化工原理英文教材-传质与分离部分cha
合集下载
化工原理英文教材传质分离过程Mass Transfer and Separation Processes

14
•How to separate a solution of salt and water?
(Problem for producing fresh water from the sea.) (1)Supply heat and boil water off; (2)Supply refrigeration and freeze out pure ice; (3)Pump the water to a higher pressure and force it through a thin solid membrane that will let water through preferentially to salt (reverse osmosis)反渗透.
•Thinking (introspection): Many environmental pollution problems are caused by separation itself?
Why? How to solve this problem?
13
3. Definition of Separation Processes
Product Streams (different in composition, at least 2 streams)
19
Example: Distillation Fractionating
column
Feed
Overhead product
Bottom product
20
•The separation is caused by the addition of a separating agent). •separating agent: matter or energy •Usually the separating agent will cause the formation of a second phase of matter. • •Separation methods(mass transfer between two immiscible phases):
•How to separate a solution of salt and water?
(Problem for producing fresh water from the sea.) (1)Supply heat and boil water off; (2)Supply refrigeration and freeze out pure ice; (3)Pump the water to a higher pressure and force it through a thin solid membrane that will let water through preferentially to salt (reverse osmosis)反渗透.
•Thinking (introspection): Many environmental pollution problems are caused by separation itself?
Why? How to solve this problem?
13
3. Definition of Separation Processes
Product Streams (different in composition, at least 2 streams)
19
Example: Distillation Fractionating
column
Feed
Overhead product
Bottom product
20
•The separation is caused by the addition of a separating agent). •separating agent: matter or energy •Usually the separating agent will cause the formation of a second phase of matter. • •Separation methods(mass transfer between two immiscible phases):
化工原理英文教材-分离设备部分chapter6

Slurry: solid liquid mixture
Slurry filtration:
Fluid flows through a filter medium by virtue of a pressure difference across the medium.
Most industrial filters are either pressure filters or vacuum filters. They are also either continuous or discontinuous.
Separation of solids from gases; cyclones
Most centrifugal separators for removing particles from gas streams contains no moving parts.
They are typified by the cyclone separator shown in Figure.
1 clarifiers 2 cake filters 3 Cros Filtrate: liquid got from solid liquid mixture by filtration
Septum (filtration media) like paper or cloth. Cake: solid got from solid liquid mixture by filtration.
Clarifying filters
Clarifying filters are also known as ―deep-bed filters‖ because the particles of solid are trapped inside the filter medium.
化工原理英文教材流体输送与计量泵设备Transportation and metering of fluids

If the suction pressure is actually less than the vapor pressure, cavitation will occur in the suction line, and no liquid can be drawn into the pump.
化工原理 Principles of Chemical Industry
Transportation and metering of fluids
The engineer is concerned with practical problems in transporting fluids from one place to another and in measuring their rates of flow.
Such devices increase the mechanical energy of the fluid.
The energy may be used to increase the velocity, the pressure, or the elevation of the fluid.
W
pb
gZb
ub2 2
pa
gZa
ua2 2
(8-1)
The equation(8-1) can be divided by g, gives
H
pb
g
Zb
ub2 2g
pa
g
Za
ua2 2g
(8-1a)
The quantity H is called developed (total) head, in which each term has the dimension of length.
天大化工原理-英文版课件-Chapter 1-11Definitions and Principles

18
4. Course objectives
• Understand the basic principles of and equipment of the unit operations. • Include designing or selection of devices. operatioroubleshooting of various unit operations.
• The international steam table calorie (calIT), used in heat power engineering, is defined by • 1 calIT=4.1868*×107 ergs = 4.1868* J (1.22)
25
• The standard gravity acceleration
gn 980.665cm / s
2
26
3. FPS Units
• The standard for mass is the avoirdupois pound (lb), • 1 lb = 0.45359237* kg (1.26) • The standard for length is the inch (in.), • 1 ft= 12× 2.54×10–2m= 0.3048* m (1.27) • The standard for time remains the second (s). • The thermodynamic temperature scale is called the Rankine scale,
9
1.1 UNIT OPERATIONS
• • • • 1. Unit operations 2. Classification of unit operations 3. Course description 4. Course objectives
4. Course objectives
• Understand the basic principles of and equipment of the unit operations. • Include designing or selection of devices. operatioroubleshooting of various unit operations.
• The international steam table calorie (calIT), used in heat power engineering, is defined by • 1 calIT=4.1868*×107 ergs = 4.1868* J (1.22)
25
• The standard gravity acceleration
gn 980.665cm / s
2
26
3. FPS Units
• The standard for mass is the avoirdupois pound (lb), • 1 lb = 0.45359237* kg (1.26) • The standard for length is the inch (in.), • 1 ft= 12× 2.54×10–2m= 0.3048* m (1.27) • The standard for time remains the second (s). • The thermodynamic temperature scale is called the Rankine scale,
9
1.1 UNIT OPERATIONS
• • • • 1. Unit operations 2. Classification of unit operations 3. Course description 4. Course objectives
化工原理英文教材-传质与分离部分chapter7

