑Co 1/2W 1/2͒O 3,11,12and Pb ͑Sc 1/2Ta 1/2͒O 3.12,13They are characterized by the satellite reflections in electron diffrac-tion patterns and the regular fringes in image contrast.The modulation wave vector represented by the satellites is along the normal direction of those fringes,and the distance of the satellites to the fundamental reflections in reciprocal space corresponds to the spacing of the fringes in real space.The wave vector is parallel to the ͗110͘direction in most cases and the wavelength lies in the range of 5ϳ20a ,where a is the lattice parameter for the parent paraelectric structure.8Transformation of the intermediate incommensurate phase to other phases can be triggered by external stimuli,such as chemical composition and temperature.6–10Sn-or Ti-content in the PZST system and La content in the PLZT system were utilized before as controlling variables for the phase transformation.8–10In the PZST system,it has been shown that decrease in Sn content or increase in Ti content leads to the incommensurate antiferroelectric-to-ferroelectric phase transformation.In the PLZT system,the modulation wavelength was observed to increase with Ti molar fraction.8Utilizing temperature as the controlled variable in the study of such transformations allows in situ TEM studies,where the evolution of the satellite reflections can be directly observed.6,7The hot-stage in situ TEM observations revealed that the modulation wavelength increases continuously with increasing temperature.Based on these studies,Viehland et al.8suggested that the competing ferroelectric and antiferro-electric ordering in these perovskites are responsible for the presence of incommensurate modulations.However,unam-biguous evidence has yet to be found.In this letter,we report the study of the incommensurate phase transformation trig-gered by controlled electric fields.The evolution of the sat-ellite spots driven by external electrical stimuli is recorded.A hybrid coprecipitation method similar to that used by Yang and Payne 14was followed to prepare the ceramic powder with a chemical formula Pb 0.99Nb 0.02͓͑Zr 0.55Sn 0.45͒0.94Ti 0.06͔0.98O 3(abbreviated as PZST 45/6/2).Pressed cylinders,15mm in diameter by 20mm thick,were formed by cold-isostatic pressing at 350MPa.The preformed pellets were then hot pressed in an Al 2O 3die at 1150°C for 2h in air.Thin slices from the hot-pressed piece were annealed at 1300°C for 2h in an atmosphere containing excess PbO.The annealed slices were then ground,polished,and electroded.Dielectric character-ization was performed with an LCR meter (HP-4284A,Hewlett-Packard )at frequency of 1kHz in conjunction with an environmental chamber.A heating rate of 3°C/min was used during measurement.Electric field-induced polariza-tions were recorded with a standardized ferroelectric test sys-tem (RT-66A,Radiant technologies ).TEM specimens were prepared from ultrasonically cut 3-mm-diam disks.The thickness of these disks was reduced to ϳ150m by grinding and polishing.The center portion was further thinned to ϳ10m by dimpling.The dimpled specimens were then annealed at 300°C for 30min to mini-mize residual stresses.An argon ion mill was used for further thinning until final perforation occurred in the center.Gold electrodes with spacing of 250µm were evaporated to the TEM specimens,and platinum wires were used to connect the electrodes to the electrical contacts on the TEM specimen holder.Figure 1shows the schematic diagram of the TEM specimen configuration.Details of the electric field in situ TEM technique can be found elsewhere.15–18TEM studies were carried out on a Phillips CM-30microscope operating at 300kV .Temperature dependence of the dielectric permittivity of PZST 45/6/2is shown in Fig.2.The dielectric constant peaks with a value of 920at the temperature of 167°C.Electric field-induced polarization measurement showed double hysteresis loops (Fig.3),indicating an electric field-induced antiferroelectric-to-ferroelectric phase transforma-tion at room temperature.These results are consistent with previous observations from other researchers.5–7In order to determine the field level needed for the in situa )Electronic mail:xtan@APPLIED PHYSICS LETTERS VOLUME 85,NUMBER 1511OCTOBER 20040003-6951/2004/85(15)/3187/3/$22.00©2004American Institute of Physics3187TEM study and to assess the sample geometry effect on the field-induced antiferroelectric-to-ferroelectric phase transfor-mation,a dimpled and annealed 3-mm-diam disk specimen was tested for the hysteresis measurement and the result is also plotted in pared to the conventional circular plate sample with electric field applied along the thickness direction,the TEM-specimen-like disk shows a more gradual phase transformation with much broader loops.Furthermore,the backward switch from the induced ferroelectric phase to the antiferroelectric phase is sluggish,and a nonzero remnant polarization is detected.In situ TEM studies were carried out on a disk specimen with a central perforation.Actual electric field in the speci-men is disturbed (intensified in some areas while diluted in others )by the presence of the perforation.Electron transpar-ent areas that are subjected to intensified electric fields in the specimen were located in the TEM and one grain within this area was focused for the successive detailed in situ study.For an ideal circular perforation,the intensification ratio is 2.19The local electric field strength in this grain was thus esti-mated by doubling the nominal field strength.The evolution of the satellite spots in a ͗112͘-zone axis selected area diffraction pattern under static electric fields is shown in Fig.4.Initially,this grain displays one set of in-commensurate modulations.In the electron diffraction pat-tern,one set of the ͑1/x ͕͒110͖satellite spots is evident,as shown in Fig.4(a ).No detectable changes to these satellite spots were observed with applied static electric field up to 40kV/cm.At the field level of 48kV/cm,these satellites become weaker in their intensity [Fig.4(b )].This field level lies in the close vicinity of the E F (the critical field to trigger the antiferroelectric-to-ferroelectric transformation )mea-sured from the bulk sample.However,careful measurement indicates that the modulation wavelength does not change with increasing field strength.When the field strength reached 56kV/cm,most satellite spots disappeared.As shown in Fig.4(c ),only very weak satellites can be barely seen surrounding the three strong fundamental reflections in the left of this micrograph.The field strength was then re-duced to 40kV/cm.The satellites reappeared,with the strongest ones sitting in the upper-left corner of Fig.4(d ).When the field level was raised again to 56kV/cm [Fig.4(e )],all the satellite spots completely disappeared,indicat-ing the complete transformation to the ferroelectric phase.After the electric field was completely removed,no satellite spots were observed to reappear,as shown in Fig.4(f ).The observation confirms the sluggish nature of the backward ferroelectric-to-antiferroelectric transformation that has been noticed by other researchers in a similar composition.20It has been suggested that in PZT-based ferroelectric per-ovskites,the presence of incommensurate modulations in the antiferroelectric phase is a result of the competition between the ferroelectric and antiferroelectric ordering.8The continu-ous increase in the modulation wavelength with increasing temperature is interpreted that there exists a ferroelectric phase within a narrow temperature range just below the paraelectric transition temperature.When temperature is raised close to this temperature range the ferroelectric order-ing is enhanced.The modulation wavelength is thus in-creased.However,our observations on the electric field-induced transformation of the incommensurate modulation show a different scenario.The intensity of the satellite reflec-tions,rather than the modulation wavelength,changes with the applied electric field strength.The wavelength stays con-stant at a value of 2.3nm.Since external electric field is known to enhance the ferroelectric ordering,we suggest that the electric field-induced antiferroelectric (incommensurate )-to-ferroelectric transformation proceeds as following.When the applied electric field reaches E F ,the phase transformation is initiated in some areas of the grain.The transformation is an abrupt one and no intermediate changes in the modulation wavelength takes place.With increasing electric field strength,the fraction of the transformed area increases and the intensity of the satellite diffraction peaks getsweaker.FIG.2.Temperature dependence of dielectric constant at 1kHz in the PZST 45/6/2ceramic.FIG.3.Electric field-induced polarization measurement at 4Hz in a bulk circular plate sample and an unperforated TEMspecimen.FIG.1.Schematic diagram of specimens for the electric field in situ TEM study.Obviously,a mechanism involving the nucleation of ferro-electric phase and the motion of the phase boundary controls the transformation process.Further studies will be focused on these issues.In addition to the ͑1/x ͕͒110͖satellite spots,12͕111͖-type superlattice reflections were also present in the ͗112͘-axis diffraction pattern,as labeled in Fig.4(e ).The structural ori-gin for these superlattice reflections is still under debate.2,5–7,9,21However,the present in situ TEM study pro-vides valuable insight into the physics mechanism for the presence of these superlattice reflections.It is clear in Fig.4that the intensity of the 12͕111͖spots does not change with the applied electric field,implying a mechanism that is quite rigid to external disturbance.This seems to favor the oxygen octohedra tilting model.21To summarize,in situ TEM technique was applied to the study of the electric field-induced antiferroelectric-to-ferroelectric phase transformation in a PZT-based ceramic.Upon application of external electric fields,the wavelength of the incommensurate modulation in the antiferroelectric phase showed no change but the intensity of the satellite reflections decreased when the field exceeds a critical value.This critical strength matches closely to the E F measured in the bulk sample.This work was supported by the Process Science Initia-tive (PSI )program at Ames Laboratory,U.S.DOE (GrantNo.10-501-115612).The authors are grateful to Dr.David Cann for the access to the dielectric characterization instru-ment in his group at Iowa State University.1Z.Xu,X.Dai,D.Viehland,and D.A.Payne,J.Am.Ceram.Soc.78,2220(1995).2J.Ricote,D.L.Corker,R.W.Whatmore,S.A.Impey,A.M.Glazer,J.Dec,and K.Roleder,J.Phys.:Condens.Matter 10,1767(1998).3S.Watanabe and Y .Koyama,Phys.Rev.B 66,134102(2002).4J.S.Speck,M.De Graef,A.P.Wilkinson,A.K.Cheetham,and D.R.Clarke,J.Appl.Phys.73,7261(1993).5D.Viehland,D.Forst,Z.Xu,and J.Li,J.Am.Ceram.Soc.78,2101(1995).6Z.Xu,D.Viehland,P.Yang,and D.A.Payne,J.Appl.Phys.74,3406(1993).7Z.Xu,D.Viehland,and D.A.Payne,J.Mater.Res.10,453(1995).8D.Viehland,X.Dai,J.Li,and Z.Xu,J.Appl.Phys.84,458(1998).9J.Knudsen,D.I.Woodward,and I.Reaney,J.Mater.Res.18,262(2003).10Z.Xu,X.Dai,and D.Viehland,Phys.Rev.B 51,6261(1995).11S.Watanabe and Y .Koyama,Phys.Rev.B 65,064108(2002).12C.A.Randall,S.A.Markgraf,A.S.Bhalla,and K.Baba-Kishi,Phys.Rev.B 40,413(1989).13K.Z.Baba-Kishi and D.J.Barber,J.Appl.Crystallogr.23,43(1990).14P.Yang and D.A.Payne,J.Appl.Phys.71,1361(1992).15Z.Xu,X.Tan,P.Han,and J.K.Shang,Appl.Phys.Lett.76,3732(2000).16X.Tan,Z.Xu,J.K.Shang,and P.Han,Appl.Phys.Lett.77,1529(2000).17X.Tan,Z.Xu,and J.K.Shang,Mater.Sci.Eng.,A 314,157(2001).18X.Tan,and J.K.Shang,Philos.Mag.A 82,1463(2002).19R.M.McMeeking,ZAMP 40,615(1989).20W.Chan,H.Chen,and E.V .Colla,Appl.Phys.Lett.82,2314(2003).21D.Viehland,Z.Xu,and D.A.Payne,J.Appl.Phys.74,7454(1993).FIG.4.Evolution of the ͗112͘selected area diffraction pattern under applied electric fields in PZST 45/6/2.(a )Original state,(b )48,(c )56,(d )40,and (e )56kV/cm,and (f )field removed.。
药用辅料中英文对照

