Amicon Ultra-4 Centrifugal Filter Devices User Guide
milipure超滤管说明书

EnglishIntroductionAmicon® Ultra-15 10K centrifugal filter devices provide fast ultrafiltration, with the capability for high concentration factors and easy concentrate recovery from dilute and complex sample matrices. Thevertical design and available membrane surface area provide fast sample processing, high sample recovery (typically greater than 90% of dilute starting solution), and the capability for 80-fold concentration. Typical processing time is 15 to 40 minutes. Solute polarization and subsequent fouling of the membrane are minimized by the vertical design, and a physical deadstop in the filter device prevents spinning todryness and potential sample loss. The concentrate is collected from the filter device sample reservoir using a pipettor, while the ultrafiltrate is collected in the provided centrifuge tube. The device can be spun in a swinging-bucket or fixed-angle rotor. Amicon® Ultra-15 10K devices are supplied non-sterile and are for single use only.Intended UseThe Amicon® Ultra-15 product line includes 5 different cutoffs (Molecular Weight Cutoff, MWCO), however, the Amicon® Ultra-15 10K device (10,000 MWCO) is the only device intended for in vitro diagnostic use. It can be used to concentrate serum, urine, cerebrospinal fluid, and other body fluids prior to analysis. For information on other Amicon® Ultra-15 cutoffs, go to and enter Amicon Ultra-15in the search box.User GuideAmicon ® Ultra-15 10K Centrifugal Filter Devicesfor volumes up to 15 mLUFC901008UFC901024UFC901096CVApplications●Concentration of biological samples containing antigens, antibodies, enzymes, nucleic acids(DNA/RNA samples, either single- or double-stranded), microorganisms, column eluates, andpurified samples●Purification of macromolecular components found in tissue culture extracts and cell lysates, removalof primer, linkers, or molecular labels from a reaction mix, and protein removal prior to HPLC●Desalting, buffer exchange, or diafiltrationMaterials SuppliedThe Amicon® Ultra-15 10K device is supplied with a cap, a filter device, and a centrifuge tube.Required Equipment●Centrifuge with swinging-bucket or fixed-angle rotor with wells/carriers that can accommodate50 mL tubesCAUTION: To avoid damage to the device during centrifugation, check clearance before spinning.●Pipettor with 200 microliter (μL) tip for concentrate recoverySuitabilityPreliminary recovery and retention studies are suggested to ensure suitability for intended use. See the “How to Quantify Recoveries” section.Shelf LifeShelf life is 3 years from date of manufacture. Refer to expiration date on package label.Rinsing Before UseThe ultrafiltration membranes in Amicon® Ultra-15 10K devices contain trace amounts of glycerine. If this material interferes with analysis, rinse the device with buffer or Milli-Q® water before use. If interference continues, rinse with 0.1 N NaOH followed by a second spin of buffer or Milli-Q® water. CAUTION: Do not allow the membrane in Amicon® Ultra filter devices to dry out once wet. If you are not using the device immediately after rinsing, leave fluid on the membrane until the device is used. How to Use Amicon® Ultra-15 Centrifugal Filter Devices1. Add up to 15 mL of sample (12 mL if using a fixed-angle rotor) to the Amicon® Ultra filter device.2. Place capped filter device into centrifuge rotor; counterbalance with a similar device.3. When using a swinging-bucket rotor, s pin the device at 4,000 × g maximum for approximately15–40 minutes.When using a fixed-angle rotor, orient the device with the membrane panel facing up and spin at 5,000 × g maximum for approximately 15–40 minutes.NOTE: Refer to Figure 1 and Table 1 for typical spin times.4. To recover the concentrated solute, insert a pipettor into the bottom of the filter device and withdrawthe sample using a side-to-side sweeping motion to ensure total recovery. The ultrafiltrate can be stored in the centrifuge tube.NOTE: For optimal recovery, remove concentrated sample immediately after centrifugation. Desalting or DiafiltrationDesalting, buffer exchange, or diafiltration are important methods for removing salts or solvents in solutions containing biomolecules. The removal of salts or the exchange of buffers can be accomplished in the Amicon® Ultra-15 device by concentrating the sample, then reconstituting the concentrate to the original sample volume with any d esired solvent. The process of “washing out” can be repeated until the concentration of the contaminating microsolute has been sufficiently reduced. See example below.1 mg/mL100 mMNaCl200 μL of75 mg/mLprotein inNaCl200 μL of75 mg/mLprotein inNaClAdd 14.8 mL of10 mM NaClor exchangebufferPerformanceFlow RateFactors affecting flow rate include sample concentration, starting vol u me, chemical nature of solute, relative centrifugal force, centrifuge rotor angle, membrane type, and temperature. Figure 1 and Table 1 can be used to estimate the time required to achieve a given volume of filtrate or concentrate. A typical spin time for a 15 mL sample is approximately 15 to 40 minutes. While most of the sample is filtered in the first 15 to 30 minutes of centrifugation, the lowest concentrate volume (100–150 μL) is reached after spinning for 15 to 40 minutes.Spin conditions: Swinging-bucket rotor (4,000 × g, 15 mL starting volume),or fixed-angle rotor, (5,000 × g, 12 mL starting volume), room temperature.Protein marker used: Cytochrome c, n=6.Table 1. Typical Concentrate Volume vs. Spin TimeSpin conditions: Room temperature.Protein marker used: Cytochrome c, n=6 (mean value of 3 membrane lots).Shaded volumes were used for the calculation of protein recovery in Table 3.Protein Retention and Concentrate RecoveryThe membranes used in Amicon® Ultra devices are characterized by a molecular weight cutoff (MWCO); that is, their ability to retain molecules above a specified molecular weight. Solutes with molecular weights close to the MWCO may be only partially retained. Membrane retention depends on the solute’s molecular size and shape. For most applications, molecular weight is a convenient parameter to use in assessing retention characteristics. For best results, use a membrane with a MWCO at least two times smaller than the molecular weight of the protein solute that one intends to concentrate. Refer to Table 2.Table 2. Typical Retention of Protein MarkersMarker/Concentration MolecularWeightDeviceMWCO% RetentionSwinging-bucket% RetentionFixed-angleSpin Time(min)α-Chymotrypsinogen (1 mg/mL)25,00010K> 95> 9530 Cytochrome c (0.25 mg/mL)12,400> 95> 9530 Vitamin B-12 (0.2 mg/mL)1,350< 5< 530 Spin Conditions: Swinging-bucket rotor (4,000 × g, 15 mL starting volume), or fixed-angle rotor, (5,000 × g,12 mL starting volume), room temperature, n=6 (mean value of 3 membrane lots).Factors that determine sample recovery include the nature of the protein solute relative to the device MWCO chosen, starting concentration, and concentration factor. Table 3 provides typical recoveries for Amicon® Ultra-15 10K device.Table 3. Typical Concentrate RecoveryConcentrate Concentration ConcentrateSpin Conditions: Swinging-bucket rotor (4,000 × g, 15 mL starting volume), or fixed-angle rotor, (5,000 × g,12 mL starting volume), room temperature, n=6 (mean value of 3 membrane lots). The shaded volumes were taken from Table 1.Maximizing Sample RecoveryLow sample recovery in the concentrate may be due to adsorptive losses, over-concentration, or passage of sample through the membrane.