USP34-NF29 911 粘度 中文翻译
USP34 41 Weights and balances 砝码和天平(中英文)

41WEIGHTS AND BALANCES砝码和天平The intent of this section is to bring the requirements for weights into conformity with American National Standard ANSI/ASTM E617,“Laboratory Weights and Precision Mass Standards.”This standard is incorporated by reference and should be consulted for full descriptions and information on the tolerances and construction of weights.[1]本节的目的是使对砝码的要求符合美国国家标准ANSI/ASTM E617“实验室砝码和精密质量标准物”。
此标准以索引的方式整合在一起,砝码的容许公差和构造应参考完整的说明和资料。
1Pharmacopeial tests and assays require balances that vary in capacity, sensitivity,and reproducibility.Unless otherwise specified,when substances are to be“accurately weighed”for Assay,the weighing is to be performed with a weighing device whose measurement uncertainty(random plus systematic error)does not exceed0.1%of the reading.Measurement uncertainty is satisfactory if three times the standard deviation of not less than ten replicate weighings divided by the amount weighed,does not exceed0.001.药典测试和检测所需的天平要求多种负荷、灵敏度和可重现性。
201404210936_美国药典34版胰蛋白酶质量标准Trypsin-USP34

Crystallized Trypsin» Crystallized Trypsin is a proteolytic enzyme crystallized from an extract of the pancreas of healthy bovine or porcine animals, or both. When assayed as directed herein, it contains not less than 2500 USP Trypsin Units in each mg, calculated on the dried basis, and not less than 90.0 percent and not more than 110.0 percent of the labeled potency.[NOTE—Determine the suitability of the substrates and check the adjustment of the spectrophotometer by performing the Assay using USP Crystallized Trypsin Reference Standard. ]Packaging and storage— Preserve in tight containers, and avoid exposure to excessive heat.USP R EFERENCE STANDARDS11—USP Trypsin Crystallized RSSolubility test— An amount, equivalent to 500,000 USP Trypsin Units, is soluble in 10 mL of water and in 10 mL of saline TS.M ICROBIAL ENUMERATION TESTS61 and T ESTS FOR SPECIFIED MICROORGANISMS62— It meets the requirements of the tests for absence of Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella species.L OSS ON DRYING731— Dry it in vacuum at 60 for 4 hours: it loses not more than5.0% of its weight.R ESIDUE ON IGNITION281: not more than 2.5%.Limit of chymotrypsin—0.067 M Phosphate buffer , pH 7.0—Dissolve 4.54 g of monobasic potassium phosphate in water to make 500 mL of solution. Dissolve 4.73 g of anhydrous dibasic sodium phosphate in water to make 500 mL of solution. Mix 38.9 mL of the monobasic potassium phosphate solution with 61.1 mL of dibasic sodium phosphate solution. Adjust dropwise, if necessary, with dibasic sodium phosphate solution to a pH of 7.0.Substrate solution— Dissolve 23.7 mg of N-acetyl-L-tyrosine ethyl ester, suitable for use in determining chymotrypsin, in about 50 mL of 0.067 M Phosphate buffer, pH 7.0 with warming. When cool, dilute with additional pH 7.0 buffer to 100 mL. (Substrate solution may be stored in the frozen state and used after thawing; it is important, however, to freeze immediately after preparation.)Crystallized Trypsin solution— Dissolve a sufficient quantity of Crystallized Trypsin, accurately weighed, in 0.0010 N hydrochloric acid to obtain a solution containing 650 USPTrypsin Units per mL.Procedure— Conduct the test in a suitable spectrophotometer equipped to maintain atemperature of 25 ± 0.1 in the cell compartment. Determine the temperature in the reaction cell before and after the measurement of absorbance to ensure that the temperature does not change by more than 0.5. Pipet 200 µL of 0.0010 N hydrochloric acid and 3.0 mL of the Substrate solution into a 1-cm cell. Place this cell in the spectrophotometer, and adjust the instrument so that the absorbance reads 0.200 at 237 nm. Pipet 200 µL of Crystallized Trypsin solution into another 1-cm cell, add 3.0 mL of the Substrate solution, and place the cell in the spectrophotometer. [NOTE—This order of addition is to be followed. ] At the time the Substrate solution is added, start a stopwatch, and read the absorbance at 30-second intervals for not less than 5 minutes. Repeat the procedure on the same dilution at least once. Absolute absorbance values are of less importance than the constancy of the