制药工艺验证实施手册缩略语
临床试验常用术语及缩略语

临床试验常用术语及缩略语监管机构和法规相关◆NMPA国家药品监督管理局(National Medical Products Administration)◆CDE国家药品监督管理局药品审评中心(CENTER FOR DRUG EVALUATION,NMPA)◆国家药品不良反应监测中心(National Center for ADR Monitoring,China)◆GCP药物临床试验质量管理规范(Good Clinical Practice)◆GMP药品生产质量管理规范(GoodManufacturingPractice)◆GLP药物非临床研究质量管理规范(Good Laboratory Practice of drug)◆ICH人用药品注册技术要求国际协调会议(InternationalConference on Harmonization)◆ICH GCP:指导原则的目的是为欧盟、日本和美国提供统一的标准,以促进这些管理当局在其权限内相互接受临床数据。
本指导原则的发展考虑了欧盟、日本、美国,以及澳大利亚、加拿大、北欧国家和世界卫生组织(GCP)的现行GCP。
◆WHO世界卫生组织(World Health Organization)◆FDA美国食品与药品管理局(Food and Drug Administration)文件相关◆CTP临床试验方案(Clinical Trial Protocol):指说明临床试验目的、设计、方法学、统计学考虑和组织实施的文件。
试验方案通常还应当包括临床试验的背景和理论基础,该内容也可以在其他参考文件中给出。
试验方案包括方案及其修订版。
◆SOP标准操作规程(Standard Operating Procedure):指为保证某项特定操作的一致性而制定的详细的书面要求。
◆TMF试验主文件夹/研究管理文件夹(Trial Master File)◆IB研究者手册(Investigator’s Brochure):指与开展临床试验相关的试验用药品的临床和非临床研究资料汇编。
GMP常见英文缩写

GMP常见英文缩写AQAI(Automated Quality Assurance Inspection Equipment):在线自动质量保证检查设备API(Active Pharmaceutical Ingredient):活性药物物质,即原料药ANDA(Abbreviated New Drug Application):简化新药申请ADR(Adverse Drug Reaction):不良反应BSE(Bovine Spongiform Encephalopathy):疯牛病BPCS(Business Planning and Control System):业务计划及控制系统BIA(Business impact assessment):商业影响评估cGMP(current Good Manufacturing Practice):现行药品生产质量管理规范CCCD(China Certification Committee for Drugs):中国药品认证委员会CIP(Cleaning In Place):在线清洁CV(Concurrent Validation):同步验证CDER(Center for Drug Evaluation and Research):药品研究与评价中心COA(Certificate Of Analysis):分析报告单CFR(Code of Federal Regulation):(美国)联邦法规CDC(Centers for Disease Control and Prevention):疾病预防控制中心COS/CEP(Certificate of Suitability for European Pharmacopeia):欧洲药典适用性证书CCD(Certification Committee for Drugs):药品认证管理中心CPMP(Committee for Proprietary Medicinal Products):欧洲专利药品委员会CTD(Common Technical Document):通用技术文件CDC(Centers for Disease Control and Prevention):疾病预防控制中心GMP(Good Manufacturing Practice):药品生产质量管理规范ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use):人用药品注册技术要求国际协调会EU(European Union):欧洲联盟EFPIA(European Federation of Pharmaceutical Industries Associations):欧洲制药工业协会联合会MHW(Ministry of Health and Welfare,Japan):日本厚生省JPMA(Japan Pharmaceutical Manufacturers Association):日本制药工业协会FDA(US Food and Drug Adminiistration):美国食品与药品管理局PRMA(Pharmaceutical Research and Manufacturers of America):美国药物研究和生产联合会WHO(World Health Organization):世界卫生组织IFPMA(International Federation of Pharmaceutical Manufacturers Associations):国际制药工业协会联合会TQC(Total Quality Control),TQM(Total Quality Management):全面质量管理PDCA(Plan,Do,Check,Action):计划,执行,检查,处理QA(Quality Assurance):质量保证QC(Quality Control):质量控制QS(Quality System):质量体系QM(Quality Management):质量管理SOP(Standard Operating Procedure):标准操作规程SMP(Standard Management Procedure):标准管理程序SOR(Standard Operating Record):标准操作记录GEP(Good Engineering Practice):工程设计规范HV AC(Heating Ventilation and Air Conditioning):空调净化系统DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认OOS(Out-Of-Specification):检验不合格;超标PFDS(Process Flow Diagrams):工艺流程图MRA(cMutual Reognition Agreements):现场检查多边认同协议DMF(Drug Master File):EDMF(European Drug Master File)欧盟药物主文件EDQM(European Directorate for Quality Medicines):欧洲药品质量管理局ORA(Office of Regulatory Affairs):药政事务办公室GGPs(Good Guidance Practices):优良指南规范MOA(Method Of Analysis):分析方法VMP(Validation Master Plan):验证主计划VP(Validation Protocol):验证方案MSDS(Material Safety Data Sheet):物料安全技术说明书NDA(New Drug Application):新药申请OTC(Over-the-counter):非处方INN(International Nonproprietary Name)国际非专有名称USP(the united state pharmacopeia):美国药典NF(National Formulary):(美国)国家药品集GAP(Good Agricultural Practice):中药材种植管理规范GCP(Good Clinical Practice):药物临床试验质量管理规范GLP(Good Laboratory Practice):药物实验室管理规范GSP(Good Supply Practice):药品经营质量管理规范GUP(Good Use Practice):药品使用质量管理规范SM(Starting Material):起始物料PMF(Plant Master File);SMF(Site Master File):工厂主文件EDL(List of Essential Drugs):基本药物目录PI(Package Insert):说明书PCT(Patent Cooperation Treaty):专利合作条约PPAC(Patent Protection Association of China):中国专利保护协会PIC(Person In Charge):负责人PDS(Pharmaceutical Development Services):整体新药研发机构SPC(Summary of Product Characteristics):产品特性摘要。
常用制药及GMP英文缩写