●Adsorptive losses depend upon solute concentration, its hydrophobic nature, temperature and timeof contact with filter device surfaces, sample composition, and pH. To minimize losses, removeconcentrated samples immediately after centrifugal spin.●If the starting sample concentration is high, monitor the centrifugation process in order to avoid over-concentration of the sample. Over-concentration can lead to precipitation and potential sample loss.●If the sample appears to be passing through the membrane, choose a lower MWCO Amicon® Ultra-15device.How to Quantify RecoveriesCalculate total recovery, percent concentrate recovery, and percent filtrate recovery using the method below. The procedure provides a close approximation of recoveries for solutions having concentrations up to roughly 20 mg/mL.NOTE: Appropriate assay techniques include absorption spectrophotometry, radioimmunoassay, refractive index, and conductivity.Direct Weighing ProcedureThe density of most dilute proteins is nearly equal to the density of water (i.e., 1 g/mL). Using this property, the concentrate and filtrate volumes can be quantified by weighing them and converting the units from grams to milliliters. This technique is valid only for solutions with concentrations of approximately20 mg/mL or less.1. Before use, separately weigh the empty filter device, the centrifuge tube, and an empty tube forconcentrate collection.2. Fill filter device with solution and reweigh.3. Assemble device and centrifuge per instructions.4. Collect the concentrate with a pipettor and dispense it into the preweighed concentrate collection tube.5. Remove the device from the centrifuge tube and weigh the centrifuge tube and concentrate collectiontube.6. Subtract weight of empty device/tubes to calculate weights of starting material, filtrate, andconcentrate.7. Assay the starting material, filtrate, and concentrate to determine solute concentration.8. Calculate recoveries using the weight/volume data and the measured concentrations as follows:% concentrate recovery = 100 × W c × C c W o × C o% filtrate recovery = 100 × W f × C f W o × C o% total recovery = % concentrate recovery + % filtrate recoveryW c = total weight of concentrate before assayW o = weight of original starting materialW f= weight of filtrateC c= concentrate concentrationC o= original starting material concentrationC f= filtrate concentrationSpecificationsMaximum initial sample volumeSwinging-bucket15.0 mLFixed-angle rotor12.0 mLTypical final concentrate volume 150–300 μLMaximum relative centrifugal forceSwinging-bucket rotor4,000 × gFixed-angle rotor5,000 × gActive membrane area7.6 cm2DimensionsFilter device in tube (capped)Length: 119 mm (4.7 in.)Diameter: 33.5 mm (1.3 in.) Filter deviceLength: 72.0 mm (2.8 in.)Diameter: 29.7 mm (1.2 in.) Materials of ConstructionFilter device Copolymer styrene/butadieneMembrane Ultracel® low binding regenerated cellulose Filtrate tube PolypropyleneFiltrate cap and liner PolyethyleneChemical CompatibilityAmicon® Ultra centrifugal devices are intended for use with biological fluids and aqueous solutions. Before use, check the sample for chemical compatibility with the device.Table 4. Chemical Compatibility of Amicon® Ultra Filter DevicesAcids Concentration Concentration Acetic acid ≤ 50%*Phosphoric acid ≤ 30% Formic acid ≤ 5%*Sulfamic acid≤ 3% Hydrochloric acid ≤ 1.0 M Sulfuric acid ≤ 3% Lactic acid ≤ 50%Trichloroacetic acid (TCA)≤ 10%* Nitric acid ≤ 10%Trifluoroacetic acid (TFA)≤ 30%* AlkalisAmmonium hydroxide ≤ 10%Sodium hydroxide ≤ 0.5 M Alcoholsn-Butanol ≤ 70%Isopropanol ≤ 70% Ethanol ≤ 70%Methanol ≤ 60% DetergentsAlconox® detergent ≤ 1%Sodium dodecyl sulfate (SDS)≤ 0.1% CHAPS detergent≤ 0.1%Tergazyme®detergent ≤ 1% Lubrol® PX detergent≤ 0.1%Triton® X-100 surfactant≤ 0.1% Nonidet™ P-40 surfactant≤ 2%Tween® 20 surfactant≤ 0.1% Sodium deoxycholate≤ 5%Organic solventsAcetone not recommended Ethyl acetate not recommended Acetonitrile ≤ 20%Formaldehyde ≤ 5% Benzene not recommended Pyridine not recommended Carbon tetrachloride not recommended Tetrahydrofuran not recommended Chloroform not recommended Toluene not recommended Dimethyl sulfoxide (DMSO)≤ 5%*MiscellaneousAmmonium sulfate Saturated Phenol≤ 1% Diethyl pyrocarbonate ≤ 0.2%Phosphate buffer (pH 8.2)≤ 1 M Dithiothreitol (DTT)≤ 0.1 M Polyethylene glycol≤ 10% Glycerine≤ 70%Sodium carbonate ≤ 20% Guanidine HCl ≤ 6 M Tris buffer (pH 8.2)≤ 1 M Imidazole≤ 100 mM Urea ≤ 8 M Mercaptoethanol≤ 0.1 M* Contact with this chemical may cause materials to leach out of the component parts. Solvent blanks are recommended to determine whether leachables represent potential assay interferences.Product Labeling SymbolsThe following table defines the symbols found on Amicon® Ultra-15 10K device labels.Product Ordering InformationThis section lists the catalogue numbers for Amicon® Ultra Ultrafiltration Devices. See the Technical Assistance section for contact information. You can purchase these products on-line at/products.* Amicon® Ultra-4 and -15 10K devices are for in vitrodiagnostic use. All other devices are for research use only.NoticeThe information in this document is subject to change without notice and should not be construed as a commitment by Merck Millipore Ltd. (“Millipore”) or an affiliate. Neither Merck Millipore Ltd. nor any of its affiliates assumes responsibility for any errors that may appear in this document.Technical AssistanceFor more information, contact the office nearest you. Up-to-date world-wide contact information is available on our web site at /offices. You can also visit the tech service page on our web site at /techservice.Standard WarrantyThe applicable warranty for the products listed in this publication may be found at/terms (“Conditions of Sale”).M Merck Millipore Ltd.Tullagreen,Carrigtwohill,Co. Cork, IrelandMade in IrelandThe M logo, Millipore, Amicon, Milli-Q, and Ultracel are registered trademarks of Merck KGaA, Darmstadt, Germany.All trademarks of third parties are the property of their respective owners.© 2014 EMD Millipore Corporation. Billerica, MA, U.S.A. All rights reserved.PR04318TR, Rev. B, English, 10/14。
碧云天 L31600 pLenti-TLR2-sgRNA 产品说明书
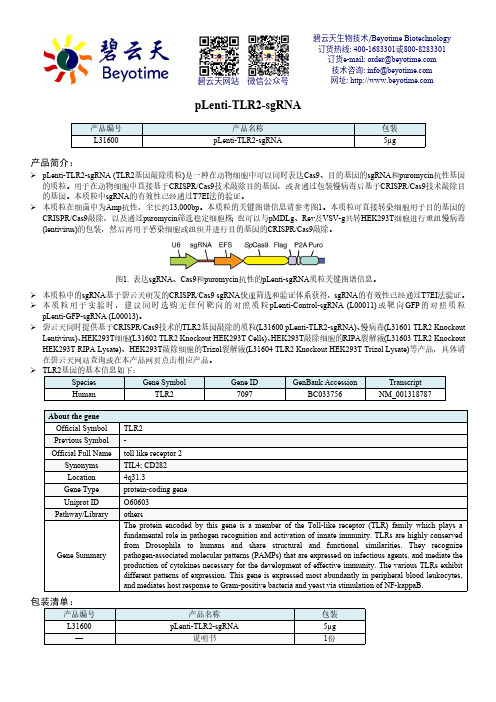
pLenti-TLR2-sgRNA产品简介:pLenti-TLR2-sgRNA (TLR2基因敲除质粒)是一种在动物细胞中可以同时表达Cas9、目的基因的sgRNA 和puromycin 抗性基因的质粒。
用于在动物细胞中直接基于CRISPR/Cas9技术敲除目的基因,或者通过包装慢病毒后基于CRISPR/Cas9技术敲除目的基因。
本质粒中sgRNA 的有效性已经通过T7EI 法的验证。
本质粒在细菌中为Amp 抗性,全长约13,000bp 。
本质粒的关键图谱信息请参考图1。
本质粒可直接转染细胞用于目的基因的CRISPR/Cas9敲除,以及通过puromycin 筛选稳定细胞株;也可以与pMDLg 、Rev 及VSV-g 共转HEK293T 细胞进行重组慢病毒(lentivirus)的包装,然后再用于感染细胞或组织并进行目的基因的CRISPR/Cas9敲除。
图1. 表达sgRNA 、Cas9和puromycin 抗性的pLenti-sgRNA 质粒关键图谱信息。
本质粒中的sgRNA 基于碧云天研发的CRISPR/Cas9 sgRNA 快速筛选和验证体系获得,sgRNA 的有效性已经通过T7EI 法验证。
本质粒用于实验时,建议同时选购无任何靶向的对照质粒pLenti-Control-sgRNA (L00011)或靶向GFP 的对照质粒pLenti-GFP-sgRNA (L00013)。
碧云天同时提供基于CRISPR/Cas9技术的TLR2基因敲除的质粒(L31600 pLenti-TLR2-sgRNA)、慢病毒(L31601 TLR2 Knockout Lentivirus)、HEK293T 细胞(L31602 TLR2 Knockout HEK293T Cells)、HEK293T 敲除细胞的RIPA 裂解液(L31603 TLR2 Knockout HEK293T RIPA Lysate)、HEK293T 敲除细胞的Trizol 裂解液(L31604 TLR2 Knockout HEK293T Trizol Lysate)等产品,具体请在碧云天网站查询或在本产品网页点击相应产品。
Agilent-GCMS培训(完整版330页)

进样口和检测器出口
气路连接口
电缆连接口
电源连接口
炉箱冷却风进口
Agilent 7890A 键盘介绍
*
运行按键
气相部件按键
数字按键
信息按键
方法存储和自动运行按键
维护按键
*
Agilent GC6890前视图
*
Agilent GC6890侧后视图
*
即时功能键 功能键 快捷键 信息键 数字键 多功能键 方法存贮与自动 运行
MSD
10-5 Torr
<2 mL/min
760 Torr
0.5 - 15 mL/min
传输线
*
气相色谱和质谱的联用技术——传输线
传输线
涡轮泵