rate of change of absorbance. If the rate of change does not remain constant for at least 3 minutes, repeat the run, and if necessary, use a lower concentration. The duplicate run at the same dilution should match the first run in rate of absorbance change. Determine the average absorbance change per minute, using only the values within the 3-minute portion of the curve where the rate of absorbance is constant. Plot a curve of absorbance against time. One USP Chymotrypsin Unit is the activity causing a change in absorbance of 0.0075 per minute under the conditions specified in this test. Calculate the number of USP Chymotrypsin Units per mg of Crystallized Trypsin taken by the formula:(A2A1) / (0.0075TW)in which A2 is the absorbance straight-line initial reading, A1is the absorbance straight-line final reading, T is the elapsed time, in minutes, between the initial and final readings, and W is the weight, in mg, of Crystallized Trypsin in the volume of solution used in determining the absorbance. Not more than 50 USP Chymotrypsin Units per 2500 USP Trypsin Units is found, indicating the presence of not more than approximately 5% of chymotrypsin.Assay—0.067 M Phosphate buffer , pH 7.6—Dissolve 4.54 g of monobasic potassium phosphate in water to make 500 mL of solution. Dissolve 4.73 g of anhydrous dibasic sodium phosphate in water to make 500 mL of solution. Mix 13 mL of the monobasic potassium phosphate solution with 87 mL of the anhydrous dibasic sodium phosphate solution.Substrate solution— Dissolve 85.7 mg of N-benzoyl-L-arginine ethyl ester hydrochloride, suitable for use in assaying Crystallized Trypsin (see N OTE), in water to make 100 mL. Dilute 10 mL of this solution with 0.067 M Phosphate buffer, pH 7.6 to 100 mL. Determine the absorbance of this solution, in a 1-cm cell, at 253 nm, in a suitable spectrophotometerequipped with thermospacers to maintain a temperature of 25 ± 0.1, using water as the blank. By the addition of 0.067 M Phosphate buffer , pH 7.6, or of the Substrate solution before dilution, adjust the absorbance so that it measures not less than 0.575 and not more than 0.585. Use this Substrate solution within 2 hours.Crystallized Trypsin solution — Dissolve a sufficient quantity of Crystallized Trypsin,accurately weighed, in 0.0010 N hydrochloric acid to obtain a solution containing about 50 to 60 USP Trypsin Units per mL.Procedure — Pipet 200 µL of 0.0010 N hydrochloric acid and 3.0 mL of the Substratesolution into a 1-cm cell. Place this cell in a spectrophotometer, and adjust the instrument so that the absorbance reads 0.050 at 253 nm. Pipet 200 µL of Crystallized Trypsin solution , containing 10 to 12 USP Trypsin Units, into another 1-cm cell, add 3.0 mL of Substrate solution , and place the cell in the spectrophotometer. At the time the Substrate solution is added, start a stopwatch, and read the absorbance at 30-second intervals for 5 minutes. Repeat the procedure on the same dilution at least once. Plot a curve ofabsorbance against time, and use only those values that form a straight line to determine the activity of the Crystallized Trypsin. If the rate of change does not remain constant for at least 3 minutes, repeat the run, and if necessary, use a lower concentration. One USP Trypsin Unit is the activity causing a change in absorbance of 0.003 per minute under the conditions specified in this Assay. Calculate the number of USP Trypsin Units per mg taken by the formula:(A 1 A 2) / (0.003TW )in which A 1 is the absorbance straight-line final reading, A 2 is the absorbance straight-line initial reading, T is the elapsed time, in minutes, between the initial and final readings, and W is the weight, in mg, of Crystallized Trypsin in the volume of solution used indetermining the absorbances.Auxiliary Information — Please check for your question in the FAQs before contactingUSP. Topic/Question Contact Expert Committee Monograph Fouad Atouf, Ph.D.Senior Scientific Liaison1-301-816-8365(BIO12010) Monographs - Biologics and Biotechnology 1Reference Standards RS Technical Services1-301-816-8129rstech@61Radhakrishna STirumalai, Ph.D.Principal Scientific Liaison1-301-816-8339(GCM2010) General Chapters - Microbiology 62Radhakrishna STirumalai, Ph.D. (GCM2010) General Chapters -MicrobiologyPrincipal Scientific Liaison1-301-816-8339USP34–NF29 Page 4535Pharmacopeial Forum: Volume No. 32(3) Page 779。
