Preparation, structural and luminescent properties of Ba2Gd2Si4O13 Eu3+ for white LEDs
Synthesis and luminescent properties of Ce~3+ doped LuAG nano-sized powders by mixed solvo-therm

J OURNAL OF RARE EARTHS,Vol.28,No.1,Feb.2010,p.16Found at ion item:Project supported by National Natural Science Foundati on of China (10774140),Knowledge Innovation Project of the Chinese Academy of Sci-(K X YW M ),S z R F f D f (6355)S F f T f ,(Z )YIN M (y @;T +65536)DOI 6S ()6Synthesis and luminescent proper ties of Ce 3+doped LuAG nano-sized powders by mixed solvo-ther mal methodWANG Linxiang (王林香)1,2,3,YIN Min (尹民)1,2,GUO Changxin (郭常新)2,ZHANG Weiping (张慰萍)2(1.Hefei Nati onal Laboratory for Physical Sciences at Microscale,Chinese Academy of Sciences,Hefei 230026,China;2.Physics Department,University of Science and Technology of C hina,Hefei 230026,China;3.School of Mathemati cs ,Physics and Information S ciences ,X injiang N ormal Univers ity,Urumqi 830054,China)Received 31April 2009;revised 8September 2009Abstract:Polycrystalline LuAG:Ce 3+(cerium 3+-doped lutetium aluminum garnet)powders were prepared by mixed solvo-thermal method.Fourier-transform IR spectroscopy (FTIR)and X-ray diffraction (XRD)measurements showed that the precursors were ethanol derivatives AlO(OH)crystal with hydroxyl and carbonate group.XRD results showed that phase of Lu 2O 3disappeared with the precursors were annealed at 400°C,cubic phase LuAG:Ce 3+appeared but only one diffraction peaks of LuAP (LuAlO 3)at calcination temperature to 700°C,and thepurified crystalline phase of LuAG:Ce 3+was obtained at 1000°C.The scanning electron microscopy (SEM)analysis revealed that the synthe-sized LuAG:Ce 3+powders were uniform and had good dispersivity with an average particle size about 100nm.Excitation and emission spec-tra of Ce 3+doped LuAG phosphors were measured.Many factors of affecting the intensity of emission spectra were discussed.Keywords:LuAG:Ce 3+phosphors;hydrothermal;mixed solvo-thermal method;photoluminescence;rare earthsAn inorganic scintillator plays an important role in radia-tion detection in many sectors of research concerning almost all medical diagnostic imaging modalities that use X-ray or gamma rays,dosimetry,nuclear medicine,high energy physics and also in many industrial measuring systems [1,2].For these different applications,scintillator is the primary radiation sensor,and scintillator material can absorb high-energy photons and then emit different energy photons [3].Recently,lutetium aluminum garnet (Lu 3Al 5O 12),because of its high density (6.73g/cm 3,94%of BGO),high lumi-nescent efficiency,and good chemical radiation stability,has been known to be one of the promising host crystals for scin-tillating materials.In rare earth ions doped Lu 3Al 5O 12host lattice,cerium (Ce 3+)as luminescent activators can yield fast decay with 5d-4f allowed transitions of the Ce 3+ion,so LuAG:Ce 3+is a fast efficient response scintillator materi-als [4–6].However,the growth of high optical quality LuAG:Ce 3+single crystal is an arduous process with high costs and long production cycle [7,8].Instead of this goal,for achieving the luminescent properties,synthesizing high density,fine dispersive and less agglomerative LuAG:Ce 3+powder is re-quired.Many methods have been used to synthesize rare earth (RE)-doped LuAG such as combustion method [8],high temperature solid-state method [9,10],sol-gel method [11],and precipitation method [12,13].However,hydrothermal method is scarcely used to synthesize LuAG:RE phosphors.In this paper,LuAG:Ce 3+nano-phosphors were synthesized by mixed solvo-thermal method.The structures and photolu-minescent properties of LuAG:Ce 3+phosphors were inves-tigated.1Experimental1.1PreparationFig.1shows the schematic flow chart for the synthesis of LuAG:Ce 3+nano-sized powders.The synthesis procedure of LuAG:Ce 3+phosphors can be divided into three steps.Firstly,lutetium oxide (Lu 2O 3,99.99%)dissolved in dilute nitric acid (HNO 3)under continuously stirring and heating.Alu-minum nitrate (Al(NO 3)39H 2O,99.99%)and cerium nitrate (Ce(NO 3)36H 2O,99.99%)were dissolved in deionized wa-ter.Then the solutions of Lu(NO 3)3,Al(NO 3)3and Ce(NO 3)3were mixed to yield a composition with general formula(Lu 1–x Ce x )3Al 5O 12(x mol.%Ce 3+),and then proper ethanol was added as dispersant.The mixture was stirred for hours at room temperature.Mixed solution was dripped into the pre-cipitation agent solution at a titration speed of 2ml/min,and stirred continuously.Three precipitants,AHC+AW (ammonium hydrogen carbonate and ammonia water),AW (ammonia water)and urea were used respectively.After the titration finished,proper amount of ammonium was dripped into the mixture to adjust the pH value (pH ≈8–9)of suspen-sion.After continuously stirring and aging for hours,the white precipitation was washed four times with distilled wa-ter and collected by filtrating.Proper amount of the resultante nc es JC 2--11pec iali ed esea rch und or the octoral Program o H ighe r Educ ation 2000804and pecia l oundati on or ale nts o A nhui Province China 2007021Corre sponding a uthor :in E-m ai l:inmi n el.:8-1-07412:10.101/1002-0721090041-7WANG Linxiang et al.,Synthesis and luminescent properties of Ce3+doped LuAG nano-sized powders by mixed solvo-thermal…17Fig.1Schematic flow chart for the synthesis of LuAG:Ce3+pow-ders by mixed solvo-thermal methodprecipitate,mixed with a certain proportion of alcohol and water,was sealed in a50ml autoclave,and then put into an oven at250°C for20h.The precursor powder was obtained by filtrating and drying at100°C,and was annealed at a higher temperature ranging from400to1100°C in a muffle furnace for2h in air.In addition,for the sake of contrast,the part of the above mentioned white precipitation was respec-tively washed four times with distilled water and anhydrous ethanol,without hydrothermal treatment,and then dried at 100°C.The obtained powders were crushed and directly calcined at800°C for2h in air.1.2Charact erizationThe phase evolution of the product was identified by X-ray diffraction(XRD,MAC Science Co.Ltd. MXP18AHF)with Cu Kαradiation in the range of2θ= 10°–70°.FTIR spectra were measured with Magra-IR750 (Nicolet Instrument Co.U.S.A)Fourier transform infrared spectrometer.The microstructure and morphology of pre-cursor and calcined powders were examined by scanning electron microscope(SEM,Sirion200,FEI Co.,Ltd.,Hol-land).The excitation and emission spectra of the samples were measured by a HITACHI(JAPAN)M850fluorospec-trophotometer using an Xe lamp as the excitation source.All the measurements were carried out at room temperature.2Results and discussions2.1FTIR analysisThe FT-IR spectra of the precursor(1)and powders(2) calcined at1000°C for2h are shown in Fig.2.In precursor (1),the peaks at488,618,737cm–1are corresponding to the Lu–OH,Al–OH and the Al–O vibration absorption[13–16].Asf,–O–f[5,],5–f Fig.2FTIR spectra of the precursor(1)and powders(2)calcined at 1000°C for2hC–H deformation mode that is attributed to coupling of O–H planar deformation angle vibration and C–OH stretching vi-bration[15,17],and the broad band from3310to3330cm–1is typical absorption caused by hydroxyl group[15,17,18].Those organic characteristic absorption peaks indicate that precur-sor powders contain a certain amount of organic ingredients. The peak at3090is assigned to the stretching and bending mode of O–H bands in pseudoboehmite[15,17,18],which refer to the poorly crystallized Al3+compound of composition Al2O3nH2O(1.0<n<2.0)[15,19].In addition,there are some weak peaks,such as the peak at about840cm–1,which is associated with the out-of-plane bending of CO32–[16],the peak at about1630cm–1from the bending of H–O–H,which overlaps the O–C–O stretching band[20,21],the absorption peaks of CO2at2350cm–122],and the weak peaks at2850 and2925cm–1corresponding to the vibrations of–CH3and –CH2–,respectively[23].As temperature increases,all the above peaks become weaker or finally disappear because of the decomposition of the precursor.As shown in the sample (2),at1000°C,the characteristic