传质与分离过程考试试卷(英文)华工
华东理工大学分离工程期末复习题及参考答案

华东理工大学网络教育学院(全部答在答题纸上,请写清题号,反面可用。
试卷与答题纸分开交)分离工程_202210_模拟卷1一、填空题(共10题,每题3分,共30分)1. 分离过程是________________的逆过程,因此需加入________________来达到分离目的。
(3分).2. 分离剂可以是____________________或____________________,有时也可两种同时应用。
(3分).3. 图解梯级法计算多组分吸收过程的理论板数,假定条件为____________________________________________________________________________ ________________________________________________________。
(3分).4. 萃取精馏塔在萃取剂加入口以上需设________________________。
(3分).5. 衡量分离的程度用________________表示,处于相平衡状态的分离程度是________________。
(3分).6. 设计变量与独立量之间的关系可用下式来表示________________________________。
(3分).7. 恒沸剂的沸点应显著比原溶液沸点________________以上。
(3分).8. 固有分离因子是根据____________________来计算的。
它与实际分离因子的差别用____________________来表示。
(3分).9. 三对角矩阵法沿塔流率分布假定为________________。
(3分).10. 吸收因子A________于平衡常数。
(3分).二、选择题(共5题,每题2分,共10分)1. 下列哪一个是速率分离过程()。
(2分)A.蒸馏B.吸收C.膜分离D.离心分离2. 下列哪一个不是均相恒沸物的特点()。
传质分离过程试卷

传质分离过程试卷一、选择题(共10题,每题2分,共20分)1.以下不属于传质分离过程的是:– A. 蒸馏– B. 气体吸附– C. 曝气– D. 结晶2.传质分离过程中,分馏是利用物质的什么性质实现的?– A. 密度差异– B. 温度差异– C. 压力差异– D. 溶解度差异3.以下哪种传质分离过程利用了膜的选择性通透性?– A. 萃取– B. 吸附– C. 渗透– D. 结晶4.下列哪种传质分离过程主要利用了溶剂的不同挥发性?– A. 蒸馏– B. 萃取– C. 气体吸附– D. 结晶5.反渗透是一种什么类型的传质分离过程?– A. 物理传质分离过程– B. 化学传质分离过程– C. 生物传质分离过程– D. 不确定6.以下哪种传质分离过程是基于物质在溶液和固体表面之间的吸附作用?– A. 吸附– B. 渗透– C. 萃取– D. 结晶7.结晶是通过什么方式实现物质之间的分离?– A. 溶解度差异– B. 密度差异– C. 温度差异– D. 压力差异8.下列哪个条件对于蒸馏过程的实现是必要的?– A. 压力大于饱和蒸汽压力– B. 温度高于沸点– C. 设备具备分离精馏的结构– D. 所有选项都对9.萃取是一种利用分散相在连续相中的亲和性实现物质分离的过程,其中分散相也称为:– A. 溶液– B. 固相– C. 气相– D. 透析10.以下哪个选项不属于传质分离过程的应用?– A. 生活中的水的净化– B. 石油炼制过程中的裂化– C. 水果的蒸馏提取– D. 医药领域中的药物合成二、简答题(共4题,每题10分,共40分)1.请简要描述传质分离过程的定义及目的。
传质分离过程是指通过运用不同物质在不同条件下的传质特性,利用物质之间的差异来实现分离纯化目标物质的过程。
其目的是根据不同物质的传质特性,使混合物中的目标物质与其他物质进行分离,以达到提纯、浓缩、分级等目的。
2.传质分离过程的分类及其基本原理有哪些?传质分离过程可以分为物理传质分离和化学传质分离两大类。
化工分离过程试卷试题库.docx

化工分离过程试题库(复习重点)第一部分填空题1、分离作用是由于加入(分离剂)而引起的,因为分离过程是(混合过程)的逆过程。
2、固有分离因子是根据(气液相平衡)来计算的。
它与实际分离因子的差别用(板效率)来表示。
3、汽液相平衡是处理(气液传质分离)过程的基础。
相平衡的条件是(所有相中的温度压力相等、每一组分的逸度也相等)。
4、精馏塔计算中每块板由于(组成)改变而引起的温度变化,可用(泡露点方程)确定。
5、多组分精馏根据指定设计变量不同可分为(设计)型计算和(操作)型计算。
6、在塔顶和塔釜同时出现的组分为(分配组分)。
7、吸收有( 1 个)关键组分,这是因为(单向传质)的缘故。
8、对多组分吸收,当吸收气体中关键组分为重组分时,可采用(吸收蒸出塔)的流程。
9、对宽沸程的精馏过程,其各板的温度变化由(进料热焓)决定,故可由(热量衡算)计算各板的温度。
10、对窄沸程的精馏过程,其各板的温度变化由(组成的改变)决定,故可由(相平衡方程)计算各板的温度。
11、为表示塔传质效率的大小,可用(级效率)表示。
12、对多组分物系的分离,应将(分离要求高)或(最困难)的组分最后分离。
13、泡沫分离技术是根据(表面吸附)原理来实现的,而膜分离是根据(膜的选择渗透作用)原理来实现的。
14、新型的节能分离过程有(膜分离)、(吸附分离)。
15、传质分离过程分为(平衡分离过程)和(速率分离过程)两大类。
16、分离剂可以是(能量)和(物质)。
17、 Lewis 提出了等价于化学位的物理量(逸度)。
18、设计变量与独立量之间的关系可用下式来表示( Ni=N v-Nc )19、设计变量分为(固定设计变量)与(可调设计变量)。
20、温度越高对吸收越(不利)21、萃取精馏塔在萃取剂加入口以上需设(萃取剂回收段)。
22、用于吸收过程的相平衡关系可表示为(L = AV )。
23、精馏有( 2)个关键组分,这是由于(双向传质)的缘故。
24、精馏过程的不可逆性表现在三个方面,即(通过一定压力梯度的动量传递),(通过一定温度梯度的热量传递或不同温度物流的直接混合)和(通过一定浓度梯度的质量传递或者不同化学位物流的直接混合)。
(完整版)传质与分离习题(含答案)

Problems for Mass Transfer and Separation ProcessAbsorption1 The ammonia –air mixture containing 9% ammonia(molar fraction) is contact with the ammonia-water liquid containing 5% ammonia (molar fraction). Under this operating condition, the equilibrium relationship is y*=0.97x. When the above two phases are contact, what will happen, absorption or stripping?Solution :09.0=y 05.0=x x y 97.0=*09.00485.005.097.0=<=⨯=*y y It is an absorption operation.2 When the temperature is 10 c 0 and the overall pressure is 101.3KPa , the solubility of oxygen in water can be represented by equation p=3.27⨯104x , where p (atm) and x refer to the partial pressure of oxygen in the vapor phase and the mole fraction of oxygen in the liquid phase, respectively. Assume that water is fully contact with the air under that condition, calculate how much oxygen can be dissolved in the per cubic meter of water?Solution: the mole fraction of oxygen in air is 0.21,hence:p = P y =1x0.21=0.21amt64410*24.610*27.321.010*27.3-===p x Because the x is very small , it can be approximately equal to molar ratio X , that is 610*42.6-=≈x XSo[])(/)(4.11)/(18*)(1)/(32*)(10*42.6lub 2322222226O H m O g O kmolH O kgH O kmolH kmolO kgO kmolO ility so ==-3 An acetone-air mixture containing 0.02 molar fraction of acetone is absorbed by water in a packed tower in countercurrent flow. And 99% of acetone is removed, mixed gas molar flow fluxis 0.03kmol ·s —1m -2 , practice absorbent flow rate L is 1.4 times as much as the min amountrequired. Under the operating condition, the equilibrium relationship is y*=1.75x. V olume totalabsorption coefficient is K y a=0.022 kmol ·s —1m -2y -1.. What is the molar flow rate of the absorbentand what height of packing will be required?solution :()0002.01=-=ηb a y y x a =0733.175.102.099.002.0*min =⨯=--=⎪⎭⎫ ⎝⎛ab a b x x y y V L 43.24.1min=⎪⎭⎫ ⎝⎛=V L V L s m kmol L 20729.003.043.2=⨯=720.043.275.1===L mV S Number of mass transfer units N oy =(y 1-y 2)/∆y=12(y b -y a )=0.02-0.0002∆y=[(y b -y*b )- (y a -y*a )]/ln[(y b -y*b )/ (y a -y*a )](y b -y*b )=0.02-1.75x b =0.0057X b =V/L (y b -y a )= (0.02-0.0002)/2.43=0.00815(y a -y*a )= y a =0.0002Or ])1)(ln[(11S S mx y mx y S N ba ab Oy +----==12 m Kya S V H OY 364.1022.003.0/=== m N H H OY OY 37.1612364.1=⨯==4 The mixed gas from an oil distillation tower contains H 2S=0.04(molar fraction). Triethanolamine (absorbent) is used as the solvent to absorb 99% H 2S in the packing tower, the equilibrium relationship is y*=1.95x, the molar flux rate of the mixed gas is 0.02kmol ·m -2·s -1,overall volumeabsorption coefficient is Kya=0.05 kmol ·s —1m -2y -1, The solvent free of H 2S enters the tower andit contains 70% of the H 2S saturation concentration when leaving the tower. Try to calculate: (a) the number of mass transfer units N oy , and (b) the height of packing layer needed, Z.solution :ya=yb(1-0.99)=0.04*1%=0.0004xb*=yb/m=0.04/1.95= 0.0205 xb=0.7xb*=0.0144yb*=1.95*0.0144=0.028yb-yb*=0.04-0.028=0.012△ym=0.0034Z=HoyNoyNoy=(yb-ya)/ △ym=11.6m a K G H y m oy 4.005.0/02.0/===Z=11.6*0.4=4.64m5 Ammonia is removed from ammonia –air mixture by countercurrent scrubbing with water in a packed tower at an atmospheric pressure. Given: the height of the packing layer Z is6 m, the mixed gas entering the tower contains 0.03 ammonia (molar fraction, all are the same below), the gas out of the tower contains ammonia 0.003; the NH 3 concentration of liquid out of the tower is 80% of its saturation concentration, and the equilibrium relation is y*=1.2x. Find:(1)the practical liquid —gas ratio and the min liquid —gas ratio L/V=?. (2) the number of overall mass transfer units.(3) if the molar fraction of the ammonia out of the tower will be reduced to 0.002 and the other operating conditions keep unchanged, is the tower suitable?solution :(1) 35.12.103.08.0003.003.0=⨯-=G L (2) 89.035.12.1==S 26.689.0003.003.011.089.011=⎥⎦⎤⎢⎣⎡+-=In N OY (3) m N Z H OY OY 958.026.66===47.889.0002.003.011.089.011=⎥⎦⎤⎢⎣⎡+⨯-='In N OY Since m N H Z OYOY 0.61.847.8958.0'>=⨯='= it is not suitable6 Pure water is used in an absorption tower with the height of the packed layer 3m to absorb ammonia in an air stream. The absorptivity is 99 percent. The operating conditions of absorber are 101.3kpa and 200c, respectively. The flux of gas V is 580kg/(m 2.h), and 6 percent (volume %) of ammonia is contained in the gas mixture. The flux of water L is 770kg/( m 2.h). The gas and liquid is countercurrent in the tower at isothermal temperature. The equilibrium equation y *=0.9x, and gas phase mass transfer coefficient k G a is proportional to V 0.8, but it has nothing to do with L. What is the height of the packed layer needed to keep the same absorptivity when the conditions of operation change as follows:(1)the operating pressure is 2 times as much as the original.