药品包装说明书-en
药品包装材料、标签、说明书

药品包装材料、标签、说明书药品包装材料、标签、说明书一、药品包装材料1.包装材料的种类和特性●塑料瓶:透明、具有一定的耐温性和防湿性,耐酸碱性强。
●铝塑泡罐:具有良好的密封性和防湿性,可以有效保护药品质量。
●玻璃瓶:透明、耐高温,对药品的稳定性有较好的保护作用,但易破碎,较重。
●泡腾片管:适用于泡腾片类型的药品包装,具有良好的密封性和防湿性。
●铝箔袋:耐光、防潮,适用于胶囊、颗粒药品的包装。
2.包装材料的选择标准●药品特性:根据药品的特性选择合适的包装材料,如对光线敏感的药品应选择不透光的包装材料。
●包装要求:根据药品的保存条件和运输特点选择合适的包装材料,如高温区域应选择耐高温的材料。
●安全性:包装材料应符合国家相关标准,不会对药品产生污染或质量变化。
●经济性:在满足药品质量要求的前提下,选择价格适中的包装材料。
二、药品标签1.标签内容●药品名称:标明药品的通用名称和商品名。
●规格和剂型:标示药品的规格(如单位含量、容量、重量等)和剂型(如片剂、颗粒剂等)。
●批号:用于追溯药品生产过程和质量监控。
●生产日期和有效期:标示药品的生产日期和有效使用期限。
●使用说明:包括适应症、用法用量、不良反应、注意事项等信息。
●生产厂商:标明药品生产厂商的名称和联系方式。
2.标签设计要求●易读性:标签上的文字和图案应清晰易读,字体大小和颜色应符合国家标准。
●耐久性:标签应具有耐久性,不易褪色、破损或掉落。
●清晰标识:标签上的信息应清晰标识,避免混淆或误解。
●尺寸要求:标签的尺寸应符合国家相关标准,能够容纳必要的信息并保证易读性。
三、药品说明书1.内容要求●药品通用名称和商品名●成分和药理作用:详细药品的成分和作用机制。
●适应症:说明药品所适用的疾病和症状。
●用法用量:指导患者正确使用药品的剂量和使用方法。
●禁忌症:使用药品的禁忌症,避免不适当的使用。
●副作用和不良反应:详细介绍可能出现的副作用和不良反应,并提供相应的应对措施。
包装文字说明

为全球女性的健康美丽而奋斗终生西安新森生物科技有限责任公司
清宫丸
『产品名称』清宫丸
『主要成份』苦参、血竭、冰片、丁香、黄连、硼砂、莪术、青黛、石膏、儿茶、百部等。
『保健作用』本品为外用保健用品,具有清热解毒、活血通经、杀虫止痒的保健作用。
对女性阴部不适具有一定的保护作用。
『用法用量』洗净阴部,擦干,用指套将丸剂送入阴道深处,线头留在阴道外部每次1丸,3天更换一次。
『注意事项』
①本品只能一次性使用,不得重复使用(中途取出,必须更新);
②处女、妊娠期、哺乳期禁用,经期停用;
③避免接触眼睛,切忌口服;
④使用期如需同房,须取出丸剂冲洗阴道。
有严重妇科疾患者使用时禁止同房;
⑤个别使用者可能出现外阴痒和痛的现象均属正常反应,可停用1-2天,同时洗剂清洗外阴,症状消失后,可继续使用;
⑥使用1-2粒后有皮屑或污血块排出,属于正常排毒现象,请放心继续使用
⑦本品不能代替药物,过敏者慎用。
『规格』1.0 g/丸
『贮藏』密封,避光,置于35oC以下阴凉干燥处保存。
『有效期』两年
『中国政府批准文号』陕卫消证字(2009)第0299号
『执行标准』Q/XAXS0029-2009
『生产单位』西安新森生物科技有限责任公司
『生产地址』西安市未央区三桥街办蔺高工业区水产路3号。
中英对照药品说明书

一、药品英文说明书的结构简介“药品说明书”的英文表达方式有Instructons,Directions,Descriiption 现在多用Package Insert,或简称Insert,也有用Leeflet或Data Sheets.Insert原意为“插入物,插页”。
药品说明书即为附在每种药品包装盒中的一份用药说明。
经过注册的进口药品一般是国家承认的有效药物,其说明书是指导医生与患者合理用药的重要依据,具有一定的法律效力。
进口药的英文说明书随药品来源的不同,有以英语为母语的国家,也有以英语为外语的国家。
说明书繁简难易不同。
短者仅百余词,长者可达上万词。
较简单的悦明书仅介绍成分、适应症、禁忌症、用法与用量等内容;较详尽的说明书中除上述内容外还包括:药品性状、药理作用、临床药理、临床前动物试验、临床经验、药代动力学、庄意事项、不良反应或副作用、用药过量、药物的相互作用、警告、有效期、包装、贮存条件、患者须知及参考文献等诸多项目。
为了顺利阅读和正确翻译进口药英文说明书,读者除应具备较好的英语基础,掌握一定的专业知识(如医学、化学、药剂学、药理学、药物代谢动力学等)外,还应熟悉英文药品说明书的结构及语言待点等。
大多数英文说明书都包括以下内容;①药品名称(Drug NameS),②性状(Descriiption),③药理作用(Pharmacological Actions),④适应症(Indications),⑤禁忌证(Contraindications),⑥用量与用法(DOsage and Administration).⑦不良反应(Adverse Reactions)。
⑧注意事项(Precautions),⑨包装(Package),⑩贮存(Storage),⑾其他项目(Others)。
现将各项专题的表述方法与翻译、结构特点、常用词语及阅读技巧等分述如下。
二、药品名称英文药品说明书中常见的药品名称有商品名(Trade Name或Proprietary Name),通用名(Generic Name)和化学名(Chemical Name),其中最常见的是商品名。
EN 868-5中文翻译版

EN 868-5:1999待灭菌医疗器械包装材料和系统第5部分:纸与塑料膜组合的热封和自封袋和卷要求和试验方法引言本系列欧洲标准的第1部分规定了预期用作医疗器械包装的包装材料和系统的通用要求和试验方法。
这些医疗器械最终在其包装内灭菌。
1 范围EN 868的本部分规定了用符合EN 868-3规定的纸和符合本部分第4章规定的塑料膜制造的热封和自封袋的专用要求和试验方法。
4.2至4.7中的专用要求可用以证实符合第1部分的一项或多项要求,但不是其全部要求。
本标准规定的热封和自封袋和卷适用于包装最终灭菌的医疗器械。
热封和自封袋和卷用作初包装能使使用者用前方便地无菌观察内装物,这一点非常重要。