ISO(International Organization for Standardization):国际标准化组织日常办事机构是中央秘书处,设在瑞士日内瓦WHO(World Health Organization):世界卫生组织是联合国属下的专门机构,国际最大的公共卫生组织,总部设于瑞士日内瓦PIC/S(Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme):国际医药品稽查协约组织由欧洲自由贸易区(EFTA)组建ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use):人用药物注册技术要求国际协调会由欧盟(EU)、欧洲制药工业协会联合会(EFPIA)、日本厚生省(MHW)、日本制药工业协会(JPMA)、美国FDA、美国药物研究生产联合会(PRMA)等机构组成WHO、EFTA、加拿大卫生保健局(CHPB)为观察员ISPE(International Society for Pharmaceutical Engineering):国际制药工程协会是致力于培训制药领域专家并提升制药行业水准的世界最大的非盈利性组织之一,在美国坦帕州设有全球总部,在布鲁塞尔设有欧洲总部,亚洲总部在新加坡HHS(United States Department of Health and Human Services):美国卫生及公共服务部(美国卫生部)FDA(Food and Drug Administration):美国食品药品监督管理局(HHS下属机构)PDA(Parenteral Drug Association):美国注射剂协会EPA(Environmental Protection Agency):美国国家环境保护局CDER(Center for Drug Evaluation and Research):FDA药物评价与研究中心EMEA(The European Agency for the Evaluation of Medicinal Products):欧洲药物评审组织MHW(Ministry of Health and Welfare):日本厚生省,现改为厚生劳动省MHLW(Ministry of Health, Labor and Welfare),负责医疗卫生和社会保障的主要部门D&B(Dun & Bradstreet):邓白氏公司DUNS(Data Universal Numbering System):邓白氏公司提供的唯一的公司代号,用于信用评级等在SMF文件中会用到GMP(Good Manufacturing Practice):药品良好生产规范cGMP(Current Good Manufacture Practices):动态药品生产管理规范,即现行的GLP(Good Laboratory Practice):药物非临床研究质量管理规范,及优良实验室规范GSP(Good Supplying Practice):药品经营质量管理规范,及良好的药品供应规范GAP(Good Agricultural Practice for Chinese Crude Drugs):中药材生产质量管理规范GDP(Good Documentation Practice):良好文件管理GEP(Good Engineering Practice):工程设计规范GAMP(Good Automated Manufacturing Practice):优良自动化生产规范USP(united states pharmacopeia):美国药典EP(European Pharmacopeia):欧洲药典JP(Japanese Pharmacopoeia):日本药典CFR(Code of Federal Regulations):美国联邦法律CFR 21 Part 11(Code of Federal Registry Part11):联邦法规法律标题21第11部分CEP/COS(Certificate of Suitability to the monographs of European Pharmacopoeia):欧洲药典适应性认证证书CEP认证,COS证书CTD(Common Technical Document):国际注册用常规技术文件CTD文件是国际公认的文件编写格式,用来制作一个向药品注册机构递交的结构完善的注册申请文件EHS(Environment、Health、Safety):环境-健康-安全管理体系HACCP(Hazard Analysis and Critical Control Point):(保健食品)危害分析和关键控制点REACH(REGULATION concerning the Registration, Evaluation, Authorization and Restriction of Chemicals):欧盟规章《化学品注册、评估、许可和限制》,欧盟建立的,并于2007年6月1日起实施的化学品监管体系ICH-Q1A:新原料药和制剂的稳定性试验ICH-Q1B:稳定性试验:新原料药和制剂的光稳定性试验ICH-Q1C:稳定性试验:新剂型的要求ICH-Q1D:新原料药和制剂的稳定性试验的括号法和矩阵法设计ICH-Q1E:稳定性数据的评价ICH-Q1F:气候带Ⅲ和Ⅳ注册申请的稳定性数据ICH-Q2A:分析步骤验证:正文ICH-Q2B:分析步骤验证:方法学ICH-Q3A:原料药中的杂质ICH-Q3B:新制剂中的杂质ICH-Q3C:杂质;残留溶剂的指导原则ICH-Q4 :药典ICH-Q4A :药典的同一化ICH-Q4B:各地区使用的药典正文评估和建议ICH-Q5A:来源于人或动物细胞系的生物技术产品的病毒安全性评价ICH-Q5B:生物技术产品的质量:rDNA衍生蛋白质产品生产细胞的表达构建体分析ICH-Q5C:生物技术产品的质量:生物制品/生物技术产品的稳定性试验ICH-Q5D:用于生物技术产品及生物制品生产的细胞基质的来源和鉴定ICH-Q5E:生物技术产品/生物制品在工艺变更时的可比性ICH-Q6A:质量标准新原料药和制剂的检测以及可接受标准:化学物质ICH-Q6B:质量标准:生物技术产品及生物制品的检测方法和可接受标准ICH-Q7 :原料药良好制造规范(ICH-Q7A的新版)ICH-Q7A:原料药的GMP规范ICH-Q8 :药物研发指南ICH-Q9 :质量风险管理ICH- Q10(PQS):药物质量体系QA(Quality Assurance):质量保证QC(Quality Control):质量控制QRM(Quality Risk Management):质量风险管理IPC(Inproceics Quality Control):制程品质控制/中控OOS(Out of Specification):检验结果超标OOT(Out of Trend):超趋势结果OOL(Out of Limit):超出极限的结果,如温湿度等OOE(Out of Expectation):超期望结果SAL(Sterility Assurance Level):无菌保证水平灭菌后微生物的存活概率的负对数,要求≥6D值:杀灭90%的微生物所需要的时间,D值越大,微生物死亡越难,D值与细菌的耐热性成正比Z值:指灭菌时间减少到原来的10%所需要升高的温度或是相同的灭菌时间内杀死99%的微生物所需要提高的温度F值:为一定温度下,给定Z值所产生的灭局效果与参比温度T0下给定Z值所产生的灭菌效果相同时所相当的时间F值用于干热灭菌F0值:为一定温度下,Z值为10℃产生的灭菌效果与120℃,Z值为10℃时产生的灭菌效果相当的时间,t分钟内的灭菌效果相当于120℃下灭菌F0分钟的效果F0被称为标准灭菌时间,用于热压灭菌LRV:除菌过滤的对数下降值LRV=lgN0-lgNSOP(Standard Operation Procedure):标准操作规程DMF(Drug Master File):药品主文件SMF(Site Master File):工厂主文件URS(User Requirement Specification):用户需求标准FS(Functional Specification):功能标准DS(Design Specification):设计标准DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认RQ(Requalification):再确认CAPA(Corrective Action & Preventive Action):纠正预防系统,Q10的四大要素之一QbD(Quality by Design):质量源于设计COA(Certificate of Analysis):分析证书/检验报告书/检验报告单BPR(Batch Production Record):批生产记录API(Active Pharmaceutical Ingredients):药物活性成分,通常指的原料药。
制药项目常见英文缩写的含义
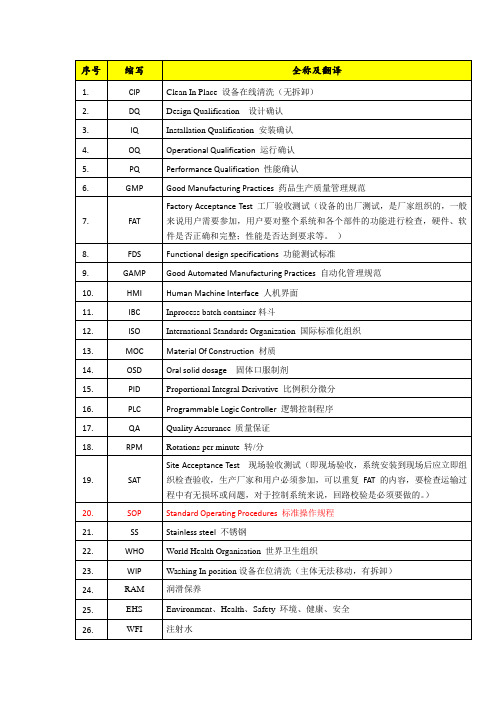
GMP
Good Manufacturing Practices药品生产质量管理规范
7.
FAT
FactoryAcceptance Test工厂验收测试(设备的出厂测试,是厂家组织的,一般来说用户需要参加,用户要对整个系统和各个部件的功能进行检查,硬件、软件是否正确和完整;性能是否达到要求等。)
8.
16.
PLC
Programmable Logic Controller逻辑控制程序
17.
QA
Quality Assurance质量保证
18.
RPM
Rotations per minute转/分
19.
SAT
Site Acceptance Test现场验收测试(即现场验收,系统安装到现场后应立即组织检查验收,生产厂家和用户必须参加,可以重复FAT的内容,要检查运输过程中有无损坏或问题,对于控制系统来说,回路校验是必须要做的。)
序号
缩写
全称及翻译
1.
CIP
Clean InPlace设备在线清洗(无拆卸)
2.
DQ
Design Qualification设计确认
3.
IQ
Installation Qualification安装确认
4.
OQ
Operational QQ
Performance Qualification性能确认
润滑保养
25.
EHS
Environment、Health、Safety环境、健康、安全
26.
WFI
注射水
27.