(2)the mass flow rate of water is one time more than the original. 3) the mass flow rate of gas is two times as much as the original Solution: 3,1,293Z m p atm T K ===1210.060.063810.06(10.99)0.000638Y Y Y ==-=-= The average molecular weight of the mixed gas M=29×0.94+17×0.06=28.2822580(10.06)19.28/()28.2877042.78/()180.919.280.405642.78V kmol m h L kmol m h mV L =-=⋅Ω==⋅Ω⨯==12221ln[()(1)]110.06380ln[()(10.4056)0.4056]10.40560.0006386.88430.43586.884OG OG OG N mV LH Y mX mX mV Y mX L L Z m N =-+-=----+-==== 1) 2p p '=''p p m m = So 1222ln[()(1)]10.90.4520.4519.280.202842.78111ln[(100)(10.2028)0.2028]10.20285.496OG mp m p m V L Y mX m X m V N mV Y mX L L L-+='==⨯=''⨯==''-'=---+-= OG r G V V H K a K aP ==ΩΩSo:OG H changes with the operating pressure10.43580.21792OG OG OG OG H H H H ρρρρ'=''=⋅=⨯='So 5.4960.2179 1.198OGOG Z N H m '''=⋅=⨯= So the height of the packed section reduce 1.802m vs the original2) 2L L '=11()0.40560.20282225.496OGmV mV mV L L L N ===⨯=''=when the mass flow rate of liquid increases,G K a has not remarkable effect0.43585.4960.4358 2.395OG OG OG OG H H m Z N H m'=='''=⋅=⨯= the height of the packed section reduce 0.605m against the original3) 2V V '=(2)2()20.40560.81161ln[(100)(10.8116)0.8116]15.8110.8116OG mV m V mV L L L N '===⨯='=-+=- when mass flow rate of gas increaes,G K a also will increase. Since it is gas film control for absorption, we have as follows:0.80.80.80.20.80.2()222220.43580.50115.810.5017.92G G G G OG OG G G OGOG K a V V K a K a K a V V V H H K aP K aP mZ N H m m ∝''==''===Ω'Ω=⨯='''==⨯= So the height of the packed section increase 4.92m against the originalDistillation1 Certain binary mixed liquid containing mole fraction of easy volatilization component F x 0.35, feeding at bubbling point, is separated through a sequence rectify column. The mole fraction in the overhead product is x D =0.96, and the mole fraction in the bottom product is x B =0.025. If the mole overflow rates are constant in the column, try to calculate(a)the flow rate ratio of overhead product to feed(D /F)?(b)If the reflux ratio R=3.2, write the operating lines for rectifying and stripping sections solution :F x =0.35;x B =0.025;x D =0.96;R=3.2。
华南理工有机化学双语综合测试题(英文版)

2011 年有机化学自测题(英文版-中英对照)Ⅲ. Choose the best answers for each of the following questions. 1. Single choice (only one choice is correct) for 1~70 For CH3CH=C=CH2, point out the hybridization of each carbon(from left to right)? 对于有机物 CH3CH=C=CH2, 请指出每个碳的杂化方式(从左至右). A. sp3 sp2 sp2 sp2 B. sp 3 sp2 sp sp 2 C. sp 3 sp 2 sp sp D. sp3 sp sp sp 2. Which of the following is electrophilic reagent? 属于亲电试剂的是: A. HNO3 B. NaHSO3 C. H2N-NH2 D. HCN 3. Which of the following is nucleophilic reagent? 属于亲核试剂的是: A. Br2 B. NaHSO3 C. H2SO4 D. HCl 4. Which of the following substituents activates an aromatic nucleus? 下列哪个取代基可以活化芳香环? A. —COOH B. —NO2 C. —OCH3 D. —SO3H 5. Which of the following does not give the iodoform test? 不能发生碘仿反应的是:A. C6H5CHO B. CH3 C O CH3 C. CH3CH2OH D. CH3C-C6H5 O6. Which of the following structural formulas has no geometrical isomers? 不存在几何异构(顺反异构)的是:A. CH3CH=CHCH3 B. C6H5CH=CHBr C. Cl Cl Cl D. Cl Cl7. Which of the following is aromatic? 具有芳香性的化合物是:A. O B. C. N H D.8. Which of the following lettered carbon-carbon bonds is the longest? 用字母标记的碳碳键中,键长最长的是:a A. B. CH3 CH b c C. CH3 C CH D. CH3 d CH3CH29. Which of the following carbocations is most stable? 最稳定的碳正离子是:+ A. CH3 C CH3 + B. CH3 CH CH3 + C. CH3 CH2 + D. CH3CH=CH210. Which of the following compounds yields a yellow oil when treated with nitrous acid? 能与亚硝酸作用生成黄色油状物的物质是:A. CH3CH2NH2 B. (CH3CH2)2NH C. (CH3CH2)3N D. NH211. Which of the following is easiest water-soluble? 最易溶于水的是:A. CH2-CH-CH2 OH OH OH B. OH C. E. CH3CHCH2CH2OH CH312. Which of the following cannot react with silver nitrate to produce silver chloride at ordinary conditions? 1通常情况下不能与硝酸银反应生成氯化银的物质是:A. CH3C-Cl O B. (CH3)3C-Cl C. CH2=CHCHCH3 Cl D. Cl13. Which one cannot be converted into amides by nucleophilic acyl substitution reaction with acid chlorides, anhydrides, or esters? 下列哪种物质不能和酰氯、酸酐、酯类通过亲核的酰基取代反应形成酰胺? A. aniline (苯胺) B. (C2H5 )3N C. C2H5NHCH3 D. (C2H5 )3C-NH2 14. Which of the following reagents cannot react with 2,4-pentadione 下列哪种试剂不能与 2,4-戊二酮(乙酰丙酮)反应? (A) Na (B) Br2 (C) NaHSO3 (D) NaHCO3 15. Which of the following shows π-π conjugate system? 具有 π-π 共轭体系的是: A. 1,3-butadiene B. ClCH=CHCH2CH3 C. +CH2CH=CH2 D.CH2=CH-CH2CH=CH2 16. Which of the following shows p-π conjugate system? 具有 p-π 共轭体系的是: A. benzaldehyde B. 1,3-cyclohexadiene C. ClCH=CH2 D. ClCH2CH=CH2 17. Which substituent on an aromatic ring is ortho-para director? 下列芳香环上的取代基,属于邻-对位定位基的是: A. -CHO 碱性最强的是: A. NH3 碱性最弱的是A.N HB. -SO3HC. -CH=CH2D. -CN18. Which of the following shows the strongest basicity? B.(CH3)2NH C. C6H5NH2 D. CH3CONH219. Which of the following shows the weakest basicity?NH2B.NC.N HD.20. Which of the following shows the strongest acidity? 酸性最强的是COOH NO2 OH C. D. H3C COOHA.COOHB.21. Which of the following can be used as Lewis base? 能用作路易斯碱的是: A. BF3 B. H2SO4 C. Br+ D. CN22. Which of the following compounds contains 1°, 2°, 3°and 4°carbon atoms? 下列哪个化合物分子中同时包含有 1°, 2°, 3° 和 4° 碳原子? A. 2, 2, 3-trimethylbutane C. 2, 3, 4-trimethylpentane 2,2,3-三甲基丁烷 2,3,4-三甲基戊烷 B. 2, 2, 3-trimethylpentane 2,3,3-三甲基戊烷 D. 3, 3-dimethylpentane 3,3-二甲基戊烷23. Which of the following carbohydrates is capable of being oxidized by bromine water? 下列哪种碳水化合物能被溴水氧化? A. fructose 果糖 B. sucrose 蔗糖 C. glucose 葡萄糖 D. cellulose 纤维素24. Which one is the most stable? 下列哪个构象最稳定?CH3 H H CH3 A. Anti H H H H H CH3 H3C H H H CH3 CH3 H H H3C CH3B. EclipsedH H C. GaucheH HD. Eclipsed225. Which one of the stability order of the following is correct? 稳定性大小排序正确的是:CH(CH3)2 H3C (a) (b)CH(CH3)2 CH3 (c) (d) H 3C CH(CH3)2H3CCH(CH3)2A. a>b>c>dB. d>a>b>cC. d>b>c>aD. d>c>a>b26. Which of the following shows the highest activity toward SN1 reaction? 在单分子亲核取代反应中活性最高的是: A.methyl bromide 甲基溴 B. ethyl bromide 乙基溴 C. 3-bromo-2-methylbutane 3-溴-2-甲基丁烷 在双分子单核取代反应中活性最高的是: A.methyl bromide 甲基溴 B. ethyl bromide 乙基溴 C. 3-bromo-2-methylbutane 3-溴-2-甲基丁烷 is reasonable? 学生们在使用泰利(Thiele)毛细管法测定萘的熔点实验中,记录了如下测定结果。
化工分离过程过程性考核试卷(一) 答案
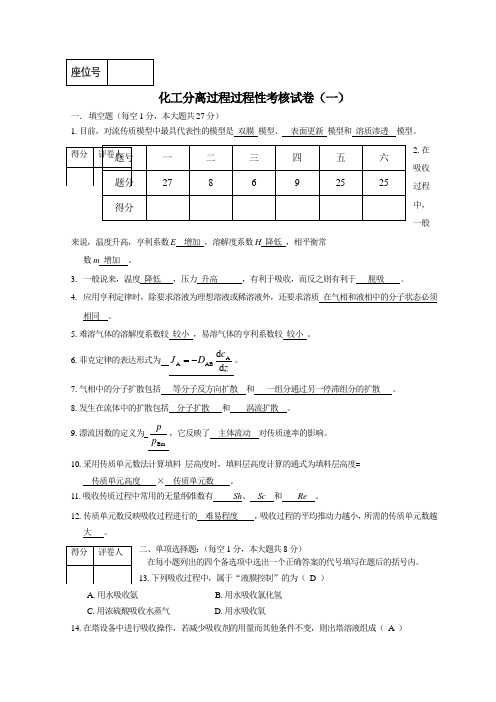
化工分离过程过程性考核试卷(一)一. 填空题(每空1分,本大题共27分)1.目前,对流传质模型中最具代表性的模型是 双膜 模型、 表面更新 模型和 溶质渗透 模型。
2.在吸收过程中,一般来说,温度升高,亨利系数E 增加 ,溶解度系数H 降低 ,相平衡常 数m 增加 。
3. 一般说来,温度 降低 ,压力 升高 ,有利于吸收,而反之则有利于 脱吸 。
4. 应用亨利定律时,除要求溶液为理想溶液或稀溶液外,还要求溶质 在气相和液相中的分子状态必须 相同 。
5.难溶气体的溶解度系数较 较小 ,易溶气体的亨利系数较 较小 。
6.菲克定律的表达形式为 zc D Jd d AABA -=。
7.气相中的分子扩散包括 等分子反方向扩散 和 一组分通过另一停滞组分的扩散 。
8.发生在流体中的扩散包括 分子扩散 和 涡流扩散 。
9.漂流因数的定义为Bmp p,它反映了 主体流动 对传质速率的影响。
10.采用传质单元数法计算填料 层高度时,填料层高度计算的通式为填料层高度= 传质单元高度 × 传质单元数 。
11.吸收传质过程中常用的无量纲准数有 Sh 、 Sc 和 Re 。
12.传质单元数反映吸收过程进行的 难易程度 ,吸收过程的平均推动力越小,所需的传质单元数越 大 。
二、单项选择题:(每空1分,本大题共8分)在每小题列出的四个备选项中选出一个正确答案的代号填写在题后的括号内。