2 规范性引用文件EN 285 灭菌蒸汽灭菌大型灭菌器EN 867-2 灭菌器中使用的非生物学系统第2部分:过程批示物(A级)EN 868-1待灭菌医疗器械包装材料和系统第1部分:通用要求和试验方法EN 868-3待灭菌医疗器械包装材料和系统第3部分:袋(EN868-4所规定的)袋和卷(EN868-5所规定的)生产用纸要求和试验方法EN 1422 医用灭菌器环氧乙烷灭菌器要求和试验方法EN 28601数据元和交换格式信息交换日期和时间表示法(ISO 8601:1988和技术修改单1:1991)GB/T 7408-1994数据元和交换格式信息交换日期和时间表示法EQV ISO 8601-88 EQV ISO 8601-88ASTM D 882:1995 塑料膜抗张性能试验方法3定义EN868-1的定义适用于本部分。
4 要求4.1 总则EN868-1的要求适用。
注:下列专用要求和试验方法可用于证实EN868-1的一项或多项要求,但不是全部要求。
4.2 材料4.2.1 纸纸应符合EN 868-3的要求。
4.2.2 塑料膜4.4.2.1 塑料膜应是由两层或多层复合而成。
按附录A试验时,塑料结合层(interplybond)应不发生分离或发白。
药品包装、标签及说明书

CHAPTER 05
药品包装、标签及说明书常 见问题及解决方案
包装破损或变形问题解决方案
总结词
药品包装的完整性和美观性对于产品形象和用户体验至 关重要。包装破损或变形会导致产品品质受损,影响用 户使用和购买决策。
详细描述
药品包装破损或变形问题通常是由于运输过程中挤压、 碰撞或振动导致的。为了解决这个问题,可以采取以下 措施
防伪标识
可在标签上加印防伪标识,以便于消 费者和监管部门识别和验证药品的真 伪。
CHAPTER 03
药品说明书编写规范
说明书基本内容要求
01
药品名称
包括通用名、商品名、英文名等 。
02
03
成分
性状
列出该药品的主要成分,包括活 性成分和非活性成分。
描述药品的外观、颜色、气味等 。
说明书基本内容要求
定期自查和整改
定期对药品包装、标签及说明书进行检查和整改,及时发现和纠正存在的问题,确保药 品质量安全。
监管部门检查要点及应对策略
检查要点
监管部门对药品包装、标签及说明书进行检查时,主要关注药品信息的真实性 、准确性、完整性,以及是否存在虚假、夸大宣传等误导性内容。
应对策略
企业应积极配合监管部门的检查,提供真实、准确的药品信息,及时整改存在 的问题,确保药品质量安全。同时,企业应加强与监管部门的沟通和联系,及 时了解法规政策动态,为企业的合规经营提供有力保障。
透明度
选择透明的包装材料,方 便用户查看药品的外观和 质量。
包装色彩与视觉效果
色彩搭配
01
根据药品的特性和目标受众,选择适当的色彩搭配,提高包装
的视觉效果和吸引力。
图案设计
02
产品包装说明和使用说明书
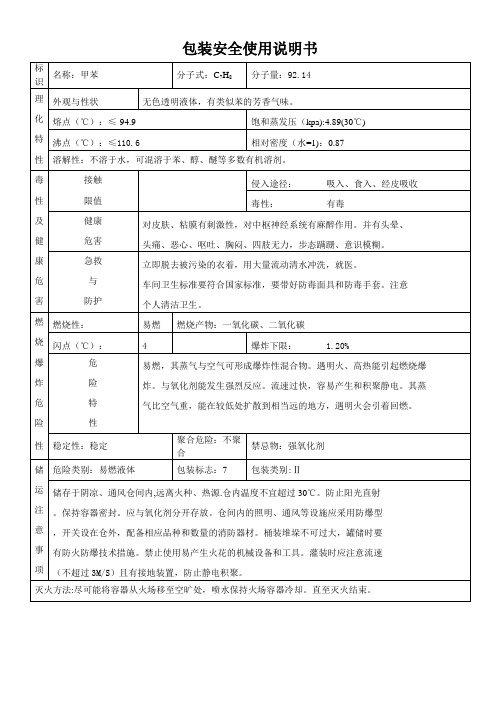
盐酸1. 标识中文名:盐酸;氢氯酸英文名:hydrochloric acid;chlorohydric acid分子式:HCl相对分子质量:36.46CAS号:7647-01-0危险性类别:第8.1类酸性腐蚀品化学类别:无机酸2. 主要组成与性状主要成分:含量工业级36%。
外观与性状:无色或微黄色发烟液体,有刺鼻的酸味。
主要用途:重要的无机化学品,广泛用于染料、医药、食品、印染、皮革、冶金等行业。
3. 健康危害侵入途径:吸入、食入。
健康危害:接触其蒸气或烟雾,可引起急性中毒,出现眼结膜炎,鼻及口腔粘膜有烧灼感,鼻衄,齿龈出血,气管炎等。
误服可引起消化道灼伤、溃疡形成,有可能引起胃穿孔、腹膜炎等。
眼和皮肤接触可致灼伤。
慢性影响:长期接触,引起慢性鼻炎、慢性支气管炎、牙齿酸蚀症及皮肤损害。
4. 急救措施皮肤接触:立即脱去被污染的衣着,用大量流动清水冲洗,至少15分钟。
就医。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。
就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
食入:误服者用水漱口,给饮牛奶或蛋清。
就医。
5. 燃爆特性与消防燃烧性:不燃危险特性:能与一些活性金属粉末发生反应,放出氢气。
遇氰化物能产生剧毒的氰化氢气体。
与碱发生中合反应,并放出大量的热。
具有较强的腐蚀性。
灭火方法:消防人员必须佩戴氧气呼吸器、穿全身防护服。
用碱性物质如碳酸氢钠、碳酸钠、消石灰等中和。
也可用大量水扑救。
6. 泄漏应急处理迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。
建议应急处理人员戴自给正压式呼吸器,穿防酸碱工作服。
不要直接接触泄漏物,尽可能切断泄漏源。
防止进入下水道、排洪沟等限制性空间。
小量泄漏:用砂土、干燥石灰或苏打灰混合。
也可以用大量水冲洗,洗水稀释后放入废水系统,大量泄漏:构筑围堤或挖坑收容;用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
药品包装标签和说明书管理规定(2篇)
药品包装标签和说明书管理规定第一条为加强药品监督管理,规范药品的包装、标签及说明书,以利于药品的运输、贮藏和使用,保证人民用药安全有效,特制定本规定。
第二条药品包装、标签及说明书必须按照国家药品监督管理局规定的要求印制,其文字及图案不得加入任何未经审批同意的内容。
第三条药品包装内不得夹带任何未经批准的介绍或宣传产品、企业的文字、音像及其他资料。
第四条凡在中国境内销售、使用的药品,其包装、标签及说明书所用文字必须以中文为主并使用国家语言文字工作委员会公布的规范化汉字。
第五条药品的通用名称必须用中文显著标示,如同时有商品名称,则通用名称与商品名称用字的比例不得小于1:2,通用名称与商品名称之间应有一定空隙,不得连用。
第六条药品商品名称须经国家药品监督管理局批准后方可在药品包装、标签及说明书上标注。
第七条提供药品信息的标志及文字说明,字迹应清晰易辨,标示清楚醒目,不得有印字脱落或粘贴不牢等现象,并不得用粘贴、剪切的方式进行修改或补充。
第八条药品的包装分内包装与外包装。
(一)内包装系指直接与药品接触的包装(如安瓿、注射剂瓶、铝箔等)。
内包装应能保证药品在生产、运输、贮藏及使用过程中的质量,并便于医疗使用。
药品内包装材料、容器(药包材)的更改,应根据所选用药包材的材质,做稳定性试验,考察药包材与药品的相容性。
(二)外包装系指内包装以外的包装,按由里向外分为中包装和大包装。
外包装应根据药品的特性选用不易破损的包装,以保证药品在运输、贮藏、使用过程中的质量。
第九条药品的标签分为内包装标签与外包装标签。
(一)内包装标签与外包装标签内容不得超出国家药品监督管理局批准的药品说明书所限定的内容;文字表达应与说明书保持一致。