6
3. Thermodynamic relationships
1)Equilibrium ratio ( or equilibrium constant or K
yA KA xA
Where KA=Equilibrium ratio KA AB yA=mole fraction of component A in vapor KB xA= mole fraction of component A in liquid
Chapter 7 Equilibrium rela
1
1. Phase Rule
•F =
C–Φ+2 F = number of degrees of freedom, or variance C= number of components Φ= number of phases 2 —— only temperature and pressure may affect th e.g.: In systems of two components, C=2;Φ=2; therefore, F = 2 - 2 + 2 = 2
The above equations can be rearranged give
(重新整理)
to
16
AB x A yA 1 ( AB 1) x A
Let xA=x;yA=y:
Phase equilibrium equation:
x y 1 ( 1) x
The above equation is used to express the concentration of component A in the vapor as a function of its concentration in the liquid and relative volatility.表示在总压一定时,气液平衡时
化工原理09-传质概论共32页

解:氨在气相的摩尔浓 度C AG 按式 8 6计算, 其中分压单位为 mmHg 时的 R由表 8 1查得 为62 .36 mmHgm 3 / kmolK , C AG p A / RT 6 / 62 .36 293 0.00328 kmol / m3 100 kg水含氨 1kg,由于氨水很稀,密度 可视为与水相同。 其体积为( 100 1)/1000 0.101 m3; 氨为1 /17 kmol; C AG (1 /17 ) / 0.101 0.582 kmol / m3
(质量分率与比质量分率) 3、摩尔分率xA与摩尔浓度cA
4、质量分率aA与摩尔浓度cA
例:8—1(P4)
浓度 换 算
aA
xA aA
MA aB
MA
MB
Y A 1 yA yA ;
yA 1 Y Y A A ;
CACmxA
CA MA aA
实验室测得在 1at总 m及压温2度0C下1, 00g水中含1g氨 时, 液面上氨的平衡6m分m压 H。为 g求气、液相组摩成皆 尔浓度表示时的关相系平。衡
化工原理
(下册)
第八章:传质过 程导论
化工原理下册——传质过程导论
第八章 传 质 过 程 导 论 第一节 概述 一、传质过程(Mass transfer process) 物质从一相(转移到) 另一相的传递过程
气体的吸收(absorption):分离气体混合物 气—液
气
空气
水
吸收
液
NH3 解吸 NH3
律)
牛顿粘性定律 t - m dur
dr
在一定的T、P及CM下,均相
混合物的分子扩散通量为:
JA - DAB
(质量分率与比质量分率) 3、摩尔分率xA与摩尔浓度cA
4、质量分率aA与摩尔浓度cA
例:8—1(P4)
浓度 换 算
aA
xA aA
MA aB
MA
MB
Y A 1 yA yA ;
yA 1 Y Y A A ;
CACmxA
CA MA aA
实验室测得在 1at总 m及压温2度0C下1, 00g水中含1g氨 时, 液面上氨的平衡6m分m压 H。为 g求气、液相组摩成皆 尔浓度表示时的关相系平。衡
化工原理
(下册)
第八章:传质过 程导论
化工原理下册——传质过程导论
第八章 传 质 过 程 导 论 第一节 概述 一、传质过程(Mass transfer process) 物质从一相(转移到) 另一相的传递过程
气体的吸收(absorption):分离气体混合物 气—液
气
空气
水
吸收
液
NH3 解吸 NH3
律)
牛顿粘性定律 t - m dur
dr
在一定的T、P及CM下,均相
混合物的分子扩散通量为:
JA - DAB
化工原理英文教材传热原理Principles of heat flow in fluids