13.下列吸收过程中,属于“液膜控制”的为( D )A .用水吸收氨B .用水吸收氯化氢C .用浓硫酸吸收水蒸气D .用水吸收氧14.在塔设备中进行吸收操作,若减少吸收剂的用量而其他条件不变,则出塔溶液组成( A )A .增加B .减少C .不变D .不确定 15.下列吸收过程中,属于“气膜控制”的为( B )A .用水吸收氮B .用水吸收氯化氢C .用水吸收硫化氢D .用水吸收氧16.在塔设备中进行吸收操作,若提高操作温度而其他条件不变,则出塔溶液组成( B )A .增加B .减少C .不变D .不确定17.在描述对流传质的表面更新模型中,对流传质系数与扩散系数的( B )成正比。
07传质与分离工程期末考试题(含答案)
,考试作弊将带来严重后果!华南理工大学期末考试2007《传质与分离工程英文》试卷A (含答案)1. 考前请将密封线内填写清楚;所有答案请直接答在试卷上(或答题纸上);.考试形式:开(闭)卷;S in the air is absorbed by NaOH solution is ( A ).2B. liquid film “controls”;C. two film “controls”.AB=1. WhenC ).>x A; B. y A<x A; C. y A=x A. D. uncertainAD ) is true.>t d; B. t>t w=t d; C. t=t w>t d; D. t=t w=t dwo C, ( C ) of reading is o C B. 77 o C C. 77.01 o C; D. 77.010 o CB ).When the water content of some material is close to its equilibrium water content X*, its drying rate will ___ C _.B. decrease;C. be close to zero;D. be uncertainFor the desorption (stripping) process, when ( B ) increases and ( A )A ) increases, it is good for the operation.D ) decreases, it is good for the operation.A.dry-bulb temperature of air;B. wet-bulb temperature of air ;C. dew point temperature of air;D. size of material to be dried(9) For absorption process, when the coefficients k x a and k y a are of the same order of magnitude and equilibrium constant m is much greater than 1, ( B ) is said to be controlling. In order to increase mass transfer rate, it is better to increase ( E ).A. gas film;B. liquid film;C. both gas film and liquid film;D. gas phase flow rate;E. liquid phase flow rate;F. both gas phase flow rate and liquid phase flow rate;(10) For absorption process, if it is the liquid film control, ( D ) is feasible to increase mass transfer rate of absorption.A to decrease gas flow rate.B to decrease liquid flow rateC to increase gas flow rate.D to increase liquid flow rate.(11)When the reflux ratio R increases and other conditions keep the same, overflow of vapor V will (increase ); the concentration of overhead product x D will (increase ); steam consumption of reboiler will (increase ), the theoretic plate numbers required will (decrease ) for the same recovery percentage of overhead product.(12) The factors to influence mass transfer coefficients of gas phase include (T, P, gas flow rate).(13) When some moist air is preheated in a preheater, its humidity will (be the same ), its relative humidity will (decrease ), its enthalpy will (increase), its dew point will (be the same ), its wet-bulb temperature will (increase ).(14) When the liquid flooding at a packed tower occurs, (pressure drop ) increases remarkably and the entire tower may fill with (liquid )..(15) Mathematic expression of Fick’s law is ( J A=-DdC A/dz ).(16) The requirements to satisfy constant molal overflow are (vaporization heat of different components will be the same, sensible heat exchange of liquid and gas phases on the plates can be ignored, heat loss of column can be igored. )(17)Some wet solids are dried by air with temperature t and humidity H.(1)When air velocity increases and air temperature t and humidity H keep constant, the drying rate at constant-rate period will (increase); If air velocity and air conditions keep unchanged, but thickness of materials increases, the critical moisture content of wet material Xc will (decrease ).2. (20 points) At a constant pressure operation a continuous distillation column with reflux is used for the separation of a binary mixture with feed rate F=1000kmol/h and concentration x F=0.36 (mol fraction of more volatile component). Feed is saturated vapor, the relative volatility ofmixture α is 3, indirect steam heating is used at a rebolier, and total condenser and bubble point reflux at the top of column are employed and the reflux ratio R is 3.2, respectively. It is required that the recovery percent of more volatile component at the top of column is 92%, and that the concentration of more volatile component at the top x D is 0.9. Calculate:(1)Operating line equation for rectifying section;(2) Overhead product flow rate D, vapor overflow V for rectifying section and vapor overflow V ’ for stripping section, in kmol/h.(3)If the practical liquid concentration leaving the first plate (x 1) is 0.825, what is this plate efficiency of plate 1L M E ,?Solution (1) y=0.762x+0.214(2) D x D /Fx F =0.92, D=0.92(1000*0.36)/0.9=368kmol/hV=(R+1)D=4.2*368=1545.6kmol/hV’=V -F=1545.6-1000=545.6kmol/h(3) E mL =(x D -x 1)/(x D -x 1*)=(0.9-0.825)/(0.9-0.75)=0.5y 1=x D =αx 1*/([α-1)x 1*+1]=0.9=3x 1*/[(3-1)x 1*+1]x 1*=0.753.(20 points) Some gas mixture contains acetone vapor with 3%(molar fraction) which is absorbed by pure water at a countercurrent operation absorber. 98% acetone of outlet gas is removed. Ratio of liquid to gas flow rate is 2 and the equilibrium relation is =*y 1.05x Find(1)what is the acetone concentration of outlet water at the tower bottom?(2) what is the overall mass transfer unit number of gas phase?(3) if the inlet compositions of gas and liquid phases keep the same and the ratio of liquid to gas flow rate is 1.04, where (in what position of tower) is the equilibrium of gas and liquid phases when the packing layer height is infinite? What is the maximum recovery percentage of acetone? Solution: (1) x 1=V(y 1-y 2)/L=0.03*0.98/2=0.0147(2) N oy =(y 1-y 1)/∆y m =0.03*0.98/0.00438=6.71∆y m =[(0.03-1.05*0.0147)-0.0006]/ln[(0.03-1.05*0.0147)/0.0006]=0.00438(3) since104.105.104.1 05.1 ='=='=VL m S VL m So the equilibrium will be reached at the bottom of tower. Max removal efficiency of acetone is121max y y y '-=η0286.005.103.005.11*1===y x 0297.00286.004.1*121=⨯=='-x VL y y %9903.00297.0121max =='-=y y y η4. (15 points) A moist material is to be dried from water content 25% to 5% (wet basis) in an adiabatic dryer (constant enthalpy drying process) under atmospheric pressure. The feed of moist material solid into the dryer is 1000 kg/h. After some fresh air with a dry-bulb temperature of 25ºC and a humidity of 0.013 kg water/kg dry air is preheated to 100ºC, it is sent to the dryer. And the air temperature leaving the dryer is 65ºC.(1) What are the bore-dry air (in kg/h) and the volume of fresh air required per unit time (in m 3/h)?(2) How much heat is obtained by air when it passes through the preheater (in kJ/h)? [Hint: 273273)244.1772.0(00+⨯+=t H v H , enthalpy of moist air=(1.01+1.88H)t+2492H] Solution: (1) W=Gc(X1-X2)=1000*0.75(1/3-5/95)=210.5kg/hI 1=I 2(1.01+1.88H 1)t 1+2492H 1=(1.01+1.88H 2)t 2+2492H 2H 1=0.013, t 1=100,t 2=65, H 2=0.0268L=W/(H 2-H 1)=210.5/(0.0268-0.013)=15253.6kgdry air/hv H =(0.772+1.244*0.013)(298/273)=0.86m 3/kgdry airV=Lv H =15253.6*0.86=13118.1 m 3/h(2)Qp=L(I 1-I 0)=15253.6(1.01+1.88*0.013)(100-25)=1183420 kJ/h5. Question (5 points)Draw the capacity performance chart of plate column and indicate the meaning of every line, describe the satisfactory operation zone. When the liquid flow rate keeps constant and vapor flow rate increases remarkably, please explain what may happen to the column.Solution: When the liquid flow rate keeps constant and vapor flow rate increases remarkably, more liquid will be taken upward the plate. So make separation efficiency of column decrease.。
化工分离过程过程性考核试卷(一)-答案
化工分离过程过程性考核试卷(一)一. 填空题(每空1分,本大题共27分) 1.目前,对流传质模型中最具代表性的模型是 双膜 模型、 表面更新 模型和 溶质渗透 模型。