甘油 USP34

Glycerin (glis' er in).C 3H 8O 392.101,2,3-Propanetriol; Glycerol [56-81-5].DEFINITIONGlycerin contains NLT 99.0% and NMT 101.0% of C 3H 8O 3, calculated on the anhydrous basis. IDENTIFICATION[N OTE —Compliance is determined by meeting the requirements for Identification tests A, B, and C . ]• A. I NFRARED A BSORPTION 197F• B. L IMIT OF D IETHYLENE G LYCOL AND E THYLENE G LYCOLStandard solution: 2.0 mg/mL of USP Glycerin RS , 0.050 mg/mL of USP Ethylene Glycol RS , 0.050 mg/mL of USP Diethylene Glycol RS , and 0.10 mg/mL of 2,2,2-trichloroethanol (internal standard) in methanolSample solution: 50 mg/mL of Glycerin and 0.10 mg/mL of 2,2,2-trichloroethanol (internal standard) in methanol Chromatographic system(See Chromatography 621, System Suitability .) Mode: GCDetector: Flame ionizationColumn: 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43 stationary phase, and a deactivated split liner with glass wool TemperatureInjector: 220 Detector: 250Column: See the temperature program table.Carrier gas: Helium Injection size: 1.0 µLInitialTemperature ()FinalTemperature ()Flow rate: 4.5 mL/minInjection type: Split ratio, about 10:1 System suitabilitySample: Standard solution[N OTE —The relative retention times for ethylene glycol, 2,2,2-trichloroethanol, diethylene glycol, and glycerin are about 0.3, 0.6, 0.8 and 1.0, respectively. ] Suitability requirementsResolution: NLT 1.5 between diethylene glycol and glycerin AnalysisSample: Sample solutionAcceptance criteria: If a peak at the retention times for the diethylene glycol or ethylene glycol is present in the Sample solution, the peak response ratio relative to 2,2,2-trichloroethanol is NMT the peak response ratio for diethylene glycol or ethylene glycol relative to 2,2,2-trichloroethanol in the Standard solution; NMT 0.10% each for diethylene glycol and ethylene glycol is found.• C. Examine the chromatograms obtained in Identification test B . The retention time of the glycerinpeak of the Sample solution corresponds to that obtained in the Standard solution . ASSAY• P ROCEDURESodium periodate solution: Dissolve 60 g of sodium metaperiodate in sufficient water containing 120 mL of 0.1 N sulfuric acid to make 1000 mL. Do not heat to dissolve theperiodate. If the solution is not clear, pass through a sintered-glass filter. Store the solution in a glass-stoppered, light-resistant container. Test the suitability of this solution as follows. Pipet 10 mL into a 250-mL volumetric flask, and dilute with water to volume. To 550 mg of Glycerin dissolved in 50 mL of water, add 50 mL of the diluted periodate solution with a pipet. For a blank, pipet 50 mL of the solution into a flask containing 50 mL of water. Allow the solutions to stand for 30 min, then to each add 5 mL of hydrochloric acid and 10 mL of potassium iodide TS, and rotate to mix. Allow to stand for 5 min, add 100 mL of water, and titrate with 0.1 N sodium thiosulfate, shaking continuously and adding 3 mL of starch TS as the endpoint is approached. The ratio of the volume of 0.1 N sodium thiosulfate required for the glycerin –periodate mixture to that required for the blank should be between 0.750 and 0.765.Analysis: Transfer 400 mg of Glycerin to a 600-mL beaker, dilute with 50 mL of water, addbromothymol blue TS, and acidify with 0.2 N sulfuric acid to a definite green or greenish yellow color. Neutralize with 0.05 N sodium hydroxide to a definite blue endpoint, free from green color. Prepare a blank containing 50 mL of water, and neutralize in the same manner. Pipet 50 mL of the Sodium periodate solution into each beaker, mix by swirling gently, cover with awatch glass, and allow to stand for 30 min at room temperature (not exceeding 35) in the dark or in subdued light. Add 10 mL of a mixture of equal volumes of ethylene glycol and water, and allow to stand for 20 min. Dilute each solution with water to 300 mL, and titrate with 0.1 N sodium hydroxide VS to a pH of 8.1 ± 0.1 for the specimen under assay and 6.5 ± 0.1 for the blank, using a pH meter. Each mL of 0.1 N sodium