化工原理 Principles of Chemical Industry
Principles of heat flow in fluids
Typical heat-exchange equipment
Single-pass shell-and-tube condenser
Expansion joint
It is clear from Fig.11-4 that Δt can vary considerably from point to point along the tube, and, therefore, the flux also varies with tube length.
The local flux dq/dA is related to the local value of Δt by the equation
because, as inspection of Figs11-4a and b will show, it is
not possible with this method of flow to bring the exit temperature of one fluid nearly to the entrance temperature of the other and the heat that can be transferred is less than that possible in countercurrent flow.
The temperatures plotted Fig11-4 are average stream temperatures.
The temperature so defined is called the average or mixing-cup stream temperature.
Principles of heat flow in fluids
Typical heat-exchange equipment
Single-pass shell-and-tube condenser
Expansion joint
It is clear from Fig.11-4 that Δt can vary considerably from point to point along the tube, and, therefore, the flux also varies with tube length.
The local flux dq/dA is related to the local value of Δt by the equation
because, as inspection of Figs11-4a and b will show, it is
not possible with this method of flow to bring the exit temperature of one fluid nearly to the entrance temperature of the other and the heat that can be transferred is less than that possible in countercurrent flow.
The temperatures plotted Fig11-4 are average stream temperatures.
The temperature so defined is called the average or mixing-cup stream temperature.
化工原理课件第七章
化工原理授课提纲
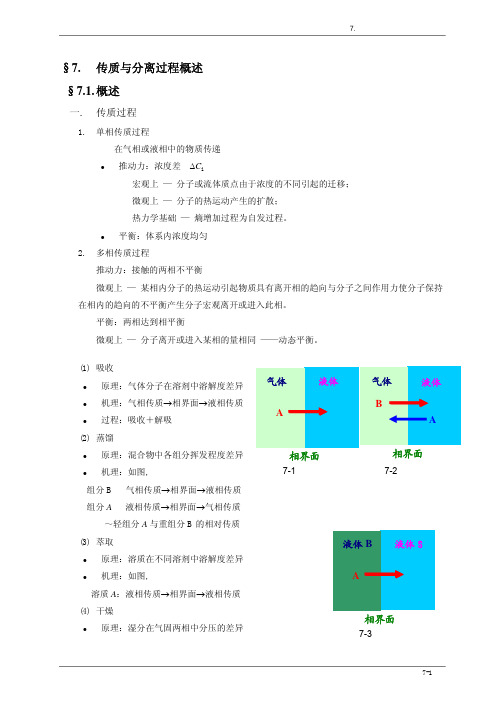
7. 传质与分离过程概述
§7.
传质与分离过程概述
§7.1. 概述
一.
1.
传质过程
单相传质过程 在气相或液相中的物质传递
z
推动力:浓度差 ∆Ci 宏观上 — 分子或流体质点由于浓度的不同引起的迁移; 微观上 — 分子的热运动产生的扩散; 热力学基础 — 熵增加过程为自发过程。
z
平衡:体系内浓度均匀
2.
液体中的扩散系数 对于很稀的非电解质溶液,有半经验式:(P14 页 7-43 式) D~溶质的体积、溶剂的粘度及分子量、溶剂的缔合参数、T 有关。由于液体压缩性小,故 忽略 P 的影响。
3.
固体中的扩散系数 z 正常扩散(或体积扩散):单相扩散 z 努森扩散:分子在孔道中的碰撞扩散+体积扩散 z 结构扩散:分子在孔道中的碰撞扩散 z 表面扩散:分子在孔道表面上吸附扩散 方程形式(略)
p DP ln B 2 RT Z p B 1 p A1 − p A 2 =1 pB 2 − pB 1
因为:P= pA1+pB1= pA2+pB2 则:pA1- pA2= pB2 -pB1 →
那么:NA=
p DP DP p − p A2 DP p A1 − p A 2 ln B 2 A1 = = (pA1-pA2) RT Z p B 1 p B 2 − p B 1 RT Z p B 2 − p B 1 RT Z p Bm p ln B 2 p B1 DC (CA1–CA2) ZCBm
液相
NA=
z
质量浓度: ci=
mi V ni V
kg/m3 kmol/m3
z
摩尔浓度: Ci = 浓度换算:
z
ρ = m/V = ∑ci ( i =A、B、…) ci = mi/V =aim/V = aiρ Ci=ni/V=xin/V=xiC (C=n/V→混合物的总摩尔浓度,kmol/m3) 对于气体混合物: Ci= ni/V=pi/RT ci= mi/V =Mini/V =Mi pi/RT …… 见 P5 页。
7. 传质与分离过程概述
§7.
传质与分离过程概述
§7.1. 概述
一.
1.
传质过程
单相传质过程 在气相或液相中的物质传递
z
推动力:浓度差 ∆Ci 宏观上 — 分子或流体质点由于浓度的不同引起的迁移; 微观上 — 分子的热运动产生的扩散; 热力学基础 — 熵增加过程为自发过程。
z
平衡:体系内浓度均匀
2.
液体中的扩散系数 对于很稀的非电解质溶液,有半经验式:(P14 页 7-43 式) D~溶质的体积、溶剂的粘度及分子量、溶剂的缔合参数、T 有关。由于液体压缩性小,故 忽略 P 的影响。
3.
固体中的扩散系数 z 正常扩散(或体积扩散):单相扩散 z 努森扩散:分子在孔道中的碰撞扩散+体积扩散 z 结构扩散:分子在孔道中的碰撞扩散 z 表面扩散:分子在孔道表面上吸附扩散 方程形式(略)
p DP ln B 2 RT Z p B 1 p A1 − p A 2 =1 pB 2 − pB 1
因为:P= pA1+pB1= pA2+pB2 则:pA1- pA2= pB2 -pB1 →
那么:NA=
p DP DP p − p A2 DP p A1 − p A 2 ln B 2 A1 = = (pA1-pA2) RT Z p B 1 p B 2 − p B 1 RT Z p B 2 − p B 1 RT Z p Bm p ln B 2 p B1 DC (CA1–CA2) ZCBm
液相
NA=
z
质量浓度: ci=
mi V ni V
kg/m3 kmol/m3
z
摩尔浓度: Ci = 浓度换算:
z
ρ = m/V = ∑ci ( i =A、B、…) ci = mi/V =aim/V = aiρ Ci=ni/V=xin/V=xiC (C=n/V→混合物的总摩尔浓度,kmol/m3) 对于气体混合物: Ci= ni/V=pi/RT ci= mi/V =Mini/V =Mi pi/RT …… 见 P5 页。