2.在吸收过程中,一般来说,温度升高,亨利系数E 增加 ,溶解度系数H 降低 ,相平衡常 数m 增加 。
3. 一般说来,温度 降低 ,压力 升高 ,有利于吸收,而反之则有利于 脱吸 。
4. 应用亨利定律时,除要求溶液为理想溶液或稀溶液外,还要求溶质 在气相和液相中的分子状态必须相同 。
5.难溶气体的溶解度系数较 较小 ,易溶气体的亨利系数较 较小 。
6.菲克定律的表达形式为 zc D Jd d A AB A -=。
7.气相中的分子扩散包括 等分子反方向扩散 和 一组分通过另一停滞组分的扩散 。
8.发生在流体中的扩散包括 分子扩散 和 涡流扩散 。
9.漂流因数的定义为 Bmp p ,它反映了 主体流动 对传质速率的影响。
10.采用传质单元数法计算填料 层高度时,填料层高度计算的通式为填料层高度= 传质单元高度 × 传质单元数 。
11.吸收传质过程中常用的无量纲准数有 Sh 、 Sc 和 Re 。
12.传质单元数反映吸收过程进行的 难易程度 ,吸收过程的平均推动力越小,所需的传质单元数越 大 。
二、单项选择题:(每空1分,本大题共8分) 在每小题列出的四个备选项中选出一个正确答案的代号填写在题后的括号内。
13.下列吸收过程中,属于“液膜控制”的为( D )A .用水吸收氨B .用水吸收氯化氢C .用浓硫酸吸收水蒸气D .用水吸收氧14.在塔设备中进行吸收操作,若减少吸收剂的用量而其他条件不变,则出塔溶液组成( A )A .增加B .减少C .不变D .不确定15.下列吸收过程中,属于“气膜控制”的为( B )A .用水吸收氮B .用水吸收氯化氢C .用水吸收硫化氢D .用水吸收氧16.在塔设备中进行吸收操作,若提高操作温度而其他条件不变,则出塔溶液组成( B )A .增加B .减少C .不变D .不确定17.在描述对流传质的表面更新模型中,对流传质系数与扩散系数的( B )成正比。
传质与分离作业题(英文)新
Chapter 20\21 Distillation1 A binary material system is to be separated in a continuous distillation column. The feed is liquid phase and mole fraction in feed is x F=0.42, Mole fraction in the overhead product is x D =0.95, Givens: The easy volatilization component’s recovery ratio of the overhead product is η=0.92, Calculate: Mole fraction in the bottom product x B(或x W)=?x=0.35, feeding at 2 Certain bianry mixed liquid containing mole fraction of easy volatilization component isFbubbling point, is separated through a sequence rectify column. The mole fraction in the overhead product isx D=0.96, and the mole fraction in the bottom product is x B(或x W) =0.025. If the mole overflow rates are constant in the column, try to calculate(a)the flow rate ratio of overhead product to feed(D/F)?(b)If the reflux ratio R=3.2, write the operating lines for rectifying and stripping sections3 A continuous distillation column is to be designed to separate an ideal binary material system,The feed whichx=0.5, feed rate 100kmol/h, is saturated vapor,the flow rate of contains easy volatilization componentFoverhead product and the flow rate of bottom product are also 50kmol/h. Suppose the operating line for rectifying section is y=0.833x+0.15, the vapor generated in the reboiler enters the column through the bottom plate, a complete condenser is used on the top of column and reflux temperature is bubbling point. Find:(1) Mole fraction of overhead product x D and the mole fraction of bottom product x B(或x W)?(2) Vapor amount condensed in the complete condenser, in mol/h?(3)The operating line for stripping section.(4) If the average relative volatility of the column is 3 and the first plate’s Murphree efficiency of the top plate is Em,L=0.6, find the constituent of gas phase leaving from the second plate of tower top.( plate’s Murphree efficiency Em,L is the liquid phase single plate efficiency of the duality liquid is rectified in continuum rectify tower)4 An ideal binary solution of a volatile component A containing 50% mole percent A is to be separated in a continuous distillation column. The feed is saturated vapor, the feed rate is 1000kmol/h, and the flow rate of overhead product and the flow rate of bottom product are also 500kmol/h. Given: the operating line for rectifying section is y=0.86x+0.12, the reboiler uses indirect vapor to heat and the complete condenser is used on the top of tower. Assume that the reflux temperature is at its bubble point. Find:(1) reflux ratio R, the mole fraction of overhead product x D and the mole fraction of bottom product x B(或x W)?(2) upward flow rate of vapor in the rectifying section(V mol/h,) and down ward flow rate of vapor in the stripping section.(L’ mol/h,) .(3) The operating line for stripping section .(4)if relative volatility a=2.4, find reflux ratio and min reflux ratio R/Rmin5 Separate component A and B of mixed liquid in a continuous distillation column, Given :raw liquid flow rate isF is 4000 kg·h-1 , mass fraction of A is 0.3. Require the mass fraction in the kettle liquid shouldn’t be beyond 0.05, recovery ratio of A is 88% on the top. Try to calculate the flow rate of the distillation liquid D and its constituent x D on the column top.( in the terms of molar flow rate and molar fraction)6 There is a continuous rectifying operation column, whose the operation line equation is as follows:Rectifying section: y=0.723x+0.263Stripping section: y=1.25x-0.0187if the feed enters the column at a dew point, find (a)the molar fraction of feed、overhead product and bottom product. (b) reflux ratio R.7 A column is to be designed to separate a liquid mixture containing 44 mole percent A and 56 mole percent B, the system to be separated can be taken as ideal. The overhead product is to contain 95.7 mole percent. given: liquid average relative volatility a=2.5, reflux ratio R=4.52, try to illustrate the thermal condition of the feed, and to calculate the value of q.8 A continuous rectifying column operated at atmospheric pressure is used to separate benzene—methylbenzene mixed liquid. The feed is saturation liquid containing 50 mole percent benzene. Given: the overhead product must contain 90 mole percent benzene and the bottom product contains 10 mole percent benzene. If reflux ratio is 4.52, try to calculate how many ideal plates are need? and locate the feed plate. Materials system equilibrium refers to Case 2—6In this situation the equilibrium materials of benzene—methylbenzenet o C 80.1 85 90 95 100 105 110.6x 1.000 0.780 0.581 0.411 0.258 0.130 0y 1.000 0.900 0.777 0.632 0.456 0.262 09 There is a rectifying column, given : mole fraction of distillation liquid from tower top x D=0.97, reflux ratio R=2, the gas-liquid equilibrium relationship y=0.86x+0.12;find: the constituent x1 of the down liquid leaving from the first plate and the constituent y2 of the up gas leaving from the second plate in the rectifying section.10.A continuous fractionating column is used to separate 4000kg/h of a mixture of 30 percent CS2and 70percent CCl4. Bottom product contains 5 percent CS2at least, and the rate of recovery of CS2in the overheadproduct is 88% by weight. Calculate (a) the moles flow of overhead product per hour. (b) the mole fractions ofCS2and CCl4in the overhead product, respectively.11. A liquid of benzene and toluene is fed to continuous distillation in a plate column. Under the total reflux ratio condition, the compositions of liquid on the close plates are 0.28, 0.41 and 0.57, respectively. Calculate the Murphree plate efficiency of relatively two low plates. The equilibrium data for benzene—toluene liquid under the operating condition are given as:x 0.26 0.38 0.51y 0.45 0.60 0.7212 Problem 20.1 , 20.2 21.8 21.1Chapter 17/18 Absorption1 At 20℃, the ammonia solubility in water is l0kgNH3/1000kgH2O, gas phase equilibrium molar fraction is 0.008, try to calculate following coefficients, solubility coefficient, Henry coefficient, equilibrium constant and equilibrium concentration when the following conditions are(1) Total pressure above the ammonia is 101.3kPa (absolute atmosphere);(2) Total pressure above the ammonia is 301.9kPa (absolute atmosphere);(3) Total pressure above the ammonia remains 301.9kPa (absolute atmosphere) but the ammonia’s temperature rises to 50℃and