(二)内包装标签可根据其尺寸的大小,尽可能包含药品名称、适应症或者功能主治、用法用量、规格、贮藏、生产日期、生产批号、有效期、生产企业等标示内容,但必须标注药品名称、规格及生产批号。
(三)中包装标签应注明药品名称、主要成分、性状、适应症或者功能主治、用法用量、不良反应、禁忌症、规格、贮藏、生产日期、生产批号、有效期、批准文号、生产企业等内容。
药品包装、标签、说明书共35页文档
36、“不可能”这个字(法语是一个字 ),只 在愚人 的字典 中找得 到。--拿 破仑。 37、不要生气要争气,不要看破要突 破,不 要嫉妒 要欣赏 ,不要 托延要 积极, 不要心 动要行 动。 38、勤奋,机会,乐观是成功的三要 素。(注 意:传 统观念 认为勤 奋和机 会是成 功的要 素,但 是经过 统计学 和成功 人士的 分析得 出,乐 观是成 功的第 三要素 。
39、没有不老的誓言,没有不变的承 诺,踏 上旅途 ,义无 反顾。 40、对时间的价值没有没有深切认识 的人, 决不❖ 丰富你的人生
71、既然我已经踏上这条道路,那么,任何东西都不应妨碍我沿着这条路走下去。——康德 72、家庭成为快乐的种子在外也不致成为障碍物但在旅行之际却是夜间的伴侣。——西塞罗 73、坚持意志伟大的事业需要始终不渝的精神。——伏尔泰 74、路漫漫其修道远,吾将上下而求索。——屈原 75、内外相应,言行相称。——韩非
英文药品说明书结构简介
英文药品说明书结构简介一、英文说明书简介“药品说明书”的英文表达方式:•Instructions •Directions •Description •Leaflet •Data Sheets •Package Insert现多用Package Insert,或简称Insert。
Insert原意为“插入物,插页”。
药品说明书即为附在每种药品包装盒中的用药说明。
经过注册的进口药品一般是国家承认的有效药物,其说明书是指导医生与患者合理用药的重要依据,具有一定的法律效力。
说明书繁简难易不同。
短者仅百余词,长者可达上万词。
较简单的说明书仅介绍成分、适应症、禁忌症、用法与用量等内容;较详尽的说明书中除上述内容外还包括:药品性状、药理作用、临床药理、临床前动物试验、临床经验、药代动力学、庄意事项、不良反应或副作用、用药过量、药物的相互作用、警告、有效期、包装、贮存条件、患者须知及参考文献等诸多项目。
2、意译:按药物名称所表达的含意译成相应的汉语。
如cholic Acid胆酸,Tetracyline四环素。
也可按其药理作用翻译,如Minidiab灭糖尿(治疗糖尿病药物),Natulan疗治癌(细胞生长抑制剂)。
3、音意合译:药物名称中的一部分采用音译,另一部分采用意译,如Coumadin香豆定(coumarin香豆素),Neo-Octin新握克丁(neo-新);Medemycin麦迪霉素(-mycin霉素),Cathinone卡西酮(-one酮)。
4、谐音译意:以音译为原则,选用谐音的汉字,既表音,又表意,音意结合。
例如:Antrenyl安胄灵,Doriden多睡丹,Legalon利肝隆,Webilin胃必灵商品名称可以这样翻洋,而法定名称则规定不可以这样翻译。
药品的化学名称反映出该药品的化学结构组成成分,可借助英汉化学化工词典进行翻译。
如果名称很长,可以分解开来,分别查出各个组成部分的名称,组合而成。
例如:Catalin(卡他林)的化学名称是1-Hvdroxy-5-oxo-5H-pyrido(3,2-a)-Phenoxazine-3-carboxylic acid,译成汉语是1-羟基-5-氧-5H-吡啶开(3,2-a)吩嗪-3-羧酸。
药品包装标签说明书(共19张PPT)
【包装】【有效期】【执行标准】【批准文号】【生产企业】 企业名称: 生产地址: 邮政编码: 电话号码: 传真号码: 注册地址:
明书”字样。 网 址:
对不合格的直接接触药品的包装材料和容器,由药品监督管理部门责令停止使用。
发运中药材必须有【包装孕。 妇及哺乳期妇女用药】【儿童用药】【老年用药】 【药物相互作用】【药物过量】【临床试验】【药理 在每件包装上,必须注明品名、产地、日期、调出单位,并附有质量合格的标志。
药品生产企业不得使用未经批准的直接接触药品的包装材料和容器。
药品通用名称应当显著、突出,其字体、字号和 颜色必须一致,并符合以下要求: (一)对于横版标签,必须在上1/3范围内显 著位置标出;对于竖版标签,必须在右1/3范围
内显著位置标出; (二)不得选用草书、篆书等不易识别的字体,
不得使用斜体、中空、阴影等形式对字体进行修饰; (三)字体颜色应当使用黑色或者白色,与相
意事项】【孕妇及哺乳期妇女用药】毒理】【药代动力学】【贮藏】
禁忌、注意事项、贮藏、生产日期、产品批号、有 【包装】【有效期】【执行标准】【批准文号】【生产企业】 企业名称: 生产地址: 邮政编码: 电话号码: 传真号码: 注册地址:
网 址:
效期、批准文号、生产企业等内容。适应症或者功 药品生产企业不得使用未经批准的直接接触药品的包装材料和容器。
【药品名称】 通用名称: 汉语拼音:【成份】【性状】 【功能主治】/【适应症】【规格】【用法用量】【不 良反应】【禁忌】【注意事项】【孕妇及哺乳期妇女用 药】【儿童用药】【老年用药】【药物相互作用】【临 床试验】【药理毒理】【药代动力学】【贮藏】【包装】 【有效期】【执行标准】【批准文号】【生产企业】
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Article 8 Pharmaceutical packages include inner packages and outer packages.(I) Inner packages refer to packages in immediate contact with drug products (e.g. ampoules, injection bottles, aluminium foils, etc.). Inner packages shall be able to ensure quality of drug products in manufacture, transportation, storage and use and be convenient to use for medical purposes.For changes of inner package materials and containers (pharmaceutical container closure systems) of drug products, stability testing shall be performed depending on material of the used container closure system to investigate compatibility of the container closure system with the pharmaceutical product.