peaks of organic ingredi-ents disappear and are replaced by the fingerprint vibrations of the isolated[AlO4]tetrahedral and[AlO6]octahedral at 809,742,708,575,519and460cm–1[14,15],which indicates that pure LuAG phase is formed[15].This result well coin-cides with the XRD patterns in Fig.3.2.2XRD patternsXRD patterns of the precursor and powders calcined at different temperatures are shown in Fig.3.For the precursor, the part of diffraction peaks were in agreement with the data of AlO(OH),which indicates that hexa-coordinate octahe-dral structure crystalline materials are formed in hydrother-mal process[24,25].It is reported that the LuAG powders obtained by co-precipitation or sol-gel combustion method were all found to be amorphous until calcined temperature to800°C[12,14].As seen in Fig.3,at calcined temperature to 800°C powders without hydrothermal treatment are stillT yff y y zf I x,fcharacteristic peaks o ethanol the strong absorption peak at 1070cm1is due to H stretching vibration o ethanol117 two absorption peaks at1420and140cm1originate rom amorphous phase.his illustrates that the h drothermal process can e ectivel reduce the cr stalli ation temperature or the precursor.n this e periment a ter precursor isannealed18JOURNAL OF RARE EARTHS,Vol.28,No.1,Feb.2010Fig.3XRD patterns of the precursor and powders calcined at vari-ous temperatures for2hat400°C for2h,phase of Lu2O3(JCPDS card86-2475)ap-pears.At600°C,diffraction peak intensities of Lu2O3be-come stronger.Until calcined temperature reaches700°C, cubic phase LuAG appears and the data are all in good agreement with those of JCPDS card(73-1368),but only one peak at2θ=34.59°is characteristic diffraction peaks of LuAP (LuAlO3).With the calcined temperature increasing,the in-tensity of LuAP diffraction peaks decreases.At calcined temperature of1000°C,pure cubic phase LuAG is ob-tained.At calcined temperature from1000to1100°C,the phase of LuAG has no change,but the width of diffraction peaks becomes narrower.It indicates that the particle di-ameter grows.2.3SEM imagesFig.4shows SEM micrograph of the precursor(a),pow-ders calcined at1000°C(b)and1100°C(c)for LuAG:Ce3+ powders.SEM result showed that the precursors(a)are crys-tal with octahedral structure,which is consistent with the XRD results seen in Fig.3.In Fig.4(b),the morphology of LuAG:Ce powders calcined at1000°C is uniform and near spherical,with an average particle size of about100nm and good dispersivity,which is generally beneficial to improving the powers luminescence efficiency and producing transpar-ent polycrystalline ceramics.When the calcined temperature reaches1100°C,agglomeration of particles becomes serious, and the average particle size of a polycrystalline material grows up,which are consistent with analysis results of XRD.2.4Formation mechanisms analysis of LuAG:CeIn the experimental process,we found that pure LuAG was relatively easy to obtain only in volume ratio from3to5 for alcohol and water in the same hydrothermal condition. When mixed ethanol was too much or too little,pure LuAG was difficult to get in the same condition.Through infrared spectra,we can obtain qualitative analy-sis of the chemical composition and molecular structure. Based on the above analysis,the formation mechanism of LuAG crystals and the effect of hydrothermal on synthesis of LuAG are as follows.The first,in the hydrothermal condi-tion,suspensions dissolve and generate Lu3+,A13+ions,and then Lu3+,A13+and OH–1will form anion coordination polyhedron[Al-(OH)6]3–,[Lu-(OH)6]3–,[Lu-(OH)9]6–,that is crystal growth units[24,25].At the same time,hydroxyl oxy-gen atoms of ethanol molecule have higher electronegativity, easily connect with above mentioned growth units,and form relevant alcohol derivatives with low stability.The alcohol derivatives and ethanol prevent OH–intervention and avoid the formation of the Boehm mine[25].With the temperature increase,alcohol derivatives molecules can be easily separated from derivatives[26,27]and the part of the OH–1is replaced by oxygen atoms.OH–1,O and A13+will constitute a hexa-coordinate octahedral AlO3(OH)3[20,24,25],which is in agreement with analysis results of XRD patterns for precur-sor.It indicates that weak crystallization has existed in the precursor.When the precursor powders are calcined at high temperature,and the weak bond Al-OH leads to dehydration of Al-(OH)6octahedral until O and Al3+form isolated[AlO4]tet-rahedral and[AlO6]octahedral.Meanwhile Lu3+will be lo-cated in dodecahedron consisted of aluminum oxygen tetrahe-dron and aluminum oxygen octahedron which indicate that pure cubic phase lutetium aluminum garnet is generated[14,24,25].2.5Luminescence properties of LuAG:CeFig.5shows the excitation spectrum and emission spec-trum of LuAG:Ce3+powders calcined at1100°C for2h.It F S M f()°()°(),yig.4E micrograph o the precursor a and powders calcined at1000C b and1100C c respectivelWANG Linxiang et al.,Synthesis and luminescent properties of Ce 3+doped LuAG nano-sized powders by mixed solvo-thermal (19)can be seen that excitation spectrum of Ce 3+contains two bands,a weak band with maximum peak at 347nm and a strong broad band with a maximum at 450nm.These excita-tion bands result from the absorption of the incident radia-tion by Ce 3+ions,which lead to the excitation of electrons from the 4f 1ground state (2F 5/2,2F 7/2)to the excited 5d 1level (2D)[8,28,29].The emission spectra display an apparent broad band covering from 450to 650nm with a maximum at 510nm,which is well in agreement with the room temperature spectra of the LuAG:Ce single crystal films [11,30,31].Accord-ing to the Gaussian fitted curves for emission spectra for λex =450nm,the photoluminescence spectra consist of two bands of Ce 3+emission centered at about 500and 534nm,respectively,which is also observed in the luminescence spectrum of the LuAG:Ce 3+single crystal films at 9K [30].This emission is ascribed to the electron transitions from the lowest crystal-splitting component of the 5d level (2D)to the ground state of Ce 3+(2F 5/2,2F 7/2)[11,30].At room temperature,two emission bands overlap.Fig.6shows intensity dependence of 5d →4f emission on concentration of Ce 3+for LuAG phosphors.As shown in Fig.6,the maximum intensity of 5d →4f emission was obtained at 1%Ce 3+doped LuAG phosphors.With doped concentration of Ce 3+gradually increase,the emission intensity of Ce 3+re-duced.Fig.7shows the emission spectrum of 1%Ce 3+doped LuAG using different precipitator.In this experiment,theFig.5Excitation spectrum and emission spectrum of 1%Ce 3+-doped LuAG phosphors calcined at 1100°C for 2hF 6I y f 5→f f3+f L G °f mixture of NH 4HCO 3and NH 3H 2O ,NH 3H 2O or urea respectively as precipitator,it is found that using mixture precipitator of NH 4HCO 3and NH 3H 2O,the emission spec-trum intensity of Ce 3+is stronger compared with using other two precipitator,and the obtained powders using mixture precipitator of NH 4HCO 3and NH 3H 2O are more loosely agglomerated and uniform.The relative large values of the solubility constants of NH 3H 2O or urea may cause rapid and thorough reaction of the precipitation,resulting in a fast growing rate and uncontrolled severe agglomeration of the resultant crystallites [32,33].It can be concluded that precipi-tant plays an important role in the morphology of the final powders.Thereby,the particle size distribution and ag-glomeration will further affect luminescent properties of powders [32].The emission spectra of 1%Ce 3+-doped LuAG phosphors sintered at different temperatures were obtained as shown in Fig.8.The emission intensity increases with increasing sin-tering temperature below 1000°C.The crystallite growth and the defects which decrease with temperature were proba-bly the reason for the above-mentioned factor [4],while the sintered temperature reaches 1100°C,the emission intensityof Ce 3+is weakened,and this may be ascribed to the oxy-genation of certain Ce 3+ions at this temperature.According to the above analysis,there are many factors that could affect the emission intensity of rare earth ion,Fig.7Emission