the equilibrium partial pressure raises 5.9 kPa above ammonia.2.The ammonia–air mixture containing 9% ammonia(molar fraction) is contact with the ammonia-water liquid containing 5% ammonia (molar fraction). Under this operating condition, the equilibrium relationship is y*=0.97x. When the above two phases are contact, what will happen, absorption or stripping? What are the max( or min) values of gas and liquid phases?3.When the temperature is 10℃and the pressure is 101.3KPa , the solubility of oxygen in water can be10x, where p (kPa) and x refer to the partial pressure of oxygen in the represented by equation p=3.31×6vapor phase and the mole fraction of oxygen in the liquid phase, respectively.Assume that water is fully contact with the air under that condition, calculate how much oxygen can be solute in the per cubic meter(立方米)of water?4.There is a packing tower whose diameter is 250mm, which contains 16 mm Rachig ring(拉西环)with the height of packing layer 2.6m. It is used to absorb the carbon dioxide in CO2-air mixture at atmospheric pressure by 2.5mol/L sodium hydroxide lye(NaOH碱液). Given: the molar flow flux of gas mixture V=0.0117kmol/m2.s, the molar flow flux of NaOH is L=0.208k kmol/m2.s. The mixed gas of inlet tower contains CO2 =3.15×10—4 (molar fraction), and outlet gas contains CO2=3.1×10—5.(molar fraction).Try to calculate the gas phase total transfer mass coefficient Kya.5 An acetone-air mixture(丙酮一空气混合物)containing 0.02 molar fraction of acetone 丙酮is absorbed by water in a packed tower in countercurrent flow. And 99%of acetone is removed, mixed gas molar flow rate is 0.03kmol·s—1m-2 , practice absorbent flow rate L is the 1.4 times more than the min amount required. Under the operating condition, the equilibrium relationship is y*=1.75x. Volume total absorption coefficient is Kya=0.022 kmol·s—1m-2y-1.. What is the molar flow rate of the absorbent and what height of packing will be required? 6.The mixed gas from petrol(石油) distillation tower contains H2S=0.04(molar fraction). Triethanolamine 三乙醇胺(absorbent) is used as the water solvent to absorb 99% H2S in the packing tower, the equilibrium relationship is y*=195x, the molar flux rate of the mixed gas is 0.02kmol·m-2·s-1,volume total absorption coefficient is Kya=0.005 kmol·s—1m-2y-1, The solvent enters the tower free of H2S and it contains 70% of the H2S saturation concentration when leaving the tower. Try to calculate: (a) the number of mass transfer units required , and (b) the height of the of packing layer needed.7.Ammonia氨is removed from ammonia氨–air mixture by countercurrent scrubbing with water in a packed tower at an atmospheric pressure. Given: the height of the packing layer is 6 m, the mixed gas entering the tower contains 0.03 ammonia (molar fraction, all are the same below), the gas out of the tower contains ammonia 0.003; the NH3 concentration of liquid out of the tower is 80% of its saturation concentration. Find:(1)the practical liquid—gas ratio and the min liquid—gas ratio.(2) the number of overall mass transfer units.(3) if the molar fraction of the ammoniac out of the tower will be reduced to 0.002 and the other operating conditions keep unchanged, is the tower suitable?8.Pure water is used in an absorption tower with the height of the packed section 3m to absorb ammonia氨in an air stream. The absorptivity is 99 percent. The operating conditions of absorber are 101.3kpa and 20℃, respectively. The flux of gas V is 580kg/(m2.h), and 6 percent (volume %) of ammonia is contained in the gas mixture. The flux of water L is 770kg/( m2.h). The gas and liquid is countercurrent in the tower at isothermal temperature. The equilibrium equation Y*=0.9X, and gas phase mass transfer coefficient ka is proportional toGV0.8, but it has nothing to do with L. Try to find what is the height of the packed tower to change in order to keepthe absorption coefficient unchanged when the conditions of operation have be changed as follows (1)the operating pressure is 2 times as much as the original.(2)the mass flow rate of water is one time more than the original. 3) the mass flow rate of gas is two times as much as the origina9. problem 17.2,17.4,17.17,18.3,18.9,Chapter 19/24 Drying1 A wet solid is dried by air from 40% to 5% moisture content (wet basis) under the convective drying conditions in 1000 kg/h. The air primary humidity H1 is 0.001(kg water/kg dry gas), and the humidity of the air leaving dryer H2 is 0.039 (kg water /kg dry gas), suppose that the materials loss in the drying process can be negligible. Find:(1) Mass rate of water vaporization W, in kg water/ h.(2) Mass rate of dry air required L, in kg dry air/h, volume flow rate of moist air, V, in kg primary air/h.(3) Mass rate of moist solids out of dryer, G2, in kg moist solids/h.2 The wet solid is to be dried from water content 20% to 5% (wet basis) in a convective dryer at atmospheric pressure. The feed of wet solid into the dryer is 1000 kg/h at a temperature of 40℃. Suppose there is no significant temperature change in dryer, Given: dry bulb temperature of air is 20℃, and wet bulb temperature is 16.5℃. After being preheated, air enters the drier. The temperature leaving dryer is 60℃, wet bulb temperature is 40℃, and heat loss is negligible, Find:(1) What is the volume of fresh air required per unit time (in m3/h)? (Based on the preheated state)(2) The temperature of the air into the dryer.(Given: Vaporization latent heat of water at 0℃is 2491.27 kJ/kg, specific heat of dry air Cg is 1.011 kJ/kg·K, specific heat of water vapor Cv is 1.88 kJ/kg·K)3 The wet solid material is to be dried from water content 42% to 4% (wet basis) in an adiabatic dryer. The solid product out of the dryer is 0.126kg/s. After the fresh air at a dry-bulb temperature of 21ºC and a relative humidity of 40%is preheated to 93ºC, it is sent to the dryer, and leaves the dryer at relative humidity of 60%. If the air is equal enthalpy in the drying process, find:(1) Determining the air state parameters from the given air state in H-I diagram.(2) If Ho=0.008(kg water/kg dry air), H2=0.03( kg water /kg dry air. Find:(a) Mass rate of dry air required L [kg dry air/s](b)How much heat is supplied to air by the preheater (in kJ/h)?4 The wet solid湿物料containing 12%(wet basis湿基) moisture is fed to a convective dryer at a temperature of 15℃and withdrawn at 28℃containing 3% moisture (wet basis). The flow rate of final moist solid (product) is 1000kg/h.(according to the dry production). After the fresh air at a dry-bulb temperature of 25℃and a humidity of 0.01 kg water/kg dry air is preheated to 70℃, it is sent to the dryer, and leaves the dryer at 45ºC. Suppose the drying process is under the constant enthalpy, heat loss in the drying system can be negligible. Find:(1) Drawing the operation process covering various air states in H-I diagram.(2) What is the volume of fresh air required per unit time (in m3/h)?(3) In order to keep the enthalpy unchanged, how much supplementary heat Q D is needed?5 Drying wet materials by hot air, primary temperature of air is t。
传质与分离习题(英文)钟理