(II) Outer packages refer to packages other than inner packages and include middle packages and large packages from the inside to the outside. Rugged packages shall be selected depending on properties of drug products to ensure quality of products in transportation, storage and use.Article 9 Pharmaceutical labels include inner package labels and outer package labels.(I) Contents of inner package labels and outer package labels shall be restricted to those specified in the pharmaceutical directions approved by State Food and Drug Administration; literal expressions shall correspond to the directions.(II) The inner package label shall, depending on the size, address, as far as possible, the drug name, indications or functions, dosage and administration, strength, manufacturing date, lot number, shelf life, manufacturer, etc. The drug name, strength and lot number are items that must be covered.(III) The middle package label shall address the drug name, main ingredients, description, indications or functions, dosage and administration, contraindications, strength, storage, manufacturing date, lot number, shelf life, approval number, manufacturer, etc.(IV) The large package label shall address the drug name, strength, storage, manufacturing date, lot number, shelf life, approval number, manufacturer and other necessary information beyond the description such as quantity in the package, announcements about transportation or other marks. (V) The shelf life on the labels shall be expressed as: Valid to: (year) (month)(VI) Where all adverse reactions, contraindications and announcements cannot be indicated in the middle package label due to size restriction, words “See the description for details”shall be indicated.Article 10 For packages of drug substances, paragraph (I) of Article 8 shall be referred to for implementation and labels shall be made as per provisions for large package labels of drug products.Article 11 The package of each minimum marketing unit of drug products shall be printed or pasted with the label according to the requirements and attached with the direction.Article 12 Pharmaceutical directions shall cover basic scientific information about the drug products such as safety and efficacy.Pharmaceutical directions shall address the following items: drug names (generic name, English name, Chinese pinyin, chemical name), chemical formula, molecular weight, structure formulae (ingredients shall be specified for compound products and biological products) ), description,pharmacology, toxicology, pharmacokinetics, indications, dosage