PW
纯化水
FDS
Functional design specifications功能测试标准
临床试验常用的英文缩略语

临床试验常用的英文缩略语TTP:time-to-progression 疾病进展时间SAE:severity Adverse Event严重不良事件AE:Adverse Event不良事件SOP:standard operation practice标准操作规程CRF: Case Report form病例报告表DLT:剂量限制毒性MTD:最大耐受剂量KPS:Karnofsky Performance Status行为状态评分CR:complete response完全缓解PR:partial response部分缓解SD:病情稳定PD:progressive disease病情进展CTC:常用药物毒性标准IEC:independent ethics committee 独立伦理委员会IRB :institutional review board 伦理委员会CRA:Clinical Research Associate临床研究助理临床监察员。
监查员是申办者与研究者之间的主要联系人。
其人数及访视的次数取决于临床试验的复杂程度和参与试验的医疗机构的数目。
监查员应有适当的医学、药学或相关专业学历,并经过必要的训练,熟悉药品管理有关法规,熟悉有关试验药物的临床前和临床方面的信息以及临床试验方案及其相关的文件。
监查的目的是为了保证临床试验中受试者的权益受到保障,试验记录与报告的数据准确、完整无误,保证试验遵循已批准的方案和有关法规。
主要负责临床监查工作,包括医院筛选、协议谈判、资料交接和管理、临床试验前、中、后期的监查工作,按照要求进行监查并填写相关资料,保证临床试验的顺利进行,并符合国家的相关法律法规和公司的利益。
CRA在中国又叫“MONITOR”,药品注册申请人或专业的临床实验代理机构均有此职位,属临床试验的最基础工作,不仅需要懂得GCP的相关要求,更要熟悉所试验药品的基本知识、临床方案、CRF表的理解,同时还应该熟悉与研究者的沟通与交流技巧。
药学英语缩略语词汇

药学英语缩略语词汇5-FU(5-fluorouracil)5-氟尿嘧啶5-HT(5-hydroxytryptamine,serotonin:5-羟色胺)5-羟色胺6-MP(6-mercaptopurine)6-巯基嘌呤A/G (albumin/ globin:珠蛋白;球蛋白;珠朊;血球蛋白)白球蛋白比率ACD (acid-citrate-dextrose)酸性枸橼酸葡萄糖ACP (acid phosphatase)酸性磷酸酶ACTH (Adrenocorticotropic Hormone)促肾上腺皮质激素ADH (antidiuretic hormone)抗利尿激素ADP (adenosine diphosphate)二磷酸腺苷AFP (alpha-fetoprotein)甲(种)胎(儿)蛋白AHF (antihemophilic factor)抗血友病因子AKP (alkaline phosphatase)碱性磷酸酶ALL (Acute lymphocytic leukemia)急性淋巴细胞性白血病ALS (anti lymphocyte serum)抗淋巴细胞血清ALT(GPT) (alanine aminotransferase/glutamic-pyruvic transaminase)丙氨酸转氨酶AMA (anti-mitoehondria antibody)抗线粒体抗体AMI (Acute Myocardial Infarction)急性心肌梗塞AML (Acute Myelogenous Leukemia)急性粒细胞性白血病AMP (adenosinemonophosphate)一磷酸腺苷ANA (antinuclear antibodies)抗核抗体APC (aspirin,phenacetin and caffeine compound)复方阿司匹林AST(GOT) (aspartate transaminase/ glutamic-oxalacetic transaminease)天冬氨酸转氨酶ATN (acute tubular necrosis)急性肾小管坏死ATP (adenosine triphosphate)三磷酸腺苷BCG (bacillus Calmette-Guerin vaccine)卡介苗BCNU(bis-chloroethyl nitrosourea carmustine,)卡氮芥BMR (Basal metabolic rate)基础代谢率BP (blood pressure)血压BSA (1 bovine serum albumin)牛血清白蛋白;(2 body surface area:体表面积;體表面積;面积;释义:人体表面积)体表面积BSP (bromsulphalein)磺溴酞钠BT (bleeding time)出血时间BUN (blood urea nitrogen)血尿素氮BV (blood volume)血容量C3 (complement-3)补体3cAMP (cyclic adenosine monophosphate)环磷酸腺苷CAPD (Continuous Ambulatory Peritoneal Dialysis)不卧床的持续性腹膜透析CB-1348 (chlorambucil)苯丁酸氮芥CBG (corticosteroid-binding globulin)皮质类固醇结合球蛋白CCNU (cyclohexyl-chloro-ethyl-nitrosourea)环己亚硝脲CCU (coronary care unit)冠心病监护病室CEA (carcinoembryonic antigen)癌胚抗原cGMP (cyclic guanosine monophosphate)环磷鸟苷CIC (circulating immune complex)循环免疫复合物CIEP (counter immunoelectrophoresis:对流免疫电泳;释义:对流免疫电泳,逆向免疫电泳)对流免疫电泳CLL (chronic lymphocytic leukemia)慢性淋巴细胞性白血病CML (Chronic myelogenous leukemia)慢性粒细胞性白血病CMV (cytomegalovirus)巨细胞病毒CNS (central nervous system)中枢神经系统CO2cp (carbon dioxide combining power)二氧化碳结合力CoA (coenzyme A)辅酶ACO (cardiac output)心输出量CPBA (competitive protein binding assay)竞争性蛋白结合分析CPC (Clinico-Pathological Conference:皮肤科临床病理讨论会)临床病理讨论会CPK (creatine phosphokinase)肌酸磷酸激酶cpm (counts per minute)每分钟计数CSF (cerebrospinal fluid)脑脊髓液CT ( Computed Tomography)电算机体层摄影CVP(Central Venous Pressure)中心静脉压DIC (disseminated intravascular coagulation)播散性血管内凝血DMSA (Dimercapto Succinic Acid)二巯基丁二酸DNA (desoxyribonucleic acid)脱氧核糖核酸DNCB (dinitrochlorobenzene)二硝基氯苯DOCA (desoxycorticosterone acetate)醋酸脱氧皮质酮dpm (disintegration per minute)每分钟衰变数DSA (digital subtraction angiography)数字减影血管造影DSS (dengue shock syndrome)登革休克综合征DTPA (diethylenetriamine pentaacetic acid)二乙三胺五乙酸ECG;EKG (electrocardiogram)心电图EDTA (Ethylene Diamine Tetraacetic Acid)乙二胺四乙酸EEG (electroencephalo-graph)脑电图EHF (epidemic hemorrhagic fiver)流行性出血热EIA (enzyme immunoassay)免疫酶法ELISA (enzyme-linked immuno sorbent assay)酶联免疫吸附试验EMCP, EMG(electromyography)肌电图ENT (ear,nose,and throat)耳鼻咽喉ERCP (Endoscopic Retrograde Cholangiopancreatography)逆行胰胆管造影ESR (erythrocyte sedimentation rate)红细胞沉降率FSH-RH (follicle stimulating hormone releasing hormone)促卵泡素释放激素FSH (Follicle-Stimulating Hormone)促卵泡素FT4I (free thyroxine index)游离甲状腺素指数FT4 (free thyroxine)游离甲状腺素GABA (γ-Aminobutyrate) γ-氨基丁酸GH (growth hormone)生长激素GTT (Glucose Tolerance Test)葡萄糖耐量试验HAI;HI (Hemagglutination-inhibition test)血凝抑制试验HA V (Hepatitis A virus)甲型肝炎病毒HBcAg (hepatitis B core antigen)乙型肝炎核心抗原HBeAg (Hepatitis B e Antigen)乙型肝炎e抗原HBsAg (epatitis B surface antigen)乙型肝炎表面抗原HBV (hepatitis B virus)乙型肝炎病毒Hb (hemoglobin)血红蛋白HCG (Human Chorionic Gonadotropin)人绒毛膜促性腺激素HCV (hepatitis C virus)丙型肝炎病毒HDL (high density lipoprotein)高密度脂蛋白HDV (hepatitis delta virus)丁型肝炎病毒HEV (Hepatitis E Virus)戊型肝炎病毒HIV (human immunodeficiency virus)人类免疫缺陷病毒HLA (human leukocyte antigen)人白细胞抗原系统HP (highpowerfield)高倍视野ICU (Intensive Care Unit)危重症病人监护病室IFA (immunoflourescence assay)免疫萤光法Ig (immunoglobulin)免疫球蛋白ITP (idiopathic thrombocytopenic purpura)特发性血小板减少性紫癜IVU (intravenous urography)静脉尿路造影JBE (Japanese B encephalitis)日本乙型脑炎LATS (long-acting thyroid stimulator)长效甲状腺刺激素LD50 (lethal dose50)半致死量LDH (lactic dehydrogenase)乳酸脱氢酶LDL (Low Density Lipoprotein)低密度脂蛋白LH-RH (Luteinizing Hormone-Releasing Hormone)黄体生成素释放激素LH (Luteinizing Hormone)黄体生成素LP (low power field)低倍视野MAA巨聚白蛋白MCHG (Mean Corpuscular Hemoglobin)平均红细胞血红蛋白浓度MCV (Mean Corpuscular V olume)平均红细胞体积MDP (methylene diphosphonic Acid)亚甲基二磷酸MRI(NMR) (Magnatic Resonance Imaging, nuclear magnetic resonance)磁共振(核磁共振)成像MTX (methotrexate)氨甲蝶呤NHL (non-hodgkin's lymphoma)非何杰金淋巴瘤NPN (non protein