spectrum (λex =450nm)for 1%Ce3+doped LuAG phosphors calcined at 1000°C for 2h using different pre-cipitationagentF f %3+LG f f ig.ntensit dependence o d 4emission on concentration o Ce or uA phosphors calcined at 1000C or 2hig.8Emission spectrum o 1Ce -doped uA calcined at di -erent temperatures20JOURNAL OF RARE EARTHS,Vol.28,No.1,Feb.2010such as the concentration of the solution,pH values of the solution,the speed of titration,the different precipitation agent and effect of titration sequence.In this study,the ex-periments have been repeated several times under strictly controlled conditions,although the results of each experi-ment come out a bit different.The detailed mechanism de-serves to be further investigated.Additionally,the emission spectra match well with the sensitivity curve of Si photodiode and CCD arrays that may be a good candidate as scintillation sensors in digital appli-cation under X-ray or blue-green light excitation[34–36].3ConclusionsThe Polycrystalline LuAG:Ce3+powders with an average particle size of about100nm were synthesized by mixed solvo-thermal method.FTIR,XRD measurements showed that the precursors were hexa-coordinate octahedral structure ethanol derivatives crystal with hydroxyl and carbonate group.The purified crystalline phase of LuAG:Ce3+was formed from the precursors materials calcined at1000°C for 2h.Intensity dependence of5d→4f emission on concentra-tion of Ce3+,different precipitation agent and sintering tem-perature was measured.It was found that the emission inten-sity reached the maximum for1%Ce3+-doped LuAG using the mixture precipitator of NH4HCO3and NH3H2O at cal-cined temperature to1000°C by mixed solvo-thermal method.The emission spectra of Ce3+doped LuAG phos-phors were located in the range of450–650nm consisting of two emission bands,because of transition from the lowest5d excited state(2D)to the4f ground state of Ce3+,which matched well with the sensitivity curve of the Si-photodiode. References:[1]Van Eijk C W E.Inorganic-scintillator development.NuclearInstruments and Methods in Physics Research A,2001,460:1.[2]Cicillini S A,Pires A M,Serra O A.Luminescent and mor-phological studies of Tm-doped Lu3Al5O12and Y3Al5O12fine powders for scintillator detector application.Journal of A lloys and Compounds,2004,374:169.[3]Greskovich C,Duclos S.Ceramic scintillators.Annu.Rev.Mater.Sci.,1997,27:69.[4]You Baogui,Yin Min,Zhang Weiping,Guo Hai,Lin Lin.Lu-minescence properties of Tb3+-doped LuAG films prepared by Pechini sol-gel method.J.Rare Earth,2006,24(6):745.[5]Van Eijk C W E.Development of inorganic scintillators.Nu-clear Instruments and Methods in Phy sics Research A,1997, 392:285.[6]Nikl M,Mihokova E,Mares J A,Vedda A,MartiniM,Nejez-chleb K,Bla ek K.Traps and timing characteristics of LuAG: Ce3+scintillator.Phys.Stat.Sol.(a),2000,181:R10.[7]Cherepy N J,Kuntz J D,Tillotson TM,Speaks D T,Payne S A,Chai B H T,Porter-Chapman Y,Derenzo S E.Cerium-doped y,N I M y R S,,5()3[8]Li Huili,Liu Xuejian,Huang Liping.Luminescent propertiesof LuAG:Ce phosphors with different Ce contents prepared bya sol-gel combustion method.Optical Materials,2007,29(9):1138.[9]Li Huili,Liu Xuejian,Huang Liping.Fabrication of transparentCe:LuAG ceramics by a solid-state reaction method.Journal ofInorganic Materials(in Chin.),2006,21(5):1161.[10]Li Huili,Liu Xuejian,Huang Liping.Fabrication of Transpar-ent cerium-doped lutetium aluminum garnet(LuAG:Ce)ce-ramics by a solid-state reaction method.Journal of the A meri-can Ceramic Society,2005,88(11):3226.[11]Li Huili,Liu Xuejian,Zhang Qitu,Huang Liping.Synthesisand characterization of cerium-doped lutetium aluminum gar-net phosphors by nitrate-citrate sol-gel combustion process.J.Rare Earths,2007,25(4):401.[12]Li Huili,Liu Xuejian,Huang Liping.Synthesis of nanocrystal-line lutetium aluminum garnet powders by co-precipitation method.Ceramics International,2006,32(3):309.[13]Xie Jianjun,Shi Ying,Hu Yaoming,Chen Qiwei,Shi Jianlin.Synthesis study of Lu3Al5O12(Ce)nanoscaled powder by co-precipitation.Journal of Inorganic Materials(in Chin.),2009, 24(1):79.[14]Li Huili,Liu Xuejian,Huang Liping.Synthesis of lutetiumaluminum garnet powders by nitrate-citrate sol-gel combustion process.Ceramics International,2007,33(6):1141.[15]Yang Ru,Qin Jie,Li Min,Liu Guoqiang.Synthesis of yttriumaluminum garnet(YAG)powder by homogeneous precipita-tion combined with supercritical carbon dioxide or ethanol fluid drying.Journal of the European Ceramic Society,2008, 28(15):2903.[16]Wang Jieqiang,Xu Hongyan,Wang Yong,Yue Yunlong.Ef-fect of sulfate ions on YAG powders synthesized by micro-wave homogeneous precipitation.Journal of Rare Earths, 2006,24(1):284.[17]Li Xia,Liu Hong,Wang Jiyang,Cui Hongmei,Yang Shunli-ang,Boughton I R.Solvothermal synthesis and luminescent properties of YAG:Tb nano-sized phosphors.Journal of Phys-ics and Chemistry ofSolids,2005,66(1):201.[18]Sato Taich.Thermal decomposition of aluminum hydroxidesto aluminas.Thermochimica A cta,1985,88(1):69.[19]Maria Lúcia Pereira Antunes,Helena de Souza Santos,Persiode Souza Santos.Characterization of the aluminum hydroxide microcrystals formed in some alcohol-water solutions.Materi-als Chemistry and Physics,2002,76(3):243.[20]Liao Yikun,Jiang Danyu,Shi Jianlin.Transparent lutetiumaluminum garnet sintered from carbonate coprecipitated pow-ders.Materials Letters,2005,59(28):3724.[21]Vera Bolis,Giuliana Magnacca,Giuseppina Cerrato,ClaudioMorterra.Microcalorimetric and IR-spectroscopic study of the room temperature adsorption of CO2on pure and sulphated t-ZrO2.Thermochimica Acta,2001,379(1-2):147.[22]Saraswati V,Rao G V N,Rama Rao G V.Structural evolutionin alumina gel.Journal of Materials Science,1987,22(7): 2529.[23]Xu Guogang,Zhang Xudong,He Wen,Liu Hong,Li Hong.The study of surfactant application on synthesis of YAG nano-sized powders.Powder Technology,2006,163(3):202. []L X,L,W y,,F, Z X,B R I R y f Y Gz y M L,,single cr stal and transparent ceramic lutetium aluminum gar-net scintillators.uclear nstruments and ethods in Ph sics esea rch.ection A2007791:8.24i ia iu Hong ang Ji ang Cui Hongmei Han eng hang udong oughton.apid s nthesis o A nano-si ed powders b a novel method.aterials etters2004WANG Linxiang et al.,Synthesis and luminescent properties of Ce3+doped LuAG nano-sized powders by mixed solvo-thermal (21)58(19):2377.[25]Li Xia,Liu Hong,Wang Jiyang,Cui Hongmei,Han Feng,Zhang Xudong.Synthesis of yttrium aluminium garnet(YAG) nano-sized powders by mixed solv-thermal method.Journal of Inorganic Materials(in Chin.),2004,19(5):1168.[26]Inoue Masashi,Otsu Hiroyuki,Kominami Hiroshi,Inui To-moyuki.Synthesis of yttrium aluminum garnet by the Glyco-thermal method.Journal of the A merican Ceramic Society, 1991,74(6):1452.[27]Zhong Weizuo,Hua Sukun.Crystal Growth Morphology.Bei-jing:Science Press(in Chin.),1999.279.[28]Zhou Yonghui,Lin Jun,Yu Min,Wang Shubin,Zhang Hong-jie.Synthesis-dependent luminescence properties of Y3Al5O12: Re3+(Re=Ce,Sm,Tb)phosphors.Materials Letters,2002, 56(5):628.[29]Liu Xuejian,Li Huili,Xie Rongjun,Zeng Yi,Huang Liping.Spectroscopic properties of nano-sized cerium-doped lutetium aluminum garnet phosphors via sol-gel combustion process.Journal ofLuminescence,2007,124(1):75.[30]Zorenko Yu,Gorbenko V,Konstankevych I,Voloshi-novskiiA,Stryganyuk G,Mikhailin V,Volobanov V,Spassky D.Sin-gle-crystalline films of Ce doped YAG and LuAG phosphors: advantages over bulk crystals analogues.J.Lumin.,2005, 114(2):85.[31]Mares J A,Nikl M,Beitlerova A,Solovieva Natalia,D’AmbrosioCarmelo,Blazek Karel,Maly Petr,Nejezchleb Karel,Fabeni Pasquale,Pazzi Gian Paolo.Ce3+-doped scintillators:status and properties of(Y,Lu)aluminium perovskites and garnets.Nuclear Instruments and Methods in Physics Research Section A,2005,537(1-2):271.[32]Wang Zhifang,Zhang Weiping,You Baogui,Yin Min.Effectsof precipitant on microstructure and luminescent properties of Lu2O3:Eu3+nanopowders and ceramics.Spectrochimica A cta Part A,2008,70(4):835.[33]Chen Qiwei,Shi Ying,An Liqiong,Wang Shiwei,Chen Ji-yang,Shi Jianlin.A novel co-precipitation synthesis of a new phosphor Lu2O3:Eu3+.Journal of the European Ceramic Soci-ety,2007,27(1):191.[34]Lempicki A,Brecher C,Szupryczynski P,Lingertat H,Na-garkar S V,Tipnis SV,Miller S R.A new lutetia-based ce-ramic scintillator for X-ray imaging.Nuclear Instruments and Methods in Physics Research Section A,2002,488(3):579. [35]Greskovich C D,Cusano D,Hoffman D,Riedner R J.Ceramicsscintillators for advanced,medical X-ray detectors.A merican Ceramic Society Bulletin,1992,71(7):1120.[36]Ambrosio C D,Notaristefani F de,Hull G,Orsolini Cencelli V,Pani R.Study of LaCl3:Ce light yield proportiona lity with a hybrid photomultiplier tube.Nuclear Instruments and Methods in Physics Research Section A,2006,556(1):187.。