药用辅料中英文对照1 阿拉伯胶(Acacia)2 乙酰舒泛钾(Acesulfame Potassium)3 冰醋酸(Acetic Acid,Glacial)4 乙酰枸橼酸三丁酯(AcetyltributylCitrate)5 乙酰枸橼酸三乙酯(AcetyltriethylCitrate)6 人血白蛋白(Albumin)7 乙醇(Alcohol)8 海藻酸(Alginic Acid)9 脂肪族聚酯(Aliphatic Polyesters)10 阿力糖(Alitame)11 杏仁油(Almond Oil)12 维生素E(Alpha Tocopherol)13 氨溶液(Ammonia Solution)14 维生素C(Ascorbic Acid)15 棕榈酸维生素C酯(Ascorbyl Palmitate)16 阿司帕坦(Aspartame)17 绿坡缕石(Attapulgite)18 皂土(Bentonite)19 苯扎氯铵(Benzalkonium Chloride)20 苄索氯铵(Benzethonium Chloride)21 苯甲酸(Benzoic Acid)22 苯甲醇(Benzyl Alcohol)23 苯甲酸苄酯(Benzyl Benzoate)24 溴硝丙二醇(Bronopol)25 丁羟茴醚(Butylated Hydroxyanisole)26 丁羟甲苯(Butylated Hydroxytoluene)27 羟苯丁酯(Butylparaben)28 碳酸钙(Calcium Carbonate)29 无水磷酸氢钙(Calcium Phosphate,Dibasic Anhydrous)30 磷酸氢钙二水合物(Calcium Phosphate,Dibasic Dihydrate)31 磷酸钙(Calcium Phosphate,Tribasic)32 硬脂酸钙(Calcium Stearate)33 硫酸钙(Calcium Sulfate)34 低芥酸菜籽油(Canola Oil)35 卡波姆(Carbomer)36 二氧化碳(Carbon Dioxide)37 羧甲纤维素钙(Carboxymethylcellulose Calcium)38 羧甲纤维素钠(Carboxymethylcellulose Sodium)39 角叉菜胶(Carrageenan)40 蓖麻油(Castor Oil)41 氢化蓖麻油(Castor Oil,Hydro-genated)42 微晶纤维素(Cellulose,Microcr ystalline)43 粉状纤维素(Cellulose,Powdered)44 微粉硅胶微晶纤维素(Cellulose, Silicified Microcrystalline)45 醋酸纤维素(Cellulose Acetate)46 纤维醋法酯(Cellulose Acetate Phthalate)47 角豆胶(Ceratonia)48 十八十六醇(Cetostearyl Alcohol)49 西曲溴铵(Cetrimide)50 十六醇(Cetyl Alcohol)51 壳聚糖(Chitosan)52 氯己定(Chlorhexidine)53 三氯叔丁醇(Chlorobutanol)54 氯甲酚(Chlorocresol)55 一氯二氟乙烷(Chlorodifluoroe-thane)56 氟里昂(Chlorofluorocabons)57 对氯间二甲酚(Chloroxylenol)58 胆固醇(Cholesterol)59 枸橼酸(Citric Acid Monohydrate)60 胶态二氧化硅(微粉硅胶)(Colloidal Silicon Dioxide)61 着色剂(Coloring Agents)62 玉米油(Corn Oil)63 棉籽油(Cottonseed Oil)64 甲酚(Cresol)65 交联羧甲纤维素钠(Croscarmellose Sodium)66 交联聚维酮(Crospovidone)67 环糊精(Cyclodextrins)68 环甲基硅酮(Cyclomethicone)69 苯甲地那铵(Denatonium Benzoate)70 葡萄糖结合剂(Dextrates)71 糊精(Dextrin)72 葡萄糖(Dextrose)73 邻苯二甲酸二丁酯(Dibutyl Phthalate)74 癸二酸二丁酯(Dibutyl Sebacate)75 二乙醇胺(Diethanolamine)76 邻苯二甲酸二乙酯(Diethyl Phthalate)77 二氟乙烷(Difluoroethane)78 二甲硅油(Dimethicone)79 二甲醚(Dimethyl Ether)80 邻苯二甲酸二甲酯(Dimethyl Phthalate)81 二甲亚砜(Dimethyl Sulfoxide)82 多库酯钠(Docusate Sodium)83 依地酸(乙二胺四乙酸)(Edetic Acid)84 乙酸乙酯(Ethyl Acetate)85 乙基麦芽酚(Ethyl Maltol)86 油酸乙酯(Ethyl Oleate)87 乙基香草醛(Ethyl Vanillin)88 乙基纤维素(Ethylcellulose)89 硬脂酸棕榈酸乙二醇酯(Ethylene Glycol Palmitostearate)90 羟苯乙酯(Ethylparaben)91 果糖(Fructose)92 富马酸(Fumaric Acid)93 明胶(Gelatin)94 液体葡萄糖(Glucose,Liquid)95 甘油(Glycerin)96 山萮酸甘油酯(Glyceryl Behenate)97 单油酸甘油酯(Glyceryl Monooleate)98 单硬脂酸甘油酯(Glyceryl Monostearate)99 硬脂酸棕榈酸甘油酯(Glyceryl Palmitostearate)100 四氢呋喃聚乙二醇醚(Glycofurol)101 瓜耳胶(Guar Gum)102 七氟丙烷(HFC)(Heptafluoro-propane)103 海克西定(Hexetidine)104 烷烃类(HC) (Hydrocarbons)105 盐酸(Hydrochloric Acid)106 羟乙纤维素(Hydroxyethyl Cellulose)107 羟乙甲纤维素(Hydroxyethylmethyl Cellulose)108 羟丙纤维素(Hydroxypropyl Cellulose)109 低取代羟丙纤维素(Hydroxypropyl Cellulose,Low-substituted) 110 羟丙甲纤维素(Hypromellose)111 羟丙甲纤维素酞酸酯(Hypromellose Phthalate)112 咪唑烷脲(Imidurea)113 异丙醇(Isopropyl Alcohol)114 肉豆蔻酸异丙酯(Isopropyl Myristate)115 棕榈酸异丙酯(Isopropyl Palmitate)116 白陶土(Kaolin)117 乳酸(Lactic Acid)118 拉克替醇(Lactitol)119 乳糖(Lactose)120 羊毛脂(Lanolin)121 含水羊毛脂(Lanolin,Hydrous)122 羊毛醇(Lanolin Alcohols)123 卵磷脂(Lecithin)124 硅酸镁铝(Magnesium Aluminum Silicate) 125 碳酸镁(Magnesium Carbonate)126 氧化镁(Magnesium Oxide)127 硅酸镁(Magnesium Silicate)128 硬脂酸镁(Magnesium Stearate)129 三硅酸镁(Magnesium Trisilicate)130 苹果酸(Malic Acid)131 麦芽糖醇(Maltitol)132 麦芽糖醇溶液(Maltitol Solution)133 麦芽糖糊精(Maltodextrin)134 麦芽酚(Maltol)135 麦芽糖(Maltose)136 甘露醇(Mannitol)137 中链脂肪酸甘油三酯(Medium-chain Triglycerides) 138 葡甲胺(Meglumine)139 薄荷脑(Menthol)140 甲基纤维素(Methylcellulose)141 羟苯甲酯(Methylparaben)142 液体石蜡(Mineral Oil)143 轻质液体石蜡(Mineral Oil,Light)144 液体石蜡羊毛醇(Mineral Oil and Lanolin Alcohols) 145 单乙醇胺(Monoethanolamine)146 谷氨酸一钠(Monosodium Glutamate)147 硫代甘油(Monothioglycerol)148 氮(Nitrogen)149 一氧化二氮(Nitrous Oxide)150 油酸(Oleic Acid)151 橄榄油(Olive Oil)152 石蜡(Paraffin)153 花生油(Peanut Oil)154 凡士林(Petrolatum)155 凡士林羊毛醇(Petrolatum and Lanolin Alcohols) 156 苯酚(Phenol)157 苯氧乙醇(Phenoxyethanol)158 苯乙醇(Phenylethyl Alcohol)159 醋酸苯汞(Phenylmercuric Acetate)160 硼酸苯汞(Phenylmercuric Borate)161 硝酸苯汞(Phenylmercuric Nitrate)162 磷酸(Phosphoric Acid)163 波拉克林钾(Polacrilin Potassium)164 泊洛沙姆(Poloxamer)165 葡聚糖(Polydextrose)166 聚乙二醇(Polyethylene Glycol)167 聚氧乙烯(Polyethylene Oxide)168 聚(甲基)丙烯酸树脂(Polymethacr-ylates)169 聚氧乙烯烷基醚(Polyoxyethylene Alkyl Ethers)170 聚氧乙烯蓖麻油衍生物(Polyoxyeth-ylene Castor Oil Derivatives) 171 聚山梨酯(Polyoxyethylene Sorbitan Fatty Acid Esters)172 硬脂酸聚氧乙烯酯(Polyoxyethylene Stearates)173 聚醋酸乙烯酞酸酯(Polyvinyl Acetate Phthalate)174 聚乙烯醇(Polyvinyl Alcohol)175 苯甲酸钾(Potassium Benzoate)176 碳酸氢钾(Potassium Bicarbonate)177 氯化钾(Potassium Chloride)178 枸橼酸钾(Potassium Citrate)179 氢氧化钾(Potassium Hydroxide)180 焦亚硫酸钾(Potassium Metabisulfite)181 山梨酸钾(Potassium Sorbate)182 聚维酮(Povidone)183 丙酸(Propionic Acid)184 没食子酸丙酯(Propyl Gallate)185 碳酸丙烯酯(Propylene Carbonate)186 丙二醇(Propylene Glycol)187 海藻酸丙二醇酯(Propylene Glycol Alginate)188 羟苯丙酯(Propylparaben)189 糖精(Saccharin)190 糖精钠(Saccharin Sodium)191 芝麻油(Sesame Oil)192 虫胶(Shellac)193 二氧化硅二甲硅油(Simethicone)194 海藻酸钠(Sodium Alginate)195 抗坏血酸钠(Sodium Ascorbate)196 苯甲酸钠(Sodium Benzoate)197 碳酸氢钠(Sodium Bicarbonate)198 氯化钠(Sodium Chloride)199 枸橼酸钠二水合物(Sodium Citrate Dihydrate)200 环拉酸钠(Sodium Cyclamate)201 氢氧化钠(Sodium Hydroxide)202 月桂硫酸钠(十二烷基硫酸钠)(Sodium Lauryl Sulfate) 203 焦亚硫酸钠(偏亚硫酸钠)(Sodium Metabisulfite) 204 磷酸氢二钠(Sodium Phosphate,Dibasic)205 磷酸二氢钠(Sodium Phosphate ,Monobasic)206 丙酸钠(Sodium Propionate)207 羧甲淀粉钠(Sodium Starch Glycolate)208 硬脂富马酸钠(Sodium Stearyl Fumarate)209 山梨酸(Sorbic Acid)210 山梨坦酯Sorbitan Esters(Sorbitan Fatty Acid Esters) 211 山梨醇(Sorbitol)212 大豆油(Soybean Oil)213 淀粉(Starch)214 预胶化淀粉(Starch,Pregelatinized)215 灭菌玉米淀粉(Starch,Sterilizable Maize)216 硬脂酸(Stearic Acid)217 硬脂醇(Stearyl Alcohol)218 羟糖氯(Sucralose)219 蔗糖(Sucrose)220 可压性蔗糖(Sugar,Compressible)221 蔗糖粉(Sugar,Confectioner’s)222 蔗糖球形颗粒(Sugar Spheres)223 硫酸(Sulfuric Acid)224 葵花籽油(Sunflower Oil)225 氢化植物油(硬脂)栓剂基质(Sup-pository Bases,Hard Fat) 226 滑石粉(Talc)227 酒石酸(Tartaric Acid)228 四氟乙烷(HFC)(Tetrafluoroe-thane)229 硫柳汞(Thimerosal)230 二氧化钛(Titanium Dioxide)231 西黄蓍胶(Tragacanth)232 海藻糖(Trehalose)233 三醋汀(Triacetin)234 枸橼酸三丁酯(Tributyl Citrate)235 三乙醇胺(Triethanolamine)236 枸橼酸三乙酯(Triethyl Citrate)237 香草醛(Vanillin)238 氢化植物油(Vegetable Oil,Hydrogenated)239 水(Water)240 阴离子乳化蜡(Wax,Anionic Emulsifying)241 巴西棕榈蜡(Wax,Carnauba)242 十六醇酯蜡(Wax,Cetyl Esters)243 微晶蜡(Wax,Microcrystalline)244 非离子乳化蜡(聚西托醇乳化蜡)(Wax,Nonionic Emulsifying) 245 白蜡(Wax,White)246 黄蜡(Wax,Yellow)247 黄原酸胶(Xanthan Gum)248 木糖醇(Xylitol)796249 玉米朊(玉米蛋白)(Zein)250 硬脂酸锌(Zinc Stearate)11 / 11。
论文列表 - 河北工业大学
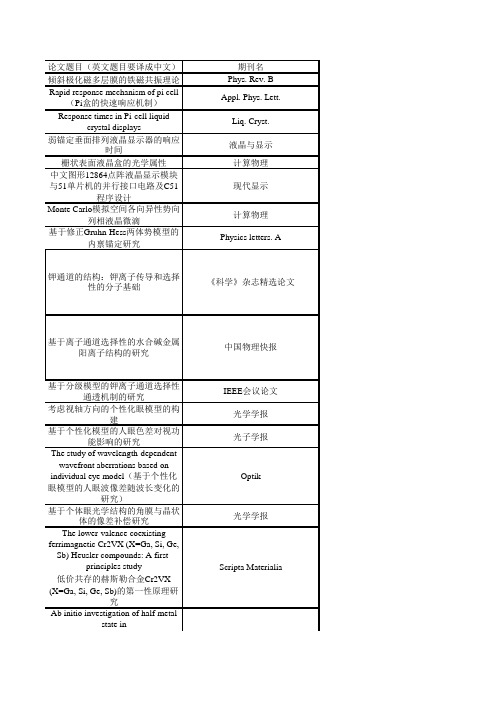
论文题目(英文题目要译成中文)期刊名倾斜极化磁多层膜的铁磁共振理论Phys. Rev. B Rapid response mechanism of pi cell(Pi 盒的快速响应机制)Appl. Phys. Lett.Response times in Pi-cell liquidcrystal displaysLiq. Cryst.弱锚定垂面排列液晶显示器的响应时间液晶与显示栅状表面液晶盒的光学属性计算物理中文图形12864点阵液晶显示模块与51单片机的并行接口电路及C51程序设计现代显示Monte Carlo 模拟空间各向异性势向列相液晶微滴计算物理基于修正Gruhn-Hess 两体势模型的内禀锚定研究Physics letters. A基于分级模型的钾离子通道选择性通透机制的研究IEEE 会议论文考虑视轴方向的个性化眼模型的构建光学学报基于个性化模型的人眼色差对视功能影响的研究光子学报The study of wavelength-dependentwavefront aberrations based onindividual eye model (基于个性化眼模型的人眼波像差随波长变化的研究)Optik 基于个体眼光学结构的角膜与晶状体的像差补偿研究光学学报The lower-valence coexistingferrimagnetic Cr2VX (X=Ga, Si, Ge,Sb) Heusler compounds: A first-principles study低价共存的赫斯勒合金Cr2VX(X=Ga, Si, Ge, Sb)的第一性原理研究Ab initio investigation of half-metalstate in 钾通道的结构:钾离子传导和选择性的分子基础《科学》杂志精选论文基于离子通道选择性的水合碱金属阳离子结构的研究中国物理快报Physica BScripta Materialiazinc-blende MnSn and MnC闪锌矿结构的MnSn和MnC的半金属态的从头计算研究用临界点附近的涨落讨论Landau相变理论的适用范围河北工业大学学报“建设国家级实验教学示范中心的探索与实践”《全国高等学校物理基础课程教育学术研讨会论文集》坚持创新教育实验教学理念建设物理实验教学示范中心《第五届全国高等学校物理实验教学研讨会论文集》创建国家级实验教学示范中心及其辐射示范作用的研究与实践《实验技术与管理》大学生自主创新教育应适应社会需求性与尊重学生选择性《第五届全国高等学校物理实验教学研讨会论文集》坚持创新教育实验教学理念建设物理实验教学示范中心《物理实验》(特刊)《关于如何提高基础物理实验课质量的探讨》《科学时代》带电粒子致细胞失活的理论模型中国原子能科学研究院年报2007Two-photon spectroscopic behaviorsand photodynamic effect on the BEL-7402 cancer cells of the newchlorophyll photosensitizer《中国科学》(一种新型叶绿素光敏剂的双光子光谱特性B 辑及其对BEL-7402肝癌细胞的双光子光动力效应)(英文版)Tunneling effect of two horizons froma Gibbons-Maeda black holeChinese Physics LettersTunneling Effect from a Non-static Black Hole with the Internal GlobalMonopole International Journal of TheoreticalPhysicsTunneling effect of two horizons from a Reissner-Nordstrom black hole International Journal of TheoreticalPhysics用新乌龟坐标计算任意直线加速带电黑洞的熵北京师范大学学报自然科学版液晶与显示Physica BTheoretical Models of Cell Inactivation by Ionizing Particles(带电粒子致细胞失活的理论模型)Annual Report of China Institute of Atomic Energy 2007片剂硬度测试仪的液晶显示界面设计克尔黑洞的内视界隧道效应北京师范大学学报自然科学版Order Parameter of theAntiferroelectric Phase Transition ofLead Zirconate,Ferroelectric LettersSymmetry of the AntiferroelectricLead ZirconateFerroelectric LettersInvestigation of Ferroelectric PhaseTransition of Rochelle SaltFerroelectrics 锆酸铅的反铁电相对称性研究人工晶体学报方硼盐本征铁电相变的研究河北工业大学学报The synchronization ofFitzHugh--Nagumo neuronnetwork coupled by gapjunctionChinese Physics BStudy of intrinsic anchoring in nematicliquid crystals based on modifiedGruhn-hess pair potential(基于修正的Gruhn-hess两体势研究向列相液晶的内禀锚定)Phys. Lett. AThe Electrod Effect in LCD Cell(LCD盒中的电极效应)电子器件聚合物稳定向列相液晶显示的上升时间常数现代显示全Heusler 合金Cr2MnAl的第一性原理研究Appl. Phys. Lett.大范围Mn掺杂Heusler-型 Cr3Al的半金属化合物的理论设计J. Appl. Phys.局域键近似下的固体材料热驱动下的弹性软化J. Phys. D: Appl. Phys. Heusler 相 Co2YBi 和半-Heusler 相CoYBi (Y=Mn, Cr)的带结构计算J. Magn. Magn. Mater含时外势作用下玻色-爱因斯坦凝聚的非自治孤子自旋极化电流导致的铁磁金属多层膜中的铁磁共振J. Appl. Phys.“液晶显示器件物理专业方向”的实验设置实验技术与管理混合排列向列相边缘场效应电光特性的模拟计算现代显示Opt. Commun.个性化视觉矫正中人眼波前像差测量数据的修正研究Photonics and Optoelectronics MeetingsDynamical Model of P53-Mdm2-P14/19ARF Network to Radiation inPopulation of Cells 电离辐射作用下多细胞P53-Mdm2-P14/19ARF 网络动力学模型机械波的半波损失条件问题探析河北工业大学成人教育学院学报地方工科高校大学物理课程建设的探索与实践2009年全国高等学校物理基础课程教育学术研讨会论文集A death-survival switch in cell: cross talk between Akt and p53International Conference on Bioinformatics and Biomedical EngineeringModeling of ATM accumulation and ATM-mediated oscillation of p53International Conference on Bioinformatics and Biomedical EngineeringFunctions of oligochitosan inducedprotein kinase in tobacco mosaic virusresistance and pathogenesis related proteins in tobacco (壳寡糖诱导蛋白激酶在烟草对烟草花叶病毒抗性和病理相关蛋白中的作用)Plant Physiology and Biochemistry Neutrino Oscillation in the Space-Time with a Global Monopole 有拓扑缺陷时空的中微子振荡Thermodynamics Properties of theInner Horizon of a Kerr-Newman Black Hole K-N 黑洞的内视界的热性质Tunneling Effect of Two Horizonsfrom a Reissner-Nordstrom Black Hole R-N 黑洞的双视界的隧穿效应太赫兹波在空芯椭圆波导中的传播特性Photonics and Optoelectronics Meetings (POEM) 2008:TerahertzScience and Technology自旋波背景下单轴各向异性铁磁中磁振子密度的新特性Ann. Phys. (New York)BIC-TA2009International Journal of TheoreticalPhysicsInternational Journal of Theoretical Physics International Journal of TheoreticalPhysicsNeutrino oscillationinterference phase in Kerrspace--time克尔时空的中微子振荡干涉相位指形场驱动垂面排列模式的响应机制,Liq. Cryst.,两块栅状表面基板构成的向列相液晶盒的阈值属性Chinese Physics B 挠曲电效应对向列相液晶盒电光效应的影响计算物理双轴向列相液晶在沟槽表面的弹性形变和稳定性Liquid Crystals用光学导波方法区分液晶盒中的预倾角与弱锚定现代显示栅状表面液晶盒的光学特性现代显示平面和高斯光束的非线性吸收系数β的微分分析Photonics and OptoelectronicsMeetingsModel for influences of magneticfields on intracellular calcium oscillations(磁场对细胞内钙振荡影响的理论研究)Commun. Theor. Phys.液晶与显示Chinese Physics B太赫兹波在空芯椭圆波导中的传播特性Photonics and Optoelectronics Meetings (POEM) 2008:Terahertz Science and Technology平行排列向列相液晶的导波研究太赫兹波在空芯镀不同介质膜圆波导中的传播特性Photonics and Optoelectronics Meetings (POEM) 2008:Terahertz Science and TechnologyStability Control System for Four-In-Wheel-Motor Drive Electric Vehicle.The Sixth International Conference onFuzzy Systems and Knowledge Discovery, Tianjin, China, 14–16August ,2009.Propagation characteristics of THzradiation in hollow ellipticalwaveguide Pro. of SPIE光电混合机器人视觉系统中光寻址空间光调制器的噪声处理光子学报A First Principles study on the fullHeusler Compond Compond利用慢度曲面研究晶体的纯模轴人工晶体学报演化网络的拓扑结构河北工业大学学报帐篷映射归宿的完整性探析天津师范大学学报用二进制讨论面包师变换的动力学特性河北工业大学学报克尔-纽曼时空的中微子振荡Class. Quantum Grav.史瓦西-德西特是空的中微子振荡干涉相位Commun. Theor. Phys.Quantitative deviation of the two-photon absorption coefficient based onthree pulse model Chinese Optics LettersTwo-photon absorption coefficientdeviation from the application of laserultra-short pulse models Chinese Physics lettersTwo-photon absorption coefficient inrelation to the typical pulse models oflaser Optics CommunicationsAppl. Phys. Lett.MnAlCr 2MnAl Cr 2罗伯特-沃克度规的中微子振荡和振荡长度的宇宙学蓝移Int J Theor Phys Domain-wall solutions of spinor Bose-Einstein condensates in an opticallattice,Phys. Rev. ANonautonomous bright and darksolitons of Bose-Einstein condensateswith Feshbach- managed time-dependent scattering lengthOpt. Commun.Formation of combined solitons intwo-component Bose-Einsteincondensates Chin. Phys. BBright and Dark Soliton Solutions inGrowing Bose-Einstein CondensatesChin. Phys. B 基于三种激光脉冲模型的双光子吸收系数偏离量研究CHINESE OPTICS LETTERS 扭曲向列相液晶显示器中的响应时间现代显示基于128_64点阵液晶显示的智能温度控制器的设计与实现实验室科学基于TRIZ 原理的航空用毛巾卷圈机折卷部件创新设计机械设计液晶全漏导模的实验研究物理实验温度对向列相液晶阈值电压的影响现代显示FlexoelectricEffectinaHAN-IPSCell Grey Solitons and Soliton interactionof Higher Nonlinear SchrödingerEquation Can. J. Phys.Screw-pitch effect and velocityoscillationof a domain wall in aferromagnetic nanowire driven byspin- polarized current J.Phys.: Condens. MatterTwo-photon absorption coefficient inrelation to the typical pulse models oflaser与若干激光典型超短脉冲模型相关的双光子吸收系数Quantitative deviation of two-photonabsorption coefficient based on threelaser pulse models基于三种激光脉冲模型的双光子吸收系数之间的量化差别Approach dealing with the pulseprofile of pump laser in Z-scanJournal of Optics Z-扫描中处理泵浦激光脉冲时域结构的一种方法曾用名:Journal of Optics A :Pure and Applied Optics双面高斯形刻槽金属纳米光栅表面等离子体传感芯片设计IEEE,Photonics and OptoelectronicA first-principles study onthelower-valencecoexisting Cr2TiX (X ¼ Al,Ga,Si, Ge, Sn,Sb) HeusleralloysJournal of Magnetism and Magnetic Materials 基于修正的Gruhn-Hess 两体势模型研究弯曲形变向列相液晶盒计算物理表面沟槽诱导双轴向列相液晶的弹性畸变液晶与显示LCD 点状缺陷分析与研究现代显示温度对向列相液晶阈值电压的影响现代显示离子与通道相互作用对NaK 通道通透特性影响的研究物理学报Extracelluar Potassium ions Play Important Roles in the Selectivity of Mutant KcsA Channel 2011 4th International Conference on Biomedical Enginerrint andInformaticsFlexibility Between the Linker of theCD and G-Loops Determines the Gating Dynamics of Hte Kir2.1ChannelBiophysical Journal Direct or Indirect Regulation ofCalcium-Activated Chloride Channel by CalciumJ Membrane Biol.离子与通道相互作用对NaK 通道通透特性影响的研究物理学报Characteristics and molecular basis ofcelecoxib modulation on Kv7potassium channelsBr. J. Pharmacol.Direct or Indirect Regulation ofCalcium-Activated Chloride Chinese Optics Letters Optics CommunicationsThe Journal of Membrane BiologyChannel by CalciumFabrication of subwavelength metallic structures using laserinterference lithographyPhotonics and Optoelectronics,IEEEENonautonomous helical motion ofmagnetization in ferromagneticnanowire driven by spin-polarizedcurrent and magnetic fieldEur. Phys. J. BMatter rogue wave in Bose-Einsteincondensates with attractive atomicinteractionEur. Phys. J. DNeutrino Oscillations in the de Sitterand the Anti-de Sitter Space-Timethe Anti-de Sitter Space-TimeSpin-Rotation Coupling in theTeleparallelism Description in HighSpeed Rotation SystemInt J Theor PhysA TETRAD DESCRIPTION ONTHE DIRACSPIN-ROTATION EFFECTOCB模式液晶器件的优化液晶与显示A high-transmittance verticalalignment liquid crystal display usinga fringe and in-plane electrical fieldLiquid CrystalsFast-response vertical alignmentliquid crystal display driven by in-plane switching, Liquid CrystalLiquid Crystals Low voltage and high transmittanceblue-phase LCDs with double-side in-plane switching electrodesLiquid CrystalsThe Journal of Membrane BiologyPhase diagram of magneticmultilayers with tilted dual spintorques J. Appl. Phys.Combined periodic wave and solitarywave solutions in two-componentBose-Einstein condensatesChin. Phys. BInt J Theor PhysInternational Journal of ModernPhysics D混和排列向列相液晶盒中挠曲电效应引起的电压漂移Commun. Theor. Phys.