自动进样器
离子源
炉箱
*
色谱柱
流量 控制器
稳压器
空气
氢气
载气
分子筛
脱水管
固定
进样口
检测器
电子部件
PC
限流器
典型的气相色谱
*
分子筛 P/N 5060-9084
(0 to 218 V)
Signal Out
EM Voltage
电子倍增器
电压有使用上限(3000伏) 电压的提高,可以提高检测器的信号
*
电子倍增器的寿命曲线
*
提供足够的平均自由程 提供无碰撞的离子轨道 减少离子-分子反应 减少背景干扰 延长灯丝寿命 消除放电 增加灵敏度
为什么MS需要真空
空气压力应为 80 psi。
推荐管线压力
氢气压力应为 60 psi。
载气必须通过控制形成恒定的压力和恒定的流量。上下游控制器压差保持1公斤以上。
5 高分子分子量与尺寸

Mz > Mw > Mv > Mn
Molecular weight distribution
分子量分布
Number-average distribution width
M M n
2 n
2
n
Mw M M 1 n
2 n
Weight-average distribution width
a
c b
样品a:可纺性很差; 样品b:有所改善;
5 10 15 M×10-4
样品c:由于分子量15~20万的大分子所占的比例较大, 可纺性很好。
Average molecular weight 平均分子量
Molecular weight
Number Weight for each type of chain
1 a i
1/ a
Example:
ni
nM n
i i
10
10 20
10 10
Mi(×10-4) 30
Mn
i
10 30 10 20 10 10 104 20 104 10 10 10
10 302 10 202 10 102 Mw 104 23.3 104 ni M i 10 30 10 20 10 10
无内干涉lightscatteringsmallparticledilutesolution小粒子稀溶液的光散射根据光的电磁波理论和涨落理论若入射光是垂直偏振光可导出每单位体积溶液中溶质的散射光强散射点至检测器的距离入射光在真空中的波长溶剂的折光率溶液浓度溶液的折光率增量溶液渗透压对浓度的偏导散射角光学常数与溶液浓度散射角溶质分子量无关rayleigh因子光学常数与溶液浓度散射角溶质分子量无关rayleigh因子smallparticles光散射法测定的是lightscatteringlargeparticledilutesolution大粒子稀溶液的光散射有内干涉时散射因子均方旋转半径无内干涉时测定不同浓度稀溶液在不同散射角的rayleigh因子将实验点外推至0c0即可求得m对高斯链无规线团illustrationlightscatteringcalculationzimmplot的测定
超滤离心管的使用

•Used for Protein Purification and Concentration•Pack of 96 filter units and corresponding 2 mL centrifuge tubes•Filter holds 0.5 mL volume (we work with such small volumes the 2, 4, and 15 mL versions are excessive, thought could be used to wash faster/ more thoroughly if you so choose) •Filter has a 100 kDa NMWL membrane (MW cut-off of 100 kDa)Rough Protocol for Buffer Exchange:1.Assemble filter column as shown in the manufacturer's manual/protocol (see User Guide).装配离心管(两个管套在一起,外管是普通离心管,内管为过滤管)2.Add volume (~5-20 uL depending on number of samples being prepared for TEM) ofconcentrated ferritin in TBS to filter unit.取5-20 μL(根据TEM样品量选择具体的量)分散铁蛋白的TBS溶液,加入内管(过滤管)3.Fill remaining filter volume (to 500 uL) with DI/ultra-purified water.加入500 μL超纯水。
4.Spin at 14,000 x g for 10-15 minutes. 内管(过滤管)套在外管内,装入离心机,14000 x g离心力下离心10-15分钟。
Thermo Scientific Heraeus Megafuge 8和8R小型实验室离心机产品介

Thermo ScientificHeraeus Megafuge 8 Centrifuge SeriesFits in.Stands out.Introducing the NEW!Thermo Scientific™Heraeus™ Megafuge™ 8 and 8R small benchtop centrifugesFits in.4t o your space, with its compact footprint.Auto-Lock ™ Rotor ExchangeSecure push-button application versatility and cleaning convenienceClickSeal ™ Biocontainment LidsOne-handed, certified 1 sample protectionMaximized Swing-Out CapacityStands out.4d esigned for solid reliability and consistent results.14Ventilated (left) and refrigerated (right)n B iocontainment sealing options, including certified ClickSeal lids for glove-friendly, one-handed operation n M anufactured with quality materials providing broad Simple solutions.Flexible processing.Reliable quality. Safe operation.1B Public Health England, Porton Down, UK Fits in. Stands out.Secure, push-button Auto-Lock rotor exchange in as little as 3 seconds delivers:n Trouble-free rotor installation and removal linical processing up to 24 x 5/7 mL blood tubes per runAccommodates up to 30 spin columns, such as DNA and RNA mini-preps, Micro-volume Solution RotorsSample-ready.User-friendly.n O ne-touch operation with pre-saved protocolsn H ighly visible backlit display for easy reading of parameters across the labn O ptional indicators at end of run, including automatic lid opening, full flashing display andadjustable audible signaln Glove- and detergent-friendlyn M ultilingual instructions – English, Dutch, French, German, Italian, Russian and Spanish — onprogramming, run conditions, alerts andservice messagesExpanded Programming Optionsn A lphanumeric program naming ,of up to 12 characters, promotes correct program selection every time.n P rogram-only mode limits thecontrol of the centrifuge to the set program and the control quadrant (Start, Stop, Pulse and Open), ideal for controlled environmentsFits in. CLINICAL APPLICATIONS.Outstanding capacity in a compact design with a smart, simple interface.n R uns up to 24 x 5/7 mL blood tubes at a time in swinging bucket configuration n A uto-Lock rotor exchange simplifies cleaning n C onforms to the latest clinical and safety standards n F ast acceleration and deceleration times for quicker spinsStands out.TX-150 Swinging Bucket RotorM10 Swinging Bucket Rotor8 x 50 mL Individually Sealed Fixed Angle RotorCLINIConic Fixed Angle RotorHematocrit Rotor1B iocontainment certification by the Public Health England, Porton Down, UKDedicated Rotors for Routine Clinical ProcessingTX-100S Clinical Swinging Bucket Rotor with Sealed Carriers TX-100 Clinical Swinging Bucket Rotor with CarriersInnovative design supports multiple users and applications to stay one step ahead of your evolving research needs.n 8 x 50 mL swinging bucket rotor capacity, 4 microplates, or 30 filtration columns for research needs n A uto-Lock rotor exchange for application flexibility – go from 50 mL tube to microplates to microtubes effortlesslyFits in. RESEARCH APPLICATIONS.Stands out.to 30,279 x g enables • quicker spins• improved separations• access to a wider range of applications while maintaining full swing-out rotor fl exibility, combined with quick, safe Auto-Lock rotor exchange.TX-150 Swinging Bucket RotorM10 Swinging Bucket RotorMT-12 Swinging Bucket RotorHIGHConic III Fixed Angle RotorCLINIConic Fixed Angle RotorMicroClick 18 x 5 RotorMicro-Volume Solution RotorsMicroClick 24 x 2Fixed Angle Rotor8 x 8 PCR Strip FixedAngle Rotor1B iocontainment certification by the Public Health England, Porton Down, UKTX-150 Swinging M10 SwingingBucket Rotor MT-12 Swinging Bucket Rotor23468Versatile Swinging Bucket RotorsMax Tube Max Speed Max RCFTX-100S Clinical Swinging Bucket Rotor TX-100 Clinical Swinging Bucket RotorClinical Swinging Bucket RotorsCat. No. DescriptionRotorCapacity(places xvolume, mL)Max TubeDimensions(O x L, mm)Max Speed(rpm)Max RCF(x g)Max Speed(rpm)Max RCF(x g)Clinical Swinging Bucket Rotors VENTILATED REFRIGERATED75005704TX-100S Clinical Swinging Bucket Rotorwith Sealed Carriers, 90°, R max 144 mm8 x 1517 x 1064,5003,2604,500 3,26075005705TX-100 Clinical Swinging Bucket Rotorwith Carriers, 90°, R max 144 mm16 x 1517 x 1214,5003,2604,5003,260Adapters for TX-100S Clinical Rotor and TX-100 Clinical Rotor (each)1120366613.5 mL Urine Tube16/8 x 13.516 x 82HIGHConic III Rotor8 x 50 mL Rotor MicroClick 18 x 5 Rotor8 x 8 PCR Strip RotorMicroClick 24 x 2 RotorMicroClick 30 x 2 RotorCat. No. Description Rotor Capacity(places xvolume, mL)Max TubeDimensions(O x L, mm)Max Speed(rpm)Hematocrit RotorThermo Scientific Heraeus Megafuge 8 Centrifuge SeriesSpecificationsSwing Bucket RotorsFixed Angle RotorsControl SystemDrive SystemRotor Locking SystemImbalance Detection SystemProgramsPulse (Short) RunAcceleration /Deceleration RatesCentrifugation ChamberMax Timer RangeTemperature RangePre-Cooling FunctionRefrigeration SystemSound LevelOther Features1 Biocontainment certification by the Public Health England, Porton Down, UKFits in. Stands out.Fits in.More centrifuge solutions for your labPerfect Fit. It’s simple. From 1 Liter bottles, to 15 and50 mL conical tubes and microplates, the versatile selection ofThermo Scientific™ Nalgene™ and Nunc™ centrifugationproducts work seamlessly with your complete centrifuge androtor system, bringing together outstanding quality and performance.Thermo Scientific Heraeus Benchtop CentrifugesAccelerate your research with high speeds, rotor versatility and outstanding safety. Fromintuitive controls, to the certified“click” in place, Thermo Scientific Heraeus benchtop centrifuges deliver the perfect blendof capabilities to support micro-volume protocols, a wide range of high volume swing-outapplications and high speed processing.Thermo ScientificSelect from two available speed options, up to 1721,000 x g for high-end research applications, and our extensive rotor portfolio, including:•1 Biocontainment certification by the Public Health England, Porton Down, UK。