and administration, adverse reactions, contraindications, announcements (administration in pregnant and lactating women, administration in children, drug interactions and other types of interactions with, e.g. smoking, drinking, etc.), overdose (symptoms, emergency measures, detoxicants), shelf life, storage, approval number, manufacturer (including address and contact telephone). If an item is not clearly known, words “Not clearly known” shall be indicated; if indeed no influence may be produced, “No ne” shall be indicated.Pharmaceutical manufacturers shall initiatively follow post-marketing safety and efficacy information of drug products. Where the pharmaceutical direction is to be revised, an application shall be made in time. Directions shall be printed in the unified format (see Annex I and Annex II for the format of directions) and the contents shall be consistent with that approved by State Food and Drug Administration.Article 13 Except units and contents, dosage and administration of drug products shall be described using expressions that can be easily understood such as “×tablets per time, ×times daily”, “×vessels per time, ×times daily”, etc. for guiding correct drug use.Article 14 For drugs under particular management such as narcotics, psychotropics, toxic drugs for medical use and radioactive drugs, drugs for external use, OTC drugs, etc., marks complying with the related requirements shall be printed on the middle package, large package, label and direction; particular requirements for storage, if any, shall be indicated at an eye-catching place on the packages and label and also in the direction.Article 15 To apply for registration of a drug product, the review and approval procedure of the package, label and direction shall be carried out according to the management regulations depending on the drug category. For revising or changing packages, labels or directions of marketed drug products, application and approval shall be performed as per the original application procedure.Article 16 Pharmaceutical manufacturers violating these provisions shall be ordered by the drug regulatory authority or department to change the package, label or direction and recall marketed non-conforming drugs and, at the same time, punished according to applicable provisions of Pharmaceutical Administration Law of the People’s Republic of China and Regulation for the Implementation of Pharmaceutical Administration Law of the People’s Republic of China.Article 17 State Food and Drug Administration shall be responsible for interpreting these provisions.Article 18 These provisions shall enter into force as of January 1, 2001.Annex I:Format of Directions of Chemical Drugs and Biological ProductsDirection of ××××[Drug Name]Generic name:Trade name:English name:Chinese spelling:The principal ingredient and chemical name of this drug:Structural formula:Molecular formula:Molecular weight:(Notes: 1. For compound drug products, it shall be described as: “This drug is a compound product and it is composed of ”;2. For biological products, this item shall be “Principal ingredients”.)