指导教师负责制的资助选课教学模式河北工业大学学报-社会科学版栅状基板表面几何参数对向列相液晶盒指向矢的影响Commun. Theor. Phys.液晶全漏导模透射率的实验研究大学物理实验离子与通道相互作用对NaK 通道通透特性影响的研究物理学报Extracelluar Potassium ions Play Important Roles in the Selectivity of Mutant KcsA Channel 2011 4th International Conference on Biomedical Enginerrint andInformaticsThe Difference Analysis of Nonlinear Absorption Coefficient β in the Beam Sections of Plain and Gaussian Distribution Nonlinear AbsorptionCoefficient βin the Beam Sections of Plain andGaussian DistributionEffects of Magnetic Fields on the Synchronization of Calcium Oscillations in Coupled Cells Journal of Computational and Theoretical NanoscienceOptimal design of hollow elliptical waveguide for THz radiationJournal of Physics: Conference Series,2011,Volume:276,No.1012229Approach dealing with the pulse profile of pump laser in Z-scantechniqueJournal of Physics: Conference Series,2011,Volume:276,No.1012229Propagation characteristics of THz radiation in hollow rectangle metalwaveguideJournal of Physics: Conference Series,2011,Volume:276,No.1012229ProcSPIE,作者(名次)时间李再东2008孙玉宝,马红梅,张志东3月孙玉宝(2)7月马红梅,王娜红,孙玉宝2月叶文江2008.9李志广2008.7第1张艳君2008.52张艳君2008安海龙(2)May-08刘铭1Feb-08刘铭1Aug-08刘铭1Jun-08刘铭1Aug-08安海龙(1)李佳120082008-3-1安海龙(1)Nov-08李佳12008王双进(第一作者)2008.1魏怀鹏(排名1)、李再东、安莉、张志东、展永,2008.7魏怀鹏(排名1)、张志东、展永2008.1魏怀鹏(排名1)、张志东、展永2008.11,温春东,魏怀鹏(排名2),段雪松,孔祥明,丁军锋,甄芳芳2008.1魏怀鹏(排名1)、张志东、展永2008.11魏怀鹏第一作者2008.4曹 天光1Jun-08ZHAO PeiDe et al第一作者(论文通讯联系人)任军12008.5任军12008.3任军1Jul-08任军12008年2月淮俊霞(第一作者)李佳120082008.6曹 天光1Jun-08Jun-08任军12008年10月周国香12008周国香12008周国香12008周国香12008周国香12008展永12008张志东,张艳君2008.1张志东,赵金良2008.1刘丽媛(学生),刘艳玲(学生),张志东2008.9盖翠丽甄晓玲王纪刚张志东李佳(1)2009李佳(1)2009李佳(1)2009李佳(1)2009李再东第二(通信作者)李再东第二2009张志东,范志新20092008.72008.6李再东第三(通信作者)刘铭(1)2009.03柳辉(1)2009.3柳辉(2)2009.7.Liu Hui (4)2009.7Liu Hui (4)2009.6Liu Hui (8)2009.8刘铭(5)2009任军(第一作者)2009年8月2009.03Liu Hui (1)2009.1任军(第一作者)2009年2月任军(第一作者)2009年7月马红梅,孙玉宝9月叶文江12009.1叶文江12009.1叶文江22009.8叶文江12009.3叶文江22009.11张勇(2)2009.03张玉红(第一)2009叶文江2任军(第一作者)Dec-092009.12张勇(1)2009.032009.03张勇(2)张玉红(第二)2009赵培德第一作者2010通讯联系人第一期赵培德第七2009赵培德第一作者通讯联系人赵培德第二作者通讯联系人周国香第二2009,1周国香第二2009,10周国香第二2009,6周国香第三2009,7周国香第三2009,8任军(1)2010年3月任军(1)2010年4月20092009周国香第三2009,5任军(1)2010年9月李再东,李秋艳,贺鹏斌,梁九卿,刘伍明,傅广生2010李秋艳,李再东,李录,傅广生2010李秋艳,李再东(*),姚淑芳,李录,傅广生2010李秋艳,李再东,贺鹏斌,宋伟为,傅广生。
DIBAL-H选择还原酯基而不影响苄溴

1250J.Med.Chem.2010,53,1250–1260DOI:10.1021/jm901530bSynthesis and Structure-Activity Relationships of Azamacrocyclic C-X-C Chemokine Receptor4 Antagonists:Analogues Containing a Single Azamacrocyclic Ring are Potent Inhibitors of T-Cell Tropic(X4)HIV-1ReplicationGary J.Bridger,*,†Renato T.Skerlj,†,)Pedro E.Hernandez-Abad,‡David E.Bogucki,†Zhongren Wang,†Yuanxi Zhou,†Susan Nan,†Eva M.Boehringer,†Trevor Wilson,†Jason Crawford,†Markus Metz,†,)Sigrid Hatse,§Katrien Princen,§Erik De Clercq,§and Dominique Schols§†AnorMED Inc.now Genzyme Corporation,500Kendall Street,Cambridge,Massachusetts02142,‡Johnson Matthey Pharmaceutical Research,1401King Road,West Chester,Pennsylvania19380,and§Rega Institute for Medical Research,Katholieke Universiteit Leuven, Minderbroedersstraat10,B-3000Leuven,Belgium.)Genzyme Corp.,153Second Avenue,Waltham,Massachusetts02451.Received October15,2009Bis-tetraazamacrocycles such as the bicyclam AMD3100(1)are a class of potent and selective anti-HIV-1agents that inhibit virus replication by binding to the chemokine receptor CXCR4,the coreceptor for entryof X4viruses.By sequential replacement and/or deletion of the amino groups within the azamacrocyclic ringsystems,we have determined the minimum structural features required for potent antiviral activity in thisclass of compounds.All eight amino groups are not required for activity,the critical amino groups on a perring basis are nonidentical,and the overall charge at physiological pH can be reduced without compromisingpotency.This approach led to the identification of several single ring azamacrocyclic analogues such asAMD3465(3d),36,and40,which exhibit EC50’s against the cytopathic effects of HIV-1of9.0,1.0,and4.0nM,respectively,antiviral potencies that are comparable to1(EC50against HIV-1of4.0nM).Moreimportantly,however,the key structural elements of1required for antiviral activity may facilitate the designof nonmacrocyclic CXCR4antagonists suitable for HIV treatment via oral administration.IntroductionThe development of antiviral agents that inhibit alternative targets in the HIV a-replicative cycle remains an important goal in order to alleviate the side effects of currently approved agents or to overcome the problem of drug resistance.In this regard,we have focused on the development of compounds that inhibit CXCR4,the coreceptor used by T-tropic(T-cell tropic)viruses for fusion and entry of HIV into target cells of the immune system.The corresponding chemokine receptor CCR5is used by M-tropic(macrophage tropic)viruses and has been associated with the early stages of infection and replication in HIV-positive patients.1,2The transition from M-tropic to T-tropic(or dual/mixed-tropic)virus during the course of HIV infection in approximately50%of patients is associated with a faster CD4þT-cell decline and a more rapid disease progression.3-5Recently,we reported the results of clinical trials with our prototype CXCR4antagonist AMD31006-8(1)and an orally bioavailable CXCR4antagonist,(S)-N0-(1H-benzimidazol-2-ylmethyl)-N0-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-dia-mine(AMD070).9-11When administered to HIV positive patients whose virus was confirmed to use CXCR4for viral entry,both agents were able to suppress the replication of CXCR4and dual-tropic strains of HIV.Similarly,the CCR5 antagonist Maraviroc suppresses replication of HIV-1that exclusively uses CCR5for entry12and was recently approved by the FDA for combined antiretroviral therapy in treatment-experienced patients.13A combination of CCR5and CXCR4 antagonists for treatment of dual/mixed-tropic HIV infection is therefore highly desirable.Beyond its use as a coreceptor for HIV,the CXCR4 chemokine receptor has a more fundamental role in the trafficking of white blood cells,which broadly express CXCR4.14,15A member of the superfamily of G-protein coupled receptors,the interaction of CXCR4and its ligand, stromal cell-derived factor-1(SDF-1),plays a central role in the homing and retention of cells within the bone marrow microenvironment.16Consistent with these observations,ad-ministration of1to healthy volunteers caused a dose-depen-dent leukocytosis6,7that in subsequent studies was shown to include the mobilization of CD34þstem and progenitor cells suitable for hematopoietic stem cell transplantation.17-20The ability of analogues of1to mobilize progenitors correlated with their in vitro capacity to inhibit SDF-1binding to CXCR4.21Because of the need for parenteral administration, 1was developed in combination with granulocyte colony-stimulating factor(G-CSF)to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin’s lymphoma(NHL)and multiple myeloma(MM).22-25Plerix-afor(1)was approved by the FDA in December2008.We have previously reported the structure-activity rela-tionships of anti-HIV bis-azamacrocycles and their transition*To whom correspondence should be addressed.Phone:617-429-7994.Fax:617-768-9809.E-mail:gary.bridger@.Ad-dress:Gary J.Bridger,Genzyme Corporation,55Cambridge Parkway,Cambridge MA02142.a Abbreviations:HIV,Human Immunodeficiency Virus;CXCR4,C-X-C chemokine receptor4;CCR5,C-C-R chemokine receptor5./jmc Published on Web12/31/2009r2009American Chemical SocietyArticle Journal of Medicinal Chemistry,2010,Vol.53,No.31251 metal complexes in detail.26-28Because of the commonstructural features between a doubly protonated cyclam(1,4,8,11-tetraazacyclotetradecane)ring present in1(at phy-siological pH)and a kinetically labile transition metal com-plex of cyclam with an overall charge ofþ2,we proposed thatboth structural motifs may bind to the CXCR4receptorthrough interactions with amino acid residues containingcarboxylate groups.29We have subsequently shown via direc-ted mutagenesis of the aspartate and glutamic acid residues inCXCR4that binding of1and related analogues to the seventransmembrane,G-protein coupled receptor is highly depen-dent upon the amino acids Asp171and Asp262,located intransmembrane region(TM)-IV and TM-VI at each end ofthe main ligand binding crevice of the receptor.30-35Mutationof either aspartic acid to aspargine significantly reduced theability of1to inhibit binding of radiolabeled stromal cellderived factor-1R(125I-Met-SDF-1R).More importantly,however,U87cells stably transfected with CD4and themutant coreceptors CXCR4[D171N]and CXCR4[D262N]were less effective at supporting infection of the CXCR4-usingHIV-1strain NL4.3compared to the wild-type receptor andthe double mutant CXCR4[D171N,D262N]completely failedas a coreceptor for HIV infection.31Correspondingly,theability of1to inhibit HIV-1infection via CXCR4[D171N]andCXCR4[D262N]was also diminished,thereby confirmingthat1binds in a region of the receptor that is critical for X4HIV-1coreceptor function.We have also reported that binding of the bis-Zn,Ni,andCu complexes of1were also dependent upon D171and D262of the receptor.36In a similar manner to1,the transitionmetal complexes were found to be less effective inhibitors of125I-Met-SDF-1R binding to the mutant receptors CXCR4-[D171N]and CXCR4[D262N]compared to the wild-typereceptor.Incorporation of Zn,Ni,or Cu into the cyclam ringsof1increased the affinity to the wild-type CXCR4receptor,but the enhancement was selectively eliminated by substitu-tion of Asp262.Supporting physiochemical evidence for theinteraction of acetate(carboxylates)with metal complexes ofazamacrocycles,including1,has been recently reported.37,38In the current study,we determine the minimum struc-tural features of1required for potent antiviral activity, leading to the identification of the single azamacrocyclic ring analogue AMD346532,33,39,40(3d)and ultimately the design of nonmacrocyclic,orally biovailable CXCR4an-tagonists.11,41,42Given the growing body of evidence that the CXCR4/SDF-1interaction is involved in regulating several human malignancies,43-45CXCR4antagonists may have additional therapeutic applications in addition to HIV treatment.ChemistryAnalogues containing a single1,4,8,11-tetraazacyclotetra-decane(cyclam)ring were prepared by modifications to previously published routes26,29as shown in Scheme1.Reac-tion of the selectively protected tris-diethylphosphoramidate (Dep)cyclam ring(2a)with R,R-dibromo-p-xylene in aceto-nitrile containing potassium carbonate gave the desired bro-momethyl intermediate(2b).Reaction of the bromide with an excess of the requisite amine,followed by deprotection of the Dep-groups with a saturated solution of hydrogen bromide in acetic acid at room temperature.gave analogues3a-i as the corresponding hydrobromide salts.To prepare analogues of3d in which the cyclam ring was replaced by a series of14-membered azamacrocyclic rings,we prepared a series of selectively protected macrocyclic ring systems containing a single(unprotected)secondary amine. This approach ensures the regiochemical outcome of the reaction with a benzylic halide during final construction (as shown in Scheme6).The syntheses of appropriate pre-cursors are shown in Schemes2-5.To incorporate fluorine groups at the desired position in the macrocyclic ring,suitably fluorinated bis-electrophiles were prepared,starting from 4-oxo-heptanedioic acid diethyl ester(4)and heptane-1,4,7-triol(8)as depicted in Scheme2.Reaction of the ketone(4) with neat(diethylamino)-sulfur trifluoride46,47(DAST)at room temperature for12days gave the corresponding di-fluoro-intermediate(5)in43%yield.Reduction of the ester groups with LAH(to give the diol6),followed by derivatiza-tion with toluenesulfonyl chloride,gave the bis-electrophile (7)required for the impending macrocyclization reaction.The corresponding monofluorinated intermediate was prepared in a similar manner.Protection of the primary alcohols in8as the acetyl group using acetic anhydride gave the secondary alcohol9,which was rapidly(and virtually quantitatively) converted to the fluorinated intermediate(10)with DAST (2.0equiv)in dichloromethane.Removal of the acetyl pro-tecting groups with saturated ammonia in methanol,followed by reaction of the diol(11)with p-toluenesulfonyl chloride, Scheme1aa Reagents:(a)R,R0-dibromo-p-xylene,K2CO3,CH3CN,reflux;(b)amine,K2CO3,CH3CN,reflux;(c)HBr,acetic acid,room temp. Scheme2aa Reagents:(a)Et2NSF3(neat),room temp;(b)LAH,Et2O;(c)Ts-Cl,Et3N,CH2Cl2;(d)acetic anhydride,pyridine;(e)Et2NSF3, CH2Cl2,-78°C,then room temp;(f)NH3/MeOH,room temp;(g)Ts-Cl,Et3N,CH2Cl2.1252Journal of Medicinal Chemistry,2010,Vol.53,No.3Bridger et al.gave the desired bis-electrophile 12containing a single fluorine group.The selectively protected azamacrocyclic rings were pre-pared via directed combinatorial macrocyclization of bis-2-nitrobenzenesulfonamides 48(Ns)(15a -c ,16a -c ,18)with bis-electrophiles (7,12,17)using previously optimized condi-tions 28(Scheme 3).To incorporate a phenyl or heterocyclic ring into the macrocycle,the corresponding bis-2-nitrobenze-nesulfonamide (15a -c )was prepared from the bis-aminoethyl intermediates 28(13a -c )by reaction with nosyl chloride (Et 3N,CH 2Cl 2).Similarly,16a ,b were obtained by reac-tion of commercially available intermediates 14a ,b