Problem for Mass Transfer and Separation ProcessesAbsorption1 At 20℃, the ammonia solubility in water is l0kgNH3/1000kgH2O, gas phase equilibrium molar fraction y* is 0.008, try to calculate following coefficients, Henry coefficient E, equilibrium constant m when the following conditions are(1) Total pressure above the ammonia is 101.3kPa (absolute atmosphere);(2) Total pressure above the ammonia is 301.9kPa (absolute atmosphere);2 The ammonia–air mixture containing 9% ammonia (molar fraction) is contact with the ammonia-water liquid containing 5% ammonia (molar fraction). Under this operating condition, the equilibrium relationship is y*=0.97x. When the above two phases are contact, what will happen, absorption or stripping?3 When the temperature is 10 o C and the overall pressure is 101.3KPa , the solubility of oxygen in water can be represented by equation p=3.27 104x, where p (atm) and x refer to the partial pressure of oxygen in the vapor phase and the mole fraction of oxygen in the liquid phase, respectively.Assume that water is fully contact with the air under that condition, calculate how much oxygen can be dissolved in per cubic meter of water?4 An acetone-air mixture containing 0.02 molar fraction of acetone is absorbed by water in a packed tower in countercurrent flow. 99%of acetone is removed when mixed gas molar flow rate is 0.03kmol·s-1m-2 and practice absorbent flow rate L is 1.4 times as much as the min amount required. Under the operating condition, the equilibrium relationship is y*=1.75x, volume total absorption coefficient is K y a=0.022 kmol·s-1m-2y-1.. What is the molar flow rate of the absorbent and how height of packing will be required?5 The mixed gas from an oil distillation tower contains H2S=0.04(molar fraction). Triethanolamine (三乙醇胺) is used as the absorbent to remove 99% H2S in the packing tower, the equilibrium relationship is y*=1.95x, the molar flux rate of the mixed gas is 0.02kmol·m-2·s-1,volume total absorption coefficient is Kya=0.05 kmol·s—1m-2, The solvent free of H2S enters the tower and it contains 70% of the H2S saturation concentration when leaving the tower. Try to calculate: (a) the number of mass transfer units N oy, and (b) the height of packing layer needed, Z.6 Ammonia is removed from ammonia–air mixture by countercurrent scrubbing with water in a packed tower at an atmospheric pressure. Given: the height of the packing layer Z is 6 m, the mixed gas entering the tower contains 0.03 ammonia (molar fraction, all are the same below), the gas out of the tower contains ammonia 0.003; the NH3 concentration of liquid out of the tower is 80% of its saturation concentration, and the equilibrium relation is y*=1.2x. Find:(1)the practical liquid—gas ratio.(2) the number of overall mass transfer units.(3) if the molar fraction of the ammonia out of the tower will be reduced to 0.002 and the other operating conditions keep unchanged, is the tower suitable?7 Pure water is used in an absorption tower with the height of the packed layer 3m to absorb ammonia in an air stream. The absorptivity is 99 percent. The operating conditions of absorber are 101.3kpa and 20o C, respectively. The flux of gas V is 580kg/(m2.h), and 6 percent (volume %) of ammonia is contained in the gas mixture. The flux of water L is 770kg/( m2.h). The gas and liquid is countercurrent in the tower at isothermal temperature. The equilibrium equation y*=0.9x, andgas phase mass transfer coefficient ka is proportional to V0.8, but it has nothing to do with L.GWhat is the height of the packed layer needed to keep the same absorptivity when the conditions of operation change as follows:(1)the operating pressure is 2 times as much as the original.(2)the mass flow rate of water is one time more than the original. 3) the mass flow rate of gas is two times as much as the originalDistillation1 A binary material system is to be separated in a continuous distillation column. The feed is liquid phase and mole fraction in feed is x F=0.42, Mole fraction in the overhead product is x D=0.95, Givens: The more volatile component’s recovery percent of the overhead product is η=0.92, Calculate: Mole fraction in the bottom product x B=?x0.35, 2 Certain binary mixed liquid containing mole fraction of easy volatilization componentF feeding at bubbling point, is separated through a sequence rectify column. The mole fraction in the overhead product is x D=0.96, and the mole fraction in the bottom product is x B =0.025. If the mole overflow rates are constant in the column, try to calculate(a)the flow rate ratio of overhead product to feed(D/F)?(b)If the reflux ratio R=3.2, write the operating lines for rectifying and stripping sections3 A continuous distillation column is to be designed to separate an ideal binary material system,x0.5, feed rate 100kmol/h, is saturated The feed which contains more volatile componentFvapor,the flow rate of overhead product and the flow rate of bottom product are also 50kmol/h. Suppose the operating line for rectifying section is y=0.833x+0.15, the vapor generated in the reboiler enters the column through the bottom plate, a complete condenser is used on the top of column and reflux temperature is bubbling point. Find:(1) Mole fraction x D of overhead product and the mole fraction x B of bottom product ?(2) Vapor amount condensed in the complete condenser, in mol/h?(3)The operating line for stripping section.(4) If the average relative volatility of the column is 3 and the first plate’s Murphree efficiencyfrom the column top is E m,L=0.6(represented by liquid mole fraction), find the constituent of gas phase leaving the second plate from the column top.4 An ideal binary solution of a volatile component A containing 50% mole percent A is to be separated in a continuous distillation column. The feed is saturated vapor, the feed rate is 1000kmol/h, and the flow rate of overhead product and the flow rate of bottom product are also500kmol/h. Given: the operating line for rectifying section is y=0.86x+0.12, indirect vapor is used in the reboiler for heating and the total condenser is used on the top of tower. Assume that the reflux temperature is at its bubble point. Find:(1) reflux ratio R, the mole fraction of overhead product x D and the mole fraction of bottom product x B?(2) upward flow rate of vapor in the rectifying section(V mol/h,) and down ward flow rate of liquid in the stripping section.(L’ mol/h,) (3) The operating line for stripping section .(4)if relative volatility α=2.4, find R/Rmin5 Separate component A and B of mixed liquid in a continuous distillation column, Given: raw liquid flow rate F is 4000 kg·h-1 , mass fraction of A is 0.3. It is required that the A mass fraction in the column bottom liquid can not be beyond 0.05, recovery ratio of A is 88% on the top. Try to calculate the flow rate of the distillated liquid D and its constituent x D at the top of column.