材料科学与工程专业英语词汇
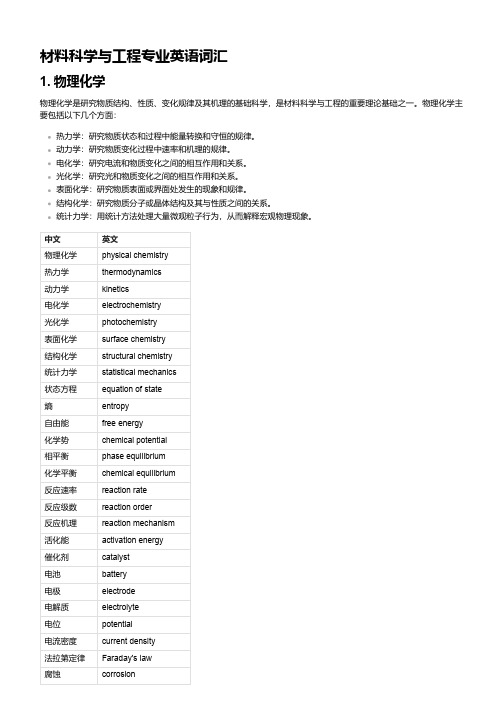
材料科学与工程专业英语词汇1. 物理化学物理化学是研究物质结构、性质、变化规律及其机理的基础科学,是材料科学与工程的重要理论基础之一。
物理化学主要包括以下几个方面:热力学:研究物质状态和过程中能量转换和守恒的规律。
动力学:研究物质变化过程中速率和机理的规律。
电化学:研究电流和物质变化之间的相互作用和关系。
光化学:研究光和物质变化之间的相互作用和关系。
表面化学:研究物质表面或界面处发生的现象和规律。
结构化学:研究物质分子或晶体结构及其与性质之间的关系。
统计力学:用统计方法处理大量微观粒子行为,从而解释宏观物理现象。
中文英文物理化学physical chemistry热力学thermodynamics动力学kinetics电化学electrochemistry光化学photochemistry表面化学surface chemistry结构化学structural chemistry统计力学statistical mechanics状态方程equation of state熵entropy自由能free energy化学势chemical potential相平衡phase equilibrium化学平衡chemical equilibrium反应速率reaction rate反应级数reaction order反应机理reaction mechanism活化能activation energy催化剂catalyst电池battery电极electrode电解质electrolyte电位potential电流密度current density法拉第定律Faraday's law腐蚀corrosion中文英文光敏材料photosensitive material光致变色photochromism光致发光photoluminescence光催化photocatalysis表面张力surface tension润湿wetting吸附adsorption膜membrane分子轨道理论molecular orbital theory晶体结构crystal structure点阵lattice空间群space group对称元素symmetry element对称操作symmetry operationX射线衍射X-ray diffraction2. 量子与统计力学量子与统计力学是物理学的两个重要分支,是材料科学与工程的重要理论基础之一。
专业英语

questions
How
do you distinguish steel from cast iron? How do you distinguish low alloy steel from high alloy steel?
1.1.1 Iron and Steel
The earth contains a large number of metals which are useful to man. One of the most important of these is iron. Modern industry needs considerable quantities of this metal, either in the form of iron or in the form of steel.
Mechanical Engineering materials
Organic polymer materials Inorganic non-metallic materials
plastic rubber Synthetic Fibers Traditional ceramics Special Ceramics Metal Matrix Composites
1.1.1 Iron and Steel
The ore becomes molten, and its oxides combine with carbon from the coke. The non-metallic constituents of the ore combine with the limestone to form a liquid slag. This floats on top of the molten iron, and passed out of the furnace through a tap. The metal which remains is pig iron.
材料试验大纲 英文

材料试验大纲英文Material Testing Outline.Introduction.The material testing outline serves as a comprehensive guide for conducting rigorous and reproducible material testing. It outlines the various stages involved in the testing process, from planning and preparation to execution and analysis. This outline aims to ensure consistency, accuracy, and safety throughout the testing procedure, ensuring reliable results that can be used for various applications, such as product development, quality control, and research.1. Testing Objectives.Before commencing the testing process, it is crucial to establish clear objectives. These objectives should be specific, measurable, achievable, relevant, and time-bound(SMART). They guide the testing team and ensure that the efforts are focused on achieving the desired outcomes. For example, the objective may be to determine the mechanical properties of a material under specific conditions or to assess the material's durability and reliability over time.2. Testing Plan.The testing plan outlines the specific steps and procedures that will be followed during the testing process. It includes information on the testing equipment and instrumentation, the test setup, the test environment, and the testing schedule. The plan also details the safety measures to be taken, ensuring the safety of the testing personnel and equipment.3. Sample Preparation.Sample preparation is a crucial step in material testing. It involves selecting the appropriate material samples, ensuring their homogeneity and consistency, and preparing them for testing. The samples should berepresentative of the material being tested and should be handled, stored, and transported according to specific guidelines to prevent any alterations in their properties.4. Test Execution.During the test execution phase, the testing plan is implemented, and the material samples are subjected to various tests. These tests may include tensile testing, compression testing, flexural testing, impact testing, fatigue testing, and more, depending on the objectives and requirements of the testing. The testing personnel should be well-trained and follow strict procedures to ensure accurate and reproducible results.5. Data Collection and Analysis.Data collection and analysis are essential for obtaining meaningful results from material testing. The testing equipment typically records various parameters, such as load, displacement, strain, stress, and time, during the testing process. These data are then analyzedusing statistical and engineering principles to derive meaningful insights about the material's properties and behavior. The analysis should be conducted by qualified personnel and should be validated to ensure its accuracy and reliability.6. Test Report and Documentation.After the completion of testing and data analysis, a detailed test report is prepared. This report summarizes the testing objectives, methods, procedures, results, and conclusions. It also includes any safety considerations, equipment calibration details, and any other relevant information. The report should be written in a clear and concise manner, enabling others to understand the testing process and results easily.7. Quality Assurance and Improvement.Quality assurance and improvement are ongoing processes in material testing. They involve monitoring the testing process, identifying any issues or discrepancies, andimplementing corrective actions to improve the accuracy and reliability of the results. Regular audits and reviews of the testing procedures and equipment are conducted to ensure their continued compliance with industry standards and best practices.In conclusion, the material testing outline provides a structured and systematic approach to material testing. It ensures that the testing process is conducted in a consistent, accurate, and safe manner, leading to reliable results that support various applications in product development, quality control, and research. By following this outline, organizations can improve their material testing capabilities, enhance product quality, and reduce risks associated with material failure.。
An elasto-plastic damage model for reinforced concrete with minimum number of material parameters