[Description][Pharmacology & Toxicology][Pharmacokinetics][Dosage and administration][Adverse reactions][Contraindications][Administration in pregnant and lactating women][Administration in children][Administration in senile patients][Drug interactions][Overdose][Strength][Shelf life][Storage][Approval number][Manufacturer (address, contact telephone)]Provisions on the Administration of Pharmaceutical Directions and Labels(SFDA Order No. 24)Chapter II Pharmaceutical DirectionsArticle 9 Pharmaceutical directions shall cover important scientific data, conclusions and information about safety and efficacy of drugs to guide safe and rational drug use. Detailed format, content and expression requirements of pharmaceutical directions are formulated and issued by the State Food and Drug Administration.Article 10 For expression of disease names, pharmaceutical terms, drug names, and names and results of clinical tests, officially issued or normalized special words and phases shall be used, and weights and measures shall comply with provisions of national standards.Article 11 Pharmaceutical directions shall indicate all active ingredients or all TCM ingredients in the prescription. For injections and OTC drugs, names of all excipients used shall also be indicated.Where the pharmaceutical prescription contains ingredients or excipients that may cause serious adverse reactions, the information shall be indicated.Article 12 Pharmaceutical manufacturers shall initiatively follow post-marketing safety and efficacy information of drugs. Where the pharmaceutical direction is to be revised, an application shall be made in time.According to data on adverse drug reaction monitoring, drug revaluation results, etc., the State Food and Drug Administration may also request pharmaceutical manufacturers to revise pharmaceutical directions.Article 13 After revision of a pharmaceutical direction is approved, the pharmaceutical manufacturer shall notify immediately related drug businesses, users and other departments of the revisions and use revised pharmaceutical direction and label timely following the requirements. Article 14 Pharmaceutical directions shall fully cover information about adverse drug reactions and specify adverse drug reactions. Where the pharmaceutical manufacturer has not timely revised the pharmaceutical direction according to post-marketing safety and efficacy information of the drug or specified adverse drug reactions in the