( in the terms of molar flow rate and molar fraction) Given: molecular weight of components A and B are 76 and 154, respectively.6 There is a continuous rectifying operation column, whose the operation line equation is as follows:Rectifying section: y=0.723x+0.263Stripping section: y=1.25x-0.0187if the feed enters the column at a dew point, find (a)the molar fraction of feed、overhead product and bottom product. (b) reflux ratio R.7 A column is to be designed to separate a liquid mixture containing 44 mole percent A and 56 mole percent B, the system to be separated can be taken as ideal. The overhead product contains 95.7 mole percent A. Given: liquid average relative volatility α=2.5, min reflux ratio Rmin =1.63, try to illustrate the thermal condition of the feed and to calculate the value of q.8 A continuous rectifying column operated at atmospheric pressure is used to separate benzene—methylbenzene (甲苯) mixed liquid. The feed is saturation liquid containing 50 mole percent benzene. Given: the overhead product must contain 90 mole percent benzene and the bottom product contains 10 mole percent benzene. If reflux ratio is 4.52, try to calculate how many ideal plates are need? and locate the feed plate. In this situation the equilibrium data of benzene—methylbenzene are as followst o C 80.1 85 90 95 100 105 110.6x 1.000 0.780 0.581 0.411 0.258 0.130 0y 1.000 0.900 0.777 0.632 0.456 0.262 09 There is a rectifying column, given : mole fraction of distillation liquid from tower top x D=0.97, reflux ratio R=2, the gas-liquid equilibrium relationship y=2.4x/(1+1.4x); find: the constituent x1 of the down liquid leaving from the first plate and the constituent y2 of the up gas leaving from the second plate in the rectifying section. Suppose the total condenser is used at the top of column.10 A continuous fractionating column is used to separate 4000kg/h of a mixture of 30 percentCS2and 70 percent CCl4. Bottom product contains 5 percent CS2, and the rate of recovery ofCS2in the overhead product is 88% by weight. Calculate (a) the moles flow of overhead productper hour. (b) the mole fraction of CS2in the overhead product, respectively.11. A liquid mixture of benzene and toluene is continuously fed to a plate column. Under the total reflux ratio condition, the compositions of liquid on the close plates are 0.28, 0.41 and 0.57, respectively. Calculate the Murphree plate efficiency of two lower plates. The equilibrium data for benzene—toluene liquid and vapor phases under the operating condition are given as follows:x 0.26 0.38 0.51y 0.45 0.60 0.72Drying1 A wet solid with 1000 kg/h is dried by air from 40% to 5% moisture content (wet basis) under the convective drying conditions. The air primary humidity H1 is 0.001(kg water/kg dry air), and the humidity of air leaving dryer H2 is 0.039 (kg water /kg dry air), suppose that the material loss in the drying process can be negligible. Find:(1) rate of water vaporization W, in kg water/ h.(2) rate of dry air G required, in kg bone dry air/h, flow rate of moist air, V, in kg fresh air/h.(3) rate of moist material out of dryer, L2, in kg moist solid/h.2 The wet solid is to be dried from water content 20% to 5% (wet basis) in a convective dryer at an atmospheric pressure. The feed of wet solid into the dryer is 1000 kg/h at a temperature of 40℃. The dry and wet bulb temperatures of air are respectively 20℃ and 16.5℃ before air enters the preheater. After being preheated, air enters the dryer. The dry and wet bulb temperatures of air leaving dryer are respectively 60℃ and 40℃. If heat loss is negligible and drying is considered as constant enthalpy process:(1) What is the fresh (wet) air required per unit time (in kg/h)?(2) The air temperature T G1 before entering the dryer?(Given: Vaporization latent heat of water at 0℃ is 2501kJ/kg, specific heat of dry air C pB is 1.005 kJ/kg·K, specific heat of water vapor C pA is 1.88 kJ/kg·K)3 The wet solid material is to be dried from water content 42% to 4% (wet basis) in an adiabatic dryer. The solid product out of the dryer is 0.126kg/s. After the fresh air at a dry-bulb temperature of 21ºC and a relative humidity of 40%is preheated to 93ºC, it is sent to the dryer, and leaves the dryer at relative humidity of 60%. If the drying is under constant enthalpy process.(1) Determining the air humidity H1.(2) If H0=0.008(kg water/kg dry air), H2=0.03( kg water /kg dry air. Find:(a) Dry air flow rate G required, in kg dry air/s.(b)How much heat is supplied to air by the preheater (in k J/h)?4 The wet solid containing 12%(wet basis) moisture is fed to a convective dryer at a temperature of 15℃ and is withdrawn at 28℃, which contains 3% moisture (wet basis). The flow rate of final moist solid (product) is 1000kg/h. After the fresh air at a dry-bulb temperature of 25℃ and a humidity of 0.01 kg water/kg dry air is preheated to 70℃, it is sent to the dryer, and leaves the dryer at 45ºC. Suppose the drying process is under constant enthalpy, heat loss in the drying system can be negligible.(1) Drawing the operation process covering various air states in T-H.(2) What is the fresh air required per unit time (in kg/h)?5 Certain wet material is dried under an ordinary pressure in a convective dryer. Given: air temperature and humidity before entering a preheater are T G0=15℃and H0=0.0073 (kg water)/ (kg dry air); air temperature before entering the dryer T G1=90℃; air temperature and humidity leaving the dryer T G2=50℃and H2=0.023(kg water)/ (kg dry air); water content contained by material entering the dryer X1=0.15 (kg water)/(kg bone dry material ); water content contained by material leaving the dryer X2=0.01 kg water/(kg bone dry material); the production capability of dryer is 273kg/h (based on the product leaving the dryer). Find:(1) flow rate of the dry air G ,in kg bone dry air/h:(2) flow rate of wet air before entering the preheater, in fresh air m3/s;(3) heat duty (quantity) provided to the preheater, in kw if heat loss can be negligible.6 Wet material is dried in a dryer at an atmosphere pressure. The operating conditions are as follows:Air conditions: air temperature before entering the preheater T G0=20ºC, air humidity before entering the preheater H0=0.01(kg water/kg dry air), air temperature before entering the dryerT G1=120℃, air temperature leaving the dryer T G2=70ºC, air humidity leaving the dryerH2=0.05(kg water/kg dry air).Material conditions: material temperature before entering the dryer T s1=30ºC, wet content before entering the dryer w1=20% based on wet basis, material temperature leaving the dryer T s2=50ºC, wet content leaving the dryer w2=5% based on wet basis, specific heat of bone-dry materialCs=1.5kJ/(kg bone-dry solids . ºC). Production capacity of dryer is 53.5 kg/h based on product leaving the dryer. Suppose heat loss in the drying system can be negligible. Find:(1) The mass of bone-dry air required per unit time.(2) How much heat is obtained by air when passing through the preheater, in kJ/h?(3) How much supplementary heat transfer rate Q D is obtained?。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