An elasto-plastic damage model for reinforced concrete with minimum number of material parametersWilfried B.Kr€a tzig a,Rainer P€o lling b,*a Institute for Statics and Dynamics,Ruhr-University Bochum,44780Bochum,Germanyb Building Contractors of Mesenbrock,Welter Straße4,48249D€u lmen,GermanyReceived1November2002;accepted4March2004Available online20April2004AbstractBased on a fully3-D elasto-plastic damage theory,the material behavior of all reinforced concrete components––concrete,reinforcement and bond––is,for biaxial loading,realistically modelled,including cyclic action.Thereby emphasis is layed on concrete in tension and compression.The presented model contains a minimum number of material parameters.It further enables to map exact uniaxial stress–strain curves as proposed by modern codes of practise,like the EC2.All material parameters of the model can be readily interpreted and determined by few standard experiments,or approximated from concrete compression strength.Finally,the concrete model is verified by numerical simulation of experiments.Ó2004Elsevier Ltd.All rights reserved.Keywords:Concrete;Reinforced concrete;Material damage;Plasticity;Crushing energy;Material model;Softening1.IntroductionIn structural engineering,reinforced concrete is considered as a2-phase composite of uniaxial steel and multi-dimensional concrete components.Hardened concrete itself,predominantly a mixture of aggregates and a cementitious matrix,exhibits a rather complicated deformation behavior,mainly because of initiation and growth of micro-cracking in the matrix.Constitutive models for concrete,the central concern of this treatise,exist on very different quality levels.Even the most advanced structural codes of practice[1]offer mainly elastic behavior,at most some monotonic non-linear stress–strain curves without any rheo-mechanical specification.On the other(research)extreme,wefind highly sophisticated constitutive attempts with few relations to engineering design practice.But for com-puter simulation of advanced structural problems,like failure analyses or life-cycle assessments,constitutive models are required,improved compared to those from codes of practice and more exact,nevertheless of lowest possible complication.Severe deficiencies of code mod-els are their uniaxial formulation and the lack of infor-mation for cyclic processes.If one attempts to set up a constitutive model for cyclic behavior of concrete as pictured in Fig.6or7,one observesfirst the residual strains after complete unloading.These generally are interpreted as‘plastic’deformations,although in concrete there will be cer-tainly no plastic slip like in metal plasticity.The second observation is the stiffness degradation along the cycles which requires a‘damage’component in the model. Obviously in constitutive modeling of concrete,an elasto-plastic component has to be combined with an elasto-damaging one.Hence the key aim here is a3-dimensional elasto-plastic description of mechanical responses of reinforced*Corresponding author.Tel.:+49-2548-9193-883;fax:+49-2548-1085.E-mail address:rainer.poelling@(R.P€o lling).0045-7949/$-see front matterÓ2004Elsevier Ltd.All rights reserved.doi:10.1016/pstruc.2004.03.002Computers and Structures82(2004)1201–1215/locate/compstrucconcrete,supplemented by a damage component.Be-cause of few cyclic experiments for concrete and the problem of reproductivity of results,a minimum number of well-defined,experimentally identifiable material parameters shall control the model[2].Classical elasto-plastic material theories[3,4]are standard in engineering,they need no explanation here. They are‘stress-based’since their yield conditions and yield potentials are formulated in stress-space.‘Strain-based’plasticity theories,with plastic stresses as internal thermodynamic variables and consequently yield con-ditions in strain-space,have their origins in the works [5,6].Remarkably early,Dougill[7]proposed a‘frac-turing theory’for concrete under compression,in our terminology a‘strain-based’damage concept.Opposite to plasticity,its basic idea required that inelastic defor-mations result merely in stiffness degradations,thus all unloading paths cross through the origin of the stress–strain diagram,and no residual strains remain after unloading.In a re-work Dougill and Rida[8]defined‘micro-fracturing’materials,equipped with stiffness degrada-tion from micro-crack evolution,linear-elastic behavior during un-/reloading,and without strain/stress residuals after complete unloading.Hence they laid the basis of ‘strain-based’continuum damage theories[9,10],in which the material stiffness tensor itself acts as internal damage variable.If instead of this theflexibility(com-pliance)tensor is applied,‘stress-based’continuum damage theories can be established[11,12].In order to properly model the constitutive behavior of concrete in compression or tension,both constitu-ents––plasticity as well as continuum damage theory––have to be combined,both either in‘stress-based’or ‘strain-based’alternatives.Suchfirst coupling work was due to Ba z ant and Kim[13].Their‘stress-based’plas-ticity combined with‘strain-based’damage theory led to an‘elasto-plastic fracturing’concept with a total of26 material parameters.Coupling concepts of both con-stituents in stress-space are due to[14],a work specified by[15]to isotropic damage of concrete under com-pression.[16]therein unified both limit surfaces for yielding and fracturing to one.Han and Chen[17]first combined both constituents in complete tensorial manifold in strain space.In order to reduce the number of material constants,they united yield and damage surface,an idea repeated in[10]. Further,[18]offered a scalar coupling of plasticity and damage theories introducing again a unified yield/dam-age condition with the key argument that micro-crack-ing in the cementitious matrix is the single source for both inelastic phenomena.Finally we mention the survey over all basic condi-tions of coupling alternatives in[19].We stress,that there only elasto-plastic-damage concepts are listed with a sound basis in continuum mechanics.Empirical models of reinforced concrete which all combine the mentioned constituents are legion[2];a prominent concept leading to excellent results is that one of Darwin and Pecknold[20].All mentioned constitutive models call,in principle, for the ability to describe material failure in compression as well as in tension,obviously both strongly localized phenomena[21].To avoid mesh sensitivity of a partic-ular FE-solution,such localized fracture processes re-quire special treatment[22].The intended constitutive law of this work is aimed to the analysis of complete structures,such that the classical‘smeared crack’con-cept shall be maintained[23].Such concept avoids the immense numerical effort of modeling hundreds of single cracks,presently to be mastered only by parallel com-puting techniques,in view of the physical irreproduc-ibility of crack-patterns in concrete structures,even under laboratory conditions.So in order to avoid modelling of localization bands or surfaces as weak or strong discontinuities,or to avoid enhancements by non-local kinematics,the use of crushing respectively fracture energy is chosen here[2]. Advantageously,such regularization requires only the determination of2additional material constants, namely the crushing and the fracture energy.Consequently,in this paper a‘stress-based’elasto-plastic damage model for reinforced concrete will be derived.It will be demonstrated,that not only the fun-damental ideas of micro-fracturing and damage theories are equal,but that both concepts lead to identical material descriptions[18],if the material stiffness tensor itself is used as internal thermodynamic variable.It hence is obvious that both phenomena––damage by tension cracks as well as by compressive micro-crack-ing––follow a unified elasto-plastic damage theory.The final constitutive law will possess a minimum number of material parameters,an important aspect for applica-tion.For this reason,the model is adapted without exact description of the response under highly triaxial com-pression.2.Theoretical basis of the elasto-plastic damage theoryThe fundamental framework of the presented con-cept is the‘stress-based’elasto-plastic damage theory. An excellent summary of this theory with the compli-ance tensor as internal variable has been given by Govindjee et al.