hydroxide, after correction for the blank, is equivalent to 9.210 mg of C 3H 8O 3.Acceptance criteria: 99.0%–101.0% on the anhydrous basis IMPURITIESInorganic Impurities• C HLORIDE AND S ULFATE , Chloride 221: A 7.0-g portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (NMT 10 ppm). • C HLORIDE AND S ULFATE , Sulfate 221: A 10-g portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid (NMT 20 ppm). • H EAVY M ETALS 231Analysis: Mix 4.0 g with 2 mL of 0.1 N hydrochloric acid, and dilute with water to 25 mL. Acceptance criteria: NMT 5 ppm • R ESIDUE ON I GNITION 281: Heat 50 g in an open, shallow 100-mL porcelain dish until it ignites,and allow it to burn without further application of heat in a place free from drafts. Cool, moisten the residue with 0.5 mL of sulfuric acid, and ignite to constant weight: the weight of the residue does not exceed 5 mg (0.01%). Organic Impurities• P ROCEDURE 1: R ELATED C OMPOUNDSSystem suitability solution: 0.5 mg/mL each of USP Diethylene Glycol RS and USP Glycerin RSSample solution: 50 mg/mL of Glycerin Chromatographic system(See Chromatography 621, System Suitability .) Mode: GCDetector: Flame ionizationColumn: 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43 stationary phase, and an inlet liner having an inverted cup or spiral structure TemperatureInjector: 220 Detector: 250Column: See the temperature program table below.Carrier gas: Helium Injection size: 0.5 µL Linear velocity: 38 cm/sInjection type: Split ratio, about 10:1 System suitabilitySample: System suitability solution Suitability requirementsTemperature Ramp (/min)Final Temperature ()Hold Time at FinalTemperature (min)100—100—1007.52204Resolution: NLT 7.0 between diethylene glycol and glycerin AnalysisSample: Sample solutionCalculate the percentage of each impurity, excluding any solvent peaks and diethylene glycol, in the portion of Glycerin taken:Result = (r U /r T) × 100Acceptance criteriaIndividual impurities: NMT 0.1% Total impurities: NMT 1.0%• P ROCEDURE 2: L IMIT OF C HLORINATED C OMPOUNDS Sample: 5 g of GlycerinAnalysis: Transfer the Sample into a dry, round-bottom, 100-mL flask. Add 15 mL ofmorpholine, and connect the flask by a ground joint to a reflux condenser. Reflux gently for 3 h. Rinse the condenser with 10 mL of water, receiving the washings in the flask, and cautiously acidify with nitric acid. Transfer the solution to a suitable comparison tube, add 0.50 mL of silver nitrate TS, and dilute with water to 50.0 mL.Acceptance criteria: The turbidity is not greater than that of a blank to which 0.20 mL of 0.020 N hydrochloric acid has been added, the refluxing being omitted (NMT 30 ppm of Cl). • P ROCEDURE 3: F ATTY A CIDS AND E STERSSample solution: Mix 50 g of Glycerin with freshly boiled water and 5 mL of 0.5 N sodium hydroxide VS. Boil the mixture for 5 min, cool, and add phenolphthalein TS.Analysis: Titrate the excess alkali with 0.5 N hydrochloric acid VS. Perform a blank determination (see Titrimetry 541, Residual Titrations ).Acceptance criteria: NMT 1 mL of 0.5 N sodium hydroxide is consumed.SPECIFIC TESTS• C OLOR : When viewed downward against a white surface in a 50-mL color-comparison tube, thecolor is not darker than the color of a standard made by diluting 0.40 mL of ferric chloride CS with water to 50 mL and similarly viewed in a color-comparison tube of approximately the same diameter and color as that containing the Glycerin. • S PECIFIC G RAVITY 841: NLT 1.249• W ATER D ETERMINATION , Method I 921: NMT 5.0% ADDITIONAL REQUIREMENTS• P ACKAGING AND S TORAGE : Preserve in tight containers. • USP R EFERENCE S TANDARDS 11 USP D IETHYLENE G LYCOL RS USP E THYLENE G LYCOL RS USP G LYCERIN RSr U == peak response of each individual impurityfrom the Sample solutionr T== sum of the responses of all the peaks fromthe Sample solution1,2,3-Propanetriol. C 3H 8O 392.10Auxiliary Information — Please check for your question in the FAQs before contacting USP. USP34–NF29 Page 2986Pharmacopeial Forum : Volume No. 28(4) Page 1245 Chromatographic Column — GLYCERINChromatographic columns text is not derived from, and not part of, USP 34 or NF 29.。
埃索美拉唑镁usp34

© 2010 USPC Official 5/1/11 - 7/31/11 USP Monographs: Esomeprazole ...