诚信应考,考试作弊将带来严重后果! 华南理工大学期末考试 《mass transfer and separation process 》试卷 A 注意事项:1. 考前请将密封线内填写清楚; 2. 所有答案请直接答在试卷上(或答题纸上); 3.考试形式:开(闭)卷;1. (40 points) Fill in the blanks or choose the correct answer (1) The diffusivities of gases are proportional to ( 1.5 ) power of absolute temperature and are ( inversely proportional to ) pressure of the system. (2) The relation between the flux and the driving force of mass transfer for the equimolar diffusion is ( )(21c c D N --=δ ) (3) Rising the pressure in an absorption process of gas, the Henry ’s constant m will ( decrease ), and the rate of absorption will ( increase ) (4)For a separation process in which relative volatility α =1, the compositions of component A would be ( the same ) in both phases, separation is ( not possible ) when this occurs. (5) At the azeotropic point,both the vapor and the liquid in equilibrium have ( the same ) composition. It is ( not possible ) to separate the azeotropic mixtures by using ordinary distillation. (6) The definition of ideal stage is: (the vapor stream and liquid stream leaving the stage are in equilibrium ) (7) The separations of binary mixtures by a distillation operation are based on differences in vapor- and liquid-phase compositions arising from the ( partial vaporization ) of a liquid mixture or the ( partial condensation ) of a vapor mixture. (8)The difference between the actual plate and the ideal plate is corrected by ( plate efficiency ) (9) In general, the liquid rate for the absorber should be ( D ) times the minimumrate, too high a value requires ( A ) and the ( E ) of recovering, a small value results in ( F)A) a large-diameter tower B) 1 C) 2.5 D) 1.1-2.0 E) cost F) a high tower(10)“G as phase is controlling” means that the major resistance through both the gas and liquid phases is in the ( D), and the solute A in a gas phase is ( A )A) very insoluble in the liquid B) very soluble in the liquid C) can’t make sure D) gas phase E) liquid phase F) the gas and liquid phases(11) Flash distillation is used most for separating components that boil at ( very large ) different temperatures(12) A single total condenser is used, the concentrations of the vapor from the top plate, of the reflux to the top plate, and of the overhead product are ( the same), replacing it by a partial condenser, then, the concentration of overhead is ( larger than) that of reflux.(13) The stream relationships for the various thermal conditions of feed•Cold feed ( C,D,G )•Feed at bubble point ( B, E)•Feed partly vapor (C,D,I )•Feed at dew point ( F, A )•Feed is a superheated vapor (C,D,H)A) L=L′B)V=V′ C) L′=L+ q F D) V=V′+(1-q)FE) L′=L+F F)V=V′+F G) q>1 H)q<0 I) 0<q<1(14) When the rates of feed and of both the overhead and bottom products are ( 0), called total reflux and the slopes of both operating lines are ( 1 ). (15)Optimum reflux ratio is the point of ( most economical ), and ( not) much larger than the minimum reflux ratio.(16)The approach of azeotropic distillation, choosing an entrainer to cause ( an new azeotropic mixture )(17) A significant entrainment will ( reduce ) the plate efficiency.(18) In the constant-rate drying period, the rate of evaporation is ( A ) ofthe solid, and is ( B ) of the properties and flow type of air.A) independent B) dependent C) uncertain(19) The relationship among dry-bulb temperature t, wet-bulb temperature t w, dew-point t d of unsaturated moist air is( t> t w > t d)(20) The equilibrium moisture content ( A ) withthe type of material for any given percent relativehumidity of air.A) varies greatly B) is the same C ) variesslightly(21)Please read off the values of bound and unboundmoisture content from curve 2 on Fig, if the woolsample contained 34 kg H 2O/100 kg dry solid,( 27 ),( 7 )Every one of the following problems is 15points2. A mixture of 50 mole percent benzene and 50 percent toluene is subjected to flash distillation at a separator pressure of 1 atm. The incoming liquid is heated to a temperature that will cause 50 percent of the feed to flash. What are the compositions of the vapor and liquid leaving the flash chamber on assuming that the relative volatility of benzene-toluene system is 2.45 ?solution:y x 5.05.05.0+= x x y 45.1145.2+=x x x 45.1145.21++= x x 245.112+=39.09.2565.12245.1245.1442=⨯±-=⨯⨯+±-=x y=0.613. A distillation column is used for the separation of a binary mixture with relative volatility equal to 2 and desired to produce an overhead product containing mol 90% low boiler. The vapor flow rate to a given plate in the rectifying section is 150kmol/h with 60%mole fraction, and liquid flow rate to this plate is 100kmol/h. Murphree efficiency defined by vapor concentration is 0.5. Find:(1) Operating line for the rectifying section,(2) What are the compositions of vapor and liquid phases leaving the plate?(Assuming that the molar flow rates of vapor and liquid are constant in the column)solution: (1)321501001==+=R R V L R=239.03211+=+++=x R x x R R y D (2) 6.039.032111=+=+++=+n D n n x R x x R R y so the composition of liquid leaving the plate45.029.036.0=-⨯=n x Murphree efficiency 6.06.0*1*1--=--=++n n n n n n y y y y y y η=0.5 62.045.19.045.0145.0212*==+⨯=+=x x y n 5.06.062.06.0=--=n y η y n =0.614. A gas stream containing 3.0 percent A is passed through a packed column to remove 99 percent of the A by absorption in water. The absorber will operate at 1 atm, and the gas and liquid rates are to be 20 mol/h·m 2 and 100 mol/h·m 2, respectively. Mass-transfer coefficients and equilibrium data are given below:Y ∗ = 3.1Xk Y a = 135 mol/h·m 3 ·unit mol fractionk X a = 540 mol/h·m 3 ·unit mol fraction.Find:(a ) What is the minimum ratio of liquid and gas(b ) N Oy , H Oy , and Z , assuming isothermal operation and neglecting changes in gas and liquid flow rates.(c) What percent of the total resistance is in the gas phase?Solution:a) 031.0310031=-=Y ()0031.099.0112=-=Y Y121)(LX Y Y V =-VL X Y Y =-121)( 069.399.01.3)(121121min =⨯==-=-=⎪⎭⎫ ⎝⎛ηm Y Y Y m mY Y Y V L b)()617.9654.3632.262.38ln 632.262.099.01162.01ln 100201.3111ln 11Noy 21=⨯==⎥⎦⎤⎢⎣⎡+--⨯-=⎥⎦⎤⎢⎣⎡+⎪⎭⎫ ⎝⎛--=L mV Y Y L mV L mVThe relative gas-film resistance is :5. A dryer is used to dry material from a moisture content of 5% to 0.5% (wet basis). The capacity of dryer is 1.5 kg dry material/s. Hot air enters at 127℃ and a humidity of 0.007kg (water)/kg (dry air) and leaves at 82℃. The temperature of the material at the inlet and outlet is 21℃ and 66℃, respectively. The specific heat of dry material is1.8kJ/(kg ⋅℃). If the heat loss of the dyer can be ignored, then calculate:a) the amount of water removed from moist material;b)the consumption of the wet air;c) the humidity of the air leaving the dryer.Solution:a) The moisture content of the material (dry basis) is:m Z m H a K a k m a k aK Oy y x y y 53.261.9263.0263.0056.7620056.7601315.05401.3135111=⨯=====+=+=percent 56or 56.0056.7611351=121250.0526950.50.0050399.5() 1.5(0.05260.0503)0.0714/C X X W G X X kg s ====∴=-=-=水 ∴ s water kg X X L W c /)(0714.0)00503.00526.0(5.1)(21=-=-= From the material balance, it can be obtained that: 0714.0)007.0(2=-H GFrom the enthalpy balance, it can be obtained that:)()(1221I I L I I G c '-'=- where,1112()(1.8 4.1870.0526)2142.49(1.8 4.1870.00503)66120.2S W I C C X Q I '=+=+⨯⨯='=+⨯⨯= 1111(1.01 1.88)2490(1.01 1.880.007)12724900.007147.4I H t H =++=+⨯⨯+⨯= 222(1.01 1.88)82249082.82644I H H 2=+⨯H ⨯+=+ (2) ∴ 7.116)43.422.120(5.1)4.147(2=-=-I G From the above, it can be obtained that: H 2=0.0178, G=6.61 kg wet air/s. s kg H G G /66.6)007.01(61.6)1(1=+=+='。