with nosyl chloride or in the case of 16c (X=S)by reduction of 3,30-thiodipropionitrile with BH 33Me 2S and reaction of the intermediate diamine (14c )with nosyl chloride to give 16c .Macrocyclization was accomplished by dropwise addition of a DMF solution of the bis-electrophile to a DMF solution of the bis-2-nitrobenzenesulfonamide containing Cs 2CO 3maintained at a temperature of 80°C.Standard workup,followed by purification of the crude product by column chromatography on silica gel,gave the desired macrocycles 19a -c ,20a -c ,and 21a ,b in yields of 19-55%.Reaction of theintermediates from above with HBr/acetic acid at room temperature gave 22a -c ,23a -c ,and 24a ,b ,respectively.Because of synthetic convenience,we also prepared the selectively protected “isomers”of 22a ,b and 23a in which the alternative secondary amine was available for the alkylation reaction.We reasoned that reaction of 19a ,b and 20a with approximately 1equiv of thiophenol 49(our reagent of choice for nosyl deprotections)may allow pseudoselective deprotec-tion of a single nosyl group,leaving the Dep group intact.After some optimization,we found that reaction of 19a ,b and 20a with 0.8equiv of thiophenol and potassium carbonate in DMF (or acetonitrile)gave the precursors 25and 26a ,b in manageable,albeit modest yields (20-50%)following col-umn purification on silica gel (Scheme 4).Finally,the inter-mediates 27a ,b and 28(Scheme 5)were synthesized as recently described by palladium(0)catalyzed coupling of organozinc iodide reagents with bromopyridines.50Having completed the series of selectively protected aza-macrocycles,we proceeded to completion of the desired analogues by straightforward installation of the right-hand portion containing the aminomethyl pyridine moiety.As shown in Scheme 6,this was accomplished in all cases by direct alkylation of the available secondary amine of the macrocycle with the benzylic chlorides 34a ,b .Intermediate 34a was prepared in four steps from 4-bromomethyl benzoic acid methyl ester (29)and 2-aminomethylpyridine (31):con-version of 31to the 2-nitrobenzenesulfonamide 32,followed by alkylation with the benzyl bromide 30(obtained by reduc-tion of 29with DIBAL-H)gave the desired alcohol 33.As previously reported,28reaction of benzylic alcohols such as 33with methanesulfonyl chloride gave the chloride 34a rather than the corresponding mesylate,presumably via in situ nucleophilic substitution of the initially formed mesylate with chloride.Intermediate 34b (Scheme 6)containing a Dep-protecting group was prepared by an alternative synthesisScheme 3aaReagents:(a)Ns-Cl,Et 3N,CH 2Cl 2;(b)Cs 2CO 3,DMF,80°C;(c)HBr(g),AcOH,room temp.Scheme4Scheme5Article Journal of Medicinal Chemistry,2010,Vol.53,No.31253(procedures in Supporting Information).Alkylation of the available secondary amine of the macrocycles with 34a (or 34b )in CH 3CN in the presence of K 2CO 3gave the penultimate intermediates 35a -n .Deprotection of the nosyl groups with thiophenol and K 2CO 3in DMF gave the free base of the desired analogues,which in the vast majority of cases were converted to the corresponding hydrobromide salts.For analogues derived from the macrocyclic precursors 25and 26a ,b ,the intermediates isolated prior to the deprotection also contained a residual Dep group in addition to nosyl groups.For compound 45,we found that conversion to the hydro-bromide salt using a saturated solution of HBr in acetic acid resulted in concomitant deprotection of the remaining Dep group to obtain compound 45.For compounds 44and 46,the residual Dep group was removed prior to nosyl deprotection and salt formation.The thioether analogue 41a was also used to prepare the corresponding sulfoxide and sulfone analogues for antiviral evaluation as shown in Scheme 7.Initially,we globally protected the amino groups of 41a with Boc and subjected this intermediate to oxidation with oxone in MeOH 51at -10°C to give a mixture of the sulfoxide and sulfone that were separated by column chromatography on silica gel.However,while deprotection of the Boc groups with simulta-neous conversion to the hydrobromide salt proceeded without incident for the sulfone (to give 41c ),we found that deprotec-tion of the corresponding sulfoxide led to substantial reduc-tion and hence recovery of the starting analogue 41a .To overcome this problem,the sulfoxide was synthesized by direct oxidation of 41a with 1equiv of oxone in MeOH to give 41b in a 21%isolated yield and was subsequently tested as the free base in antiviral assays.Finally,we prepared a short series of analogues containing a carbon atom in place of a tertiary nitrogen group at the ring junction.To economize on the number of synthetic steps,weelected to synthesize the dimesylate 54(Scheme 8),an inter-mediate that could be commonly used for the synthesis of multiple analogues via macrocylization with the bis-2-nitro-benzenesulfonamide precursors already in our possession (namely 15a ,16a ,b from Scheme 3).Intermediate 54was prepared from the commercially available starting material bromo-p -tolunitrile via a double one-carbon homologation of the malonate 51,followed by derivatization to gave the requisite bis-methanesulfonate 54.Macrocyclizations of 54with bis-sulfonamides 15a and 16a ,b were performed as described above.Deprotection of the nosyl groups followed by conversion to the corresponding hydrobromide salts gave analogues 56and 58a ,b .DiscussionHaving previously established the optimum ring size and distance between the amines of both aliphatic andScheme 6a aReagents:(a)DIBAL-H,CH 2Cl 2;(b)Ns-Cl,Et 3N,CH 2Cl 2;(c)K 2CO 3,CH 3CN,60°C;(d)Ms-Cl,Et 3N,CH 2Cl 2;(e)K 2CO 3,CH 3CN,80°C;(f)R =Ns:thiophenol,K 2CO 3,DMF,or R =Dep:HBr(g),AcOH,room temp.Scheme 7aaReagents:(a)oxone,MeOH,-10°C;(b)(Boc)2O,THF;(c)HBr(g),AcOH,room temp.Scheme 8aaReagents:(a)NaH,R -bromo-tolunitrile,THF;(b)LiAlH 4,THF;(c)Ns-Cl,Et 3N,CH 2Cl 2;(d)2-picolyl chloride,Et 3N,K 2CO 3,KBr,CH 3CN,reflux;(e)Ms-Cl,Et 3N,CH 2Cl 2;(f)cetyltrimethyammonium bromide,NaCN,benzene,H 2O,reflux;(g)conc HCl/AcOH (4:1),reflux;(h)BH 3.Me 2S,THF;(i)Ms-Cl,Et 3N,CH 2Cl 2;(j)Cs 2CO 3,DMF,80°C;(k)thiophenol,K 2CO 3,CH 3CN (or DMF),40°C.1254Journal of Medicinal Chemistry,2010,Vol.53,No.3Bridger et al.pyridine-fused bis-tetraazamacrocycles required for potent X4anti-HIV activity,we designed a series of compounds to address the question of structural redundancy.The prototype bis-macrocycle 1has a center of symmetry and contains eight amino groups,of which four are positively charged at phy-siological pH.In the current study,we aimed to answer two specific questions:(1)Are all four positive charges required for potent anti-HIV activity?(2)On a per ring basis,what are the minimum structural requirements for activity?Assuming that the structural requirements are not iden-tical for both rings of 1,we reasoned that the simplest replacement for a single tetraaza-macrocyclic ring would be a pseudo diamine-segment,representing the first two amino groups of the macrocyclic ring from the point of attachment at the benzylic position.A judicious choice of “diamine”would also reduce the overall charge to þ1.Having previously established that the optimum distance between the first two amino groups was a two-carbon unit,we prepared a series of aminomethyl-substituted analogues in which the second amino group was a substituent upon an aromatic ring or part of a heterocyclic ring.In either case,the second p K a would be sufficiently low to prevent a second protonation at physiological pH.The compounds were tested for their ability to inhibit replication of HIV-1III B in MT-4cells,a strain of HIV-1that uses exclusively CXCR4for fusion and viral entry into target cells.The results are shown in Table 1.Compared to 1,the introduction of a benzylamine group (3a )in place of the azamacrocyclic ring substantially reduced anti-HIV potency,although the compound remained active at submicromolar concentrations.The concentration of 3a re-quired to inhibit HIV-1replication by 50%(the EC 50)was 0.49μM,which was approximately 100-fold higher than the 50%inhibitory concentration of 1.Aromatic amino groups at the 2-position (3b )or 4-position (3c )did not affect antiviral potency.Both 3b ,c exhibited comparable EC 50’s to the un-substituted benzyl group (3a ).However,we observed a sub-stantial increase in anti-HIV potency when the benzyl group was replaced by a pyridyl group (3d ).Compound 3d exhibited a 50%inhibitory concentration of 0.009μM,which was only ca.2-fold higher than the EC 50of 1.Furthermore,the 50%cytotoxic concentration (CC 50)of compound 3d in MT-4cells was greater than 112μM.Thus 3d exhibits a selectivity index of greater than 12000.The positional specificity of the pyridine-N in 3d was also examined.Replacement of the 2-pyridyl group with the 3-pyridyl (3e )or 4-pyridyl (3f )group had a detrimental effect on anti-HIV potency.For example,the EC 50’s of analogues 3e ,f were approximately 3orders of magnitude higher than the concentration of 3d required to inhibit HIV-1replication by 50%(the EC 50’s of 3e and 3f were 8.470and 9.977μM,respectively).Methylation of the amine in 3d (to give 3g )or extension of the connectivity to an aminoethyl pyridine group (to give 3h )also adversely affected the anti-HIV potency.Finally,we replaced the pyridine moiety with a comparable heterocycle of lower p K a than pyridine,namely the pyrazine group (3i ).Perhaps not surprisingly,the antiviral potency of analogue 3i was approximately comparable to the benzyl analogue 3a ,which did not contain a vicinal heterocycle nitrogen atom.With the optimized “right-hand”replacement for the aza-macrocycle ring of 1fixed as the 2-aminomethyl pyridine group,we then turned our attention to the “left-hand”ring.Needless to say,the mandatory synthesis of the symmetrical analogue in which both rings were replaced by a 2-amino-methyl pyridine group turned out to be a predictably fruitless exercise (EC 50was >250μM,data not shown).We therefore focused on systematically replacing individual amine groups of the left ring.As shown in Table 2,we first prepared an analogue in which the [14]aneN 4(cyclam)ring had been replaced by the optimized and equally suitable,py[iso -14]-aneN 4ring (to give compound 36).Consistent with the structure -activity relationship of py[iso -14]aneN 4bis-azama-crocycles,compound 36proved to be a potent inhibitor of HIV-1replication,exhibiting an EC 50of 0.001μM,that is,around 9-fold and 4-fold lower,respectively,than the con-centration of 3d or 1required to inhibit viral replication by 50%.Although the pyridine-N of the macrocyclic ring in 36was previously found to be critical for high antiviral potency,we reasoned that a precise determination of the pyridine-N contribution to potency could help redesign a less basic pounds 37and 38were then prepared to answer this question.Both analogues 37,containing a phenyl replacement and 38,containing an “exocyclic”pyridine fused group,retained reasonable anti-HIV potency (the EC 50’s of 37and 38were 0.040and 0.104μM,respectively)but were at least 40-to 100-fold less potent than analogue 36.So what role does the pyridine group play?At physiological pH,the overall charge of the py[iso -14]-aneN 4ring in 36is also þ2(in a similar manner to cyclam 52)and the likely protonation sequence is indicated in Figure 1A,based on the sequence reported by Delgado et al.53for similar 14-membered tetraazamacrocyclic rings contain-ing pyridine.Presumably,the secondary amino groups are predominantly protonated and the overall structure is stabi-lized by intramolecular hydrogen bond interactions from the adjacent hydrogen-bond acceptors,the pyridine and tertiary benzylic amine groups (while minimizing the elec-trostatic repulsion of two positive charges in a confined macrocyclic ring).This is confirmed by a conformational analysis of 36on B3LYP/6-31G*level followed by single point energy calculations.In the energetically most stable ring conformation (LMP2/6-311þG*þZPE),the pyridine nitro-gen forms two six-membered intramolecular hydrogen bond interactions with the two adjacent protonated nitrogens as shown in Figure 2.Potential five-membered intramolecular hydrogen bond interactions are formed with the tertiary amine.Table 1.Antiviral Activity of Single RingAzamacrocyclesnR 1R 2HIV-1(III B )EC 50(μM)MT-4cells CC 50(μM)3a 1H Ph0.4911603b 1H 2-amino-Ph 1.825243c 1H 4-amino-Ph 0.7172273d 1H 2-pyridine 0.009>1123e 1H 3-pyridine 8.470373f 1H 4-pyridine 9.977>2793g 1Me 2-pyridine 0.416383h 2H 2-pyridine 49.135>1103I 1H5-Me-pyrazine1.8957810.004>421ArticleJournal of Medicinal Chemistry,2010,Vol.53,No.31255The stabilization provided by this “shared”protonated structure could account for the high basicity of azamacrocyc-lic rings,as suggested by Kimura et al.54It did not seem unreasonable,therefore,that a potential role of the pyridine group is the contribution of a single intramolecular hydrogen-bond,which locks the conformation of the protonated aza-macrocyclic ring in manner that is beneficial to antiviral potency.To test this hypothesis,we prepared a series of analogues (depicted in Figure 1B,data in Table 2)in which the fused aromatic group had been removed and replaced by an aliphatic group,in some cases containing a hydrogen-bondacceptor at the key position “x,”the position occupied by the pyridine nitrogen in compound 36.Consistent with the hydrogen-bonding hypothesis,the alkyl analogue 39exhibited an anti-HIV potency that was compar-able to the phenyl and exocyclic pyridine analogues 37and 38(the EC 50’s of 37and 39,were 0.040and 0.043μM,re-spectively).This result categorically rules out the possibility