页码,Байду номын сангаас/7
prepared atomic absorption standard solution. [NOTE—Store the solution in a plastic bottle. ] Standard solution A: Transfer 10.0 mL of Standard stock solution to a 500-mL volumetric flask, add 50 mL of 1 N hydrochloric acid, and dilute with water to volume. Transfer 20.0 mL of this solution to a 200-mL volumetric flask, and dilute with water to volume. [NOTE —This solution contains 2 µg/mL of magnesium. ] Standard solution B: Combine 5.0 mL of Standard solution A and 4.0 mL of Lanthanum solution , and dilute with water to 100.0 mL (0.1 µg/mL). Standard solution C: Combine 10.0 mL of Standard solution A and 4.0 mL of Lanthanum solution , and dilute with water to 100.0 mL (0.2 µg/mL). Standard solution D: Combine 15.0 mL of Standard solution A and 4.0 mL of Lanthanum solution , and dilute with water to 100.0 mL (0.3 µg/mL). Standard solution E: Combine 20.0 mL of Standard solution A and 4.0 mL of Lanthanum solution , and dilute with water to 100.0 mL (0.4 µg/mL). Standard solution F: Combine 25.0 mL of Standard solution A and 4.0 mL of Lanthanum solution , and dilute with water to 100.0 mL (0.5 µg/mL). [NOTE— Concentrations of the Standard solutions and the Sample solution may be modified to fit the linear or working range of the instrument. When using instruments with a linear calibration graph, the number of Standard solutions can be reduced. ] Blank solution: Transfer 4.0 mL of Lanthanum solution to a 100-mL volumetric flask, and dilute with water to volume. Sample solution: Transfer 250 mg of Esomeprazole Magnesium to a 100-mL volumetric flask, add 20 mL of 1 N hydrochloric acid, swirl until dissolved, and dilute with water to volume. Allow to stand for 30 min. Transfer 10.0 mL of this solution to a 200-mL volumetric flask, and dilute with water to volume. Transfer 10.0 mL of the solution to another 100 -mL volumetric flask, add 4.0 mL of Lanthanum solution , and dilute with water to volume. Spectrometric conditions (See Spectrophotometry and Light-Scattering 851 .) Mode: Atomic absorption spectrophotometer Flame: Air–acetylene Analytical wavelength: 285.2 nm Analysis Samples: Standard solution B, Standard solution C, Standard solution D, Standard solution E , Standard solution F, Blank solution , and Sample solution Determine the concentration, C s , in µg/mL, of magnesium in the Sample solution using the calibration graph. Calculate the percentage of magnesium in the portion of Esomeprazole Magnesium taken:
USP34-NF29 911 粘度 中文翻译

<911> VISCOSITYViscosity is a property of liquids that is closely related to the resistance to flow. It is defined in terms of the force required to move one plane surface continuously past another under specified steady-state conditions when the space between is filled by the liquid in question. It is defined as the shear stress divided by the rate of shear strain. The basic unit is the poise; however, viscosities commonly encountered represent fractions of the poise, so that the centipoise (1 poise = 100 