pharmaceutical direction, adverse results incurred thereby shall be assumed by the manufacturer.Article 15 Approval date and revision date of pharmaceutical directions shall be clearly indicated in the pharmaceutical directions.Chapter III Pharmaceutical LabelsArticle 16 A pharmaceutical label means content printed or pasted on a pharmaceutical package and may be an inner label or an outer label. An inner label means the label in immediate contact with the pharmaceutical package, and an outer label means a label of the package other than the inner label.Article 17 An inner pharmaceutical label shall cover items such as generic name, indications or functions, strength, dosage and administration, manufacture date, product lot number, shelf life, manufacturer, etc. of the drug.Where the package size is too small to specify all of the above items, at least generic name, strength, product lot number, shelf life, etc. shall be indicated.Article 18 An outer pharmaceutical label shall cover items such as generic name, ingredients, description, indications or functions, strength, dosage and administration, adverse reactions, contraindications, precautions, storage, manufacture date, product lot number, shelf life, approval document number, manufacturer, etc. of the drug. Where all information of indications or functions, dosage and administration, adverse reactions, contraindications and precautions cannot be specified, primary content of these items shall be given and “See Direction for details” shall be indicated.Article 19 The label for shipment and storage packages shall cover at least generic name, strength, storage, manufacture date, product lot number, shelf life, approval document number and manufacturer of the drug and, as necessary, may also include quantity in the package, cautions for shipment or other signs.Article 20 Labels of drug substances shall indicate drug name, storage, manufacture date, product lot number, shelf life, executive standard, approval document number and manufacturer of the drug and also necessary items such as quantity in the package, precautions for shipment, etc. Article 21 As regards the same drug produced by the same pharmaceutical manufacturer, content, format and color of labels shall be consistent for the same drug strength and package strength, and labels shall differ notably or the strength shall be notably indicated for different drug strengths or package strengths.As regards the same drug produced by the same manufacturer, package colors shall notably differ for separate administration as a prescribed drug and an OTC drug.Article 22 Special storage requirements of drugs, if any, shall be indicated at an eye-catching position of the labels.Article 23 The shelf life in pharmaceutical labels shall be indicated in a