[12]in complete analogy to plasticity theory.Recently a scalar combination of both damage and plasticity theory has been performed by[16].We start with a brief summary of the underlying concept.As in[12]our starting point is the assumption of a Helmholtz-free energy W and the definition of internal thermo-dynamical variables.To consider both,plastic deformation and stiffness reduction,we introduce as1202W.B.Kr€a tzig,R.P€o lling/Computers and Structures82(2004)1201–1215internal thermo-dynamical variables the plastic strain e plas in ordinary plasticity theory and the change D da of the compliance tensor like in [12].Then the Helmholtz-free energy reads:q 0W ðe ;e pl ;D da ;q Þ¼1ðe Àe pl Þ:ðD 0þD da|fflfflfflfflfflffl{zfflfflfflfflfflffl}DÞÀ1:ðe Àe plÞþq 0W inðq Þ;ð1Þwhere r and e denote the stress and strain tensors,respectively.D 0abbreviates the initial compliance ten-sor,and q as further internal variable describes the hardening/softening behavior of the material.W in ðq Þcontains the free energy associated with progressive degradation and plastic deformations.Finally,with q 0as initial density we identify the thermodynamically associated variables Àr ¼q 0o W =o e pl ;Àa ðq Þ¼q 0o W =o q ;ð2ÞÀ12r r ¼q 0o W =o D da :ð3ÞApplying the C LAUSIUS –D UHEM inequality q 0_W6r :_e ,we arrive on one hand at the compliance rela-tione ¼ðD 0þD da Þ:r þe pl !r ¼ðD 0þD da ÞÀ1:ðe Àe pl Þ;ð4Þand on the other hand at the inequality of dissipation P dis ¼12r :_D da :r þr :_e pl þa _q P 0:ð5ÞAfter introducing the elastic region E in the space of the associated variables (stresses):E :¼fðr ;a Þj U ðr r ;a Þ60g ;ð6Þwe deduce from the principle of maximum inelastic dissipation the following normality rules:_epl :o r o ðr r Þþ12_D da ¼o U o ðr r ÞÁ_k ;ð7Þ_a¼Ào U Á_k :ð8ÞAt this point we have to separate the inelastic strains into those of plastic and damaged origin.Because of few available cyclic experiments for later parameter fitting,the use of just one scalar b ,as proposed in [16]for separation of plastic from damaged parts,is adopted.Thus we assume _Dda ¼2_kb o U o ðr r Þand_epl ¼_k ð1Àb Þo U o r:ð9ÞObviously with (34),the normality rule (7)is fulfilled.With the consistency condition _U¼0one finally derives the tangential stiffness relation and the evolution equa-tions for the internal variables as summarized in Table 1.3.Unified elasto-plastic damage model for reinforced concrete3.1.Concrete under compression3.1.1.Yield/damage potentialA yield/damage potential of Drucker–Prager-type has been selected for description of concrete in com-pression.Such type of potential can be handled rela-tively easy,it enables a sufficient modelling quality at least for uniaxial and biaxial proportional loading.This potential reads U c ðr ;a c Þ¼11ffiffi3p Àl l I 1ÂþffiffiffiffiJ 2p ÃÀa c ðq c Þ;ð10Þwith I 1and J 2as first invariant of r and as secondinvariant of its deviator s ,respectively.The parameter l herein controls the influence of the hydrostatic stress on damage or yield.Further,the term left of the brackets guarantees that during plastic/damage loading a c always corresponds to the negative uniaxial compression stress.Differentiation of this potential with respect to r deliversTable 1Tangential stiffness relation and evolution equations of internal variables of the stress-based elasto-plastic damage theoryElastic loading/unloading _k ¼0Plastic-damaging loading k>0,U ¼0Tangential stiffness relation:_r ¼D À1:_e _r ¼ D À1ÀD À1:o U o r o Uo r:D À1o U :D :o U Ào U a ;q o U!:_e Equations of evolution of internal variables:_D da ¼0_D da ¼2b o U Á_k or ð_D da :r Þ¼b o U _k _e pl ¼0_e pl ¼ð1Àb Þo U o rÁ_k _q¼0_q¼o U o aÁ_k with _k ¼o Uo r:D À1o U:D :o U Ào Ua ;q o U:_eW.B.Kr €a tzig,R.P €o lling /Computers and Structures 82(2004)1201–12151203o U c o r ¼11ffiffi3p Àl l o I 1o r þ12ffiffiffiffiJ 2p o J 2o r¼11ffiffi3p Àl l I þs þs T4ffiffiffiffiJ 2p :ð11Þ3.1.2.Modification of the internal variable,yield anddamage ruleWe now introduce a new internal variable q Ãc ,replacing the original one q c :_q Ãc ¼À2b w ðr Þ_q c ¼2b w ðr Þ_k c with w ðr Þ¼21ffiffi3p Àl l1þ1ffiffiffi2p:ð12ÞThen a c in (10)shall be only a function of q Ãc .Due toSection 3.1.4this transformation allows an analytical solution for the hardening/softening function a c ðq Ãc Þfrom a given uniaxial stress–strain curve [2],one of our goals.The rate of this variable a c reads_a c ¼d a c Ãc _q Ãc ¼d a c Ãc 2b _k c :ð13ÞFurthermore we assume,that the parameter b ,which separates plastic from damage parts,shall also be a function of q Ãc .The equations of evolution of the plastic strain _epl and the strain _eda ¼_D da :r can directly be determined from the potential (10).With respect to (11)and (12)we gain:_e pl ¼ð1Àb ðq Ãc ÞÞo U c o r _k c ð14Þ¼w ðr Þ121b ðq Ãc Þ À111ffiffi3p Àl l I þ14ffiffiffiffiJ 2p s Àþs TÁ _q Ãc ð15Þand_e da ¼_D da :r ¼b ðq ÃcÞo U c o r _k c ð16Þ¼b ðq Ãc Þ11ffiffi3p Àl l I þ14ffiffiffiffiJ 2p s Àþs T Á_k c :ð17ÞAssuming now an isotropic damage evolution due to compression,we find the following compliance evolu-tion law,the correctness of which can be verified by an double scalar contraction with r :_Dda ;c ¼b ðq Ãc Þ1ffiffi3p Àl l 1 À16ffiffiffiffiJ 2p I I þ14ffiffiffiffiJ 2p ðI þI Þ!Á_k c ð18Þ¼w ðr Þ1211ffiffi3p Àl l I 1À16ffiffiffiffiJ 2pI I þ14ffiffiffiffiJ 2p ðI þI Þ!Á_q Ãc :ð19ÞThe tensor D da ;c describes the compliance evolution and can be represented by two scalars in the isotropic case.Consequently,we replace the fourth-order tensor D da ;cin the set of internal variables by D da ;c s1and D da ;cs2as fol-lows:D da ;c ¼D da ;c s1I I þD da ;c s2ðI þI Þ:ð20ÞBy comparison with the coefficients in (18)and (20),onearrives at evolution equations for D da ;c s1and D da ;cs2:_D da ;c s1¼b ðq Ãc Þ1ffiffi3p Àl l 1 À16ffiffiffiffiJ 2p _k c ;ð21Þ_D da ;c s2¼b ðq ÃcÞ1ffiffi3p Àl14ffiffiffiffiJ 2p_k c :ð22Þ3.1.3.Tangential stiffness relationBecause of all above assumptions the tangential stiffness relation slightly differs from the standard form in Table 1,and one arrives with q Ãc at:_r ¼D À1"ÀD À1:o U c o r o U c o r:D À1o U c o r :D À1:o U c o r þd a c d q ÃcÃc w ðr Þ#:_e ð23Þin the case of plastic-damaging loading.3.1.4.Concept of determination of the hardening/softeningfunctionWe now consider uniaxial loading with respect to an orthogonal cartesian frame,where the unit base vector i 1coincides with the loading direction.Then the compli-ance relation (4)becomese ¼1E cþD da ;c !r þe pl with e ¼e 11;e pl ¼e pl 11;r ¼r 11;1E c¼D 1111;D da ;c ¼D da ;c 1111ð24Þand the evolution equation (19)for the compliance boilsdown to_D da ;c ¼w ðr Þ1ffiffi3p Àl l r "À16ffiffi3p r !þ14ffiffi3p r Á2#Á_q Ãc ¼_q Ãc :ð25ÞBy integration of this relation one arrives at theimportant statement D da ;c ¼q Ãc ;ð26Þinterpreting the internal variable q Ãc as the uniaxial change of the compliance due to damage.Furthermore we assume the split of inelastic strains into plastic and damaging parts by the scalar parameter b ,as illustrated in Fig.1.From all previous transformations follows for the plastic strain e pl :1204W.B.Kr €a tzig,R.P €o lling /Computers and Structures 82(2004)1201–1215e pl¼b e pl h þD da ;cÁ rðe Þi!e pl ¼b1ÀbD da ;c Á rðe Þ¼b1Àb q ÃcÁ r ðe Þ:ð27ÞWe then obtain by substituting (26)and the latter identity into (24)e ¼1c þ1q Ãc ! r ðe Þ;ð28Þwhich equation solves with given function rðe Þfor e as e ¼e ðq Ãc Þ,yielding together with the stress–strain instruction finally to a c ðq Ãc Þ¼ r ðe ðq Ãc ÞÞ:ð29ÞFurthermore we need the differential quotient d a c =d q Ãcand findd a c d q Ãc ¼Àd r de d e d q Ãc ¼a c d r d e ð1Àb Þ1À1E c þq Ãc 1Àb dr d eh i ;ð30Þwith d e =d q Ãc gained by total differentiation of Eq.(28).Finally we need the explicit form of b ðq Ãc Þ.Starting point is the evolution equation (15),which reduces under uniaxial loading conditions to_e pl¼1Ãc À1r Á_q Ãc ;ð31Þfrom which we find directly b ðq Ãc Þ¼11þo e =o q cc c :ð32ÞFor further transformation we can conclude from (27)o e pl o q Ãc ¼b 1Àb a c ðq Ãc Þþq Ãcd a c d q Ãc !;ð33Þleading finally to b ðq Ãc Þ¼11þb 1þq Ãcc Ãcd a cÃchi :ð34Þ3.1.5.Mapping to uniaxial stress–strain curveFor convenience the uniaxial stress–strain curve of concrete,assumed here as given function,shall be sub-divided into three parts as illustrated in Fig.2.Region 1:Elastic.Below the initial yield/damage stress f c y we assume linear-elastic behavior,defined by Young’s modulus E c and Poisson’s ratio m c .The initial yield stress f c y is taken as one third of the failure strength f c ,such that we obtain the initial conditiona c ðq Ãc ¼0Þ¼f c y ¼13f c :ð35ÞRegion 2:Hardening.In this region the stress grows until failure strength f c ,consequently the tangential stiffness decreases from initial stiffness to zero (hori-zontal tangent).An analytical function of this behavior,which fits well with experiments is found in [2].Eq.