Amicon Ultra-15 Centrifugal Filter Devices

English IntroductionAmicon® Ultra-15 centrifugal filter devices provide fast ultrafiltration, with the capability for high concentration factors and easy concentrate recovery from dilute and complex sample matrices. Thevertical design and available membrane surface area provide fast sample processing, high sample recovery (typically greater than 90% of dilute starting solution), and the capability for 80-fold concentration.Typical processing time is 15 to 60 minutes depending on Nominal Molecular Weight Limit (NMWL). Solute polarization and subsequent fouling of the membrane are minimized by the vertical design, and a physical deadstop in the filter device prevents spinning to dryness and potential sample loss. The concentrate is collected from the filter device sample reservoir using a pipettor, while the ultrafiltrate is collected in the provided centrifuge tube. The device can be spun in a swinging bucket or fixed angle rotor. Amicon® Ultra-15 devices are supplied non-sterile and are for single use only.The Amicon® Ultra-15 product line includes 5 different cutoffs (Nominal Molecular Weight Limit, NMWL, or Molecular Weight Cutoff, MWCO):●Amicon® Ultra 3K device — 3,000 NMWL ●Amicon® Ultra 10K device — 10,000 NMWL ●Amicon® Ultra 30K device — 30,000 NMWL ●Amicon® Ultra 50K device — 50,000 NMWL ●Amicon® Ultra 100K device — 100,000 NMWLAmicon® Ultra-15 10K filter devices are for in vitro diagnostic use and can be used to concentrate serum, urine, cerebrospinal fluid, and other body fluids prior to analysis.Amicon® Ultra-15 3K, 30K, 50K, and 100K filter devices are for research use only and not for use in diagnostic procedures.User GuideAmicon ® Ultra-15Centrifugal Filter Devicesfor volumes up to 15 mLAmicon® Ultra-15 10K device for in vitro diagnostic use Amicon® Ultra-15 3K, 30K, 50K, and 100K devices forresearch use only; not for use in diagnostic proceduresMaterials SuppliedThe Amicon® Ultra-15 device is supplied with a cap, a filter device, and a centrifuge tube.Applications●Concentration of biological samples containing antigens, antibodies, enzymes, nucleic acids (DNA/RNA samples, either single- or double-stranded), microorganisms, column eluates, and purified samples ●Purification of macromolecular components found in tissue culture extracts and cell lysates, removal of primer, linkers, or molecular labels from a reaction mix, and protein removal prior to HPLC ●Desalting, buffer exchange, or diafiltrationRequired Equipment●Centrifuge with swinging bucket or fixed angle rotor with wells/carriers that can accommodate50 mL tubes CAUTION : To avoid damage to the device during centrifugation, check clearance before spinning. ●Pipettor with 200 microliter (μL) tip for concentrate recoverySuitabilityPreliminary recovery and retention studies are suggested to ensure suitability for intended use. See the “How to Quantify Recoveries” section.Device Storage and Shelf LifeStore at 15–30 °C. Shelf life is 3 years from date of manufacture. For cat. nos. UFC901008, UFC901024, and UFC901096, refer to expiration date on package label.PrerinsingThe ultrafiltration membranes in Amicon® Ultra-15 devices contain trace amounts of glycerine. If this material interferes with analysis, prerinse the device with buffer or Milli-Q® water. If interference continues, rinse with 0.1 N NaOH followed by a second spin of buffer or Milli-Q® water. CAUTION: Do not allow the membrane in Amicon® Ultra filter devices to dry out once wet. If you are not using the device immediately after prerinsing, leave fluid on the membrane until the device isused.How to Use Amicon® Ultra-15 Centrifugal Filter Devices1. Add up to 15 mL of sample (12 mL if using a fixed angle rotor) to the Amicon® Ultra filter device.2. Place capped filter device into centrifuge rotor; counterbalance with a similar device.3. When using a swinging bucket rotor, spin the device at 4,000 × g maximum for approximately15–60 minutes.When using a fixed angle rotor, orient the device with the membrane panel facing up and spin at 5,000 × g maximum for approximately 15–60 minutes.NOTE: Refer to Figures 1 and 2, and Tables 1 and 2 for typical spin times.4. To recover the concentrated solute, insert a pipettor into the bottom of the filter device and withdrawthe sample using a side-to-side sweeping motion to ensure total recovery. The ultrafiltrate can be stored in the centrifuge tube.NOTE: For optimal recovery, remove concentrated sample immediately after centrifugation. Desalting or DiafiltrationDesalting, buffer exchange, or diafiltration are important methods for removing salts or solvents in solutions containing biomolecules. The removal of salts or the exchange of buffers can be accomplished in the Amicon® Ultra-15 device by concentrating the sample, then reconstituting the concentrate to the original sample volume with any d esired solvent. The process of “washing out” can be repeated until the concentration of the contaminating microsolute has been sufficiently reduced. See example below.1 mg/mL100 mMNaCl200 μL of75 mg/mLprotein inNaCl200 μL of75 mg/mLprotein inNaClAdd 14.8 mL of10 mM NaClor exchangebufferSpin conditions: 4,000 × g, room temperature, 15 mL starting volume.Protein markers used: Cytochrome c for 3K and 10K, BSA for 30K and 50K, and IgG for 100K, n=6.Spin conditions: 5,000 × g, room temperature, 12 mL starting volume.Protein markers used: Cytochrome c for 3K and 10K, BSA for 30K and 50K, and IgG for 100K, n=6.PerformanceFlow RateFactors affecting flow rate include sample concentration, starting vol u me, chemical nature of solute, relative centrifugal force, centrifuge rotor angle, membrane type, and temperature. Figures 1 and 2, and Tables 1 and 2 can be used to estimate the time required to achieve a given volume of filtrate orconcentrate for a variety of protein markers. A typical spin time for a 15 mL sample is approximately 15 to 60 minutes (depending on device nominal molecular weight limit). While most of the sample is filtered in the first 15 to 30 minutes of centrifugation, the lowest concentrate volume (150–300 μL) is reached after spinning for 15 to 60 minutes.Flowrate, continuedTable 1. Typical Concentrate Volume vs. Spin Time (Swinging bucket rotor)Spin conditions: 4,000 × g, room temperature, 15 mL starting volume. Protein markers used: Cytochrome cfor 3K and 10K, BSA for 30K and 50K, and IgG for 100K, n=6 (mean value of 3 membrane lots). Shaded volumes were used for the calculation of protein recovery in Table 4.Table 2. Typical Concentrate Volume vs. Spin Time (Fixed angle rotor)Spin conditions: 5,000 × g, room temperature, 12 mL starting volume. Protein markers used: Cytochrome cfor 3K and 10K, BSA for 30K and 50K, and IgG for 100K, n=6 (mean value of 3 membrane lots). Shaded volumes were used for the calculation of protein recovery in Table 4.Protein Retention and Concentrate RecoveryThe