nitrogen)非蛋白氮OT (old tyberculin)旧结核菌素PaCO2 (arterial partial pressure of carbon dioxide:动脉血二氧化碳分压)动脉血二氧化碳分压PAGE (polyacrylamide gel electrophoresis)聚丙烯酰胺凝胶电泳PaO2 (arterial partial pressure of oxygen)动脉血氧分压PAS (para amino salicylic acid)对氨基水杨酸PBI蛋白结合碘(protein-bound iodine)PBS (phosphate buffer solution)磷酸盐缓冲液PB (phosphate buffer)磷酸盐缓冲剂PCR (polymerase chain reaction)聚合酶链反应PG (prostaglandin)前列腺素PHA (phytohemagglutinphytolectin)植物血凝素:被动血凝试验PPD (Purified Protein Derivative)结核菌素纯蛋白衍生物PSP (phenolsulfonphthalein)酚磺酞(酚红)PTC (percutaneous transhepatic cholangiography)经皮肝穿刺胆道造影PTH (parathyroid hormone)甲状旁腺激素PT (protrombin time)凝血酶原时间RBC(red blood cell)红细胞RES (reticuloendothelial system)网状内皮系统RF (rheumatoid factor)类风湿因子RIA (radioimmunoassay)放射免疫分析RNA (Ribonucleic Acid)核糖核酸rpm (Revolutions Per Minute)每分钟转速(现改用r/min)RV (Rotavirus)轮状病毒SaO2 (Oxygen Saturation)氧饱和度SBE (subacute bacterial endocarditis)亚急性细菌性心内膜炎SLE (systemic lupus erythematosus)系统性红斑狼疮SPECT (single photon emission computed tomography)单光子发射计算机断层图像检查T3 (triiodothyronine)三碘甲状腺原氨酸T4 (thyroxine)甲状腺素TBG (thyroxine binding globulin)甲状腺素结合球蛋白TG (triglyceride/)甘油三酯Tg (thyroglobulin)甲状腺球蛋白TRH (Thyrotropin-releasing Hormone)促甲状腺激素释放激素TSH (Thyroid stimulating hormone)促甲状腺激素TTT (thymol turbidity test)麝香草酚浊度试验U (unit)单位VLDL (very low density lipoprotein)极低密度脂蛋白Wbc (white blood cell)白细胞WBC ()白细胞计数WHO (world health organization)世界卫生组织A.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会(美国)ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics uation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug uation amd Research 药物评价与研究中心CI Corporate Identity (Image) 企业识别(形象)CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局(美国)GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HV AC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter (Drug) 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USP Uneted States Pharmacopeia 美国药典FDA(Food and Drug Administration)和EDQM(European Directoratefor the Quality of Medicines)术语:CLINICAL TRIAL:临床试验ANIMAL TRIAL:动物试验ACCELERATED APPROV AL:加速批准STANDARD DRUG:标准药物INVESTIGATOR:研究人员;调研人员PREPARING AND SUBMITTING:起草和申报SUBMISSION:申报;递交BENIFIT(S):受益RISK(S):受害DRUG PRODUCT:药物产品DRUG SUBSTANCE:原料药ESTABLISHED NAME:确定的名称GENERIC NAME:非专利名称PROPRIETARY NAME:专有名称;INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称ADVERSE EFFECT:副作用ADVERSE REACTION:不良反应PROTOCOL:方案ARCHIV AL COPY:存档用副本REVIEW COPY:审查用副本OFFICIAL COMPENDIUM:法定药典(主要指USP、NF).USP(THE UNITED STATES PHARMACOPEIA):美国药典NF(NATIONAL FORMULARY):(美国)国家处方集OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门(指FDA)IDENTITY:真伪;鉴别;特性STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)LABELED AMOUNT:标示量REGULATORY SPECIFICATION:质量管理规格标准(NDA提供)REGULATORY METHODOLOGY:质量管理方法REGULATORY METHODS V ALIDATION:管理用分析方法的验证制药企业名称中英对照Pfizer 辉瑞美国Johnson & Johnson 强生美国GlaxoSmithKline 葛兰素史克英国Novartis 诺华瑞士Roche Group 罗氏瑞士Merck 默克美国22485.9Bristol-Myers Squibb 百时美施贵宝美国Aventis 安万特法国Abbott Laboratories 雅培美国AstraZeneca 阿斯利康英国Wyeth 惠氏美国Eli Lilly 礼来大药厂美国BASF 巴斯夫德国Dow Chemical 道化学美国Bayer 拜耳德国Akzo Nobel 阿克苏诺贝尔荷兰。
常用制药及GMP英文缩写

ISO(International Organization for Standardization):国际标准化组织日常办事机构是中央秘书处,设在瑞士日内瓦WHO(World Health Organization):世界卫生组织是联合国属下的专门机构,国际最大的公共卫生组织,总部设于瑞士日内瓦PIC/S(Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme):国际医药品稽查协约组织由欧洲自由贸易区(EFTA)组建ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use):人用药物注册技术要求国际协调会由欧盟(EU)、欧洲制药工业协会联合会(EFPIA)、日本厚生省(MHW)、日本制药工业协会(JPMA)、美国FDA、美国药物研究生产联合会(PRMA)等机构组成WHO、EFTA、加拿大卫生保健局(CHPB)为观察员ISPE(International Society for Pharmaceutical Engineering):国际制药工程协会是致力于培训制药领域专家并提升制药行业水准的世界最大的非盈利性组织之一,在美国坦帕州设有全球总部,在布鲁塞尔设有欧洲总部,亚洲总部在新加坡HHS(United States Department of Health and Human Services):美国卫生及公共服务部(美国卫生部)FDA(Food and Drug Administration):美国食品药品监督管理局(HHS下属机构)PDA(Parenteral Drug Association):美国注射剂协会EPA(Environmental Protection Agency):美国国家环境保护局CDER(Center for Drug Evaluation and Research):FDA药物评价与研究中心EMEA(The European Agency for the Evaluation of Medicinal Products):欧洲药物评审组织MHW(Ministry of Health and Welfare):日本厚生省,现改为厚生劳动省MHLW (Ministry of Health, Labor and Welfare),负责医疗卫生和社会保障的主要部门D&B(Dun & Bradstreet):邓白氏公司DUNS(Data Universal Numbering System):邓白氏公司提供的唯一的公司代号,用于信用评级等在SMF文件中会用到GMP(Good Manufacturing Practice):药品良好生产规范cGMP(Current Good Manufacture Practices):动态药品生产管理规范,即现行的GLP(Good Laboratory Practice):药物非临床研究质量管理规范,及优良实验室规范GSP(Good Supplying Practice):药品经营质量管理规范,及良好的药品供应规范GAP(Good Agricultural Practice for Chinese Crude Drugs):中药材生产质量管理规范GDP(Good Documentation Practice):良好文件管理GEP(Good Engineering Practice):工程设计规范GAMP(Good Automated Manufacturing Practice):优良自动化生产规范USP(united states pharmacopeia):美国药典EP(European Pharmacopeia):欧洲药典JP(Japanese Pharmacopoeia):日本药典CFR(Code of Federal Regulations):美国联邦法律CFR 21 Part 11(Code of Federal Registry Part11):联邦法规法律标题21第11部分CEP/COS(C ertificate o f S uitability to the monographs of E uropean P harmacopoeia):欧洲药典适应性认证证书CEP认证,COS证书CTD(Common Technical Document):国际注册用常规技术文件CTD文件是国际公认的文件编写格式,用来制作一个向药品注册机构递交的结构完善的注册申请文件EHS(Environment、Health、Safety):环境-健康-安全管理体系HACCP(Hazard Analysis and Critical Control Point):(保健食品)危害分析和关键控制点REACH(REGULATION concerning the Registration, Evaluation, Authorization and Restriction of