that the conformational restrictions imposed by the fused aromatic groups in compounds 37,38were even partially responsible for the high potency of 36.However,incorpora-tion of a hydrogen-bond acceptor at position x (Figure 1B)in some cases restored activity comparable to 36.For example,the oxygen analogue 40exhibited an EC 50that was only 4-fold higher than the concentration of 36required to inhibit HIV-1replication by 50%(the EC 50of 40was 0.004μM).The corresponding thioether analogue 41a exhibited an EC 50of 0.013μM,which is approximately 3-fold higher than com-pound 40.Although the antiviral potency of the thioether analogue 41a compared to the ether analogue 41is greater than one would predict from the strength of the hydrogen-bond acceptor acceptor capabilities (thioether groups are considerably weaker H-bond acceptors than the oxygen inTable 2.Antiviral Activity of Single RingAzamacrocyclesFigure 1.Proposed hydrogen-bond structure of protonated aza-macrocycles.1256Journal of Medicinal Chemistry,2010,Vol.53,No.3Bridger et al.40),this result can be reconciled by considering the nature of the H-bond required;a six-membered intramolecular H-bond constrained by the macrocyclic ring (Figure 2).With the thioether compound 41a in hand,we also pre-pared the sulfoxide (41b )and sulfone (41c )analogues by direct oxidation of 41a .We reasoned that the oxygen atoms of the sulfoxide and sulfone are stronger H-bond acceptors than the sulfur atom of 41a and may consequently improve the anti-HIV potency.However,both 41b and 41c were considerably weaker antiviral agents,exhibiting 50%effective concentra-tions for inhibition of HIV-1replication that were at least 79-fold higher than the EC 50of 41a (the EC 50’s of 41b and 41c were 0.485and 11.878μM,respectively).The precise reason for the poor antiviral activity exhibited by analogues 41b ,c was unclear;although the sulfoxide and sulfone are more sterically demanding than the thioether and could induce a ring conformation that is detrimental to antiviral activity,we could not rule out the possibility that the H-bond acceptor oxygen is now “one-bond”outside of the ring,and the intramolecular H-bond itself induces an unfavorable confor-mation (a seven-membered ring H-bond in 41b ,c (Figure 2)compared to a six-membered in 41a ).To complete this series of compounds therefore,we decided to introduce the fluoro and difluoro substituents at position x (Figure 1B).Several reports have demonstrated that the fluoro group can partici-pate as an acceptor for intramolecular H-bonds,particularly within highly constrained ring structures.55-57This is also confirmed by our calculations,as shown in Figure 2.The fluoro (43)and difluoro (42)analogues were also attractive substituents for two other reasons:(1)the substituents would be situated at the fourth carbon from the adjacent amine group,thereby minimizing the affect on p K a ;(2)in a similar manner to the sulfoxide and sulfone,the H-bond acceptor would be one-bond outside of the macrocyclic ring.However in this case,because the fluorine atom in C -F groups is isostructural with hydrogen,a negative effect of the fluoro substituents on antiviral activity can only be attributed to an inappropriately positioned H-bond rather than steric requirements (that is,in the absence of an H-bond,we would expect the fluoro or difluoro analogues to exhibit an EC 50comparable to the methylene analogue 39).In antiviral test-ing,the fluoro (43)and difluoro (42)analogues displayed EC 50’s that were greater than 20-fold higher than the methy-lene analogue 39(the EC 50’s of 39,42,and 43were 0.043,0.920,and 1.239μM,respectively),confirming the negative consequences of an incorrectly positioned hydrogen-bond (Figure 2).Next,we focused on the sequence of aliphatic amine groups in the macrocyclic ring required for potent antiviral activity.By straightforward synthetic manipulation of our collection of ring systems,we prepared the structural isomers of analo-gues 36,37,and 39in which the side-chain (R,in Table 2)was connected to the alternative secondary amine group to give compounds 44,45,and 46.In antiviral testing,analogue 44was substantially less potent than its corresponding regioi-somer 39:the EC 50of 44was 11.131μM,which was approxi-mately 260-fold higher than the EC 50of 39.A similar loss of antiviral potency was observed with the phenyl analogue 46and its isomer 37(the EC 50’s of 46and 37were 14.106and 0.040μM,respectively).Interestingly,the loss of antiviral potency with the pyridine-fused isomer 45compared to 36was significant but not as substantial;the EC 50of 45was 0.063μM,around 60-fold higher than the concentration of 36required to inhibit HIV-1replication by 50%.There was a possibility,therefore,that while the “tri-aza”ring configura-tion required for potent antiviral activity is clearlyrepresentedFigure 2.Lowest energy conformations of compounds 36,40,41c ,and 42.View from top on a plane defined by three nitrogens and X (see Figure 1).Dashed lines indicate hydrogen bond interactions:the hydrogen bond acceptors in 36and 40are in one plane with the three nitrogens.This is not the case for 41c and 42.Bond angles:36:—(N 333H -N þ)=140.5°,122.4°,102.1°,108.4°.40:—(O 333H -N þ)=135.1°,141.5°;—(N 333H -N þ)=104.6°,102.8°.41c :—(O 333H -N þ)=112.8°,112.8°;—(N 333H -N þ)=108.2°,108.0°.42:—(F 333H -N þ)=142.2°,142.2°;—(N 333H -N þ)=114.7°,114.7°.。
生物医药专业英语词汇知识整合

⽣物医药专业英语词汇知识整合⽣物制药专业英语词汇Aabsolute lethal dose;LD100绝对致死剂量absorption rate constant吸收速率常数accelerated testing加速试验acetylcholinesterase⼄酰胆碱酯酶acetylcholine⼄酰胆碱acrylic acid resin丙烯酸树酯activation激活作⽤activator激活剂active targeting preparation主动靶向制剂acute toxicity test急性毒性实验additive effect累加效应additive附加剂adenosine phosphate腺苷磷酸adhersive strength粘附⼒adhesion粘附性adhesives粘合剂adjuvant佐剂adrenergic nerve肾上腺素能神经adrenergic receptor肾上腺素能受体adverse reaction不良反应aerogel⽓凝胶aerosil微粉硅胶aerosol of micropowders for inspiration吸⼊粉雾剂aethylis oleas油酸⼄酯agglomerate聚结物aggregation聚集air suspension空⽓悬浮法albumin microballoons⽩蛋⽩微球制剂alkaloid⽣物碱alkalosis;alkali-poisoning碱中毒allergy;allergic reaction变态反应allotted date of drug quality ensuring by manufacturer药品负责期all-trans全反式alterntae addition method两相交替加⼊法amebocyte lysate变形细胞溶解物amorphous forms⽆定型anaphylactic drug reaction过敏性药物反应anaphylatoxin过敏毒素anatoxin;toxoid类毒素angle of repose休⽌⾓antagonism拮抗作⽤antiadherent抗粘剂antibacterial spectrum抗菌谱antibody抗体antigen抗原antioxidants抗氧剂antipode对映体antisepesis防腐antiserum抗⾎清antitoxin抗毒素apparent solubility表现溶解度aprotinin抑酞酶aromatic compound芳族化合物aromatic waters芳⾹⽔剂Arrhenius⽅程阿仑尼乌斯⽅程artificial antigen⼈⼯合成抗原artificial immunization⼈⼯免疫aseptic technique⽆菌操作法astringent收敛药autoimmunity⾃⾝免疫Bbactericidal activity杀菌活性bactericidal effect杀菌作⽤bacteriophage噬菌体bacteriostatic activity抑菌活性bactriostasis抑菌作⽤ball mill球磨机base adsorption基质吸附率bases基质beeswax蜂蜡bending弯曲⼒bioavailability⽣物利⽤度bioavailability⽣物利⽤度biochemical approach⽣物学⽅法biochemistry⽣物化学biogenic amine⽣物胺biological half life⽣物半衰期biological product⽣物制品biometrics;biometry⽣物统计学biopharmacy⽣物药剂学blood coagulation⾎液凝固blood concentration⾎药浓度blood products⾎液制品blood volume expander⾎容量扩充剂blood-cerebral barrier⾎脑屏障body fluid体液body surface area体表⾯积bound water结合⽔分breakage(Bk)脆碎度broad-spectrum antibiotic⼴谱抗⽣素bulk density松密度、堆密度burst effect突释效应Ccaking结饼capillary state⽑细管状capsules胶囊剂carcinogenic test致癌实验carcinogen致癌物carrier载体catecholamine⼉茶酚胺CD圆⼆⾊谱法cellular immunity细胞免疫cellulose acetate(CA)醋酸纤维素chelating agent螯合剂chemical analysis化学分析chemical disinfection化学消毒法chemical physics化学物理学chemotherapy化学药物治疗chewable tablets咀嚼⽚chiral drug⼿性药物Chitosan壳聚糖chlinical pharmacy临床药学cholinesterase胆碱酯酶chronaxia;chronaxy时值chronic toxicity test;long term toxicity test慢性毒性实验chronopathology时⾠病理学chronopharmacology时⾠药理学chronosusceptability时间感受性chronotherapy时间治疗cipher prescription协定处⽅Clausius-Clapeyron⽅程克劳修斯-克拉珀龙⽅程clinical pharmaceutics临床药剂学clinical pharmacology临床药理学cloud point对聚氧⼄烯型⾮离⼦表⾯活性剂CMC-Na羧甲基纤维素纳CMS-Na羧甲基淀粉钠coagulation聚沉coated tablets包⾐⽚coating material表材cocoa butter可可⾖脂coefficient of diffusion扩散系数coenzyme辅酶cohesion凝聚性、粘着性cohesive strength内聚⼒cold compression method汽压法cold-homogenization冷却⼀匀化法cold-storage冷藏colon-targeted capsules结肠靶向胶囊剂compactibility成形性complement system补体系统complement补体complete antigen完全抗原complex coacervation复凝聚法complex solubilizer助溶剂compliance顺应性compressed tablets普通⽚compressibility压缩度compressibility压缩性compression压缩⼒compressive work压缩功concentration浓度cone and plate viscometer圆椎平板粘度计consistency curve稠度曲线content uniformity含量均匀度controllability可控性controlled release preparation控释制剂controlled release tablets控释⽚controlled-release preparation控释制剂convective mixing对流混合convective transport传递透过coordination number配位数core material表⼼物cosolvency潜溶cosolvent潜溶剂coulter counter method库尔特计数法count basis个数基准covalent bond共价键cracemization外消旋作⽤critical relative humidity(CRH)临界相对湿度critical velocity临界速度crude drugs;natural drugs天然药物crude drugs⽣药crushing粉碎crystal form晶型crystal habit晶态、晶癖、结晶习性cumulative size distribution累积分布cumulative urinary excretion curves累积尿排泄曲线cutting剪切⼒cyclodextrin(CYD)环糊精cylinder model圆栓体模型cytotoxic hexitols⼰糖醇细胞毒剂cytotoxicity细胞素Ddecoction汤剂degree of circularity圆形度degree of sphericility球形度delipidization⾓质层去脂质化desiccant;drying agent⼲燥剂detoxication解毒作⽤dextrin糊精dextrorotatory form右旋体dextrose右旋糖dialysis cell method渗析池法dicetyl phosphate磷酸⼆鲸蜡脂dielectric constant介电常数differential scanning calorimetry DSC差⽰扫描显热法Differential thermal analysis DTA差⽰热分析法diffusion扩散diffusive mixing扩散混合dilatant flow胀性流动diluents稀释剂、填充剂dimethicone(silicones)⼆甲基硅油、硅油、硅酮directed pharmaceutical preparations定向药物制剂discontinuous sterilization 间歇灭菌法disinfection消毒disintegrants崩解剂disintegration崩解度disk assemble method圆盘法dispensing pharmacy调剂学disperse medium分散介质disperse phase分散相disperse system分散体系dispersed phase分散相、内相、⾮连续相dispersible tablets分散⽚displacement value(DV)置换价dissolution;dissolving溶解distilled water蒸馏⽔DLVO理论引⼒势能与斥⼒势能DME⼆甲醚DMSO⼆甲基亚矾dosage form剂型dosage regimen or dose rate给药⽅案或给药速度dosage;dose剂量dose or concentration dependency剂量或浓度的依存性dosing interval给药间隔double-blind technique双盲法drop dentifrices滴⽛剂drug absorption药物吸收drug accumulation药物蓄积drug administration law药品管理法drug batch number药品批号drug carrier药物载体drug combination合并⽤药drug distribution药物分布drug elimination药物消除drug excretion药物排泄drug interaction药物相互作⽤drug metablic enzyme药物代谢酶drug metabolism药物代谢drug reaction药物反应drug sensitive test药敏试验Drug Standard of Ministry of Public Health of the People's Republic of China中华⼈民共和国卫⽣部药品标准drug standard药品质量标准drug tolerance耐药性drug-induced diseases药源性疾病drug-loading rate载药量drug-time curve药—时曲线dry bulb temperature⼲球温度dumping effect突释效应Eear drops滴⽿剂effective concentration有效浓度effective halt有效半衰期effective rate有效率effectiveness有效性effector效应器effector效应物effect效应effervescent disintegrants泡腾崩解剂effervescent tablets泡腾⽚elastic deformation弹性变形elastic recovery(ER)弹性复原率elastic work弹性功elasticity弹性electrolyte电解质electrolyzation电解electroporesis电致孔法electuary煎膏剂elimination rate constant消除速率常数emulsifer in water method⽔中乳化剂法、湿胶法emulsifier in oil method油中乳化剂法、⼲胶法emulsions乳剂emulsion普通乳enamine烯胺endocytosis内呑endotoxin内毒素enteric coated tablets肠溶⾐⽚enteric coating肠溶⾐enteric controlled release tablets肠溶控释⽚enterohepatic circulation肠肝循环entrapment rate包封率environmental pharmacology环境药理学epidermis表⽪epimerization差向异构作⽤equilibrium solubility平衡溶解度equilibrium water平衡⽔分essential aminoacid必需氨基酸essential drugs基本药物essential fatty acid必需脂肪酸ethical(prescription)drug处⽅药ethnopharmacology⼈种药理学ethycellulose(EC)⼄基纤维素etiological treatment对因治疗evaporation蒸发excipients辅料excitability兴奋性exotoxin外毒素expiry date;date of expiration药品有效期external phase分散介质、外相、连续相extracts浸膏剂extravascular administration⾎管外给药eye drop滴眼剂eye ointments眼膏剂Ffactorial design析因设计fatal dose;lethal dose致死量fatty oils脂肪油fermentation发酵fillers填充剂film coated tablets薄膜⾐⽚film dispersion method薄分散法film-coating薄膜⾐films膜剂filter aid助滤剂first pass effect of hepar肝⾸过效应first-pass effect⾸过效应fliud extracts流浸膏剂flocculation value絮凝度flocculation絮凝flow curve流动曲线flow velocity流出速度flowability流动性fluid-energy mills流能磨、⽓流式粉碎机fluidity buffer流动性缓冲剂fluidized bed coating流化床包⾐法free water⾃由⽔分freely movable liquid⾃由流动液体freezing;refrigeration冷冻frequency size distribution频率分布funicular state索带状fusion融合Ggas analysis⽓体分析gas permeability method⽓体透过法GCP药物临床试验管理规范gelatin glycerin⽢油胫胶gelatinization糊化gelatin明胶general acid-base catalysis⼴义酸碱催化Geneva nomenclature⽇内⽡命名法geometric diameter⼏何学粒⼦径geometric isomerization⼏何异构ghost cell影细胞glidants助流剂GLP药物⾮临床研究管理规范gluconeogenesis糖异⽣作⽤glycerins⽢油剂glyceryl monostearate硬脂酸、⽢油酯glycolic acid羟基⼄酸glycolysis酵解GMP药品⽣产质量管理规范granule density颗粒密度granules颗粒剂growth curve⽣长曲线guest molecules客分⼦Hhalf lethal dose;median lethal dose;LD50半数致死剂量half-life period;half life time半衰期halogenide卤化物hard capsules硬胶囊剂hardness硬度hemolysis溶⾎histamine组胺holonzyme and prosthetic group全酶与辅基hormone激素host molecules主分⼦humidity湿度humoral immunity体液免疫hydration of stratum corneum⾓质层的⽔化作⽤hydrogel⽔性凝胶hydrolysis⽔解(作⽤)hydrophile-lipophile balance亲⽔亲油平衡值hydrotropy agent助溶剂hydrotropy助溶hydroxypropyl methylcellulose羟丙甲纤维素hygroscopicity吸湿性hyperreactivity⾼敏性hypodermic tablets⽪下注射⽤⽚IIDDS植⼊给药系统IEC离⼦交换⾊谱法IEF等电点聚焦immobile liquid不可流动液体immunoenhancement免疫增强剂immunogenicity免疫原性immunosuppressant;immuno inhibitor免疫抑制剂impact mill冲击式粉碎机impact冲击⼒implant tablets植⼊⽚implants埋植剂inclusion compound包含物incomplete antigen不完全抗原indirect carcinogenesis间接致癌individual differences;individual variation个体差异性industrial pharmacy⼯业药剂学。
免疫球蛋白(Ig)
正常血清蛋白电泳图
Albumin
-globin -globin -globin
Immune sera Normal sera
Two basic definitions
Antibody (Ab) IApmlatysmpmeuaonf cogegllollbaousbliuanlirwnehssiuc(lhtIiogsf)ptrhoeduced by
6. Secretory Piece,SP IgA dimer
(1)chemical nature:
Polypeptide(70kD)
(2)function: ①. enabling IgA to be transported across mucosal tissues into secretions. ② . protecting sIgA from being proteolytic attack.