membranes used in Amicon® Ultra devices are characterized by a nominal molecular weight limit (NMWL); that is, their ability to retain molecules above a specified molecular weight. Solutes with molecular weights close to the NMWL may be only partially retained. Membrane retention depends on the solute’s molecular size and shape. For most applications, molecular weight is a convenient parameter to use in assessing retention characteristics. Merck Millipore Ltd. recommends using a membrane with a NMWL at least two times smaller than the molecular weight of the protein solute that one intends to concentrate. Refer to Table 3.Table 3. Typical Retention of Protein Markers Spin Conditions for Tables 3 and 4: Swinging bucket rotor (4,000 × g, 15 mL starting volume), or fixed angle rotor,(5,000 × g, 12 mL starting volume), room temperature, n=6 (mean value of 3 membrane lots).Protein Retention and Concentrate Recovery, continuedFactors that determine sample recovery include the nature of the protein solute relative to the device NMWL chosen, starting concentration, and concentration factor. Table 4 provides typical recoveries forAmicon® Ultra-15 devices.Table 4. Typical Concentrate Recovery Concentrate Concentration Concentrate Shaded volumes were taken from Tables 1 and 2.How to Quantify RecoveriesCalculate total recovery, percent concentrate recovery, and percent filtrate recovery using the method below. The procedure provides a close approximation of recoveries for solutions having concentrations up to roughly 20 mg/mL.NOTE : Appropriate assay techniques include absorption spectrophotometry, radioimmunoassay, refractiveindex, and conductivity.Direct Weighing ProcedureThe density of most dilute proteins is nearly equal to the density of water (i.e., 1 g/mL). Using this property, the concentrate and filtrate volumes can be quantified by weighing them and converting the units from grams to milliliters. This technique is valid only for solutions with concentrations of approximately 20 mg/mL or less.1. Before use, separately weigh the empty filter device, the centrifuge tube, and an empty tube forconcentrate collection.2. Fill filter device with solution and reweigh.3. Assemble device and centrifuge per instructions.4. Collect the concentrate with a pipettor and dispense it into the preweighed concentrate collectiontube.5. Remove the device from the centrifuge tube and weigh the centrifuge tube and concentrate collectiontube.6. Subtract weight of empty device/tubes to calculate weights of starting material, filtrate, andconcentrate.7. Assay the starting material, filtrate, and concentrate to determine solute concentration.8. Calculate recoveries using the weight/volume data and the measured concentrations as follows:Maximizing Sample RecoveryLow sample recovery in the concentrate may be due to adsorptive losses, over-concentration, or passage of sample through the membrane.●Adsorptive losses depend upon solute concentration, its hydrophobic nature, temperature and timeof contact with filter device surfaces, sample composition, and pH. To minimize losses, remove concentrated samples immediately after centrifugal spin.●If starting sample concentration is high, monitor the centrifugation process in order to avoid over-concentration of the sample. Over-concentration can lead to precipitation and potential sample loss. ●If the sample appears to be passing through the membrane, choose a lower NMWL Amicon® Ultra-15device.Direct Weighing Procedure, continued% concentrate recovery = 100 × % filtrate recovery = 100 × % total recovery = % concentrate recovery + % filtrate recovery W c = total weight of concentrate before assay W o = weight of original starting material W f = weight of filtrate C c = concentrate concentrationC o = original starting material concentration C f = filtrate concentrationW c × C c W o × C oW f × C fW o × C oSpecificationsMaximum initial sample volumeSwinging bucket 15.0 mL Fixed angle rotor 12.0 mL Typical final concentrate volume 150–300 μL Maximum relative centrifugal forceSwinging bucket rotor 4,000 × g Fixed angle rotor 5,000 × g Active membrane area 7.6 cm 2DimensionsFilter device in tube (capped)Length: 122 mm (4.8 in.)Diameter: 29.7 mm (1.2 in.)Filter deviceLength: 72.0 mm (2.8 in.)Diameter: 29.6 mm (1.2 in.)Materials of ConstructionFilter device Copolymer styrene/butadiene Membrane Ultracel® low binding regenerated cellulose Filtrate tube Polypropylene Filtrate cap and liner PolyethyleneChemical CompatibilityAmicon® Ultra centrifugal devices are intended for use with biological fluids and aqueous solutions. Before use, check the sample for chemical compatibility with the device.Table 5. Chemical Compatibility of Amicon® Ultra Filter DevicesAcids Concentration ConcentrationAcetic acid ≤ 50%*Phosphoric acid ≤ 30%Formic acid ≤ 5%*Sulfamic acid≤ 3% Hydrochloric acid ≤ 1.0 M Sulfuric acid ≤ 3%Lactic acid ≤ 50%Trichloroacetic acid (TCA)≤ 10%*Nitric acid ≤ 10%Trifluoroacetic acid (TFA)≤ 30%*AlkalisAmmonium hydroxide ≤ 10%Sodium hydroxide ≤ 0.5 M Alcoholsn-Butanol ≤ 70%Isopropanol ≤ 70%Ethanol ≤ 70%Methanol ≤ 60% DetergentsAlconox® detergent ≤ 1%Sodium dodecyl sulfate (SDS)≤ 0.1% CHAPS detergent≤ 0.1%Tergazyme®detergent ≤ 1%Lubrol® PX detergent≤ 0.1%Triton® X-100 surfactant≤ 0.1% Nonidet™ P-40 surfactant≤ 2%Tween® 20 surfactant≤ 0.1%Sodium deoxycholate≤ 5%Organic solventsAcetone not recommended Ethyl acetate not recommended Acetonitrile ≤ 20%Formaldehyde ≤ 5% Benzene not recommended Pyridine not recommended Carbon tetrachloride not recommended Tetrahydrofuran not recommended Chloroform not recommended Toluene not recommended Dimethyl sulfoxide (DMSO)≤ 5%*MiscellaneousAmmonium sulfate Saturated Phenol≤ 1%Diethyl pyrocarbonate ≤ 0.2%Phosphate buffer (pH 8.2)≤ 1 M Dithiothreitol (DTT)≤ 0.1 M Polyethylene glycol≤ 10% Glycerine≤ 70%Sodium carbonate ≤ 20% Guanidine HCl ≤ 6 M Tris buffer (pH 8.2)≤ 1 M Imidazole≤ 100 mM Urea ≤ 8 M Mercaptoethanol≤ 0.1 M* Contact with this chemical may cause materials to leach out of the component parts. Solvent blanks are recommended to determine whether leachables represent potential assay interferences.In Vitro Diagnostic Product LabelingThe following table defines the symbols found on Amicon® Ultra-15 10K device labels.Product Ordering InformationThis section lists the catalogue numbers for Amicon® Ultra Ultrafiltration Devices. See the Technical Assistance section for contact information. You can purchase these products on-line at/products.* Amicon® Ultra-4 and -15 10K devices are for in vitrodiagnostic use. All other devices are for research use only.11Amicon® Ultra-15 Centrifugal Filter Devices NoticeThe information in this document is subject to change without notice and should not be construed as a commitment by Merck Millipore Ltd. (“Millipore”) or an affiliate. Neither Merck Millipore Ltd. nor any of its affiliates assumes responsibility for any errors that may appear in this document.Technical AssistanceFor more information, contact the office nearest you. Up-to-date world-wide contact information is available on our web site at /offices. You can also visit the tech service page on our web site at /techservice.Standard WarrantyThe applicable warranty for the products listed in this publication may be found at /terms (within the “Terms and Conditions of Sale” applicable to your purchase transaction).The M logo is a trademark of Merck KGaA, Darmstadt, Germany. Millipore, Amicon, Milli-Q, and Ultracel are registered trademarks of Merck KGaA. Alconox and Tergazyme are registered trademarks of Alconox, Inc. Lubrol is a registered trademark of Imperial Chemical Industries PLC. Nonidet is a trademark of Royal Dutch/Shell Group. Triton is a registered trademark of Union Carbide Corp. Tween is a registered trademark of ICI Americas Inc.© 2012 EMD Millipore Corporation. All rights reserved.PR03520TR, Rev. A, English, 03/12Made in IrelandMerck Millipore Ltd. Tullagreen, Carrigtwohill, Co. Cork, IRL。