Chemicals):欧盟规章《化学品注册、评估、许可和限制》,欧盟建立的,并于2007年6月1日起实施的化学品监管体系ICH-Q1A:新原料药和制剂的稳定性试验ICH-Q1B:稳定性试验:新原料药和制剂的光稳定性试验ICH-Q1C:稳定性试验:新剂型的要求ICH-Q1D:新原料药和制剂的稳定性试验的括号法和矩阵法设计ICH-Q1E:稳定性数据的评价ICH-Q1F:气候带Ⅲ和Ⅳ注册申请的稳定性数据ICH-Q2A:分析步骤验证:正文ICH-Q2B:分析步骤验证:方法学ICH-Q3A:原料药中的杂质ICH-Q3B:新制剂中的杂质ICH-Q3C:杂质;残留溶剂的指导原则ICH-Q4:药典ICH-Q4A:药典的同一化ICH-Q4B:各地区使用的药典正文评估和建议ICH-Q5A:来源于人或动物细胞系的生物技术产品的病毒安全性评价ICH-Q5B:生物技术产品的质量:rDNA衍生蛋白质产品生产细胞的表达构建体分析ICH-Q5C:生物技术产品的质量:生物制品/生物技术产品的稳定性试验ICH-Q5D:用于生物技术产品及生物制品生产的细胞基质的来源和鉴定ICH-Q5E:生物技术产品/生物制品在工艺变更时的可比性ICH-Q6A:质量标准新原料药和制剂的检测以及可接受标准:化学物质ICH-Q6B:质量标准:生物技术产品及生物制品的检测方法和可接受标准ICH-Q7:原料药良好制造规范(ICH-Q7A的新版)ICH-Q7A:原料药的GMP规范ICH-Q8:药物研发指南ICH-Q9:质量风险管理ICH- Q10(PQS):药物质量体系QA(Quality Assurance):质量保证QC(Quality Control):质量控制QRM(Quality Risk Management):质量风险管理IPC(Inproceics Quality Control):制程品质控制/中控OOS(Out of Specification):检验结果超标OOT(Out of Trend):超趋势结果OOL(Out of Limit):超出极限的结果,如温湿度等OOE(Out of Expectation):超期望结果SAL(Sterility Assurance Level):无菌保证水平灭菌后微生物的存活概率的负−lgN0对数,要求≥6SAL=−lg存活率=F0DD值:杀灭90%的微生物所需要的时间,D值越大,微生物死亡越难,D值与细菌的耐热性成正比Z值:指灭菌时间减少到原来的10%所需要升高的温度或是相同的灭菌时间内杀死99%的微生物所需要提高的温度F值:为一定温度下,给定Z值所产生的灭局效果与参比温度T0下给定Z值所产生的灭菌效果相同时所相当的时间F值用于干热灭菌F0值:为一定温度下,Z值为10℃产生的灭菌效果与120℃,Z值为10℃时产生的灭菌效果相当的时间,t分钟内的灭菌效果相当于120℃下灭菌F0分钟的效果F0被称为标准灭菌时间,用于热压灭菌LRV:除菌过滤的对数下降值LRV=lgN0-lgNSOP(Standard Operation Procedure):标准操作规程DMF(Drug Master File):药品主文件SMF(Site Master File):工厂主文件URS(User Requirement Specification):用户需求标准FS(Functional Specification):功能标准DS(Design Specification):设计标准DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认RQ(Requalification):再确认CAPA(Corrective Action & Preventive Action):纠正预防系统,Q10的四大要素之一QbD(Quality by Design):质量源于设计COA(Certificate of Analysis):分析证书/检验报告书/检验报告单BPR(Batch Production Record):批生产记录API(Active Pharmaceutical Ingredients):药物活性成分,通常指的原料药PMC(Product Material Control):生产物料控制PC生产控制;MC物料控制CMC(Chemistry and manufacture control):生产和化学控制APR(Annual Products Review):年度质量回顾KPI(Key Performance Indicators):关键业绩指标P&ID(Piping and Instrument Diagram):工艺管道仪表流程图PFD(Process Flow Diagram):工艺流程图UFD(Utility Flow Diagram):公用工程流程图CIP(Cleaning in Place):原位清洗(全自动,如针剂配制系统)WIP(Washing in Place):在线清洁(半自动,需要手动的拆卸,如流化床)SIP(Sterilization in Place):在线灭菌WFI(Water for Injection):注射用水HVAC(Heating Ventilation Air Conditioning):供热空气调节净化系统HEPA(High Efficiency Particulate Air Filter):高效过滤器DOP:为邻苯二甲酸二辛酯,HEPA检漏用的气溶胶PAO:聚-α-烯烃,HEPA检漏用的气溶胶IBC(I ntermediate Bulk Container):中型散装容器BFS(Blowing Filling and Sealing):吹-灌-封PAT(Process Analytical Technology):过程分析技术PLC(Programmable Logic Controller):可编程逻辑控制CPP(Critical Process Parameters):关键工艺参数FBD(Fluid Bed Dryer):流化床AHU(Air Handling Unit):空气处理单元SAT(Site Acceptance Test):现场验收测试FAT(Factory Acceptance Test):工厂验收测试。
制药工程常用英文缩写,缩略语

1GMP Good Manufacturing Practices药品生产质量管理规范2GxP各种药品规范的统称3GCP Good Clinical Practice药物临床试验质量管理规范4GLP Good Laboratory Practice药物非临床试验(实验室)质量管理规范5GSPGDPGood Supplg practiceGood Distribute Practice(美)药品经营质量管理规范6GDP Good Dossier practice申报资料质量管理规范7GPP Good Pharmacy practice药房质量管理规范8GQP Good Quality Practice 药品质量管理规范9GRP Good Rearch Practice药品研究质量管理规范10GUPGPPGood Use PracticeGood Preparation Practice(欧美)药品使用质量管理规范11GVP Good Validation Practice验证管理规范12GAP Good Agricultural Practice中药材生产质量管理规范13GEP Good Engineering Practice工程管理规范14GWP Good Warehousing Practice药品仓储规范15GMPC Good Manufacture Practice of Cosmetic Products化学品生产质量管理规范16cGMP Current Good Manufacturing Practice现行药品生产质量管理规范17EU-GMP European –Good Manufacturing Practice欧洲GMP18CFR Code of Federal Regulations美国联邦法规19ChP Chinese Pharmacopoeia中国药典20USP United States Pharmacopoeia美国药典21EP European Pharmacopoeia欧洲药典22JP Japanese Pharmacopoeia日本药典23BP British Pharmacopoeia英国药典24IP Indian Pharmacopoeia印度药典25EN European Norm欧洲规范,欧洲标准26ANSI American National Standards Institute美国国家标准学会27ASME American Society of Mechanical Engineers美国机械工程师学会28ASTM American Society for Testing and Materials美国材料实验学会29ISPE International Society for Pharmaceutical Engineering国际制药工程学会30WHO World Health Organization世界卫生组织31ISO International Standards Organization国际标准组织32EEC European Economic Community欧洲共同体、欧共体33EU European Union欧盟34ES European Commission欧洲委员会35CFDA China Food and Drug Administration中国食品和药品监督管理局36FDA Food and Drug Administration(美国)食品和药品管理局37MHRA Medicines & Healthcare Products Regulatory Agency(英国)药品和健康产品管理局38EHX Environment Health Safety环境、职业健康、安全管理体系39BPE Bioprocessing Equipment生物处理设备403A美国卫生行业协会、美国卫生论证标识41NBST National Bureau of Standards and Technology美国国家标准研究院42EMA European Medicines Agency欧洲药监局43EMEA European Agency for the evaluationof medicinal欧洲药品评价局44EDQM European Directorate for the Quality of Medicines欧洲药品理事会45EQDM European Directorate for the Quality of Medicines & Healthcare欧洲药品与健康理事会46EHEDG European Hygienic Equipment Design Group欧洲卫生设备设计组织47ICH International Conference on Harmonization of TechnicalRequirements for Registration of Pharmaceuticals for Human人用药物注册技术要求国际协调会议48IEC International Electrotechnical Commission国际电工委员会通用及组织49NEMA National Electrical Manufacturers Association美国电器制造商协会50CEP Certificate of Suitability for European Pharmacopeia欧洲药典适用性证书51CE Conformite Europeenne欧洲电气安全论证52PIC/S Pharmaceutical Inspection ConventionPharmaceutical Inspection Cooperation Scheme国际医药品稽查协约组织53HHS United States Department of Health and Human Services美国卫生及公共服务部、美国卫生部54PDA Parenteral Drug Association(美国)注射剂协会55EPA Environmental Protection Agency(美国国家)环境保护局56CDER Center for Drug Evaluation and Research药物评价与研究中心57MHWMHLWMinistry of Health and WelfareMinistry of Health, Labor and Welfare(日本)厚生省(日本)厚生劳动省5821 CFR Title 21―Food and Drugs美国联邦法规,第21篇,食品与药品59Part11Electronic Records; Electronic