centipoises) proves to be the more convenient unit. The specifying of temperature is important because viscosity changes with temperature; in general, viscosity decreases as temperature is raised. While on the absolute scale viscosity is measured in poises or centipoises, for convenience the kinematic scale, in which the units are stokes and centistokes (1 stoke = 100 centistokes) commonly is used. To obtain the kinematic viscosity from the absolute viscosity, the latter is divided by the density of the liquid at the same temperature, i.e., kinematic viscosity = (absolute viscosity)/(density). The sizes of the units are such that viscosities in the ordinary ranges are conveniently expressed in centistokes. The approximate viscosity in centistokes at room temperature of ether is 0.2; of water, 1; of kerosene, 2.5; of mineral oil, 20 to 70; and of honey, 10,000.粘度是液体的属性之一,它与流动阻力紧密相关。
美国药典(USP)规定的色谱柱编号模板

美国药典(USP)规定的色谱柱编号L1和L8是美国药典(USP)规定的色谱柱编号,其实就是ODS柱和NH2柱。
下面是USP规定的编号所对应的色谱柱类型。
L1:十八烷基键合多孔硅胶或无机氧化物微粒固定相,简称ODS柱L2:30~50m m表面多孔薄壳型键合十八烷基固定相,简称C18柱L3:多孔硅胶微粒,即一般的硅胶柱L4:30~50m m表面多孔薄壳型硅胶柱L5:30~50m m表面多孔薄壳型氧化铝柱L6:30~50m m实心微球表面包覆磺化碳氟聚合物,强阳离子交换柱L7:全多孔硅胶微粒键合C8官能团固定相,简称C8柱L8:全多孔硅胶微粒键合非交联NH2固定相,简称NH2柱L9:强酸性阳离子交换基团键合全多孔不规则形硅胶固定相,即SCX柱L10:多孔硅胶微球键合氰基固定相(CN),简称CN柱L11:键合苯基多孔硅胶微球固定相,简称苯基柱L12:无孔微球键合季胺功能团的强阴离子交换柱L13:三乙基硅烷化学键合全多孔硅胶微球固定相(C1),简称C1柱L14:10m m硅胶化学键合强碱性季铵盐阴离子交换固定相,简称SAX柱L15:已基硅烷化学键合全多孔硅胶微球固定相,简称C6柱L16:二甲基硅烷化学键合全多孔硅胶微粒固定相 C2柱L17:氢型磺化交联苯乙烯-二乙烯基苯共聚物,强阳离子交换柱L18:3~10m m全多孔硅胶化学键合胺基(NH2)和氰基(CN)柱L19:钙型磺化交联苯乙烯-二乙烯基苯共聚物,强阳离子交换柱L20:二羟基丙烷基化学键合多孔硅胶微球固定相(Diol),简称二醇基柱L21:刚性苯乙烯-二乙烯基苯共聚物微球填料柱L22:带有磺酸基团的多孔苯乙烯阳离子交换柱L23:带有季胺基团的聚甲基丙烯酸甲酯或聚丙烯酸酯多孔离子交换柱L24:表面含有大量羟基的半刚性聚乙烯醇亲水凝胶柱L25:聚甲基丙烯酸酯树脂交联羟基醚(表面含有残余羧基功能团)树脂。
能分离分子量100~5000MW范围的水溶性中性、阳离子型及阴离子型聚合物(用聚氧乙烯测定)的固定相L26:丁基硅烷化学键合全多孔硅胶微球固定相,即C4柱L27:30~50m m的全多孔硅胶微粒L28:多功能载体,100Å的高纯硅胶加以氨基键合以及C8反相键合的官能团L29:氧化铝,反相键合,含碳量低,氧化铝基聚丁二稀小球,5m m,孔径80ÅL30:全多孔硅胶键合乙基硅烷固定相L31:季胺基改性孔径2000Å的交联苯乙烯和二乙烯基苯(55%)强阴离子交换树脂L32: L-脯氨酸铜配合物共价键合于不规则形硅胶微粒的配位体的交换手性色谱填料L33:能够分离分子量4000~40000MW范围蛋白质分子的球形硅胶固定相, pH稳定性好L34:铅型磺化交联苯乙烯-二乙烯基苯共聚物强阳离子交换树脂,9m m球形L35:锆稳定的硅胶微球键合二醇基亲水分子单层固定相,孔径150ÅL36:5m m胺丙基硅胶键合L-苯基氨基乙酸-3,5二硝基苯甲酰L37:适合分离分子量2000~40000MW的聚甲基丙烯酸酯凝胶L38:水溶性甲基丙烯酸酯基质SEC色谱柱L39:亲水全多孔聚羟基甲基丙烯酸酯色谱柱L40:Tris 3,5-二甲基苯基氨基甲酸酯纤维素涂覆多孔硅胶微球L41:球形硅胶表面固定α1酸糖蛋白固定相L42: C8和C18硅烷化学键合多孔硅胶固定相L43:硅胶微球键合五氟代苯基固定相L44:多功能固定相,60 Å高纯硅胶基质键合磺酸阳离子交换功能团和C8反相功能团L45: β-环糊精键合多孔硅胶微球L46:季胺基改性苯乙烯-二乙烯基苯聚合物微球L1 Octadecyl silane chemically bonded to porous silica or ceramic.L1 十八烷基键合硅烷化学键合于多孔硅胶或陶瓷微粒,3-10u。
USP34–NF29 Lamivudine
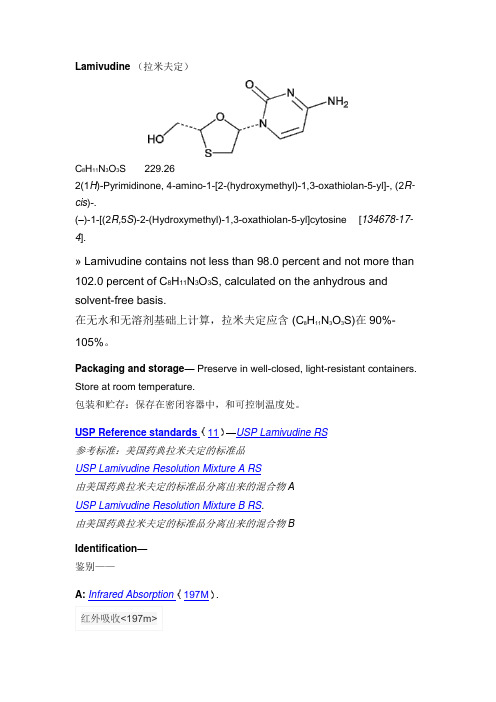
Lamivudine (拉米夫定)C 8H 11N 3O 3S229.26 2(1H )-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-, (2R-cis )-.(–)-1-[(2R,5S )-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine [134678-17-4]. » Lamivudine contains not less than 98.0 percent and not more than 102.0 percent of C 8H 11N 3O 3S, calculated on the anhydrous and solvent-free basis.在无水和无溶剂基础上计算,拉米夫定应含 (C 8H 11N 3O 3S)在90%-105%。
Packaging and storage — Preserve in well-closed, light-resistant containers. Store at room temperature.包装和贮存:保存在密闭容器中,和可控制温度处。