Radioimmunoassay
Koler,Milstein Monoclonal Ab
& Jerne
Tonegawa Ig gene rearrangement
Discovery of Ab
1890: von Behring and Kitasato -ANTIBODIES
in the serum of vaccinated individual bind to pathogens
Fab(Ag-binding Fragment)
Fc(crystallizable Fragment):
pepsin
Fc
pFc
(Fab’)2 pFc(peptides of Fc)
蛋白酶水解片段
木瓜蛋白酶 (papain) 2 Fab,1 Fc
抗蚀剂 英语
抗蚀剂英语Corrosion InhibitorsCorrosion is a ubiquitous phenomenon that affects a wide range of materials and industries. It is a natural process in which materials, primarily metals, undergo gradual deterioration and degradation due to chemical or electrochemical reactions with their surrounding environment. Corrosion can have devastating consequences, leading to the failure of critical infrastructure, the loss of valuable assets, and the potential for environmental damage. To mitigate the detrimental effects of corrosion, the use of corrosion inhibitors has become a crucial strategy in various applications.Corrosion inhibitors are chemical substances that, when added in small concentrations to a corrosive environment, effectively reduce or prevent the rate of corrosion. These inhibitors work by forming a protective layer on the surface of the metal, which acts as a barrier against the corrosive agents. The mechanism by which corrosion inhibitors function can be categorized into three main types: anodic, cathodic, and mixed inhibitors.Anodic inhibitors work by suppressing the anodic reaction, which isthe oxidation of the metal. They form a passivating film on the metal surface, preventing the dissolution of the metal ions. Examples of anodic inhibitors include chromates, phosphates, and silicates. Cathodic inhibitors, on the other hand, target the cathodic reaction, which is the reduction of oxygen or hydrogen ions. They form a protective layer on the cathode, hindering the cathodic process and slowing down the overall corrosion rate. Common cathodic inhibitors include zinc salts, magnesium salts, and rare earth compounds. Mixed inhibitors, as the name suggests, employ a combination of both anodic and cathodic inhibition mechanisms to provide comprehensive protection against corrosion.The selection of an appropriate corrosion inhibitor depends on various factors, such as the nature of the metal, the corrosive environment, the desired level of protection, and the cost-effectiveness of the inhibitor. In some cases, a single inhibitor may not be sufficient, and a combination of different inhibitors is used to achieve the desired level of corrosion protection.One of the most widely used corrosion inhibitors is chromate, which has been extensively employed in various industries due to its excellent inhibition properties. Chromate inhibitors form a stable, adherent oxide film on the metal surface, effectively preventing the initiation and propagation of corrosion. However, concerns have been raised about the environmental and health impacts ofchromate-based inhibitors, as they contain hexavalent chromium, which is a known carcinogen. This has led to the development of alternative, more environmentally friendly corrosion inhibitors.In recent years, there has been a growing interest in the use of organic corrosion inhibitors, which are derived from natural sources or synthetic compounds. These inhibitors are often less toxic and more environmentally friendly compared to traditional inorganic inhibitors. Organic inhibitors can be further classified into several categories, including amine-based, imidazoline-based, and heterocyclic compounds. These inhibitors work by adsorbing onto the metal surface, forming a protective film that prevents the access of corrosive agents.Another emerging class of corrosion inhibitors is the use of nanoparticles and nanomaterials. Nanoparticle-based inhibitors offer unique advantages, such as enhanced surface coverage, targeted delivery, and the ability to incorporate multiple active components within a single nanostructure. These nanoparticle-based inhibitors have shown promising results in various applications, including the protection of steel, aluminum, and copper alloys.The development and application of corrosion inhibitors are not limited to traditional industries. In recent years, there has been a growing interest in the use of corrosion inhibitors in the renewableenergy sector, particularly in the protection of solar panels and wind turbine components. Corrosion can significantly reduce the efficiency and lifespan of these critical infrastructure, making the use of effective corrosion inhibitors essential for the long-term sustainability of renewable energy systems.In addition to their industrial applications, corrosion inhibitors also play a crucial role in the protection of cultural heritage artifacts and historical monuments. These valuable assets are often exposed to various environmental factors, such as humidity, pollutants, and salts, which can accelerate the deterioration of the materials. The use of corrosion inhibitors, combined with other conservation techniques, helps to preserve the integrity and longevity of these irreplaceable cultural treasures.The future of corrosion inhibitors holds great promise, as researchers and industries continue to explore new and innovative approaches to address the challenge of corrosion. The development of smart, self-healing corrosion inhibitors, the integration of corrosion inhibitors with advanced materials, and the exploration of sustainable and eco-friendly inhibitor formulations are just a few examples of the ongoing advancements in this field.In conclusion, corrosion inhibitors are essential tools in the fight against the detrimental effects of corrosion. They provide a cost-effective and efficient means of protecting a wide range of materials and assets, from industrial infrastructure to cultural heritage. As the demand for corrosion protection continues to grow, the development and application of innovative corrosion inhibitors will play a crucial role in ensuring the long-term sustainability and resilience of our built environment and technological systems.。
二氟尼柳美国药典
Diflunisal Tablets» Diflunisal Tablets contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of C13H8F2O3.Packaging and storage— Preserve in well-closed containers.USP Reference standards 11—USP Diflunisal RS.Identification—A: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that of the Standard preparation, obtained as directed in the Assay.B: Transfer a quantity of finely ground Tablets, equivalent to about 100 mg of diflunisal, to a 10-mL volumetric flask, add 2 mL of water, and sonicate for 5 minutes. Dilute with methanol to volume, sonicate for an additional 5 minutes, mix, and filter. Separately apply 10 µL each of the filtrate and a Standard solution of USP Diflunisal RS in methanol solution (4 in 5) containing 10 mg per mL to a thin-layer chromatographic plate (see Chromatography 62) coated with a 0.25-mm layer of chromatographic silica gel mixture.Develop the chromatogram in a solvent system consisting of n-hexane, glacial acetic acid, and chloroform (17:3:2) until the solvent front has moved aboutthree-fourths of the length of the plate. Remove the plate from the chamber, air-dry, and examine under long-wavelength UV light: the RF value of the principal spot in the chromatogram of the test solution corresponds to that obtained from the Standard solution.Dissolution 71—pH 7.20, 0.1 M Tris buffer— Dissolve 121 g of tris (hydroxymethyl) aminome thane (THAM) in 9 liters of water. Adjust the solution with a 7 in 100 solution of anhydrous citric acid in water to a pH of 7.45, at 25. Dliters, equilibrate to 37, a H of 7.20, if necessary.Medium: pH 7.20, 0.1 M Tris buffer; 900 mL.Apparatus 2: 50 rpm.Time: 30 minutes.Procedure— Determine the amount of C13H8F2O3 dissolved from UV absorbances at the wavelength of maximum absorbance at about 306 nm of filtered portions of the solution under test, suitably diluted with pH 7.20, 0.1 M Tris buffer, in comparison with a Standard solution having a known concentration of USP Diflunisal RS in the same Medium.Tolerances— Not less than 80% (Q) of the labeled amount of C13H8F2O3 is dissolved in 30 minutes.Uniformity of dosage units 90: mProcedure for content uniformity—Transfer 1 finely powdered Tablet to a200-mL volumetric flask, add 50 mL of water, shake by mechanical means for 30 minutes, and sonicate for 2 minutes. Add 100 mL of alcohol to the flask, shake by mechanical means for 15 minutes, and sonicate for 2 minutes. Dilute with alcohol to volume, mix, and centrifuge a portion of the solution. Quantitatively dilute an accurately measured volume of the resultant clear supernatant with alcohol, if necessary, to obtain a test solution containing about 1.25 mg per mL. Transfer about 125 mg of USP Diflunisal RS, accurately weighed, to a 100-mL volumetric flask, add 75 mL of alcohol to dissolve, dilute with water to volume, and mix to obtain the Standard solution. Transfer 3.0 mL each of the Standard solution and the test solution to separate 50-mL volumetric flasks. To each flask add 5.0 mL of a solution containing 1 g of ferric nitrate in 100 mL of 0.08 N nitric acid, dilute with water to volume, and mix. Concomitantly determine the absorbances of the solutions at the wavelength of maximum absorbance at about 550 nm, with a suitable spectrophotometer, using water as the blank. Calculate the quantity, in mg, of C13H8F2O3 in the Tablet by the formula:(TC / D)(AU / AS)in which T is the labeled quantity, in mg, of diflunisal in the Tablet; C is the concentration, in µg per mL, of USP Diflunisal RS in the Standard solution; D is the concentration, in µg per mL, of diflunisal in the test solution, based uponthe labeled quantity per Tablet and the extent of dilution; and AU and AS are the absorbances of the solutions from the test solution and the Standard solution, respectively.Assay—Mobile phase—Prepare a suitable degassed mixture of water, methanol, acetonitrile, and glacial acetic acid (45:40:17:6) such that the retention time of diflunisal is about 8 minutes.Standard preparation— Dissolve a suitable quantity of USP Diflunisal RS in a mixture of acetonitrile and water (60:40) to obtain a solution having a known concentration of about 1.0 mg per mL.Assay preparation—Weigh and finely powder not fewer than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 100 mg of diflunisal, to a 100-mL volumetric flask containing about 5 mL of water. Sonicate for 5 minutes, add 60.0 mL of acetonitrile, sonicate for an additional 5 minutes, dilute with water to volume, mix, and filter.Chromatographic system (see Chromatography 62)—The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm × 30-cm column that contains packing L1.The flow rate is about 2.0 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor for the analyte peak is not more than 2.0, and the relative standard deviation for replicate injections is not more than 2.0%.Procedure— Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of diflunisal (C13H8F2O3) in the portion of Tablets taken by the formula:100C(rU / rS)in which C is the concentration, in mg per mL, of USP Diflunisal RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.。
欧洲药典索引版3
EUROPEAN PHARMACOPOEIA5.5INDEXTo aid users the index includes a reference to the supplement where the latest version of a text can be found.For example:Acetone...............................................5.1-2875means the monograph Acetone can be found on page2875of Supplement5.1.Note that where no reference for a supplement is made,the text can be found in the principal volume.Monographs deleted from the5th edition are not included in the index;the list of deleted texts is found in the Contents of this supplement,page xxx.EUROPEAN PHARMACOPOEIA5.5Numerics1.1.General statements (5)1.2.Other provisions applying to general chapters and monographs (5)1.3.General chapters (6)1.4.Monographs (7)1.5.Abbreviations and symbols (9)1.6.Units of the International System(SI)used in the Pharmacopoeia and equivalence with other units (10)1.General notices (5)2.1.1.Droppers (17)parative table of porosity of sintered-glass filters (17)2.1.3.Ultraviolet ray lamps for analytical purposes (17)2.1.4.Sieves (18)2.1.5.Tubes for comparative tests (19)2.1.6.Gas detector tubes (19)2.1.Apparatus (17)2.2.10.Viscosity-Rotating viscometer method.........5.3-3337 2.2.11.Distillation range (30)2.2.12.Boiling point (31)2.2.13.Determination of water by distillation (32)2.2.14.Melting point-capillary method (32)2.2.15.Melting point-open capillary method (33)2.2.16.Melting point-instantaneous method (33)2.2.17.Drop point (33)2.2.18.Freezing point (34)2.2.19.Amperometric titration (34)2.2.1.Clarity and degree of opalescence of liquids (23)2.2.20.Potentiometric titration (35)2.2.21.Fluorimetry (35)2.2.22.Atomic emission spectrometry (35)2.2.23.Atomic absorption spectrometry (36)2.2.24.Absorption spectrophotometry,infrared (37)2.2.25.Absorption spectrophotometry,ultraviolet and visible.................................................................................5.2-3089 2.2.26.Paper chromatography. (40)2.2.27.Thin-layer chromatography...............................5.2-3090 2.2.28.Gas chromatography.. (42)2.2.29.Liquid chromatography (43)2.2.2.Degree of coloration of liquids (24)2.2.30.Size-exclusion chromatography (45)2.2.31.Electrophoresis (45)2.2.32.Loss on drying (50)2.2.33.Nuclear magnetic resonance spectrometry (51)2.2.34.Thermal analysis (52)2.2.35.Osmolality (54)2.2.36.Potentiometric determination of ionic concentration using ion-selective electrodes (55)2.2.37.X-ray fluorescence spectrometry (56)2.2.38.Conductivity.........................................................5.1-2783 2.2.39.Molecular mass distribution in dextrans (57)2.2.3.Potentiometric determination of pH (26)2.2.40.Near-infrared spectrophotometry (59)2.2.41.Circular dichroism (63)2.2.42.Density of solids (64)2.2.43.Mass spectrometry (65)2.2.44.Total organic carbon in water for pharmaceutical use (68)2.2.45.Supercritical fluid chromatography (68)2.2.46.Chromatographic separation techniques (69)2.2.47.Capillary electrophoresis (74)2.2.48.Raman spectrometry (79)2.2.49.Falling ball viscometer method (80)2.2.4.Relationship between reaction of solution, approximate pH and colour of certain indicators (27)2.2.54.Isoelectric focusing (81)2.2.55.Peptide mapping (82)2.2.56.Amino acid analysis.......................................................862.2.5.Relative density.. (27)2.2.6.Refractive index (28)2.2.7.Optical rotation......................................................5.4-3695 2.2.8.Viscosity (29)2.2.9.Capillary viscometer method (29)2.2.Physical and physicochemical methods (23)2.3.1.Identification reactions of ions and functional groups...............................................................................5.5-4101 2.3.2.Identification of fatty oils by thin-layer chromatography. (98)2.3.3.Identification of phenothiazines by thin-layer chromatography (99)2.3.4.Odour (99)2.3.Identification (95)2.4.10.Lead in sugars (107)2.4.11.Phosphates (108)2.4.12.Potassium (108)2.4.13.Sulphates (108)2.4.14.Sulphated ash......................................................5.3-3341 2.4.15.Nickel in polyols.. (108)2.4.16.Total ash (108)2.4.17.Aluminium (108)2.4.18.Free formaldehyde (109)2.4.19.Alkaline impurities in fatty oils (109)2.4.1.Ammonium (103)2.4.21.Foreign oils in fatty oils by thin-layer chromatography (109)position of fatty acids by gas chroma-tography (110)2.4.23.Sterols in fatty oils..............................................5.1-2787 2.4.24.Identification and control of residual solvents (113)2.4.25.Ethylene oxide and dioxan (118)2.4.26.N,N-Dimethylaniline (119)2.4.27.Heavy metals in herbal drugs and fatty oils (119)2.4.28.2-Ethylhexanoic acid (120)position of fatty acids in oils rich inomega-3-acids...................................................................5.5-4107 2.4.2.Arsenic (103)2.4.30.Ethylene glycol and diethylene glycol in ethoxylated substances........................................................................5.2-3095 2.4.3.Calcium.. (103)2.4.4.Chlorides (104)2.4.5.Fluorides (104)2.4.6.Magnesium (104)2.4.7.Magnesium and alkaline-earth metals (104)2.4.8.Heavy metals (104)2.4.9.Iron (107)2.4.Limit tests (103)2.5.10.Oxygen-flask method (130)plexometric titrations (130)2.5.12.Water:semi-micro determination (130)2.5.13.Aluminium in adsorbed vaccines (131)2.5.14.Calcium in adsorbed vaccines (131)2.5.15.Phenol in immunosera and vaccines (131)2.5.16.Protein in polysaccharide vaccines (131)2.5.17.Nucleic acids in polysaccharide vaccines (132)2.5.18.Phosphorus in polysaccharide vaccines (132)2.5.19.O-Acetyl in polysaccharide vaccines (132)2.5.1.Acid value................................................................5.2-3099 2.5.20.Hexosamines in polysaccharide vaccines. (132)2.5.21.Methylpentoses in polysaccharide vaccines (133)2.5.22.Uronic acids in polysaccharide vaccines (133)2.5.23.Sialic acid in polysaccharide vaccines (133)2.5.24.Carbon dioxide in gases (134)2.5.25.Carbon monoxide in gases (134)2.5.26.Nitrogen monoxide and nitrogen dioxide in gases (135)2.5.27.Oxygen in gases (136)2.5.28.Water in gases (136)2.5.29.Sulphur dioxide (136)2.5.2.Ester value (127)2.5.30.Oxidising substances (137)2.5.31.Ribose in polysaccharide vaccines (137)2.5.32.Water:micro determination (137)2.5.33.Total protein (138)2.5.34.Acetic acid in synthetic peptides (141)2.5.35.Nitrous oxide in gases (141)2.5.36.Anisidine value (142)2.5.3.Hydroxyl value (127)2.5.4.Iodine value (127)2.5.5.Peroxide value (128)2.5.6.Saponification value (129)2.5.7.Unsaponifiable matter (129)2.5.8.Determination of primary aromaticamino-nitrogen (129)2.5.9.Determination of nitrogen by sulphuric acid digestion (129)2.5.Assays (127)2.6.10.Histamine (153)2.6.11.Depressor substances (153)2.6.12.Microbiological examination of non-sterile products (total viable aerobic count) (154)2.6.13.Microbiological examination of non-sterile products (test for specified micro-organisms) (156)2.6.14.Bacterial endotoxins (161)2.6.15.Prekallikrein activator........................................5.5-4111 2.6.16.Tests for extraneous agents in viral vaccines for human use (169)2.6.17.Test for anticomplementary activity of immunoglobulin (170)2.6.18.Test for neurovirulence of live virus vaccines (172)2.6.19.Test for neurovirulence of poliomyelitis vaccine (oral) (172)2.6.1.Sterility (145)2.6.20.Anti-A and anti-B haemagglutinins(indirect method) (174)2.6.21.Nucleic acid amplification techniques............5.5-4111 2.6.22.Activated coagulation factors...........................5.5-4115 2.6.24.Avian viral vaccines:tests for extraneous agents in seed lots............................................................................5.4-3699 2.6.25.Avian live virus vaccines:tests for extraneous agents in batches of finished product.....................................5.3-3345 2.6.26.Test for anti-D antibodies in human immunoglobulin for intravenous administration....................................5.3-3348 2.6.2.Mycobacteria. (149)2.6.7.Mycoplasmas (149)2.6.8.Pyrogens (152)2.6.9.Abnormal toxicity (153)2.6.Biological tests (145)2.7.10.Assay of human coagulation factor VII (203)2.7.11.Assay of human coagulation factor IX............5.5-4120 2.7.12.Assay of heparin in coagulation factors (204)2.7.13.Assay of human anti-D immunoglobulin (205)2.7.14.Assay of hepatitis A vaccine..............................5.1-2795 2.7.15.Assay of hepatitis B vaccine(rDNA). (207)2.7.16.Assay of pertussis vaccine(acellular) (208)2.7.17.Assay of human antithrombin III (209)2.7.18.Assay of human coagulation factor II (209)2.7.19.Assay of human coagulation factor X (210)2.7.1.Immunochemical methods (187)2.7.20.In vivo assay of poliomyelitis vaccine (inactivated) (210)2.7.21.Assay of human von Willebrand factor...........5.5-4120 2.7.22.Assay of human coagulation factor XI............5.5-4121 2.7.2.Microbiological assay of antibiotics. (188)2.7.4.Assay of human coagulation factor VIII...........5.5-4119 2.7.5.Assay of heparin.. (195)2.7.6.Assay of diphtheria vaccine(adsorbed).....................1962.7.7.Assay of pertussis vaccine (197)2.7.8.Assay of tetanus vaccine(adsorbed)..................5.1-2791 2.7.9.Test for Fc function of immunoglobulin. (202)2.7.Biological assays (187)2.8.10.Solubility in alcohol of essential oils (216)2.8.11.Assay of1,8-cineole in essential oils (216)2.8.12.Determination of essential oils in vegetable drugs (217)2.8.13.Pesticide residues (218)2.8.14.Determination of tannins in herbal drugs (221)2.8.15.Bitterness value (221)2.8.16.Dry residue of extracts (222)2.8.17.Loss on drying of extracts (222)2.8.1.Ash insoluble in hydrochloric acid (215)2.8.2.Foreign matter (215)2.8.3.Stomata and stomatal index (215)2.8.4.Swelling index (215)2.8.5.Water in essential oils (216)2.8.6.Foreign esters in essential