Signatures第11节,电子记录与电子签名60Part210Current Good Manufacturing Practice in Manufacturing,Processing,Packing,or Holding of Drugs;General第210节,药品生产、加工、包装、储存质量规范部分61Part211Current Good Manufacturing Practice for Finished第211节,制剂药物生产质量规范部分62Part314Applications for FDA Approval to Market a New Drug第314节,新药上市申请部分63Part320Bioavailability and Bioequivalence Requirements第320节,生物利用度和等效性要求1QMS Quality Management System质量管理体系2QRS Quality Regulation System质量控制体系3QA Quality Assurance质量保证4QC Quality Control质量控制5QM Quality Management质量管理6QI Quality Inspection质量检验7QP Quality Plan质量计划8QRM Quality Risk Management质量风险管理9URS User Requirement Specification用户需求10DQ Design Qualification设计确认11IQ Installation Qualification安装确认12OQ Operational Qualification操作确认13PQ Performance Qualification性能确认14VIT Vendor Internal Test供应商内部测试15FAT Factory Acceptance Test工厂验收测试16SAT Site Acceptance Test现场验收测试17SOP Standard of Operation标准操作规程18FDS Functional and Design Specifications功能设计说明、功能设计规范19FS Functional Specifications功能说明20DS Design Specifications设计说明21TS Technical Specification技术说明、技术规范22RTM(TM)Requirement Traceability Matrix需求追溯矩阵23ITR Inspection Test Reports检查测试报告24QOR Quality Observation Report质量检查报告25QR Quality Requirements质量要求26QR Quality Records质量记录27RA Risk Assessment风险评估28SIA System Impact Assessment系统影响性评估29CCA CriticalComponents Assessment部件关键性评估30PV Process Validation工艺验证31CV Cleaning Validation清洁验证32CSV Computer System Validation计算机验证33VMP Validation Master Plan 验证主计划质量、验证34VP Validation Plan 验证计划35VP Validation Protocol验证方案36VR Validation Report验证报告37PVP Project Validation Plan项目验证计划38PVR Project Validation Report项目验证报告39QbD Quality by Design质量源于设计40DMF Drug Master File药品主文件、药物管理档案41FMEA Failure Mode and Effects Analysis失效模式和效果分析42SST System Suitability Test系统适应性测试43CAL Calibration校验、校准44CAPA Corrective Action and Preventive Action纠正预防措施45RCA Root Cause Analysis根本原因分析46ERES Electronic Record and Electronic Signature电子记录与电子签名47AQL Acceptable Quality Level可接受质量水平48CQA Critical Quality Attribut关键质量属性49CPP Critical Process Pararneter关键工艺参数50CTD Common Technical Document通用技术文件51IA Impact Assessment影响评估52PQR Procut Quality Review产品质量回顾53COA Certification of Analysis分析合格证书、检验报告54BPR Batch Production Records批生产记录55BR Batch Records批记录56CC Change Control变更控制57DR Deviation Records偏差记录58COM Commissioning试车59BAR Batch Analysis Record批检验记录60PP Process Procedure工艺规程61OOS Out of Specification超出标准(限度)62LAL Limulus Smoebocyte Lysate鲎试剂63AQL Acceptable Quality Level可接受质量水平64SMF Site Master File工厂主文件65PM Preventive Maintenance预防性维修66QP Qualified Person质量授权人67R&D Research and Development研发部门68NDA New Drug Application新药申请电气及自控1GAMP Good Automated Manufacturing Practices设备自动化生产管理规范2HMI Human Machine Interface人机界面3OIT Operator Interface Terminals操作员界面终端4OIP Operator Interface Panel操作员界面面板5PLC Programmable Logic Controller可编程序控制器6PCS Process Control System过程控制系统7DCS Distributed Control System集散控制系统8PCS Process Control System工艺控制系统9DDC Direct Digital Controller直接数字控制器10IPC Industrial Personal Computer工业控制计算机,工控机11PAC Programmable Automation Controller可编程自动化控制器12PCC programmable computer controller可编程计算机控制器13MCU Microcontroller Unit单片机14CPU Central Process Unit中央处理器15PC Personal Computer个人电脑16SCADA Supervisory Control And Data Acquisition监控及数据采集17SDS Software design specification软件设计说明18HDS Hardware Design Specification硬件设计说明19FL Functional Logic功能逻辑说明20I/O Input / Output输入/输出21AI Analog Input模拟量输入22AO Analog Output模拟量输出23DI Digital Input数字量输入24DO Digital Output数字量输出25RTD Resistance Temperature Detector热电阻26T/C Thermocouple热电偶27RTU Remote Terminal Unit远程终端单元28ARS Automation Requirement Specification自动化需求规范29VFD Variable Frequency Drive变频驱动30EMC Electromagnetic Compatibility电磁兼容31UPS Uninterrupted Power supply不间断电源32EPS Emergency Power supply应急电源33FL Functional Logic功能逻辑说明34ER and Electronic Signature电子记录35ES Electronic Signature电子签名36AT Audit Trail审计踪迹37NO Normally Open常开38NC Normally Close常关39FO Fault Open故障开40FC Fault Close故障关41AC Alternating Current交流42DC Direct Current直流43PID Proportional Integral Derivative比例积分微分44LED Light Emitting Diode发光二极管45LCD Liquid Crystal Display液晶显示器46LIMS Laboratory Information Management System实验室信息管理系统 47LECP Laboratory Equipment Calibration Program实验室仪器校准程序48WMS Warehouse Management System仓库管理系统49MES Manufacturing Execution System制造执行系统50ERP Enterprise Resource Planning企业资源计划其它1N/A Not Applicable不适用2NLT Not Less Than不少于3NMT Not More Than不多于4NB Nominal Bore公称管径5PED Pressure Equipment Directive压力设备指令(欧洲) 6PW Purified Water纯化水7WFI Water for Injections注射用水8PS Pure Steam纯蒸汽发生器9PWG PW Generator Unit纯化水制备机组10WFIG WFI Generator注射用水制备机组11MEWD Multi-effect Water Distillator 多效蒸馏水机12PSG PS Generator纯蒸汽发生器13PAC Poly Alumina Chlorine聚合氯化铝14DW Demineralized Water脱盐水,去离子水15MF Micro-Filter微滤16UF Ultra-Filter 超滤17NF Nano-Filter纳滤18MMF Multi-Media Filter多介质过滤器19ACF Activated Carbon Filter活性炭过滤器20SF Softener软化器21DG Degasifier脱气塔22RO Reverse Osmosis 反渗透23EDI Electrodeionization电法去离子24MB Mixed Bed混床25MDG Membrane Degasifier膜脱气26COP Clean out Place离线清洗27CEB Chemical Enhanced Backwash化学增强反冲洗28CIP Clean In Place在线清洗29SIP Sterilization in Place在线灭菌30POU Point Of Use使用点31PH Potential of Hydrogen酸碱度32TOC Total Organic Carbon总有机碳33ORP Oxidation-Reduction Potential氧化还原电位34COD Chemical Oxygen Demand化学耗氧量35BOD Biological Oxygen Demand生物耗氧量36SDI Silt Density Index污染密度指数37TUB Turbidity浊度38TSS Suspended Solid总悬浮固体39DO