USP Reference standards 11—USP Lamivudine RS参考标准:美国药典拉米夫定的标准品 USP Lamivudine Resolution Mixture A RS由美国药典拉米夫定的标准品分离出来的混合物A USP Lamivudine Resolution Mixture B RS .由美国药典拉米夫定的标准品分离出来的混合物BIdentification —鉴别——A: Infrared Absorption 197M . 红外吸收<197m>B: The retention time of the major peak in the chromatogram of the Test solution corresponds to that in the chromatogram of the Resolution solution, as obtained in the test for Limit of lamivudine enantiomer.供试品溶液的主要色谱峰的保留时间与拉米夫定对映体的相对应。
USP34–NF29 Stavudine capsules

Stavudine Capsules(司他夫定胶囊)» Stavudine Capsules contain not less than 90.0 percent and not more than 105.0 percent of the labeled amount of stavudine(C10H12N2O4).司他夫定胶囊应含司他夫定(C10H12N2O4)在90%-105%.Packaging and storage— Preserve in tightly closed containers, and store at controlled room temperature.包装和贮存:保存在密闭容器中,和可控制温度处。
USP Reference standards 11—USP Stavudine RS.参考标准:美国药典司他夫定的标准品Identification—鉴别:A: Thin-Layer Chromatographic Identification Test 201—A: 薄层色谱鉴别实验 <201>Test solution— Using sonication, dissolve a portion of Capsule contents in enough water to obtain a solution having a concentration of 0.2 mg of stavudine per mL, filter, and mix. Use the filtrate as the Test solution.试验方案:取胶囊内容物溶于水中,使溶液浓度为0.2mg/ml,超声,过滤。
滤液作为供试品溶液。
Application volume: 10 µL, applied in two 5-µL portions.用量:共10μL,取两个平行,每个5μL。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
<911> VISCOSITYViscosity is a property of liquids that is closely related to the resistance to flow. It is defined in terms of the force required to move one plane surface continuously past another under specified steady-state conditions when the space between is filled by the liquid in question. It is defined as the shear stress divided by the rate of shear strain. The basic unit is the poise; however, viscosities commonly encountered represent fractions of the poise, so that the centipoise (1 poise = 100 centipoises) proves to be the more convenient unit. The specifying of temperature is important because viscosity changes with temperature; in general, viscosity decreases as temperature is raised. While on the absolute scale viscosity is measured in poises or centipoises, for convenience the kinematic scale, in which the units are stokes and centistokes (1 stoke = 100 centistokes) commonly is used. To obtain the kinematic viscosity from the absolute viscosity, the latter is divided by the density of the liquid at the same temperature, i.e., kinematic viscosity = (absolute viscosity)/(density). The sizes of the units are such that viscosities in the ordinary ranges are conveniently expressed in centistokes. The approximate viscosity in centistokes at room temperature of ether is 0.2; of water, 1; of kerosene, 2.5; of mineral oil, 20 to 70; and of honey, 10,000.粘度是液体的属性之一,它与流动阻力紧密相关。
在规定的条件下,待测液体充满平面间的空隙,在不断转动过程中,所产生的力定义为粘度。
该粘度等于剪切应力除以剪切应变率。
基本单位是泊;但是经常遇到泊的分数表示的粘度,因此厘泊(1泊=100厘泊)是更常用的单位。
由于粘度随温度变化明显,需指明温度。
通常情况下,粘度随温度的升高而减小。
尽管粘度的测量是以绝对粘度形式表示,其常用单位是泊或厘泊,但为方便期间常常得到的是运动粘度,常用单位是司和厘司(1司=100厘司)。