Amicon Pro Affinity Concentration Kit - GST 产品说明书

Amicon® Pro AffinityConcentration Kit - GSTPurification of GST-tagged recombinant proteins.Catalog Nos. ACR5000GS, ACK5003GS, ACK5010GS, ACK5030GS,ACK5050GS, ACK5100GSFOR RESEARCH USE ONLYNot for use in diagnostic procedures.USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209Australia +61 3 9839 2000IntroductionThe expression and purification of recombinant proteins is central to protein regulation, structure and function studies. Purification has been greatly facilitated by the addition of small affinity tags to protein sequences such as the glutathione S-transferase domain (GST). GST agarose resin is an affinity chromatography matrix, when used in concert with the Amicon®Pro Affinity Concentration device, offers rapid purification of GST fusion proteins, native GST, or glutathione-binding proteins. GST-tagged proteins bind specifically to reduced glutathione in near-neutral, non-denaturing conditions (e.g., phosphate buffer). Following resin capture of the target protein, unbound lysate components are removed by spin-based clearing and washing steps. Bound protein is recovered by centrifugation following competitive displacement with buffer containing free, reduced glutathione.By condensing the entire purification workflow into one device, the Amicon® Pro device eliminates the need for multiple sample transfers thereby minimizing protein loss. The large exchange device reservoir (up to 9ml) accommodates a range of sample capacities as well as reduces the need for multiple spin steps during the wash and elution phases. Direct coupling to an Amicon® Ultra-0.5 device further provides simultaneous concentration during the elution phase. Lastly, the Amicon® Pro device offers highly efficient diafiltration in a single 15 minute spin.The kit contains sufficient GST resin, optimized buffers, and Amicon® Pro devices for 12 standard reactions.Sample Preparation GuidelinesOptimizing lysis parameters is critical for protein extraction and downstream purification. The Amicon®Pro Affinity Concentration GST kit is compatible with a range of conditions, including reducing agents (β-mercaptoethanol, DTT), chelating agents (EDTA), non-ionic detergents, and BugBuster®Protein Extraction Reagent (Cat. No. 70584), a proprietary mixture of non-ionic detergents offering a rapid cost-effective alternative to mechanical cell lysis methods such as sonication.Irrespective of extraction method, we recommend inclusion of rLysozyme™ Solution (Cat. No.71110) and Benzonase®Nuclease (Cat. No. 70746) and during protein extraction for increased cell lysis (protein yield) and a reduction in sample viscosity (improved sample handling), respectively.Protease inhibitors may also be added to the lysis buffers to protect against degradative enzymes.A listing of individual protease inhibitors and cocktails is available at /search on key words “Protease Inhibitor.” Note: Serine proteases should be used with caution if the GST fusion tag is to be cleaved via Thrombin or Factor Xa digestion. If used during protein extraction, we strongly recommend the eluted fraction be buffer exchanged prior to performing the cleavage reaction.In certain systems, high protein expression can lead to aggregation in the form of inclusion bodies.Strong denaturants such as 6 M guanidine or 8 M urea can be used to solubilize aggregates greatly enhancing yield. However, only properly folded, functional GST is capable of binding GST resin. GST fractions recovered from denaturation of inclusion bodies must be refolded to reconstitute active GST fusion constructs. To a degree, this can be accomplished through use of the Protein Refolding Kit (Cat. No. 70123).Cat. No. BWEGSFor more information, consult the GST•Bind™ Resin User Guide available at /chemicals. Search using the Cat. No. 70541.Kit ComponentsCS211421-GST Resin (3 mL) – Crosslinked agarose with immobilized reduced glutathione supplied as 50% slurry in 50 mM Phosphate Buffer pH 7.5, 0.15 M NaCl, 0.1% NaN3. The resin utilizes an 11-atom spacer arm (~16 Å) for covalent attachment of reduced glutathione via a sulfide linkage. The binding capacity is typically 5-8 mg/mL of settled resin.Buffers:o CS211416-10X GST Bind/Wash Buffer (25 mL) – 43 mM Na2HPO4, 14.7 mM KH2PO4,1.37 M NaCl, 27 mM KCl pH7.2o CS211354-10X Glutathione Reconstitution buffer (5 mL) – 500 mM Tris, pH 8.0o CS211418-Glutathione, reduced, free acid (125 mg)Amicon®Pro Devices – The kit includes 12 complete assemblies. Each device consists of an exchange device, holder tube, 50 mL collection tube, and Amicon®Ultra-0.5 centrifugal filter device. A 2 mL collection tube is included for sample recovery from the AU-0.5 device by reverse spin. The kit is available in five formats based on the nominal molecular weight limit (NMWL) of the AU-0.5: 3 (ACK5003GS), 10 (ACK5010GS), 30 (ACK5030GS), 50 (ACK5050GS), and 100 kDa (ACK5100GS). Consult the Amicon®Pro (/psp, search keywords “Amicon Pro”) and Amicon®Ultra-0.5 centrifugal filter device User Guides (/catalogue/module/c82301) for proper assembly/disassembly and additional product information.All reagents should be stored at 2° to 8°C (do not freeze). The Amicon Pro devices can be stored separately at room temperature.Procedures for using the Amicon® Pro Affinity Concentration Kit - GSTThe protocol is based on purification of GST-tagged protein from 1 mL of E. coli lysate using 200 µl of GST resin slurry (100 µL packed resin). The protocol is linearly scalable for 50-1000 µL of resin slurry. Due to large variability among sample preps, parameters which may require optimization include bead input, binding time, wash, and elution parameters. This protocol includes steps for simultaneous concentration during the elution step as well as buffer exchange using the Amicon®Ultra-0.5 centrifugal filter device.Note: Given the collection tube’s capacity, it is not necessary to remove filtrate between the various centrifugation steps. However, if process samples need be retained for analytical purposes, the collection tube should be cleared.Buffer Preparation10X Bind/Wash Buffer should be diluted to 1X with sterile deionized H2O; 1X Bind/Wash solution may be stored at 4°C for 1 week.Prepare 10X GST Elution Buffer containing 100 mM reduced glutathione as follows: add 4ml 10X Reconstitution buffer directly to the glutathione powder vial. Pipette repeatedly then incubate for30 minutes at room temperature. Divide buffer into working volumes and store at -20o C (400Cat. No. BWEGSµl/standard reaction); 10X GST Elution Buffer is stable for 6 months at -20o C. We do not recommend repeated freeze/thaw cycles. To minimize glutathione oxidation, dilute 10X buffer to 1X using sterile deionized water and pH to 8.0 immediately before use. Discard any unused 1X Elution Buffer.Bead Preparation1. To ensure uniform suspension, vortex the GST resin thoroughly before adding it to the device.2. Remove the collection tube cap and open the