oils (216)2.8.7.Fatty oils and resinified essential oils in essential oils (216)2.8.8.Odour and taste of essential oils (216)2.8.9.Residue on evaporation of essential oils (216)2.8.Methods in pharmacognosy (215)2.9.10.Ethanol content and alcoholimetric tables (237)2.9.11.Test for methanol and2-propanol...................5.3-3362 2.9.12.Sieve test (239)2.9.13.Limit test of particle size by microscopy (239)2.9.14.Specific surface area by air permeability (239)2.9.15.Apparent volume (241)2.9.16.Flowability (242)2.9.17.Test for extractable volume of parenteral preparations.....................................................................5.3-3363 2.9.18.Preparations for inhalation:aerodynamic assessment of fine particles...............................................................5.2-3103 2.9.19.Particulate contamination:sub-visible particles (253)2.9.1.Disintegration of tablets and capsules..............5.3-3351 2.9.20.Particulate contamination:visible particles. (255)2.9.22.Softening time determination of lipophilic suppositories (256)2.9.23.Pycnometric density of solids (257)2.9.24.Resistance to rupture of suppositories and pessaries (258)2.9.25.Dissolution test for medicated chewing gums..................................................................................5.2-3116 2.9.26.Specific surface area by gas adsorption.........5.1-2811 2.9.27.Uniformity of mass of delivered doses from multidose containers. (263)2.9.28.Test for deliverable mass or volume of liquid and semi-solid preparations (263)2.9.29.Intrinsic dissolution............................................5.4-3705 2.9.2.Disintegration of suppositories and pessaries (227)2.9.36.Powder flow..........................................................5.3-3363 2.9.37.Optical microscopy..............................................5.3-3366 2.9.38.Particle-size distribution estimation by analytical sieving...............................................................................5.3-3368 2.9.3.Dissolution test for solid dosage forms............5.3-3353 2.9.40.Uniformity of dosage units................................5.3-3370 2.9.42.Dissolution test for lipophilic solid dosage forms..................................................................................5.3-3373 2.9.4.Dissolution test for transdermal patches (231)2.9.5.Uniformity of mass of single-dose preparations (233)2.9.6.Uniformity of content of single-dose preparations..234 2.9.7.Friability of uncoated tablets..............................5.2-3103 2.9.8.Resistance to crushing of tablets.. (235)2.9.9.Measurement of consistency by penetrometry (235)2.9.Pharmaceutical technical procedures (225)3.1.10.Materials based on non-plasticised poly(vinyl chloride) for containers for non-injectable,aqueous solutions (289)3.1.11.Materials based on non-plasticised poly(vinyl chloride)for containers for dry dosage forms for oral administration..........................................................................2913.1.1.1.Materials based on plasticised poly(vinyl chloride)for containers for human blood and blood components. (269)3.1.1.2.Materials based on plasticised poly(vinyl chloride)for tubing used in sets for the transfusion of blood andblood components (272)3.1.13.Plastic additives (293)3.1.14.Materials based on plasticised poly(vinyl chloride)for containers for aqueous solutions for intravenous infusion......................................................................................2963.1.15.Polyethyleneterephthalatefor containers forpreparations not for parenteral use.....................................2983.1.1.Materials for containers for human blood and blood components. (269)3.1.3.Polyolefines (274)3.1.4.Polyethylene without additives for containers for parenteral preparations and for ophthalmic preparations..............................................................................2783.1.5.Polyethylene with additives for containers for parenteral preparations and for ophthalmicpreparations..............................................................................2793.1.6.Polypropylene for containers and closures for parenteral preparationsand ophthalmic preparations (282)3.1.7.Poly(ethylene -vinyl acetate)for containers and tubing for total parenteral nutrition preparations........................2853.1.8.Silicone oilused as a lubricant (287)3.1.9.Silicone elastomer for closures and tubing..............2883.1.Materials used for the manufacture of containers.....2693.2.1.Glass containers for pharmaceutical use..................3033.2.2.1.Plastic containers for aqueous solutions for parenteral infusion..................................................................3093.2.2.Plastic containers and closures for pharmaceuticaluse...............................................................................................3083.2.3.Sterile plastic containers for human blood and bloodcomponents...............................................................................3093.2.4.Empty sterile containers of plasticised poly(vinylchloride)forhuman blood and blood components...........3113.2.5.Sterile containers of plasticisedpoly (vinylchloride)for human blood containing anticoagulant solution.......3123.2.6.Sets for the transfusion of blood and blood components................................................................................3133.2.8.Sterile single-use plastic syringes................................3143.2.9.Rubber closures for containers for aqueous parenteral preparations,for powders and for freeze-dried powders..3163.2.Containers...........................................................................3034.1.1.Reagents..................................................................5.4-37094.1.1.Reagents..................................................................5.5-41254.1.2.Standard solutions for limit tests.......................5.4-38174.1.2.Standard solutions for limit tests.......................5.5-41264.1.3.Buffer solutions.....................................................5.4-38214.1.3.Buffer solutions.....................................................5.5-41264.1.Reagents,standard solutions,buffer solutions..5.4-37094.2.1.Primary standards for volumetric solutions....5.4-38274.2.2.Volumetric solutions.............................................5.4-38274.2.2.Volumetric solutions.............................................5.5-41274.2.Volumetric analysis...................................................5.4-38274.Reagents.........................................................................5.4-37095.10.Control of impurities in substances for pharmaceuticaluse......................................................................................5.5-41455.11.Characters section in monographs (565)5.1.1.Methods of preparation of sterile products..............4455.1.2.Biological indicators of sterilisation (447)5.1.3.Efficacy of antimicrobial preservation.......................4475.1.4.Microbiological quality of pharmaceuticalpreparations (449)5.1.5.Application of the F 0concept to steam sterilisation of aqueous preparations....................................................5.1-2821 5.1.6.Alternative methods for control of microbiological quality................................................................................5.5-41315.1.Generaltexts onsterility..................................................4455.2.1.Terminology used in monographs on vaccines (453)5.2.2.Chicken flocks free from specified pathogens for the production and quality control of vaccines...............5.1-28255.2.3.Cell substrates for the production of vaccines for human use.................................................................................4555.2.4.Cell cultures for the production of veterinaryvaccines (458)5.2.5.Substances of animal origin for the production ofveterinary vaccines (460)5.2.6.Evaluation of safety of veterinary vaccines andimmunosera ....................................................................5.1-28275.2.7.Evaluation of efficacy of veterinary vaccines and immunosera.....................................................................5.1-28295.2.8.Minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products (463)5.2.9.Evaluation of safety of each batch of veterinary vaccines and immunosera.............................................5.1-28305.2.General texts on vaccines (453)5.3.Statistical analysis of results of biological assays andtests (475)5.4.Residual solvents...............................................................5075.5.Alcoholimetric tables.........................................................5195.6.Assay of interferons..................................................5.3-33815.7.Table of physical characteristics of radionuclidesmentioned in the European Pharmacopoeia.....................5395.8.Pharmacopoeial harmonisation.....................................5515.9.Polymorphism (555)AAbbreviationsand symbols (1.5.) (9)Abnormal toxicity (2.6.9.) (153)Absinthiiherba ........................................................................2710Absorption spectrophotometry,infrared (2.2.24.). (37)Absorption spectrophotometry,ultraviolet and visible (2.2.25.).............................................................................5.2-3089Acacia (905)Acaciae gummi (905)Acaciae gummi dispersione desiccatum .............................905Acacia,spray-dried (905)Acamprosate calcium................................................................906Acamprosatum calcicum (906)Acarbose..............................................................................5.1-2873Acarbosum .........................................................................5.1-2873Acebutololhydrochloride................................................5.4-3889Acebutololi hydrochloridum .........................................5.4-3889Aceclofenac (909)Aceclofenacum (909)Acesulfame potassium.....................................................5.4-3890Acesulfamum kalicum ....................................................5.4-3890Acetazolamide (912)Acetazolamidum (912)Acetic acid,glacial (913)Acetic acid in synthetic peptides (2.5.34.) (141)Acetone................................................................................5.1-2875Acetonum ...........................................................................5.1-2875Acetylcholine chloride...............................................................914Acetylcholini chloridum .. (914)Acetylcysteine..............................................................................915Acetylcysteinum (915)β-Acetyldigoxin..................................................................5.5-4185β-Acetyldigoxinum ...........................................................5.5-4185Acetylsalicylic acid (917)Acetyltryptophan,N - (918)Acetyltyrosine,N - (920)Aciclovir..............................................................................5.3-3436Aciclovirum.......................................................................5.3-3436 Acidum4-aminobenzoicum (973)Acidum aceticum glaciale (913)Acidum acetylsalicylicum (917)Acidum adipicum (926)Acidum alginicum (942)Acidum amidotrizoicum dihydricum (967)Acidum aminocaproicum (974)Acidum ascorbicum (1025)Acidum asparticum (1029)Acidum benzoicum (1072)Acidum boricum (1117)Acidum caprylicum (1172)Acidum chenodeoxycholicum (1247)Acidum citricum anhydricum (1306)Acidum citricum monohydricum (1307)Acidum edeticum.............................................................5.4-3933 Acidum etacrynicum.. (1542)Acidum folicum (1630)Acidum fusidicum (1645)Acidum glutamicum (1670)Acidum hydrochloridum concentratum (1755)Acidum hydrochloridum dilutum (1756)Acidum iopanoicum (1824)Acidum iotalamicum (1825)Acidum ioxaglicum (1826)Acidum lacticum..............................................................5.2-3227 Acidum lactobionicum.. (1885)Acidum maleicum (1966)Acidum malicum (1966)Acidum mefenamicum (1984)Acidum methacrylicum et ethylis acrylas polymerisatum 1:1 (2005)Acidum methacrylicum et ethylis acrylas polymerisatum 1:1dispersio30per centum (2005)Acidum methacrylicum et methylis methacrylas polymerisatum1:1 (2006)Acidum methacrylicum et methylis methacrylas polymerisatum1:2 (2007)Acidum nalidixicum (2080)Acidum nicotinicum (2097)Acidum nitricum (2105)Acidum oleicum (2132)Acidum oxolinicum (2165)Acidum palmiticum (2179)Acidum phosphoricum concentratum (2237)Acidum phosphoricum dilutum (2238)Acidum pipemidicum trihydricum (2249)Acidum salicylicum.........................................................5.1-3007 Acidum(S)-lacticum........................................................5.2-3227 Acidum sorbicum.. (2467)Acidum stearicum (2490)Acidum sulfuricum (2520)Acidum tartaricum (2534)Acidum thiocticum...........................................................5.5-4312 Acidum tiaprofenicum.. (2578)Acidum tolfenamicum (2601)Acidum tranexamicum (2609)Acidum trichloraceticum (2620)Acidum undecylenicum (2658)Acidum ursodeoxycholicum (2662)Acidum valproicum (2669)Acid value(2.5.1.)..............................................................5.2-3099 Acitretin. (922)Acitretinum (922)Acriflavinii monochloridum (924)Acriflavinium monochloride (924)Actinobacillosis vaccine(inactivated),porcine (784)Activated charcoal....................................................................1246Activated coagulation factors(2.6.22.).........................5.5-4115 Additives,plastic(3.1.13.) (293)Adenine (924)Adeninum (924)Adenosine (925)Adenosinum (925)Adeps lanae.......................................................................5.2-3285 Adeps lanae cum aqua.. (2709)Adeps lanae hydrogenatus (2708)Adeps solidus (1711)Adipic acid (926)Adrenaline tartrate (927)Adrenalini tartras (927)Aer medicinalis (929)Aer medicinalis artificiosus (932)Aerodynamic assessment of fine particles in preparations for inhalation(2.9.18.).........................................................5.2-3103 Aether.. (1548)Aether anaestheticus (1549)Agar (928)Agni casti fructus.............................................................5.4-3892 Agnus castus fruit.............................................................5.4-3892 Agrimoniae herba (929)Agrimony (929)Air,medicinal (929)Air,synthetic medicinal (932)Alanine (933)Alaninum (933)Albendazole (934)Albendazolum (934)Albumini humani solutio...............................................5.3-3511 Albumin solution,human................................................5.3-3511 Alchemilla (935)Alchemillae herba (935)Alcohol benzylicus...........................................................5.5-4197 Alcohol cetylicus...............................................................5.3-3475 Alcohol cetylicus et stearylicus....................................5.3-3474 Alcohol cetylicus et stearylicus emulsificans A.. (1239)Alcohol cetylicus et stearylicus emulsificans B (1241)Alcoholes adipis lanae (2703)Alcoholimetric tables(2.9.10.) (237)Alcoholimetric tables(5.5.) (519)Alcohol isopropylicus (1841)Alcohol oleicus (2134)Alcohol stearylicus...........................................................5.3-3621 Alcuronii chloridum.. (935)Alcuronium chloride (935)Alexandrian senna pods (2404)Alfacalcidol (937)Alfacalcidolum (937)Alfadex (938)Alfadexum (938)Alfentanil hydrochloride (939)Alfentanili hydrochloridum (939)Alfuzosin hydrochloride (941)Alfuzosini hydrochloridum (941)Alginic acid (942)Alkaline-earth metals and magnesium(2.4.7.) (104)Alkaline impurities in fatty oils(2.4.19.) (109)Allantoin (942)Allantoinum (942)Allergen products (569)Allii sativi bulbi pulvis (1651)Allium sativum ad praeparationes homoeopathicas (897)Allopurinol (943)Allopurinolum (943)all-rac-α-Tocopherol..........................................................5.5-4313 all-rac-α-Tocopheryl acetate...........................................5.5-4314 Almagate.............................................................................5.2-3169Almagatum.........................................................................5.2-3169 Almond oil,refined...........................................................5.4-3893 Almond oil,virgin.............................................................5.3-3437 Aloe barbadensis.. (947)Aloe capensis (948)Aloes,barbados (947)Aloes,Cape (948)Aloes dry extract,standardised (949)Aloes extractum siccum normatum (949)Alphacyclodextrin (938)Alprazolam (950)Alprazolamum (950)Alprenolol hydrochloride (952)Alprenololi hydrochloridum (952)Alprostadil (953)Alprostadilum (953)Alteplase for injection (956)Alteplasum ad iniectabile (956)Alternative methods for control of microbiological quality (5.1.6.)................................................................................5.5-4131 Althaeae folium (1974)Althaeae radix...................................................................5.2-3232 Alum. (959)Alumen (959)Aluminii chloridum hexahydricum (960)Aluminii hydroxidum hydricum ad adsorptionem..5.5-4186 Aluminii magnesii silicas (961)Aluminii oxidum hydricum (962)Aluminii phosphas hydricus (963)Aluminii phosphatis liquamen.....................................5.3-3438 Aluminii sulfas (964)Aluminium(2.4.17.) (108)Aluminium chloride hexahydrate (960)Aluminium hydroxide,hydrated,for adsorption........5.5-4186 Aluminium in adsorbed vaccines(2.5.13.).. (131)Aluminium magnesium silicate (961)Aluminium oxide,hydrated (962)Aluminium phosphate gel...............................................5.3-3438 Aluminium phosphate,hydrated.. (963)Aluminium sulphate (964)Amantadine hydrochloride (964)Amantadini hydrochloridum (964)Ambroxol hydrochloride (965)Ambroxoli hydrochloridum (965)Amfetamine sulphate (966)Amfetamini sulfas (966)Amidotrizoic acid dihydrate (967)Amikacin (968)Amikacini sulfas...............................................................5.4-3894 Amikacin sulphate............................................................5.4-3894 Amikacinum. (968)Amiloride hydrochloride..................................................5.3-3439 Amiloridi hydrochloridum.............................................5.3-3439 Amino acid analysis(2.2.56.).. (86)Aminobenzoic acid,4- (973)Aminocaproic acid (974)Aminoglutethimide (975)Aminoglutethimidum (975)Amiodarone hydrochloride (977)Amiodaroni hydrochloridum (977)Amisulpride (978)Amisulpridum (978)Amitriptyline hydrochloride (980)Amitriptylini hydrochloridum (980)Amlodipine besilate (981)Amlodipini besilas (981)Ammonia(13N)injection (817)Ammoniae(13N)solutio iniectabilis (817)Ammoniae solutio concentrata.............................................983Ammonia solution,concentrated. (983)Ammonii bromidum (985)Ammonii chloridum (986)Ammonii glycyrrhizas....................................................5.1-2876 Ammonii hydrogenocarbonas.. (988)Ammonio methacrylate copolymer(type A) (983)Ammonio methacrylate copolymer(type B) (984)Ammonio methacrylatis copolymerum A (983)Ammonio methacrylatis copolymerum B (984)Ammonium(2.4.1.) (103)Ammonium bromide (985)Ammonium chloride (986)Ammonium glycyrrhizate................................................5.1-2876 Ammonium hydrogen carbonate.. (988)Amobarbital (988)Amobarbital sodium (989)Amobarbitalum (988)Amobarbitalum natricum (989)Amoxicillin sodium (990)Amoxicillin trihydrate......................................................5.3-3440 Amoxicillinum natricum (990)Amoxicillinum trihydricum...........................................5.3-3440 Amperometric titration(2.2.19.).. (34)Amphotericin B (995)Amphotericinum B (995)Ampicillin,anhydrous (996)Ampicillin sodium (998)Ampicillin trihydrate (1001)Ampicillinum anhydricum (996)Ampicillinum natricum (998)Ampicillinum trihydricum (1001)Amygdalae oleum raffinatum.......................................5.4-3893 Amygdalae oleum virginale..........................................5.3-3437 Amylum pregelificatum (2490)Anaesthetic ether (1549)Analysis,thermal(2.2.34.) (52)Analytical sieving,particle-size distribution estimation by (2.9.38.).............................................................................5.3-3368 Angelicae radix (1003)Angelica root (1003)Anhydrous silica,hydrophobic colloidal......................5.5-4297 Animal anti-T lymphocyte immunoglobulin for human use (1010)Animal spongiform encephalopathies,products with risk of transmitting agents of (577)Animal spongiform encephalopathy agents,minimising the risk of transmitting via human and veterinary medicinal products(5.2.8.) (463)Aniseed (1006)Anise oil (1004)Anisi aetheroleum (1004)Anisidine value(2.5.36.) (142)Anisi fructus (1006)Anisi stellati aetheroleum (2488)Anisi stellati fructus.........................................................5.5-4297 Antazoline hydrochloride. (1006)Antazolini hydrochloridum (1006)Anthrax spore vaccine(live)for veterinary use (715)Anti-A and anti-B haemagglutinins(indirect method)(2.6.20.) (174)Antibiotics,microbiological assay of(2.7.2.) (188)Anticoagulant and preservative solutions for human blood (1007)Anticomplementary activity of immunoglobulin(2.6.17.)