year-month-day format, where the year shall be expressed with four figures; the month and day shall be expressed with two figures respectively. The indication format is “valid to XXXX year XX month” or valid to “XXX year XX month XX day”. Expressions using figures and other symbols such as “valid to XXXX.XX.” or “valid to XXXX/XX/XX” also apply.Indication of shelf life of prophylactic biological products shall be implemented in accordance with registration specifications approved by the State Food and Drug Administration. Indication of shelf life of curative biological products shall be calculated as of the date of repackaging, and indication of shelf life of other drugs shall be calculated as of the manufacture date.Shelf life indicated to day shall be the day before the corresponding year-month-day of the calculation starting date; shelf life indicated to month shall be the month before the corresponding year-month of the calculation starting month.Chapter IV Use of Pharmaceutical Names and Registered TrademarksArticle 24 Pharmaceutical names indicated in pharmaceutical directions and labels shall comply with nomenclatures of generic names and trade names of drugs published by the State Food and Drug Administration and be consistent with corresponding content in certificates of approval of drugs.Article 25 Generic names of drugs shall be notable and eye-catching; characters shall be in the same style, size and color and meet the following the requirements:(I) Generic names shall be indicated at a notable position in the upper 1/3 of labels in cross layout or in the right 1/3 of labels in vertical layout;(II) Character styles difficult to identify, e.g. cursive script, seal script, etc. shall not be used; characters shall not be modified into italic, hollow or shadowed forms;(III) Characters shall be black or white with intense contrast against the corresponding light or dark background.(IV) The generic name shall not be indicated in multiple lines unless restricted by the package size.Article 26 Trade name and generic name of a drug shall not be indicated in the same line; the character size and color of the trade name shall not be more notable and eye-catching than those of the generic name. Calculated by area of single characters, the character size shall not be half larger than character size of the generic name.Article 27 Unregistered trademarks and other pharmaceutical names having not been approved by the State Food and Drug Administration shall not be used in pharmaceutical directions or labels. Registered trademarks, if used in pharmaceutical labels, shall be printed in corners of the pharmaceutical labels. Where words are contained in the registered trademark, the character size shall not be 1/4 larger than character size of the generic name.。