exchange device cap.3. Add 200 µL of resin slurry to the base of the exchange device. Close the exchange cap.Up to 500 µl packed resin (1000 µl slurry volume) may be added per device We recommend using wide-bore tips (Cat. No. 02-707-134, Fisher Scientific) for resin transfer.4. To remove storage buffer, centrifuge in a swinging bucket rotor at 1000 g X 1 min.5. Add 500 µL of 1X Bind Buffer. Centrifuge at 1000 g X 1 min.Protein Binding1. Add 500 µL of sample to the exchange device.Up to 9 mL of sample can be added. The volume loaded is determined by the target protein’s expression level and resin’s binding capacity.2. Incubate for 60 min at room temp with gentle agitation.We recommend upright agitation on a plate shaker at low setting.End-over-end mixing, particularly with small volumes or for extended time, may result in substantial bead loss to the sides of the feeder tube.The duration of binding time may vary with application.3. Centrifuge the device at 1000 g X 1 min in a swinging bucket rotor. Recover the sample flow-through from the 50 mL collection tube (optional).To ensure maximal protein capture, collect all resin into solution prior to centrifugation.4. Add1.5 mL of Wash Buffer. Centrifuge at 1000 g X 1 min. Recover the wash fraction from the 50mL collection tube (optional).Due to the large capacity of the exchange device, the volume of the wash can be increased for greater sample purity. There is no need for multiple wash steps.Sample ElutionSamples can be eluted without concentration by adding elution buffer and centrifuging (1000g X 2 minute) directly into a clean 50 ml collection tube. Given the limited volume processing capacity of the AU-0.5 device, we recommend this protocol if elution volumes > 1.5 ml are required.For simultaneous elution with concentration, attach the Amicon® Ultra-0.5 device and follow the steps outlined below.1. Remove the exchange device and insert it into the AU0.5 device.2. Place the exchange device/AU-0.5 assembly back in the holder and return the device to thecollection tube.3. Add up to 1.5 mL of Elution Buffer, gently resuspend the resin, and incubate for 5 min.Under standard conditions, one elution is sufficient for recovery of 90-95% of captured protein.4. Close the exchange device cap and screw on the collection tube cap to ensure a proper seal.5. Centrifuge at 4000 g X 15 min in a swinging bucket rotor. Concentrated samples can be bufferexchanged or recovered from the AU-0.5 device by reverse spin (see below).Cat. No. BWEGSDepending on the starting elution volume, NMWL of AU0.5 device employed, and the degree of concentration desired, the length of the spin time can range for 10-30 minutes. Please consult the Performance Characteristics section in the Amicon®Pro Affinity Concentration System User Guide (/psp, and search keywords "Amicon Pro”) for recommended guidelines., consult.6. Recover the concentrated fraction by reverse spin or proceed to Buffer Exchange (see below).Buffer Exchange (Optional if samples have been collected in the Amicon® Ultra-0.5 device)1. After sample concentration, add 1.5ml desired buffer to the exchange device/AU-0.5 assembly.2. Centrifuge device at 4000g X 15 minutes in a swinging bucket rotor. Concentrated samples canbe recovered from the AU-0.5 device by reverse spin (see below).Collect sample from the AU0.5 device by Reverse Spin(following Concentration or Buffer Exchange)1. Disassemble the exchange device/AU-0.5 assembly from the holder tube.2. Using a gentle twisting motion, detach the AU-0.5 from the exchange device.3. If there is residual sample in the exchange device tip, depress the exchange device cap to expelthe remaining sample volume into the AU-0.5.4. Hold AU-0.5 upright and slide the 2 ml collection tube on top of it.5. Invert the assembly and centrifuge (in a microcentrifuge) with a fixed angle rotor 1000g X 2min.Sample protein yield can be determined by Mid IR-based spectrometry using the DirectDetect™ biomolecular quantitation system and DirectDetect™ Assay Free Sample Cards.TroubleshootingCat. No. BWEGSIssue: Recombinant Protein is present in low amount in eluatePossible Cause SolutionProtein is insoluble and may have formed inclusion bodies. After lysate clearance, check both the supernatant and pellet for protein. Perform lysis and binding procedures under denaturing conditions.The GST fusion protein is not in the proper three dimensional conformation. Attempt to reconstitute native protein structure using Protein Refolding Kit (Cat. No. 70532).The GST-tag is not exposed for binding to the affinityresin.The protein may require denaturing conditions for binding.The GST-tag is not present. Sequence the ligation junctions to ensure that the readingframe is correct. Check for possible internal translation starts(N-terminal tag) or premature termination sites (C-terminal tag). Recombinant protein is degraded during cell lysis. Add protease inhibitors during cell lysis.Protein forms aggregates. Add solubilizing agents such as detergents (0.1% Triton® X-100, TWEEN®-20) or increase salt concentration.pH of the Lysis or Binding buffers is incorrect. Check buffer pH; the acceptable range is 7-8. Acidic bufferswill prevent binding.Protein expression is insufficient. Optimize the growth/induction conditions.Cell Lysis is incomplete. Optimize the Cell Lysis Protocol.Cell Lysate is too viscous. If possible, dilute the lysate in Bind Buffer. Alternatively,include Benzonase® Nuclease during lysis to remove freeRNA/DNA.Protein precipitates during sample concentration while using the AU0.5 device due to over-concentration. Reduce the duration of the centrifugation time during the elution/concentration step.Protein is lost during sample concentration while using AU0.5 device. Check the protein's expected size and MWCO of AU0.5 device used. AU0.5 device is offered in 5 different MWCO formats - 3, 10, 30, 50, and 100 kDa.Issue: High Non-specific bindingPossible Cause SolutionInsufficient washing. Increase the volume of the Wash Buffer used or the number ofwash steps. Alternatively, supplement the Bind/Wash Buffers.Contaminants interact directly with the GST fusion protein. Add reducing agents such as DTT or β-mercaptoethanol to reduce disulfide bonds. Add detergents to disrupt non-specific interactions.GST fusion protein is degraded. Degraded/truncated forms of the recombinant protein will stillbind to the GST resin and appear as contaminating bands inSDS-PAGE. Perform a lysis procedure on ice and includeprotease inhibitors.Cell lysate is too concentrated. Dilute the lysate in Bind Buffer before purification.Cat. No. BWEGSCat. No. BWEGSPerformanceProduct Ordering InformationNo Devices Amicon ® Pro + AU 0.5 ml with MWCO: 3k 10k 30k 50k 100k Amicon ® Pro Affinity Concentration Kit - GSTACR5000GS ACK5003GS ACK5010GS ACK5030GS ACK5050GS ACK5100GS Amicon ® Pro AffinityConcentration System 12PKACS500312 ACS501012 ACS503012 ACS505012 ACS510012 Amicon ® Pro AffinityConcentration System 24PK ACS500324 ACS501024 ACS503024 ACS505024 ACS510024The Amicon ® Pro Affinity Concentration Kit contains reagents and devices sufficient for 12 standard reactions. Amicon ® Pro devices are also sold separately in 12 and 24 packs.Description Catalogue Number Qty/Pack GST•Bind™ Resin 70541-3/4/5 10/50/25 mL GST•Bind™ Buffer Kit 70534 1 KitPurification using Amicon Pro Affinity Concentration Kit – GST purifications of a 50 kDa GST-tagged protein the Amicon ® Pro Affinity Concentration Kit numbered