..170 Anticorpora monoclonalia ad usum humanum......5.2-3127 Anti-D antibodies in human immunoglobulins for intravenous administration,test for(2.6.26.)..................................5.3-3348 Anti-D immunoglobulin,human. (1732)Anti-D immunoglobulin,human,assay of(2.7.13.) (205)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Materials Science and Engineering B64(1999)54–59Antiferroelectric lead zirconate thinfilms by pulsed laser ablationS.S.N.Bharadwaja,S.B.Krupanidhi*Materials Research Centre,Indian Institute of Science,Bangalore560012,IndiaReceived19March1999AbstractLead Zirconate(PbZrO3)thinfilms were deposited by pulsed laser ablation method.Pseudocubic(110)oriented in-situfilms were grown at low pressure.Thefield enforced anti-ferroelectric(AFE)to ferroelectric(FE)phase transformation behaviour was investigated by means of a modified Sawyer Tower circuit as well as capacitance versus applied voltage measurements.The maximum polarisation obtained was36m C cm−2and the criticalfield to induce ferroelectric state and to reverse the antiferroelectric states were65and90kV cm−1respectively.The dielectric properties were investigated as a function of frequency and temperature.The dielectric constant of the AFE lead zirconate thinfilm was190at100kHz which is more than the bulk ceramic value(120)with a dissipation factor of less than0.07.The polarisation switching kinetics of the antiferroelectric PbZrO3 thinfilms showed that the switching time to be around275ns between antipolar state to polar states.©1999Elsevier Science S.A. All rights reserved.Keywords:Antiferroelectric;Lead zirconate;Pulsed laser ablation1.IntroductionFerroclectric(FE)thinfilms have a wide range of applications in areas such as memory systems,infrared detectors and piezoelectric transducers.More recently, much attention is being focused on the piezoelectric, electrostrictive and charge storage properties of fer-roelectric materials for applications in the areas of microelectronics and microelectromechanical systems (MEMS)[1]and high charge storage devices[2].Ultra-sonic micromotors have been fabricated using lead zirconate titanate(PZT)micromachined thinfilms[3]. However,even higher strain values could be produced using phase switching compositions based on antifer-roelectric(AFE)materials[4,5].Recently,Lathanum modified AFE sol gel thinfilms materials were reported by Cross et al.[6].Thefield enforced AFE to FE phase transition could be utilised to produce discrete strain levels for the applications in the areas such as actuators and shape memory devices[7].AFE materials are char-acterised by rows of dipoles with the dipole moment of adjacent rows equal but anti-parallel so that in equi-librium there is no net spontaneous polarisation and lead zirconate(PbZrO3)was thefirst compound to be identified as an antiferroelectric material[8].The small free energy between the AFE and FE phases makes such switching possible[9],while thefield induced switching is accompanied by large strain changes[10]. The AFE phase can be recovered by the removal of the external appliedfield.Antiferroelectric PbZrO3thinfilms fabrication was reported by earlier workers[11–13]and both polycrys-talline and oriented PbZrO3thinfilms[14]exhibited excellent electromechanical response.Besides these me-chanical properties,it was suggested that the AFE materials could be used as very high charge storage capacitors[2].Whenever the applied electricfield is removed from the poled FE phase of lead zirconate,it reverts back to the low energy AFE phase,thereby releasing all of the polarisation charge at afixed voltage,and AFE materials act like a constant current source.In the present work,a detailed study was done on the processing of PbZrO3thinfilms deposited by laser ablation technique and it’s structural and dielec-tric properties in order to investigate reproducibility and compatibility in fabrication of microactuators,mi-crosensors and charge storage capacitors.*Corresponding author.Tel.:+91-80-331-1330;fax:+91-80-334-1683.E-mail address:sbk@mrc.iisc.ernet.in(S.B.Krupanidhi)0921-5107/99/$-see front matter©1999Elsevier Science S.A.All rights reserved. PII:S0921-5107(99)00163-4S.S.N.Bharadwaja,S.B.Krupanidhi/Materials Science and Engineering B64(1999)54–59552.ExperimentalLead Zirconate thinfilms were deposited by a KrF (248nm)pulsed excimer laser system(Lambda Physik). The schematic diagram of the deposition set-up was given elsewhere[15].The laser beam at a repetition frequency of7Hz was focused using a UV grade plano-convex lens of50cm focal length and was made incident on the ceramic target through a quartz window at an angle of45°.The energyfluence on the target surface was kept in the range of3–3.5J cm−2by adjusting the laser spot size on the target.The operat-ing pressure inside the chamber was controlled precisely with a MKS massflow meter,whereas the substrate temperature was monitored with an external biased heaterfixed inside the deposition chamber.A lead zirconate target was prepared by mixing the oxides,PbO(Aldrich,99.9%)and ZrO2(Aldrich, 99.9%)in molar ratio like in the conventional solid state reaction method.The calcinated PbZrO3powder was pressed into a pellet of diameter of2in.with PVA as binder and sintered at1100°C for2h to achieve above96%of theoretical density.The structural and compositional analysis of the target was confirmed prior to deposition by X-ray diffraction technique (XRD)and energy disperse analysis of X-rays(EDAX).A copper plate was glued on the rear side of the PbZrO3target in order to avoid thermal shock effects during ablation.The front surface of the target was polished and mounted on a motor driven rotator shaft with a continuous rotation at a constant speed,to ensure uniform ablation rate during deposition. (111)Pt/TiO2/SiO2/(100)Si wafers were cleanedfirstly with boiled water and then with isopropanol and they were then placed parallel to the target at a distance of 3cm on a substrate holder with a mechanical clamping system.The temperature of the substrate was controlled by means of an external voltage regulator.The lead zirconate thinfilms were deposited around550°C with various oxygen partial pressures.The structure of the films was confirmed by XRD.The microstructures and chemical composition of thefilms were examined by means of SEM and EDAX,respectively.To study the electrical and dielectric properties,gold electrodes with a radius of0.4mm were deposited on the top surface of thefilms by means of an evaporation technique.The dielectric properties were investigated at different temperatures by a computer controlled Keith-ley3000LCZ meter in conjunction with a Eurotherm temperature controller.The hysteresis properties were studied by a utilizing modified Sawyer Tower circuit. The switching kinetics of AFE lead zirconate was also investigated in order to evaluate the switching speed of field induced transition between AFE and FE phases.3.Results and discussion3.1.Structure and morphologyLead Zirconatefilms were deposited on Pt coated Si substrates.Fig.1shows the X-ray diffraction pattern for thefilms deposited at different pressures with a repetition rate of7Hz and3–3.5J cm−2fluence on the target for30min.Thefilm thickness was determined by means of cross sectional SEM and verified by stylus method and was found to be around1.0m m.At a low pressure of10mTorr,thefilms showed initiation of perovskite peaks with very less intensity of main peaks. As the pressure was increased to50mTorr,the peak intensities increased and further increase in the partial pressure to100mTorr tends to show a dominant pyrochlore phase along with perovskite phase.As the pressure was increased from50to100mTorr the scattering of cationic species also increased which in turn,led to off-stoichiometric cationic ratio in thefilms, thus leading to the dominance of the pyrochlore phase. At a very low pressure like10mTorr the oxidation of the cationic species matters in the enhancement of the perovskite phase.Earlier workers reported this type of behaviour in he case of pulsed excimer laser deposition of PZTfilms[16].The semi-quantitative analysis also revealed that the cationic ratio deviated from the stoi-chiometric ratio(1:1)in thefilms deposited at10and 100mTorr.Films deposited25°C below and25°C above550°C also showed only pyrochlore phase.This Fig.1.X-ray diffraction pattern of in-situ grown lead zirconate thin films with a substrate temperature of550°C,(a)10mTorr,(b)50 mTorr,(c)100mTorr.S.S.N.Bharadwaja,S.B.Krupanidhi/Materials Science and Engineering B64(1999)54–59 56Fig.2.(a)Scanning electron micrographs of surface image of lead zirconate thinfilm deposited at550°C with50mTorr.(b)Scanning electron micrograph of cross sectional image of lead zirconate thin film deposited at550°C with50mTorr.Fig.3.Dielectric constant and loss factor dispersion with frequency at room temperature.at100kHz and did not show much dispersion with frequency.This value was little higher than the bulk value( 120)reported by Cross et al.[17].The dielec-tric constant of the stoichiometric lead zirconatefilms showed a temperature dependent broad phase transi-tion around235°C,which is very close to the bulk crystal( 230°C),[8]as shown in Fig.4.The observed broadness of the peak and the higher transition temper-ature than the bulk ceramic could be attributed to either:(1)the stress betweenfilm and electrodes;(2) defects such as Pb vacancies and/or oxygen vacancies,explains that optimal operating parameters are very much necessary to get reproducible100%perovskite phase in the PvZrO3films and thefilms that were deposited at550°C with50mTorr were taken to study dielectric properties of the same,while this set of growth condition offered stoichiometricfilms.Fig.2(a)and(b)show the SEM data of the surface and cross sectional features of thefilm deposited at a temperature of550°C with50mTorr pressure.The micrographs of thefilm showed a very dense,fine grained columnar structure with less number of particu-lates.Micrographs of thefilms that were deposited at 10and100mTorr showed notable discontinuities with off stoichiometry in cationic ratio which was also re-vealed earlier from the XRD patterns.3.2.Electrical propertiesThe variation in the dielectric constant(K)and dissi-pation(tan d)factor were measured with50mV oscilla-tion level for1.0m m PbZrO3films deposited at550°C is shown in Fig.3.The dielectric constant was above190Fig. 4.Variation of dielectric constant with temperature in lead zirconate thinfilm.S .S .N .Bharadwaja ,S .B .Krupanidhi /Materials Science and Engineering B 64(1999)54–5957Fig.5.Polarisation versus voltage hysteresis behaviour of anti ferroelectric lead zirconate thin film.The thickness of the film is 1m m.associated with the film during deposition;or (3)to the finer grain size of the films or combinations of all the above mentioned factors.3.3.Hysteresis beha 6iourThe polarisation–voltage (P–V)measurements were done using a computer automated modified Sawyer Tower circuit on the lead zirconate thin films and results were shown in Fig.5.The forward AFE [FE and backward switching FE [AFE fields were 64and 90kV cm −1respectively.The saturated polarisation was 36m C cm −2which was a comparable to that reported by earlier workers [18,19].This behaviour confirmed the presence of the AFE phase in the laser ablated PbZrO 3films.The other way to demonstrate phase switching in the antiferroelectric material is by recording the variation of the capacitance of the film as a function of the d.c.bias.The change in the polarisation of a polar material with the applied electric field changes the dielectric constant as follows.K h(P(1)where K is the dielectric constant of the materials and (#P /#E )is the polarisation change with the change in the d.c.field.For a constant geometrical factor,(m 0A )/d (where m 0is the permitivity of the free space,A is the electrode area,d is the thickness of the film),the capacitance is proportional to the dielectric constant of the material.Thus,the typical capacitance versus voltage (C–V)curve for an AFE composition exhibits a double peak butterfly type characteristics [2].The capacitance variation with the d.c.field is shown in the Fig.6.the observation of ‘double butterfly’loop in the C–V curve clearly establishes the evidence for the field induced ferroelectric transition in the AFE leadzirconate films.The values of forward AFE [FE (E AFE )switching field and backward FE [AFE (E FE )switching field are 64and 90kV cm −1respectively and may be found in concurrence with the values observed from the P–V double hysteresis loop.The asymmetry in E AFE and E FE values in the forward and reverse directions might be attributed to:(1)the differences in work functions between top and bottom surfaces of the films,there by leading to the formation of the space charge layer between the electrode and the film sur-faces;(2)the lateral mechanical interaction between the film and the substrate which leads to a 2-D clamping effect of the dipoles [20];and (3)the growth and /or nucleation of ferroelectric and ferroelastic domain wall reorientations in the grain remains because of the possi-ble presence of space charges at the grain boundaries.Fig.6.Capacitance versus applied voltage in lead zirconate thin film.S.S.N.Bharadwaja,S.B.Krupanidhi/Materials Science and Engineering B64(1999)54–59 583.4.D.C.leakage propertiesIt is worth understanding the leakage current mecha-nism through the antiferroelectric PbZrO3thinfilms,since the leakage current directly effects the chargestorage in these materials.The leakage current mecha-nism can be explained by the following relation[21];I8V n(2)for a dielectric slab in between two electrodes,where Iis the current density and V is the applied voltage.Thevalue of the exponent varies depending up on thefieldstrength and microstructural inhomogeneities in thefilms.In lower voltage regime there is a linear relation-ship between leakage current and the d.c.bias,whichfalls under ohmic region.The ohmic regime is given byI8V(3)i.e.for n=1,the leakage current varies linearly with theapplied d.c.voltage,which is generally observed in thelow voltage regime,like in many other dielectrics.For,although at any voltage V,however small,there will beexcess charge injected into the insulator,from impuri-ties and structural defects present in thefilm.Thesecharged species excite into the conduction band even atlower voltages and are responsible for the leakagecurrent.For the values of n higher than1the leakagecurrent varies non-linearly with the applied d.c.stressand if the dielectric possesses discrete traps with differ-ent activation energies it follows space charge limitedconduction(SCLC)given by[22];I8V(s+1)d(2s+1)(4)where s=T t/T and d is the thickness of thefilm,T t is a temperature parameter characterising the trap distribu-tion and T is the absolute temperature.Fig.7shows the log I−log V characteristics of AFE lead zirconate thin films sandwiched between the Au and the Pt electrode. It may be seen that at lower temperatures(30–50°C) there was a sudden jump to the higher order in the magnitude of the current.As the temperature was increased to above a certain critical temperature,the abruptness in the change of order slowly vanished and followed a non-linear curvature.At higher temperature and higher voltages,the curves are showing a slope of approximately2,which means the current conduction mechanism through these PbZrO3thinfilms follow a square law.At higher voltages all the curves are merg-ing with one another this in turn indicates that the temperature does not have a critical role on the conduc-tion mechanism,and is in concurrence with the Space Charge Limited Conduction theory.The sudden shoot-ing up in the current below certain characteristic tem-perature in lowfield regime could be attributed to the releasing of certain discrete inherent traps with lesser Fig.7.Log current−log voltage(log I−log V)curves measured for lead zirconate thinfilms.activation energy as shallow traps present are in the film.As the temperature increased to above that char-acteristic temperature,the onset of distributed trap space charge limited conduction(SCLC)was holding in the moderate and highfield regimes.Further detailed studies are required to confirm this type of behaviour with thefilms of different thickness’which are in progress.3.5.Field induced switching kinetics from AFE to FE phaseAnother important parameter worth studying in the AFE thinfilms for microactuator applications is the switching speed offield induced phase transition be-tween the AFE to FE phases.In Fig.8,the second current maximum shows the switching behaviour of PbZrO3thinfilms under voltage pulses.The initial peak is the surge of capacitor at the time offield applied.The switching time is defined as the time at which the current decreases to10%of the value at the second maximum[23].With the applied signal voltage in-creased(see Fig.8),the switching time also increased. The shift in the discharge peak(first peak)with the voltage increase might be due to d.c.stress between the film and the electrode surfaces and/or the accumulation of the space charges between the electrode and thefilm. The switching time betweenfield induced FE to AFE phases was found to be about275ns,which was much less when compared to the speed offield induced transi-tion in the bulk La-modified antiferroelectric ceramics ( 2m s)[24]and comparable to that reported forS .S .N .Bharadwaja ,S .B .Krupanidhi /Materials Science and Engineering B 64(1999)54–5959Fig.8.Room temperature switching characteristics in lead zirconate thin films.AcknowledgementsAuthors acknowledge the financial support from De-partment of Science and Technology to carry out the present work.One of the authors (SSNB )also acknowl-edges UGC for the financial assistance.References[1]D.L.Polla,Microelec.Eng.29(1995)51–58.[2]B.Jaffe,Proc.of the IRE,(1960)1264–1267.[3]K.R.Udayakumar,J.Chen,K.G.Brooks,L.E.Cross,A.M.Flymn,D.J.Ehrlich,Mater.Res.Soc.Symp.Proc.49(1992)243.[4]W.Y.Pan,C.Q.Dam,Q.M.Zhang,L.E.Cross,J.Appl.Phys.66(1989)6014.[5]W.Pan,Q.Zhang,A.Bhalla,L.E.Cross,J.Am.Ceram.Soc.72(1998)571–578.[6]K.G.Brooks,J.Chen,K.R.Udayakumar,L.E.Cross,J.Appl.Phys.75(1994)1699.[7]K.Uchino,Proc.of the Materials Research Society Internationalmetting on Advanced Materials,vol.9,MRS,1989pp.489–503.[8]G.Shirane,S.Swaguchio,T.Tokagi,Phy.Rev.B 84(1951)476.[9]D.Berlincourt,IEEE Trans.Sonics Ultrason.SU-13(1966)116.[10]D.Berlincourt,H.H.A.Krueger,B.Jaffe,J.Phys.Chem.Solids.25(1964)659–674.[11]T.Tani,J.F.Li,D.Viehland,D.A.Payne,J.Appl.Phys.75(1994)3017.[12]K.Yamakawa,S.Trolier-McKinstry,J.P.Dougherty,S.B.Kru-panidhi,Appl.Phys.Lett.67(1995)2014.[13]K.D.Budd,S.K.Dey,D.A.Payne,Br.Ceram.Soc.Proc.36(1985)107.[14]K.Yamakawa,S.Trolier-McKinstry,J.P.Dougherty,Proc.ofthe 10th IEEE International Symposium of the Application of Ferroelectrics (ISAF 96),vol.II,1996,405–408.[15]S.B Krupanidhi,J.Vac.Sci.Tech.A 10(1992)1569.[16]D.Roy,S.B.Kruapnidhi,J.Mater.Res.7(1992)2521–2529.[17]M.J.Haun,T.J.Harvin,nagan,Z.Q.Zhuang,S.J.J.ang,L.E.Cross,J.Appl.Phys.65(1989)3173.[18]K.K.Li,F.Wang,G.H.Haertling,J.Mater.Sci.30(1995)1386.[19]T.Tani,J.F.Li,D.Viehland,D.A.Payne,J.Appl.Phys.75(1994)3017.[20]N.A.Pertsev,A.G.Zembilgotov,A.K.Tagantsev,Phys.Rev.Lett.80(1998)1988.[21]J.J.O’Dwyer,The theory of Electrical Conduction and Break-down in Solid Dielectrics,Clarendon Press,Oxford,1973.[22]mpert,P.Mark,Current Injection In Solids,AcademicPress,New York,1970.[23]W.Mertz,Phy.Rev.95(1954)690.[24]W.Y.Pan,W.Y.Gu,L.E.Cross,Ferroelectrics 99(1989)185–194.[25]K.G.Brooks,J.Chen,K.R.Udayakumar,L.E.Cross,J.Appl.Phys.75(1994)1699.sol-gel thin films [25].The kinetics is controlled by nucle-ation of new domains,grain size and defects present in the films.Further studies are in progress with the varia-tion of the film thickness,electrode padding to gain fur-ther understanding.4.ConclusionsIn-situ antiferroelectric (AFE)lead zirconate (PbZrO 3)thin films were successfully fabricated using pulsed laser ablation at a low pressure.PbZrO 3thin films with pseu-docubic (110)orientation were prepared on Pt coated Si substrate with TiO 2buffer layer.Films exhibited a dielectric constant of 190at 100kHz frequency.The field enforced ferroelectric (FE)–antiferroelectric (AFE)transition was confirmed at room temperature,both in polarisation versus voltage (P–V)and capaci-tance versus voltage (C–V)curves.The maximum polar-isation obtained was 36m C cm −2and the energies required for the both forward AFE [FE and backward FE [AFE switching field was 64and 90kV cm −1respectively.The switching time of field induced transi-tion between AFE and FE phase was about 275ns..。