Dissoved Cxygn溶解氧40TDS Total dissolved solids总溶解固体41TH Total Hardness总硬度42PAT Process Analytical & Measurement Technology过程分析技术43IRS Installation Requirement Specification安装要求说明44OEM Original Equipment Manufacturer 原始设备制造商45GDS General Design Specification总体设计说明46DDS Detailed Design Specification详细设计说明47PCP Preparation of Construction Plan施工组织设计48WMS Work Method Statement施工方案49BOQ Bill of Quantities工程量清单50BOM Bill of Material材料清单51P&ID Process and Instrumentation Diagram工艺与仪表流程图52PFD Process Flow Diagram工艺流程示意图53ANDA Abbreviation New Drug Application仿制药或仿制新药申请54OPQ Operational Personnel Qualification操作人员资格鉴定55MBT Microbiologic Test微生物测定56ADR Adverse Drug Reaction药物副作用报告,药品不良报告57OMM Operating and Maintenance Manual操作和维护保养手册58HACCP Hazard Analysis and Critical Control Point危害分析及关键环节控制点59CCP Critical Control Point关键环节控制点60IPC In Process Control过程控制61IPC Intermediate Production Control中间生产控制62CIPC Critical In-Process Control关键中间控制点63MBR Master Batch Record主生产批记录64PPM Parts Per Million百万分之一65OC Organizational Charts组织结构图66FIT Filter Integrity Test过滤器完整性测试67WIT Water Intergrity Test水侵入测试68GA General Arrangement总平面图69RPM Rotations per minute转/分70PD Prescription Drug处方药71Rx Receptor x处方药72NPD Nonprescription Drug非处方药73OTC Over The Counter非处方药74API Active Pharmaceutical Ingredient原料药、活性药75BPC Bulk Pharmaceutical Chemical原料药(原简称)76DS Drug Substance原料药77DP Drug Product成品药78RO Restriction orifice限流孔板79SG Sight Glass视镜80LG Lamp Glass,Light Glass灯镜81RD Rupture Disk爆破片材料1MOC Material Of Construction建造材质2SS Stainless Steel不锈钢3CI Cast iron铸铁4NCI Nodular east iron球墨铸铁5CS Carbon Steel碳钢6 C.Stl Cast Steel铸钢7 F.Stl Freezing Steel锻钢8PA Polyamide聚酰胺9PB Polybutylene聚丁烯10PC Polycarbonate聚碳酸酯11PE Polyethylene聚乙烯12PEX Cross-linked PolyEthylene交联聚乙烯13HDPE High-density polyethylene plastics高密度聚乙烯14MDPE Medium-density polyethylene plastics中密度聚乙烯15PO Polyolefin聚烯烃16PP Polypropylens聚丙烯17FRPP Polypropylens玻纤增强聚丙烯18PPR Polypropyla无规共聚聚丙烯19PPS PolyPhenylene Sulfide聚苯硫醚20PS Polystrene聚苯乙烯21PU Polyurethane,或者缩写为PUR聚氨酯22POM PolyOxyMethylene or Polyacetal聚甲醛,聚氧化亚甲基23HIPS High impact polystyrene高抗冲聚苯乙烯26PFA Polyfluoroalkoxy四氟乙烯—全氟烷氧基乙烯基醚共聚物27PTFE Polytetrafluoroethylene聚四氟乙烯28PVDF Poly vinylidene fluofide聚偏二氟乙烯29PVC Polyvinyl chloride聚氯乙烯30UPVC Unplasticised Polyvinyl Chloride硬聚氯乙烯,增强聚氯乙烯31CPVC Chlorinated polyvinyl chloride,或者缩写为PVCC氯化聚氯乙烯32PA Nylon,Polyamide尼龙,聚酰胺33PES PolyEtherSulfone聚醚砜,聚酯34AAS Acrylonirile butadiene styrene丙烯腈-丙烯酸酌-苯乙烯35ABS Acrylonitrile-Butadiene-Styrene丙烯腈-丁二烯-苯乙烯共聚物36ACS Acrylonitrile Chlorinated polyethylene Styrene丙烯胯-氯化聚乙烯-苯乙烯37ASB Asbestos石棉38PMMA Polymethel methacrylate聚甲基丙烯酸甲酯39SR Styrene-rubber苯乙烯橡胶24EPDM Ethylene Propylene Diene Monomer三元乙丙橡胶25EPM Ethylene Propylene Methylene乙丙橡胶,乙烯/丙烯共聚物40SR Silicone rubber硅橡胶40HTV High Temperature Vulcanization高温硫化(硅橡胶)40RTV Room Temperature Vulcanization室温硫化(硅橡胶)40MQ Silicone rubber甲基硅橡胶40VMQ Silicone rubber甲基乙烯基硅橡胶40PVMQ Silicone rubber甲基乙烯基苯基硅橡胶41FPMFKMFluororubberFluorocarbon Rubber氟橡胶42NBR Vulcanized nitrile rubber丁腈橡胶43FRP Glass Fibre Reinforced Plastic玻璃钢,玻璃纤维增强塑料1HVAC Heating Ventilation and Conditioning供热通风空调2AC Air Conditioner空调3AHU Air Handling Unit空气处理单元4BMS Building Monitoring System建筑管理系统、楼宇检测系统5CFU Colony Forming Unit菌落形成单位6CNC Controlled Non-Classified控制但未分级7FFU Fan Filter Unit风机过滤单元8FMS Factory Monitoring System车间监控系统9HEPA High Efficiency Particulate Air高效空气过滤器10LAF Laminar Air Flow层流、单向流11UDF Unidirectional Flow单向流12RABS Restricted Access Barrier Systems限制通过隔离系统13DP Differential Pressure压差14SDP Static Differential Pressure静压差15RH Relative Humidity相对湿度16CHWs Chilled Water (Supply)冷冻水(供给)17CWr Cooling Water (Return)冷却水(回流)18HW Hot Water热水19FS Factory Steam工厂蒸汽20SC Steam Condensate蒸汽冷凝水21WD Waste Drain废水排放22PWW Process Wastewater工艺污水23CA Compressed Air压缩空气24PA Process Air工艺压缩空气25IA Instrument Air仪表压缩空气26RW Raw Water原水27SW Soft Water软水28MW Middle Water中水29DW Domestic Water生活用水30CW City Water市政供水、自来水31DK Drinking Wat 饮用水32LPG Liquefied Petroleum Gas液化石油气33LNG Liquefied Natural Gas液化天然气34CNG Compressed natural gas压缩天然气35VE Visual Examination外观检查36UT Ultrasonic inspection Test超声探伤37RT Radiographic inspection Test射线探伤38MT Magnetic particle inspection Test磁粉探伤39PT liquid Penterant inspection Test液体渗透探伤40AutoclaveSterilizer灭菌柜公用工程41FBD Fluid Bed Dryer流化床42BFS Blowing Filling and Sealing吹灌封43HPLC High Pressure Liquid Chromatograph高效液相色谱44TLC Thin Layer Chromatograph薄层色谱45GC Gas Chromatograph气相色谱46UV Ultra-Violet紫外线47IR InfraRed红外线48RFQ Request for Quotations报价征询书49NPT American standard taper pipe thread美国标准锥管螺纹50NPS American standard straight pipe thread美国标准直管螺纹51NF American national fine thread美国标准细牙螺纹52NC American national coarse thread美国标准粗牙螺纹53Union Union活接头,由宁。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