(2.1-18)therein readsr2ðe Þ¼E ci e cþe c21ÀE ci e c fcÀ2e e cf c ;ð36Þwith f c and e c as failure stress and accompanying strain,respectively.The modulus E ci ;originally denotes the initial modulus of elasticity.But in order to guarantee the stress–strain curve to cross the point ðÀe c y ;Àf c y Þ,we define E ci ;with respect to (35)as secant modulus[2]Fig.1.Definition of material parameter b.Fig.2.Assumed uniaxial stress–strain curve of concrete undercompression.W.B.Kr €a tzig,R.P €o lling /Computers and Structures 82(2004)1201–12151205E ci¼12E cf ce c2Àf ce cþ32E c:ð37ÞRegion3:Softening.After exceeding the compression strain e c,localization of damage occurs in this softening region.A suitable concept to overcome possible ill-po-sedness there,originally derived for tension softening in smeared crack idealization,is the use of fracture energy: Then the softening branch depends on the fracture en-ergy,a material parameter,and on the characteristic length l eq[24].Use of fracture energy as material parameter instead of a softening function is generally accepted for tension cracks.The transfer of this concept to softening under compression has beenfirst proposed by Feenstra[25] and accepted since that,i.e.[26].To distinguish fracture energy under tension from that one under compression, the latter will be denoted as‘crushing energy’.But one should notice,that because of nonlinear hardening also a diffuse crushing energy g cu;exists[27],not included in the presently described localized one.Thus one sets thevolume specific localized crushing energy gÃcl (compareFig.2)equal to G cl=l eq,where G cl is the material parameter‘crushing energy’and l eq the characteristic length of the respective FE integration point.Clearly,l eq depends on type,quadrature rule and form of the ele-ment[28].Consequently,the following function has been cho-sen for the softening branch of region3:rðeÞ¼À12þc c f c e ccþc c eþc ccÁe2with c c>0ð38Þin which c c is the only free parameter controlling thearea under the stress–strain curve,corresponding to gÃcl .Note,that this area isfinite.Determination delivers the following relation to the localized crushing energy G cl,as explained in detail in[19]:c c¼p2f c e c2G cleq À1f c e cð1ÀbÞþb f cch i2:ð39ÞRemark.If the term in square brackets becomes nega-tive,the above equation renders invalid,since this would describe a‘snap-back’-behavior in the considered material point.To avoid this,one should elude elements leading tol eq6G clf c e cð1ÀbÞþb f cE c:ð40ÞFor determination of a cðqÃc Þ(29)from re-branches,seeAppendix A.3.2.Concrete under tensionFor description of concrete behavior in tension we apply the stress-based continuum damage theory again. But for simplicity,all inelastic deformations shall be due to pure damaging,i.e.b¼1.Also,opposite to com-pression,we exclude nonlinear hardening before soft-ening.Thus the initial damage surface and the failure surface are identical.3.2.1.Damage potentialFor tension failure,we use the widely accepted damage potential of Rankine type,a criterium in good accordance with biaxial experiments[29].To allow for softening in different directions to model cracks in dif-ferent directions at least a kinematic softening rule is required.Hence introducing the back-stress a t of the stress state r as internal variable,the damage potential readsU tð1Þðr;a tÞ¼nð1ÞÀf ct60;ð41Þabbreviating with nð1Þthefirst eigenvalue of n¼rÀa t.The derivatives of this potential with respect to r and a t areo U to r¼Mð1Þnando U to a t¼ÀMð1Þn;ð42Þwhere Mð1Þndenotes the eigenvalue basis of thefirst eigenvalue of n.3.2.2.Modification of internal variable,kinematic soften-ing and damage ruleIf one follows strictly the thermodynamic concept of the continuum damage theory,one has to introduce asecond-order tensor qt,thermodynamically conjugate to a t.Furthermore one has tofind an evolution law as normality condition for this tensor and a tensor-valued function a tðq tÞas softening rule.The evolution law then reads_a t¼o a t=o q t:_q t.Instead of such complexity determination,we replace the fourth-order tensoro a t=o q t by a scalar function Zða1t nÞ,with a1t n¼a t:Mð1Þnas normal component of the back-stress in crack-direction.Introduction of an explicit conjugate variable q1t ncan be omitted,because no hardening regime has been as-sumed,and the function q1t nða1t nÞtherefore is bijective. These simplifications lead to the softening rule,illus-trated in Fig.3:_a t¼Zða1t nÞo U to a t_ktð1Þ¼À_k tð1ÞZða1t nÞMð1Þnð43Þcorresponding to Prager’s hardening law.(43)guaran-tees crack formation in each principal stress direction, independent of other existing cracks.Now the normality rule can be derived from the potential U tð1Þ(41)reading1206W.B.Kr€a tzig,R.P€o lling/Computers and Structures82(2004)1201–1215_e da ;t :¼_D da ;t :r ¼_k t ð1Þo U t ð1Þ¼_k t ð1ÞM ð1Þn :ð44ÞThe anisotropic damage rule:_Dda ;t ¼1n_k t ð1ÞM ð1Þn M ð1Þnwithr 1n¼M ð1Þn:r ð45Þhas been taken over from [12].This rule guarantees the accomplishment of (44),and preserves symmetry of the compliance tensor.3.2.3.Consideration of further cracksAs well known,a second crack in 2D-plane and a third one in 3D-space can appear,each orthogonal to the existing ones.Therefore two more damage potentials are introduced analogously to the original one (41):U t ð2Þðr ;a t Þ¼n ð2ÞÀf ct ;60;ð46ÞU t ð3Þðr ;a t Þ¼n ð3ÞÀf ct ;60:ð47ÞThe complete theory thus develops to a non-smoothmulti-surface continuum damage theory.According to Koiter’s rule [30],we then obtain _at ¼ÀX 3i ¼1_k t ði ÞZ ða i t nÞM ði Þn ;ð48Þ_Dda ;t ¼X 3i ¼11r i n _k t ði ÞM ði Þn M ði Þn with r i n ¼M ði Þn :r :ð49ÞLoading conditions have to been introduced for each crack direction separately:_kt ði ÞP 0;U t ði Þ60;_kt ði ÞÁU t ði Þ¼0:ð50Þ3.2.4.Closure of cracksCrack closures results in instantaneous local re-stiff-ening of the concrete,where the cracks remain as ‘pas-sive’ones.Following an idea of Ortiz [11]we thusdisregard in the compliance tensor D the constituent D da ;t ,which represents all cracks,but maintain D da ;ta of all ‘active’cracks:D ¼D 0þD da ;c þD da ;ta :ð51ÞKraj c inovi c [31]obtained the following relation between D da ;ta and D da ;t ,assembling only those active cracks in principal directions,which belong to positive principal stresses:D da ;ta ¼P þ:D da ;t :P þ;ð52Þwhere P þdenotes a fourth-order projection tensor.According to [31],one possible form of P þwith H ðÞas Heaviside-function is:P þ¼X 3i ¼1H r ði ÞÀÁM ði Þr M ði Þr :ð53Þ3.2.5.Tangential stiffness relationWith all these assumptions the tangential stiffness relation will expectedly slightly differ from that one in Section 2.For its evaluation,we determine the consis-tency parameters by use of the consistency condition _Ut ði Þ¼0,the damage rule (49)and the kinematic soft-ening rule (48),finally arriving at:_r ¼D À1"ÀX 3i ¼1H _k t ði Þ D À1:M ði Þn M ði Þn :D À1M ði Þn :D À1:M ði ÞnÀZ a i t n ÀÁ#:_e :ð54Þ3.2.6.Assumed uniaxial stress–strain curve of concreteunder tensionIn order to relate our theory to experiments,the softening function Z a it n ÀÁwill be determined directly from a given uniaxial curve analogously to the proce-dure in Section 3.1.5.Due to Fig.4we hence distinguish:Region 1:Elastic region.Below the tension strength,the initial damage stress f ct ,linear-elastic behavior isassumed.Fig.3.Illustration of the assumed kinematic softening rule.W.B.Kr €a tzig,R.P €o lling /Computers and Structures 82(2004)1201–12151207。
2024年考研英语一完形填空详解

Roots and affixes memory method
Using knowledge of roots and affixes to expand vocabulary and improve memory efficiency.
• Problem solving technique: First, read the entire text thoroughly to understand the main idea of the article; Analyze sentence by sentence and choose the best answer based on context and logical relationships; Finally, reread the entire text and check if the answers are reasonable.
Associative memory method
associating new vocabulary with known things or images to form interesting associations and help with memory.
The recognition and application of phrase collocation in articles
02
Analyze clauses
Identify the types of clauses and understand how clauses modify or supplement the main clause.