lanes are: 2 – flowthrough fraction 3 – wash fraction 4 – eluted fractionNoticeThe information in this document is subject to change without notice and should not be construed as a commitment by EMD Millipore Corporation (“Millipore”) or an affiliate. Neither EMD Millipore nor any of its affiliates assumes responsibility for any errors that may appear in this document. Technical AssistanceFor more information, contact the office nearest you. Up-to-date world-wide contact information is available on our web site at /offices. You can also visit the tech service page on our web site at /techserves.WarrantyEMD Millipore Corporation(“EMD Millipore”) warrants its products will meet their applicable published specifications when used in accordance with their applicable instructions for a period of one year from shipment of the products. EMD MILLIPORE MAKES NO OTHER WARRANTY, EXPRESSED OR IMPLIED. THERE IS NO WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. The warranty provided herein and the data, specifications and descriptions of EMD Millipore products appearing in EMD Millipore’s published catalogues and product literature may not be altered except by express written agreement signed by an officer of EMD Millipore. Representations, oral or written, which are inconsistent with this warranty or such publications are not authorized and if given, should not be relied upon.In the event of a breach of the foregoing warranty, EMD Millipore Corporation’s sole obligation shall be to repair or replace, at its option, the applicable product or part thereof, provided the customer notifies EMD Millipore Corporation promptly of any such breach. If after exercising reasonable efforts, EMD Millipore Corporation is unable to repair or replace the product or part, then EDM Millipore shall refund to the Company all monies paid for such applicable Product. EMD MILLIPORE CORPORATION SHALL NOT BE LIABLE FOR CONSEQUENTIAL, INCIDENTAL, SPECIAL OR ANY OTHER DAMAGES RESULTING FROM ECONOMIC LOSS OR PROPERTY DAMAGE SUSTAINED BY ANY COMPANY CUSTOMER FROM THE USE OF ITS PRODUCTS.Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.(c) 2009 - 2012: Merck KGaA, Darmstadt. All rights reserved. No part of these works may be reproduced in any form without permission in writing.Benzonase®, BugBuster®, and Amicon® are registered trademarks of Merck KGaA, Darmstadt, Germany. DirectDetect™ and GST•Bind™ is a trademark of Merck KGaA, Darmstadt, Germany. Triton® is a registered trademark of Dow Chemical Company. Tween® is a registered trademark of ICI Americas, Inc.Cat. No. BWEGS。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Contents
Introduction. .........................................................................................................................................................1 Applications..........................................................................................................................................................2 Materials Supplied................................................................................................................................................3 Required Equipment. ...........................................................................................................................................4 Suitability..............................................................................................................................................................4 Device Storage.....................................................................................................................................................4 Specifications.......................................................................................................................................................5 Chemical Compatibility.........................................................................................................................................7 How to Use Amicon Ultra-4 Centrifugal Filter Devices..........................................................................................9 How to Quantitate Recoveries............................................................................................................................10 Performance - DNA Concentration.....................................................................................................................13 Performance - Protein Concentration.................................................................................................................14 Flow Rate...................................................................................................................................................14 Protein Retention and Concentrate Recovery............................................................................................19 Maximizing Sample Recovery............................................................................................................................22 Desalting or Diafiltration.....................................................................................................................................23 Centrifugal Product Ordering Information...........................................................................................................24 Technical Assistance..........................................................................................................................................25 In Vitro Diagnostic Product Labeling...................................................................................................................26 Standard Warranty.............................................................................................................................................27
Introduction
Millipore’s Amicon® Ultra-4 centrifugal filter devices provide fast ultrafiltration, with the capability for high concentration factors and easy concentrate recovery from dilute and complex sample matrices. The vertical design and available membrane surface area provide fast sample processing, high sample recovery (typically greater than 90% of dilute starting solution), and the capability for 80-fold concentration. Typical processing time is 10 to 40 minutes depending on Nominal Molecular Weight Limit (NMWL). Solute polarization and subsequent fouling of the membrane are minimized by the vertical design, and a physical deadstop in the filter device prevents spinning to dryness and potential sample loss. The concentrate is collected from the filter device sample reservoir using a pipettor, while the ultrafiltrate is collected in the provided centrifuge tube. The device can be spun in a swinging bucket (for optimal performance) or fixed angle rotor. Amicon Ultra-4 devices are supplied non-sterile and are for single use only.
Notice
The information in this document is subject to change without notice and should not be construed as a commitment by Millipore Corporation or an affiliated corporation. Neither Millipore Corporation nor any of its affiliated corporations assumes responsibility for any errors that may appear in this document. This manual is believed to be complete and accurate at the time of publication. In no event shall Millipore Corporation be liable for incidental or consequential damages in connection with or arising from the use of this manual. © 2009 MILLIPORE CORPORATION. ALL RIGHTS RESERVED. PR02843 Rev. B, 10/09