术语/缩略语英文全拼中文AHU Air Handing Unit 空调机组AOAC Association of Official AnalyticalChemists美国公职化学家协会AMV Analytical Method Validation 分析方法验证API Active Pharmaceutical Ingredient 药物活性成分ASME American Society of MechanicalEngineers美国机械工程学会ASME BPE American Society of MechanicalEngineers Bioprocessing Equipment美国机械工程/生物工程设备学会ASTM American Society for Testing andMaterials美国材料与试验协会BD Bowie-Dick 布维-狄克BI Biological indicator 生物指示剂BMS Building Management System 楼宇控制系统CAPA Corrective and Preventative Action 纠正和预防措施CCA Component Criticality Assessment 部件关键性评估CCP Critical Control Point 关键控制点CD Cycle Development 程序开发CEHT Clean equipment hold time 干净设备保留时间CFR Code For Federal Regulations 美国联邦法规CFU Colony Forming Unit 菌落形成单位cGMP Current Good Manufacturing Practice 现行药品生产质量管理规范CHO Chinese Hamster Ovary 中国仓鼠卵巢细胞CIP Clean In Place 在线清洗ChP Chinese Pharmacopeia 中国药典C p/C pk Process Capability Index 工序能力指数CPP Critical Process Parameter 关键工艺参数CQA Critical Quality Attribute 关键质量属性CSV Computer System Validation 计算机系统验证CVP Cleaning Validation Plan 清洁验证计划DCS Distributed Control System 分布式控制系统DDS Detailed Design Specification 详细设计说明DEHT Dirty equipment hold time 脏设备保留时间DNA Deoxyribonucleic acid 脱氧核糖核酸DOE Design of Experiment 实验设计DOP Dioctyl Phthalate(Or Equivalent,I.E.,Dispersed Oil Particulate)邻苯二甲酸二辛脂(或等同品,例如分散油颗粒)DQ Design Qualification 设计确认DS Design Specification 设计说明ED 50 50% Effective Dose 半数有效量EDI Electrodeionization Deionization (USFilter)电级法去离子(美国滤材)EHS Environment Health Safety 环境健康安全ELISA Enzyme-Linked Immuno Sorbent Assay 酶联免疫吸附测定EMA European Medicines Agenc 欧洲药品管理局EMS Environmental Monitoring System 环境监测系统EP European Pharmacopoeia 欧洲药典EPA Environmental Protection Agency 美国环境保护署ERP Enterprise Resource Planning 企业资源计划ETOP Engineering turnover packages 工程交付包EU European Union 欧盟FAT Factory Acceptance Testing 工厂验收测试FDA Food and Drug Administration 美国食品与药品监督管理局FDS Functional Design Specification 功能设计说明FMEA Failure Modes And Effects Analysis 失效模式和影响分析FS Function Specification 功能说明FTA Fault Tree Analysis 故障树分析FQCP Field Quality Control Plan 现场质量控制计划GAMP Good Automated ManufacturingPractice良好自动化生产实践GDP Good Document Practice 良好文件管理规范GEP Good Engineering Practice 良好工程管理规范GLP Good Laboratory Practice 良好实验室管理规范GMP Good Manufacturing Practice 良好药品生产管理规范GxP Good x Practice 药品质量管理规范HACCP Hazard Analysis and Critical ControlPoints危害分析和关键控制点HAZOP Hazard and Operability Analysis 危险与可操作性分析HDS Hardware Design Specification 硬件设计说明HEPA High Efficiency Particulate Air 高效空气过滤器HBV Hepatitis B Virus 乙型肝炎病毒HIV Human Immunodeficiency Virus 人类免疫缺陷病毒HMI Human Machine Interface 人机界面HPLC High Performance Liquid Chromatography高效液相色谱HVAC Heating, Ventilation, And AirConditioning采暖通风和空调系统I/O Input and Output 输入/输出IC 50 The Half maximal inhibitory concentration半抑制浓度ICH International Conference onHarmonization of TechnicalRequirements for Registration ofPharmaceuticals for Human Use人用药品注册技术要求国际协调会IEC International Electro technicalCommission国际电工委员会IQ Installation Qualification 安装确认ISO International Standards Organization 国际标准化组织ISPE The International Society forPharmaceutical Engineering国际制药工程协会IUPAC International Union of Pure and AppliedChemistry国际理论(化学)与应用化学联合会LIMS Laboratory Information ManagementSystem实验室信息管理系统LOD Limits of Detection 检测限度LOQ Limit of Quantizatity 含量限度MB/L Methyleneblue 亚甲蓝光敏法MCB Master Cell Bank 主细胞库MES Manufacturing Execution System 生产执行系统MTDD minimum treatment daily dosage 最低日治疗剂量OOS Out of Specification 检验结果偏差OQ Operational Qualification 运行确认OSD Oral Solid Dosage 口服固体制剂P&ID Piping and Instrumentation Diagrams 管道和仪表图PAO Poly-Alpha-Olefin 聚Α-烯烃PAT Process Analytical Technology 过程分析技术PBS phosphate buffer 磷酸缓冲液PCB Primary Cell Bank 原始细胞库PCR Polymerase Chain Reaction 聚合酶链反应PDA Parenteral Drug Association 美国注射剂协会PDI Pre-delivery Inspection 发货前检查PEP Project Execution Plan 项目执行计划PFD Process Flow Diagrams 工艺流程图PHA Preliminary Hazard Analysis 初步危害分析PIC/S Pharmaceutical Inspiration ConventionAnd Pharmaceutical InspectionCo-Operation Scheme国际药品检查协会组织PLC Programmable Logic Controller 可编程逻辑控制器PM Project Managemnet 项目管理PP Polypropylene 聚丙烯PPE Personal Protective Equipment 人员保护装备PPQ Process Performance Qualification 工艺性能确认PQ Performance Qualification 性能确认PS Pure Steam 纯蒸汽PTFE Polytetrafluoroethylene 聚四氟乙烯PV Process Validation 工艺验证PVC Polyvinyl Chloride 聚氯乙烯PVP Process Validation Plan 工艺验证计划PW Purified Water 纯化水QA Quality Assurance 质量保证QbD Quality by Design 质量源于设计QC Quality Control 质量控制QMS Quality Management System 质量管理体系QPP Quality and Project Plan 质量及项目计划QRM Quality Risk Management 质量风险管理RA Risk Assessment 风险分析RABS Restricted Access Barrier System 限制进出隔离系统RH Relative Humidity 相对湿度RNA Ribonucleic Acid 核糖核酸RO Reverse Osmosis 反渗透RPN Risk Priority Number 风险优先性RSD Relative Standard Deviation 相对标准偏差RTM Requirements Traceability Matrix 需求追溯性矩阵RTP Rapid Transfer Port 快速运转接口SAL Sterility Assurance Level 灭菌保证水平SAT Site Acceptance Testing 现场验收测试SCADA Supervisory Control And DataAcquisition检测控制和数据收集SCR Source Code Review 源代码审核SDA-PAGE Sodium DodecylSulfate-polyacrylamide gel十二烷基硫酸钠-聚丙烯酰胺凝胶SDI Silt Density Index 淤泥指数SDS Software Design Specification 软件设计说明SFDA State Food and Drug Administration 国家食品药品监督管理局SIA System Impact Assessment 系统影响性评估SIP Sterilize In Place 在线灭菌SME Subject Matter Expert 主题专家SMS Software Module Specifications 软件模块说明SMT Software Module Test 软件模块测试SOP Standard Operating Procedure 标准操作规程SV Sindbis Virus 辛德毕斯病毒TM Traceability Matrix 可追溯矩阵TOC Total Organic Carbon 总有机碳TR Technical Report 技术报告UAF Unidirectional Airflow 单向气流UCL Upper confidence limit 置信上限UPS Uninterruptable Power Supply 不间断电源URB User Requirements Brief 用户需求简介URS User Requirements Specification 用户需求说明USP United States Pharmacopoeia 美国药典UV Ultraviolet Light 紫外灯VHP Vaporized Hydrogen Peroxide 汽化过氧化氢灭菌技术VMP Validation Master Plan 验证主计划/验证总计划VP Validation Plan 验证计划VSR Validation Summary Report 验证总结报告VSV Vesicular Stomatitis Virus 水疱性口炎病毒WCB Working Cell Bank 工作细胞库WFI Water for Injection 注射用水WHO World Health Organization 世界卫生组织WIP Wetting In Place 在线加湿。