为了从绝对粘度得到运动粘度,绝对粘度需要除以同温度下的液体密度。
如,运动粘度=绝对粘度/密度。
单位的大小便于表示常规范围内的粘度为厘司。
在室温情况下,以厘司估计的粘度,醚为0.2;水为1;煤油为2.5;矿物油为20~70;蜂蜜为10,000。
Absolute viscosity can be measured directly if accurate dimensions of the measuring instruments are known, but it is more common practice to calibrate the instrument with a liquid of known viscosity and to determine the viscosity of the unknown fluid by comparison with that of the known.如果已知测量仪器的准确尺寸,可以直接测得绝对粘度。
但是更常用的做法是使用已知粘度的液体校准仪器,通过与已知粘度的液体相对比,测定未知粘度的液体。
Many substances, such as the gums employed in pharmacy, have variable viscosity, and most of them are less resistant to flow at higher flow rates. In such cases, a given set of conditions is selected for measurement, and the measurement obtained is considered to be an apparent viscosity. Since a change in the conditions of measurement would yield a different value for the apparent viscosity of such substances, the instrument dimensions and conditions for measurement must be closely adhered to by the operator.许多物质,如制药中用到的胶,具有可变粘度。
它们中的大多数在高流速的情况下,流动阻力较低。
在这种情况下,为了测量粘度选定一个特定条件,得到的测量值被认为是表观粘度。
因此测量条件的改变会造成这类物质的表观粘度值的不同。
操作者必须严格遵守测量时的仪器尺寸和条件。
Measurement of Viscosity— The usual method for measurement of viscosity involves the determination of the time required for a given volume of liquid to flow through a capillary. Many capillary-tube viscosimeters have been devised, but Ostwald and Ubbelohde viscosimeters are among the most frequently used. Several types are described, with directions for their use, by the American Society for Testing and Materials (ASTM, D-445). The viscosity of oils is expressed on arbitrary scales that vary from one country to another, there being severalcorresponding instruments. The most widely used are the Redwood No. I and No. II, the Engler, the Saybolt Universal, and the Saybolt Furol. Each of these instruments uses arbitrary units that bear the name of the instrument. Standard temperatures are adopted as a matter of convenience with these instruments. For the Saybolt instruments, measurements usually are made at 100°F and 210F; Redwood instruments may be used at several temperatures up to 250°F; and values obtained on the Engler instrument usually are reported at 20°C and 50°C. A particularly convenient and rapid type of instrument is a rotational viscosimeter, which utilizes a bob or spindle immersed in the test specimen and measures the resistance to movement of the rotating part. Different spindles are available for given viscosity ranges, and several rotational speeds generally are available. Other rotational instruments may have a stationary bob and a rotating cup. The Brookfield, Rotouisco, and Stormer viscosimeters are examples of rotating-bob instruments, and the MacMichael is an example of the rotating-cup instrument. Numerous other rotational instruments of advanced design with special devices for reading or recording, and with wide ranges of rotational speed, have been devised.粘度的测量方法- 测量粘度的常用方法涉及测定指定体积的液体流经毛细管所需的时间。