材料科学与工程_专业英语_Unit_2_Classification_of_Materials译文

Solid materials have been conveniently grouped into three basic classifications: metals, ceramics, and polymers. This scheme is based primarily on chemical makeup and atomic structure, and most materials fall into one distinct grouping or another, although there are some intermediates. In addition, there are three other groups of important engineering materials—composites, semiconductors, and biomaterials.译文:固体材料被便利的分为三个基本的类型:金属,陶瓷和聚合物。
这个分类是首先基于化学组成和原子结构来分的,大多数材料落在明显的一个类别里面,尽管有许多中间品。
除此之外,有三类其他重要的工程材料-复合材料,半导体材料和生物材料。
Composites consist of combinations of two or more different materials, whereas semiconductors are utilized because of their unusual electrical characteristics; biomaterials are implanted into the human body. A brief explanation of the material types and representative characteristics is offered next.译文:复合材料由两种或者两种以上不同的材料组成,然而半导体由于它们非同寻常的电学性质而得到使用;生物材料被移植进入人类的身体中。
给学生上体育课英语作文

Teaching physical education to students is a crucial aspect of their overall development.It not only helps in building a strong physique but also in fostering teamwork,discipline,and a competitive spirit.Here are some key points to consider when writing an essay on teaching physical education to students:1.Introduction to Physical Education:Begin by explaining the importance of physical education in a students life.Discuss how it contributes to their physical,mental,and social wellbeing.2.Curriculum Design:Describe the structure of a typical physical education curriculum. Include activities such as team sports,individual sports,gymnastics,dance,and fitness training.3.Skill Development:Highlight the various skills that students can learn through physical education,such as coordination,balance,agility,and strength.4.Health Benefits:Discuss the longterm health benefits of regular physical activity, including improved cardiovascular health,weight management,and prevention of chronic diseases.5.Mental Health Aspects:Explain how physical education can help reduce stress, improve mood,and increase selfesteem among students.6.Social Skills:Emphasize the role of physical education in teaching students social skills like communication,cooperation,and leadership.7.Inclusivity and Adaptability:Address the need for physical education programs to be inclusive,catering to students of all abilities and adapting activities to suit individual needs.8.Safety Measures:Mention the importance of safety in physical education classes, including the use of appropriate equipment,warmup exercises,and injury prevention techniques.9.Technological Integration:Discuss how technology can be integrated into physical education to enhance learning experiences,such as using fitness trackers or virtual reality for simulations.10.Assessment and Feedback:Explain the methods of assessment used in physical education,including both physical performance and personal development,and theimportance of constructive feedback.11.Challenges and Solutions:Identify common challenges faced in physical education classes,such as lack of interest,physical limitations,or inadequate facilities,and propose solutions to overcome these issues.12.Conclusion:Conclude by reiterating the significance of physical education in holistic student development and the role of educators in making it an engaging and beneficial part of the school curriculum.Remember to use descriptive language and provide examples to illustrate your points effectively.Additionally,ensure that your essay is wellstructured,with a clear introduction,body,and conclusion.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
19. X. Li, Z. Yang, L. Guan, C. Liu, and P. Li, “Luminescent properties of Eu3+-doped La2Mo2O9 red phosphor by the flux method,” J. Cryst. Growth 310(12), 3117–3120 (2008). 20. M. Wierzbicka-Wieczorek, U. Kolitsch, and E. Tillmanns, “Ba2Gd2(Si4O13): a silicate with finite Si4O13 chains,” Acta Crystallogr. C 66(3), i29–i32 (2010). 21. G. Blasse, “Energy transfer in oxidic phosphors,” Phys. Lett. A 28(6), 444–445 (1968).
©2011 Optical Society of America
OCIS codes: (160.2540) Fluorescent and luminescent materials; (160.5690) Rare-earth-doped materials.
References and Links
Preparation, structural and luminescent properties of Ba2Gd2Si4O13:Eu3+ for white LEDs
Hai Guo,1,* Hao Zhang,1, 2 RongFei Wei,1 MengDi Zheng,1 LiHong Zhang1
#140475 - $15.00 USD
Received 4 Jaቤተ መጻሕፍቲ ባይዱ 2011; revised 28 Feb 2011; accepted 1 Mar 2011; published 11 Mar 2011
(C) 2011 OSA
14 March 2011 / Vol. 19, No. S2 / OPTICS EXPRESS A201
Abstract: To find out efficient red phosphors used for white light-emitting diodes (LEDs), a new Ba2Gd2Si4O13:Eu3+ phosphor was prepared by conventional solid-state reaction method. The effect of Li2CO3 flux and Eu3+ doping concentrations on structural and luminescent properties of Ba2Gd2Si4O13 phosphors was studied in detail. The phosphors show intense absorption in near ultraviolet-blue region and exhibit intense red emissions with CIE coordinates of (0.66, 0.34) under 393 nm excitation. The integrated emission intensity of Ba2(Gd0.4Eu0.6)2Si4O13 excited at 393 nm, 362 nm and 464 nm is about 3.5, 4.0 and 3.1 times as that of Y 2O3:Eu3+ commercial phosphors, respectively. The excellent luminescent properties and good color saturation make it a promising red phosphor for white LEDs.
1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. E. F. Schubert and J. K. Kim, “Solid-state light sources getting smart,” Science 308(5726), 1274–1278 (2005). S. Nakamura, T. Mukai, and M. Senoh, “Candela-class high-brightness InGaN/AlGaN double-heterostructure blue-light-emitting diodes,” Appl. Phys. Lett. 64(13), 1687–1689 (1994). S. Ye, F. Xiao, Y. X. Pan, Y. Y. Ma, and Q. Y. Zhang, “Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties,” Mater. Sci. Eng. Rep. 71(1), 1–34 (2010). X. Piao, T. Horikawa, H. Hanzawa, and K. I. MacHida, “Characterization and luminescence properties of Sr2Si5N8:Eu2+ phosphor for white light-emitting-diode illumination,” Appl. Phys. Lett. 88(16), 161908 (2006). H. Guo, X. Wang, X. Zhang, Y. Tang, L. Chen, and C. Ma, “Effect of NH4F flux on structural and luminescent properties of Sr2SiO4:Eu2+ phosphors prepared by solid-state reaction method,” J. Electrochem. Soc. 157(8), J310–J314 (2010). T. W. Kuo, W. R. Liu, and T. M. Chen, “High color rendering white light-emitting-diode illuminator using the red-emitting Eu(2+)-activated CaZnOS phosphors excited by blue LED,” Opt. Express 18(8), 8187–8192 (2010). S. Lee and S. Y. Seo, “Optimization of yttrium aluminum garnet:Ce3+ phosphors for white light-emitting diodes by combinatorial chemistry method,” J. Electrochem. Soc. 149(11), J85–J88 (2002). Y. Zhou, J. Liu, X. Yang, X. Yu, and J. Zhuang, “A promising deep red phosphor AgLaMo2O8:Pr 3+ with blue excitation for white LED application,” J. Electrochem. Soc. 157(3), H278–H280 (2010). X. Yang, J. Liu, H. Yang, X. Yu, Y. Guo, Y. Zhou, and J. Liu, “Synthesis and characterization of new red phosphors for white LED applications,” J. Mater. Chem. 19(22), 3771–3774 (2009). W. R. Liu, C. C. Lin, Y. C. Chiu, Y. T. Yeh, S. M. Jang, and R. S. Liu, “ZnB2O4:Bi3+,Eu3+:a highly efficient, redemitting phosphor,” Opt. Express 18(3), 2946–2951 (2010). J. S. Kim, P. E. Jeon, Y. H. Park, J. C. Choi, H. L. Park, G. C. Kim, and T. W. Kim, “White-light generation through ultraviolet-emitting diode and white-emitting phosphor,” Appl. Phys. Lett. 85(17), 3696–3698 (2004). H. Guo, X. Wang, J. Chen, and F. Li, “Ultraviolet light induced white light emission in Ag and Eu3+ co-doped oxyfluoride glasses,” Opt. Express 18(18), 18900–18905 (2010). H. Guo, H. Zhang, J. Li, and F. Li, “Blue-white-green tunable luminescence from Ba2Gd2Si4O13:Ce3+,Tb3+ phosphors excited by ultraviolet light,” Opt. Express 18(26), 27257–27262 (2010). G. Gundiah, Y. Shimomura, N. Kijima, and A. K. Cheetham, “Novel red phosphors based on vanadate garnets for solid state lighting applications,” Chem. Phys. Lett. 455(4-6), 279–283 (2008). J. Zhang and Y. Wang, “Eu3+-doped Ba3Bi(PO4)3: A red phosphor for white light-emitting diodes,” Electrochem. Solid-State Lett. 13(4), J35–J37 (2010). W. R. Liu, C. C. Lin, Y. C. Chiu, Y. T. Yeh, S. M. Jang, R. S. Liu, and B. M. Cheng, “Versatile phosphors BaY2Si3O10:RE (RE = Ce3+, Tb3+, Eu3+) for light-emitting diodes,” Opt. Express 17(20), 18103–18109 (2009). H. Yang, G. Lakshminarayana, S. Zhou, Y. Teng, and J. Qiu, “Cyan-white-red luminescence from europium doped Al2O3-La2O3-SiO2 glasses,” Opt. Express 16(9), 6731–6735 (2008). G. Blasse and B. C. Grabmaier, Luminescent Materials (Springer, Berlin) (1994).
